Abstract
2D nanomaterials have long been considered for development of high permeability membranes. However, current processes have yet to yield a viable membrane for practical use due to the lack of scalability and substantial performance improvements over existing membranes. Herein, an ultrathin graphene oxide (GO) membrane with a permeability of 1562 mL h−1 mmHg−1 m−2, two orders of magnitude higher than the existing nanofiltration membranes, and a tight molecular weight cut-off is presented. To build such a membrane, a new process involving self-assembly and optimization of GO nanoplatelet physicochemical properties is developed. The process produces a highly organized mosaic of nanoplatelets enabling ultra-high permeability and selectivity. An adjustable molecular interlinker between the layers enables absolute nanometer-scale size cut-offs. These characteristics promise significant improvements to many nanoparticle and biological separation applications. In this work, the performance of the membrane in blood dialysis scenarios is evaluated. Urea and cytochrome-c sieving coefficients of 0.5 and 0.4 are achieved while retaining 99% of albumin. Hemolysis, complement activation, and coagulation studies exhibit a performance on par or superior to the existing dialysis membrane materials.
Keywords: biological filtration, graphene oxide, hemocompatibility, hemodialysis, membranes
1. Introduction
Fabrication of high-throughput and selective separation media at low cost has been the main objective of the membrane industry for decades. Recent advancements in nanomaterials have opened new opportunities for development of such membranes. Graphene and graphene oxide (GO), the pinnacle of ultrathin mechanically strong 2D nanomaterials,[1–3] have long been considered for separation applications. Theoretical studies and simulations have demonstrated that nanoporous graphene and GO exhibit superior sieving potential in water separation applications.[4,5] However, despite their significant promise, their use has not been realized due to the lack of scalable fabrication processes.[6] Development of a facile and scalable process demands new approaches and a holistic understanding of the relation between physicochemical properties of these 2D nanomaterials and the membrane transport characteristics.
A number of fabrication processes have been proposed to produce nanoporous graphene and GO membranes. Porous single layer graphene membranes patterned via ion bombardment,[7,8] focused-ion beam irradiation,[9] or focused electron beam writing[10,11] with pore sizes of 0.4–0.6 nm and pore densities in the 1012 cm−2 range have been fabricated. While these efforts demonstrate the potential of graphene separation media, implementation as a viable membrane is limited by fabrication challenges. The limited membrane area produced through graphene synthesis[12] combined with defects occurring during the transfer/assembly process[13,14] presents significant scalability challenges. GO laminate membranes prepared through vacuum filtration[15,16] have been pursued as scalable alternatives. This process produces uniquely selective membranes due to a precise interlayer spacing between the GO nanoplatelets. However, GO membranes prepared through this method are generally thick, severely increasing the species transport path length (i.e., transport resistance) compared to a single-layer graphene membrane.
Layer-by-layer (L-b-L) assembly of GO[17–19] has been pursued in an attempt to reduce the membrane thickness and transport resistance, while maintaining an acceptable selectivity. L-b-L assembly frameworks have achieved minimum thicknesses of ≈100 nm. These assemblies have demonstrated water permeabilities of ≈25–60 mL h−1 mmHg−1 m−2 with improved retention of charged molecules compared to typical nanofiltration (NF) membranes.[20,21] However, L-b-L assemblies have largely ignored the impact of critical GO nanoplatelet physicochemical properties on the membrane transport characteristics. For example, as depicted in Figure 1, the nanoplatelet size dictates the overall transport path length of species through the membrane—large nanoplatelets reduce the permeability.
Figure 1.
Illustration of species transport across GO laminate through nanoplatelet-edge and defects. Primary transport pathways through self-assembled GO nanoplatelet layers, dependent on nanoplatelet size and interlayer spacing.
2. GO Nanoplatelets Assembly
An ideal GO membrane should consist of the fewest number of GO layers while avoiding defects that diminish the membrane selectivity. The current GO assembly methods yield a wide array of nanoplatelet assemblies, ranging from sparsely packed to overly condensed layers hardly exhibiting any order and planarity necessary for minimizing through-plane defects, and consequently the number of GO layers. We initially attempted to mimic the common immersion L-b-L or dip-draw techniques [22,23] to form an organized ultrathin GO structure but were ultimately unsuccessful. Resulting structures had large open defect areas for dip-draw processing (Figure 2a) or yielded multiple GO nanoplatelet layers stacked atop one another during each immersion step (Figure 2b). Both approaches yielded poor sieving necessitating deposition of a larger number of layers, as in prior studies, to improve coverage at the expense of permeability (see Figure 1).
