Highlights
-
•
Macrophages serve as replicative niche, antimicrobial effectors or immunoregulators.
-
•
Functional diversity results from cytokines, micromilieu factors and metabolites.
-
•
Micro milieu factors include hypoxia, tonicity and amino acid availability.
-
•
Leishmania reprogram the transcription, translation and metabolism of macrophages.
Keywords: Macrophages, Leishmaniasis, Interferon-γ, Interferon-α/β, Nitric oxide, Arginase, Phagocyte NADPH oxidase, Hypoxia, Tonicity, Metabolism
Abbreviations: AHR, aryl hydrocarbon receptor; AMP, antimicrobial peptide; Arg, arginase; CAMP, cathelicidin-type antimicrobial peptide; CR, complement receptor; DC, dendritic cells; DCL, diffuse cutaneous leishmaniasis; IDO, indoleamine-2,3-dioxygenase; IFN, interferon; IFNAR, type I IFN (IFN-α/β) receptor; IL, interleukin; HO-1, heme oxygenase 1; JAK, Janus kinase; (L)CL, (localized) cutaneous leishmaniasis; LPG, lipophosphoglycan; LRV1, Leishmania RNA virus 1; mTOR, mammalian/mechanistic target of rapamycin; NCX1, Na+/Ca2+ exchanger 1; NFAT5, nuclear factor of activated T cells 5; NK cell, natural killer cell; NOS2 (iNOS), type 2 (or inducible) nitric oxide synthase; NO, nitric oxide; NOX2, NADPH oxidase 2 (gp91 or cytochrome b558 β-subunit of Phox); OXPHOS, mitochondrial oxidative phosphorylation; Phox, phagocyte NADPH oxidase; PKDL, post kala-azar dermal leishmaniasis; RNS, reactive nitrogen species; ROS, reactive oxygen species; SOCS, suppressor of cytokine signaling; STAT, signal transducer and activator of transcription; TGF-β, transforming growth factor-beta; Th1 (Th2), type 1 (type2) T helper cell; TLR, toll-like receptor; VL, visceral leishmaniasis
Abstract
Leishmania are protozoan parasites that predominantly reside in myeloid cells within their mammalian hosts. Monocytes and macrophages play a central role in the pathogenesis of all forms of leishmaniasis, including cutaneous and visceral leishmaniasis. The present review will highlight the diverse roles of macrophages in leishmaniasis as initial replicative niche, antimicrobial effectors, immunoregulators and as safe hideaway for parasites persisting after clinical cure. These multiplex activities are either ascribed to defined subpopulations of macrophages (e.g., Ly6ChighCCR2+ inflammatory monocytes/monocyte-derived dendritic cells) or result from different activation statuses of tissue macrophages (e.g., macrophages carrying markers of of classical [M1] or alternative activation [M2]). The latter are shaped by immune- and stromal cell-derived cytokines (e.g., IFN-γ, IL-4, IL-10, TGF-β), micro milieu factors (e.g., hypoxia, tonicity, amino acid availability), host cell-derived enzymes, secretory products and metabolites (e.g., heme oxygenase-1, arginase 1, indoleamine 2,3-dioxygenase, NOS2/NO, NOX2/ROS, lipids) as well as by parasite products (e.g., leishmanolysin/gp63, lipophosphoglycan). Exciting avenues of current research address the transcriptional, epigenetic and translational reprogramming of macrophages in a Leishmania species- and tissue context-dependent manner.
1. Introduction
Leishmania are protozoan parasites that are transmitted as flagellated, infective (so-called metacyclic) promastigotes to mammalian organisms by the bites of sand flies. Within the mammalian host, the promastigotes are rapidly taken up by different types of myeloid cells, notably neutrophils, macrophages and dendritic cells, in which the parasites transform into a non-flagellated, amastigote form. Depending on the species, strain and inoculated dose of Leishmania, the site of infection as well as on the genetic background, immune and health status, age and sex of the host, the transferred parasites will either lead to an asymptomatic or symptomatic infection. The latter can manifest itself as a localized, frequently self-healing skin lesion (localized cutaneous leishmaniasis [LCL]) or as a systemic, non-healing disease (mucocutaneous leishmaniasis, diffuse cutaneous leishmaniasis [DCL], disseminated cutaneous leishmaniasis, visceral leishmaniasis [kala azar, VL], post-kala azar dermal leishmaniasis [PKDL]) [1], [2], [3].
Amongst the myeloid host cells for Leishmania parasites, macrophages undoubtedly play a paramount role, both from a historical as well as from a clinical, diagnostic and immunological perspective. The description of the close alliance between macrophages and Leishmania dates back to the early work of the Russian borne military doctor Peter Borovsky (1863–1932) from Taschkent, who in 1898 provided the first histological account on the pathogenesis of cutaneous leishmaniasis (CL; termed “sart sore” in his country). He not only correctly classified the underlying infectious agent as a protozoan parasite, but also recognized and graphically illustrated its size (on average 1.5 to 2 μm) and localization within host cells, which he described as “lymphoid and epithelioid cells” as he presumably was unaware of Metschnikoẃs characterization of macrophages [4]. The microscopical detection of oval-shaped Leishmania amastigotes (with the typical disc-formed kinetoplast adjacent to the flagellar basal body) within tissue macrophages (“histiocytes”) of cutaneous, splenic, hepatic or bone marrow biopsies is still a central pillar of the microbiological diagnosis of both CL and VL.
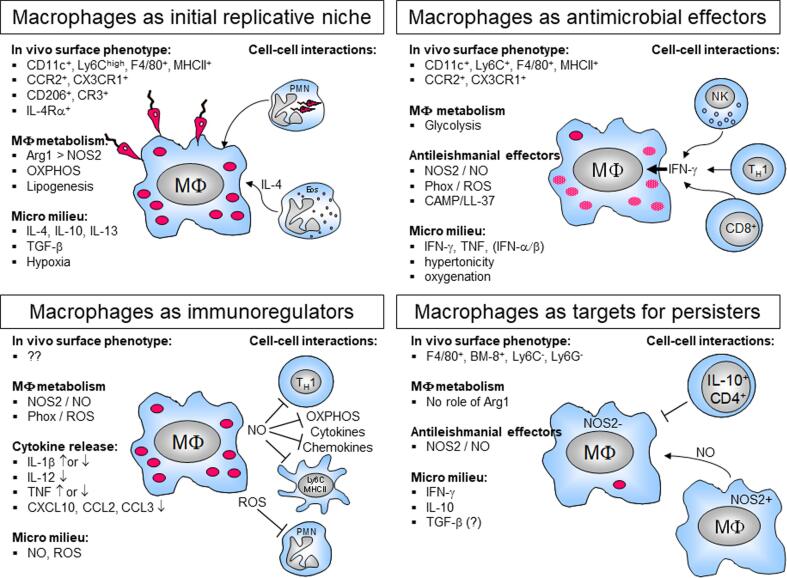
From an immunological point of view, macrophages proved to be much more than simple target cells for Leishmania parasites. In leishmaniasis, macrophages not only function as a replicative niche during the acute phase of infection, but also operate as anti-leishmanial effector cells, as immunoregulators and as permissive host cells for long-term survival of persistent parasites (Fig. 1). These diverse activities are carried out by defined subpopulations of macrophages or result from different activation statuses of tissue macrophages that are shaped by immune and stromal cell-derived cytokines, micro milieu factors, host cell-derived metabolites as well as by parasite products.
Fig. 1.
Schematic overview of macrophage functions in cutaneous leishmaniasis. Most of the functions depicted have been established in the L. major mouse infection model of cutaneous leishmaniasis. For details see the respective sections in the text.
In the following, I will review some key findings that illustrate the phenotypic and functional heterogeneity of macrophages in leishmaniasis and also document the impact of microenvironmental and parasite-derived factors. Results on myeloid cells other than macrophages will also be discussed, if they are of principal conceptual relevance. For detailed summaries of the role of neutrophils and dendritic cells in leishmaniasis the reader is referred to previous review articles [5], [6], [7], [8].
2. Macrophages as replicative niche to establish Leishmania infection
2.1. Experimental cutaneous leishmaniasis
When Leishmania promastigotes are inoculated into the dermis of mammalian hosts by the bite of sand flies or – experimentally – by intradermal needle injection, they immediately have to cope with the humoral and cellular antimicrobial machinery of the host. Especially in the case of natural infection, the promastigotes initially end up in a blood pool due to the laceration of capillaries in the stratum papillare of the dermis [9]. In the case of mouse infections with L. major, one of the species causing CL, promastigotes were rapidly taken up by neutrophils that served as vehicles for subsequent infection of monocyte-derived or tissue-resident macrophages [10], [11]. Although L. major promastigotes were reported to delay the apoptosis of neutrophils [12], L. major-infected neutrophils ultimately died and became a target of macrophages. This process was facilitated by the release of the monocyte-attracting chemokine CCL4 (MIP-1β) by neutrophils. Phagocytosis of apoptotic, infected neutrophils that still carry viable promastigotes, not only shuttled the parasite into macrophages, but at the same time suppressed their anti-leishmanial effector function by the release of transforming-growth factor (TGF)-β [10], [13]. TGF-β is a known inhibitor of the production of reactive oxygen species (ROS) by the phagocyte NADPH oxidase (Phox) [14] and of nitric oxide (NO) by type 2 (or inducible) NO synthase (NOS2, iNOS) [15] and secures at least the transient survival of Leishmania by these and other mechanisms [16]. It is important to emphasize that the role of neutrophils as “trojan horses” causing susceptibility to infection is neither observed with all Leishmania species nor in all mouse models [17] and reflects only part of their function in leishmaniasis. In addition to serving as replicative niche, neutrophils activately impede the recruitment of monocyte-derived dendritic cells, thereby impair the development of a protective T cell response and lead to disease chronicity as seen in L. mexicana-infected mice [18]. Furthermore, as reviewed in detail elsewhere, neutrophils also act as leishmanicidal effector cells during the innate phase of infection (see also below) [6], [7], [17], [19].
The inoculated Leishmania parasites can also be directly endocytosed by host cells located in the epidermis or dermis. It has been long-standing question whether Leishmania promastigotes sneak into host cells that are particularly permissive to Leishmania replication and survival. Early in vitro studies identified dermal macrophages as targets of L. major promastigotes [20], whereas epidermal Langerhans cells could only be infected by L. major amastigotes [20], [21]. Neither the dermal macrophages nor the epidermal Langerhans cells produced leishmanicidal effector molecules (ROS or NO) in response to L. major infection [20], [21] and were therefore considered as potential niches for the initiation of the skin infection.
More recent studies characterized the phenotype of dermal macrophages that allow for parasite replication. Lee et al. observed that parasites of the highly virulent L. major Seidman strain that cause non-healing CL despite development of a type 1T-helper (Th1) cell immune response, were preferentially taken up by dermal macrophages expressing high levels of the C-type lectin receptor CD206 (mannose receptor) [22], which is a known receptor for Leishmania parasites [23]. The CD206high dermal macrophages (a) were positive for additional markers (e.g., CD36, CD209, CD301) that signify alternatively activated or M2 macrophages [24], [25], but were negative for arginase 1 as well as NOS2 [22]; (b) were not replenished by recruited monocytes or other bone-marrow-derived cells, suggesting an embryonic origin; (c) required IL-10 and eosinophil-derived IL-4 for their CD206 expression and local proliferation (Fig. 1); and (d) accounted for the non-healing course of infection as shown by depletion experiments [22], [26]. Importantly, CD206high dermal macrophages were not a preferential target of a less virulent L. major strain (Friedlin) [22], which offers an explanation why the course of cutaneous infection with L. major Friedlin was comparable in wild-type and CD206-deficient mice [27].
In CL, the susceptibility of macrophages to Leishmania and the development of a non-healing course of infection has also been linked to M2 markers other than CD206, such as arginase (Arg) 1 (Fig. 1), which is induced by IL-4, IL-13, TGF-β1 or IL-10 [28,29]. Arg1 is a cytosolic enzyme found in both non-hematopoietic (e.g., hepatocytes) and hematopoietic cells (e.g., macrophages). Arg1 converts L-arginine into urea and ornithine, a precursor of polyamines that promote parasite proliferation and the synthesis of trypanothione, a dithiol required for parasite protection against oxidants [30]. At the same time, Arg1 competes with NOS2 for the common substrate L-arginine (see below) and thereby impedes NO production [29], [31]. Earlier studies that relied on global arginase inhibitors (blocking Arg1 as well as the Arg2 isoform without cell-type selectivity) provided first evidence for a disease-promoting role of Arg1 in L. major-infected BALB/c mice, a mouse strain that develops visceral and lethal disease following cutaneous infection with L. major [32], [33]. Hölscher et al. showed that conditional deletion of the IL-4 receptor alpha chain (IL-4Rα) on neutrophils and macrophages using a lysozyme M promoter-driven Cre-recombinase (LysMCre) delayed the skin swelling and reduced the parasite burden in L. major-infected susceptible BALB/c mice, but ultimately still allowed for progressive disease as seen in the respective BALB/c wild-type controls. Resident peritoneal macrophages isolated from L. major-infected LysMCreIL-4Rαflox/− mice and co-cultured with draining lymph node cells showed a reduced arginase activity in response to in vitro stimulation with lipopolysaccharide (LPS) [34]. Direct genetic proof that Arg1 expression accounts for progressive disease in L. major-infected BALB/c mice came from a study by Schleicher et al., who observed that Tie2CreArg1flox/flox BALB/c mice lacking Arg1 in endothelial cells and all hematopoietic cells were protected from a non-healing course of L. major infection. Importantly, Arg1 expression in the skin and draining lymph nodes of L. major-infected BALB/c wild-type mice (3–4 weeks post infection [p.i.]) was largely dependent on IL-4 and IL-13, restricted to CD45+ (i.e. hematopoietic) cells and exclusively found within a myeloid population that carried surface markers of both monocytes/macrophages and dendritic cells (CD11b+CD11c+Ly6C+CCR2+MHCII+Ly6G+F4/80+CD64+). These Arg1+ cells were also positive for additional M2 markers (CD206, resistin-like molecule alpha [also termed RELM-alpha or Fizz1], programmed death ligand 2 [PDL2]), but deficient for CD207 (langerin) and CCR7. Although approximately 75% of the Arg1+ cells co-expressed intracellular NOS2 protein, the enzymatic activity of Arg1 largely suppressed the generation of NO by NOS2 and thereby allowed for parasite survival [29], [35]. In accordance with our findings, two subsequent studies identified CD11b+Ly6C+CCR2+CX3CR1+CD64+CD24low cells (which were termed “inflammatory monocytes”; [36]) and CD11c+Ly6C+CCR2+F4/80+ cells (categorized as “monocytes” or “monocyte-derived dendritic cells”; [37]) as central host cells during the innate (≤4 days p.i.) and acute phase of L. major infection (3 weeks p.i.). Using an elegant photoconversion-based biosensor for measuring in vivo proliferation, Heyde et al. demonstrated that the CD11c+Ly6C+CCR2+F4/80+ cells were highly permissive for L. major replication [37]. A recently published third study, applying the L. amazonensis mouse model of chronic CL, again came to the conclusion that CD11b+Ly6C+CCR2+CX3CR1+CD64+ monocytes, coexpressing MHC class II, CD206, PDL2 and Arg1, were the primary reservoir for Leishmania parasites during the first 3 weeks of infection. Paradoxically, the recruitment and expansion of these permissive host cells turned out to be dependent on IFN-γ from day 4 p.i. onwards [38]. Together, all these studies convincingly show that blood monocyte-derived phagocytes in the infected skin form a replicative niche for Leishmania parasites as has been proposed by early analyses many years ago (reviewed in Ref. [39]).
Other macrophage molecules or products that support the establishment of a Leishmania infection include the complement receptor type 3 (CR3) and IL-10. CR3, which was originally identified to be a major receptor for the phagocytosis of L. major promastigotes by peritoneal macrophages in vitro [40], later turned out to be non-essential for parasite uptake, but critical for delaying phagosome maturation [41]. In vivo, however, mice deficient for CD11b and, thus, for the heterodimeric protein CR3 (CD1b/CD18) showed an unaltered or only slightly ameliorated course of infection in C57BL/6 or BALB/c mice, respectively [42]. IL-10, a known co-inducer of Arg1 [28] and inhibitor of NO, ROS and TNF production [43], was released by macrophages in response to antibody-opsonized L. major promastigotes in an Fcγ-receptor-dependent manner both in vitro and in vivo [44], [45]. In a L. mexicana mouse model, in contrast, the LysMCre-mediated deletion of IL-10 in macrophages and neutrophils had no impact on the chronic course of infection, whereas a CD4Cre-mediated deletion of IL-10 in T cells caused healing of the skin lesions [46]. Thus, in CL the cellular source of IL-10 that is critical for macrophage deactivation appears to be dependent on the parasite species.
2.2. Experimental visceral leishmaniasis
In mouse VL, due to L. donovani or L. infantum infections or resulting from the systemic dissemination of L. major in highly susceptible BALB/c mice, immature monocytes and macrophage precursors as well as certain phagocyte subpopulations have been suspected early on as “safe targets” for the parasite in spleen, liver or bone marrow [47]. In the bone marrow, L. donovani caused the expansion of cells resembling hematopoietic stem cells as well as of Sca1+ emergency myeloid progenitor cells that gave rise to CD11c+Ly6C+ monocytes exhibiting a permissive and immunosuppressive phenotype [48], [49]. Kupffer cells in the liver and macrophages of the red pulp and of the marginal zone of the spleen (marginal zone macrophages [MZM] and marginal metallophilic macrophages [MMM]) have been known for a long time to be host cells for Leishmania parasites (Refs. [50], [51], [52], [53], [54] and references therein). Hepatic Kupffer cells showed a defective ROS production in response to IFN-γ and consequently a reduced anti-leishmanial activity [51]. Splenic red pulp and white pulp macrophages infected with L. donovani amastigotes expressed CD68 (macrosialin) and MOMA-2, but also MHC class II, the latter suggesting their possible interaction with CD4+ T cells [53]. In the bone marrow, stromal macrophages were highly susceptible to infection by L. donovani, allowed for parasite replication, and generated TNF and granulocyte macrophage-colony stimulating factor (GM-CSF), which further stimulated myelopoiesis and therefore potential host cells for Leishmania [55].
More recently, it was observed that following intravenous infection of mice with L. donovani amastigotes blood monocytes were newly recruited into the liver and spleen, where they became target for the parasites. The surface phenotype of these inflammatory monocytes (CD11b+CD11c−Ly6ChighLy6G−CCR2+MHCIIintF4/80int) closely resembled the monocytes mobilized into the infected dermis during CL (see above), except for the lack of CD11c [56]. The recruitment of the cells was not only strictly dependent on C-C chemokine receptor type 2 (CCR2), but also required the signal transducer and activator of transcription 1 (STAT1), a key transcription factor of the IFN-γ signaling cascade. Treatment of L. donovani-infected mice with a CCR2-antagonist that blocked the influx of inflammatory monocytes, led to parasite and disease control in both liver and spleen [56]. Another study on L. donovani-infected mice came to the conclusion that during the chronic phase of infection (>day 14 p.i.) the percentage and absolute number of CD11b+CD11c+Ly6Chigh inflammatory monocytes increased in the spleen. The vast majority of these monocytes co-expressed CCR2, F4/80, and MHC II. Total CD11b+ myeloid cells purified from chronically infected spleens strongly suppressed Th1 cell differentiation in vitro and therefore functionally resembled myeloid-derived suppressor cells [49].
