Abstract
Aims: Doxorubicin cardiomyopathy is a lethal pathology characterized by oxidative stress, mitochondrial dysfunction, and contractile impairment, leading to cell death. Although extensive research has been done to understand the pathophysiology of doxorubicin cardiomyopathy, no effective treatments are available. We investigated whether monoamine oxidases (MAOs) could be involved in doxorubicin-derived oxidative stress, and in the consequent mitochondrial, cardiomyocyte, and cardiac dysfunction.
Results: We used neonatal rat ventricular myocytes (NRVMs) and adult mouse ventricular myocytes (AMVMs). Doxorubicin alone (i.e., 0.5 μM doxorubicin) or in combination with H2O2 induced an increase in mitochondrial formation of reactive oxygen species (ROS), which was prevented by the pharmacological inhibition of MAOs in both NRVMs and AMVMs. The pharmacological approach was supported by the genetic ablation of MAO-A in NRVMs. In addition, doxorubicin-derived ROS caused lipid peroxidation and alterations in mitochondrial function (i.e., mitochondrial membrane potential, permeability transition, redox potential), mitochondrial morphology (i.e., mitochondrial distribution and perimeter), sarcomere organization, intracellular [Ca2+] homeostasis, and eventually cell death. All these dysfunctions were abolished by MAO inhibition. Of note, in vivo MAO inhibition prevented chamber dilation and cardiac dysfunction in doxorubicin-treated mice.
Innovation and Conclusion: This study demonstrates that the severe oxidative stress induced by doxorubicin requires the involvement of MAOs, which modulate mitochondrial ROS generation. MAO inhibition provides evidence that mitochondrial ROS formation is causally linked to all disorders caused by doxorubicin in vitro and in vivo. Based upon these results, MAO inhibition represents a novel therapeutic approach for doxorubicin cardiomyopathy.
Keywords: doxorubicin, cardiomyopathy, mitochondria, monoamine oxidase, reactive oxygen species (ROS)
Introduction
Doxorubicin (Adriamycin®) is a very efficient antineoplastic drug used in cancer treatment that belongs to the anthracycline family (44). Unfortunately, its therapeutic value is very much limited by a remarkable cardiotoxicity (21). The doxorubicin-derived cardiac dysfunction is dose dependent, and the resulting doxorubicin cardiotoxicity (DC) may arise even at low doses in an individual-dependent manner (33). Many mechanisms (21) have been suggested to explain doxorubicin anticancer properties, such as DNA intercalation (65), sarcopenia (40), adverse signaling (38), impairment of intracellular [Ca2+] homeostasis (32), and, above all, oxidative stress (65).
The two main metabolic pathways leading to anthracyclines-dependent formation of reactive oxygen species (ROS) are one-electron reduction, which generates superoxide (44), and two-electron reduction, which generates a secondary alcohol, DOXol (54). These two mechanisms are of great importance for the anticancer properties of doxorubicin, since the one-electron reduction occurs especially at the mitochondrial level, while the two-electron reduction is achieved mainly in the cytoplasm (54). However, all these mechanisms that are essential for cancer therapy become harmful when they are observed also in the myocardium, in which they were mostly reported as independent events by different laboratories, with hardly any attempt to achieve a unifying mechanism of DC (21).
In this respect, the major focus has been given to doxorubicin-induced oxidative stress (21, 62). Although doxorubicin induces ROS formation by different pathways, such as interacting with free iron (44), interacting with NAD(P)H oxidases (15), or binding to the endothelial nitric oxide synthase (44), this anthracycline is retained within the mitochondria as a result of its nearly irreversible binding to cardiolipin (69), making mitochondria both the source (20, 47) and the target of ROS (20, 73).
Doxorubicin-dependent formation of ROS within the mitochondria is related to its redox cycling activity at Complex I (6, 25) and to the consequent dysfunction of the electron transport chain (ETC) (25). Notably, we have recently demonstrated that another redox cycler at Complex I (MitoParaquat) causes a primary increase in mitochondrial ROS formation that is upstream of both mitochondrial and cellular abnormalities (2). The relevance of oxidative stress supports the use of the iron chelator and topoisomerase II inhibitor dexrazoxane, which is the only drug approved by the U.S. Food and Drug Administration against DC. Anthracycline–iron complexes are believed to promote myocyte-damaging free radicals, and thus dexrazoxane reduces cardiac toxicity in subjects treated with anthracyclines. However, far from enhancing the antitumor efficacy of doxorubicin, the therapeutic value of dexrazoxane is limited by the risk of secondary malignancies (41, 64).
Innovation
Monoamine oxidase (MAO)-dependent reactive oxygen species (ROS) formation contributes to cardiomyocyte dysfunction by exacerbating doxorubicin-derived mitochondrial ROS. MAO inhibition demonstrates that mitochondrial ROS formation is upstream of doxorubicin-induced injury in cardiomyocytes and doxorubicin-treated hearts. This study suggests that MAOs may represent a promising target for novel therapeutic approaches for doxorubicin cardiotoxicity, which should be validated by clinical testing as well as by additional experimental studies.
Although doxorubicin acts as a redox cycler at the ETC (6, 25), mitochondria contain many other sources of ROS. Among those, we have characterized the relevant role of monoamine oxidases (MAOs) in several cardiac pathologies (17, 18, 34, 36). MAOs are located at the outer mitochondrial membrane, and exist in two different isoforms (i.e., MAO-A and MAO-B), the crystal structures of which have been completely solved (7, 57). MAOs catalyze the deamination of biogenic amines that are relevant for cardiac function. The catalytic products are H2O2, aldehydes, and ammonia (17), which can synergize in inducing mitochondrial and cardiac dysfunction (34, 36). Besides these biochemical features, MAOs are the only mitochondrial sources of ROS that can be targeted by clinically available drugs (17). In addition, doxorubicin-induced neurotoxicity has been linked to an increase in MAO activity (49), and several cancerous tissues (e.g., prostate cancer, Hodgkin's lymphomas) display a decrease in cell growth upon MAO inhibition alone (27) or in combination with doxorubicin treatment (39).
Chronic doxorubicin toxicity is well documented (21, 33, 44). However acute DC may occur during doxorubicin administration or immediately afterward in up to 40% of treated patients, leading to abnormal changes in the left ventricle (LV), cardiac rhythm disturbances, and alterations in cell metabolism (23, 48, 60). Here, we tested the hypothesis that a primary increase in mitochondrial ROS elicited by doxorubicin could be modulated by MAOs, thus leading to cardiac dysfunction. In particular, we investigated whether (i) MAOs are involved in mitochondrial ROS formation induced by doxorubicin; (ii) MAO-dependent oxidative stress triggered by doxorubicin can impair both mitochondrial and cell functions, eventually causing cardiomyocyte death; (iii) MAO inhibition can prevent doxorubicin-induced cardiotoxicity in vivo.
Results
MAO inhibition reduces mitochondrial oxidative stress induced by doxorubicin in cardiomyocytes
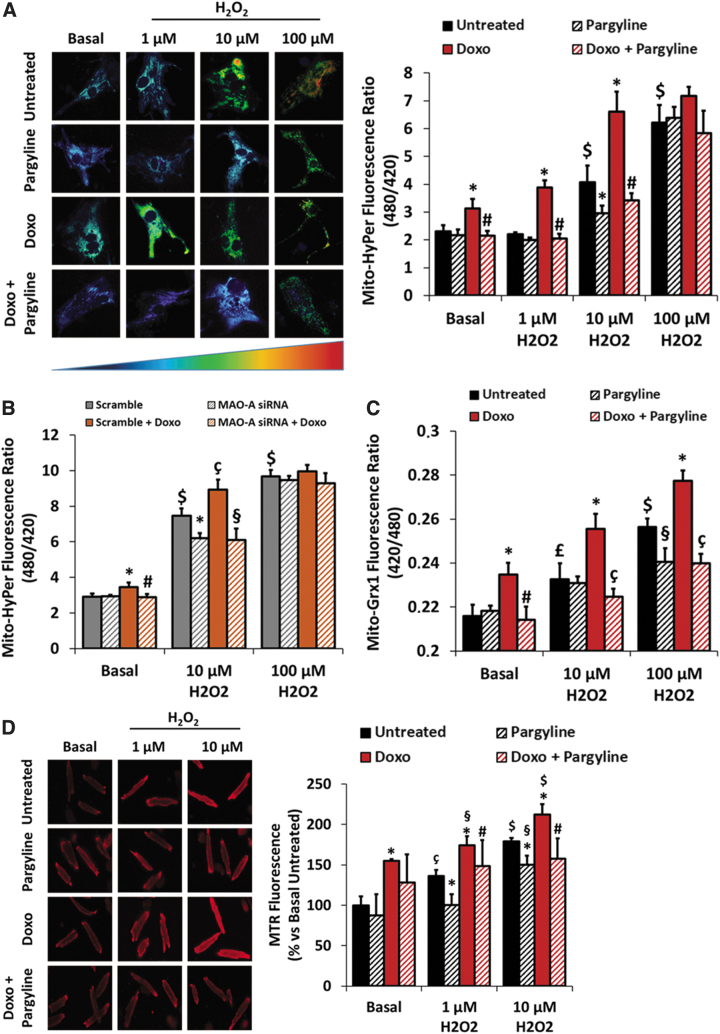
Despite the cardiotoxicity elicited by doxorubicin being well documented (10, 25, 69), the connection between this drug and the deleterious effects elicited by the elevation in mitochondrial ROS levels has been described mostly using supratherapeutic concentrations of doxorubicin (6). To verify that doxorubicin could increase ROS levels in neonatal rat ventricular myocytes (NRVMs), we treated cells with a clinically relevant concentration (6, 44, 62) of doxorubicin (i.e., 0.5 μM) for 24 h. Moreover, to test possible involvement of MAOs, we incubated cells with 100 μM pargyline, a selective inhibitor of both MAO isoforms (18). Mitochondrial and cytosolic ROS levels were monitored using either Mito-HyPer or Cyto-HyPer, genetically encoded sensors highly specific for H2O2 (4).
Doxorubicin-treated NRVMs displayed increased mitochondrial ROS levels that were totally prevented by pargyline (fluorescence ratio: untreated 2.30 ± 0.22; Doxo 3.12 ± 0.34; Doxo+Pargyline 2.14 ± 0.17; Fig. 1A). Of note, doxorubicin did not induce any increase in cytosolic ROS levels (Supplementary Fig. S1A). The pharmacological evidence was strengthened by a genetic approach. Indeed, in NRVMs knocked down (KD) for MAO-A (Supplementary Fig. S1B, full blot in Supplementary Fig. S2A) (18) mitochondrial ROS levels were reduced in the presence of doxorubicin (fluorescence ratio: scramble 2.90 ± 0.19; Doxo 3.44 ± 0.25; scramble+Doxo 2.89 ± 0.18; Fig. 1B). The protective efficacy of pargyline and the results obtained in MAO-A KD NRVMs demonstrate that MAOs can promote the formation of ROS caused by doxorubicin. This also implies that the redox cycling activity at the ETC results in the generation of a very limited amount of ROS, which becomes significant only when MAOs are involved.
FIG. 1.
Effects of doxorubicin and MAO inhibition on mitochondrial oxidative status. NRVMs have been treated with 0.5 μM doxorubicin for 24 h, in the presence or absence of 100 μM pargyline. AMVMs have been treated with 0.5 μM doxorubicin for 24 h, in the presence or absence of 200 μM pargyline. Cells have been further stimulated with increasing concentrations of H2O2 (i.e., 1–10–100 μM) for 10 min. (A) Mitochondrial H2O2 formation measured by Mito-HyPer in isolated NRVMs. *p < 0.001 versus Untreated, #p < 0.001 versus Doxo, $p < 0.001 versus Basal Untreated by one-way ANOVA with post hoc Tukey's multiple comparison test. (B) Mitochondrial H2O2 formation measured by Mito-HyPer in isolated NRVMs. Cells have been previously transfected with scramble or MAO-A siRNA. *p < 0.05 versus Scramble, #p < 0.01 versus Scramble+Doxo, çp < 0.01 versus Scramble, §p < 0.001 versus Scramble+Doxo, $p < 0.001 versus Scramble Basal by one-way ANOVA with post hoc Tukey's multiple comparison test. (C) Mitochondrial GSSG/GSH ratio measured by Mito-Grx1-roGFP in isolated NRVMs. *p < 0.01 versus Untreated, #p < 0.01 versus Doxo, çp < 0.001 versus Doxo, §p < 0.05 versus Untreated, £p < 0.05 versus Basal Untreated, $p < 0.001 versus Basal Untreated by one-way ANOVA with post hoc Tukey's multiple comparison test. (D) Mitochondrial ROS formation measured by MTR in AMVMs. *p < 0.05 versus Untreated, #p < 0.05 versus Doxo, §p < 0.05 versus Basal Doxo, çp < 0.01 versus Basal Untreated, $p < 0.001 versus Basal Untreated by one-way ANOVA with post hoc Tukey's multiple comparison test. Approximately 30 cells were analyzed per condition in each experiment, and all the experiments were performed at least three times using three different animal or cell preparations. Data are expressed as mean ± SEM. AMVM, adult mouse ventricular myocyte; GSSG/GSH, glutathione disulfide/glutathione; MAO, monoamine oxidase; MTR, MitoTracker Red; NRVM, neonatal rat ventricular myocyte; ROS, reactive oxygen species; SEM, standard error of the mean.
