Abstract
Objective
Milk holds an anti-inflammatory response that is particularly important to protecting infants against necrotizing enterocolitis. Milk might also exert anti-inflammatory effects in adulthood, including the oral cavity where macrophages of the oral mucosal control innate immunity defense. It remains unknown, however, whether milk can modulate the local inflammatory response by affecting the polarization of macrophages.
Material and Methods
To determine whether pasteurized human milk and pasteurized cow milk can provoke macrophage polarization, murine bone marrow macrophages and RAW264.7 cells were exposed to human saliva or the inflammatory cytokines IL1β and TNFα. Activation of pro-(M1) inflammatory response is indicated by the expression of IL1 and IL8. To determine polarization towards a M2 phenotype, the expression of arginase 1 (ARG1) and chitinase-like 3 (Chil3) was determined by reverse transcriptase PCR and immunoassay. Western blot was done on phosphorylated p38 and JNK.
Results
Aqueous fractions of human milk and cow milk from different donors, respectively, significantly decreased the inflammatory response of primary macrophages and RAW264.7 cells when exposed to saliva or IL1 and TNFα. Similar to IL4, human milk and cow milk caused a robust expression of ARG1 and Chil3 in primary macrophages. The polarization of macrophages by pasteurized milk occurred independent of the phosphorylation of p38 and JNK.
Conclusion
These data suggest that pasteurized milk, independent of the origin, can cause the polarization of macrophages from a pro-inflammatory M1 towards a pro-resolving M2 phenotype. Thus, milk might have a protective role for the oral cavity by modulation of the macrophage-based innate immune system.
Keywords: Oral, Mucositis, Milk, Inflammation, Macrophages, Polarisation
1. Introduction
The oral mucosa is a barrier that is permanently exposed to the commensal microbiom but also dietary and airborne antigens [1]. Apart from the mechanical barrier of the oral mucosa, local immunity including resident and recruited monocytes and macrophages is required for tissue homeostasis [2]. It is particular the thin junctional epithelium at the basis of the gingival sulcus that is constantly exposed to the local triggers keeping innate immunity activated [3]. However, this immune response can shift from a physiological towards a pathological situation, the latter indicated as gingivitis, which can progress into the irreversible periodontitis [4], or peri-implantitis [5]. Moreover, patients with systemic chronic inflammatory diseases, including inflammatory bowel diseases have oral manifestations [6]. In addition, oral ulcerating mucositis is a severe complication of chemotherapy and radiation therapy in oncologic patients, raising the need for interventions based on foods and natural products [7] and administration of radioprotectors and/or chemoprotectors [8]. These interventions might target macrophages.
Considering macrophages are gatekeepers of innate immunity, they are highly abundant in the oral mucosa. For example, the majority of antigen-presenting cells in gingiva include resident macrophages and migratory monocytes [2]. Moreover, macrophages are pleiotropic cells that can undergo polarization changes [9]. Macrophage polarization is of critical importance as the traditional inflammatory response upon activation of pattern recognition receptors. For example, endotoxins in saliva [10], or activation by inflammatory cytokines including IL1β and TNFα causes a M1 polarization, while other molecules such as IL4 increase markers of M2 polarization such as arginase 1 (ARG1) and chitinase-like 3 (Chil3) [11]. The importance of macrophage polarization in oral tissue homeostasis is supported by preclinical research suggesting that manipulation of endogenous M2 macrophages can prevent alveolar bone loss in periodontitis models [12]. There is a demand for interventions causing a polarization shift of M1 towards M2 macrophages.
Milk being a hallmark of mammalian evolution is a rich source of nutrients but also has the capacity to control inflammation. For example, milk can reduce necrotizing enterocolitis in newborns [13], [14]. In preclinical studies, milk reduces tissue injury and mortality in chemically-induced endotoxemia models [15]. In vitro, milk can decrease TNFα-induced inflammation in intestinal epithelial cells [16]. We have recently shown that aqueous fractions of pasteurized human milk and pasteurized cow milk greatly diminished the inflammatory response to cytokines in oral fibroblasts and oral epithelial cells [17]. Moreover, human milk contains pro-resolving signals that can enhance phagocytosis [18] and milk fat globule—epidermal growth factor—factor VIII (MFGE8)/lactadherin [19] supporting pro-resolving M2 macrophages [20]. The question arises if milk can modulate macrophage polarization in vitro. The aim of the current study was to investigate the impact of aqueous fractions of pasteurized human and cow milk on in vitro polarization of mouse macrophages.
