Abstract
Purpose
FGFR3-TACC3 (fibroblast growth factor receptor 3–transforming acidic coiled coil-containing protein 3) fusions have recently been identified as driver mutations that lead to the activation of FGFR3 in bladder cancer and other tumor types and are associated with sensitivity to tyrosine kinase inhibitors. We examined the clinical and molecular characteristics of patients with FGFR3-TACC3 fusions and hypothesized that they are enriched in a subset of patients with bladder cancer.
Materials and Methods
We correlated somatic FGFR3-TACC3 fusions with clinical and molecular features in two cohorts of patients with bladder cancer. The first cohort consisted of the muscle-invasive bladder cancer (MIBC) data set (n = 412) from The Cancer Genome Atlas. The second cohort consisted of patients with MIBC or high-grade non-MIBC at the Dana-Farber Cancer Institute that had targeted capture sequencing of a selected panel of cancer genes (n = 356). All statistical tests were two sided. The clinical response of one patient with FGFR3-TACC3 bladder cancer to an FGFR3 inhibitor was investigated.
Results
Overall, 751 patients with high-grade bladder cancer without FGFR3-TACC3 fusions and 17 with FGFR3-TACC3 fusions were identified in the pooled analysis of the data sets from The Cancer Genome Atlas and the Dana-Farber Cancer Institute. FGFR3-TACC3 fusions were enriched in patients age ≤ 50 years versus age 51 to 65 years versus those older than 65 years (pooled, P = .002), and were observed in four (12%) of 33 patients age ≤ 50 years in the pooled analysis. Similarly, FGFR3-TACC3 fusions were significantly more common in Asians (13%) compared with African Americans (4%) and whites (2%; pooled, P < .001), as well as in never smokers (5.6%) compared with ever smokers (1.1%; pooled, P < .001). One patient with the fusion who was treated with an FGFR3 inhibitor achieved complete remission for 10 months.
Conclusion
Clinical testing to identify FGFR3 fusions should be prioritized for patients with bladder cancer who are younger, never smokers, and/or Asian.
INTRODUCTION
Bladder cancer remains a major contributor to cancer-related morbidity and mortality. In 2017, 79,030 new cases of bladder cancer are expected to be diagnosed, and approximately 16,870 deaths are predicted to occur from the disease in the United States.1,2 Compared with other cancer subtypes, advances in the management of bladder cancer have been limited in the past three decades, and there is an unmet need to develop novel therapeutic agents that target potentially actionable alterations.3,4
Genomic alterations in fibroblast growth factor receptors (FGFRs) are among the most frequent events during bladder cancer development. FGFRs are receptor tyrosine kinases that orchestrate various cellular processes, including cell proliferation, differentiation, and survival.5 FGFR mutations lead to developmental syndromes when present in the germline, and contribute to cancer growth when acquired somatically.6 FGFR fusions with an intact kinase domain have been identified in several cancer types, including cervical cancer, bladder carcinoma, glioblastoma multiforme, squamous lung carcinoma, and head and neck cancer.7-15 FGFR3, a member of this family, has been reported to be involved in fusions with several genes in bladder carcinoma, including TACC3 (transforming acidic coiled coil-containing protein 3). The TACC3 gene is located just 48 kb away from FGFR3 on 4p16.3, which likely predisposes FGFR3 and TACC3 to fusion events. TACC3 normally is thought to mediate the stabilization and organization of the mitotic spindle during mitosis.14