Although DC attributed to excessive ROS production is well documented (44), the onset of cardiomyopathy in doxorubicin-treated patients may occur as a consequence of a further increase in ROS due to an additional event (i.e., ischemia/reperfusion, exercise, infection) (1, 12), eventually leading to heart failure. To simulate the consequences derived from a secondary source of oxidative stress, NRVMs treated with 0.5 μM doxorubicin for 24 h, in the presence or absence of 100 μM pargyline, were further stimulated with increasing concentrations of exogenous H2O2. The coadministration of H2O2 (i.e., 1–10–100 μM) with doxorubicin resulted in a further increase of mitochondrial ROS levels, which was prevented by MAO inhibition (fluorescence ratio after 10 μM H2O2: Untreated 4.06 ± 0.60; Doxo 6.60 ± 0.72; Doxo+Pargyline 3.41 ± 0.26; Fig. 1A). However, the protective effect of pargyline was lost when cells were exposed to 100 μM H2O2, probably due to the excessive oxidative stress and/or sensor saturation.
This result was strengthened by the genetic approach. Indeed, NRVMs KD for MAO-A cotreated with doxorubicin and H2O2 display decreased mitochondrial ROS levels compared with the scramble RNA-treated cells (fluorescence ratio after 10 μM H2O2: scramble 7.46 ± 0.40; Doxo 8.91 ± 0.56; scramble+Doxo 6.09 ± 0.65; Fig. 1B). Notably, cardiomyocytes incubated with doxorubicin and H2O2 displayed a severe increase of cytosolic ROS levels, which was not affected by MAO inhibition (Supplementary Fig. S1A). These results could suggest that the combination of MAO activation and doxorubicin may interfere with the mitochondrial antioxidant capacity leading to oxidative stress. To this aim, we monitored the intramitochondrial glutathione redox potential by means of Mito-Grx1-roGFP2, a genetically encoded sensor that detects changes in the mitochondrial ratio of oxidized to reduced glutathione (GSSG/GSH) (28). The coadministration of H2O2 (i.e., 10–100 μM) with doxorubicin led to a dramatic increase in the GSSG/GSH ratio (fluorescence ratio after 100 μM H2O2: Untreated 0.25 ± 0.003; Doxo 0.28 ± 0.004; Fig. 1C), likely affecting the mitochondrial antioxidant capacity of NRVMs in these conditions. Importantly, MAO inhibition significantly preserved mitochondrial GSSG/GSH ratio (fluorescence ratio after 100 μM H2O2: Doxo+Pargyline 0.23 ± 0.004; Fig. 1C). A similar outcome was observed when the glutathione redox potential was monitored in the whole cell (Supplementary Fig. S1C), although the total thiol oxidation state was not affected by doxorubicin (Supplementary Fig. S1D). Since MAO inhibition prevented the rise in the mitochondrial/cell GSSG/GSH ratio without affecting cytosolic H2O2 levels, it is plausible that a secondary source of ROS [e.g., NAD(P)H oxidases] is also at play here and that the excessive increase in H2O2-derived ROS levels in the cytosol was not correlated with MAO activity.
To strengthen the significance of the results obtained in NRVMs, we tested our hypothesis also in adult mouse ventricular myocytes (AMVMs). Of note, besides a more mature metabolic phenotype (i.e., high dependency on fatty acid oxidation), AMVMs express prevalently MAO-B, while NRVMs contain mostly MAO-A (34). Since AMVMs are stable in cell culture for only up to 2 days, making the transfection of the cells with a sensor such as Mito-HyPer impossible, mitochondrial ROS levels were monitored by the reduced form of MitoTracker Red (MTR) (16).
AMVMs incubated with 0.5 μM doxorubicin for 24 h displayed an increase in mitochondrial ROS levels (Untreated 100% ± 11.08%; Doxo 154.74% ± 2.51%; Fig. 1D), which were further elevated by different concentrations of H2O2 (i.e., 1–10 μM) (Fig. 1D). Similarly to what observed in NRVMs, the rise in mitochondrial ROS levels was prevented by treatment with 200 μM pargyline (after 10 μM H2O2: Untreated 178.79% ± 4.55%; Doxo 212.24% ± 12.69%; Doxo+Pargyline 157.82% ± 25.14%; Fig. 1D). Although after treatment with 100 μM H2O2 mitochondrial ROS could not be measured by MTR due to mitochondrial depolarization (18), pargyline was still protective. Indeed, the cotreatment with doxorubicin and H2O2 led to the transformation of rod-shaped cells into rounded dysfunctional cardiomyocytes. Conversely, pargyline treatment maintained the rod-shaped morphology of AMVMs (Supplementary Fig. S1E). These results demonstrate that the oxidative stress elicited by doxorubicin alone or by the cotreatment with doxorubicin and H2O2 is exacerbated by MAOs that act as ROS enhancers in cardiomyocytes.
To understand how doxorubicin treatment resulted in an increased MAO-dependent ROS formation, we evaluated MAO expression levels in both NRVMs and AMVMs. After 24 h of treatment with doxorubicin, both MAO-A in NRVMs and MAO-B in AMVMs expression levels were comparable with the untreated control (Supplementary Fig. S1F, G, full blots shown in Supplementary Fig. S2B, C). Hence, we investigated whether doxorubicin treatment could increase MAO activity in NRVMs. H2O2 formation was monitored by means of Mito-HyPer in cells pretreated with 0.5 μM doxorubicin for 24 h, in the presence or absence of 100 μM pargyline, and then incubated with 20 μM of the MAO substrate tyramine for 2 h. Treatment with tyramine significantly increased H2O2 levels within mitochondria (Supplementary Fig. S1H), while the cotreatment with tyramine and doxorubicin did not induce any further increase (Supplementary Fig. S1H). In both cases, MAO inhibition completely prevented this increase in H2O2 formation. This result suggests that, when the MAO enzyme is working at its maximum (i.e., in the presence of its substrate tyramine), doxorubicin cannot further enhance MAO-dependent ROS generation.
MAO inhibition ameliorates both mitochondrial function and morphology altered by cotreatment with doxorubicin and H2O2 in cardiomyocytes
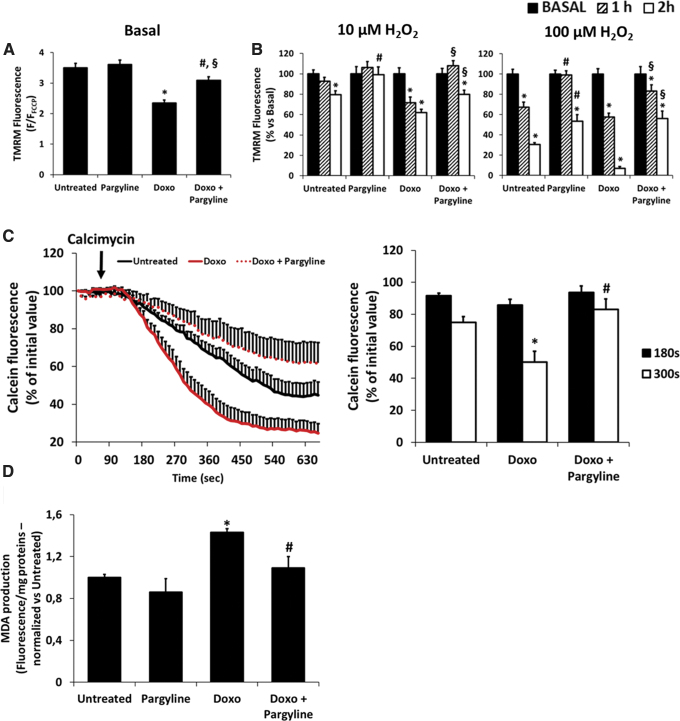
The increase in mitochondrial ROS production causes mitochondrial dysfunction, characterized by mitochondrial depolarization (37). Hence, we monitored mitochondrial membrane potential (ΔΨm) using tetramethylrhodamine (TMRM). NRVMs treated with 0.5 μM doxorubicin for 24 h displayed a decrease in ΔΨm that was partially prevented by MAO inhibition (F/FFCCP: Untreated 3.49 ± 0.14; Doxo 2.34 ± 0.10; Doxo+Pargyline 3.08 ± 0.11; Fig. 2A).
FIG. 2.
Effects of doxorubicin and MAO inhibition on mitochondrial function and lipid peroxidation. NRVMs have been treated with 0.5 μM doxorubicin for 24 h, in the presence or absence of 100 μM pargyline. (A) ΔΨm monitored by TMRM fluorescence in isolated NRVMs. TMRM fluorescence was normalized using 5 μM FCCP. *p < 0.001 versus Untreated, §p < 0.05 versus Untreated, #p < 0.001 versus Doxo by one-way ANOVA with post hoc Tukey's multiple comparison test. (B) ΔΨm monitored by TMRM fluorescence in isolated NRVMs. TMRM fluorescence was evaluated before and after 1 and 2 h of treatment with increasing concentrations of H2O2 (i.e., 10–100 μM). TMRM fluorescence was normalized using 5 μM FCCP. *p < 0.001 versus Basal, #p < 0.001 versus Untreated, §p < 0.001 versus Doxo by one-way ANOVA with post hoc Tukey's multiple comparison test. (C) PTP opening monitored by decrease of calcein fluorescence in isolated NRVMs. PTP opening was triggered by 5 μM calcimycin. Data were quantified after 180 and 300 s from the beginning of the experiment. *p < 0.001 versus Untreated, #p < 0.01 versus Doxo by one-way ANOVA with post hoc Tukey's multiple comparison test. (D) MDA formation in isolated NRVMs. Raw fluorescence values were normalized to milligram proteins, and then normalized versus Untreated. *p < 0.01 versus Untreated, #p < 0.05 versus Doxo by one-way ANOVA with post hoc Tukey's multiple comparison test. Approximately 30 cells were analyzed per condition in each experiment, and all the experiments were performed three times using three different animal preparations. MDA assay was performed three times using three different animal preparations. Data are expressed as mean ± SEM. ΔΨm, mitochondrial membrane potential; FCCP, carbonyl cyanide-p-trifluoromethoxyphenylhydrazone; MDA, malondialdehyde; PTP, permeability transition pore; TMRM, tetramethylrhodamine.
Subsequently, doxorubicin-treated cells were coincubated with increasing concentrations of exogenous H2O2 (i.e., 10–100 μM), and ΔΨm was monitored up to 2 h after the treatment, in the presence or absence of 100 μM pargyline. H2O2 induced a progressive decrease in ΔΨm, which was exacerbated by the presence of doxorubicin (Fig. 2B). This effect was prevented by pargyline, and the protection was observed even 2 h after the addition of 100 μM H2O2, both in control and in doxorubicin-treated cells (after 2 h: Untreated 30.56% ± 1.59%; Doxo 6.96% ± 1.63%; Doxo+Pargyline 56.20% ± 7.40%; Fig. 2B).
It has been reported that doxorubicin-derived ROS can sensitize the propensity for the mitochondrial permeability transition pore (PTP) to open (14). Thus, we treated NRVMs with 0.5 μM doxorubicin, in the presence or absence of 100 μM pargyline, and monitored the opening of the PTP using the calcein assay (50). After triggering the PTP opening by means of the Ca2+ ionophore calcimycin, doxorubicin-treated NRVMs displayed a gradual increase in PTP opening compared with the control, an effect that was prevented by MAO inhibition (after 300 s: Untreated 74.86% ± 3.71%; Doxo 50.06% ± 6.89%; Doxo+Pargyline 82.99% ± 6.56%; Fig. 2C).
Since lipid peroxidation is one of the downstream effects of an increase in ROS levels induced by doxorubicin (44), we assessed malondialdehyde (MDA) production in NRVMs (Supplementary Fig. S3A) (67, 72). Cells treated for 24 h with 0.5 μM doxorubicin displayed a significant increase in MDA production compared with the control (Fluorescence/mg protein normalized to control: Untreated 1 ± 0.03, Doxo 1.43 ± 0.04; Fig. 2D). Notably, the inhibition of MAO by pargyline reduced doxorubicin-induced lipid peroxidation (Fluorescence/mg protein normalized to control: Doxo+Pargyline 1.09 ± 0.10; Fig. 2D). Therefore, doxorubicin-induced mitochondrial dysfunction and lipid peroxidation require MAO involvement, whereas doxorubicin per se (i.e., in the presence of MAO inhibition) causes only a slight impairment of mitochondria.