Similar to chronic CL, alternative macrophage activation also occurs in experimental VL. In the spleen of L. donovani-infected mice, arginase enzyme activity and the expression of Arg1 protein, one of the prototypic M2 markers, closely paralleled the steadily increasing parasite burden in the spleen. Treatment of the mice with an arginase inhibitor or with anti-IL-4 (+/- anti-IL-10) interrupted disease progression, restored NOS2 expression and induced parasite control. IL-4 and IL-10 synergized in the induction of Arg1, which was partly due to the upregulation of IL-4Rα by IL-10 [57]. These findings were largely confirmed by Terrazas et al. [56], who reported that the Arg1/NOS2 mRNA ratio was ca. 15-fold higher in the spleen than in the liver of L. donovani-infected mice, correlating with the progressive and self-limiting organ manifestation, respectively. Similarly, in L. donovani-infected Syrian hamsters, which is considered the best model for human VL, high Arg1 and low NOS2 levels characterized the infected splenic tissue and macrophages [58], [59].
An unusual intraperitoneal mouse infection model investigated the role of the macrophage mannose receptor (CD206) in VL. LysMCre+CD206flox/flox mice, which lack mannose receptor in myeloid cells, had a significantly higher parasite burden in the spleen than wild-type mice at day 14 after intraperitoneal injection of L. infantum. In vitro, CD206-deficient peritoneal macrophages showed, despite a slightly reduced initial binding and uptake of L. infantum or L. donovani promastigotes, an enhanced parasite content after 48 h of infection, which correlated with a reduced production of ROS and IL-1β as compared to wild-type macrophages [60]. These data suggest that in VL, unlike to L. major Seidman infections described above, crosslinking of CD206 by Leishmania parasites cause macrophage activation rather than deactivation.
2.3. Human leishmaniasis
The potential role of (inflammatory) monocytes as host cells for Leishmania parasites has not only been investigated in mice, but also in humans. While peripheral blood monocytes from healthy volunteers were activated by IFN-γ for antileishmanial activity (see below), a series of studies revealed that prior infection of normal human monocytes with L. donovani impaired their ability to produce ROS or cytokines (e.g., IL-1) or to express MHC class II in response to IFN-γ [61], [62], [63]. In line with these results, peripheral blood monocytes from Indian patients with VL showed an attenuated generation of ROS and NO, a reduced expression of MHC class II and certain chemokine receptors and co-stimulatory molecules, and a decreased production of IL-1 and IL-6 as compared to healthy endemic controls [64], [65]. At the same time, the plasma concentrations of IL-4, IL-10 and IL-13 were elevated [64]. Patients with early, non-ulcerated CL caused by L. braziliensis exhibited increased frequencies of intermediate (CD14+CD16+) and non-classical monocytes (CD14dimCD16+) in the peripheral blood, whereas the percentage of classical monocytes (CD14+CD16−) was similar to healthy controls. Importantly, the intermediate monocytes were positive for the chemokine receptor CCR2, and one of its ligands, the chemokine CCL2, was elevated in the skin lesions as compared to normal skin, suggesting that these monocytes will be attracted to the site of infection and contribute to pathology via their ability to produce TNF [66].
To date, there is no direct proof that alternatively activated or M2 monocytes or macrophages represent the preferential host cell reservoir for Leishmania parasites in humans. However, several studies provided evidence that Arg1 activity is associated with disease progression in human leishmaniasis. In Ethiopian patients with LCL, Arg1 activity in lesional tissue was significantly higher than in control skin biopsies, but was present in granulocytes and not in monocytes or macrophages. Whether Arg1+ granulocytes harboured Leishmania, was not analysed [67]. Similarly, in an Iranian study population, skin lesions of patients with acute LCL (<1 year duration) showed increased levels of Arg1 compared to biopsies from healthy controls [68], with higher Arg1 activities detected in L. tropica-infected than in L. major-infected patients. mRNA and immunohistochemical analyses of skin lesions from L. amazonensis-infected patients with DCL not only revealed a significant reduction of IFN-γ, TNF and IL-1β, but also higher levels of Arg1 and ornithine decarboxylase and a preponderance of transcripts that are characteristic for regulatory macrophages [69] when compared to lesions of patients with LCL [70], [71]. Skin lesions from L. mexicana-infected patients with DCL were reported to contain macrophages with more prominent Arg1-staining than lesions of LCL patients [72], whereas the frequency of NOS2-positive macrophages was significantly lower in DCL lesions [73]. The Arg1 activity of peripheral blood mononuclear cells from Ethiopian patients with VL was again restricted to granulocytes, elevated compared to uninfected controls, and declined after treatment, suggesting that Arg1 expression is associated with disease [74]. In Indian patients with VL, blood monocytes showed an enhanced expression of certain “anti-inflammatory” markers (e.g., transglutaminase-2, vitamin D signalling receptor, peroxisome proliferator-activated receptor [PPAR] γ) compared to healthy endemic controls [65].
Finally, in patients with PKDL, there was an increase of the M2 markers Arg1 (associated with CD68+ macrophages), CD206 and PPARγ in the skin lesions and in peripheral blood monocytes, which returned to normal levels upon treatment. Similarly, the reduced generation of NO and ROS by peripheral blood monocytes of PKDL patients was partly restored by successful therapy [75].
3. Macrophages as antileishmanial effector cells in leishmaniasis
The transformation of macrophages from permissive host cells into leishmanicidal effector cells during the course of Leishmania infection requires activation by cytokines, notably by IFN-γ, which is produced by a variety of cell types (e.g., natural killer [NK] cells, CD4+ or CD8+ T cells, certain types of NKT cells; Fig. 1) and already released during the early phase of infection (reviewed in [76], [77], [78], [79], [80]). The canonical activity of IFN-γ not only causes transcriptional induction of antimicrobial effector pathways in both uninfected and infected macrophages (reviewed in [81]), but also primes the responsiveness of macrophages by epigenetic remodelling of pre-existing enhancers and latent enhancers to subsequent stimuli (reviewed in [82]), including exposure to Leishmania parasites [62].
The current immunological concept for the control of intracellular Leishmania amastigotes entails the differentiation of Th1 cells, following the immunosynaptic interaction between infected and antigen-presenting dendritic cells and macrophages and naïve CD4+ T cells. The IFN-γ released by Th1 cells then triggers various effector mechanisms in neighbouring phagocytes (see below) and was proven to be essential for healing of experimental and human leishmaniasis [83]. The dominant role of Th1 cells, IFN-γ, dendritic cells and macrophages was mainly elucidated in the L. major mouse model of CL. A number of elegant studies using adoptive cell transfers, conditional cell ablations, bone marrow chimeric as well as transgenic mice, revealed that (1) IFN-γ release by CD4+ T cells was necessary and sufficient for achieving parasite control and could not be replaced by IFN-γ production by non-T cells [84]; (2) different populations of dendritic cells were required for a protective T cell response [85], [86], [87]; (3) IFN-γ responsiveness of CD68+ macrophages was critical for parasite containment in vivo [88]; and (4) the effect of IFN-γ was not restricted to macrophages with direct contact to the IFN-γ-producing CD4+ T cells but also reached more distant by-stander macrophage targets [89].
It is important to point out that IFN-γ can also exert paradoxical effects on macrophages and other myeloid cells, as already discussed for acute CL (see above, Ref. [38]). In mouse macrophages infected with L amazonensis amastigotes, stimulation with IFN-γ alone failed to induce NOS2, but increased the uptake of L-arginine as well as the intracellular parasite burden. Both effects were dependent on the host cell cationic amino acid transporter (CAT) 2B, indicating that L-arginine supported the growth of the parasites [90], e.g., via enhanced generation of polyamines [30], [91]. Likewise, in cultures of mouse bone marrow-derived immature monocytes infected with L. donovani amastigotes, the addition of IFN-γ caused an increase of the percentage of infected cells [49]. Similarly, IFN-γ promoted parasite replication in a STAT3-and Arg1-dependent manner when added to splenic macrophages isolated from L. donovani-infected hamsters [59]. Conversely, there is compelling evidence that IL-4 and IL-4Rα, which normally drive M2 gene expression [92], block classical macrophage activation [93] and characterize Th2 responses, can also synergize with IFN-γ and promote Leishmania control by macrophages and inflammatory dendritic cells [94], [95], [96]. These and other findings (reviewed in Refs. [97], [26]) point to the coexistence of classical and alternative macrophage activation during leishmaniasis and question the conventional Th1/Th2 scheme for protection versus susceptibility to disease.
3.1. Reactive oxygen species
The instant generation of ROS by myeloid cells after priming by cytokines and exposure to microbial products (e.g., N-formylmethionyl-leucyl-phenylalanine [FMLP]) or protein kinase C activators (e.g., phorbol myristate acetate) is termed “oxidative burst” and is due to the activation of the multimeric enzyme phagocyte NADPH oxidase (Phox) [19], [98]. The seminal discovery that recombinant IFN-γ primed human monocytes for ROS production and antileishmanial activity and was superior to 12 other cytokine preparations tested [99], [100], [101] was the starting point for a series of studies documenting the dominant role of IFN-γ in monocyte and macrophage activation [102], [103]. Later analyses demonstrated that mouse and human blood monocytes as well as inflammatory monocytes newly recruited into infected tissues (Ly6C+CCR2+CX3CR1+F4/80int) functioned as antileishmanial effectors and that the killing of different Leishmania species (e.g., L. major, L. donovani, L. braziliensis) in vitro was dependent on ROS formation (reviewed in Refs. [39], [19], [104], [105], [106]). The use of mouse strains deficient in the 91 kDa heme-containing β-subunit (NOX2) of the catalytic flavocytochrome b558 of Phox (gp91phox−/−) revealed an organ-specific contribution of Phox to parasite control in vivo, with absent or weak effects in the skin (L. major, L. amazonensis), draining lymph node (L. major, L. amazonensis) and liver (L. donovani), but strong effects in the spleen (L. major) [107], [108], [109], [110]. Several case reports on VL in patients with NOX2-deficiency indicate a protective role of ROS in humans (Refs. [111], [112] and references therein).
3.2. Reactive nitrogen species
IFN-γ is the key inducer of NOS2 in macrophages [113], [114]. Several observations in mouse models have provided convincing evidence for a critical role of NOS2-derived NO in controlling Leishmania parasites. These include (a) the inverse correlation between tissue expression of NOS2 and disease severity [115]; (b) the prevention of parasite killing by macrophages in the presence of NOS2 inhibitors [116]; (c) the development of progressive disease in mice lacking NOS2 or treated with NOS2 inhibitors [107], [117], [118], [119]; and (d) the reactivation of disease in clinically cured mice, when NOS2 activity was blocked [120]. More recent in vivo analyses, using elegant reporter systems for tracing Leishmania replication, suggested that during the acute phase of L. major infection NO restricts parasite metabolism and proliferation in the absence of killing and that it acts not only cell-intrinsically in NOS2-positive cells, but also reaches parasites residing in NOS2-negative cells [121], [122]. An equivalent concept was previously established for the chronic (latent) phase of L. major infection based on detailed in situ studies [120], [123], [124].
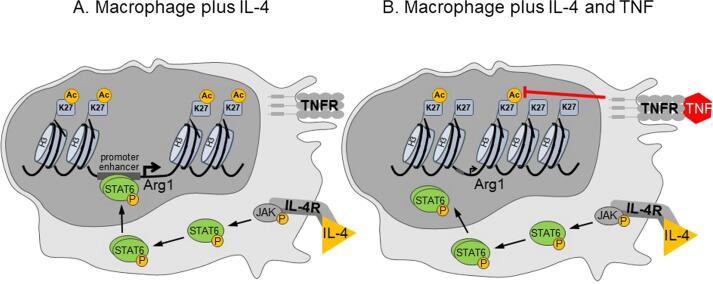
The induction of NOS2 in mouse macrophages by IFN-γ was critically dependent on endogenous TNF [94], [125], [126]. TNF supports the induction of NOS2 and the production of NO by activating mitogen-activated protein kinases and the transcription factors NF-κB and AP-1 [127], [128]. A new facet of TNF action was recently discovered by Schleicher et al., who found that TNF not only supported the expression of NOS2, but also inhibited the IL-4-induced expression of Arg1 in both uninfected and L. major-infected mouse macrophages or dendritic cells as well as in L. major-infected mice. Mechanistically, TNF reduced the acetylation of lysine 27 of histone H3 and thereby hindered the accessibility of phosphorylated STAT6 to the Arg1 enhancer and promoter region (Fig. 2). The TNF-mediated suppression of Arg1 increased the availability of L-arginine and thereby promoted the production of NO by NOS2 at the site of infection in vivo. TNF also counteracted the IL-4-mediated increase of mitochondrial oxidative phosphorylation (OXPHOS) and shifted the macrophage metabolism towards glycolysis [35]. The implications of this metabolic shift will be discussed in Section 5.4.
Fig. 2.
TNF downregulates Arg1 gene transcription by inhibiting the access of the transcription factor STAT6. IL-4, an alternative macrophage activator, caused tyrosine phosphorylation and nuclear translocation of STAT6, which, together with other transcription factors (not shown), bound to the promoter and enhancer regions regulating the transcription of Arg1 (and other M2 genes; not shown). The binding required prior remodelling and opening of the chromatin, which was associated with IL-4-induced acetylation of lysine 27 of histone 3. Co-stimulation of macrophages with IL-4 plus TNF reduced the histone acetylation and impaired the opening of the gene locus and the binding of phosphorylated STAT6 (Ref. [35] and data not shown).
In addition to IFN-γ, there is also evidence for a protective role of type I IFNs (IFN-α/β) in leishmaniasis. In mouse CL following L. major infection, the expression of IFN-α/β by dermal macrophages was associated with early NOS2 expression, NK cell activation and parasite containment [119], but was dispensable for long-term parasite control and clinical cure during primary or secondary L. major infection in self-healing C57BL/6 mice [129]. In L. major-infected BALB/c mice, deletion of IFN-β or of the type I IFN receptor (IFNAR) did not alter the non-healing course of disease [129], although exogenous IFN-β was able to convey protection in these mice [130], and macrophages exposed simultaneously or sequentially to L. major promastigotes and IFN-α/β expressed NOS2 and antileishmanial activity [78], [131]. Besides these protective effects of IFN-α/β, a series of studies indicated that an exuberant production of type I IFNs can also cause aggravation of CL or VL (see Section 5.3. below). As discussed elsewhere, the function of type I IFNs is clearly concentration-dependent [130], [131], [132].
3.3. Impact of microenvironmental and metabolic factors on oxygen-dependent parasite defense
The activity of both Phox and NOS2 is dependent on the presence of sufficient amounts of oxygen. In many tissues, the oxygen concentration is already low under steady-state conditions, e.g., in the skin (pO2 ~ 5–6%) [133], liver (portal vein pO2 ~ 7%) [134] and the bone marrow (pO2 ~ 1.3–4%) [135]. In situ analyses of L. major-infected mice revealed that the transcutaneously measured oxygen tension (ptcO2) in skin lesions dropped to levels (~2.8%) which in bone marrow-derived macrophages caused upregulation of IL-4/IL-10-driven Arg1 expression and impeded the oxygen-dependent generation of NO and parasite control [133]. Clinical and parasitological resolution of CL was accompanied by recovery of the ptcO2 concentrations. When mice were kept under a normobaric, but hypoxic atmosphere (pO2 ~ 9%), the ptcO2 levels were significantly reduced compared to control mice exposed to ambient air (pO2 ~ 20%), the tissue parasite burdens were increased and the resolution of the skin lesions delayed [133]. In vitro, hypoxia led to the expression of hypoxia-inducible factor (HIF) 1α which was enhanced in the presence of L. major and further increased by the addition of TNF and IFN-γ [136]. HIF-1α is a transcription factor that regulates the expression of genes necessary to cope with reduced oxygen conditions, such as vascular endothelial growth factor (VEGF)-A (that promotes angiogenesis and lymphangiogenesis) [137] or NOS2 (that leads to the production of vasodilatory NO) [138], [139]. During L. major-induced CL, macrophages were the dominant cell type expressing HIF-1α and VEGF-A [137]. In L. major-infected mice lacking HIF-1α in myeloid cells (LysMCre HIF-1αflox/flox), the tissue expression of NOS2 was reduced and the skin lesion sizes and parasite loads were increased [136].
The findings made in the L. major model of CL contrast with results obtained in another model of experimental CL. In L. amazonensis-infected J774 macrophage-like cells or in L. amazonensis-infected peritoneal exudate macrophages from both wild-type and NOS2-deficient mice, hypoxia (pO2 ~ 3%) led to an NOS2/NO-independent reduction of the number of intracellular parasites as compared to normoxic culture conditions [140], [141]. Unexpectedly, hypoxia not only upregulated the HIF-1α-dependent production of the cytokine migration inhibitory factor (MIF) and the protein expression of gp91phox (NOX2), but also enhanced the generation of ROS detected by 2́,7́-dichlorodihydrofluorescein diacetate. Silencing of HIF-1α or addition of diphenylene iodonium chloride, an inhibitor of Phox (as well as of NOS2), prevented the improved parasite control under hypoxia [141].
In experimental VL, the analysis of the role of HIF-1α yielded discrepant results, depending on the conditional knock-out mouse system used. Deletion of HIF-1α in granulocytes, monocytes and mature macrophages (using LysMCre HIF-1αflox/flox mice) led to increased susceptibility to L. donovani, which resulted from a mTOR (mammalian target of rapamycin)- and SREBP-1c (sterol regulatory element-binding protein 1c)-driven intracellular accumulation of lipids following the upregulation of lipogenic enzymes. Lipids may serve as energy source for amastigotes or protect them against microbicidal effector molecules of macrophages [142]. In contrast, deletion of HIF-1α in CD11c+ myeloid cells (CD11cCreHIF-1αflox/flox mice) increased the expansion, but reduced the immunosuppressive potential of Ly6Chigh inflammatory monocytes, caused an upregulation of NOS2 and ROS production by CD11b+ splenic cells, promoted the release of IL-12 by dendritic cells, and supported the differentiation of CD4+ Th1 cells and the expansion of CD8+ T cells. Consequently, the splenic parasite burden in CD11cCreHIF-1αflox/flox mice was significantly lower than in the respective wild-type controls [49], [143], [144]. These results argue for a fundamentally different role of HIF-1α in lysozyme-positive versus CD11c-positive myeloid cells.
Tissue osmolarity is a micro milieu factor that has only recently attracted the interest of infectious disease immunologists. Pioneering studies had provided evidence that hypertonicity, as it, for example, prevails in the skin and inflamed tissues [145], has a strong regulatory impact on the secretion of cytokines [146], the function of macrophages [147], [148] and the differentiation of different T cell subsets [149], [150], [151], [152]. In the context of CL, it was shown that mice lacking the tonicity-responsive enhancer binding protein (TonEBP) (also termed nuclear factor of activated T-cells [NFAT] 5) displayed reduced levels of NOS2 in dermal macrophages and increased parasite numbers in skin lesions, draining lymph nodes and spleen of L. major-infected mice. However, whether these findings reflected the impact of tissue tonicity on macrophage responses, was not addressed [153]. Jantsch et al. provided direct and firm evidence that increased concentrations of Na+ in the tissue following a high-salt diet improved control of L. major in vivo via NFAT5-dependent induction of NOS2 [154]. The Na+/Ca2+ exchanger 1 (NCX1, also known as solute carrier family 8 member A1 [SLC8A1]) accounted for the Na+ influx during exposure of macrophages to high salt conditions [155]. Recent data indicate that the effect of tissue osmolarity on phagocyte functions might not be restricted to classically activated (M1) macrophages, but could also pertain to alternatively activated (M2) macrophages [156].