In The Cancer Genome Atlas (TCGA) muscle-invasive bladder cancer (MIBC) cohort, in-frame activating FGFR3-TACC3 fusions—observed in 10 (2.4%) of 412 patients—were the most common gene fusions identified.7 FGFR3-TACC3 fusion proteins consist of the immunoglobulin, transmembrane, and tyrosine kinase domains of FGFR3, fused to the coiled-coil domain of TACC3. Through the promotion of dimerization, these fusions lead to a constitutively active FGFR3 kinase protein that has been demonstrated to promote cell proliferation in vivo and in vitro.7-9,13 Phase I and II trials of FGFR inhibitors have reported promising antitumor activity in patients with FGFR genetic alterations, especially bladder cancer.16
Certain genetic alterations, particularly gene fusion events, are enriched in clinical subsets of patients with cancer. For example, never-smoking patients with lung adenocarcinoma have more frequent EGFR mutations and ALK and ROS1 fusions17,18; therefore, we hypothesized that a similar association may exist between somatic FGFR3-TACC3 fusions and patient characteristics in bladder cancer.17-19
METHODS
TCGA and Dana-Farber Cancer Institute Data
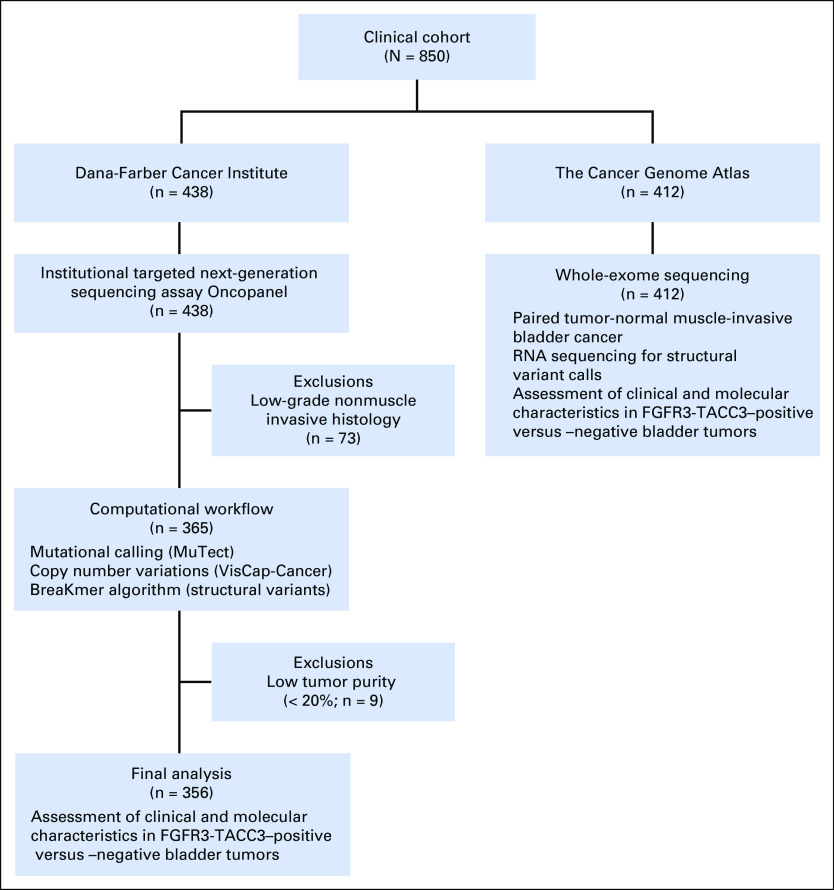
We tested our hypotheses in two cohorts, one from TCGA and one from the Dana-Farber Cancer Institute (DFCI). For the TCGA MIBC cohort (n = 412), we examined the clinical and molecular characteristics of the 10 FGFR3-TACC3 fusion patients who were identified on the basis of analysis of RNA sequencing data compared with the remaining 402 patients.7 For the DFCI cohort (n = 356), we identified 240 patients who were diagnosed with MIBC and 116 with high-grade20 non-MIBC (n = 116). Patients with MIBC and high-grade non-MIBC were pooled together in the DFCI cohort as there is substantial evidence that the two subtypes are biologically and genomically similar.21-23 Overall, seven patients with FGFR3-TACC3 fusions were identified in the DFCI cohort using an institutional targeted next-generation sequencing assay24 (Oncopanel). Figure 1 shows the sample inclusion and exclusion criteria and workflow.
Fig 1.
CONSORT diagram for 850 patients with bladder cancer.
Tissue Collection and DNA Extraction
Tumor specimens and clinicopathologic information were collected with institutional review board approval at DFCI. Board-certified genitourinary pathologists at DFCI reviewed and verified the diagnosis, tumor grade, stage, and histology. Tumor areas that contained at least 20% of tumor cells (mean tumor purity, 58%; range, 20% to 100%) were isolated from normal tissue and chosen for DNA extraction. DNA was then isolated using the QIAamp DNA formalin-fixed, paraffin-embedded tissue kit (Qiagen, Wetzlar, Germany) according to manufacturer instructions. DNA was quantified by Nanodrop and pico-Green assays.
Targeted Sequencing
Two hundred nanograms of genomic DNA from each sample was subjected to targeted exon capture and sequencing using Oncopanel_v1 to v3 cancer gene panels at Brigham and Women’s Hospital (Boston, MA). The Oncopanel gene panel includes capture probes for 275 to 560 cancer-associated genes, as well as intronic portions of 60 genes for rearrangement detection, including FGFR3.24 Sample DNA was captured using Oncopanel_v1 to v3 bait sets using a solution-phase Agilent SureSelect hybrid capture kit (Agilent Technologies, Santa Clara, CA). Sequencing libraries were prepared from captured DNA as described in detail elsewhere. Paired-end sequencing was performed on an Illumina HiSEquation 2500 sequencer (Illumina, San Diego, CA). Reads were demultiplexed using Picard tools (http://picard.sourceforge.net) and aligned to human reference genome b37 using the Burrows-Wheeler Aligner25 (http://bio-bwa.sourceforge.net/bwa.shtml). Low-quality reads and duplicates were filtered out and eliminated using Picard. Single-nucleotide variants and small indels were analyzed using MuTect version 1 0.27200 (https://confluence.broadinstitute.org/display/CGATools/MuTect; accessed May 2013) and annotated by Oncotator (http://www.broadinstitute.org/oncotator; accessed May 2013). Copy number alterations were analyzed using a custom R-based tool26,27 (VisCap-Cancer).