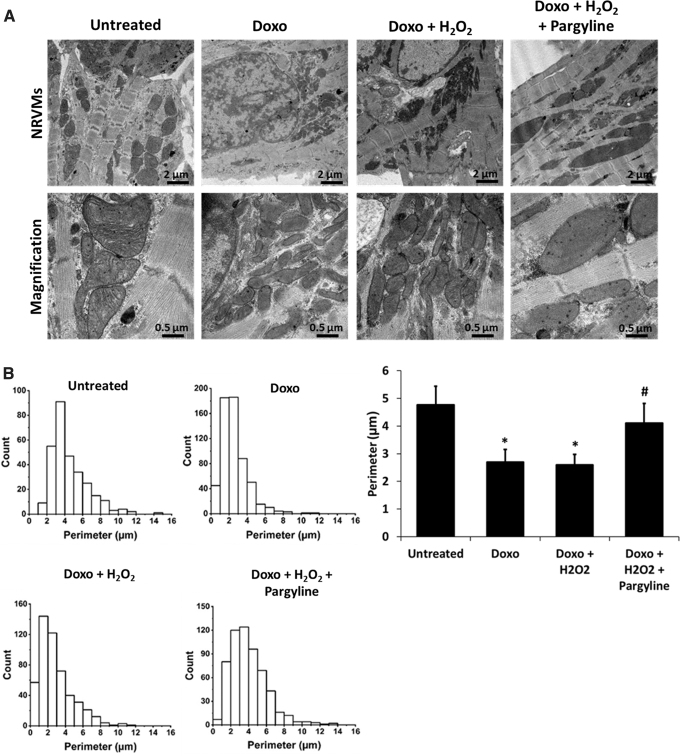
Alterations in mitochondrial bioenergetics induced by an increase in mitochondrial ROS are associated with alterations in the structure of mitochondria (5). Thus, mitochondrial morphology was analyzed by conventional transmission electron microscopy (TEM) in NRVMs treated with 0.5 μM doxorubicin for 24 h, with 10 μM H2O2 for 1 h, in the presence or absence of 100 μM pargyline. Doxorubicin-treated cardiomyocytes displayed mitochondria with highly reduced size and irregular shape (Fig. 3A), confirming a previous report (73). Mitochondrial morphological alterations were further supported by the analysis of mitochondrial perimeter, which was reduced in doxorubicin-treated cells, in a process prevented by pargyline (Fig. 3B).
FIG. 3.
Effects of doxorubicin and MAO inhibition on mitochondrial morphology. (A) TEM of isolated NRVMs treated with 0.5 μM doxorubicin for 24 h, in the presence or absence of 100 μM pargyline. Cells have been further stimulated with 10 μM H2O2 for 1 h. (B) Probability histograms of mitochondrial perimeter, representing values count on the y axis and mitochondrial perimeter values on the x axis. The bar graph displays the average of the mitochondrial perimeter. *p < 0.001 versus Untreated, #p < 0.01 versus Doxo+H2O2 by one-way ANOVA with post hoc Tukey's multiple comparison test. Where data were not normally distributed, the Kruskal–Wallis test has been applied. All the experiments were performed three times using three different animal preparations. Untreated: N = 15 cells, 297 mitochondria. Doxo: N = 14 cells, 588 mitochondria. Doxo+H2O2: N = 11 cells, 463 mitochondria. Doxo+H2O2+pargyline: N = 15 cells, 536 mitochondria. Data are expressed as mean ± SEM. TEM, transmission electron microscopy.
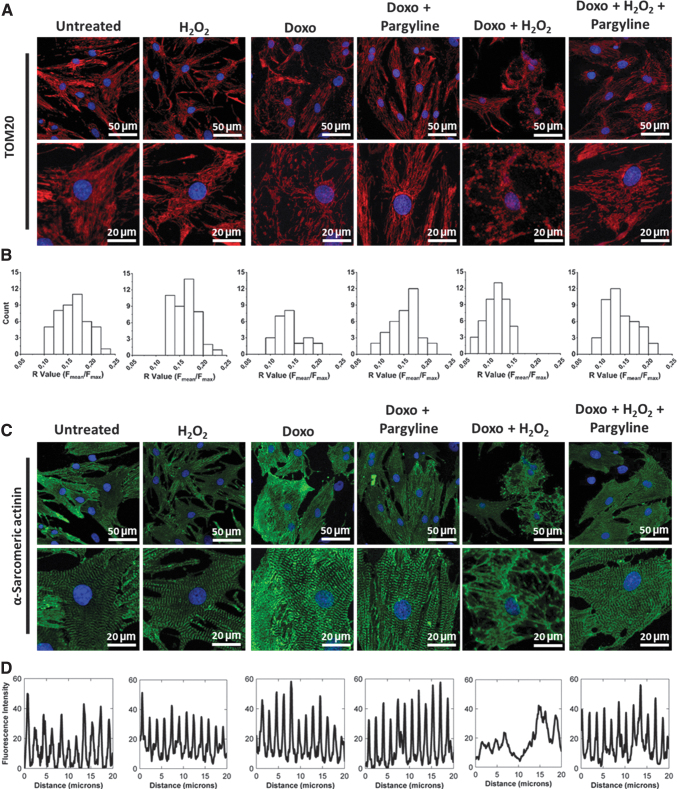
The TEM analysis was paralleled by confocal microscopy. To this aim, NRVMs were treated as described above (i.e., 0.5 μM doxorubicin; 10 μM H2O2; 100 μM pargyline), and mitochondria were stained for TOM20. Doxorubicin or H2O2 alone did not affect mitochondrial distribution (Fig. 4A), while their combination induced the disruption of the mitochondrial network (Fig. 4A). The alteration in mitochondrial distribution was validated by quantitative analysis expressed by the R parameter (59), defined as the ratio between average and maximal fluorescence on a given cell. The distribution of this parameter was significantly decreased in NRVMs incubated with both doxorubicin and H2O2, while it was restored by the treatment with pargyline (Fig. 4B), reflecting a decrease in dysfunctional mitochondria (R value: Untreated 0.165 ± 0.004; Doxo+H2O2 0.114 ± 0.003; Doxo+H2O2+Pargyline 0.141 ± 0.004; Supplementary Fig. S3B).
FIG. 4.
Effects of doxorubicin and MAO inhibition on mitochondria and sarcomere alignment. NRVMs have been treated with 0.5 μM doxorubicin for 24 h, in the presence or absence of 100 μM pargyline. Cells have been further stimulated with 10 μM H2O2 for 1 h. (A) Mitochondrial morphology assessed by confocal microscopy in isolated NRVMs. Confocal images were processed for immunofluorescent labeling of TOM20 (red), while nuclei were stained with DAPI (blue). Both representative images (upper row) and regions of interests (lower row) are provided. (B) Probability histograms of mitochondrial distribution, representing values count on the y axis and R values on the x axis. (C) Sarcomere arrangement assessed by confocal microscopy in isolated NRVMs. Confocal images were processed for immunofluorescent labeling of α-sarcomeric actinin (green), while nuclei were stained with DAPI (blue). Both representative images (upper row) and regions of interests (lower row) are provided. (D) The fluorescence intensity of the α-sarcomeric actinin was plotted and reported on the graph to show the effect of the treatments on the distribution of the sarcomeres within the cell. Approximately 30 cells were analyzed per condition in each experiment, and all the experiments were performed at least three times using three different animal preparations.
These data demonstrate that MAO inhibition prevents the alterations of mitochondrial bioenergetics and morphology in NRVMs treated with doxorubicin and subjected to an additional stress induced by exogenous H2O2.
MAO inhibition ameliorates cardiomyocyte contractility altered by doxorubicin-induced oxidative stress
Besides mitochondria, doxorubicin has been reported to alter the entire ultrastructure of cardiomyocytes (61). Therefore, we investigated whether treatment with pargyline could prevent doxorubicin-induced derangement of sarcomeric organization in cardiomyocytes. NRVMs were incubated with 0.5 μM doxorubicin for 24 h, in the presence or absence of 100 μM pargyline, and subsequently treated with 10 μM H2O2 for 1 h. The distribution of the α-sarcomeric actinin was evaluated by immunocytochemistry. Similarly to what was observed with mitochondrial morphology, doxorubicin or H2O2 alone was not sufficient to modify sarcomeric structure (Fig. 4C), while their combination altered the distribution of the α-sarcomeric actinin in cardiomyocytes (Fig. 4C, D). In contrast, NRVMs pretreated with pargyline displayed a distribution of the α-sarcomeric actinin similar to control, indicating a well-aligned sarcomeric pattern (Fig. 4C, D).
In respect to the excitation–contraction (EC) coupling (66) and in parallel with the evaluation of morphological alteration in cardiomyocytes, we analyzed doxorubicin-derived changes in intracellular [Ca2+] homeostasis.
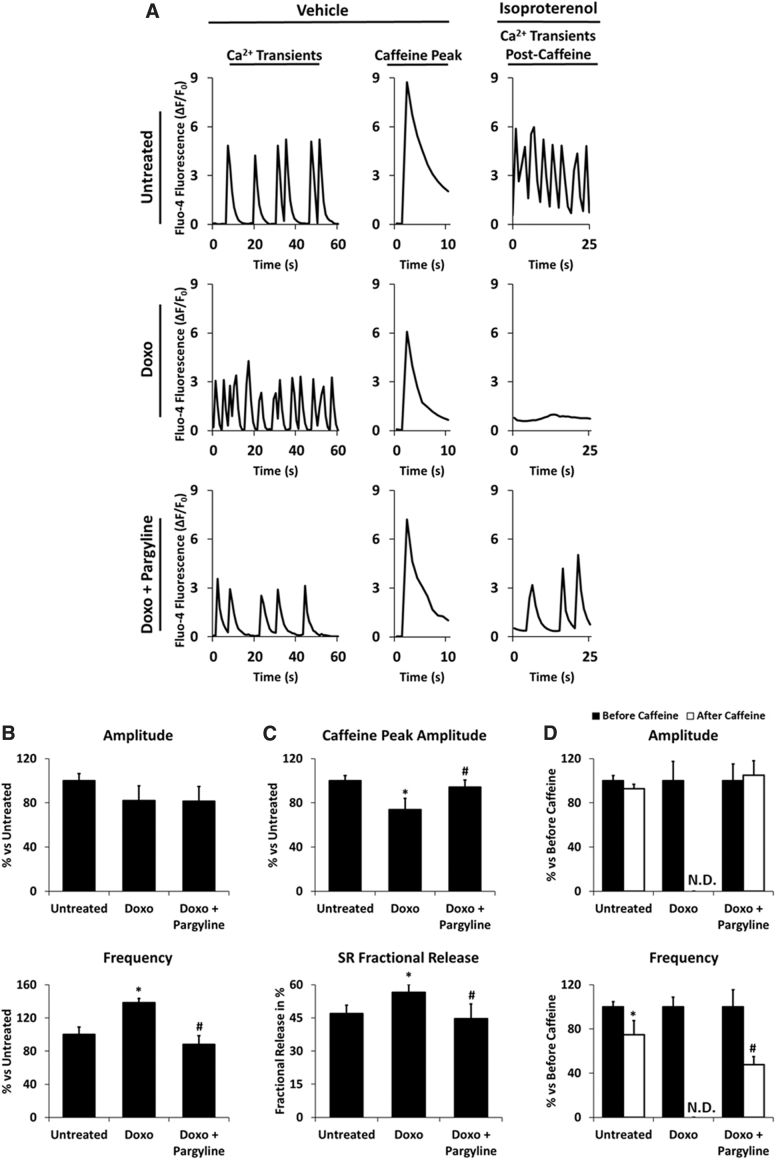
NRVMs were treated with 0.5 μM doxorubicin for 24 h in the presence or absence of 100 μM pargyline, and intracellular [Ca2+] transients were monitored by means of Fluo-4 fluorescence. Doxorubicin did not induce significant changes in transient amplitude, while it increased transient frequency, which was prevented by pargyline (Untreated 100% ± 8.90%, Doxo 138.34% ± 5.18%, Doxo+Pargyline 87.93% ± 10.76%; Fig. 5A, B). In addition, we evaluated sarcoplasmic reticulum (SR) Ca2+ content by stimulating the release of Ca2+ from the SR using 10 mM caffeine. Doxorubicin-treated cardiomyocytes displayed a decrease in response to caffeine (Untreated 100% ± 4.68%, Doxo 73.80% ± 10.12%, Doxo+Pargyline 94.11% ± 6.58%; Fig. 5A, C) and an increase in the fractional release of Ca2+ (Untreated 46.93% ± 3.80%, Doxo 56.59% ± 3.59%, Doxo+Pargyline 44.68% ± 6.63%; Fig. 5C). Similar results were obtained by Sag et al. (53). Their interpretation of this phenomenon was that an increase in both NCX activity and RyR2 opening would lead to an increase of trans-sarcolemmal Ca2+ extrusion, and to SR Ca2+ leak. Of note, the doxorubicin-dependent SR Ca2+ load reduction was prevented by MAO inhibition (Fig. 5C).
FIG. 5.