3.4. Other antileishmanial effector mechanisms
In human monocytes and monocyte-derived macrophages, degradation of the amino acid L-tryptophan by the IFN-γ-inducible enzyme indoleamine 2,3-dioxygenase (IDO) was found to partially contribute to the killing of intracellular L. donovani. As the effect was reversed by the addition of L-tryptophan, depletion of the amino acid rather than accumulation of potentially toxic tryptophan metabolites (kynurenines) were thought to account for the anti-leishmanial activity of IDO. No IDO activity was detectable in mouse macrophages [157], presumably due to the inactivation of IDO by high levels of NOS2-derived NO [158]. Later studies established a role for IDO during the interaction of dendritic cells with T cells in mouse and human leishmaniasis. IDO-mediated tryptophan depletion suppressed T cell proliferation and stimulated the generation of regulatory T cells, thereby promoting the chronicity of disease [159], [160], [161], [162] (see also Table 1). This immunomodulatory function of IDO is dependent on the generation of superoxide anion by Phox [158], [163].
Table 1.
Examples of evasion strategies and underlying molecular mechanisms by which Leishmania parasites promote their survival in macrophages.
| Step of defense | Process elicited by Leishmania infection | Molecular mechanism(s) | Leishmania species studied | References |
|---|---|---|---|---|
| Macrophage polarization towards activated (M1) macrophages | Transcriptional reprogramming towards permissive macrophages | 1. Metabolic reprogramming of the host cell | ||
|
L. donovani | [260] | ||
|
L. amazonensis | [261] | ||
|
L. major, L. mexicana | [262], [263] | ||
|
L. infantum, L. donovani | [240], [264] | ||
| 2. Suppression of the production of proinflammatory cytokines | ||||
|
L. donovani | [177] | ||
|
L. amazonensis | [181] | ||
|
L. donovani | [265] | ||
|
L. major, L. donovani etc. | [236], [266] | ||
| 3. Skewing of CD40-signaling to ERK-1/2 and IL-10 induction | L. major | [267] | ||
| 4. LRV1-/TLR3-dependent induction of microRNA155 and PI3Kinase/Akt promoting macrophage survival and parasite persistence | L. guyanensis | [227] | ||
| Translational reprogramming | 1. Cleavage of the mammalian/ mechanistic target of rapamycin (mTOR) by the metalloprotease gp63 (leishmanolysin; expressed on the surface of pro- and amastigotes) → activation of the translational repressor 4E-BP1 → reduced type I IFN-production, increased parasite proliferation | L. major | [248] | |
| 2. Enhancement of mTOR- and eIF4A-sensitive mRNA-translation (inhibition of eIF4A promoted parasite elimination, whereas inhibition of mTOR supported parasite persistence) | L. donovani | [247] | ||
| Macrophage uptake of parasites | Inhibition of alternative complement activation | Conversion of C3b into iC3b by gp63 → parasite uptake by macrophages via CR1 and CR3; resistance to complement-mediated lysis |
L. major, L. mexicana, L. amazonensis and others |
[235], [268], [269] |
| Macrophage antimicrobial activity | Inhibition of phagolysosomal fusion by LPG | Alteration of physical properties of phagosomal membrane by LPG (expressed on the surface of promastigotes)? Inhibition of recruitment of the small GTPase rab7? |
L. donovani, L. major |
[270], [271], [272] |
| Inhibition of phagocyte NADPH oxidase (NOX2) activity |
|
L. mexicana pifanoi, L. donovani | [273], [274] | |
|
L. amazonensis, L. braziliensis | [228] | ||
| Inhibition of NOS2 expression |
|
L. major | Reviewed in [235] | |
|
L. amazonensis | [275] | ||
|
L. donovani | [265] | ||
| Inhibition of iron export, thereby increased iron availability for the parasite | Translational suppression of the iron exporter ferroportin-1 via upregulation of iron-regulatory-protein-2 (IRP-2) | L. amazonensis, L. donovani | [276], [277] | |
| Macrophage antigen presentation | Inhibition of antigen presentation and T cell activation |
|
L. donovani | [278] and ref. therein |
|
L. donovani | [279] | ||
| Macrophage-/ dendritic cell-mediated generation and expansion of antigen-specific effector T cells (Th1) | Upregulation of indoleamine-2,3-dioxygenase (IDO) by Leishmania parasites | IDO-mediated depletion of L-tryptophan and generation of immunosuppressive kynurenines → suppression of T cell proliferation; generation of regulatory, FoxP3+ T cells |
L. major; L. donovani; L. infantum; L. guyanensis |
[159], [160], [161], [162] |
Neutrophils are known to generate extracellular traps (so-called NETs) that consist of DNA decorated with histones, neutrophil elastase, myeloperoxidase and other antimicrobial molecules and serve to contain infections with bacteria and fungi [164]. Human neutrophils also formed NETs in response to Leishmania infection in both ROS-dependent and ROS-independent processes [165], [166], [167]. Whereas L. amazonensis turned out to be susceptible to NETs [165], [167], other Leishmania species (e.g., L. mexicana, L. major, L. infantum, L. donovani) resisted killing by NETs via their expression of surface lipophosphoglycan (LPG) [166], the secretion of 3́-nucleotidase/nuclease [168] or by unknown mechanisms [18]. Although macrophages have been reported to produce extracellular DNA traps [169], there is no published evidence to date that they play a role in the defense against Leishmania parasites.
In the past, various antimicrobial peptides (AMP) from natural sources showed cytotoxic activity against cultured extracellular Leishmania promastigotes [170], [171], [172]. While these findings are potentially relevant for novel therapeutic approaches, only few AMPs are endogenously produced by macrophages [173] and therefore could contribute to the control of intracellular amastigotes. Kulkarni et al. found that cathelicidin-type antimicrobial peptides (CAMP) exerted cytolytic effects on L. amazonensis promastigotes and that CAMP-deficient mice developed significantly more severe skin lesions with higher parasite burdens [171], [174]. Crauwels et al. recently found an increased CAMP gene expression in skin lesions of L. aethiopica-infected patients as compared to skin biopsies from healthy controls. LL37, the bioactive cleavage product of human cationic antimicrobial protein (hCAP18) that is encoded by CAMP, caused apoptosis of L. aethiopica and L. major promastigotes in vitro. Treatment of human macrophages (derived from rhGM-CSF-treated monocytes) with 1α,25-dihydroxyvitamin D3 enhanced CAMP mRNA expression and the production of hCAP18, which was paralleled by reduced survival of intracellular Leishmania [175].
4. Macrophages as immunoregulators in leishmaniasis
During the innate and acute phase of Leishmania infections, macrophages not only serve as host cells and antileishmanial effector cells, but also fulfil immunoregulatory functions due to their expression of MHC class II and costimulatory molecules, presentation of antigens, secretion of cytokines and release of RNS and ROS (Fig. 1). These processes can be positively or negatively affected by the infection with Leishmania, depending on the parasite species, its developmental stage and the experimental set-up, as illustrated by the following examples (see also Table 1).
4.1. Macrophage cytokine production
The effects of Leishmania parasites on macrophage cytokine production are far from uniform. L. major or L. donovani promastigotes impaired the IL-12 production of macrophages in vitro [176], even in the presence of IFN-γ [177]. Similarly, L. major or L. mexicana promastigotes attenuated the production of IL-1β by bone marrow-derived macrophages after stimulation with synthetic hemozoin, an agonist of the inflammasome NLRP3; the effect resulted from the proteolytic activity of leishmanolysin, a 63 kDa surface metalloprotease of Leishmania parasites [178]. On the other hand, L. major, L. braziliensis or L. amazonensis promastigotes were reported to trigger the activation of NLRP3, the production of IL-1β and the generation of NO by bone marrow-derived macrophages primed with lipopolysaccharide (LPS), Pam3CSK4 or IFN-γ [179], [180], [280]. When, however, the macrophages were infected with L. amazonensis amastigotes in the presence of the NLRP3 agonists LPS and ATP, the activation of NLRP3 and the release of IL-1β and IL-18 were prevented [181]. At the same time, the production of TNF was enhanced [181] as previously also seen with IFN-γ-stimulated macrophages following infection with L. major amastigotes [94], [125], [126].
The analysis of the functional role of NLRP3 or IL-1β during CL in vivo, using C57BL/6 mice deficient for the IL-1 receptor or its adaptor molecule MyD88, for NLRP3 or ASC (an adaptor molecule of NLRP3) or for caspase 1 (processing pro-IL-1β to mature IL-1β), also yielded discrepant results: intradermal infections with (i) L. amazonensis or L. braziliensis, (ii) L. major (LV39 strain) or (iii) L. major (Seidman strain) led to aggravated, unaltered or reduced skin lesion pathology, respectively, compared to wild-type controls [179], [180]. Subcutaneous infection of NLRP3-, ASC- or caspase 1/11-deficient mice on a non-healing BALB/c background with L. major (IR173 strain) ameliorated the course on infection [182], whereas in a L. guyanensis footpad infection model the inflammasome activation was without impact on lesion pathology or parasite burden [281]. In a natural mouse model of VL, transmission of L. donovani by sand fly bites along with gut bacteria of the vector caused NLRP3 activation, IL-1β production by neutrophils rather than macrophages, and parasite dissemination to the spleen, which was prevented by antibiotic treatment of the sand flies or anti-IL-1β treatment of the host [183]. In contrast, intraperitoneal infections of caspase 1- or ASC-deficient mice with L. infantum led to increased parasite loads in spleen and liver [179]. Together, these data illustrate that in CL and VL endogenous IL-1β can be protective, disease-promoting or without clinical effect, depending on the parasite species and strain and the mouse genetic background.
4.2. Immunoregulation by RNS and ROS
One of the functional characteristics of NO is its ability to positively or negatively regulate a broad spectrum of immune responses due to its chemical reactivity with enzymes, signaling molecules and transcriptions factors [114], [184], [185], [186], [187]. Early studies already reported that NOS2-derived NO exerts negative feedback-regulatory effects on the differentiation and proliferation of Th1 cells [188] as well as on the production of numerous cytokines (reviewed in [189]). Consequently, L. major-infected mice with a reduced but not absent NOS2 gene expression showed a striking expansion of Th1 cells and upregulation of IFN-γ production [118], [190], and in L. major-infected wild-type treated with an NOS2-inhibitor the expression of NOS2 protein in the infected tissue was strongly upregulated [120], [191]. More recently, it was demonstrated that in L. major-infected mice the production of NO by NOS2+ macrophages impeded mitochondrial respiration and ATP generation (a long-known effect of NO [192], [193], [194]), thereby limited the production of proinflammatory cytokines (e.g., IL-1β, TNF) and chemokines (e.g., CCL2, CCL3) and inhibited the further recruitment of inflammatory cells [195]. In vitro co-culture experiments with NOS2+/+ and NOS2−/− macrophages as well as mixed bone marrow chimeric mice revealed that the negative regulatory activity of NO required a certain density of NOS2+ cells and, due to the diffusion capacity of NO, affected both NOS2+ as well as NOS2− cells in the microenvironment [195].
Similar to NOS2-derived NO, ROS generated by Phox (NOX2) function as stimulatory or inhibitory signaling molecules and secondary messengers in the immune system [196], [197], [198], [199]. Recent studies with L. amazonensis-infected mice illustrated that in this model of CL Phox-derived ROS operate mainly as inhibitors of neutrophil influx and as executers of neutrophil apoptosis rather than as antileishmanial effector molecules [200].
The ability of ROS as well as RNS to restrict ongoing immune responses is critical for the termination and resolution of inflammatory processes and for preventing immunopathologies. However, it is important to emphasize that their effects vary between stimulatory and inhibitory, depending on the in situ concentrations. Therefore, it is not surprising that during the innate phase of L. major infection the small amounts of NO produced by macrophages mainly serve to initiate IL-12-signaling, NK cell activation and early parasite containment [78], [119], [201], whereas later during the acute clinical phase of infection the antiparasitic and T cell-dampening of NO effect prevails [195].
5. Macrophages as niche for long-term parasite persistence
5.1. Immunological consequences of Leishmania persistence
One of the enigmas in both CL and VL is the persistence of parasites even after treatment and clinical cure of the disease. From an immunological point of view, parasite persistence has two major consequences, as revealed by mouse studies and clinical observations. First, in the L. major mouse model surviving amastigotes lead to continuous stimulation of Ly6C+T-bet+CD4+ and IFN-γ-producing effector T cells [202] that mediate induction of NOS2 [120] and protect against secondary disease [202], [203], although secondary infections are not prevented [204]. Immunity following primary infection has been the basis for the use of leishmanization (injection of parasites at cosmetically non-critical locations) to avoid ulcerative and scar-forming CL, e.g., in the face (reviewed in Ref. [205]). Second, persisting parasites can be the starting point for the reemergence of clinical disease once their containment by the immune system is impaired (e.g., following iatrogenic immunosuppression due to an autoimmune or malignant disease) (reviewed in Refs. [2], [123], [124], [206]). The underlying mechanisms of parasite persistence and reactivation have been of major interest to Leishmania researchers.
5.2. Host cells for persisting Leishmania
In mouse CL, parasites persisted in mature tissue macrophages (positive for F4/80 [syn. EMR1, EGF-like module-containing mucin-like hormone receptor-like 1], BM-8 and MOMA-2, negative for Gr-1 and Ly6C; [120], [123], [207], [208]), dendritic cells (positive for CD205 [DEC-205, NLDC-145] and CD11c; [120], [207], [209]) and in reticular fibroblasts of the lymph node draining the former skin lesion (positive for ER-TR7; [120], [123], [207], [208]. In three independent studies [120], [207], [208], persisting L. major parasites were associated with both NOS2+ cells (mostly macrophages and dendritic cells) and NOS2− cells (macrophages, dendritic cells, reticular fibroblasts). Although the percentages of L. major amastigotes residing in NOS2+ and NOS2− cells ranged from 15% to 60% and from 85% to 40%, respectively, and the quantitative distribution of the parasites across the different host cell types significantly varied between these studies, the data obtained uniformly support the following scenario: (1) The persisting parasites are under continuous control by NOS2. The prompt reappearance of clinical disease upon inhibition of NOS2 and the absence of spontaneous episodes of reactivation strongly argue against the development of genuine NO resistance amongst the persisting parasite population [120], [123], [124], [208]. (2) The persisting parasites are not dormant, but are metabolically active and proliferate [207]. Persistence is a dynamic process and characterized by ongoing parasite replication, killing (by NOS2+ cells) and evasion (via retreat into NOS2− cells) that occur in parallel [120], [123]. (3) NO-mediated control of Leishmania is not restricted to parasites located in NOS2+ cells, but also reaches parasites in NOS2− cells [122], due to the known diffusion capacity of NO [210].
In VL, the chronicity of infection is accompanied by remarkable structural changes in the spleen changes, notably the loss of marginal zone macrophages, which form an important splenic macrophage population phagocytosing Leishmania, and the loss of stromal cells in the periarteriolar lymphoid sheath, which impairs the generation of lymphocyte-attracting chemokines such as CCL19 and CCL21 (reviewed in: [211]). In the bone marrow, L. donovani infections cause secondary hemophagocytosis (erythrophagocytosis) by macrophages, which is at least partially due to the downregulation of signal regulatory protein (SIRP) α, a receptor transmitting “don’t eat me signals”, on macrophages [212]. This process contributes to the anemia observed in VL [2], but also leads to increased susceptibility to secondary infections because heme (released from phagocytosed erythrocytes) inhibits endocytosis, oxidative burst and migration of mouse and human neutrophils and macrophages [213], [214], [215]. Importantly, macrophages that have taken up erythrocytes also showed improved survival of intracellular L. donovani amastigotes [212].
5.3. Molecular mechanisms of Leishmania persistence and survival: Role of cytokines
Persistence of L. major parasites in experimental CL was dependent on regulatory CD25+CD4+ T cells and IL-10 [203], [216], which led to the hypothesis that IL-10-induced Arg1 might deprive macrophages of L-arginine and thereby impede NO production or even NOS2 protein expression [29], [124]. However, the analysis of mice lacking Arg1 expression in all hematopoietic and endothelial cells unequivocally showed that L. major parasites persisted in Arg1-deficient mice to the same degree and in the same host cells as in wild-type mice [208]. Arg2 is also unlikely to be decisive for the long-term survival of L. major because, similar to Arg1, Arg2 was not detectable in the draining lymph nodes after clinical cure of the infection [208]. With respect to the arginase of the parasite, Mou et al. reported that an arginase-deficient mutant of L. major showed an unaltered ability to persist in long term-infected mice [217].
As IL-10 does not seem to cause Leishmania persistence via induction of arginases, other mechanisms of action need to be considered. IL-10 is known to suppress the production of NO, ROS, TNF and IL-1 by primary macrophages [43], [218], the production of IL-1, IL-6 and TNF by macrophage cell lines [219] and the cytokine-mediated activation of Th1 cells by antigen-presenting cells [220]. It is conceivable that all these activities of IL-10 support the survival of Leishmania in macrophages and other host cells.
In addition to IL-10, other cytokines might facilitate Leishmania persistence in macrophages. Several studies with L. tropica-, L. infantum- or L. donovani-infected mice and with humans from Leishmania-endemic areas suggested a pathogenic role for TGF-β [16], [221], [222], [223], a known suppressor of macrophage functions and Th1 differentiation [93], [224], [225]. For type I IFNs (IFN-α/β), at least five different categories of mechanisms of action have been described that could support parasite survival in vivo. (a) Resident or inflammatory peritoneal macrophages exposed to IFN-α/β for as little as 30 min showed a strongly reduced expression of NOS2 mRNA, protein and activity in response to subsequent infection with L. major promastigotes. The effect of IFN-α/β was due to an impaired activation and nuclear translocation of STAT1α and NF-κB, prevented parasite killing and could not be reverted by IFN-γ treatment [131]. However, in L. major-infected C57BL/6 mice deficient for IFNAR1 or IFN-β, parasite persistence was fully maintained [129]. (b) Bone marrow-derived macrophages infected with L. guyanensis promastigotes carrying an endogenous Leishmania RNA virus 1 (LRV1) expressed reduced levels of IFN-γ-receptor (IFN-γR) on their surface compared to macrophages infected with LRV1-negative Leishmania. As the double-stranded RNA of LRV1 is a ligand of TLR3 and known inducer of IFN-α/β, the downregulation of IFN-γR was also seen with macrophages infected with LRV1-negative parasites and treated with IFN-α or IFN-β. In vivo, LRV1+ L. guyanensis caused more severe skin lesions than LRV1− Leishmania in wild-type, but not in IFNAR1-deficient mice [226]. An additional disease-promoting effect of LRV1 originated from the TLR3-dependent induction of microRNA155 and the activation of the phosphatidylinositol 3-kinase/Akt signaling pathway that fosters macrophage survival and thereby parasite persistence [227]. Furthermore, it was observed that LRV1-induced type I IFNs triggered autophagy, which led to the degradation of NLRP3 and the inhibition of inflammasome activation, thereby supporting parasite survival [282]. (c) IFN-β promoted the growth of L. amazonensis or L. braziliensis in human monocyte-derived macrophages, which was due to the induction of superoxide dismutase 1 and an ensuing suppression of ROS production [228]. (d) Stimulation of bone-marrow-derived macrophages with IFN-α raised the uptake of L. donovani or L. infantum promastigotes, which was causally linked to an increased surface expression of sialoadhesin (Siglec-1/CD169) [229]. (e) In VL due to L. donovani or L. infantum, type I IFNs, which were mainly produced by plasmacytoid DCs and not by myeloid DCs or macrophages [78], [230], strongly acted on myeloid DCs to suppress the production of IFN-γ and to enhance the expression of IL-10 by parasite-specific CD4+ T cells, leading to increased parasite loads in liver and spleen [231].