Mean depth of read coverage for the targeted genes was ×283. Mean, median, and range of percentage of target bases with read depth > ×30 was 98%, 99%, and 78% to 99%, respectively.
Identification of Rearrangements and Analysis of Genomic Breakpoints
FGFR3 fusion sequences were identified using the BreaKmer algorithm28 and were manually reviewed using Integrated Genomic Viewer29 to exclude sequencing or alignment artifacts. All analyses of sequencing data and mutation and fusion calls were performed blinded to clinical data.
Clinical Response to Anti-FGFR3 Therapy
One patient with FGFR3-TACC3 MIBC received anti-FGFR3 therapy along with docetaxel and the clinical response was monitored.
Statistical Analysis
We used Fisher's exact test for categorical data and the Wilcoxon rank-sum test for quantitative data. All statistical tests were two sided and a P value ≤ .05 was considered statistically significant. Statistical correction for multiple comparisons was not performed, as we considered these analyses exploratory.
RESULTS
Formalin-fixed, paraffin-embedded tumor specimens were obtained from 438 patients at DFCI. We excluded 82 tumors from the analysis because they were of low-grade nonmuscle invasive histology (n = 73) or had low (< 20%) tumor purity (n = 9). Three hundred fifty-six patients from the DFCI data set were analyzed, including three Asian patients, five African American patients, 339 white patients, and nine of unknown race.
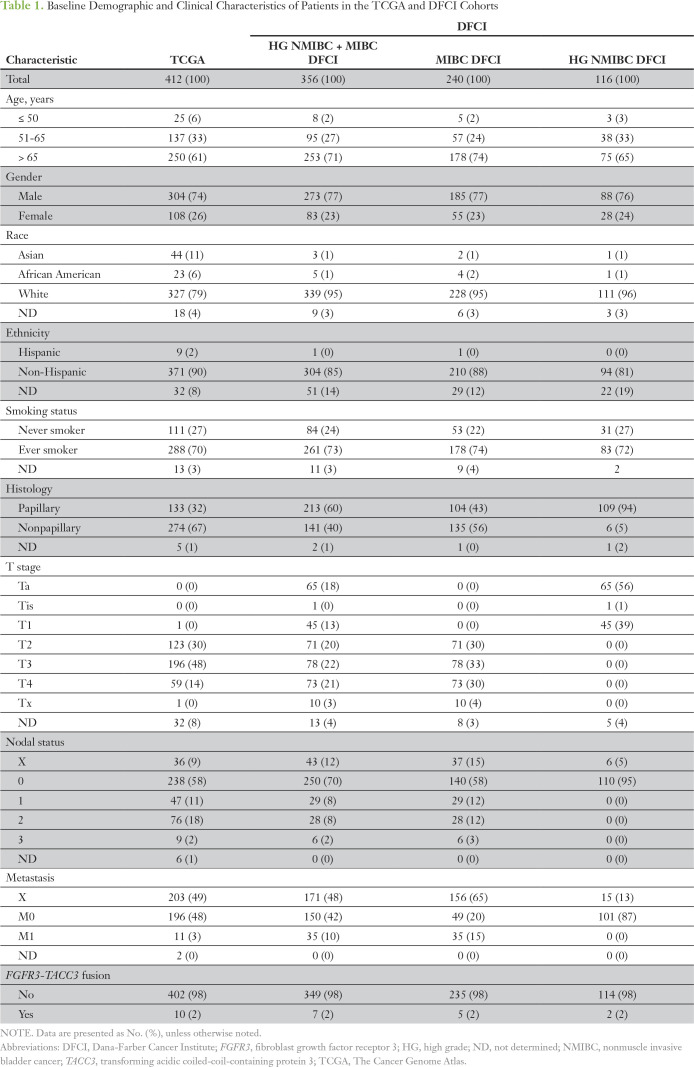
Ten (2.4%) of 412 patients in the TCGA cohort (mean age at diagnosis, 68 years [range 34 to 90 years]; median age at diagnosis, 69 years), and seven (2.0%; MIBC, n = 5; non-MIBC, n = 2) of 356 patients in the DFCI cohort (mean age at diagnosis, 71 years [range, 12 to 96 years]; median age at diagnosis, 72 years) harbored FGFR3-TACC3 fusions. Table 1 lists the baseline clinicopathologic characteristics of the 768 patients. We mapped the genomic breakpoints of FGFR3 and its corresponding fusion partners that were identified in the DFCI cohort, which included four non-TACC3 fusions (Fig 2). All FGFR3-TACC3 fusions occurred in the exon 17 to 18 intron (n = 6) or in exon 18 (n = 1) of FGFR3, which led to a small C-terminal truncation of FGFR3 with preservation of the kinase domain. FGFR3 was fused to various exons of TACC3, most commonly exon 11, all of which maintain the TACC3 coiled-coil domain in the fusion protein.