Effects of doxorubicin and MAO inhibition on cytosolic [Ca2+] homeostasis. NRVMs have been treated with 0.5 μM doxorubicin for 24 h, in the presence or absence of 100 μM pargyline. Cytosolic [Ca2+] transients have been monitored by Fluo-4 AM. (A) Representative intracellular [Ca2+] transients, intracellular [Ca2+] peaks after stimulation with 10 mM caffeine, and intracellular [Ca2+] transients after cotreatment with 10 mM caffeine and 10 μM isoproterenol. (B) Transient amplitude and transient frequency average in spontaneous beating NRVMs. *p < 0.05 versus Untreated, #p < 0.01 versus Doxo by one-way ANOVA with post hoc Tukey's multiple comparison test. (C) Caffeine peak average and SR fractional release of Ca2+ in spontaneous beating NRVMs. *p < 0.05 versus Untreated, #p < 0.05 versus Doxo by one-way ANOVA with post hoc Tukey's multiple comparison test. (D) Transient amplitude and transient frequency average in the presence of isoproterenol, before and after caffeine stimulus. *p < 0.05 versus before caffeine, #p < 0.001 versus before caffeine by one-way ANOVA with post hoc Tukey's multiple comparison test. Approximately 30 cells were analyzed per condition in each experiment, and all the experiments were performed three times using three different animal preparations. Data are expressed as mean ± SEM. AM, acetoxymethyl; SR, sarcoplasmic reticulum.
To further investigate SR function and Ca2+ cycling, the response to caffeine was monitored in isoproterenol-treated NRVMs. Doxorubicin is reported to alter the regulation of β-adrenergic receptors (22), thereby worsening the latent DC. Administration of isoproterenol increases mitochondrial ROS levels (51), allowing us to further investigate the effect elicited by pargyline. Doxorubicin-treated cardiomyocytes incubated with 10 μM isoproterenol for 15 min did not recover the spontaneous beating activity after caffeine (Fig. 5A). Conversely, MAO inhibition resulted in the recovery of Ca2+ transients after caffeine (Fig. 5A–D).
Taken together, these data demonstrate that pargyline prevents doxorubicin-induced derangement in EC coupling by preserving both cardiomyocyte structure and intracellular [Ca2+] homeostasis. Moreover, the protection against doxorubicin-induced oxidative stress is maintained even in the presence of an additional stress, such as exogenous H2O2 or β-adrenergic stimulation.
MAO inhibition reduces cell death induced by doxorubicin and oxidative stress in cardiomyocytes
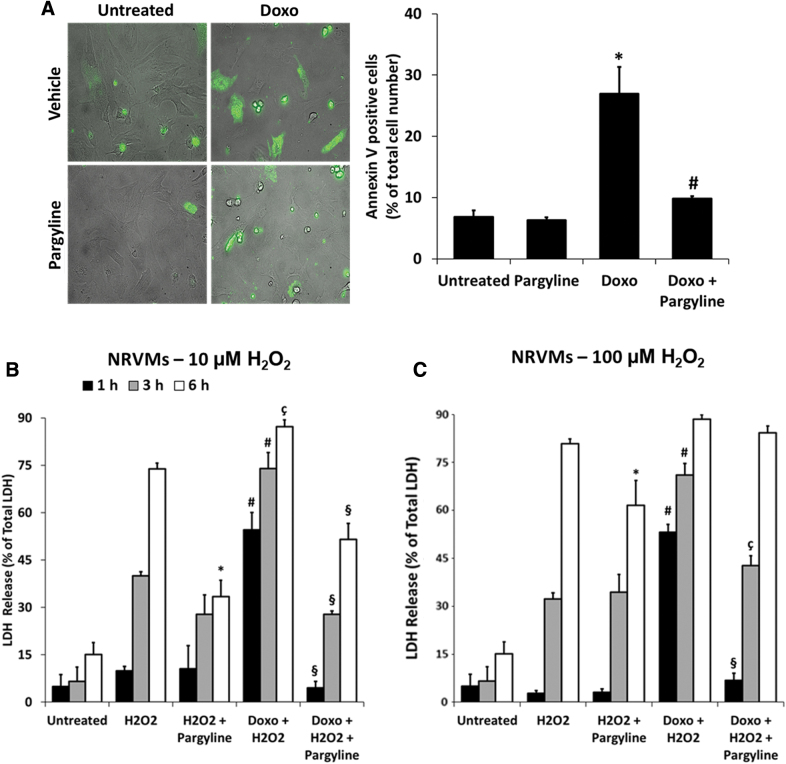
A severe oxidative stress results in cell death in any pathological condition, including DC (21). It has been reported that doxorubicin-induced mitochondrial dysfunction and oxidative stress could synergize in promoting apoptosis (37). To test whether MAO inhibition affects this process, NRVMs were treated with 0.5 μM doxorubicin, in the presence or absence of 100 μM pargyline, and stained with Annexin-V-Fluos that recognizes exposed phosphatidylserines, a typical feature of apoptosis (Supplementary Fig. S3C) (13). Doxorubicin induced a marked increase in Annexin-V positive cells, which was prevented by pargyline treatment (Untreated 6.84% ± 1.06%, Doxo 26.90% ± 4.41%, Doxo+Pargyline 9.79% ± 0.43%; Fig. 6A). In addition, we wondered whether autophagy processes could be involved. At baseline, LC3B-II and p62 levels were not significantly different between the groups (Supplementary Fig. S3D, E, full blots in Supplementary Fig. S2D, E). Upon inhibition of lysosomal degradation, LC3B-II abundance increased to the same extent in control versus doxorubicin-treated cells (Supplementary Fig. S3D), suggesting that autophagy levels and autophagic flux were not affected by the 24 h-long 0.5 μM doxorubicin treatment. Thus, doxorubicin-induced alterations in our model appear to occur independently of autophagy.
FIG. 6.
Effects of doxorubicin and MAO inhibition on cardiomyocyte viability. NRVMs have been treated with 0.5 μM doxorubicin for 24 h, in the presence or absence of 100 μM pargyline. (A) Apoptosis evaluated by Annexin-V-Fluos staining in isolated NRVMs. Histogram displays the percentage of Annexin V-positive cells normalized to the total number of cells identified by bright-field images. *p < 0.01 versus Untreated, #p < 0.01 versus Doxo by the Kruskal–Wallis test. (B) Cell death measured by LDH release from isolated NRVMs. Cells have been further stimulated with 10 μM H2O2, and supernatants were collected at different time points. *p < 0.001 versus H2O2; #p < 0.001 versus H2O2; çp < 0.01 versus H2O2; §p < 0.001 versus Doxo+H2O2 by one-way ANOVA with post hoc Tukey's multiple comparison test. (C) Cell death measured by LDH release from isolated NRVMs. Cells have been further stimulated with 100 μM H2O2, and supernatants were collected at different time points. *p < 0.05 versus H2O2; #p < 0.001 versus H2O2; §p < 0.001 versus Doxo+H2O2; çp < 0.01 versus Doxo+H2O2 by one-way ANOVA with post hoc Tukey's multiple comparison test. For the evaluation of apoptosis, ∼100 cells were analyzed per condition in each experiment, and all the experiments were performed three times using three different animal preparations. For LDH release approximately three to four wells were analyzed per condition in each experiment. All the experiments were performed at least three times using three different animal preparations. Data are expressed as mean ± SEM. LDH, lactic dehydrogenase.
Since the induction of apoptosis induced by doxorubicin might be upstream of the rupture of cell membrane, leading to necrosis, we investigated whether pargyline could decrease doxorubicin-induced cell death in cardiomyocytes. NRVMs were treated with 0.5 μM doxorubicin for 24 h, in the presence or absence of 100 μM pargyline, and cell death was monitored by lactic dehydrogenase (LDH) release after the administration of different concentrations of H2O2 (i.e., 10–100 μM). H2O2 per se induced a dose-dependent increase in cell death in NRVMs (after 6 h: 73.88% ± 1.82% with 10 μM H2O2, 80.89% ± 1.44% with 100 μM H2O2; Fig. 6B, C), which was prevented by pargyline (after 6 h: 33.37% ± 5.23% with 10 μM H2O2, 61.61% ± 7.74% with 100 μM H2O2; Fig. 6B, C). The injury elicited by H2O2 was accelerated in the presence of doxorubicin, with cell death occurring after only 1 h of treatment (after 1 h: 54.51% ± 5.55% with 10 μM H2O2; after 3 h: 73.94% ± 5.05% with 10 μM H2O2; Fig. 6B, C). Notably, pargyline reduced significantly the extent of cell death in doxorubicin-treated NRVMs, in the presence of either 10 μM H2O2 (after 1 h: 4.53% ± 2.03%; after 3 h: 27.77% ± 1.08%, Fig. 6B) or 100 μM H2O2 (after 1 h: 6.85% ± 2.22%; after 3 h: 42.70% ± 3.11%, Fig. 6C). However, the protection by pargyline against cell death was not observed when the exposure to oxidative stress was massive (i.e., treatment with doxorubicin and 6 h of exposure to 100 μM H2O2) (Fig. 6C).
Taken together, these results demonstrate the protective effect elicited by MAO inhibition in cardiomyocytes coincubated with doxorubicin and H2O2.
MAO inhibition prevents LV dilation and dysfunction in doxorubicin-treated mice
Given the protective effect exerted by MAO inhibition against doxorubicin-induced cellular derangements and death in vitro, we next investigated whether pharmacological MAO inhibition could protect against doxorubicin-induced cardiotoxicity in vivo. To this aim, mice were injected with doxorubicin and immediately randomized to receive either vehicle or pargyline. LV function was monitored by echocardiography.
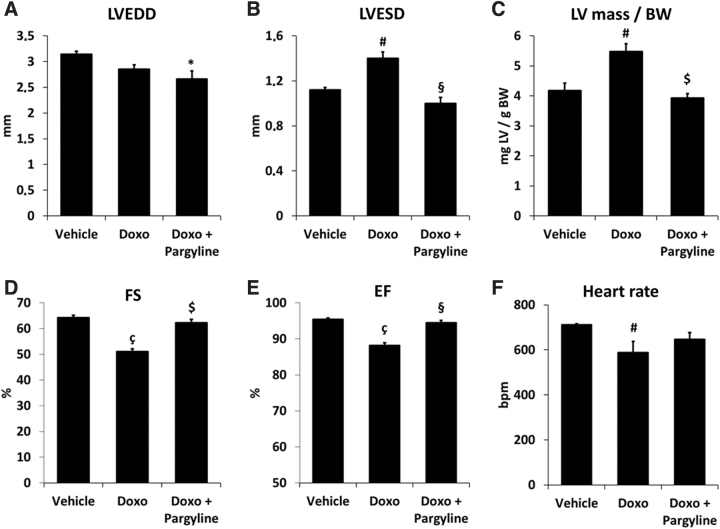
After 5 weeks, doxorubicin-treated mice exhibited no change in LV dimension in end-diastole (Fig. 7A), but showed increased LV dilation in end-systole (Fig. 7B) and an increase in LV mass normalized to body weight (Fig. 7C and Table 1). In addition, significant decrease in fractional shortening (FS) and ejection fraction (EF), common features of the doxorubicin-induced cardiomyopathy, was clearly evident (Fig. 7D, E and Table 1). Remarkably, LV dilation and function were fully preserved in doxorubicin-treated mice receiving pargyline (Fig. 7 and Table 1). Doxorubicin-treated mice displayed a significant decrease in heart rate, although pargyline treatment did not afford a significant improvement (Fig. 7F).
FIG. 7.
Changes in cardiac morphology and function in doxorubicin-treated mice. (A–C) Changes in cardiac morphology and (D–F) function in vehicle- (n = 5), doxorubicin- (n = 7), and doxorubicin and pargyline-treated mice (n = 5). *p < 0.05, #p < 0.01, çp < 0.001 versus Vehicle; §p < 0.01, $p < 0.001 versus Doxo by one-way ANOVA with post hoc Tukey's multiple comparison test. Where data were not normally distributed, the Kruskal–Wallis test has been applied. Data are expressed as mean ± SEM. EF, ejection fraction; FS, fractional shortening; LV mass/BW, left ventricle mass/body weight; LVEDD, left ventricular end-diastolic dimension; LVESD, left ventricular end-systolic dimension.
Table 1.
Changes in Cardiac Morphology and In Vivo Ventricular Function Induced by Doxorubicin in Vehicle- and Pargyline-Treated Mice
| Vehicle | Doxo | Doxo+Pargyline | |
|---|---|---|---|
| BW, g | 31.40 ± 0.47 | 22.38 ± 0.91a | 24.28 ± 1.20a |
| LV mass/BW | 4.18 ± 0.25 | 5.48 ± 0.26b | 3.93 ± 0.15c |
| IVS, cm | 0.96 ± 0.02 | 1.02 ± 0.03 | 0.94 ± 0.04 |
| LVEDD, cm | 3.14 ± 0.06 | 2.85 ± 0.09 | 2.66 ± 0.16d |
| LVESD, cm | 1.12 ± 0.02 | 1.40 ± 0.05b | 1.00 ± 0.05c |
| LVPW, cm | 0.9 ± 0.03 | 1.01 ± 0.03d | 0.9 ± 0.03e |
| Heart rate, bpm | 712.00 ± 4.06 | 587.15 ± 50.67b | 646.00 ± 30.71 |
| FS, % | 64.27 ± 0.88 | 51.01 ± 1.09a | 62.20 ± 1.38c |
| EF, % | 95.40 ± 0.33 | 88.14 ± 0.78a | 94.51 ± 0.64f |
Data are expressed as mean ± standard error of the mean. Vehicle n = 5; Doxo n = 7; Doxo+Pargyline n = 5.
p < 0.001 versus Vehicle, bp < 0.01 versus Vehicle, cp < 0.001 versus Doxo, dp < 0.05 versus Vehicle, ep < 0.05 versus Doxo, fp < 0.01 versus Doxo.