5.4. Molecular mechanisms of Leishmania persistence and survival: Role of metabolism
Over the years, numerous further strategies of immune evasion and subversion have been identified by which Leishmania parasites transform macrophages into a niche for initial infection, but possibly also for long-term survival. The underlying molecular mechanisms include reprogramming of the transcriptome by interference with signaling cascades and/or by epigenetic remodelling, cleavage of host proteins and inhibition of macrophage translation, and remodelling of the metabolome including the degradation of substrates and cofactors required for anti-leishmanial effector pathways (reviewed in [232], [233], [234], [235], [236], [237], [238], [239]). Some pertinent examples are listed in Table 1.
A particularly exciting area of research is the effect of Leishmania parasites on host cell metabolism and, conversely, the impact of host cell microenvironment on Leishmania metabolism and survival. It has become evident that the metabolic reprogramming of macrophages by Leishmania parasites differs between the species and developmental stage of the parasite, but also depends on the time-point of infection and the genetic background of the host cell (Table 1). In bone marrow-derived macrophages of BALB/c mice, infection with L. infantum promastigotes caused an early peak (6 h p.i.) of glycolysis due to the upregulation of glycolytic enzymes, which was followed by increased mitochondrial respiration (OXPHOS) (18 h p.i.), paralled by an enhanced expression of the PPAR-γ coactivator-1α (PGC-1α) [240]. This metabolic switch was dependent on sirtuin-1 deacetylase, the serin-threonine-kinase 11 (LKB1) and the AMP-activated kinase (AMPK). Importantly, macrophages or mice deficient for any of these enzymes showed reduced numbers of L. infantum, indicating that OXPHOS, which signifies alternatively activated macrophages (M2) [241], favours parasite survival [240]. Why macrophage skewing towards OXPHOS supports parasite persistence, has not yet been studied in detail. One possibility is that ATP generation by the host cells via mitochondrial respiration reduces the consumption of glucose and thereby increases the availability of glucose for intracellular Leishmania. The importance of glucose for Leishmania virulence in vivo has been demonstrated for L. major and L. mexicana using mutants defective for gluconeogenesis [242] or glucose uptake [243], respectively. Data obtained in the L. mexicana model suggest that parasites which maintain glycolysis may become less sensitive to the action of NO by avoiding the NO-sensitive Krebs cycle [244] and mitochondrial respiration [193].
One of the central hubs of macrophage carbon metabolism is mTOR, which functions as a sensor of amino acid concentrations, promotes mRNA translation and lipid synthesis [245]. Several studies found that L. donovani activated the mTOR pathway in macrophages, which was associated with increased parasite survival [142], [246], M2 macrophage polarization [246] and lipid synthesis [142]. Recently, it was demonstrated that Leishmania parasites express a 2,4-dienoyl-CoA reductase (DECR), an enzyme that is essential for β-oxidation of polyunsaturated fatty acids. A DECR-deletion mutant of L. major failed to survive in bone marrow-derived macrophages and did not cause disease in BALB/c mice, which are highly susceptible to wild-type L. major. These data suggest that Leishmania parasites thrive on lipids accumulating in macrophages. However, the metabolic role of mTOR appears to be far more complex in leishmaniasis, because other studies (using mTOR inhibitors) came to the conclusion that activation of mTOR contributes to the control of L. donovani [247] and L. major [248] (see Table 1).
Leishmania and macrophages also compete for amino acids, best exemplified by L-arginine. As detailed in 2.1, 3.2, macrophages express Arg1 and/or NOS2 depending on their activation status. Both Arg1 and NOS2 are L-arginine metabolizing enzymes that are capable to deprive Leishmania parasites of L-arginine. However, Leishmania promastigotes, axenic amastigotes as well as intracellular amastigotes residing in macrophages were shown to respond to arginine deprivation by upregulation of a high-affinity, monospecific L-arginine transporter (amino acid permease, AAP3) [249], [250], [251]. Thereby, the parasites secure their L-arginine supply for the parasite-arginase-dependent synthesis of L-ornithine and polyamines even in an L-arginine-deficient environment.
6. Concluding remarks
-
•
A common theme in both CL and VL in mice and humans is the function of Ly6ChighCCR2+ inflammatory monocytes as initial reservoir for parasite replication. These and other myeloid cells such as M2 macrophages qualify as replicative niche for Leishmania parasites, either due to the expression of specific uptake receptors or an impaired antimicrobial activity. The latter results from the remodelling of the host cell by the parasites or from specific microenvironmental conditions in the infected tissues such as hypoxia or the prevailing cytokine milieu.
-
•
Markers of classical (M1) and alternative (M2) macrophage activation are not mutually exclusive. The co-expression of NOS2 and Arg1 on a single cell level, e.g., during acute L. major CL, supports the concept that a continuous and bidirectional transition of macrophage activation statuses rather than the development of macrophage subpopulations with an irreversible function and fate determines the outcome of Leishmania infections. “M2 → M1 plasticity” of macrophages has been seen during therapy with conventional anti-leishmanial drugs (e.g. Ref. [75]).
-
•
While the effect of cytokines and tissue milieu factors on macrophage metabolism has been studied quite extensively in recent years (reviewed in [252] and [253]), our understanding of the mutual impact of Leishmania and macrophage metabolites on each other in vitro and in vivo is still limited [254], [255]. Recently applied innovative techniques for the detection of host cell and parasite metabolites, such as in vivo heavy water labelling [256] or in vitro surface-enhanced Raman scattering [257], will certainly help to further unravel the intimate metabolic relationship between macrophages and Leishmania.
-
•
In experimental mouse models, host cytokine factors that are required for long-term Leishmania persistence in vivo have been identified, but the exact molecular mechanisms that enable Leishmania to survive still remain to be defined.
-
•
The application of the knowledge of different macrophage phenotypes for therapeutic purposes will be a major challenge. Many of the cytokine, effector and metabolic pathways discussed fulfil diverse functions, depending on the responsible parasite species, the clinical form and disease stage of leishmaniasis. Ideally, macrophage-modulatory as well as anti-parasitic therapeutics should act in a tissue- and host cell-specific, non-toxic manner. Possible concepts and prototypic drugs have been put forward [258], [259].
Credit authorship contribution statement
Christian Bogdan: Conceptualization, search and analysis of published literature, writing and editing of the entire manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
I apologize to all authors, whose work could only be acknowledged by referring to previous review articles because of space limitations. I am grateful to Dr. Katrin Paduch for the graphical design of Figure 2.
Funding
The preparation of the manuscript and some of the studies reviewed were supported by the Deutsche Forschungsgemeinschaft, Germany (project C04 within the CRC 1181 “Checkpoints for resolution of inflammation”; priority program SPP1937, grant BO996/5-1 and 5-2), the Interdisciplinary Center for Clinical Research (IZKF) of the Universitätsklinikum Erlangen, Germany (project grants A61 and A63), and the Bundesministerium für Bildung und Forschung, Germany (BMBF; Infect-Era “EpiCross”, grant 031L0126).
References
- 1.Murray H.W., Berman J.D., Davies C.R., Saravia N.G. Advances in leishmaniasis. Lancet. 2005;366:1561–1577. doi: 10.1016/S0140-6736(05)67629-5. [DOI] [PubMed] [Google Scholar]
- 2.Bogdan C. Leishmaniasis in Rheumatology, Hematology, and Oncology: Epidemiological, Immunological, and Clinical Aspects and Caveats. Ann. Rheumatic Diseases. 2012;71(suppl. 2):i60–i66. doi: 10.1136/annrheumdis-2011-200596. [DOI] [PubMed] [Google Scholar]
- 3.Burza S., Croft S.L., Boelaert M. Leishmaniasis. Lancet. 2018;392(10151):951–970. doi: 10.1016/S0140-6736(18)31204-2. [DOI] [PubMed] [Google Scholar]
- 4.C.A. Hoare, Early discoveries regarding the parasite of oriental sore (with an English translation of the memoir by P.F. Borovsky: “On Sart Sore” 1898), Trans. Roy. Soc. Trop. Med. Hyg. 32(1) (1938) 67–93.
- 5.Schmid M., Wege A.K., Ritter U. Characteristics of “Tip-DCs and MDSCs” and Their Potential Role in Leishmaniasis. Front Microbiol. 2012;3:74. doi: 10.3389/fmicb.2012.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlsen E.D., Liang Y., Shelite T.R., Walker D.H., Melby P.C., Soong L. Permissive and protective roles for neutrophils in leishmaniasis. Clin. Exp. Immunol. 2015;182(2):109–118. doi: 10.1111/cei.12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hurrell B.P., Regli I.B., Tacchini-Cottier F. Different Leishmania Species Drive Distinct Neutrophil Functions. Trends Parasitol. 2016;32(5):392–401. doi: 10.1016/j.pt.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 8.von Stebut E., Tenzer S. Cutaneous leishmaniasis: Distinct functions of dendritic cells and macrophages in the interaction of the host immune system with Leishmania major. Int. J. Med. Microbiol.: IJMM. 2018:206–214. doi: 10.1016/j.ijmm.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Bates P.A. Transmission of Leishmania metacyclic promastigotes by phlebotomine sand flies. Int. J. Parasitol. 2007;37(10):1097–1106. doi: 10.1016/j.ijpara.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Zandbergen G., Klinger M., Mueller A., Dannenberg S., Gebert A., Solbach W., Laskay T. Cutting edge: neutrophil granulocyte serves as a vector for Leishmania entry into macrophages. J. Immunol. 2004;173(11):6521–6525. doi: 10.4049/jimmunol.173.11.6521. [DOI] [PubMed] [Google Scholar]
- 11.Peters N.C., Egen J.G., Secundino N., Debrabant A., Kimblin N., Kamhawi S., Lawyer P., Fay M.P., Germain R.N., Sacks D. In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies. Science. 2008;321(5891):970–974. doi: 10.1126/science.1159194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aga E., Katschinski D.M., van Zandbergen G., Laufs H., Hansen B., Müller K., Solbach W., Laskay T. Inhibition of the spontaneous apoptosis of neutrophil granulocytes by the intracellular parasite Leishmania major. J. Immunol. 2002;169:898–905. doi: 10.4049/jimmunol.169.2.898. [DOI] [PubMed] [Google Scholar]
- 13.Ribeiro-Gomes F.L., Otero A.C., Gomes N.A., Moniz-De-Souza M.C., Cysne-Finkelstein L., Arnholdt A.C., Calich V.L., Coutinho S.G., Lopes M.F., DosReis G.A. Macrophage interactions with neutrophils regulate Leishmania major infection. J. Immunol. 2004;172(7):4454–4462. doi: 10.4049/jimmunol.172.7.4454. [DOI] [PubMed] [Google Scholar]
- 14.Tsunawaki S., Sporn M., Ding A., Nathan C. Deactivation of macrophages by transforming growth factor-β. Nature. 1988;334:260–262. doi: 10.1038/334260a0. [DOI] [PubMed] [Google Scholar]
- 15.Vodovotz Y., Bogdan C., Paik J., Xie Q.-W., Nathan C. Mechanisms of suppression of macrophage nitric oxide release by transforming growth factor-β. J. Exp. Med. 1993;178:605–613. doi: 10.1084/jem.178.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gantt K.R., Schultz-Cherry S., Rodriguez N., Jeronimo S.M., Nascimento E.T., Goldman T.L., Recker T.J., Miller M.A., Wilson M.E. Activation of TGF-beta by Leishmania chagasi: importance for parasite survival in macrophages. J. Immunol. 2003;170(5):2613–2620. doi: 10.4049/jimmunol.170.5.2613. [DOI] [PubMed] [Google Scholar]
- 17.Ribeiro-Gomes F.L., Peters N.C., Debrabant A., Sacks D.L. Efficient capture of infected neutrophils by dendritic cells in the skin inhibits the early anti-leishmania response. PLoS Pathog. 2012;8(2) doi: 10.1371/journal.ppat.1002536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurrell B.P., Schuster S., Grun E., Coutaz M., Williams R.A., Held W., Malissen B., Malissen M., Yousefi S., Simon H.U., Muller A.J., Tacchini-Cottier F. Rapid Sequestration of Leishmania mexicana by Neutrophils Contributes to the Development of Chronic Lesion. PLoS Pathog. 2015;11(5) doi: 10.1371/journal.ppat.1004929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bogdan C. Phagocyte effector functions against Leishmania parasites. In: Denkers E.Y., Gazzinelli R.T., editors. Protozoans in macrophages. Landes Bioscience; Austin, Texas: 2007. pp. 193–206. [Google Scholar]
- 20.Locksley R.M., Heinzel F.P., Fankhauser J.E., Nelson C.S., Sadick M.D. Cutaneous host defense in leishmaniasis: interaction of isolated dermal macrophages and epidermal Langerhans cells with the insect-stage promastigote. Infect. Immun. 1988;56:336–342. doi: 10.1128/iai.56.2.336-342.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blank C., Fuchs H., Rappersberger K., Röllinghoff M., Moll H. Parasitism of epidermal Langerhans cells in experimental cutaneous Leishmaniasis with Leishmania major. J. Infect. Dis. 1993;167:418–425. doi: 10.1093/infdis/167.2.418. [DOI] [PubMed] [Google Scholar]
- 22.Lee S.H., Charmoy M., Romano A., Paun A., Chaves M.M., Cope F.O., Ralph D.A., Sacks D.L. Mannose receptor high, M2 dermal macrophages mediate nonhealing Leishmania major infection in a Th1 immune environment. J. Exp. Med. 2018;215(1):357–375. doi: 10.1084/jem.20171389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blackwell J.M., Ezekowitz R.A., Roberts M.B., Channon J.Y., Sim R.B., Gordon S. Macrophage complement and lectin-like receptors bind Leishmania in the absence of serum. J. Exp. Med. 1985;162(1):324–331. doi: 10.1084/jem.162.1.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stein M., Keshav S., Harris N., Gordon S. IL-4 potently enhances murine macrophage receptor activity, a marker of alternative immunologic macrophage activation. J. Exp. Med. 1992;176:287–292. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gordon S. Alternative activation of macrophages. Nature Reviews Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 26.Lee S.H., Chaves M.M., Kamenyeva O., Gazzinelli-Guimaraes P.H., Kang B., Pessenda G., Passelli K., Tacchini-Cottier F., Kabat J., Jacobsen E.A., Nutman T.B., Sacks D.L. M2-like, dermal macrophages are maintained via IL-4/CCL24-mediated cooperative interaction with eosinophils in cutaneous leishmaniasis. Sci Immunol. 2020;5(46):eaaz4415. doi: 10.1126/sciimmunol.aaz4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akilov O.E., Kasuboski R.E., Carter C.R., McDowell M.A. The role of mannose receptor during experimental leishmaniasis. J Leukoc Biol. 2007;81(5):1188–1196. doi: 10.1189/jlb.0706439. [DOI] [PubMed] [Google Scholar]
- 28.Munder M., Eichmann M., Moran J.M., Centeno F., Soler G., Modolell M. Th1/Th2-regulated expression of arginase isoforms in murine macrophages and dendritic cells. J. Immunol. 1999;163:3771–3777. [PubMed] [Google Scholar]
- 29.El-Gayar S., Thüring-Nahler H., Pfeilschifter J., Röllinghoff M., Bogdan C. Translational control of inducible nitric oxide synthase by IL-13 and arginine availability in inflammatory macrophages. J. Immunol. 2003;171:4561–4568. doi: 10.4049/jimmunol.171.9.4561. [DOI] [PubMed] [Google Scholar]
- 30.Colotti G., Ilari A. Polyamine metabolism in Leishmania: from arginine to trypanothione. Amino Acids. 2010;40(2):269–285. doi: 10.1007/s00726-010-0630-3. [DOI] [PubMed] [Google Scholar]
- 31.Wu G., Morris S.M. Arginine metabolism: nitric oxide and beyond. Biochem. J. 1998;336:1–17. doi: 10.1042/bj3360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iniesta V., Carcelen J., Molano I., Peixoto P.M.V., Redondo E., Parra P., Mangas M., Monroy I., Campo M.L., Nieto C.G., Corraliza I.M. Arginase I induction during Leishmania major infection mediates the development of disease. Infect. Immun. 2005;73:6085–6090. doi: 10.1128/IAI.73.9.6085-6090.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kropf P., Fuentes J.M., Fahnrich E., Arpa L., Herath S., Weber V., Soler G., Celada A., Modolell M., Müller I. Arginase and polyamine synthesis are key factors in the regulation of experimental leishmaniasis in vivo. FASEB J. 2005;19:1000–1002. doi: 10.1096/fj.04-3416fje. [DOI] [PubMed] [Google Scholar]
- 34.Holscher C., Arendse B., Schwegmann A., Myburgh E., Brombacher F. Impairment of alternative macrophage activation delays cutaneous leishmaniasis in nonhealing BALB/c mice. J. Immunol. 2006;176(2):1115–1121. doi: 10.4049/jimmunol.176.2.1115. [DOI] [PubMed] [Google Scholar]
- 35.Schleicher U., Paduch K., Debus A., Obermeyer S., Konig T., Kling J.C., Ribechini E., Dudziak D., Mougiakakos D., Murray P.J., Ostuni R., Korner H., Bogdan C. TNF-Mediated Restriction of Arginase 1 Expression in Myeloid Cells Triggers Type 2 NO Synthase Activity at the Site of Infection. Cell Rep. 2016;15(5):1062–1075. doi: 10.1016/j.celrep.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Romano A., Carneiro M.B.H., Doria N.A., Roma E.H., Ribeiro-Gomes F.L., Inbar E., Lee S.H., Mendez J., Paun A., Sacks D.L., Peters N.C. Divergent roles for Ly6C+CCR2+CX3CR1+ inflammatory monocytes during primary or secondary infection of the skin with the intra-phagosomal pathogen Leishmania major. PLoS Pathog. 2017;13(6) doi: 10.1371/journal.ppat.1006479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heyde S., Philipsen L., Formaglio P., Fu Y., Baars I., Hobbel G., Kleinholz C.L., Seiss E.A., Stettin J., Gintschel P., Dudeck A., Bousso P., Schraven B., Muller A.J. CD11c-expressing Ly6C+CCR2+ monocytes constitute a reservoir for efficient Leishmania proliferation and cell-to-cell transmission. PLoS Pathog. 2018;14(10) doi: 10.1371/journal.ppat.1007374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carneiro M.B., Lopes M.E., Hohman L.S., Romano A., David B.A., Kratofil R., Kubes P., Workentine M.L., Campos A.C., Vieira L.Q., Peters N.C. Th1-Th2 Cross-Regulation Controls Early Leishmania Infection in the Skin by Modulating the Size of the Permissive Monocytic Host Cell Reservoir. Cell Host Microbe. 2020;27(5):752–768 e7. doi: 10.1016/j.chom.2020.03.011. [DOI] [PubMed] [Google Scholar]