2. Material and Methods
2.1. Milk preparation
Human milk samples were collected at the Department of Paediatrics and Adolescent Medicine, Division of Neonatology, of the Medical University of Vienna. The milk of mothers was prepared in daily totals for the premature babies and delivered to the feeding stations. The milk tested in this study was leftover residual amounts that could not be refrozen. Mothers were not asked to donate milk for study purposes (Ethical committee of the Medical University of Vienna Nr. 1021/2017). Human milk was centrifuged at 20,000 g for 10 min at room temperature. The aqueous fraction was pooled and heated to 80 °C for one hour before freezing. For cow milk, three different batches of pasteurized cow milk (Billa Bergbauern Heumilch; Spar Halbfett Milch; Hofer Milfina Halbfett Milch) were centrifuged and the aqueous fractions pooled and frozen until testing. Samples were subjected to not more than two freeze-thaw cycles. In the indicated experiments, 1% of the most prevalent human milk oligosaccharide 2′-Fucosyllactose [21] (2FL; Jennewein Biotechnologie GmbH, Rheinbreitbach, Germany) was used.
2.2. Saliva preparation
Whole human saliva was collected from the authors (L.P., R.G.) who are non-smokers and gave their informed consent. Saliva flow was stimulated by chewing paraffin wax (Ivoclar Vivadent AG, Schaan, Liechtenstein) without eating and drinking for 1 h prior to collection. Immediately after collection, saliva was centrifuged at 4000 g for 5 min. The saliva supernatant was passed through a filter with a pore diameter of 0.2 µm (Diafil PS, DIA-Nielsen GmbH, Düren, Germany). The saliva from the two donors was pooled and frozen stocks were used.
2.3. Murine bone marrow-derived macrophages and RAW264.7 cells
Bone marrow cells were collected from the femora and tibiae of Balb/c mice aged 6–8 weeks old. Bone marrow cells were seeded at 3 × 106 cells/cm2 into 12-well plates and grown for 7 days in alpha Minimum Essential Medium (αMEM) supplemented with 10% fetal bovine serum, antibiotics (all Invitrogen, Grand Island, NY) and 30 ng/ml M-CSF (Prospec, Ness-Ziona, Israel). RAW264.7 macrophage-like cells (ATCC, Manassas, VA) were expanded in growth medium and seeded 1 × 106 cells/cm2 into 12-well plates under standard conditions at 37 °C, 5% CO2, and 95% humidity.
2.4. Cell viability
Primary macrophages were incubated overnight with 1 to 100% aqueous fractions of milk. MTT (3-[4,5-dimethythiazol-2-yl]-2,5-diphenyltetrazolium bromide; Sigma) solution at a final concentration of 0.5 mg/ml was added to each well of a microtiter plate (CytoOne) for 2 h at 37 °C. The medium was removed and formazan crystals were solubilized with dimethyl sulfoxide. Optical density was measured at 570 nm. Data were expressed as percentage of optical density in the treatment groups normalized to unstimulated control values.
2.5. qRT-PCR analysis and immunoassay
Primary macrophages and RAW264.7 cells were pre-exposed to 5% aqueous fractions of milk for 1 h before subjected to IL1β 5 ng/ml and TNFα 5 ng/ml, or to 5% saliva for 24 h. Primary macrophages and RAW264.7 cells were also exposed to 5% milk alone for 24 h. In indicated experiments, 1% 2FL was used instead of milk. The supernatant was harvested. Total RNA was isolated with the ExtractMe total RNA kit (Blirt S.A., Gdańsk, Poland). Reverse transcription was performed with SensiFASTTM cDNA (Bioline, London, UK). Polymerase chain reaction was done with the SensiFASTTM SYBR ROX Kit (Bioline). Amplification was monitored on the CFX Connect™ Real-Time PCR Detection System (Bio-Rad Laboratories, CA, USA). Primer sequences were mIL1β_F AAGGGCTGCTTCCAAACCTTTGAC, mIL1β_R ATACTGCCTGCCTGAAGCTCTTGT; mIL8_F GAAGCACACTTTACAATTACGTGAG, mIL8_R CTCCACCTCTTCCTTTACTGCT; m YM 1_F GGGCATACCTTTATCCTGAG, m YM 1_R CCACTGAAGTCATCCATGT; mARG1_F GAATCTGCATGGGCAACC, mARG1_R GAATCCTGGTACATCTGGGAAC. mGAPDH_F AACTTTGGCATTGTCGAACG, mGAPDH_R GGATGCAGGGATGATGTTCT; mACTIN_F CTAAGGCCAACCGTGAAAAG, mACTIN_R ACCAGAGGCATACAGGGACA. The mRNA levels were calculated by normalizing to the housekeeping gene GAPDH and actin using the ΔΔCt method. For the immunoassay, the mouse IL1β Quantikine ELISA kit was used (R&D Systems, Minneapolis, MN).