BW, body weight; EF, ejection fraction.; FS, fractional shortening; IVS, interventricular septum; LVEDD, left ventricular end-diastolic dimension; LVESD, left ventricular end-systolic dimension; LVmass/BW, left ventricular mass normalized to body weight (expressed as mg of left ventricle/gram of body weight mg/g); LVPW, left ventricular posterior wall.
Discussion
This study shows that MAOs are involved in the oxidative stress induced by doxorubicin treatment in cardiomyocytes. This increase in ROS formation causes mitochondrial dysfunction, which is causally related to all the alterations induced by doxorubicin, including sarcomere disorganization, intracellular [Ca2+] impairment, and eventually cell death. Interestingly, DC is further exacerbated by H2O2 or isoproterenol. The causal role of mitochondrial ROS formation and dysfunction is demonstrated by the protective efficacy of MAO inhibition. Remarkably, MAO inhibition proves to be effective also in the in vivo model of DC, and completely prevents LV dilation and cardiac dysfunction in doxorubicin-treated mice.
Our data demonstrate that the treatment with doxorubicin increases H2O2 levels only in the mitochondrial compartment. However, the observed elevation in ROS levels is not simply a direct consequence of doxorubicin interaction with Complex I, since MAO inhibition significantly reduced mitochondrial ROS generation. Therefore, MAOs modulate the initial oxidative stress caused by doxorubicin, resulting in a major increase in mitochondrial ROS levels. The beneficial role of MAO inhibition is observed at a doxorubicin concentration (i.e., 0.5 μM) comparable with the one used in clinics. However, it is likely that at higher concentrations, such as those used previously in in vitro studies (6, 62), doxorubicin causes a major increase of ROS that is no longer affected by MAO inhibition. The fact that pargyline prevented not only doxorubicin-induced derangements but also those exacerbated by administration of H2O2 appears relevant from a pathophysiological and clinical point of view, since a latent DC is likely to be precipitated by comorbidities occurring with doxorubicin (1, 12).
DC is not limited to mitochondria. Indeed, as also reported previously (10, 32, 66), we observed profound alterations in both cardiomyocyte structure and intracellular [Ca2+] homeostasis, which are likely to impair the EC coupling. These changes obviously occur in viable cells. Therefore, DC is accompanied by both cell death and functional changes, which are however both prevented by MAO inhibition.
The absence of an increase in cytosolic ROS levels rules out the possibility that mitochondrial ROS can diffuse to the cytosol, as expected by the abundance of peroxidases and presence of superoxide dismutases (20). Nevertheless, redox communication between mitochondria and cytosol is likely to exist to explain the beneficial effects of pargyline on doxorubicin-induced derangements at the cytosolic level (11, 31). While additional studies are necessary to identify the mechanisms by which mitochondrial oxidative stress is extended to the cytosol, mitochondrial ROS formation appears to be sufficient to explain all the derangements caused by DC. For instance, a recent work highlighted the role of PI3Kγ (38) and also in that case, the triggering factor was suggested to be represented by mitochondrial disruption.
Given the important role played by mitochondrial ROS in DC, various antioxidant treatments have been investigated (e.g., Vitamin A and E, polyunsaturated fatty acids, flavonoids) (9, 43). However, only few of them entered clinical trials, with minor amelioration of cardiac function (9). This failure of antioxidant treatment, which can be extended also to other pathologies (24), can be explained by ROS involvement in both pathological responses and cell defense mechanisms (2, 52). For instance, ischemic preconditioning appears to involve generation of low levels of ROS (20, 56). Indeed, preconditioning protection can be abrogated by antioxidants (29) or replicated by the addition of exogenous oxidants (30). Therefore, the indiscriminate buffer of ROS is potentially able to decrease beneficial responses of tissues (20, 52). In fact, we have recently demonstrated that high levels of selective mitochondrial ROS are deleterious, while low levels of mitochondrial ROS can contribute to cardioprotection in ischemia/reperfusion, both in vitro and in vivo (2). Thus, a more efficacious approach should identify specific sources of ROS that do not affect other cellular functions. This is especially the case for mitochondria. For instance, if the respiratory complexes were the only sources of ROS, it would not be possible to develop therapeutic approaches since the inhibition of ETC would inevitably impair ATP production and cell viability.
In this respect, MAO inhibition is a valuable tool to demonstrate the involvement of mitochondrial ROS formation in pathophysiology. We have already provided evidence for MAO involvement in cardiac injury induced by postischemic reperfusion, pressure overload, and diabetes, as well as in muscular dystrophy (17, 34, 36, 58). This study further supports and extends this notion, and identifies MAOs as key mediators of doxorubicin-induced cardiotoxicity. This was confirmed in animals injected with doxorubicin, in which concomitant treatment with pargyline prevented LV dilation, increase in LV mass and preserved LV function. Indeed, results from our in vivo study strongly suggest that MAO inhibition could prevent cardiac dysfunction and heart failure due to DC also in patients. Nevertheless, our model is more representative of the acute cardiotoxicity that may occur with higher doses of doxorubicin. Future studies will establish if the results obtained from this study apply also to a chronic model of doxorubicin cardiomyopathy.
Growing evidence obtained from animal studies indicates that females might be protected compared with males in relation to DC (42). Interestingly, mitochondrial dysfunction and energy metabolism signaling pathways seem to be associated with early cardiotoxicity in males but not in females (45). In addition, female cardiomyocytes are more resistant to oxidant-induced death (70), while 17-β-estradiol affords cardioprotection in ovariectomized rats treated with doxorubicin (46). In patients, female subjects seem to be more at increased risk of DC, especially when treated with doxorubicin before puberty and adolescence, or after menopause (8). In our in vivo study, we used only male mice, and it remains to be established whether MAO inhibition would equally afford protection also in female mice with DC.
Overall, the causal relationship between MAO activity and doxorubicin-induced cardiac derangements suggests that mitochondrial oxidative stress is likely to represent a unifying mechanism of DC. In addition, our data pave the way for the development of MAO inhibitors to prevent cardiac failure in patients treated with doxorubicin.
Limitations of the study
The findings of this study have to be seen in light of some limitations. The first is that the protection elicited by pargyline gives a cytoprotective and cardioprotective role to MAO inhibition, a feature that hardly applies to antitumor interventions. Therefore, it could be hypothesized that MAO inhibition might counteract the antineoplastic efficacy of doxorubicin. This is likely not the case since MAO-A downregulation by shRNA has been shown to protect against tumorigenesis and cancer metastasis (39, 71). In addition, unlike dexrazoxane, MAO inhibitors have no effect on topoisomerase II. Furthermore, they have been used in the clinic for many years, and have never been associated with the development of secondary malignancies. Nevertheless, future studies are necessary to prove that MAO-A and/or B inhibition, while protecting against DC, does not affect or partially favor the antitumor effect of doxorubicin in cancer patients and across cancer types.
The second limitation is that although our study provides clear evidence of the ability of MAOs to modulate an initial oxidative stress, the underlying mechanism is not yet defined. Additional studies are necessary to clarify whether doxorubicin affects directly the structural–functional relationship of MAO or its interaction with the lipid components of the outer mitochondrial membrane. In addition, besides H2O2 formation, MAOs are a source of reactive aldehydes and ammonia (35). Since DC is also associated with changes in cytotoxic aldehydes, and mitochondrial aldehyde dehydrogenase (ALDH2) ameliorates DC (63), it is plausible that MAO inhibition in DC abolishes the detrimental effects of these reactive aldehydes as well. Additional studies are necessary to quantitate the relative contribution to DC given by ROS and aldehydes produced by MAOs.
Materials and Methods
Animals and procedures
All animal studies were performed using male C57BL6/J mice at 12 weeks of age. Animals were injected intraperitoneally with 10 mg/kg doxorubicin (Sigma) using saline solution as vehicle. The same day of the doxorubicin injection, mice were randomized and subjected to a daily treatment with either vehicle or MAO inhibitor pargyline (50 mg/kg/day, i.p.; Sigma). Animals were observed and weighed daily for 5 weeks, and were sacrificed when they exhibited distress and/or when weight loss approached 25%.
Experiments were carried out in the animal facility of the Department of Experimental Oncology of the European Institute of Oncology (Ministry of Health authorization: DM No. 86/2005-17/06/2005). All aspects of animal care and experimentation were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and the Italian Laws (D.L.vo 116/92 and after additions), which enforces EU86/609 Directive. Experiments were approved by the local Ethical Committee and notified to the Ministry of Health (Project No. 13/2010).
Echocardiography
In vivo cardiac morphology and function were assessed by serial M-mode echocardiography (Acuson Sequioa C256, 13 MHz transducer; Siemens, PA) performed in conscious mice. LV end-systolic and end-diastolic dimensions were averaged from three to five beats. LV percent FS, EF, and LV mass were calculated as described previously (36). Thickness of posterior free wall and interventricular septum was averaged.
Cell culture and treatments
NRVMs were isolated from 1- to 3-day-old Wistar rats as previously described (34). Cardiomyocytes were plated in 0.1% porcine gelatin (Sigma)-coated plates at variable densities (∼2.5 × 105 cells/mL) in MEM supplemented with 10% fetal bovine serum (FBS; Thermo Fisher Scientific), 1% penicillin/streptomycin (Thermo Fisher Scientific), 1% nonessential amino acids (Thermo Fisher Scientific), 1 mM 5-bromo-2-deoxyuridine (Sigma). Cells were maintained at 37°C in the presence of 5% CO2. The medium was changed to MEM supplemented with 1% FBS, 1% penicillin/streptomycin, and 1% nonessential amino acids after 24 h of plating.
AMVMs were isolated from the hearts of 12-week-old C57Bl6/J mice, as previously described (36). Collagenase-digested isolated myocytes were incubated in buffer with increasing concentrations of Ca2+, achieving a final concentration of 1.2 mM Ca2+ as in Dulbecco's modified Eagle's medium (DMEM) plating media. Cells were seeded at 6 × 104 rod-shaped myocytes per milliliter in DMEM plating media supplemented with 10 mM 2,3-butanedione monoxime (Sigma) in plates coated with laminin. Cells were maintained at 37°C in the presence of 5% CO2. The medium was replaced after 1 h incubation to remove unattached cells.
To evaluate the effect of doxorubicin, cells were pretreated with 0.5 μM doxorubicin (32, 44), in the presence or the absence of 100 μM pargyline (Sigma), unless differently specified in the results. To induce oxidative stress, cells were treated with different concentrations of exogenous H2O2, ranging from 1 to 100 μM. To stimulate MAO-A in intact NRVMs, cells were treated with 20 μM tyramine for 2 h (36).
Live imaging
Experiments were carried out in HBSS at pH 7.4 (adjusted with NaOH) and at 37°C. Images were acquired using an inverted fluorescence microscope (Leica DMI6000B equipped with DFC365FX camera) with PL APO 40 × /1.25 oil objective. Fluorescence intensity was quantified using the Fiji distribution of the Java-based image processing program ImageJ (55), and background signal was subtracted from all analyzed regions of interest. For Ca2+ imaging, traces were analyzed using the “Peak Analyzer” tool of Origin Pro 9.1.
To silence MAO-A expression, NRVMs were transfected with scramble or MAO-A siRNA (Ambion) as previously described (18). The efficiency of MAO-A knockdown was assessed by Western blotting (Supplementary Fig. S1B).
To monitor mitochondrial and cytosolic ROS formation in NRVMs, cells were transfected with the genetically encoded H2O2 sensors Mito-HyPer and Cyto-HyPer (Evrogen) (4). To monitor mitochondrial GSSG/GSH ratio in NRVMs, cells were transfected with the genetically encoded sensor Mito-Grx1-roGFP. pLPCX mito Grx1-roGFP2 was a gift from Tobias Dick (Addgene Plasmid No. 64977; RRID:Addgene_64977) (28). NRVMs were plated on μ-Plate 96-well black plates (Ibidi) at a density of 4 × 104 cells/well, and transfected with Lipofectamine 3000 reagent (Sigma). For each transfection, 0.1 μg of plasmid was diluted in 5 μL of Opti-MEM medium (Thermo Fisher Scientific) in the presence of 0.2 μL of P3000™ reagent (Life Technologies), and later combined with 0.3 μL of Lipofectamine™ 3000 (Life Technologies). The DNA–lipid complexes were added to the cells and incubated at 37°C in the presence of 5% CO2. Transfected cells were used for experiments after 48 h. Fluorescence values were expressed as HyPer ratio (488/410 nm) and Grx1-roGFP ratio (410/488).