- 39.Murray H.W. Blood monocytes: differing effector role in experimental visceral versus cutaneous leishmaniasis. Parasitol. Today. 1994;10:220–223. doi: 10.1016/0169-4758(94)90117-1. [DOI] [PubMed] [Google Scholar]
- 40.Mosser D.M., Edelson P.J. The mouse macrophage receptor for C3bi (CR3) is a major mechanism in the phagocytosis of Leishmania promastigotes. J. Immunol. 1985;135(4):2785–2789. [PubMed] [Google Scholar]
- 41.Polando R., Dixit U.G., Carter C.R., Jones B., Whitcomb J.P., Ballhorn W., Harintho M., Jerde C.L., Wilson M.E., McDowell M.A. The roles of complement receptor 3 and Fcgamma receptors during Leishmania phagosome maturation. J Leukoc Biol. 2013;93(6):921–932. doi: 10.1189/jlb.0212086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carter C.R., Whitcomb J.P., Campbell J.A., Mukbel R.M., McDowell M.A. Complement receptor 3 deficiency influences lesion progression during Leishmania major infection in BALB/c mice. Infect. Immun. 2009;77(12):5668–5675. doi: 10.1128/IAI.00802-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bogdan C., Vodovotz Y., Nathan C. Macrophage deactivation by IL-10. J. Exp. Med. 1991;174:1549–1555. doi: 10.1084/jem.174.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kane M.M., Mosser D.M. The role of IL-10 in promoting disease progression in leishmaniasis. J. Immunol. 2001;166:1141–1147. doi: 10.4049/jimmunol.166.2.1141. [DOI] [PubMed] [Google Scholar]
- 45.Miles S.A., Conrad S.M., Alves R.G., Jeronimo S.M.B., Mosser D.M. A role for IgG immune complexes during infection with the intracellular pathogen Leishmania. J. Exp. Med. 2005;201:747–754. doi: 10.1084/jem.20041470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buxbaum L.U. Interleukin-10 from T cells, but not macrophages and granulocytes, is required for chronic disease in Leishmania mexicana infection. Infect. Immun. 2015;83(4):1366–1371. doi: 10.1128/IAI.02909-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mirkovich A.M., Galelli A., Allison A.C., Modabber F.Z. Increased myelopoiesis during Leishmania major infection in mice: generation of “safe targets”, a possible way to evade the effector immune mechanism. Clin. Exp. Immunol. 1986;64:1–7. [PMC free article] [PubMed] [Google Scholar]
- 48.Abidin B.M., Hammami A., Stager S., Heinonen K.M. Infection-adapted emergency hematopoiesis promotes visceral leishmaniasis. PLoS Pathog. 2017;13(8) doi: 10.1371/journal.ppat.1006422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hammami A., Abidin B.M., Charpentier T., Fabie A., Duguay A.P., Heinonen K.M., Stager S. HIF-1alpha is a key regulator in potentiating suppressor activity and limiting the microbicidal capacity of MDSC-like cells during visceral leishmaniasis. PLoS Pathog. 2017;13(9) doi: 10.1371/journal.ppat.1006616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gutierrez Y., Maksem J.A., Reiner N.E. Pathologic changes in murine leishmaniasis (Leishmania donovani) with special reference to the dynamics of granuloma formation in the liver. Am J Pathol. 1984;114(2):222–230. [PMC free article] [PubMed] [Google Scholar]
- 51.Lepay D.A., Nathan C.F., Steinman R.M., Murray H.W., Cohn Z.A. Murine Kupffer cells. Mononuclear phagocytes deficient in the generation of reactive oxygen intermediates. J. Exp. Med. 1985;161(5):1079–1096. doi: 10.1084/jem.161.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McElrath M.J., Murray H.W., Cohn Z.A. The dynamics of granuloma formation in experimental visceral leishmaniasis. J. Exp. Med. 1988;167(6):1927–1937. doi: 10.1084/jem.167.6.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lang T., Ave P., Huerre M., Milon G., Antoine J.C. Macrophage subsets harbouring Leishmania donovani in spleens of infected BALB/c mice: localization and characterization. Cell. Microbiol. 2000;2(5):415–430. doi: 10.1046/j.1462-5822.2000.00070.x. [DOI] [PubMed] [Google Scholar]
- 54.Beattie L., Peltan A., Maroof A., Kirby A., Brown N., Coles M., Smith D.F., Kaye P.M. Dynamic imaging of experimental Leishmania donovani-induced hepatic granulomas detects Kupffer cell-restricted antigen presentation to antigen-specific CD8 T cells. PLoS Pathog. 2010;6(3) doi: 10.1371/journal.ppat.1000805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cotterell S.E., Engwerda C.R., Kaye P.M. Leishmania donovani infection of bone marrow stromal macrophages selectively enhances myelopoiesis, by a mechanism involving GM-CSF and TNF-alpha. Blood. 2000;95(5):1642–1651. [PubMed] [Google Scholar]
- 56.Terrazas C., Varikuti S., Oghumu S., Steinkamp H.M., Ardic N., Kimble J., Nakhasi H., Satoskar A.R. Ly6C(hi) inflammatory monocytes promote susceptibility to Leishmania donovani infection. Sci. Rep. 2017;7(1):14693. doi: 10.1038/s41598-017-14935-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Biswas A., Bhattacharya A., Kar S., Das P.K. Expression of IL-10-triggered STAT3-dependent IL-4Ralpha is required for induction of arginase 1 in visceral leishmaniasis. Eur J. Immunol. 2011;41(4):992–1003. doi: 10.1002/eji.201040940. [DOI] [PubMed] [Google Scholar]
- 58.Osorio E.Y., Zhao W., Espitia C., Saldarriaga O., Hawel L., Byus C.V., Travi B.L., Melby P.C. Progressive visceral leishmaniasis is driven by dominant parasite-induced STAT6 activation and STAT6-dependent host arginase 1 expression. PLoS Pathog. 2012;8(1) doi: 10.1371/journal.ppat.1002417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kong F., Saldarriaga O.A., Spratt H., Osorio E.Y., Travi B.L., Luxon B.A., Melby P.C. Transcriptional Profiling in Experimental Visceral Leishmaniasis Reveals a Broad Splenic Inflammatory Environment that Conditions Macrophages toward a Disease-Promoting Phenotype. PLoS Pathog. 2017;13(1) doi: 10.1371/journal.ppat.1006165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lefevre L., Lugo-Villarino G., Meunier E., Valentin A., Olagnier D., Authier H., Duval C., Dardenne C., Bernad J., Lemesre J.L., Auwerx J., Neyrolles O., Pipy B., Coste A. The C-type lectin receptors dectin-1, MR, and SIGNR3 contribute both positively and negatively to the macrophage response to Leishmania infantum. Immunity. 2013;38(5):1038–1049. doi: 10.1016/j.immuni.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 61.Engelhorn S., Bruckner A., Remold H.G. A soluble factor produced by inoculation of human monocytes with Leishmania donovani promastigotes suppresses IFN-γ-dependent monocyte activation. J. Immunol. 1990;145:2662–2668. [PubMed] [Google Scholar]
- 62.Reiner N.E., Ng W., Wilson C.B., McMaster W.R., Burchett S.K. Modulation of in vitro monocyte cytokine responses to Leishmania donovani. Interferon-γ prevents parasite-induced inhibition of interleukin-1 production and primes monocytes to respond to Leishmania by producing both tumor necrosis factor-α and interleukin 1. J. Clin. Invest. 1990;85:1914–1924. doi: 10.1172/JCI114654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Passwell J.H., Shor R., Smolen J., Jaffe C.L. Infection of human monocytes by Leishmania results in a defective oxidative burst. Int. J. Exp. Pathol. 1994;75:277–284. [PMC free article] [PubMed] [Google Scholar]
- 64.Roy S., Mukhopadhyay D., Mukherjee S., Ghosh S., Kumar S., Sarkar K., Pal D., Bhowmik P., Mandal K., Modak D., Guha S.K., Pramanik N., Goswami R.P., Saha B., Chatterjee M. A Defective Oxidative Burst and Impaired Antigen Presentation are Hallmarks of Human Visceral Leishmaniasis. J. Clin. Immunol. 2015;35(1):56–67. doi: 10.1007/s10875-014-0115-3. [DOI] [PubMed] [Google Scholar]
- 65.Singh N., Kumar R., Chauhan S.B., Engwerda C., Sundar S. Peripheral Blood Monocytes With an Antiinflammatory Phenotype Display Limited Phagocytosis and Oxidative Burst in Patients With Visceral Leishmaniasis. J. Infect. Dis. 2018;218(7):1130–1141. doi: 10.1093/infdis/jiy228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Passos S., Carvalho L.P., Costa R.S., Campos T.M., Novais F.O., Magalhaes A., Machado P.R., Beiting D., Mosser D., Carvalho E.M., Scott P. Intermediate monocytes contribute to pathologic immune response in Leishmania braziliensis infections. J. Infect. Dis. 2015;211(2):274–282. doi: 10.1093/infdis/jiu439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.T. Abebe, A. Hailu, M. Woldeyes, W. Mekonen, K. Bilcha, T. Cloke, L. Fry, N.K. Seich Al Basatena, K. Corware, M. Modolell, M. Munder, F. Tacchini-Cottier, I. Muller, P. Kropf, Local increase of arginase activity in lesions of patients with cutaneous leishmaniasis in Ethiopia, PLoS Negl. Trop. Dis. 6(6) (2012) e1684. [DOI] [PMC free article] [PubMed]
- 68.Mortazavi H., Sadeghipour P., Taslimi Y., Habibzadeh S., Zali F., Zahedifard F., Rahmati J., Kamyab K., Ghandi N., Zamanian A., Reza Tohidinik H., Muller I., Kropf P., Rafati S. Comparing acute and chronic human cutaneous leishmaniasis caused by Leishmania major and Leishmania tropica focusing on arginase activity. J. Eur. Acad. Dermatol. Venereol. 2016;30(12):2118–2121. doi: 10.1111/jdv.13838. [DOI] [PubMed] [Google Scholar]
- 69.Chandrasekaran P., Izadjoo S., Stimely J., Palaniyandi S., Zhu X., Tafuri W., Mosser D.M. Regulatory Macrophages Inhibit Alternative Macrophage Activation and Attenuate Pathology Associated with Fibrosis. J. Immunol. 2019;203(8):2130–2140. doi: 10.4049/jimmunol.1900270. [DOI] [PubMed] [Google Scholar]
- 70.J. Franca-Costa, J. Van Weyenbergh, V.S. Boaventura, N.F. Luz, H. Malta-Santos, M.C. Oliveira, D.C. Santos de Campos, A.C. Saldanha, W.L. dos-Santos, P.T. Bozza, M. Barral-Netto, A. Barral, J.M. Costa, V.M. Borges, Arginase I, polyamine, and prostaglandin E2 pathways suppress the inflammatory response and contribute to diffuse cutaneous leishmaniasis, J. Infect. Dis. 211(3) (2015) 426–35. [DOI] [PubMed]
- 71.Christensen S.M., Belew A.T., El-Sayed N.M., Tafuri W.L., Silveira F.T., Mosser D.M. Host and parasite responses in human diffuse cutaneous leishmaniasis caused by L. amazonensis. PLoS Negl. Trop. Dis. 2019;13(3) doi: 10.1371/journal.pntd.0007152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wilkins-Rodriguez A.A., Perez-Torres A., Escalona-Montano A.R., Gutierrez-Kobeh L. Differential Regulation of l-Arginine Metabolism through Arginase 1 during Infection with Leishmania mexicana Isolates Obtained from Patients with Localized and Diffuse Cutaneous Leishmaniasis. Infect. Immun. 2020;88(7) doi: 10.1128/IAI.00963-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Qadoumi M., Becker I., Donhauser N., Röllinghoff M., Bogdan C. Expression of inducible nitric oxide synthase in skin lesions of patients with American cutaneous leishmaniosis. Infect. Immun. 2002;70:4638–4642. doi: 10.1128/IAI.70.8.4638-4642.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abebe T., Takele Y., Weldegebreal T., Cloke T., Closs E., Corset C., Hailu A., Hailu W., Sisay Y., Corware K., Corset M., Modolell M., Munder M., Tacchini-Cottier F., Muller I., Kropf P. Arginase activity - a marker of disease status in patients with visceral leishmaniasis in ethiopia. PLoS Negl. Trop. Dis. 2013;7(3) doi: 10.1371/journal.pntd.0002134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mukhopadhyay D., Mukherjee S., Roy S., Dalton J.E., Kundu S., Sarkar A., Das N.K., Kaye P.M., Chatterjee M. M2 Polarization of Monocytes-Macrophages Is a Hallmark of Indian Post Kala-Azar Dermal Leishmaniasis. PLoS Negl. Trop. Dis. 2015;9(10) doi: 10.1371/journal.pntd.0004145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bogdan C., Gessner A., Röllinghoff M. Cytokines in Leishmaniasis: a complex network of stimulatory and inhibitory interactions. Immunobiol. 1993;189:356–396. doi: 10.1016/S0171-2985(11)80366-9. [DOI] [PubMed] [Google Scholar]
- 77.Scharton-Kersten T.M., Sher A. Role of natural killer cells in innate resistance to protozoan infections. Curr. Opin. Immunol. 1997;9:44–51. doi: 10.1016/s0952-7915(97)80157-4. [DOI] [PubMed] [Google Scholar]
- 78.Liese J., Schleicher U., Bogdan C. The innate immune response against Leishmania parasites. Immunobiology. 2008;213(3–4):377–387. doi: 10.1016/j.imbio.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 79.Bogdan C. Natural killer cells in experimental and human leishmaniasis. Front. Cell. Infect. Microbiol. 2012;2(69):1–9. doi: 10.3389/fcimb.2012.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Prajeeth C.K., Haeberlein S., Sebald H., Schleicher U., Bogdan C. Leishmania-infected macrophages are targets of NK cell-derived cytokines, but not of NK cell cytotoxicity. Infect. Immun. 2011;79:2699–2708. doi: 10.1128/IAI.00079-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Decker T., Stockinger S., Karaghiosoff M., Müller M., Kovarik P. IFNs and STATs in innate immunity to microorganisms. J. Clin. Invest. 2002;109:1271–1277. doi: 10.1172/JCI15770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ivashkiv L.B. IFNgamma: signalling, epigenetics and roles in immunity, metabolism, disease and cancer immunotherapy. Nat. Rev. Immunol. 2018;18(9):545–558. doi: 10.1038/s41577-018-0029-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sacks D.L., Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nat. Rev. Immunol. 2002;2:845–858. doi: 10.1038/nri933. [DOI] [PubMed] [Google Scholar]
- 84.Wakil A.E., Wang Z.E., Ryan J.C., Fowell D.J., Locksley R.M. Interferon gamma derived from CD4(+) T cells is sufficient to mediate T helper cell type 1 development. J. Exp. Med. 1998;188(9):1651–1656. doi: 10.1084/jem.188.9.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Suzue K., Kobayashi S., Takeuchi T., Suzuki M., Koyasu S. Critical role of dendritic cells in determining the Th1/Th2 balance upon Leishmania major infection. Int. Immunol. 2008;20(3):337–343. doi: 10.1093/intimm/dxm147. [DOI] [PubMed] [Google Scholar]
- 86.Brewig N., Kissenpfennig A., Malissen B., Veit A., Bickert T., Fleischer B., Mostbock S., Ritter U. Priming of CD8+ and CD4+ T cells in experimental leishmaniasis is initiated by different dendritic cell subtypes. J. Immunol. 2009;182(2):774–783. doi: 10.4049/jimmunol.182.2.774. [DOI] [PubMed] [Google Scholar]
- 87.Ashok D., Schuster S., Ronet C., Rosa M., Mack V., Lavanchy C., Marraco S.F., Fasel N., Murphy K.M., Tacchini-Cottier F., Acha-Orbea H. Cross-presenting dendritic cells are required for control of Leishmania major infection. Eur J. Immunol. 2014;44(5):1422–1432. doi: 10.1002/eji.201344242. [DOI] [PubMed] [Google Scholar]
- 88.Lykens J.E., Terrell C.E., Zoller E.E., Divanovic S., Trompette A., Karp C.L., Aliberti J., Flick M.J., Jordan M.B. Mice with a selective impairment of IFN-gamma signaling in macrophage lineage cells demonstrate the critical role of IFN-gamma-activated macrophages for the control of protozoan parasitic infections in vivo. J. Immunol. 2010;184(2):877–885. doi: 10.4049/jimmunol.0902346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Muller A.J., Filipe-Santos O., Eberl G., Aebischer T., Spath G.F., Bousso P. CD4(+) T Cells Rely on a Cytokine Gradient to Control Intracellular Pathogens beyond Sites of Antigen Presentation. Immunity. 2012;37(1):147–157. doi: 10.1016/j.immuni.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 90.Wanasen N., MacLeod C.L., Ellies L.G., Soong L. L-arginine and cationic amino acid transporter 2B regulate growth and survival of Leishmania amazonensis amastigotes in macrophages. Infect. Immun. 2007;75(6):2802–2810. doi: 10.1128/IAI.00026-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wanasen N., Soong L. L-arginine metabolism and its impact on host immunity against Leishmania infection. Immunol. Res. 2008;41(1):15–25. doi: 10.1007/s12026-007-8012-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Murray P.J., Allen J.E., Biswas S.K., Fisher E.A., Gilroy D.W., Goerdt S., Gordon S., Hamilton J.A., Ivashkiv L.B., Lawrence T., Locati M., Mantovani A., Martinez F.O., Mege J.L., Mosser D.M., Natoli G., Saeij J.P., Schultze J.L., Shirey K.A., Sica A., Suttles J., Udalova I., van Ginderachter J.A., Vogel S.N., Wynn T.A. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41(1):14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bogdan C., Nathan C. Modulation of macrophage function by transforming growth factor-β, interleukin 4 and interleukin 10. Ann. New York Acad. Sci. 1993;685:713–739. doi: 10.1111/j.1749-6632.1993.tb35934.x. [DOI] [PubMed] [Google Scholar]
- 94.Stenger S., Solbach W., Röllinghoff M., Bogdan C. Cytokine interactions in experimental cutaneous leishmaniasis. II. Endogenous tumor necrosis factor-α production by macrophages is induced by the synergistic action of interferon (IFN)-γ and interleukin (IL) 4 and accounts for the antiparasitic effect mediated by IFN-γ and IL-4. Eur. J. Immunol. 1991;21:1669–1675. doi: 10.1002/eji.1830210713. [DOI] [PubMed] [Google Scholar]
- 95.Hurdayal R., Nieuwenhuizen N.E., Revaz-Breton M., Smith L., Hoving J.C., Parihar S.P., Reizis B., Brombacher F. Deletion of IL-4 receptor alpha on dendritic cells renders BALB/c mice hypersusceptible to Leishmania major infection. PLoS Pathog. 2013;9(10) doi: 10.1371/journal.ppat.1003699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hurdayal R., Nieuwenhuizen N.E., Khutlang R., Brombacher F. Inflammatory Dendritic Cells, Regulated by IL-4 Receptor Alpha Signaling, Control Replication, and Dissemination of Leishmania major in Mice. Front. Cell Infect. Microbiol. 2019;9:479. doi: 10.3389/fcimb.2019.00479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Alexander J., Brombacher F. T helper1/t helper2 cells and resistance/susceptibility to leishmania infection: is this paradigm still relevant? Front. Immunol. 2012;3:80. doi: 10.3389/fimmu.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bogdan C. Reactive oxygen and reactive nitrogen intermediates in the immune system. In: Kaufmann S.H.E., Rouse B.T., Sacks D.L., editors. The Immune response to Infection. ASM Press; Washington, D.C.: 2011. pp. 69–84. [Google Scholar]