Figure 2.
GO nanoplatelet assembly utilizing standard L-b-L techniques. Initial layer processing results in poor substrate coverage, observing A) large defect areas (circled in red) for standard dip-draw techniques or B) over-compacted structures (circled in blue) after typical L-b-L immersion techniques. C) Utilization of optimized nanoplatelet and assembly conditions outlined in this study yield highly ordered ultrathin structures.
We have discovered that if small GO nanoplatelets (≈100s nm) are given sufficient diffusion time to assemble, rather than the common dip-and-rise step used in the L-b-L assembly process, they can produce a closely packed, ordered GO mosaic (Figure 2c) enabling a highly selective membrane with only a few GO layers. This approach has been inspired by our studies of clay nanoplatelets self-assembly.[24] The GO nanoplatelet organized self-assembly is dictated by the electrostatic interactions between the nanoplatelets, particularly their edge functional groups, and the interlinking molecules charged end group. In a solution with suitable pH, GO nanoplatelets tend to avoid overlapping due to electrostatic repulsion of their negatively charged functionalities, while being attracted by the interlinker. The net result of this interaction is edge-to-edge and surface-to-surface repulsion between the GO nanoplatelets, and surface-to-surface attraction between GO nanoplatelets and the underlying substrate functionalized with charged molecules. In this case, a positive interlinker is selected to attract negative functionalities of the GO nanoplatelets. This process fundamentally differs from GO laminates prepared through vacuum filtration and dip-and-rise L-b-L assembly, where GO nanoplatelet orientation is wholly random due to mechanical forces overcoming the relatively weaker functional group repulsive forces. Providing nanoplatelets with sufficient diffusion time allows them to settle/orient and assemble. The noninterlinked GO nanoplatelets remaining on the assembled/locked platelets while the sample is pulled out of the solution are then removed through submerging in a clean deionized (DI) water bath followed by a gentle DI water rinse. This planar GO layer formed is subsequently functionalized with another layer of interlinking molecule, for assembly of the next GO layer.
Nanoplatelet size is often not reported in studies that have utilized L-b-L assembly and the few studies that do have reported a size on the order of micrometers.[25–28] The Hummers’ and Marcano’s methods universally used to exfoliate GO produced nanoplatelets ranging from a few micrometers to 10s μm.[29] By breaking the GO nanoplatelets (Figure 3a–c) through sonication, they can better diffuse and rotate/orient while forming smaller open areas (i.e., defects) in between themselves. Combining this closely packed nanoplatelet assembly with an appropriate interlinking molecule (polyallylamine hydrochloride, PAH) enables highly permeable membranes with a precise molecular weight cut-off (MWCO). Figure 3d provides an atomic force microscope (AFM) image of the GO mosaic showing a precise 5 nm distance from the underlying substrate.
Figure 3.
GO nanoplatelet size distribution and PAH interlinker characterization across tested devices. Nanoplatelet size distribution for A) 500 nm support, B) 250 nm support, and C) 100 nm support. D) AFM characterization of GO anchored on PMMA support.