To monitor mitochondrial ROS in AMVMs, cells were incubated with 250 nM MTR CM-H2XRos (Thermo Fisher Scientific) for 30 min at 37°C, in the presence of 5% CO2. Since the accumulation of MTR in cells can vary from different preparations, data were normalized to the dimethyl sulfoxide (DMSO) control.
To monitor ΔΨm, cells were incubated with 25 nM TMRM (Thermo Fisher Scientific) and 1.6 μM cyclosporin H for 30 min at 37°C, in the presence of 5% CO2. TMRM fluorescence intensity was monitored, and images were acquired before and after the addition of 4 μM carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP; Sigma) (34). To obtain a reliable value for TMRM, fluorescence values were expressed as ΔF (F0/FFCCP), and results were normalized to the DMSO control basal value.
To monitor PTP opening, cells were incubated with 1 μM calcein acetoxymethyl (AM) ester (Thermo Fisher Scientific) in the presence of 2 mM cobalt chloride for 15 min at 37°C in a humidified incubator, followed by 20 min of de-esterification as previously described (50). PTP opening was induced by 5 μM of the Ca2+ ionophore calcimycin. To evaluate the extent of pore opening, data were normalized to the basal value.
To monitor intracellular [Ca2+] homeostasis, cells were incubated with 5 μM Fluo-4 AM ester (Thermo Fisher Scientific), 0.01% w/v pluronic F-127 (Sigma), and 250 μM sulfinpyrazone (Sigma), for 20 min at 37°C in a humidified incubator, followed by 20 min of de-esterification. Caffeine stimulus was applied to NRVMs in the presence or absence of 10 μM isoproterenol (Sigma) (74). Since the accumulation of Fluo-4 in NRVMs can vary from different preparations, data were normalized to the DMSO control. The fractional release of Ca2+ was evaluated to define the fraction of SR Ca2+ released at every twitch (3).
Glutathione redox state estimation in cell lysates
NRVMs were plated in six-well plates at a density of 5 × 105 cells/well. At day 4, cells were treated with 0.5 μM doxorubicin for 24 h, in the presence or the absence of 100 μM pargyline. The day after, NRVMs were collected, washed twice with cold phosphate-buffered saline (PBS), and then lysed and deproteinized with 6% meta-phosphoric acid. After 10 min at 4°C, samples were centrifuged, and supernatants were neutralized with 15% Na3PO4 and assayed for total glutathione as previously described (68). Samples were derivatized with 2-vinylpyridine to block reduced glutathione, and oxidized glutathione was then estimated. Total glutathione was normalized to protein level, and data expressed as the percentage of oxidized glutathione (GSSG) normalized to total glutathione (GSH tot).
Total thiol estimation
NRVMs well plated in six-well plates at a density of 5 × 105 cells/well. At day 4, cells were treated with 0.5 μM doxorubicin for 24 h, in the presence or the absence of 100 μM pargyline. After the treatment, cells were washed with 1 mL of PBS 1 × and then dissolved with 1 mL of 0.2 M Tris/HCl (pH 8.1), 5 mM EDTA, and 7.2 M guanidine. The reaction was started by the addition of 0.1 M DTNB [5,5′-dithiobis(2-nitrobenzoic acid)]. The absorbance of the DTNB reduction product was followed at 412 nm at 25°C (68).
Lipid peroxidation assay
Lipid peroxidation was estimated as previously described (67). NRVMs were plated in six-well plates at a density of 5 × 105 cells/well. At day 4, cells were treated with 0.5 μM doxorubicin for 24 h, in the presence or the absence of 100 μM pargyline. Cells were washed with 1 mL of PBS 1 × . Subsequently, cells were disrupted with 1 mL of 0.1 N sulfuric acid and 0.15 mL of 10% phosphotungstic acid (PTA), and incubated for 10 min at room temperature. Then, samples were centrifuged at 11,600 g for 10 min. The supernatant was removed, and 1 mL of 0.1 N H2SO4 and 0.15 mL of 10% PTA were added to the pellets. After 5 min of incubation at room temperature, samples were centrifuged as described above. The supernatant was removed, and the dried pellet was dissolved in 0.35% NONIDET, 0.014% butylated hydroxytoluene (BHT), and 0.23% thiobarbituric acid in a final volume of 0.25 mL. Subsequently, samples were incubated at 95°C for 60 min. After this time, samples were cooled on ice for 5 min and centrifuged at 11,600 g for 10 min. The pellets were discarded, and 0.4 mL of n-butanol were added to the supernatants, vigorously mixed, and centrifuged at 11,600 g for 15 min. MDA is present in the upper phase. The fluorescence of the latter was analyzed at 530 nm (Ex) and at 590 nm (Em) using a Tecan plate reader. Incubation with 25 mM cumene hydroperoxide and 50 μM hemin for 15 min, or with 100 μM H2O2 for 1 h was used as the positive control (Supplementary Fig. S3A). Data are expressed as fluorescence per milligram proteins normalized versus control.
Western blot analysis
NRVMs were plated in 6-well plates at a density of 4 × 105 cells/mL, while AMVMs were plated in 12-well plates at a density of 6 × 104 rod-shaped myocytes per milliliter. Cells were incubated for 24 h with 0.5 μM doxorubicin, in the presence or absence of 100 μM pargyline. For the assessment of autophagy flux, cells were treated with 20 mM ammonium chloride and 100 μM leupeptin 4 h before cell lysis. Cells were homogenized in RIPA Lysis Buffer (1 × ; EMD Millipore) containing protease (1 × , cOmplete mini protease inhibitor cocktail; Roche) and phosphatase (1 × , PhosStop; Roche) inhibitors. Protein concentration was determined using BCA protein assay (BCA Kit; Euroclone). Proteins were separated using 4%–12% Bis-Tris gels (Life Technologies) in 2-(N-Morpholino) ethanesulfonic acid (MES)/SDS (sodium dodecyl sulfate) running buffer 1 × (i.e., 1 M MES, 1 M Tris Base, 69.3 mM SDS, 20.5 mM EDTA, pH 7.3). Proteins were transferred to nitrocellulose membrane (Bio-Rad) by Western blotting in transfer buffer (i.e., 250 mM Tris Base, 1.92 M glycine, 0.1% SDS) overnight at 4°C. Membranes were blocked for 1 h with 3% bovine serum albumin (BSA) in TBS-Tween, and incubated overnight with the following primary antibodies diluted in 3% BSA:
Anti-MAO-A → 1:1000 (ab126751, Rabbit; Abcam)
Anti-MAO-B → 1:1000 (M1821, Rabbit; Sigma)
Anti-LC3B → 1:1000 (#2775, Rabbit; Cell Signaling)
Anti-p62 → 1:1000 (P0067, Rabbit; Sigma)
Antiactin →1:1000 (sc-56459, Mouse; Santa Cruz Biotechnology)
After incubation with primary antibodies, membranes were washed three times in TBS-Tween, 5 min each, and incubated for 1 h with the following secondary antibodies diluted in 3% BSA:
Antimouse (m-IgGκ BP-HRP) →1:3000 (#sc-516102; Santa Cruz Biotechnology)
Antirabbit (mouse antirabbit IgG-HRP) →1:3000 (#sc-2357; Santa Cruz Biotechnology)
After incubation with primary and secondary HRP-conjugated antibodies, bands were detected by the Pierce™ ECL Western Blotting Substrate using the UVITEC Cambridge, mini-HD instrument. Bands were quantified by densitometry analysis performed using the Fiji distribution of the Java-based image processing program ImageJ (55).
Immunocytochemistry
NRVMs were plated in 24-well plates on gelatin-coated coverslips at a density of 5 × 104 cells/well. At day 4, cells were treated with 0.5 μM doxorubicin for 24 h, in the presence or absence of 100 μM pargyline. The day after, NRVMs were treated with 10 μM H2O2 for 1 h. NRVMs were fixed with 4% paraformaldehyde in PBS for 30 min at room temperature, permeabilized with PBS supplemented with 1% saponin for 15 min, and blocked with 3% BSA for 1 h at 4°C. After blocking, mitochondria were stained using rabbit anti-TOM20 antibody (1:500; Santa Cruz Biotechnology), while sarcomeres were stained using mouse anti-α-sarcomeric actinin antibody (1:500; Life Technologies) overnight at 4°C. The day after, samples were incubated with Alexa Fluor 594-conjugated antirabbit (1:250; Life Technologies) and Alexa Fluor 488-conjugated antimouse (1:250; Life Technologies) secondary antibodies for 1 h at room temperature. Coverslips were mounted using ProLong Diamond Antifade Mountant with DAPI to stain nuclei (Life Technologies). Images were collected at Zeiss LSM 700 confocal system equipped with a PlanApo 40 × /1.2 oil objective, and an Argon laser was used to excite the fluorophores at the appropriate wavelength. Images were collected at 2048 × 2048 pixels per image with a 100 Hz acquisition rate. Images were analyzed using the Fiji distribution of the Java-based image processing program ImageJ (55).
Analysis of mitochondrial subcellular distribution
The evaluation of mitochondrial subcellular distribution was obtained based on the method described by Spinazzi et al. (59). In brief, mitochondria were stained using rabbit anti-TOM20 antibody, sarcomeres were stained using mouse anti-α-sarcomeric actinin, and images were collected and analyzed as described in the Immunocytochemistry Section. For each cell, a region of interest (ROI) was drawn based on the contours identified by the α-sarcomeric staining. For a given ROI, we computed a distribution parameter R defined as R = F/Fmax, where F is TOM20 mean fluorescence of the ROI, and Fmax is the highest fluorescence value. The R parameter was comprised between 0 and 1, and it was expected to have higher values in cells with homogeneous mitochondrial distribution (i.e., F will be closer to Fmax) compared with cells displaying alterations in mitochondrial distribution.
Transmission electron microscopy
TEM imaging of cells was performed as described before (26). In brief, cells were plated at a density of 105 cells/well in a 24-well plate, and they were treated with 0.5 μM doxorubicin for 24 h in the presence or absence of 100 μM pargyline. The day after, NRVMs were treated with 10 μM H2O2 for 1 h. Cells were fixed for 1 h at 37°C with freshly prepared 1.25% (v/v) glutaraldehyde in 0.1 M sodium cacodylate, pH 7.4. After washing with 0.1 M sodium cacodylate, cells were postfixed in 1% OsO4, 1.5% K4Fe(CN)6 in 0.1 M sodium cacodylate, pH 7.4, stained with 0.5% uranyl acetate, dehydrated in ethanol, and embedded in Embed 812. Sample sections were imaged on a Tecnai G2 (FEI) transmission electron microscope operating at 100 kV at the EM Facility of the University of Padova. Images were captured using a Veleta (Olimpus Imaging System) digital camera (pixel size 13 × 13 μm; pixel size at a 46,000 × magnification with screen magnification of 3 × 0.1 × 0.1 nm). Mitochondrial perimeter was quantified using the ROI Manager plug-in of the Fiji distribution of the Java-based image processing program ImageJ (55).
Assessment of cell death
To monitor apoptosis, NRVMs were seeded in μ-Slide eight-well plates (Ibidi) at a density of 4 × 104 cells/well, and incubated with 0.5 μM doxorubicin for 24 h in the presence or absence of 100 μM pargyline. The day after, cells were incubated with Annexin-V-Fluos (1:200; Sigma) following manufacturer's instructions for 15 min at room temperature in the dark (13). Positive cells have been evaluated in relation to the total number of cells identified in bright field in every field of view analyzed. Incubation with 2 μM Staurosporine for 1 h was used as positive control (Supplementary Fig. S3C). Data are expressed as percentage of the total cell number.
The release of LDH from NRVMs was measured to evaluate cell death as described before (19). NRVMs were seeded in 24-well plates at a density of 1.5 × 105 cells/well, and incubated with 0.5 μM doxorubicin for 24 h in the presence or absence of 100 μM pargyline. The day after, medium was replaced with HBSS at pH 7.4 (adjusted with NaOH), and cells were incubated with different concentrations of H2O2 (i.e., 10–100 μM H2O2). Supernatant aliquots were collected at different time points after the incubation with H2O2 (i.e., 1–3–6 h). At the end of every experiment, intact cells were lysed by incubating with 1% Triton X-100 (Sigma) for 30 min, and supernatants were collected to evaluate the total amount of LDH. LDH enzymatic activity was measured spectrophotometrically by the absorbance of nicotinamide adenine dinucleotide (Roche) at 340 nm, indicative of the reduction of pyruvate to lactate.
Data analysis
All values are expressed as mean ± standard error of the mean. Comparison between groups was performed by one-way ANOVA, followed by Tukey's post hoc multiple comparison where data were normally distributed. Data that did not follow the normal distribution were statistically analyzed by Kruskal–Wallis ANOVA. Comparisons between two groups were performed using two-tailed Student's t-test. A value of p < 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank Dr. Djahida Bedja for expert technical assistance and Dr. Federico Caicci (EM Facility, Department of Biology, University of Padova) for the TEM analysis.