- 99.Nathan C.F., Murray H.W., Wiebe M.E., Rubin B.Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J. Exp. Med. 1983;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Murray H.W., Rubin B.Y., Rothermel C.D. Killing of intracellular Leishmania donovani by lymphokine-stimulated human mononuclear phagocytes: evidence that IFN-γ is the activating lymphokine. J. Clin. Invest. 1983;72:1506–1510. doi: 10.1172/JCI111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nathan C.F., Prendergast T.J., Wiebe M.E., Stanley E.R., Platzer E., Remold H.G., Welte K., Rubin B.Y., Murray H.W. Activation of human macrophages. Comparison of other cytokines with interferon-γ. J. Exp. Med. 1984;160:600–605. doi: 10.1084/jem.160.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Farrar M.A., Schreiber R.D. The molecular cell biology of interferon-γ and its receptor. Ann. Rev. Immunol. 1993;11:571–611. doi: 10.1146/annurev.iy.11.040193.003035. [DOI] [PubMed] [Google Scholar]
- 103.Murray H.W. Interferon-g and host antimicrobial defense: current and future clinical applications. Amer. J. Med. 1994;97(5):459–467. doi: 10.1016/0002-9343(94)90326-3. [DOI] [PubMed] [Google Scholar]
- 104.Conrad S.M., Strauss-Ayali D., Field A.E., Mack M., Mosser D.M. Leishmania-derived murine monocyte chemoattractant protein 1 enhances the recruitment of a restrictive population of CC chemokine receptor 2-positive macrophages. Infect. Immun. 2007;75(2):653–665. doi: 10.1128/IAI.01314-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Goncalves R., Zhang X., Cohen H., Debrabant A., Mosser D.M. Platelet activation attracts a subpopulation of effector monocytes to sites of Leishmania major infection. J. Exp. Med. 2011;208(6):1253–1265. doi: 10.1084/jem.20101751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Novais F.O., Nguyen B.T., Beiting D.P., Carvalho L.P., Glennie N.D., Passos S., Carvalho E.M., Scott P. Human classical monocytes control the intracellular stage of Leishmania braziliensis by reactive oxygen species. J. Infect. Dis. 2014;209(8):1288–1296. doi: 10.1093/infdis/jiu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Murray H.W., Nathan C.F. Macrophage microbicidal mechanisms in vivo: reactive nitrogen vs. oxygen intermediates in the killing of intracellular visceral Leishmania donovani. J. Exp. Med. 1999;189:741–746. doi: 10.1084/jem.189.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Blos M., Schleicher U., Rocha F.J., Meissner U., Röllinghoff M., Bogdan C. Organ-specific and stage-dependent control of Leishmania major infection by inducible nitric oxide synthase and phagocyte NADPH oxidase. Eur. J. Immunol. 2003;33:1224–1234. doi: 10.1002/eji.200323825. [DOI] [PubMed] [Google Scholar]
- 109.White J.K., Mastroeni P., Popoff J.-F., Evans C.A.W., Blackwell J.M. Slc11a1-mediated resistance to Salmonella enterica serovar typhimurium and Leishmania donovani infections does not require functional inducible nitric oxide synthase or phagocyte oxidase activity. J. Leukocyt. Biol. 2005;77:311–320. doi: 10.1189/jlb.0904546. [DOI] [PubMed] [Google Scholar]
- 110.Roma E.H., Macedo J.P., Goes G.R., Goncalves J.L., Castro W., Cisalpino D., Vieira L.Q. Impact of reactive oxygen species (ROS) on the control of parasite loads and inflammation in Leishmania amazonensis infection. Parasites Vectors. 2016;9:193. doi: 10.1186/s13071-016-1472-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Finocchi A., Palma P., Di Matteo G., Chiriaco M., Lancella L., Simonetti A., Rana I., Livadiotti S., Rossi P. Visceral leishmaniasis revealing chronic granulomatous disease in a child. Int. J. Immunopathol. Pharmacol. 2008;21(3):739–743. doi: 10.1177/039463200802100330. [DOI] [PubMed] [Google Scholar]
- 112.Martin A., Marques L., Soler-Palacin P., Caragol I., Hernandez M., Figueras C., Espanol T. Visceral leishmaniasis associated hemophagocytic syndrome in patients with chronic granulomatous disease. Pediatr. Infect. Dis. J. 2009;28(8):753–754. doi: 10.1097/INF.0b013e31819c6f3a. [DOI] [PubMed] [Google Scholar]
- 113.Ding A.H., Nathan C.F., Stuehr D.J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J. Immunol. 1988;141:2407–2412. [PubMed] [Google Scholar]
- 114.Bogdan C. Nitric oxide synthase in innate and adaptive immunity: an update. Trends Immunol. 2015;36(3):161–178. doi: 10.1016/j.it.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 115.Stenger S., Thüring H., Röllinghoff M., Bogdan C. Tissue expression of inducible nitric oxide synthase is closely associated with resistance to Leishmania major. J. Exp. Med. 1994;180:783–793. doi: 10.1084/jem.180.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liew F.Y., Li Y., Millott S. Tumor necrosis factor-α synergizes with IFN-γ in mediating killing of Leishmania major through the induction of nitric oxide. J. Immunol. 1990;145:4306–4310. [PubMed] [Google Scholar]
- 117.F.Y. Liew, S. Millott, C. Parkinson, R. Palmer, M. J., S. Moncada, Macrophage killing of Leishmania parasite in vivo is mediated by nitric oxide from L-arginine, J. Immunol. 144 (1990) 4794–4797. [PubMed]
- 118.Wei X.-Q., Charles I.G., Smith A., Ure J., Feng G.-J., Huang F.-P., Xu D., Müller W., Moncada S., Liew F.Y. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995;375:408–411. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- 119.Diefenbach A., Schindler H., Donhauser N., Lorenz E., Laskay T., MacMicking J., Röllinghoff M., Gresser I., Bogdan C. Type 1 interferon (IFN-α/β) and type 2 nitric oxide synthase regulate the innate immune response to a protozoan parasite. Immunity. 1998;8:77–87. doi: 10.1016/s1074-7613(00)80460-4. [DOI] [PubMed] [Google Scholar]
- 120.Stenger S., Donhauser N., Thüring H., Röllinghoff M., Bogdan C. Reactivation of latent leishmaniasis by inhibition of inducible nitric oxide synthase. J. Exp. Med. 1996;183:1501–1514. doi: 10.1084/jem.183.4.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Muller A.J., Aeschlimann S., Olekhnovitch R., Dacher M., Spath G.F., Bousso P. Photoconvertible pathogen labeling reveals nitric oxide control of Leishmania major infection in vivo via dampening of parasite metabolism. Cell Host Microbe. 2013;14(4):460–467. doi: 10.1016/j.chom.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 122.Olekhnovitch R., Ryffel B., Muller A.J., Bousso P. Collective nitric oxide production provides tissue-wide immunity during Leishmania infection. J. Clin. Investig. 2014;124(4):1711–1722. doi: 10.1172/JCI72058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bogdan C., Donhauser N., Döring R., Röllinghoff M., Diefenbach A., Rittig M.G. Fibroblasts as host cells in latent leishmaniosis. J. Exp. Med. 2000;191:2121–2129. doi: 10.1084/jem.191.12.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bogdan C. Mechanisms and consequences of persistence of intracellular pathogens: leishmaniasis as an example. Cell. Microbiol. 2008;10:1221–1234. doi: 10.1111/j.1462-5822.2008.01146.x. [DOI] [PubMed] [Google Scholar]
- 125.Green S.J., Crawford R.M., Hockmeyer J.T., Meltzer M.S., Nacy C.N. Leishmania major amastigotes initiate the L-arginine-dependent killing mechanism in IFN-γ stimulated macrophages by induction of tumor necrosis factor-α. J. Immunol. 1990;145:4290–4297. [PubMed] [Google Scholar]
- 126.Wilhelm P., Ritter U., Labbow S., Donhauser N., Röllinghoff M., Bogdan C., Körner H. Rapidly fatal leishmaniasis in resistant C57BL/6 mice lacking tumor necrosis factor. J. Immunol. 2001;166:4012–4019. doi: 10.4049/jimmunol.166.6.4012. [DOI] [PubMed] [Google Scholar]
- 127.Bogdan C. Regulation and antimicrobial function of inducible nitric oxide synthase in phagocytes. In: Russell D., Gordon S., editors. Phagocyte-pathogen interaction. ASM Press; Washington, D.C.: 2009. pp. 367–378. [Google Scholar]
- 128.Kalliolias G.D., Ivashkiv L.B. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat. Rev. Rheumatol. 2016;12(1):49–62. doi: 10.1038/nrrheum.2015.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Schleicher U., Liese J., Justies N., Mischke T., Haeberlein S., Sebald H., Kalinke U., Weiss S., Bogdan C. Type I Interferon Signaling Is Required for CpG-Oligodesoxynucleotide-Induced Control of Leishmania major, but Not for Spontaneous Cure of Subcutaneous Primary or Secondary L. major Infection. Front. Immunol. 2018;9:79. doi: 10.3389/fimmu.2018.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mattner J., Wandersee-Steinhäuser A., Pahl A., Röllinghoff M., Majeau G.R., Hochman P.S., Bogdan C. Protection against progressive leishmaniasis by IFN-β. J. Immunol. 2004;172:7574–7582. doi: 10.4049/jimmunol.172.12.7574. [DOI] [PubMed] [Google Scholar]
- 131.Mattner J., Schindler H., Diefenbach A., Röllinghoff M., Gresser I., Bogdan C. Regulation of type 2 NO synthase by type I interferons in macrophages infected with Leishmania major. Eur. J. Immunol. 2000;30:2257–2267. doi: 10.1002/1521-4141(2000)30:8<2257::AID-IMMU2257>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 132.Bogdan C., Mattner J., Schleicher U. The role of type I interferons in non-viral infections. Immunol. Rev. 2004;202:33–48. doi: 10.1111/j.0105-2896.2004.00207.x. [DOI] [PubMed] [Google Scholar]
- 133.Mahnke A., Meier R.J., Schatz V., Hofmann J., Castiglione K., Schleicher U., Wolfbeis O.S., Bogdan C., Jantsch J. Hypoxia in Leishmania major Skin Lesions Impairs the NO-Dependent Leishmanicidal Activity of Macrophages. J. Invest. Dermatol. 2014;134(9):2339–2346. doi: 10.1038/jid.2014.121. [DOI] [PubMed] [Google Scholar]
- 134.Martinez I., Nedredal G.I., Oie C.I., Warren A., Johansen O., Le Couteur D.G., Smedsrod B. The influence of oxygen tension on the structure and function of isolated liver sinusoidal endothelial cells. Comp. Hepatol. 2008;7:4. doi: 10.1186/1476-5926-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Spencer J.A., Ferraro F., Roussakis E., Klein A., Wu J., Runnels J.M., Zaher W., Mortensen L.J., Alt C., Turcotte R., Yusuf R., Cote D., Vinogradov S.A., Scadden D.T., Lin C.P. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature. 2014;508(7495):269–273. doi: 10.1038/nature13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Schatz V., Strussmann Y., Mahnke A., Schley G., Waldner M., Ritter U., Wild J., Willam C., Dehne N., Brune B., McNiff J.M., Colegio O.R., Bogdan C., Jantsch J. Myeloid Cell-Derived HIF-1alpha Promotes Control of Leishmania major. J. Immunol. 2016;197(10):4034–4041. doi: 10.4049/jimmunol.1601080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Weinkopff T., Roys H., Bowlin A., Scott P. Leishmania Infection Induces Macrophage Vascular Endothelial Growth Factor A Production in an ARNT/HIF-Dependent Manner. Infect. Immun. 2019;87(11) doi: 10.1128/IAI.00088-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Melillo G., Musso T., Sica A., Taylor L.S., Cox G.W., Varesio L. A hypoxia-responsive element mediates a novel pathway of activation of the inducible nitric oxide synthase promoter. J. Exp. Med. 1995;182(6):1683–1693. doi: 10.1084/jem.182.6.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jantsch J., Schodel J. Hypoxia and hypoxia-inducible factors in myeloid cell-driven host defense and tissue homeostasis. Immunobiology. 2015;220(2):305–314. doi: 10.1016/j.imbio.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 140.Degrossoli A., Arrais-Silva W.W., Colhone M.C., Gadelha F.R., Joazeiro P.P., Giorgio S. The influence of low oxygen on macrophage response to Leishmania infection. Scand. J. Immunol. 2011;74(2):165–175. doi: 10.1111/j.1365-3083.2011.02566.x. [DOI] [PubMed] [Google Scholar]
- 141.Alonso D., Serrano E., Bermejo F.J., Corral R.S. HIF-1alpha-regulated MIF activation and Nox2-dependent ROS generation promote Leishmania amazonensis killing by macrophages under hypoxia. Cell Immunol. 2019;335:15–21. doi: 10.1016/j.cellimm.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 142.I. Mesquita, C. Ferreira, D. Moreira, G.E.G. Kluck, A.M. Barbosa, E. Torrado, R.J. Dinis-Oliveira, L.G. Goncalves, C.J. Beauparlant, A. Droit, L. Berod, T. Sparwasser, N. Bodhale, B. Saha, F. Rodrigues, C. Cunha, A. Carvalho, A.G. Castro, J. Estaquier, R. Silvestre, The Absence of HIF-1alpha Increases Susceptibility to Leishmania donovani Infection via Activation of BNIP3/mTOR/SREBP-1c Axis, Cell Rep. 30(12) (2020) 4052–4064 e7. [DOI] [PubMed]
- 143.Hammami A., Charpentier T., Smans M., Stager S. IRF-5-Mediated Inflammation Limits CD8+ T Cell Expansion by Inducing HIF-1alpha and Impairing Dendritic Cell Functions during Leishmania Infection. PLoS Pathog. 2015;11(6) doi: 10.1371/journal.ppat.1004938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hammami A., Abidin B.M., Heinonen K.M., Stager S. HIF-1alpha hampers dendritic cell function and Th1 generation during chronic visceral leishmaniasis. Sci. Rep. 2018;8(1):3500. doi: 10.1038/s41598-018-21891-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Schatz V., Neubert P., Schroder A., Binger K., Gebhard M., Muller D.N., Luft F.C., Titze J., Jantsch J. Elementary immunology: Na(+) as a regulator of immunity. Pediatr. Nephrol. 2017;32(2):201–210. doi: 10.1007/s00467-016-3349-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shapiro L., Dinarello C.A. Osmotic regulation of cytokine synthesis in vitro. Proc. Natl. Acad. Sci. USA. 1995;92(26):12230–12234. doi: 10.1073/pnas.92.26.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Machnik A., Neuhofer W., Jantsch J., Dahlmann A., Tammela T., Machura K., Park J.K., Beck F.X., Muller D.N., Derer W., Goss J., Ziomber A., Dietsch P., Wagner H., van Rooijen N., Kurtz A., Hilgers K.F., Alitalo K., Eckardt K.U., Luft F.C., Kerjaschki D., Titze J. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat. Med. 2009;15(5):545–552. doi: 10.1038/nm.1960. [DOI] [PubMed] [Google Scholar]
- 148.Ip W.K., Medzhitov R. Macrophages monitor tissue osmolarity and induce inflammatory response through NLRP3 and NLRC4 inflammasome activation. Nat. Commun. 2015;6:6931. doi: 10.1038/ncomms7931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kleinewietfeld M., Manzel A., Titze J., Kvakan H., Yosef N., Linker R.A., Muller D.N., Hafler D.A. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature. 2013;496(7446):518–522. doi: 10.1038/nature11868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wu C., Yosef N., Thalhamer T., Zhu C., Xiao S., Kishi Y., Regev A., Kuchroo V.K. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature. 2013;496(7446):513–517. doi: 10.1038/nature11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hernandez A.L., Kitz A., Wu C., Lowther D.E., Rodriguez D.M., Vudattu N., Deng S., Herold K.C., Kuchroo V.K., Kleinewietfeld M., Hafler D.A. Sodium chloride inhibits the suppressive function of FOXP3+ regulatory T cells. J. Clin. Investig. 2015;125(11):4212–4222. doi: 10.1172/JCI81151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Binger K.J., Gebhardt M., Heinig M., Rintisch C., Schroeder A., Neuhofer W., Hilgers K., Manzel A., Schwartz C., Kleinewietfeld M., Voelkl J., Schatz V., Linker R.A., Lang F., Voehringer D., Wright M.D., Hubner N., Dechend R., Jantsch J., Titze J., Muller D.N. High salt reduces the activation of IL-4- and IL-13-stimulated macrophages. J. Clin. Investig. 2015;125(11):4223–4238. doi: 10.1172/JCI80919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Buxade M., Lunazzi G., Minguillon J., Iborra S., Berga-Bolanos R., Del Val M., Aramburu J., Lopez-Rodriguez C. Gene expression induced by Toll-like receptors in macrophages requires the transcription factor NFAT5. J. Exp. Med. 2012;209(2):379–393. doi: 10.1084/jem.20111569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jantsch J., Schatz V., Friedrich D., Schroder A., Kopp C., Siegert I., Maronna A., Wendelborn D., Linz P., Binger K.J., Gebhardt M., Heinig M., Neubert P., Fischer F., Teufel S., David J.P., Neufert C., Cavallaro A., Rakova N., Kuper C., Beck F.X., Neuhofer W., Muller D.N., Schuler G., Uder M., Bogdan C., Luft F.C., Titze J. Cutaneous Na+ storage strengthens the antimicrobial barrier function of the skin and boosts macrophage-driven host defense. Cell Metab. 2015;21(3):493–501. doi: 10.1016/j.cmet.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Neubert P., Homann A., Wendelborn D., Bar A.L., Krampert L., Trum M., Schroder A., Ebner S., Weichselbaum A., Schatz V., Linz P., Veelken R., Schulte-Schrepping J., Aschenbrenner A.C., Quast T., Kurts C., Geisberger S., Kunzelmann K., Hammer K., Binger K.J., Titze J., Muller D.N., Kolanus W., Schultze J.L., Wagner S., Jantsch J. NCX1 represents an ionic Na+ sensing mechanism in macrophages. PLoS Biol. 2020;18(6) doi: 10.1371/journal.pbio.3000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tellechea M., Buxade M., Tejedor S., Aramburu J., Lopez-Rodriguez C. NFAT5-Regulated Macrophage Polarization Supports the Proinflammatory Function of Macrophages and T Lymphocytes. J. Immunol. 2018;200(1):305–315. doi: 10.4049/jimmunol.1601942. [DOI] [PubMed] [Google Scholar]
- 157.Murray H.W., Szuro-Sudol A., Wellner D., Oca M.J., Granger A.M., Libby D.M., Rothermel C.D., Rubin B.Y. Role of tryptophan degradation in respiratory burst-independent antimicrobial activity of gamma interferon-stimulated human macrophages. Infect. Immun. 1989;57:845–849. doi: 10.1128/iai.57.3.845-849.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Thomas S.R., Stocker R. Redox reactions related to indoleamine 2,3-dioxygenase and tryptophan metabolism along the kynurenine pathway. Redox Rep. 1999;4(5):199–220. doi: 10.1179/135100099101534927. [DOI] [PubMed] [Google Scholar]