Table 1.
Baseline Demographic and Clinical Characteristics of Patients in the TCGA and DFCI Cohorts
Fig 2.
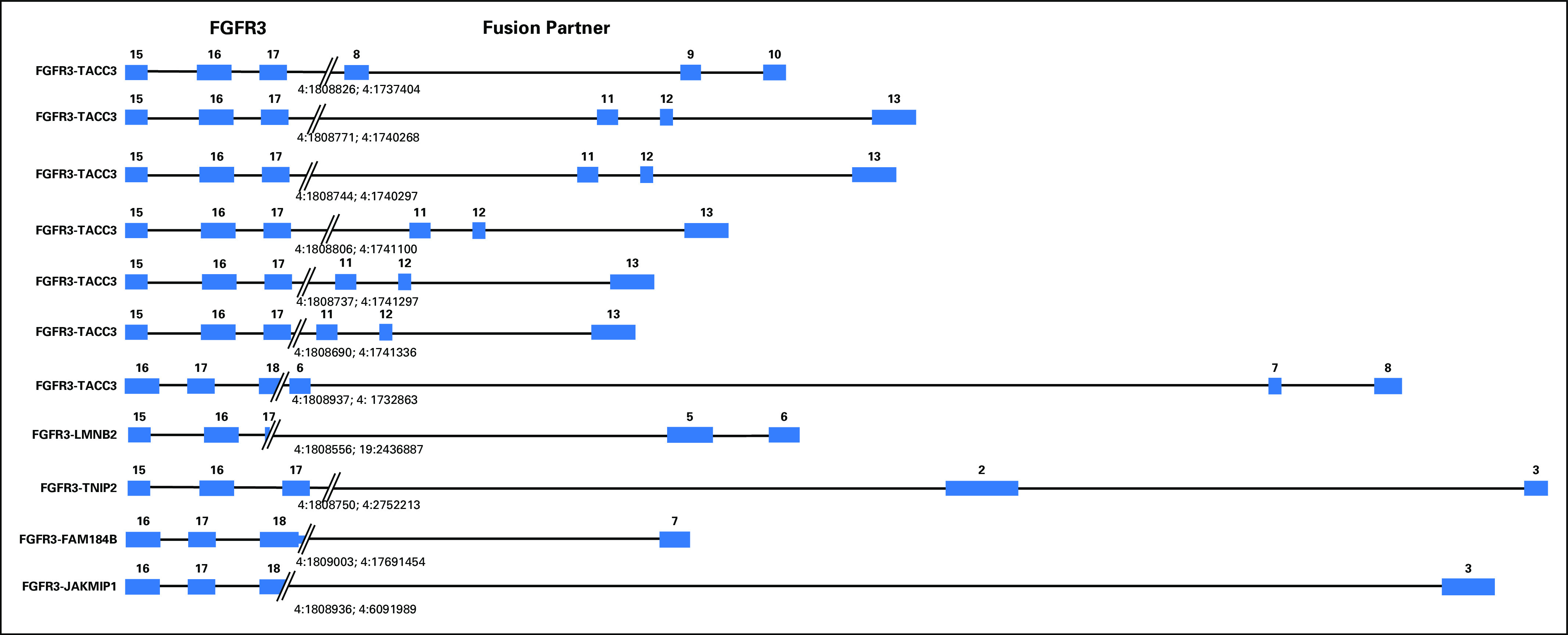
Schematic representation of the genomic rearrangements observed in 11 tumor samples that harbor fibroblast growth factor receptor 3 (FGFR3) fusion variants identified using the Oncopanel assay in the Dana-Farber Cancer Institute cohort. All exons and introns are drawn to scale. FAM184B, family with sequence similarity 184 member B; LMNB2, lamin B2; JAKMIP1, Janus kinase and microtubule interacting protein 1; TACC3, transforming acidic coiled-coil-containing protein 3; TNIP2, TNFAIP3 interacting protein 2.
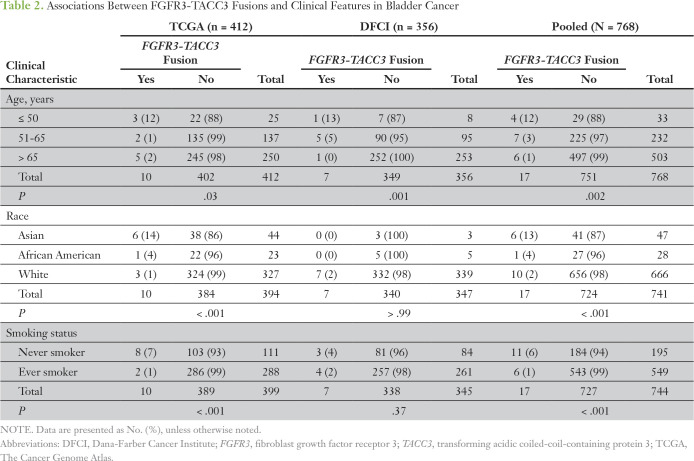
FGFR3-TACC3 fusions were enriched in the TCGA cohort in patients age ≤ 50 years compared with those age 51 to 65 years and those older than 65 years, with three (12%) of 25 patients age ≤ 50 harboring a fusion (P = .03; Table 2). FGFR3-TACC3 fusions in TCGA were also more frequent in Asians (six [14%] of 44 patients) compared with other races (P < .001), as well as in never smokers (eight [7.2%] of 111 patients) compared with ever smokers (P < .001; Table 2). Similarly, FGFR3-TACC3 fusions were more common in DFCI patients age ≤ 50 years (one [12%] of eight patients) compared with other age groups (P = .001; Table 2). Race and smoking status were not associated with fusions in the DFCI cohort as a result of small numbers of patients in these categories and lack of statistical power.