2.6. Western blot
RAW264.7 cells were serum-starved overnight and then preincubated for 10 min with 5% aqueous fractions of milk or 1% 2FL, before being exposed for 25 min to 5% saliva. Cell extracts containing SDS buffer and protease inhibitors (PhosSTOP with cOmplete; Sigma, St. Louis, MO) were separated by SDS-PAGE and transferred onto nitrocellulose membranes (Whatman, GE Healthcare, General Electric Company, Fairfield, CT). Membranes were blocked and the binding of the first antibody raised against phospho-ERK, phospho-p38 and phospho-JNK (Cell Signaling Technology, Danvers, MA) and beta-actin (Santa Cruz Biotechnology, Santa Cruz, CA) was detected with the appropriate secondary antibody linked to a peroxidase. Chemiluminescence signals were visualized with the ChemiDoc imaging system (Bio-Rad Laboratories, Inc., Hercules, CA).
2.7. Statistical analysis
All experiments were repeated at least three times. Data from individual experiments are shown as dot-blots. If not otherwise indicated, data are described as percentage inflammation remaining compared to stimulated controls. Stimulated controls are cells exposed to either saliva or IL1β/TNFα that initiated an inflammatory response. An inflammatory response is defined by a minimum of at least a 10-fold increase in the expression of IL1 and IL8. Statistical analysis was based on Wilcoxon matched-pairs signed rank test with p < 0.05 and the Prism 7.0e software (GraphPad Software; San Diego, CA).
3. Results
3.1. Milk suppress saliva-induced inflammation in primary macrophages and RAW264.7 cells
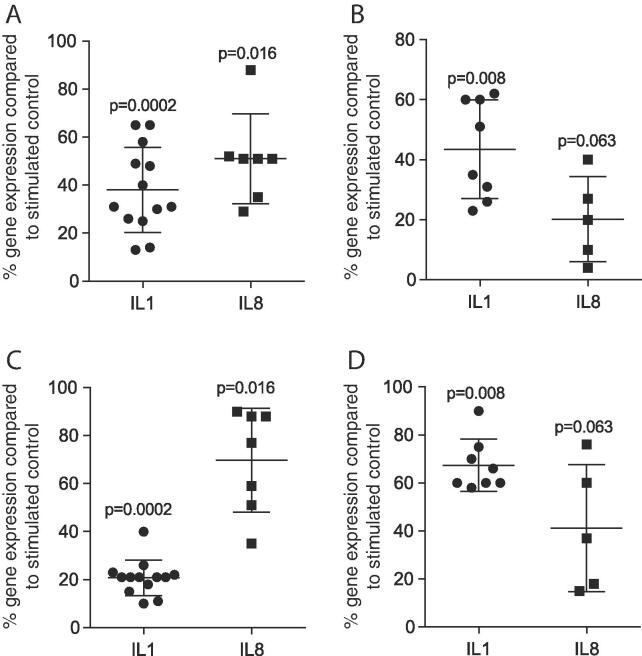
Based on our recent results that saliva induced an inflammation in macrophages [10] and our observations that milk reduced the inflammatory response on oral fibroblasts and epithelial cells [17], we now investigate the possible anti-inflammatory activity of milk in macrophages. Results suggest that aqueous fractions of pasteurized human milk reduced the saliva-induced inflammatory response of IL1 and IL8 to mean value of 38% (p = 0.0002) and 51% (p = 0.016) – and 43% (p = 0.008) and 20% (p = 0.063) of the respective controls, in primary macrophages and RAW264.7 cells, respectively (Fig. 1A, B). Pasteurized cow milk also reduced IL1 and IL8 expression to 21% (p = 0.0002) and 70% (p = 0.016) of the particular saliva groups in primary macrophages (Fig. 1C) and to 67% (p = 0.0008) and 41% (p = 0.063) in RAW264.7 cells (Fig. 1D). In support of this finding, 5% human milk reduced the protein levels of saliva-induced IL1β in the supernatant of primary macrophages to a mean of 65% (Table 1) and RAW264.7 cells to 33% (Table 2), respectively.
Fig. 1.
Milk suppress saliva-induced inflammation in primary macrophages and RAW264.7 cells. Murine bone marrow-derived macrophages (A, C) and RAW264.7 cells (B, D) were exposed to 5% saliva with and without 5% aqueous fractions of pasteurized human (HM; A, B) and cow milk (CM; C, D). Expression of inflammatory genes is indicated in percentage (%) compared to stimulated controls (100%). Dot-blots represent independent experiments. p-values are based on a Wilcoxon matched-pairs signed rank test.
Table 1.