- 159.Bourreau E., Ronet C., Darcissac E., Lise M.C., Sainte Marie D., Clity E., Tacchini-Cottier F., Couppie P., Launois P. Intralesional regulatory T-cell suppressive function during human acute and chronic cutaneous leishmaniasis due to Leishmania guyanensis. Infect. Immun. 2009;77(4):1465–1474. doi: 10.1128/IAI.01398-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Makala L.H., Baban B., Lemos H., El-Awady A.R., Chandler P.R., Hou D.Y., Munn D.H., Mellor A.L. Leishmania major attenuates host immunity by stimulating local indoleamine 2,3-dioxygenase expression. J. Infect. Dis. 2011;203(5):715–725. doi: 10.1093/infdis/jiq095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Donovan M.J., Tripathi V., Favila M.A., Geraci N.S., Lange M.C., Ballhorn W., McDowell M.A. Indoleamine 2,3-dioxygenase (IDO) induced by Leishmania infection of human dendritic cells. Parasite Immunol. 2012;34(10):464–472. doi: 10.1111/j.1365-3024.2012.01380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gangneux J.P., Poinsignon Y., Donaghy L., Amiot L., Tarte K., Mary C., Robert-Gangneux F. Indoleamine 2,3-dioxygenase activity as a potential biomarker of immune suppression during visceral leishmaniasis. Innate Immun. 2013;19(6):564–568. doi: 10.1177/1753425912473170. [DOI] [PubMed] [Google Scholar]
- 163.Romani L., Fallarino F., De Luca A., Montagnoli C., D'Angelo C., Zelante T., Vacca C., Bistoni F., Fioretti M.C., Grohmann U., Segal B.H., Puccetti P. Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature. 2008;451(7175):211–215. doi: 10.1038/nature06471. [DOI] [PubMed] [Google Scholar]
- 164.Boeltz S., Amini P., Anders H.J., Andrade F., Bilyy R., Chatfield S., Cichon I., Clancy D.M., Desai J., Dumych T., Dwivedi N., Gordon R.A., Hahn J., Hidalgo A., Hoffmann M.H., Kaplan M.J., Knight J.S., Kolaczkowska E., Kubes P., Leppkes M., Manfredi A.A., Martin S.J., Maueroder C., Maugeri N., Mitroulis I., Munoz L.E., Nakazawa D., Neeli I., Nizet V., Pieterse E., Radic M.Z., Reinwald C., Ritis K., Rovere-Querini P., Santocki M., Schauer C., Schett G., Shlomchik M.J., Simon H.U., Skendros P., Stojkov D., Vandenabeele P., Berghe T.V., van der Vlag J., Vitkov L., von Kockritz-Blickwede M., Yousefi S., Zarbock A., Herrmann M. To NET or not to NET:current opinions and state of the science regarding the formation of neutrophil extracellular traps. Cell Death Differ. 2019;26(3):395–408. doi: 10.1038/s41418-018-0261-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Guimaraes-Costa A.B., Nascimento M.T., Froment G.S., Soares R.P., Morgado F.N., Conceicao-Silva F., Saraiva E.M. Leishmania amazonensis promastigotes induce and are killed by neutrophil extracellular traps. Proc. Natl. Acad. Sci. USA. 2009;106(16):6748–6753. doi: 10.1073/pnas.0900226106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gabriel C., McMaster W.R., Girard D., Descoteaux A. Leishmania donovani promastigotes evade the antimicrobial activity of neutrophil extracellular traps. J. Immunol. 2010;185(7):4319–4327. doi: 10.4049/jimmunol.1000893. [DOI] [PubMed] [Google Scholar]
- 167.N.C. Rochael, A.B. Guimaraes-Costa, M.T. Nascimento, T.S. DeSouza-Vieira, M.P. Oliveira, L.F. Garcia e Souza, M.F. Oliveira, E.M. Saraiva, Classical ROS-dependent and early/rapid ROS-independent release of Neutrophil Extracellular Traps triggered by Leishmania parasites, Sci. Rep. 5 (2015) 18302. [DOI] [PMC free article] [PubMed]
- 168.Guimaraes-Costa A.B., DeSouza-Vieira T.S., Paletta-Silva R., Freitas-Mesquita A.L., Meyer-Fernandes J.R., Saraiva E.M. 3'-nucleotidase/nuclease activity allows Leishmania parasites to escape killing by neutrophil extracellular traps. Infect. Immun. 2014;82(4):1732–1740. doi: 10.1128/IAI.01232-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Doster R.S., Rogers L.M., Gaddy J.A., Aronoff D.M. Macrophage Extracellular Traps: A Scoping Review. J. Innate Immun. 2018;10(1):3–13. doi: 10.1159/000480373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Stenger S., Hanson D.A., Teitelbaum R., Dewan P., Niazi K.R., Froelich C.J., Ganz T., Thoma-Uszynski S., Melian A., Bogdan C., Porcelli S.A., Bloom B.R., Kresnky A.M., Modlin R.L. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science. 1998;282:121–125. doi: 10.1126/science.282.5386.121. [DOI] [PubMed] [Google Scholar]
- 171.Kulkarni M.M., McMaster W.R., Kamysz E., Kamysz W., Engman D.M., McGwire B.S. The major surface-metalloprotease of the parasitic protozoan, Leishmania, protects against antimicrobial peptide-induced apoptotic killing. Mol. Microbiol. 2006;62(5):1484–1497. doi: 10.1111/j.1365-2958.2006.05459.x. [DOI] [PubMed] [Google Scholar]
- 172.Zahedifard F., Rafati S. Prospects for antimicrobial peptide-based immunotherapy approaches in Leishmania control. Expert Rev. Anti Infect. Ther. 2018;16(6):461–469. doi: 10.1080/14787210.2018.1483720. [DOI] [PubMed] [Google Scholar]
- 173.Cole J.N., Nizet V. Bacterial Evasion of Host Antimicrobial Peptide Defenses. Microbiol. Spectr. 2016;4(1) doi: 10.1128/microbiolspec.VMBF-0006-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kulkarni M.M., Barbi J., McMaster W.R., Gallo R.L., Satoskar A.R., McGwire B.S. Mammalian antimicrobial peptide influences control of cutaneous Leishmania infection. Cell. Microbiol. 2011;13(6):913–923. doi: 10.1111/j.1462-5822.2011.01589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Crauwels P., Bank E., Walber B., Wenzel U.A., Agerberth B., Chanyalew M., Abebe M., Konig R., Ritter U., Reiling N., van Zandbergen G. Cathelicidin Contributes to the Restriction of Leishmania in Human Host Macrophages. Front. Immunol. 2019;10:2697. doi: 10.3389/fimmu.2019.02697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Carrera L., Gazzinelli R.T., Badolato R., Hieny S., Müller W., Kühn R., Sacks D.L. Leishmania promastigotes selectively inhibit interleukin-12 induction in bone marrow-derived macrophages from susceptible and resistant mice. J. Exp. Med. 1996;183:515–526. doi: 10.1084/jem.183.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chandrakar P., Parmar N., Descoteaux A., Kar S. Differential Induction of SOCS Isoforms by Leishmania donovani Impairs Macrophage-T Cell Cross-Talk and Host Defense. J. Immunol. 2020;204(3):596–610. doi: 10.4049/jimmunol.1900412. [DOI] [PubMed] [Google Scholar]
- 178.Shio M.T., Christian J.G., Jung J.Y., Chang K.P., Olivier M. PKC/ROS-Mediated NLRP3 Inflammasome Activation Is Attenuated by Leishmania Zinc-Metalloprotease during Infection. PLoS Negl. Trop. Dis. 2015;9(6) doi: 10.1371/journal.pntd.0003868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lima-Junior D.S., Costa D.L., Carregaro V., Cunha L.D., Silva A.L., Mineo T.W., Gutierrez F.R., Bellio M., Bortoluci K.R., Flavell R.A., Bozza M.T., Silva J.S., Zamboni D.S. Inflammasome-derived IL-1beta production induces nitric oxide-mediated resistance to Leishmania. Nat. Med. 2013;19(7):909–915. doi: 10.1038/nm.3221. [DOI] [PubMed] [Google Scholar]
- 180.Charmoy M., Hurrell B.P., Romano A., Lee S.H., Ribeiro-Gomes F., Riteau N., Mayer-Barber K., Tacchini-Cottier F., Sacks D.L. The Nlrp3 inflammasome, IL-1beta, and neutrophil recruitment are required for susceptibility to a nonhealing strain of Leishmania major in C57BL/6 mice. Eur J. Immunol. 2016;46(4):897–911. doi: 10.1002/eji.201546015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lecoeur H., Prina E., Rosazza T., Kokou K., N'Diaye P., Aulner N., Varet H., Bussotti G., Xing Y., Milon G., Weil R., Meng G., Spath G.F. Targeting Macrophage Histone H3 Modification as a Leishmania Strategy to Dampen the NF-kappaB/NLRP3-Mediated Inflammatory Response. Cell Rep. 2020;30(6):1870–1882 e4. doi: 10.1016/j.celrep.2020.01.030. [DOI] [PubMed] [Google Scholar]
- 182.Gurung P., Karki R., Vogel P., Watanabe M., Bix M., Lamkanfi M., Kanneganti T.D. An NLRP3 inflammasome-triggered Th2-biased adaptive immune response promotes leishmaniasis. J. Clin. Investig. 2015;125(3):1329–1338. doi: 10.1172/JCI79526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.R. Dey, A.B. Joshi, F. Oliveira, L. Pereira, A.B. Guimaraes-Costa, T.D. Serafim, W. de Castro, I.V. Coutinho-Abreu, P. Bhattacharya, S. Townsend, H. Aslan, A. Perkins, S. Karmakar, N. Ismail, M. Karetnick, C. Meneses, R. Duncan, H.L. Nakhasi, J.G. Valenzuela, S. Kamhawi, Gut Microbes Egested during Bites of Infected Sand Flies Augment Severity of Leishmaniasis via Inflammasome-Derived IL-1beta, Cell Host Microbe. 23(1) (2018) 134–143 e6. [DOI] [PMC free article] [PubMed]
- 184.Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992;6:3051–3064. [PubMed] [Google Scholar]
- 185.Bogdan C. Nitric oxide and the immune response. Nat. Immunol. 2001;2:907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 186.Bogdan C. Nitric oxide and the regulation of gene expression. Trends Cell Biol. 2001;11:66–75. doi: 10.1016/s0962-8924(00)01900-0. [DOI] [PubMed] [Google Scholar]
- 187.Nathan C. Specificity of a third kind: reactive oxygen and nitrogen intermediates in cell signaling. J. Clin. Invest. 2003;111:769–778. doi: 10.1172/JCI18174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Taylor-Robinson A.W., Liew F.Y., Severn A., Xu D., McScorley S.J., Garside P., Padron J., Phillips R.S. Regulation of the immune response by nitric oxide differentially produced by T helper type 1 and T helper type 2 cells. Eur. J. Immunol. 1994;24:980–984. doi: 10.1002/eji.1830240430. [DOI] [PubMed] [Google Scholar]
- 189.Bogdan C. The function of nitric oxide in the immune system. In: Mayer B., editor. Handbook of Experimental Pharmacology. Volume: Nitric Oxide. Springer; Heidelberg: 2000. pp. 443–492. [Google Scholar]
- 190.Niedbala W., Wei X.-Q., Piedrafita D., Xu D., Liew F.Y. Effects of nitric oxide on the induction and differentiation of Th1 cells. Eur. J. Immunol. 1999;29:2498–2505. doi: 10.1002/(SICI)1521-4141(199908)29:08<2498::AID-IMMU2498>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 191.Stenger S., Thüring H., Röllinghoff M., Manning P., Bogdan C. L-N6-(1-iminoethyl)lysine potently inhibits inducible nitric oxide synthase and is superior to NG-monomethyl-arginine in vitro and in vivo. Eur. J. Pharmacol. 1995;294:703–712. doi: 10.1016/0014-2999(95)00618-4. [DOI] [PubMed] [Google Scholar]
- 192.Drapier J.-C., Hibbs J.B. Differentiation of murine macrophages to express nonspecific cytotoxicity for tumor cells results in L-arginine-dependent inhibition of mitochondrial iron-sulfur enzymes in the macrophage effector cells. J. Immunol. 1988;140:2829–2838. [PubMed] [Google Scholar]
- 193.Albina J.E., Mastrofrancesco B. Modulation of glucose metabolism in macrophages by products of nitric oxide synthase. Am. J. Physiol. 1993;264(6 Pt 1):C1594–C1599. doi: 10.1152/ajpcell.1993.264.6.C1594. [DOI] [PubMed] [Google Scholar]
- 194.Everts B., Amiel E., van der Windt G.J., Freitas T.C., Chott R., Yarasheski K.E., Pearce E.L., Pearce E.J. Commitment to glycolysis sustains survival of NO-producing inflammatory dendritic cells. Blood. 2012;120(7):1422–1431. doi: 10.1182/blood-2012-03-419747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Postat J., Olekhnovitch R., Lemaitre F., Bousso P. A Metabolism-Based Quorum Sensing Mechanism Contributes to Termination of Inflammatory Responses. Immunity. 2018;49(4):654–665 e5. doi: 10.1016/j.immuni.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 196.Bogdan C., Röllinghoff M., Diefenbach A. Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Curr. Opin. Immunol. 2000;12:64–76. doi: 10.1016/s0952-7915(99)00052-7. [DOI] [PubMed] [Google Scholar]
- 197.Reth M. Hydrogen peroxide as second messenger in lymphocyte activation. Nat. Immunol. 2002;3:1129–1134. doi: 10.1038/ni1202-1129. [DOI] [PubMed] [Google Scholar]
- 198.Nathan C., Cunningham-Bussel A. Beyond oxidative stress: an immunologist's guide to reactive oxygen species. Nat. Rev. Immunol. 2013;13(5):349–361. doi: 10.1038/nri3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sies H. Role of metabolic H2O2 generation: redox signaling and oxidative stress. J. Biol. Chem. 2014;289(13):8735–8741. doi: 10.1074/jbc.R113.544635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Carneiro M.B.H., Roma E.H., Ranson A.J., Doria N.A., Debrabant A., Sacks D.L., Vieira L.Q., Peters N.C. NOX2-Derived Reactive Oxygen Species Control Inflammation during Leishmania amazonensis Infection by Mediating Infection-Induced Neutrophil Apoptosis. J. Immunol. 2018;200(1):196–208. doi: 10.4049/jimmunol.1700899. [DOI] [PubMed] [Google Scholar]
- 201.Diefenbach A., Schindler H., Röllinghoff M., Yokoyama W., Bogdan C. Requirement for type 2 NO-synthase for IL-12 signaling in innate immunity. Science. 1999;284:951–955. doi: 10.1126/science.284.5416.951. [DOI] [PubMed] [Google Scholar]
- 202.Peters N.C., Pagan A.J., Lawyer P.G., Hand T.W., Henrique Roma E., Stamper L.W., Romano A., Sacks D.L. Chronic parasitic infection maintains high frequencies of short-lived Ly6C+CD4+ effector T cells that are required for protection against re-infection. PLoS Pathog. 2014;10(12) doi: 10.1371/journal.ppat.1004538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Belkaid Y., Piccirillo C.A., Mendez S., Shevach E.M., Sacks D.L. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420(6915):502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 204.Mandell M.A., Beverley S.M. Concomitant Immunity Induced by Persistent Leishmania major Does Not Preclude Secondary Re-Infection: Implications for Genetic Exchange, Diversity and Vaccination. PLoS Negl. Trop. Dis. 2016;10(6) doi: 10.1371/journal.pntd.0004811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Khamesipour A., Dowlati Y., Asilian A., Hashemi-Fesharki R., Javadi A., Noazin S., Modabber F. Leishmanization: use of an old method for evaluation of candidate vaccines against leishmaniasis. Vaccine. 2005;23(28):3642–3648. doi: 10.1016/j.vaccine.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 206.Conceicao-Silva F., Morgado F.N. Leishmania Spp-Host Interaction: There Is Always an Onset, but Is There an End? Front. Cell Infect. Microbiol. 2019;9:330. doi: 10.3389/fcimb.2019.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Mandell M.A., Beverley S.M. Continual renewal and replication of persistent Leishmania major parasites in concomitantly immune hosts. Proc. Natl. Acad. Sci. USA. 2017;114(5):E801–E810. doi: 10.1073/pnas.1619265114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Paduch K., Debus A., Rai B., Schleicher U., Bogdan C. Resolution of Cutaneous Leishmaniasis and Persistence of Leishmania major in the Absence of Arginase 1. J. Immunol. 2019;202(5):1453–1464. doi: 10.4049/jimmunol.1801249. [DOI] [PubMed] [Google Scholar]
- 209.Moll H., Flohé S., Röllinghoff M. Dendritic cells in Leishmania major-immune mice harbor persistent parasites and mediate an antigen-specific T cell immune response. Eur. J. Immunol. 1995;25:693–699. doi: 10.1002/eji.1830250310. [DOI] [PubMed] [Google Scholar]
- 210.Lancaster J.R.J. A tutorial on the diffusibility and reactivity of free nitric oxide. Nitric Oxide. 1997;1:18–30. doi: 10.1006/niox.1996.0112. [DOI] [PubMed] [Google Scholar]
- 211.Engwerda C.R., Ato M., Kaye P.M. Macrophages, pathology and parasite persistence in experimental visceral leishmaniasis. Trends Parasitol. 2004;20(11):524–530. doi: 10.1016/j.pt.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 212.Morimoto A., Uchida K., Chambers J.K., Sato K., Hong J., Sanjoba C., Matsumoto Y., Yamagishi J., Goto Y. Hemophagocytosis induced by Leishmania donovani infection is beneficial to parasite survival within macrophages. PLoS Negl. Trop. Dis. 2019;13(11) doi: 10.1371/journal.pntd.0007816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Hand W.L., King-Thompson N.L. Effect of erythrocyte ingestion on macrophage antibacterial function. Infect. Immun. 1983;40:917–923. doi: 10.1128/iai.40.3.917-923.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kim Y.M., Yamazaki I., Piette L.H. The effect of hemoglobin, hematin, and iron on neutrophil inactivation in superoxide generating systems. Arch. Biochem. Biophys. 1994;309(2):308–314. doi: 10.1006/abbi.1994.1118. [DOI] [PubMed] [Google Scholar]
- 215.Martins R., Maier J., Gorki A.D., Huber K.V., Sharif O., Starkl P., Saluzzo S., Quattrone F., Gawish R., Lakovits K., Aichinger M.C., Radic-Sarikas B., Lardeau C.H., Hladik A., Korosec A., Brown M., Vaahtomeri K., Duggan M., Kerjaschki D., Esterbauer H., Colinge J., Eisenbarth S.C., Decker T., Bennett K.L., Kubicek S., Sixt M., Superti-Furga G., Knapp S. Heme drives hemolysis-induced susceptibility to infection via disruption of phagocyte functions. Nat. Immunol. 2016;17(12):1361–1372. doi: 10.1038/ni.3590. [DOI] [PubMed] [Google Scholar]
- 216.Belkaid Y., Hoffmann K.F., Mendez S., Kamhawi S., Udey M.C., Wynn T.A., Sacks D.L. The role of interleukin-10 in the persistence of Leishmania major in the skin after healing and the therapeutic potential of anti-IL-10 receptor antibody for sterile cure. J. Exp. Med. 2001;194:1497–1506. doi: 10.1084/jem.194.10.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Mou Z., Muleme H.M., Liu D., Jia P., Okwor I.B., Kuriakose S.M., Beverley S.M., Uzonna J.E. Parasite-derived arginase influences secondary anti-Leishmania immunity by regulating programmed cell death-1-mediated CD4+ T cell exhaustion. J. Immunol. 2013;190(7):3380–3389. doi: 10.4049/jimmunol.1202537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Bogdan C., Paik J., Vodovotz Y., Nathan C. Contrasting mechanisms for suppression of macrophage cytokine release by transforming growth factor-β and interleukin-10. J. Biol. Chem. 1992;267:23301–23308. [PubMed] [Google Scholar]
- 219.D.F. Fiorentino, A. Zlotnik, T.R. Mosmann, M. Howard, A. ÓGarra, IL-10 inhibits cytokine production by activated macrophages, J. Immunol. 147 (1991) 3815–3822. [PubMed]
- 220.D.F. Fiorentino, A. Zlotnik, P. Vieira, T.R. Mosmann, M. Howard, K.W. Moore, A. ÓGarra, IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells, J. Immunol. 146 (1991) 3444–3451. [PubMed]
- 221.Anderson C.F., Lira R., Kamhawi S., Belkaid Y., Wynn T.A., Sacks D. IL-10 and TGF-beta control the establishment of persistent and transmissible infections produced by Leishmania tropica in C57BL/6 mice. J. Immunol. 2008;180(6):4090–4097. doi: 10.4049/jimmunol.180.6.4090. [DOI] [PubMed] [Google Scholar]