Table 2.
Associations Between FGFR3-TACC3 Fusions and Clinical Features in Bladder Cancer
Analysis of the pooled TCGA and DFCI cohorts (N = 768) confirmed significant associations between FGFR3-TACC3 fusions and age ≤ 50 years (12%; P = .002), Asian race (13%; P < .001), and never-smoking status (5.6%; P < .001; Table 2). Eleven (65%) of 17 patients with FGFR3-TACC3 fusions were associated with least one of these three clinical characteristics, and three (18%) of the 17 patients were Asian never smokers age ≤ 50 years.
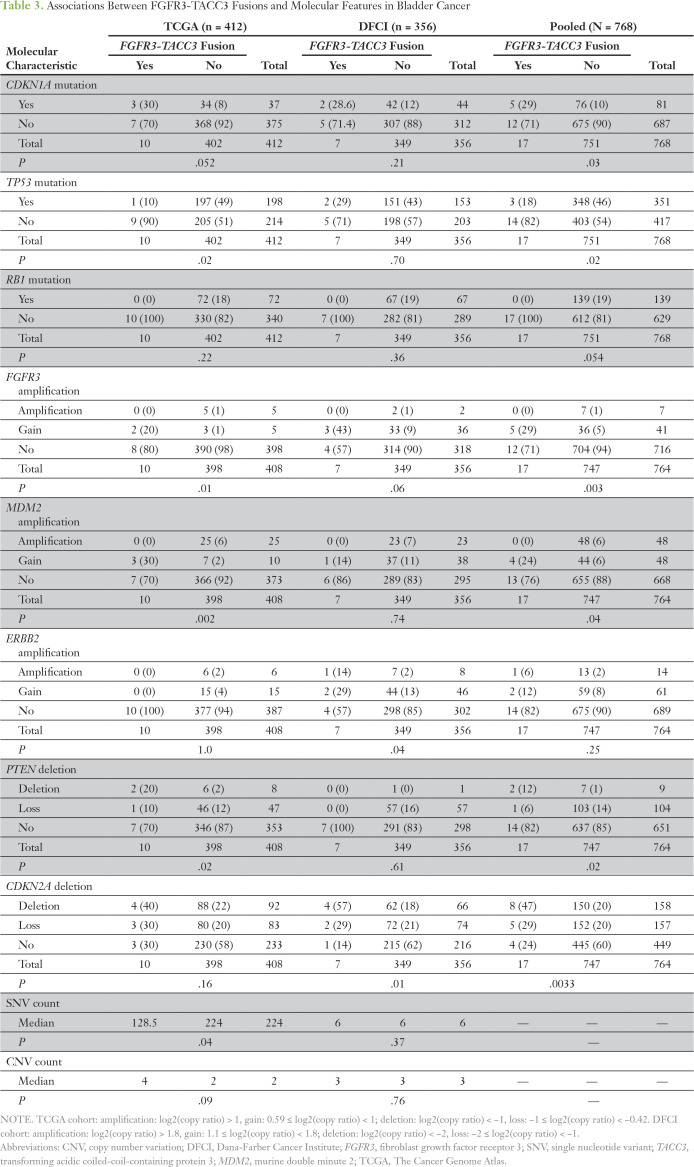
We next examined whether tumors with FGFR3-TACC3 fusions had molecular features that distinguished them from other tumors. We examined 33 genes that were defined as being significantly mutated in the TCGA analysis and were also tested in the Oncopanel assay. As the Oncopanel analysis was performed on tumor samples only, we excluded variants that were observed at any frequency in the Exome Aggregation Consortium database,30 as they were considered likely germline variants. The 17 patients whose tumors harbored FGFR3-TACC3 fusions were enriched for CDKN1A mutations (5 [29%] of 17 v 76 [10%] of 751; P = .03; Table 3). Conversely, FGFR3-TACC3 fusion-positive tumors had significantly fewer TP53 mutations (P = .02), and none had RB1 mutations (P = .054; Table 3). Somatic copy number alterations were also analyzed in both cohorts using criteria for loss, deletion, gain, and amplification that were developed and applied independently in the two cohorts (Table 3). Analysis of the pooled cohorts demonstrated significant associations between FGFR3-TACC3 fusions and FGFR3 gain (P = .003), MDM2 (murine double minute 2) gain (P = .04), deletion of PTEN (P = .02), and deletion of CDK2NA (P = .0033; Table 3).