Human milk suppresses saliva-induced IL1β in primary macrophages. Cells were exposed to 5% saliva (SAL) with and without 5% of aqueous fractions of pasteurized human milk (HM). Production of IL1β is expressed in pg/ml. Data of two independent experiments (EXP) are shown.
| wo | SAL | HM + SAL | |
|---|---|---|---|
| EXP1 | 16.9 | 708.4 | 429.3 |
| EXP2 | 14.7 | 714.4 | 492.8 |
Table 2.
Human milk suppresses saliva-induced IL1β in RAW264.7 cells. Cells were exposed to 5% saliva (SAL) with and without 5% of aqueous fractions of pasteurized human milk (HM). Production of IL1β is expressed in pg/ml. Data of tree independent experiments (EXP) are shown.
| wo | SAL | HM + SAL | |
|---|---|---|---|
| EXP1 | 123.5 | 1675.9 | 485.3 |
| EXP2 | 160.9 | 1465.9 | 384.0 |
| EXP3 | 81.1 | 780.2 | 416.2 |
3.2. Milk but not 2FL suppress IL1β-TNFα-induced inflammation in primary macrophages and RAW264.7 cells
To confirm if the anti-inflammatory activity of milk is independent of saliva, cells were exposed to IL1β-TNFα. As indicated in Fig. 2A, aqueous fractions of pasteurized human milk reduced the IL1β-TNFα-induced inflammatory reaction of IL1 and IL8 to 55% (p < 0.0001) and 42% (p = 0.063) of the respective controls, respectively in primary macrophages, and to 42% (p = 0.004) and 57% (p = 0.063) in RAW264.7 cells (Fig. 2B). Correspondingly, cow milk decreased the inflammatory answer of IL1 and IL8 to 43% (p < 0.0001) and 63% (p = 0.063) in primary macrophages (Fig. 2C), and to 53% (p = 0.004) and 64% (p = 0.063) in RAW264.7 cells (Fig. 2D). Dose-response experiments indicated that even 0.05% pasteurized human milk has an anti-inflammatory in our in vitro model (Table 3, Table 4). The milk oligosaccharide 2FL, however, failed to reduce saliva or IL1β-TNFα-induced IL1 and IL8 expression in primary macrophages and RAW264.7 cells (Fig. 3A–D). Taken together, pasteurized milk can suppress the M1 inflammatory response in macrophages.
Fig. 2.
Milk suppress IL1β-TNFα-induced inflammation in primary macrophages and RAW264.7 cells. Murine bone marrow-derived macrophages (A, C) and RAW264.7 cells (B, D) were exposed to IL1β-TNFα with and without 5% aqueous fractions of pasteurized human (HM; A, B) and cow milk (CM; C, D). Expression of inflammatory genes is indicated in percentage (%) compared to stimulated controls (100%). Dot-blots represent independent experiments. p-values are based on a Wilcoxon matched-pairs signed rank test.
Table 3.
Dose-response of human milk to suppress saliva- and cytokine-induced IL1β in RAW264.7 cells. Cells were exposed to 5% saliva (SAL) or IL1β-TNFα with and without various concentrations of aqueous fractions of pasteurized human milk (HM). Expression of IL1β is indicated in x-fold change of stimulated controls. Data of at least three independent experiments are indicated as mean and standard deviation.
| HM 5% | HM 0.5% | HM 0.05% | |
|---|---|---|---|
| SAL | 42.0 ± 22.2 | 86.3 ± 17.2 | 64.2 ± 33.3 |
| IL1β-TNFα | 19.0 ± 11.8 | 39.7 ± 23.3 | 53.8 ± 25.5 |
Table 4.
Dose-response of human milk to suppress saliva- and cytokine-induced IL8 in RAW264.7 cells. Cells were exposed to 5% saliva (SAL) or IL1β-TNFα with and without various concentrations of aqueous fractions of pasteurized human milk (HM). Expression of IL8 is indicated in x-fold change of stimulated controls. Data of at least three independent experiments are indicated as mean and standard deviation.
| HM 5% | HM 0.5% | HM 0.05% | |
|---|---|---|---|
| SAL | 13.0 ± 2.2 | 26.3 ± 12.3 | 20.5 ± 12.5 |
| IL1β-TNFα | 28.5 ± 18.3 | 20.3 ± 3.7 | 26.0 ± 6.0 |
Fig. 3.
2FL does not suppress saliva or IL1β-TNFα-induced inflammation in primary macrophages and RAW264.7 cells. Murine bone marrow-derived macrophages and RAW264.7 cells were exposed to (A) 5% saliva or (B) IL1β-TNFα with and without 1% 2FL. Expression of IL1 is indicated in x-fold change of stimulated controls. Dot-blots represent independent experiments. p-values are based on a Wilcoxon matched-pairs signed rank test.