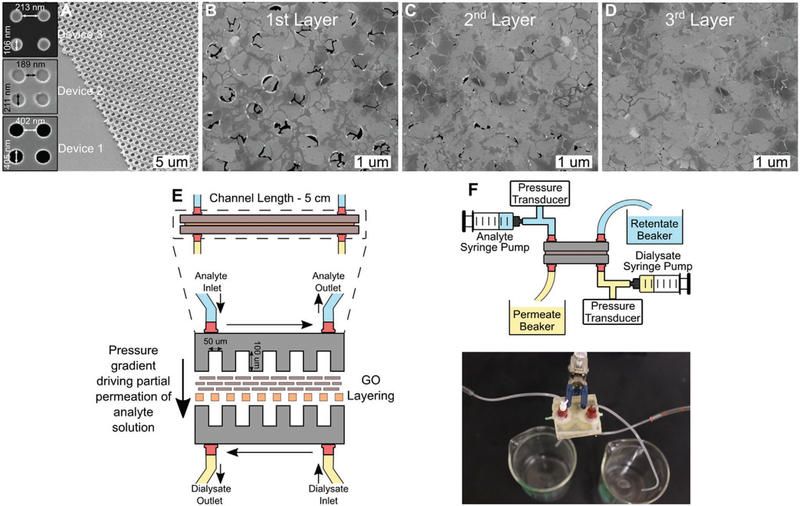
An ideal assembly fabricated using the aforementioned process would only require two layers of GO, but this has yet to be achieved without incurring defects that compromise permselectivity. Herein, we demonstrate the efficacy of a membrane utilizing three GO layers. To achieve a high selectivity with only three GO layers, it was critical to use a near-atomically smooth underlying substrate. Polyethersulfone (PES) membranes used in prior studies[30,31] are very rough (Figure S1, Supporting Information), requiring lamination of layers of large GO nanoplatelets to establish planarity. Furthermore, the pore size of the support membrane must be smaller than the GO nanoplatelets and uniform to prevent nanoplatelets clogging of the pores. PES membranes also have a wide range of pore size (Figures S1 and S2, Supporting Information). For these reasons, three polymethyl methacrylate (PMMA) membranes with pore sizes of ≈100, 200, and 400 nm (insets in Figure 4a) were nanoimprinted and then hydrolyzed for the GO nanoplatelet sizes presented in Figure 3a–c. Figure 4b shows that the first GO assembled layer provides significant coverage of the underlying PMMA membrane, but had large defects left between the nanoplatelets. The second layer (Figure 4c) substantially reduced the defects but a third layer was needed to eliminate visible defects (Figure 4d).
Figure 4.
Visualization of PMMA support and impact of subsequent GO layering, with permeability and permselectivity results for tested device iterations. A–D) SEM of support PMMA imprint and subsequent GO layering. E) Schematic representation of the characterized GO device. F) System schematic for sieving characterization and actual device.
2.1. Permselective Characteristic of Ultrathin GO Membrane
In order to determine the membrane transport properties, microfluidic test devices were developed. Figure 4e,f shows a device. A set of tests was conducted to determine the membrane permeability and selectivity of different species. First, water permeability of the membrane was measured, provided in Figure 5a. Reducing the nanoplatelets size directly improved the permeability, through shortening the effective transport path length. A permeability of 1562 mL h−1 mmHg−1 m−2 was measured with Device 3, two orders of magnitude higher than existing NF membranes. The permeability of this membrane is nearly 40-fold higher than the commercial high flux hemodialysis membranes (e.g., EVODIAL 2.2 and ELISIO-9H manufactured by Baxter Healthcare Ltd. and Nipro Medical Corp., respectively) and nearly five times greater than that of the glomerular membrane of the kidney,[32] demonstrating the unique capability of nanomaterials to exceed their biological equivalent. Another unique transport characteristic of the membrane is its precise MWCO that offers ultimate selectivity relative to polymer membranes that have a range of pore sizes. With such a high permeability, a small membrane area can be built into a multilayer microfluidic cartridge for dialysis of a human adult to reduce the extracorporeal blood circuit by an order of magnitude, preventing loss of 100–150 mL blood in each dialysis session.[33]
Figure 5.
Preliminary permeability and sieving performance of ultrathin GO surfaces across multiple device iterations. A) Device iteration characteristics and permeability compared to typical commercial membranes. B) Sieving efficiency for characteristic low and middle-weight uremic toxins with minimal albumin loss. C) Sieving characterization of small-weight Dextran T500 along with β-g and P-b, exhibiting molecular weights much larger than the expected MWCO of these devices. The negligible loss of both β-g and P-b demonstrates the lack of large defect areas across the GO surface, providing a continuous sieving structure along the device.