Table 3.
Associations Between FGFR3-TACC3 Fusions and Molecular Features in Bladder Cancer
As a result of differences in the extent of genome sequencing in the TCGA and DFCI cohorts, we analyzed the overall mutational burden in each cohort separately. In the TCGA cohort, the nonsynonymous somatic mutation rate across 18,862 genes was significantly higher in patients without FGFR3-TACC3 fusions compared with those with fusions (median 224 v 128; P = 0.04; Table 3). In the DFCI cohort, which analyzed a smaller number of genes, no significant difference in mutational burden was observed (Table 3). In addition, there were no significant differences in the frequency of somatic copy number alterations in either the TCGA or DFCI cohorts (Table 3).
One patient who harbored the FGFR3-TACC3 fusion in MIBC in the DFCI cohort was treated with an FGFR3 inhibitor and docetaxel and experienced complete remission for approximately 10 months.
DISCUSSION
Our results demonstrate that patients with bladder cancer with FGFR3-TACC3 fusions have distinct clinical and molecular features compared with the general population of patients with bladder cancer. We observed significant enrichment for these fusions in patients age ≤ 50 years (12% of patients), of Asian race (13%), and who were never smokers (5.6%). In addition, FGFR3-TACC3 fusions were associated with a low frequency of TP53 and RB1 mutations and a higher frequency of CDKN1A mutations, FGFR3 and MDM2 amplifications, and PTEN deletions. Because FGFR3-TACC3 fusion-positive tumors can be sensitive to FGFR inhibitors,9,31,32 these observations suggest that molecular testing to detect FGFR3-TACC3 fusions in bladder cancer should be prioritized for patients who are young (age ≤ 50 years), of Asian race, and/or who have never smoked. Most strikingly, we observed that all patients with bladder cancer who were Asian never smokers younger than age 50 years (n = 3) had FGFR3-TACC3 fusions.
We emphasize that our study has significant limitations as a result of the small number of patients with FGFR3-TACC3 fusions included (n = 17), which reflects that this is a relatively rare molecular subset of bladder cancer. We began this study with a specific hypothesis about associations between clinical features and FGFR3-TACC3 fusion mutations, and that hypothesis was validated; however, we recognize that there may be other associations of clinical and pathologic features with FGFR3-TACC3 fusion mutations that we did not explore here. Most importantly, we strongly advocate additional studies of this association to extend and confirm these findings.
In conclusion, FGFR3-TACC3 fusion-positive bladder cancer is highly enriched in Asians, never smokers, and those age ≤ 50 years. This association suggests that patients in these demographic categories should be prioritized for molecular testing, and, if the FGFR3-TACC3 fusion is found, enrolled in appropriate clinical trials that are using emerging targeted therapies against FGFR3.
A directly related abstract was accepted as a poster presentation in the 2018 Genitourinary Cancer Symposium of the American Society of Oncology, San Francisco, CA, February 8-10, 2018.
SUPPORT
D.J.K. was supported by National Cancer Institute Grant No. 1P01CA120964: Molecular Pathogenesis of the Hamartoma Syndromes.
AUTHOR CONTRIBUTIONS
Conception and design: Amin H. Nassar, Kevin Lundgren, Mark Pomerantz, Toni K. Choueiri, Joaquim Bellmunt, David J. Kwiatkowski, Guru P. Sonpavde
Financial support: Toni K. Choueiri
Administrative support: Toni K. Choueiri, David J. Kwiatkowski
Provision of study material or patients: Amin H. Nassar, Lauren Harshman, Graeme S. Steele, Toni K. Choueiri, Joaquim Bellmunt, Guru P. Sonpavde
Collection and assembly of data: Amin H. Nassar, Kevin Lundgren, Graeme S. Steele, Toni K. Choueiri, Joaquim Bellmunt, David J. Kwiatkowski, Guru P. Sonpavde
Data analysis and interpretation: Amin H. Nassar, Eliezer Van Allen, Lauren Harshman, Atish D. Choudhury, Mark A. Preston, Kent W. Mouw, Xiao X. Wei, Bradley A. McGregor, Toni K. Choueiri, Joaquim Bellmunt, David J. Kwiatkowski, Guru P. Sonpavde
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Amin H. Nassar
No relationship to disclose
Kevin Lundgren
No relationship to disclose
Mark Pomerantz
Honoraria: Bayer
Eliezer Van Allen
Stock and Other Ownership Interests: Synapse, Tango Therapeutics, Genome Medical