3.3. Milk does not modulate saliva-induced phosphorylation of p38 and JNK in RAW264.7 cells
Considering that saliva-induced inflammation causes the phosphorylation of p38 and JNK in RAW264.7 cells [10], both being central mediators of inflammatory signal transduction [22], questions arise if milk can lower the respective phosphorylation signals. Western blot analysis supported the expected increase in the phosphorylation of p38 and JNK in RAW264.7 cells by saliva; milk, however, failed to cause considerable changes (Fig. 4). Saliva only moderately increased phosphorylation of ERK being substantially increased by human and cow milk (Fig. 4). 2FL failed to change phosphorylation of p38 but considerably increased pJNK (Fig. 3E). Thus, the anti-inflammatory activity of milk is not caused by the lowering of p38 and JNK phosphorylation but possibly involves activation of ERK signaling in RAW264.7 cells.
Fig. 4.
Milk but not 2FL modulate saliva-induced phosphorylation of p38 and JNK in RAW264.7 cells. Serum-starved RAW264.7 cells were exposed to (A) 5% aqueous fractions of pasteurized human (HM) and cow milk (CM) or (B) 2FL for 10 min before being exposed to saliva for 25 min. Targets were detected by antibodies raised against phospho-ERK (pERK), phosphor-p38 (pp38) and phospho-JNK (pJNK).
3.4. Milk increases ARG1 and Chil3 in primary macrophages and RAW264.7 cells
The reduction of the saliva- and IL1β-TNFα-induced M1 polarization raises the question if milk can shift the macrophages towards a M2 phenotype. Fig. 5A denotes that in primary macrophages, IL4 but also pasteurized human and cow’s milk increased ARG1 to a mean of 66-fold, 21-fold, and 20-fold (all p = 0.063), respectively. Also in RAW264.7 cells (Fig. 5B), IL4, human and cow’s milk increased ARG1 to 15-fold, 15-fold, and 16-fold (all p = 0.016). When focusing on Chil3 in primary macrophages (Fig. 5C), IL4 pasteurized human and cow’s milk all increased gene expression to a mean of 57-fold, 21-fold, and 16-fold (all p = 0.016), respectively. Finally, Chil3 expression in RAW264.7 cells was increased by IL4, human and cow’s milk to 32-fold, 16-fold, and 26-fold (all p = 0.063) (Fig. 5D). The increase of ARG1 by human milk and cow milk was confirmed by Western blot analysis (Fig. 5E). These data suggest that milk can provoke a shift of macrophages towards the M2 lineage.
Fig. 5.
Milk increases ARG1 and Chil3 in primary macrophages and RAW264.7 cells. Murine bone marrow-derived macrophages (A, C) and RAW264.7 cells (B, D) were exposed to IL4, 5% aqueous fractions of pasteurized human (HM), and cow milk (CM). Expression of M2 marker genes is indicated in x-fold change of unstimulated control. Dot-blots represent independent experiments. p-values are based on a Wilcoxon matched-pairs signed rank test. (E) Increased arginase 1 in RAW264.7 cells was confirmed by Western blot.
3.5. Milk at 10% does not affect the viability of in primary macrophages and RAW264.7 cells
To rule out that milk affects the viability of macrophages, the NAD(P)H-dependent cellular oxidoreductase enzymes was determined. Results suggest that aqueous fractions of undiluted milk caused a significant reduction in cell viability, whereas 10% aqueous fractions of pasteurized human or cow milk had no impact on the formazan formation in primary macrophages (Fig. 6A, B) and RAW264.7 cells (Fig. 6C, D). These findings support the use of 5% of aqueous fractions of pasteurized human or cow milk for the experiments on macrophage polarization.
Fig. 6.
Milk at 10% does not affect the viability of in primary macrophages and RAW264.7 cells. Murine bone marrow-derived macrophages (A, B) and RAW264.7 cells (C, D) were incubated overnight with 1 to 100% pasteurized human (A, C; HM), and cow milk (B, D; CM). MTT conversion into solubilized formazan crystals was determined on a photometer. Mean values from at least four independent experiments are expressed as percentage of optical density in the treatment groups normalized to unstimulated control values.
4. Discussion
Uncontrolled inflammation can lead to irreversible periodontitis [4] and peri-implantitis [5], and it is particular the oral mucositis, being a side effect of chemotherapy and radiation therapy in oncologic patients, that dramatically reduces the quality of life [7], [8]. Considering that macrophages are central to each inflammation, it is reasonable to assume that macrophage polarization plays a key role in the pathogenesis of oral inflammation. For example, there is a role played by accumulating monocytes in ascorbic acid-induced gastrointestinal mucositis [23]. Moreover, no mouth rinses and hydrogels target the acute oral inflammation caused by chemotherapy or radiation therapy [24], [25]. Thus, there is a great demand for easily available mouth rinses with an anti-inflammatory activity with the overall goal to reduce painful symptoms of oral mucositis. With respect to milk-based products, topical application of whey growth factor extract-A reduced chemotherapy-induced oral mucosal ulceration in hamsters [26] but kefir consumption had no impact on the incidence of mucositis development in cancer patients [27]. Thus, today’s evidence for a beneficial role of milk in oral mucositis is scarce.