A second set of tests was conducted to determine the transport rate and selectivity of different species. The membrane sieving capability of urea, cytochrome-c, and Dextran T500 as representative small and middle-weight uremic toxins, while monitoring the retention of albumin, is provided in Figure 5b,c. β-Galactosidase and phosphorylase-b were utilized to evaluate the presence of large defects across the GO surface in Figure 5c, as the MW of each molecule is much larger than the MWCO of this assembly. 0.5 mL samples were collected for analysis from the analyte and permeate outlets every 30 min over a 2 h span, monitoring 10–15 mL solution per test. The GO test device was a 5 cm long single serpentine polydimethylsiloxane (PDMS) microchannel with a 50 × 100 μm2 cross-section. PX-26 pressure transducers were utilized in-line to monitor the pressures within the system. A maximum urea sieving coefficient of 0.5 is achieved for Device 3 while the human serum albumin (HSA), with a size of <66 kDa, retention was >99% across all devices (Figure 5b), which would be necessary for any hemodialysis membrane. Retention of large molecule β-galactosidase and phosphorylase-b (>99.8% in both cases) indicates the lack of open tears or large defects across the surface commonly found in graphene or GO surfaces. For comparison, sparse and compact GO devices (shown in Figure 2a,b) were evaluated under similar test conditions. As demonstrated in Figure S3a in the Supporting Information, the sparse configuration provides no retention of small or large molecules throughout testing, attributed to the presence of large defects across the device. Likewise, the compact GO configuration provides improved retention of characteristic molecules yet demonstrates an order of magnitude decline in permeability (Figure S3b, Supporting Information) due to the extended transport pathway of larger GO flakes. Both configurations demonstrate inferior permselectivity compared to the devices presented within this study. In order to accommodate slow rate nocturnal dialysis on the order of 100 mL min−1, we anticipate as little as 0.015 m2 effective sieving area would be sufficient, considering the membrane surface area of 2.5 mm2 used in our 0.017 mL min−1 test device. This membrane area can be incorporated in a microfluidic membrane module with a 5 × 5 cm2 footprint consisting of 15 microchannel layers.
3. Evaluation of Ultrathin GO Hemocompatibility
Next, we investigated the hemocompatibility of GO-based membranes compared to commercially available hemodialyzer materials. The GO–blood interactions were investigated, utilizing careful control on oxidation extent and GO nanoplatelet size to identify their hemocompatibility. GO was synthesized,[34,35] sonicated in a bath sonicator,[36,37] and then deposited onto glass substrates, where complete coverage of the glass substrate with GO nanoplatelets is critical in preventing the substrate contribution toward hemolysis. Scanning electron microscope (SEM) imaging showed close packing of GO nanoplatelets on the glass substrate with minimal exposure of the underlying glass, where layers of GO-PAH fully cover the surface.
First, we investigated hemolysis, the rupture of red blood cells, following 1 h of exposure to membrane surfaces. We found that diethylaminoethyl cellulose (DEAE) and regenerated cellulose (RC) membranes, which fell out of favor as hemodialysis materials, induced a slight level of hemolysis (≈2%), as expected. For these tests, 1 mL of the hematocrit-adjusted blood (Hct = 36%) was pipetted onto each sample. With 1 mL of blood, the total surface area to blood volume ratio is ≈5.4 cm2 mL−1, following the specifications recommended by ASTM F756.[38] The 1 mL blood, when spread over the 5 × 1 cm2 area, did not confer sufficient hydrostatic pressure to drive the seepage of blood through DEAE, RC, or PES membranes, enabling straightforward recovery for analysis similarly to the GO-coated test substrates. These membranes produced notably higher hemolysis compared to all four tested GO oxidation assemblies (Figure 6a). Teflon, silicone, and glass, which were used in the GO testing apparatus, showed comparable hemolysis levels to the different GO substrates indicating that the actual GO contribution to hemolysis may be even lower than that measured.
Figure 6.
Hemolytic and C5b-9 complement activity for GO suspension and laminate scenarios. A) Hemolysis results for GO membrane conformation with varying oxidation factors and comparative commercial substrates. B) Complement activation results for GO membrane layout (each bar and the associated error bar represent the mean and the standard error of six independent samples (n = 6)). For positive control (LPS) and the negative controls at 4 and 37 °C, n = 3. C) Coagulation results for GO and standard membrane material.
We then investigated substrate-induced immunogenicity, as assessed by the production of C5b-9 complement. The test samples were incubated for 15 min in a manner similar to that described in the hemolysis testing. 10 μL of the plasma sample was retrieved and diluted 1:30 in phosphate buffered saline (PBS). 100 μL of the diluted sample was then assessed for complement activation using a C5b-9 ELISA (enzyme-linked immunosorbent assay) kit. C5b-9 ELISA revealed (Figure 6b) that none of the test substrates promoted significant C5b-9 production, with all GO samples falling within the physiological range. We induced complement activation using 100 ng mL−1 of lipopolysaccharide (LPS) from E. coli O55: B for a positive control, which resulted in markedly increased levels of C5b-9 production. There was no statistical significance between GO substrates and PES membranes.