- 222.Asad M., Sabur A., Shadab M., Das S., Kamran M., Didwania N., Ali N. Corrigendum: EBI-3 Chain of IL-35 Along With TGF-beta Synergistically Regulate Anti-leishmanial Immunity. Front. Immunol. 2019;10:2409. doi: 10.3389/fimmu.2019.02409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Weirather J.L., Duggal P., Nascimento E.L., Monteiro G.R., Martins D.R., Lacerda H.G., Fakiola M., Blackwell J.M., Jeronimo S.M., Wilson M.E. Comprehensive candidate gene analysis for symptomatic or asymptomatic outcomes of Leishmania infantum infection in Brazil. Ann Hum Genet. 2017;81(1):41–48. doi: 10.1111/ahg.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Laouar Y., Sutterwala F.S., Gorelik L., Flavell R.A. Transforming growth factor-b controls T helper type 1 cell development through regulation of natural killer cell interferon-γ. Nat. Immunol. 2005;6(6):600–607. doi: 10.1038/ni1197. [DOI] [PubMed] [Google Scholar]
- 225.Batlle E., Massague J. Transforming Growth Factor-beta Signaling in Immunity and Cancer. Immunity. 2019;50(4):924–940. doi: 10.1016/j.immuni.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Rossi M., Castiglioni P., Hartley M.A., Eren R.O., Prevel F., Desponds C., Utzschneider D.T., Zehn D., Cusi M.G., Kuhlmann F.M., Beverley S.M., Ronet C., Fasel N. Type I interferons induced by endogenous or exogenous viral infections promote metastasis and relapse of leishmaniasis. Proc Natl Acad Sci U S A. 2017;114(19):4987–4992. doi: 10.1073/pnas.1621447114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Eren R.O., Reverte M., Rossi M., Hartley M.A., Castiglioni P., Prevel F., Martin R., Desponds C., Lye L.F., Drexler S.K., Reith W., Beverley S.M., Ronet C., Fasel N. Mammalian Innate Immune Response to a Leishmania-Resident RNA Virus Increases Macrophage Survival to Promote Parasite Persistence. Cell Host Microbe. 2016;20(3):318–328. doi: 10.1016/j.chom.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.R. Khouri, A. Bafica, P. Silva Mda, A. Noronha, J.P. Kolb, J. Wietzerbin, A. Barral, M. Barral-Netto, J. Van Weyenbergh, IFN-beta impairs superoxide-dependent parasite killing in human macrophages: evidence for a deleterious role of SOD1 in cutaneous leishmaniasis, J Immunol 182(4) (2009) 2525–2531. [DOI] [PubMed]
- 229.Van Bockstal L., Bulte D., Van den Kerkhof M., Dirkx L., Mabille D., Hendrickx S., Delputte P., Maes L., Caljon G. Interferon Alpha Favors Macrophage Infection by Visceral Leishmania Species Through Upregulation of Sialoadhesin Expression. Front. Immunol. 2020;11:1113. doi: 10.3389/fimmu.2020.01113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Schleicher U., Liese J., Knippertz I., Kurzmann C., Hesse A., Heit A., Fischer J.A., Weiss S., Kalinke U., Kunz S., Bogdan C. NK cell activation in visceral leishmaniasis requires TLR9, myeloid DCs, and IL-12, but is independent of plasmacytoid DCs. J. Exp. Med. 2007;204(4):893–906. doi: 10.1084/jem.20061293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.R. Kumar, P.T. Bunn, S.S. Singh, S.S. Ng, M. Montes de Oca, F. De Labastida Rivera, S.B. Chauhan, N. Singh, R.J. Faleiro, C.L. Edwards, T.C.M. Frame, M. Sheel, R.J. Austin, S.W. Lane, T. Bald, M.J. Smyth, G.R. Hill, S.E. Best, A. Haque, D. Corvino, N. Waddell, L. Koufariotis, P. Mukhopadhay, M. Rai, J. Chakravarty, O.P. Singh, D. Sacks, S. Nylen, J. Uzonna, S. Sundar, C.R. Engwerda, Type I Interferons Suppress Anti-parasitic Immunity and Can Be Targeted to Improve Treatment of Visceral Leishmaniasis, Cell Rep 30(8) (2020) 2512–2525 e9. [DOI] [PMC free article] [PubMed]
- 232.Bogdan C., Röllinghoff M. The immune response to Leishmania: mechanisms of parasite control and evasion. Int. J. Parasitol. 1998;28:121–134. doi: 10.1016/s0020-7519(97)00169-0. [DOI] [PubMed] [Google Scholar]
- 233.Kima P.E. The amastigote forms of Leishmania are experts at exploiting host cell processes to establish infection and persist. Int. J. Parasitol. 2007;37(10):1087–1096. doi: 10.1016/j.ijpara.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Shio M.T., Hassani K., Isnard A., Ralph B., Contreras I., Gomez M.A., Abu-Dayyeh I., Olivier M. Host cell signalling and leishmania mechanisms of evasion. J. Trop. Med. 2012;2012 doi: 10.1155/2012/819512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Olivier M., Atayde V.D., Isnard A., Hassani K., Shio M.T. Leishmania virulence factors: focus on the metalloprotease GP63. Microbes. Infect. 2012;14(15):1377–1389. doi: 10.1016/j.micinf.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 236.Atayde V.D., Hassani K., da Silva Lira A., Filho A.R., Borges A., Adhikari C., Martel M. Olivier. Leishmania exosomes and other virulence factors: Impact on innate immune response and macrophage functions. Cell Immunol. 2016;309:7–18. doi: 10.1016/j.cellimm.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 237.McMaster W.R., Morrison C.J., Kobor M.S. Epigenetics: A New Model for Intracellular Parasite-Host Cell Regulation. Trends Parasitol. 2016;32(7):515–521. doi: 10.1016/j.pt.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 238.Rossi M., Fasel N. How to master the host immune system? Leishmania parasites have the solutions! Int. Immunol. 2018;30(3):103–111. doi: 10.1093/intimm/dxx075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Marr A.K., MacIsaac J.L., Jiang R., Airo A.M., Kobor M.S., McMaster W.R. Leishmania donovani infection causes distinct epigenetic DNA methylation changes in host macrophages. PLoS Pathog. 2014;10(10) doi: 10.1371/journal.ppat.1004419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Moreira D., Rodrigues V., Abengozar M., Rivas L., Rial E., Laforge M., Li X., Foretz M., Viollet B., Estaquier J., Cordeiro da Silva A., Silvestre R. Leishmania infantum modulates host macrophage mitochondrial metabolism by hijacking the SIRT1-AMPK axis. PLoS Pathog. 2015;11(3) doi: 10.1371/journal.ppat.1004684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.O'Neill L.A., Kishton R.J., Rathmell J. A guide to immunometabolism for immunologists. Nat. Rev. Immunol. 2016;16(9):553–565. doi: 10.1038/nri.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Naderer T., Ellis M.A., Sernee M.F., De Souza D.P., Curtis J., Handman E., McConville M.J. Virulence of Leishmania major in macrophages and mice requires the gluconeogenic enzyme fructose-1,6-bisphosphatase. Proc. Natl. Acad. Sci. USA. 2006;103(14):5502–5507. doi: 10.1073/pnas.0509196103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Saunders E.C., Naderer T., Chambers J., Landfear S.M., McConville M.J. Leishmania mexicana can utilize amino acids as major carbon sources in macrophages but not in animal models. Mol. Microbiol. 2018;108(2):143–158. doi: 10.1111/mmi.13923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Richardson A.R., Payne E.C., Younger N., Karlinsey J.E., Thomas V.C., Becker L.A., Navarre W.W., Castor M.E., Libby S.J., Fang F.C. Multiple targets of nitric oxide in the tricarboxylic acid cycle of Salmonella enterica serovar typhimurium. Cell Host Microbe. 2011;10(1):33–43. doi: 10.1016/j.chom.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Weichhart T., Hengstschlager M., Linke M. Regulation of innate immune cell function by mTOR. Nat. Rev. Immunol. 2015;15(10):599–614. doi: 10.1038/nri3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Kumar A., Das S., Mandal A., Verma S., Abhishek K., Kumar A., Kumar V., Ghosh A.K., Das P. Leishmania infection activates host mTOR for its survival by M2 macrophage polarization. Parasite Immunol. 2018;40(11) doi: 10.1111/pim.12586. [DOI] [PubMed] [Google Scholar]
- 247.Chaparro V., Leroux L.P., Masvidal L., Lorent J., Graber T.E., Zimmermann A., Arango Duque G., Descoteaux A., Alain T., Larsson O., Jaramillo M. Translational profiling of macrophages infected with Leishmania donovani identifies mTOR- and eIF4A-sensitive immune-related transcripts. PLoS Pathog. 2020;16(6) doi: 10.1371/journal.ppat.1008291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Jaramillo M., Gomez M.A., Larsson O., Shio M.T., Topisirovic I., Contreras I., Luxenburg R., Rosenfeld A., Colina R., McMaster R.W., Olivier M., Costa-Mattioli M., Sonenberg N. Leishmania repression of host translation through mTOR cleavage is required for parasite survival and infection. Cell Host Microbe. 2011;9(4):331–341. doi: 10.1016/j.chom.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 249.Castilho-Martins E.A., Laranjeira da Silva M.F., dos Santos M.G., Muxel S.M., Floeter-Winter L.M. Axenic Leishmania amazonensis promastigotes sense both the external and internal arginine pool distinctly regulating the two transporter-coding genes. PLoS ONE. 2011;6(11) doi: 10.1371/journal.pone.0027818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Goldman-Pinkovich A., Balno C., Strasser R., Zeituni-Molad M., Bendelak K., Rentsch D., Ephros M., Wiese M., Jardim A., Myler P.J., Zilberstein D. An Arginine Deprivation Response Pathway Is Induced in Leishmania during Macrophage Invasion. PLoS Pathog. 2016;12(4) doi: 10.1371/journal.ppat.1005494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Aoki J.I., Muxel S.M., Zampieri R.A., Acuna S.M., Fernandes J.C.R., Vanderlinde R.H., Sales M., Floeter-Winter L.M. L-arginine availability and arginase activity: Characterization of amino acid permease 3 in Leishmania amazonensis. PLoS Negl. Trop. Dis. 2017;11(10) doi: 10.1371/journal.pntd.0006025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.O'Neill L.A., Pearce E.J. Immunometabolism governs dendritic cell and macrophage function. J. Exp. Med. 2016;213(1):15–23. doi: 10.1084/jem.20151570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Saha S., Shalova I.N., Biswas S.K. Metabolic regulation of macrophage phenotype and function. Immunol Rev. 2017;280(1):102–111. doi: 10.1111/imr.12603. [DOI] [PubMed] [Google Scholar]
- 254.McConville M.J., Saunders E.C., Kloehn J., Dagley M.J. Leishmania carbon metabolism in the macrophage phagolysosome- feast or famine? F1000Res. 2015;4(F1000 Faculty Rev):938. doi: 10.12688/f1000research.6724.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Russell D.G., Huang L., VanderVen B.C. Immunometabolism at the interface between macrophages and pathogens. Nat. Rev. Immunol. 2019;19(5):291–304. doi: 10.1038/s41577-019-0124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.McConville M.J. Metabolic Crosstalk between Leishmania and the Macrophage Host. Trends Parasitol. 2016;32(9):666–668. doi: 10.1016/j.pt.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 257.Zivanovic V., Semini G., Laue M., Drescher D., Aebischer T., Kneipp J. Chemical Mapping of Leishmania Infection in Live Cells by SERS Microscopy. Anal. Chem. 2018;90(13):8154–8161. doi: 10.1021/acs.analchem.8b01451. [DOI] [PubMed] [Google Scholar]
- 258.Lamotte S., Spath G.F., Rachidi N., Prina E. The enemy within: Targeting host-parasite interaction for antileishmanial drug discovery. PLoS Negl. Trop. Dis. 2017;11(6) doi: 10.1371/journal.pntd.0005480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Valdez C.A., Saavedra J.E., Showalter B.M., Davies K.M., Wilde T.C., Citro M.L., Barchi J.J., Jr., Deschamps J.R., Parrish D., El-Gayar S., Schleicher U., Bogdan C., Keefer L.K. Hydrolytic Reactivity Trends among Potential Prodrugs of the O(2)-Glycosylated Diazeniumdiolate Family. Targeting Nitric Oxide to Macrophages for Antileishmanial Activity. J. Med. Chem. 2008;51(13):3961–3970. doi: 10.1021/jm8000482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Beattie L., d'El-Rei Hermida M., Moore J.W., Maroof A., Brown N., Lagos D., Kaye P.M. A transcriptomic network identified in uninfected macrophages responding to inflammation controls intracellular pathogen survival. Cell Host Microbe. 2013;14(3):357–368. doi: 10.1016/j.chom.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.J. Osorio y Fortea, E. de La Llave, B. Regnault, J.Y. Coppee, G. Milon, T. Lang, E. Prina, Transcriptional signatures of BALB/c mouse macrophages housing multiplying Leishmania amazonensis amastigotes, BMC Genomics 10 (2009) 119. [DOI] [PMC free article] [PubMed]
- 262.Rabhi I., Rabhi S., Ben-Othman R., Rasche A., Daskalaki A., Trentin B., Piquemal D., Regnault B., Descoteaux A., Guizani-Tabbane L., Sysco C. Transcriptomic signature of Leishmania infected mice macrophages: a metabolic point of view. PLoS Negl. Trop. Dis. 2012;6(8) doi: 10.1371/journal.pntd.0001763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Dillon L.A., Okrah K., Hughitt V.K., Suresh R., Li Y., Fernandes M.C., Belew A.T., Corrada Bravo H., Mosser D.M., El-Sayed N.M. Transcriptomic profiling of gene expression and RNA processing during Leishmania major differentiation. Nucleic Acids Res. 2015;43(14):6799–6813. doi: 10.1093/nar/gkv656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.H.A. Ospina, A. Descoteaux, Leishmania donovani modulates host macrophage mitochondrial metabolism, integrity and function, J. Immunol. 204 (Supplement 1) (2020) 82.26 (Abstract).
- 265.Parmar N., Chandrakar P., Kar S. Leishmania donovani Subverts Host Immune Response by Epigenetic Reprogramming of Macrophage M(Lipopolysaccharides + IFN-gamma)/M(IL-10) Polarization. J. Immunol. 2020;204(10):2762–2778. doi: 10.4049/jimmunol.1900251. [DOI] [PubMed] [Google Scholar]
- 266.Silverman J.M., Reiner N.E. Exosomes and other microvesicles in infection biology: organelles with unanticipated phenotypes. Cell. Microbiol. 2011;13(1):1–9. doi: 10.1111/j.1462-5822.2010.01537.x. [DOI] [PubMed] [Google Scholar]
- 267.Mathur R.K., Awasthi A., Wadhone P., Ramanamurthy B., Saha B. Reciprocal CD40 signals through p38MAPK and ERK-1/2 induce counteracting immune responses. Nat. Med. 2004;10(5):540–544. doi: 10.1038/nm1045. [DOI] [PubMed] [Google Scholar]
- 268.Brittingham A., Morrison C.J., McMaster W.R., McGwire B.S., Chang K.-P., Mosser D.M. Role of the Leishmania surface protease gp63 in complement fixation, cell adhesion and resistance to complement-mediated lysis. J. Immunol. 1995;155:3102–3111. [PubMed] [Google Scholar]
- 269.Brittingham A., Mosser D.M. Exploitation of the complement system by Leishmania promastigotes. Parasitol. Today. 1996;12:444–447. doi: 10.1016/0169-4758(96)10067-3. [DOI] [PubMed] [Google Scholar]
- 270.Desjardins M., Descoteaux A. Inhibition of phagolysosomal biogenesis by the Leishmania lipophosphoglycan. J. Exp. Med. 1997;185:2061–2068. doi: 10.1084/jem.185.12.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Dermine J.F., Scianimanico S., Prive C., Descoteaux A., Desjardins M. Leishmania promastigotes require lipophosphoglycan to actively modulate the fusion properties of phagosomes at an early step of phagocytosis. Cell. Microbiol. 2000;2(2):115–126. doi: 10.1046/j.1462-5822.2000.00037.x. [DOI] [PubMed] [Google Scholar]
- 272.Späth G.F., Garraway L.A., Turco S.J., Beverley S.M. The role(s) of lipophosphoglycan (LPG) in the establishment of Leishmania major infections in mammalian hosts. Proc. Natl. Acad. Sci. USA. 2003;100:9536–9541. doi: 10.1073/pnas.1530604100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Pham N.-K., Mouriz J., Kima P.E. Leishmania pifanoi amastigotes avoid macrophage production of superoxide by inducing heme degradation. Infect. Immun. 2005;73:8322–8333. doi: 10.1128/IAI.73.12.8322-8333.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Saha S., Basu M., Guin S., Gupta P., Mitterstiller A.M., Weiss G., Jana K., Ukil A. Leishmania donovani Exploits Macrophage Heme Oxygenase-1 To Neutralize Oxidative Burst and TLR Signaling-Dependent Host Defense. J. Immunol. 2019;202(3):827–840. doi: 10.4049/jimmunol.1800958. [DOI] [PubMed] [Google Scholar]
- 275.Muxel S.M., Laranjeira-Silva M.F., Zampieri R.A., Floeter-Winter L.M. Leishmania (Leishmania) amazonensis induces macrophage miR-294 and miR-721 expression and modulates infection by targeting NOS2 and L-arginine metabolism. Sci. Rep. 2017;7:44141. doi: 10.1038/srep44141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Ben-Othman R., Flannery A.R., Miguel D.C., Ward D.M., Kaplan J., Andrews N.W. Leishmania-mediated inhibition of iron export promotes parasite replication in macrophages. PLoS Pathog. 2014;10(1) doi: 10.1371/journal.ppat.1003901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.N.K. Das, S. Sandhya, V.V. G, R. Kumar, A.K. Singh, S.K. Bal, S. Kumari, C.K. Mukhopadhyay, Leishmania donovani inhibits ferroportin translation by modulating FBXL5-IRP2 axis for its growth within host macrophages, Cell. Microbiol. 20(7) (2018) e12834. [DOI] [PubMed]
- 278.Meier C.L., Svensson M., Kaye P.M. Leishmania-induced inhibition of macrophage antigen presentation at the single-cell level. J. Immunol. 2003;171:6706–6713. doi: 10.4049/jimmunol.171.12.6706. [DOI] [PubMed] [Google Scholar]
- 279.Chakraborty D., Banerjee S., Sen A., Banerjee K.K., Das P., Roy S. Leishmania donovani affects antigen presentation of macrophage by disrupting lipid rafts. J. Immunol. 2005;175:3214–3224. doi: 10.4049/jimmunol.175.5.3214. [DOI] [PubMed] [Google Scholar]
- 280.de Carvalho R.V.H., Andrade W.A., Lima-Junior D.S., Dilucca M., de Oliveira C.V., Wang K., Nogueira P.M., Rugani J.N., Soares R.P., Beverley S.M., Shao F., Zamboni D.S. Leishmania lipophosphoglycan triggers caspase-11 and the non-canonical activation of the NLRP3 inflammasome. Cell Rep. 2019;26:429–437. doi: 10.1016/j.celrep.2018.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Hartley M.-A., Eren R.O., Rossi M., Prevel F., Castiglioni P., Isorce N., Desponds C., Lye L.-F., Beverley S.M., Drexler S.K., Fasel N. Leishmania guyanensis parasites block the activation of the inflammasome by inhibiting maturation of IL-1beta. Microbial Cell. 2018;5:137–149. doi: 10.15698/mic2018.03.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.de Carvalho R.V.H., Lima-Junior D.S., da Silva M.V.G., Dilucca M., Rodrigues T.S., Horta C.V., Silva A.L.N., da Silva P.F., Frantz F.G., Lorenzon L.B., Souza M.M., Almeido F., Cantanhede L.M., de Godoi R., Ferreira M., Cruz A.K., Zamboni D.S. Leishmania RNA virus exacerbates leishmaniasis by subverting innate immunity via TLR3-mediated NLRP3 inflammasome inhibition. Nat. Commun. 2019;10:5273. doi: 10.1038/s41467-019-13356-2. [DOI] [PMC free article] [PubMed] [Google Scholar]