Consulting or Advisory Role: Synapse, Roche, Third Rock Ventures, Takeda, Novartis, Genome Medical, InVitae
Speakers' Bureau: Illumina
Research Funding: Bristol-Myers Squibb, Novartis
Lauren Harshman
Consulting or Advisory Role: Medivation, Astellas Pharma, Pfizer, Genentech, Theragene, KEW, Corvus Pharmaceuticals, Merck, Exelixis, Bayer
Research Funding: Medivation, Astellas Pharma (Inst), Bayer (Inst), Sotio (Inst), Genentech (Inst), Dendreon (Inst), Bristol-Myers Squibb (Inst), Takeda (Inst), Merck (Inst), Janssen Oncology (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: Bayer
Atish D. Choudhury
Employment: LeMaitre Vascular (I)
Honoraria: Bayer, Astellas Pharma
Research Funding: Janssen Pharmaceuticals (Inst)
Mark A. Preston
No relationship to disclose
Graeme S. Steele
No relationship to disclose
Kent W. Mouw
Consulting or Advisory Role: Pfizer, EMD Serono
Xiao X. Wei
No relationship to disclose
Bradley A. McGregor
Consulting or Advisory Role: Bayer, Seattle Genetics, Astellas Pharma, Exelixis, AstraZeneca, Genentech, Nextar
Toni K. Choueiri
Honoraria: National Comprehensive Cancer Network, UpToDate
Consulting or Advisory Role: Pfizer, Bayer, Novartis, GlaxoSmithKline, Merck, Bristol-Myers Squibb, Genentech, Eisai, Foundation Medicine, Cerulean Pharma, AstraZeneca, Peloton Therapeutics, Exelixis, Prometheus Laboratories, Alligent, Ipsen, Corvus Pharmaceuticals
Research Funding: Pfizer (Inst), Novartis (Inst), Merck (Inst), Exelixis (Inst), TRACON Pharma (Inst), GlaxoSmithKline (Inst), Bristol-Myers Squibb (Inst), AstraZeneca (Inst), Peloton Therapeutics (Inst), Genentech (Inst), Celldex (Inst), Agensys (Inst), Eisai (Inst)
Joaquim Bellmunt
Honoraria: UpToDate
Consulting or Advisory Role: Pierre Fabre, Astellas Pharma, Pfizer, Merck, Genentech, Novartis, AstraZeneca, MedImmune, Bristol-Myers Squibb
Research Funding: Millennium Pharmaceuticals (Inst), Sanofi (Inst)
Travel, Accommodations, Expenses: Pfizer, MSD Oncology
David J. Kwiatkowski
Consulting or Advisory Role: AstraZeneca, Genentech
Research Funding: AADi
Guru P. Sonpavde
Honoraria: UpToDate
Consulting or Advisory Role: Bayer, Genentech, Sanofi, Merck, Novartis, Pfizer, Argos Therapeutics, Agensys, Eisai, AstraZeneca, Janssen Pharmaceuticals, Amgen, Bristol-Myers Squibb, Exelixis
Speakers' Bureau: Clinical Care Options, National Comprehensive Cancer Network, Physician Education Resource, Onclive, Research to Practice
Research Funding: Onyx Pharmaceuticals (Inst), Bayer (Inst), Boehringer Ingelheim (Inst), Celgene (Inst), Merck (Inst), Pfizer (Inst)
Other Relationship: Boehringer Ingelheim, AstraZeneca
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A.Cancer Statistics, 2017 CA Cancer J Clin 677–302017 [DOI] [PubMed] [Google Scholar]
- 2.Chen CH, Changou CA, Hsieh TH, et al. Dual inhibition of PIK3C3 and FGFR as a new therapeutic approach to treat bladder cancer Clin Cancer Res 241176–11892018 [DOI] [PubMed] [Google Scholar]
- 3.Acquaviva J, He S, Zhang C, et al. FGFR3 translocations in bladder cancer: Differential sensitivity to HSP90 inhibition based on drug metabolism Mol Cancer Res 121042–10542014 [DOI] [PubMed] [Google Scholar]
- 4.Felsenstein KM, Theodorescu D.Precision medicine for urothelial bladder cancer: Update on tumour genomics and immunotherapy Nat Rev Urol 1592–1112018 [DOI] [PubMed] [Google Scholar]
- 5.Turner N, Grose R.Fibroblast growth factor signalling: From development to cancer Nat Rev Cancer 10116–1292010 [DOI] [PubMed] [Google Scholar]
- 6.Nelson KN, Meyer AN, Siari A, et al. Oncogenic gene fusion FGFR3-TACC3 is regulated by tyrosine phosphorylation Mol Cancer Res 14458–4692016 [DOI] [PubMed] [Google Scholar]
- 7.Robertson AG, Kim J, Al-Ahmadie H, et al. Comprehensive molecular characterization of muscle-invasive bladder cancer Cell 171540.e25–556.e252017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang R, Wang L, Li Y, et al. FGFR1/3 tyrosine kinase fusions define a unique molecular subtype of non–small-cell lung cancer Clin Cancer Res 204107–41142014 [DOI] [PubMed] [Google Scholar]
- 9.Capelletti M, Dodge ME, Ercan D, et al. Identification of recurrent FGFR3-TACC3 fusion oncogenes from lung adenocarcinoma Clin Cancer Res 206551–65582014 [DOI] [PubMed] [Google Scholar]