Thrombogenicity, or the tendency of a material to induce clotting, of the GO surface was evaluated based on coagulation time after post-thrombin addition, where shorter times for coagulation onset corresponded with higher thrombogenicity. No statistically significant differences were observed across all GO variants (Figure 6c) compared to the control substances. Compared to PES membranes and Teflon, noted for their highly hemocompatible characteristics, GO membranes exhibit no significant variance in hemolytic, coagulation, or complement activation characteristics (Figure 6a–c).
3.1. Hemocompatibility Comparison between GO Nanoplatelet and Laminate Structure
These hemocompatibility results are contradictory to prior GO compatibility studies,[39,40] which indicated that GO induces high levels of hemolysis and complement activation.[41–43] Comparison between GO suspension and membrane platforms suggest that the interactions between red blood cells (RBCs) and GO platelets in these two cases fundamentally differ. The ability of GO nanoplatelets in suspension to freely diffuse leads to an increased interaction rate with other species. A nanoplatelet size distribution ranging from 150 to 500 nm was analyzed through nanoparticle tracking analysis (NTA). When freely diffusing, a randomly oriented GO nanoplatelet with an estimated disk diameter of 150 nm has a diffusion coefficient of ≈2.9 μm2 s−1. At an RBC concentration of 5 × 108 mL−1 and a GO concentration of 3.5 × 1010 mL−1, this translates to a GO–RBC encounter frequency of ≈82 times every 20 s. This interaction frequency is drastically higher compared to the laminate scenario that we present in this study, as RBC sedimentation tends to occur, limiting the number of RBCs which can actively interact with the surface. Even when accounting for recirculation of the RBCs atop a GO laminate at 10 dyn cm−2 that might occur in a wearable hemodialyzer, the hemolytic activity is comparable to polymer baselines and well below GO suspensions (Figure 7c). These results suggest that the primary hemolytic mechanism found in suspension studies is absent in GO laminates or occurs on a much less pronounced magnitude.
Figure 7.
Characterization of GO suspension behavior and quantification of hemolytic behavior after perfusion at physiological conditions. A) GO suspension hemolysis using GO 60 min sonication at varied concentrations. B) GO aggregation in DI water and PBS solutions based on number of particles present with higher aggregation in PBS. C) Hemolysis observed after perfusion across GO surfaces, which falls in the nonhemolytic regime (<2%).
4. Conclusion
In summary, we demonstrated a unique self-assembled GO nanoplatelet ordered mosaic, greatly advancing a decade-old effort on development of graphene-based membranes. Careful control of GO nanoplatelet characteristics (through utilization of small, highly sonicated GO nanoplatelets) and the self-assembly process (by providing a sufficient diffusion time during immersion with a subsequent step to dislodge noninterlinked platelets) along with the use of a planar and smooth support layer enabled formation of our ordered GO layers, where previous processes have not. The new membrane requires only three layers of GO atop a PMMA support, achieving permeabilities as high as 1562 ± 30 mL h−1 mmHg−1 m−2, nearly two orders of magnitude greater than existing NF membranes. A precise effective pore size of 5 nm represents a great advantage over the polymer membranes with a range of pore sizes. This GO laminate has also shown vastly improved hemolytic and biocompatible properties compared to previous studies concerning GO nanoplatelets in suspension. Even under recirculation conditions of 10 dyn cm−2, hemolytic activity of GO laminates remains at or below the commercially available dialyzers. The membrane provides a viable platform for miniaturized dialysis devices that could enhance in-home low flow rate nocturnal dialysis.
5. Experimental Section
Fabrication of Microchannel Test Device:
The GO nanoplatelets herein were sonicated for up to 10 h in a bath sonicator to achieve the 100–500 nm nanoplatelet sizes reported in this study. PMMA film (≈200 nm thickness) was spin-coated onto a silicon wafer for nanoimprinting. 400, 200, and 100 nm holes were introduced on PMMA via an in-house nanoimprint lithography setup (NIL) (Figure S4, Supporting Information) using 3 × 3 mm2 silicon stamps patterned with electron beam lithography.[44] These silicon master stamps were pretreated with a hydrophobic perfluorodecyltrichlorosilane (FDTS) coating to lower surface adhesion between PMMA and silicon surfaces, eliminating any tearing which might occur when removing the silicon stamp after imprinting was complete. The silicon stamp and PMMA wafer were inserted into the NIL setup then heated to ≈170–180 °C while under vacuum for 45 min. This setup was then cooled to below PMMA glass transition temperature while maintaining pressure on the stamp, after which both wafers were removed and separated. Any residual PMMA remaining in the nanofeature pores was removed through O2 reactive ion etching for ≈30 s after imprinting.