Abbreviations Used
- ΔΨm
mitochondrial membrane potential
- AM
acetoxymethyl
- AMVMs
adult mouse ventricular myocytes
- BSA
bovine serum albumin
- BW
body weight
- DC
doxorubicin cardiotoxicity
- DMEM
Dulbecco's modified Eagle's medium
- DMSO
dimethyl sulfoxide
- DTNB
5,5′-dithiobis(2-nitrobenzoic acid)
- EC
excitation-contraction
- EF
ejection fraction
- ETC
electron transport chain
- FBS
fetal bovine serum
- FCCP
carbonyl cyanide-p-trifluoromethoxyphenylhydrazone
- FS
fractional shortening
- GSSG/GSH
glutathione disulfide/glutathione
- HRP
horseradish peroxidase
- IVS
interventricular septum
- KD
knock down
- LDH
lactic dehydrogenase
- LV
left ventricle
- LVEDD
left ventricular end-diastolic dimension
- LVESD
left ventricular end-systolic dimension
- LVmass/BW
left ventricular mass normalized to body weight
- LVPW
left ventricular posterior wall
- MAOs
monoamine oxidases
- MDA
malondialdehyde
- MES
2-(N-Morpholino) ethanesulfonic acid
- MTR
MitoTracker Red
- NRVMs
neonatal rat ventricular myocytes
- PBS
phosphate-buffered saline
- PTA
phosphotungstic acid
- PTP
permeability transition pore
- ROI
region of interest
- ROS
reactive oxygen species
- SDS
sodium dodecyl sulfate
- SEM
standard error of the mean
- SR
sarcoplasmic reticulum
- TEM
transmission electron mycroscopy
- TMRM
tetramethylrhodamine
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported by the Leducq Transatlantic Network of Excellence 16CVD04 (F.D.L.) and the COST Action EU-CARDIOPROTECTION CA16225 (F.D.L.); S.A., M.D.S., and N.K. are Fellows of the Leducq Transatlantic Network of Excellence.
Supplementary Material
References
- 1. Ali MK, Ewer MS, Gibbs HR, Swafford J, and Graff KL. Late doxorubicin-associated cardiotoxicity in children. The possible role of intercurrent viral infection. Cancer 74: 182–188, 1994 [DOI] [PubMed] [Google Scholar]
- 2. Antonucci S, Mulvey JF, Burger N, Di Sante M, Hall AR, Hinchy EC, Caldwell ST, Gruszczyk AV, Deshwal S, Hartley RC, Kaludercic N, Murphy MP, Di Lisa F, and Krieg T. Selective mitochondrial superoxide generation in vivo is cardioprotective through hormesis. Free Radic Biol Med 134: 678–687, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bassani JW, Bassani RA, and Bers DM. Twitch-dependent SR Ca accumulation and release in rabbit ventricular myocytes. Am J Physiol 265: C533–C540, 1993 [DOI] [PubMed] [Google Scholar]
- 4. Belousov VV, Fradkov AF, Lukyanov KA, Staroverov DB, Shakhbazov KS, Terskikh AV, and Lukyanov S. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat Methods 3: 281–286, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Benard G, Bellance N, James D, Parrone P, Fernandez H, Letellier T, and Rossignol R. Mitochondrial bioenergetics and structural network organization. J Cell Sci 120: 838–848, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Berthiaume JM and Wallace KB. Adriamycin-induced oxidative mitochondrial cardiotoxicity. Cell Biol Toxicol 23: 15–25, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Binda C, Newton-Vinson P, Hubalek F, Edmondson DE, and Mattevi A. Structure of human monoamine oxidase B, a drug target for the treatment of neurological disorders. Nat Struct Biol 9: 22–26, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Cadeddu Dessalvi C, Pepe A, Penna C, Gimelli A, Madonna R, Mele D, Monte I, Novo G, Nugara C, Zito C, Moslehi JJ, de Boer RA, Lyon AR, Tocchetti CG, and Mercuro G. Sex differences in anthracycline-induced cardiotoxicity: the benefits of estrogens. Heart Fail Rev 24: 915–925, 2019 [DOI] [PubMed] [Google Scholar]
- 9. Cappetta D, De Angelis A, Sapio L, Prezioso L, Illiano M, Quaini F, Rossi F, Berrino L, Naviglio S, and Urbanek K. Oxidative stress and cellular response to doxorubicin: a common factor in the complex milieu of anthracycline cardiotoxicity. Oxid Med Cell Longev 2017: 1521020, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cardinale D, Colombo A, Lamantia G, Colombo N, Civelli M, De Giacomi G, Rubino M, Veglia F, Fiorentini C, and Cipolla CM. Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol 55: 213–220, 2010 [DOI] [PubMed] [Google Scholar]
- 11. Chandel NS. Mitochondria as signaling organelles. BMC Biol 12: 34, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen MH, Colan SD, and Diller L. Cardiovascular disease: cause of morbidity and mortality in adult survivors of childhood cancers. Circ Res 108: 619–628, 2011 [DOI] [PubMed] [Google Scholar]
- 13. Ciscato F, Filadi R, Masgras I, Pizzi M, Marin O, Damiano N, Pizzo P, Gori A, Frezzato F, Chiara F, Trentin L, Bernardi P, and Rasola A.. Hexokinase 2 displacement from mitochondria-associated membranes prompts Ca(2+) -dependent death of cancer cells. EMBO Rep e49117, 2020. [Epub ahead of print]; DOI: 10.15252/embr.201949117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ciscato F, Sciacovelli M, Villano G, Turato C, Bernardi P, Rasola A, and Pontisso P. SERPINB3 protects from oxidative damage by chemotherapeutics through inhibition of mitochondrial respiratory complex I. Oncotarget 5: 2418–2427, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Deng S, Kruger A, Kleschyov AL, Kalinowski L, Daiber A, and Wojnowski L. Gp91phox-containing NAD(P)H oxidase increases superoxide formation by doxorubicin and NADPH. Free Radic Biol Med 42: 466–473, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Deshwal S, Antonucci S, Kaludercic N, and Di Lisa F. Measurement of mitochondrial ROS formation. Methods Mol Biol 1782: 403–418, 2018 [DOI] [PubMed] [Google Scholar]
- 17. Deshwal S, Di Sante M, Di Lisa F, and Kaludercic N. Emerging role of monoamine oxidase as a therapeutic target for cardiovascular disease. Curr Opin Pharmacol 33: 64–69, 2017 [DOI] [PubMed] [Google Scholar]
- 18. Deshwal S, Forkink M, Hu CH, Buonincontri G, Antonucci S, Di Sante M, Murphy MP, Paolocci N, Mochly-Rosen D, Krieg T, Di Lisa F, and Kaludercic N. Monoamine oxidase-dependent endoplasmic reticulum-mitochondria dysfunction and mast cell degranulation lead to adverse cardiac remodeling in diabetes. Cell Death Differ 25: 1671–1685, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Di Lisa F, Menabo R, Canton M, Barile M, and Bernardi P. Opening of the mitochondrial permeability transition pore causes depletion of mitochondrial and cytosolic NAD+ and is a causative event in the death of myocytes in postischemic reperfusion of the heart. J Biol Chem 276: 2571–2575, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Egea J, Fabregat I, Frapart YM, Ghezzi P, Gorlach A, Kietzmann T, Kubaichuk K, Knaus UG, Lopez MG, Olaso-Gonzalez G, Petry A, Schulz R, Vina J, Winyard P, Abbas K, Ademowo OS, Afonso CB, Andreadou I, Antelmann H, Antunes F, Aslan M, Bachschmid MM, Barbosa RM, Belousov V, Berndt C, Bernlohr D, Bertran E, Bindoli A, Bottari SP, Brito PM, Carrara G, Casas AI, Chatzi A, Chondrogianni N, Conrad M, Cooke MS, Costa JG, Cuadrado A, My-Chan Dang P, De Smet B, Debelec-Butuner B, Dias IHK, Dunn JD, Edson AJ, El Assar M, El-Benna J, Ferdinandy P, Fernandes AS, Fladmark KE, Forstermann U, Giniatullin R, Giricz Z, Gorbe A, Griffiths H, Hampl V, Hanf A, Herget J, Hernansanz-Agustin P, Hillion M, Huang J, Ilikay S, Jansen-Durr P, Jaquet V, Joles JA, Kalyanaraman B, Kaminskyy D, Karbaschi M, Kleanthous M, Klotz LO, Korac B, Korkmaz KS, Koziel R, Kracun D, Krause KH, Kren V, Krieg T, Laranjinha J, Lazou A, Li H, Martinez-Ruiz A, Matsui R, McBean GJ, Meredith SP, Messens J, Miguel V, Mikhed Y, Milisav I, Milkovic L, Miranda-Vizuete A, Mojovic M, Monsalve M, Mouthuy PA, Mulvey J, Munzel T, Muzykantov V, Nguyen ITN, Oelze M, Oliveira NG, Palmeira CM, Papaevgeniou N, Pavicevic A, Pedre B, Peyrot F, Phylactides M, Pircalabioru GG, Pitt AR, Poulsen HE, Prieto I, Rigobello MP, Robledinos-Anton N, Rodriguez-Manas L, Rolo AP, Rousset F, Ruskovska T, Saraiva N, Sasson S, Schroder K, Semen K, Seredenina T, Shakirzyanova A, Smith GL, Soldati T, Sousa BC, Spickett CM, Stancic A, Stasia MJ, Steinbrenner H, Stepanic V, Steven S, Tokatlidis K, Tuncay E, Turan B, Ursini F, Vacek J, Vajnerova O, Valentova K, Van Breusegem F, Varisli L, Veal EA, Yalcin AS, Yelisyeyeva O, Zarkovic N, Zatloukalova M, Zielonka J, Touyz RM, Papapetropoulos A, Grune T, Lamas S, Schmidt H, Di Lisa F, and Daiber A. European contribution to the study of ROS: a summary of the findings and prospects for the future from the COST action BM1203 (EU-ROS). Redox Biol 13: 94–162, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eschenhagen T, Force T, Ewer MS, de Keulenaer GW, Suter TM, Anker SD, Avkiran M, de Azambuja E, Balligand JL, Brutsaert DL, Condorelli G, Hansen A, Heymans S, Hill JA, Hirsch E, Hilfiker-Kleiner D, Janssens S, de Jong S, Neubauer G, Pieske B, Ponikowski P, Pirmohamed M, Rauchhaus M, Sawyer D, Sugden PH, Wojta J, Zannad F, and Shah AM. Cardiovascular side effects of cancer therapies: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 13: 1–10, 2011 [DOI] [PubMed] [Google Scholar]
- 22. Fajardo G, Zhao M, Powers J, and Bernstein D. Differential cardiotoxic/cardioprotective effects of beta-adrenergic receptor subtypes in myocytes and fibroblasts in doxorubicin cardiomyopathy. J Mol Cell Cardiol 40: 375–383, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ferrans VJ, Clark JR, Zhang J, Yu ZX, and Herman EH. Pathogenesis and prevention of doxorubicin cardiomyopathy. Tsitologiia 39: 928–937, 1997 [PubMed] [Google Scholar]
- 24. Fortmann SP, Burda BU, Senger CA, Lin JS, and Whitlock EP. Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer: an updated systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med 159: 824–834, 2013 [DOI] [PubMed] [Google Scholar]
- 25. Geisberg CA and Sawyer DB. Mechanisms of anthracycline cardiotoxicity and strategies to decrease cardiac damage. Curr Hypertens Rep 12: 404–410, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Glytsou C, Calvo E, Cogliati S, Mehrotra A, Anastasia I, Rigoni G, Raimondi A, Shintani N, Loureiro M, Vazquez J, Pellegrini L, Enriquez JA, Scorrano L, and Soriano ME. Optic atrophy 1 is epistatic to the core MICOS component MIC60 in mitochondrial cristae shape control. Cell Rep 17: 3024–3034, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gordon RR, Wu M, Huang CY, Harris WP, Sim HG, Lucas JM, Coleman I, Higano CS, Gulati R, True LD, Vessella R, Lange PH, Garzotto M, Beer TM, and Nelson PS. Chemotherapy-induced monoamine oxidase expression in prostate carcinoma functions as a cytoprotective resistance enzyme and associates with clinical outcomes. PLoS One 9: e104271, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gutscher M, Pauleau AL, Marty L, Brach T, Wabnitz GH, Samstag Y, Meyer AJ, and Dick TP. Real-time imaging of the intracellular glutathione redox potential. Nat Methods 5: 553–559, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Hao J, Li WW, Du H, Zhao ZF, Liu F, Lu JC, Yang XC, and Cui W. Role of vitamin C in cardioprotection of ischemia/reperfusion injury by activation of mitochondrial KATP channel. Chem Pharm Bull (Tokyo) 64: 548–557, 2016 [DOI] [PubMed] [Google Scholar]
- 30. Hegstad AC, Antonsen OH, and Ytrehus K. Low concentrations of hydrogen peroxide improve post-ischaemic metabolic and functional recovery in isolated perfused rat hearts. J Mol Cell Cardiol 29: 2779–2787, 1997 [DOI] [PubMed] [Google Scholar]