Based on our previous observation that aqueous fractions of pasteurized human milk and pasteurized cow's milk can lower the expression of inflammatory cytokines in gingival fibroblasts and the oral epithelial cell line HSC2 [17], and milk can provoke a TLR-4 dependent M1 shift of macrophages in vitro [10], we report here that human and cow's milk not only decreased the inflammatory response of primary macrophages but also RAW264.7 cells when exposed to saliva or IL1 and TNFα. Milk also caused a strong increase in the expression of ARG1 and Chil3 in primary macrophages and RAW264.7 cells, suggesting cellular polarization towards a M2 phenotype. These findings support previous observations that human milk lipid mediators support inflammation resolution in a peritonitis model and enhance human macrophage efferocytosis in vitro [18]. Moreover, milk is rich in MFGE8 supporting M2 polarization [20]. It remains, however, unclear if milk lipid mediator, MFGE8 or other molecules cause the effects we have observed.
The data presented here and our previous approach with oral fibroblasts and epithelial cells [28] suggest that milk holds the potential to diminish inflammation. What remains unclear is how milk can penetrate the epithelial barrier to reach the macrophage population in vivo. Considering that the inflammation impairs the integrity of the epithelial barrier, milk might penetrate into deeper layers of the oral mucosa and target the macrophage population. On the other hand, mounting inflammatory response to foreign antigens is crucial for oral mucosal innate immunity [1] and the question arises how such subdued acute immunity caused by milk can be beneficial? At least in theory, milk could reduce the oral mucosal immune defense making the oral cavity less susceptive to a proper innate response to foreign factors. Thus, the overall question if macrophage polarization in response to milk under healthy conditions supports or even reduces oral mucosal innate immunity remains unknown.
Future research should focus on the role of macrophages in pathological chemotherapy and radiation-induced mucositis in rodent models [29]. Based on this preclinical evidence, future research is needed to determine whether patients suffering from severe forms of oral mucositis benefit from rinsing the mouth with milk. This a critical issue as there is the risk that, even though in vitro milk reduces the M1 inflammatory response of macrophages and pushes the M2 phenotype, milk might have unwanted side effects that cannot be simulated in vitro. For example, detrimental effects from topical administration of bovine lactoferrin to the wounded oral mucosa of immunocompromised hamsters were reported [30]. Thus, the clinical relevance of the present investigation needs to be evaluated considering the complexity of the living organism and the pathological changes of oral mucositis.
There are a number of study limitations. First, it is unclear what molecular mechanisms are responsible for the macrophage polarization; particular if the same anti-inflammatory cues also cause the M2 shift of macrophages. We have not ruled out so far that it is the lipid mediators [18] or MFGE8 [20] being responsible for the observations presented here. What we can rule out however, is the involvement of the main milk oligosaccharide 2FL, which has an anti-inflammatory activity in human enterocytes [31], but failed to inhibit the inflammatory response of macrophages to saliva. Second, as already stated, it remains to be determined if the robust milk-induced macrophage polarization can be translated in vivo reaching macrophages in oral mucositis models. Finally, we have a xenogeneic model based on mouse macrophages and cow and human milk, respectively. Further research is needed to understand whether the inflammatory response to saliva can be reduced by human milk.
In conclusion, our research suggests that in vitro pasteurized milk, independent of the origin, can support the polarization of macrophages from a pro-inflammatory M1 towards a pro-resolving M2 phenotype. This research provides a scientific basis to further evaluate the possible beneficial effect of milk serving as a mouth rinse to ease the symptoms in oral mucositis or even reduce the oral mucosal immune defense.