- 10.Stransky N, Cerami E, Schalm S, et al. The landscape of kinase fusions in cancer. Nat Commun. 2014;5:4846. doi: 10.1038/ncomms5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu YM, Su F, Kalyana-Sundaram S, et al. Identification of targetable FGFR gene fusions in diverse cancers Cancer Discov 3636–6472013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costa R, Carneiro BA, Taxter T, et al. FGFR3-TACC3 fusion in solid tumors: Mini-review Oncotarget 755924–559382016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh D, Chan JM, Zoppoli P, et al. Transforming fusions of FGFR and TACC genes in human glioblastoma Science 3371231–12352012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams SV, Hurst CD, Knowles MA.Oncogenic FGFR3 gene fusions in bladder cancer Hum Mol Genet 22795–8032013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carneiro BA, Elvin JA, Kamath SD, et al. FGFR3-TACC3: A novel gene fusion in cervical cancer Gynecol Oncol Rep 1353–562015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nogova L, Sequist LV, Perez Garcia JM, et al. Evaluation of BGJ398, a fibroblast growth factor receptor 1-3 kinase inhibitor, in patients with advanced solid tumors harboring genetic alterations in fibroblast growth factor receptors: Results of a global phase I, dose-escalation and dose-expansion study J Clin Oncol 35157–1652017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non–small-cell lung cancer who harbor EML4-ALK J Clin Oncol 274247–42532009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bergethon K, Shaw AT, Ou SH, et al. ROS1 rearrangements define a unique molecular class of lung cancers J Clin Oncol 30863–8702012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers J Natl Cancer Inst 97339–3462005 [DOI] [PubMed] [Google Scholar]
- 20.Humphrey PA, Moch H, Cubilla AL, et al. The 2016 WHO classification of tumours of the urinary system and male genital organs-part B: Prostate and bladder tumours Eur Urol 70106–1192016 [DOI] [PubMed] [Google Scholar]
- 21.Balbás-Martínez C, Sagrera A, Carrillo-de-Santa-Pau E, et al. Recurrent inactivation of STAG2 in bladder cancer is not associated with aneuploidy Nat Genet 451464–14692013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Höglund M.The bladder cancer genome; chromosomal changes as prognostic makers, opportunities, and obstacles Urol Oncol 30533–5402012 [DOI] [PubMed] [Google Scholar]
- 23.Lindgren D, Frigyesi A, Gudjonsson S, et al. Combined gene expression and genomic profiling define two intrinsic molecular subtypes of urothelial carcinoma and gene signatures for molecular grading and outcome Cancer Res 703463–34722010 [DOI] [PubMed] [Google Scholar]
- 24.Sholl LM, Do K, Shivdasani P, et al. Institutional implementation of clinical tumor profiling on an unselected cancer population. JCI Insight. 2016;1:e87062. doi: 10.1172/jci.insight.87062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li H, Durbin R.Fast and accurate long-read alignment with Burrows-Wheeler transform Bioinformatics 26589–5952010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia EP, Minkovsky A, Jia Y, et al. Validation of OncoPanel: A targeted next-generation sequencing assay for the detection of somatic variants in cancer Arch Pathol Lab Med 141751–7582017 [DOI] [PubMed] [Google Scholar]
- 27.Pugh TJ, Amr SS, Bowser MJ, et al. VisCap: Inference and visualization of germ-line copy-number variants from targeted clinical sequencing data Genet Med 18712–7192016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abo RP, Ducar M, Garcia EP, et al. BreaKmer: Detection of structural variation in targeted massively parallel sequencing data using kmers. Nucleic Acids Res. 2015;43:e19. doi: 10.1093/nar/gku1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thorvaldsdóttir H, Robinson JT, Mesirov JP.Integrative Genomics Viewer (IGV): High-performance genomics data visualization and exploration Brief Bioinform 14178–1922013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lek M, Karczewski KJ, Minikel EV, et al. Analysis of protein-coding genetic variation in 60,706 humans Nature 536285–2912016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tabernero J, Bahleda R, Dienstmann R, et al. Phase I dose-escalation study of JNJ-42756493, an oral pan-fibroblast growth factor receptor inhibitor, in patients with advanced solid tumors J Clin Oncol 333401–34082015 [DOI] [PubMed] [Google Scholar]
- 32.Di Stefano AL, Fucci A, Frattini V, et al. Detection, characterization, and inhibition of FGFR-TACC fusions in IDH wild-type glioma Clin Cancer Res 213307–33172015 [DOI] [PMC free article] [PubMed] [Google Scholar]