- 31. Hinchy EC, Gruszczyk AV, Willows R, Navaratnam N, Hall AR, Bates G, Bright TP, Krieg T, Carling D, and Murphy MP. Mitochondria-derived ROS activate AMP-activated protein kinase (AMPK) indirectly. J Biol Chem 293: 17208–17217, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ikeda S, Matsushima S, Okabe K, Ikeda M, Ishikita A, Tadokoro T, Enzan N, Yamamoto T, Sada M, Deguchi H, Morimoto S, Ide T, and Tsutsui H. Blockade of L-type Ca(2+) channel attenuates doxorubicin-induced cardiomyopathy via suppression of CaMKII-NF-kappaB pathway. Sci Rep 9: 9850, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jain D. Cardiotoxicity of doxorubicin and other anthracycline derivatives. J Nucl Cardiol 7: 53–62, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Kaludercic N, Carpi A, Nagayama T, Sivakumaran V, Zhu G, Lai EW, Bedja D, De Mario A, Chen K, Gabrielson KL, Lindsey ML, Pacak K, Takimoto E, Shih JC, Kass DA, Di Lisa F, and Paolocci N. Monoamine oxidase B prompts mitochondrial and cardiac dysfunction in pressure overloaded hearts. Antioxid Redox Signal 20: 267–280, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kaludercic N, Mialet-Perez J, Paolocci N, Parini A, and Di Lisa F. Monoamine oxidases as sources of oxidants in the heart. J Mol Cell Cardiol 73: 34–42, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kaludercic N, Takimoto E, Nagayama T, Feng N, Lai EW, Bedja D, Chen K, Gabrielson KL, Blakely RD, Shih JC, Pacak K, Kass DA, Di Lisa F, and Paolocci N. Monoamine oxidase A-mediated enhanced catabolism of norepinephrine contributes to adverse remodeling and pump failure in hearts with pressure overload. Circ Res 106: 193–202, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kokoszka JE, Coskun P, Esposito LA, and Wallace DC. Increased mitochondrial oxidative stress in the Sod2 (+/-) mouse results in the age-related decline of mitochondrial function culminating in increased apoptosis. Proc Natl Acad Sci U S A 98: 2278–2283, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li M, Sala V, De Santis MC, Cimino J, Cappello P, Pianca N, Di Bona A, Margaria JP, Martini M, Lazzarini E, Pirozzi F, Rossi L, Franco I, Bornbaum J, Heger J, Rohrbach S, Perino A, Tocchetti CG, Lima BHF, Teixeira MM, Porporato PE, Schulz R, Angelini A, Sandri M, Ameri P, Sciarretta S, Lima-Junior RCP, Mongillo M, Zaglia T, Morello F, Novelli F, Hirsch E, and Ghigo A. Phosphoinositide 3-kinase gamma inhibition protects from anthracycline cardiotoxicity and reduces tumor growth. Circulation 138: 696–711, 2018 [DOI] [PubMed] [Google Scholar]
- 39. Li PC, Siddiqi IN, Mottok A, Loo EY, Wu CH, Cozen W, Steidl C, and Shih JC. Monoamine oxidase A is highly expressed in classical Hodgkin lymphoma. J Pathol 243: 220–229, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lim CC, Zuppinger C, Guo X, Kuster GM, Helmes M, Eppenberger HM, Suter TM, Liao R, and Sawyer DB. Anthracyclines induce calpain-dependent titin proteolysis and necrosis in cardiomyocytes. J Biol Chem 279: 8290–8299, 2004 [DOI] [PubMed] [Google Scholar]
- 41. Lipshultz SE, Scully RE, Lipsitz SR, Sallan SE, Silverman LB, Miller TL, Barry EV, Asselin BL, Athale U, Clavell LA, Larsen E, Moghrabi A, Samson Y, Michon B, Schorin MA, Cohen HJ, Neuberg DS, Orav EJ, and Colan SD. Assessment of dexrazoxane as a cardioprotectant in doxorubicin-treated children with high-risk acute lymphoblastic leukaemia: long-term follow-up of a prospective, randomised, multicentre trial. Lancet Oncol 11: 950–961, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Meiners B, Shenoy C, and Zordoky BN. Clinical and preclinical evidence of sex-related differences in anthracycline-induced cardiotoxicity. Biol Sex Differ 9: 38, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Milei J, Boveris A, Llesuy S, Molina HA, Storino R, Ortega D, and Milei SE. Amelioration of adriamycin-induced cardiotoxicity in rabbits by prenylamine and vitamins A and E. Am Heart J 111: 95–102, 1986 [DOI] [PubMed] [Google Scholar]
- 44. Minotti G, Menna P, Salvatorelli E, Cairo G, and Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev 56: 185–229, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Moulin M, Piquereau J, Mateo P, Fortin D, Rucker-Martin C, Gressette M, Lefebvre F, Gresikova M, Solgadi A, Veksler V, Garnier A, and Ventura-Clapier R. Sexual dimorphism of doxorubicin-mediated cardiotoxicity: potential role of energy metabolism remodeling. Circ Heart Fail 8: 98–108, 2015 [DOI] [PubMed] [Google Scholar]
- 46. Munoz-Castaneda JR, Montilla P, Munoz MC, Bujalance I, Muntane J, and Tunez I. Effect of 17-beta-estradiol administration during adriamycin-induced cardiomyopathy in ovariectomized rat. Eur J Pharmacol 523: 86–92, 2005 [DOI] [PubMed] [Google Scholar]
- 47. Murphy MP. How mitochondria produce reactive oxygen species. Biochem J 417: 1–13, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Octavia Y, Tocchetti CG, Gabrielson KL, Janssens S, Crijns HJ, and Moens AL. Doxorubicin-induced cardiomyopathy: from molecular mechanisms to therapeutic strategies. J Mol Cell Cardiol 52: 1213–1225, 2012 [DOI] [PubMed] [Google Scholar]
- 49. Pal S, Ahir M, and Sil PC. Doxorubicin-induced neurotoxicity is attenuated by a 43-kD protein from the leaves of Cajanus indicus L. via NF-kappaB and mitochondria dependent pathways. Free Radic Res 46: 785–798, 2012 [DOI] [PubMed] [Google Scholar]
- 50. Petronilli V, Miotto G, Canton M, Brini M, Colonna R, Bernardi P, and Di Lisa F. Transient and long-lasting openings of the mitochondrial permeability transition pore can be monitored directly in intact cells by changes in mitochondrial calcein fluorescence. Biophys J 76: 725–734, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Riccio G, Antonucci S, Coppola C, D'Avino C, Piscopo G, Fiore D, Maurea C, Russo M, Rea D, Arra C, Condorelli G, Di Lisa F, Tocchetti CG, De Lorenzo C, and Maurea N. Ranolazine attenuates trastuzumab-induced heart dysfunction by modulating ROS production. Front Physiol 9: 38, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ristow M. Unraveling the truth about antioxidants: mitohormesis explains ROS-induced health benefits. Nat Med 20: 709–711, 2014 [DOI] [PubMed] [Google Scholar]
- 53. Sag CM, Kohler AC, Anderson ME, Backs J, and Maier LS. CaMKII-dependent SR Ca leak contributes to doxorubicin-induced impaired Ca handling in isolated cardiac myocytes. J Mol Cell Cardiol 51: 749–759, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Salvatorelli E, Guarnieri S, Menna P, Liberi G, Calafiore AM, Mariggio MA, Mordente A, Gianni L, and Minotti G. Defective one- or two-electron reduction of the anticancer anthracycline epirubicin in human heart. Relative importance of vesicular sequestration and impaired efficiency of electron addition. J Biol Chem 281: 10990–11001, 2006 [DOI] [PubMed] [Google Scholar]
- 55. Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, and Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods 9: 676–682, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sena LA and Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Mol Cell 48: 158–167, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Son SY, Ma J, Kondou Y, Yoshimura M, Yamashita E, and Tsukihara T. Structure of human monoamine oxidase A at 2.2-A resolution: the control of opening the entry for substrates/inhibitors. Proc Natl Acad Sci U S A 105: 5739–5744, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sorato E, Menazza S, Zulian A, Sabatelli P, Gualandi F, Merlini L, Bonaldo P, Canton M, Bernardi P, and Di Lisa F. Monoamine oxidase inhibition prevents mitochondrial dysfunction and apoptosis in myoblasts from patients with collagen VI myopathies. Free Radic Biol Med 75: 40–47, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Spinazzi M, Cazzola S, Bortolozzi M, Baracca A, Loro E, Casarin A, Solaini G, Sgarbi G, Casalena G, Cenacchi G, Malena A, Frezza C, Carrara F, Angelini C, Scorrano L, Salviati L, and Vergani L. A novel deletion in the GTPase domain of OPA1 causes defects in mitochondrial morphology and distribution, but not in function. Hum Mol Genet 17: 3291–3302, 2008 [DOI] [PubMed] [Google Scholar]
- 60. Steinberg JS, Cohen AJ, Wasserman AG, Cohen P, and Ross AM. Acute arrhythmogenicity of doxorubicin administration. Cancer 60: 1213–1218, 1987 [DOI] [PubMed] [Google Scholar]
- 61. Steinberg SF. Oxidative stress and sarcomeric proteins. Circ Res 112: 393–405, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sterba M, Popelova O, Vavrova A, Jirkovsky E, Kovarikova P, Gersl V, and Simunek T. Oxidative stress, redox signaling, and metal chelation in anthracycline cardiotoxicity and pharmacological cardioprotection. Antioxid Redox Signal 18: 899–929, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sun A, Cheng Y, Zhang Y, Zhang Q, Wang S, Tian S, Zou Y, Hu K, Ren J, and Ge J. Aldehyde dehydrogenase 2 ameliorates doxorubicin-induced myocardial dysfunction through detoxification of 4-HNE and suppression of autophagy. J Mol Cell Cardiol 71: 92–104, 2014 [DOI] [PubMed] [Google Scholar]
- 64. Tebbi CK, London WB, Friedman D, Villaluna D, De Alarcon PA, Constine LS, Mendenhall NP, Sposto R, Chauvenet A, and Schwartz CL. Dexrazoxane-associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin's disease. J Clin Oncol 25: 493–500, 2007 [DOI] [PubMed] [Google Scholar]
- 65. Thorn CF, Oshiro C, Marsh S, Hernandez-Boussard T, McLeod H, Klein TE, and Altman RB. Doxorubicin pathways: pharmacodynamics and adverse effects. Pharmacogenet Genomics 21: 440–446, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Timolati F, Ott D, Pentassuglia L, Giraud MN, Perriard JC, Suter TM, and Zuppinger C. Neuregulin-1 beta attenuates doxorubicin-induced alterations of excitation-contraction coupling and reduces oxidative stress in adult rat cardiomyocytes. J Mol Cell Cardiol 41: 845–854, 2006 [DOI] [PubMed] [Google Scholar]
- 67. Tonolo F, Folda A, Cesaro L, Scalcon V, Marin O, Ferro S, Bindoli A, and Rigobello MP. Milk-derived bioactive peptides exhibit antioxidant activity through the Keap1-Nrf2 signaling pathway. J Funct Foods 64: 2020 [Google Scholar]
- 68. Tonolo F, Sandre M, Ferro S, Folda A, Scalcon V, Scutari G, Feller E, Marin O, Bindoli A, and Rigobello MP. Milk-derived bioactive peptides protect against oxidative stress in a Caco-2 cell model. Food Funct 9: 1245–1253, 2018 [DOI] [PubMed] [Google Scholar]
- 69. Wallace KB, Sardao VA, and Oliveira PJ. Mitochondrial determinants of doxorubicin-induced cardiomyopathy. Circ Res 126: 926–941, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wang F, He Q, Sun Y, Dai X, and Yang XP. Female adult mouse cardiomyocytes are protected against oxidative stress. Hypertension 55: 1172–1178, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wu JB, Shao C, Li X, Li Q, Hu P, Shi C, Li Y, Chen YT, Yin F, Liao CP, Stiles BL, Zhau HE, Shih JC, and Chung LW. Monoamine oxidase A mediates prostate tumorigenesis and cancer metastasis. J Clin Invest 124: 2891–2908, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Yagi K. Assay for blood plasma or serum. Methods Enzymol 105: 328–331, 1984 [DOI] [PubMed] [Google Scholar]
- 73. Zhou S, Starkov A, Froberg MK, Leino RL, and Wallace KB. Cumulative and irreversible cardiac mitochondrial dysfunction induced by doxorubicin. Cancer Res 61: 771–777, 2001 [PubMed] [Google Scholar]
- 74. Zou Y, Yao A, Zhu W, Kudoh S, Hiroi Y, Shimoyama M, Uozumi H, Kohmoto O, Takahashi T, Shibasaki F, Nagai R, Yazaki Y, and Komuro I. Isoproterenol activates extracellular signal-regulated protein kinases in cardiomyocytes through calcineurin. Circulation 104: 102–108, 2001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.