Acknowledgments
Acknowledgements
Authors thank Martina Wiederstein and Gabriele Haar for technical assistance. Authors declare no conflicts of interest. Layla Panahipour and Reinhard Gruber were supported by a grant from the Osteology Foundation.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
References
- 1.Moutsopoulos N.M., Konkel J.E. Tissue-specific immunity at the oral mucosal barrier. Trends Immunol. 2018;39(4):276–287. doi: 10.1016/j.it.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dutzan N., Konkel J.E., Greenwell-Wild T., Moutsopoulos N.M. Characterization of the human immune cell network at the gingival barrier. Mucosal Immunol. 2016;9(5):1163–1172. doi: 10.1038/mi.2015.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delima A.J., Van Dyke T.E. Origin and function of the cellular components in gingival crevice fluid. Periodontology. 2003;2000(31):55–76. doi: 10.1034/j.1600-0757.2003.03105.x. [DOI] [PubMed] [Google Scholar]
- 4.Darveau R.P. Periodontitis: a polymicrobial disruption of host homeostasis. Nat. Rev. Microbiol. 2010;8(7):481–490. doi: 10.1038/nrmicro2337. [DOI] [PubMed] [Google Scholar]
- 5.Berglundh T., Zitzmann N.U., Donati M. Are peri-implantitis lesions different from periodontitis lesions? J. Clin. Periodontol. 2011;38(Suppl 11):188–202. doi: 10.1111/j.1600-051X.2010.01672.x. [DOI] [PubMed] [Google Scholar]
- 6.Mortada I., Leone A., Gerges Geagea A., Mortada R., Matar C., Rizzo M., Hajj Hussein I., Massaad-Massade L., Jurjus A. Oral manifestations of inflammatory bowel disease. J. Biol. Regul. Homeost Agents. 2017;31(3):817–821. [PubMed] [Google Scholar]
- 7.Thomsen M., Vitetta L. Adjunctive treatments for the prevention of chemotherapy- and radiotherapy-induced mucositis. Integr. Cancer Ther. 2018;17(4):1027–1047. doi: 10.1177/1534735418794885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oronsky B., Goyal S., Kim M.M., Cabrales P., Lybeck M., Caroen S., Oronsky N., Burbano E., Carter C., Oronsky A. A review of clinical radioprotection and chemoprotection for oral mucositis. Transl. Oncol. 2018;11(3):771–778. doi: 10.1016/j.tranon.2018.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garlet G.P., Giannobile W.V. Macrophages: the bridge between inflammation resolution and tissue repair? J. Dent. Res. 2018;97(10):1079–1081. doi: 10.1177/0022034518785857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pourgonabadi S., Muller H.D., Mendes J.R., Gruber R. Saliva initiates the formation of pro-inflammatory macrophages in vitro. Arch. Oral Biol. 2017;73:295–301. doi: 10.1016/j.archoralbio.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 11.Jablonski K.A., Amici S.A., Webb L.M., Ruiz-Rosado Jde D., Popovich P.G., Partida-Sanchez S., Guerau-de-Arellano M. Novel markers to delineate murine M1 and M2 Macrophages. PLoS One. 2015;10(12):e0145342. doi: 10.1371/journal.pone.0145342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhuang Z., Yoshizawa-Smith S., Glowacki A., Maltos K., Pacheco C., Shehabeldin M., Mulkeen M., Myers N., Chong R., Verdelis K., Garlet G.P., Little S., Sfeir C. Induction of M2 Macrophages Prevents Bone Loss in Murine Periodontitis Models. J. Dent. Res. 2018;22034518805984 doi: 10.1177/0022034518805984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sisk P.M., Lovelady C.A., Dillard R.G., Gruber K.J., O'Shea T.M. Early human milk feeding is associated with a lower risk of necrotizing enterocolitis in very low birth weight infants. J. Perinatol. 2007;27(7):428–433. doi: 10.1038/sj.jp.7211758. [DOI] [PubMed] [Google Scholar]
- 14.Namachivayam K., Blanco C.L., Frost B.L., Reeves A.A., Jagadeeswaran R., MohanKumar K., Safarulla A., Mandal P., Garzon S.A., Raj J.U., Maheshwari A. Preterm human milk contains a large pool of latent TGF-beta, which can be activated by exogenous neuraminidase. Am. J. Physiol. Gastrointest. Liver Physiol. 2013;304(12):G1055–G1065. doi: 10.1152/ajpgi.00039.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ozawa T., Miyata M., Nishimura M., Ando T., Ouyang Y., Ohba T., Shimokawa N., Ohnuma Y., Katoh R., Ogawa H., Nakao A. Transforming growth factor-beta activity in commercially available pasteurized cow milk provides protection against inflammation in mice. J. Nutr. 2009;139(1):69–75. doi: 10.3945/jn.108.092528. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y., Patel A., Meier P.P., Fantuzzi G. Digested early preterm human milk suppresses tumor necrosis factor-induced inflammation and cytotoxicity in intestinal epithelial cells. J. Pediatr. Gastroenterol. Nutr. 2018 doi: 10.1097/MPG.0000000000001932. [DOI] [PubMed] [Google Scholar]
- 17.Panahipour L., Nasserzare S., Amer Z., Brucke F., Stahli A., Kreissl A., Haiden N., Gruber R. The anti-inflammatory effect of milk and dairy products on periodontal cells: an in vitro approach. Clinical Oral Investigations. 2018 doi: 10.1007/s00784-018-2642-4. [DOI] [PubMed] [Google Scholar]
- 18.Arnardottir H., Orr S.K., Dalli J., Serhan C.N. Human milk proresolving mediators stimulate resolution of acute inflammation. Mucosal Immunol. 2016;9(3):757–766. doi: 10.1038/mi.2015.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor M.R., Couto J.R., Scallan C.D., Ceriani R.L., Peterson J.A. Lactadherin (formerly BA46), a membrane-associated glycoprotein expressed in human milk and breast carcinomas, promotes Arg-Gly-Asp (RGD)-dependent cell adhesion. DNA Cell Biol. 1997;16(7):861–869. doi: 10.1089/dna.1997.16.861. [DOI] [PubMed] [Google Scholar]
- 20.Laplante P., Brillant-Marquis F., Brissette M.J., Joannette-Pilon B., Cayrol R., Kokta V., Cailhier J.F. MFG-E8 reprogramming of macrophages promotes wound healing by increased bFGF production and fibroblast functions. J. Invest. Dermatol. 2017;137(9):2005–2013. doi: 10.1016/j.jid.2017.04.030. [DOI] [PubMed] [Google Scholar]
- 21.Bode L. Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology. 2012;22(9):1147–1162. doi: 10.1093/glycob/cws074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arthur J.S., Ley S.C. Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 2013;13(9):679–692. doi: 10.1038/nri3495. [DOI] [PubMed] [Google Scholar]
- 23.Takaba J., Mishima Y., Hatake K., Kasahara T. Role of bone marrow-derived monocytes/macrophages in the repair of mucosal damage caused by irradiation and/or anticancer drugs in colitis model. Mediators Inflamm. 2010;2010:634145. doi: 10.1155/2010/634145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hashemi A., Bahrololoumi Z., Khaksar Y., Saffarzadeh N., Neamatzade H., Foroughi E. Mouth-rinses for the prevention of chemotherapy induced oral mucositis in children: a systematic review. Iran J. Ped. Hematol. Oncol. 2015;5(2):106–112. [PMC free article] [PubMed] [Google Scholar]
- 25.McGuire D.B., Fulton J.S., Park J., Brown C.G., Correa M.E., Eilers J., Elad S., Gibson F., Oberle-Edwards L.K., Bowen J., Lalla R.V., Mucositis Study O. Group of the multinational association of supportive care in cancer/international society of oral, systematic review of basic oral care for the management of oral mucositis in cancer patients. Support Care Cancer. 2013;21(11):3165–3177. doi: 10.1007/s00520-013-1942-0. [DOI] [PubMed] [Google Scholar]
- 26.Clarke J., Butler R., Howarth G., Read L., Regester G. Exposure of oral mucosa to bioactive milk factors reduces severity of chemotherapy-induced mucositis in the hamster. Oral Oncol. 2002;38(5):478–485. doi: 10.1016/s1368-8375(01)00107-5. [DOI] [PubMed] [Google Scholar]
- 27.Topuz E., Derin D., Can G., Kurklu E., Cinar S., Aykan F., Cevikbas A., Disci R., Durna Z., Sakar B., Saglam S., Tanyeri H., Deniz G., Gurer U., Tas F., Guney N., Aydiner A. Effect of oral administration of kefir on serum proinflammatory cytokines on 5-FU induced oral mucositis in patients with colorectal cancer. Invest New Drugs. 2008;26(6):567–572. doi: 10.1007/s10637-008-9171-y. [DOI] [PubMed] [Google Scholar]
- 28.Panahipour L., Nasserzare S., Amer Z., Brucke F., Stahli A., Kreissl A., Haiden N., Gruber R. The anti-inflammatory effect of milk and dairy products on periodontal cells: an in vitro approach. Clinic. Oral Investigations. 2019;23(4):1959–1966. doi: 10.1007/s00784-018-2642-4. [DOI] [PubMed] [Google Scholar]
- 29.Gruber S., Dorr W. Tissue reactions to ionizing radiation-Oral mucosa. Mutat. Res. 2016;770(Pt B):292–298. doi: 10.1016/j.mrrev.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Clarke J., Edwards B., Srpek L., Regester G. Evaluation of bovine lactoferrin as a topical therapy for chemotherapy-induced mucositis in the golden Syrian hamster. Oral Oncol. 1999;35(2):197–202. doi: 10.1016/s1368-8375(98)00087-6. [DOI] [PubMed] [Google Scholar]
- 31.He Y., Liu S., Kling D.E., Leone S., Lawlor N.T., Huang Y., Feinberg S.B., Hill D.R., Newburg D.S. The human milk oligosaccharide 2'-fucosyllactose modulates CD14 expression in human enterocytes, thereby attenuating LPS-induced inflammation. Gut. 2016;65(1):33–46. doi: 10.1136/gutjnl-2014-307544. [DOI] [PubMed] [Google Scholar]