After introduction of 400, 200, and 100 nm nanofeatures on PMMA, the substrate was bonded to a PDMS film with imprinted microchannels for fluid delivery through oxygen plasma treatment. This process first exposed the imprinted PMMA structure under oxygen plasma to introduce surface reactive functionalities, followed by immediate immersion in 10%, v/v (3-aminopropyl)triethoxysilane (3-APTES) solution for 15 min. After removal from 3-APTES, the Si-NH2 functional group was again treated with oxygen plasma, then bonded to PDMS under light pressure. The resulting silane bond between PMMA and PDMS provided sufficient bond strength to withstand further flow testing. This PDMS–PMMA structure was removed from the underlying silicon wafer through etching of the aluminum sacrificial layer after immersion in Aluminum Etchant (Transene Co., MA) for ≈1 h. After complete lift-off from the supporting silicon wafer, the PDMS–PMMA structure was thoroughly rinsed in DI water to remove any residual etchant.
The PMMA surface was then hydrolyzed in 1.5 m sodium hydroxide solution for 30 min at 45 °C to achieve an electronegative surface, to enable interlinking with PAH through electrostatic attraction. The PMMA–PDMS device was immersed in alternating GO (1 g L−1) or PAH (1 g L−1) solutions for 15 min to allow for ordered nanoplatelet orientation, before being removed and rinsed with DI water for ≈30 s to remove any weakly bonded nanoplatelets. PAH immersion times of 5 min (Figure S5a, Supporting Information) and 10 min (Figure S5b, Supporting Information) were initially employed, but ultimately yielded noticeable defects across the GO layer. PAH-immersion for 15 min at room conditions with no shocks/movement to the sample was found to yield the highly ordered layer observed in Figure 2b. This process limited the size of defect areas or overlapping associated with prior L-b-L assembly techniques. This dipping process was repeated until three GO-PAH layers were assembled atop the PMMA–PDMS device. After successful GO assembly atop the PMMA support, an upper and lower PDMS microchannel was epoxy bonded to complete the device fabrication.
Once the GO device was fully fabricated, permeate and sieving characteristics were assessed in triplicate for each device/test molecule using an in-house setup. Pressure transducer data were monitored by data acquisition system (Agilent Technologies Inc., CA) at room temperature (T = 20–25 °C). The device was connected to two syringe pumps (Figure 4f) used to deliver the analyte and dialysate at the same flow rate of 1 mL h−1 (i.e., 0.017 mL min−1). Sieving performance of the device was assessed using urea (4.6 mmol L−1), cytochrome-c (0.08 mmol L−1), and human serum albumin (0.075 mmol L−1). Urea sample concentrations were assessed using urea colorimetric assay kit (Biovision Inc., CA) combined with micro-plate spectrophotometry. Cytochrome-c and albumin concentrations were determined through UV-vis spectroscopy at 405 and 280 nm, respectively. Dextran T500 (0.191 mmol L−1), phosphorylase-b (0.0102 mmol L−1), and β-galactosidase (0.0105 mmol L−1) assays were used to evaluate each test device for the presence of large defects which could compromise sieving efficacy.
Fabrication of Microfluidic Device for Hemocompatibility Evaluation:
A simple microfluidic device was created by bonding a PMMA cap to the substrate (GO or Teflon) using double-sided adhesive transfer tape (3M 468MP, by 3M, St. Paul, MN). The PMMA cap was cut from 0.56 cm thick PMMA sheets using a 90W CO2 laser cutter (Full Spectrum P48–36, by Full Spectrum, Las Vegas, NV) at the RIT Maker Space. The PMMA top piece contained 2.85 mm holes to host the insertion of E-3603 tygon tubing (ID/OD = 1/16”/3 mm, by Saint-Gobain, Malvern, PA), which enabled fluidic access to the substrate. Rectangles (2 mm × 5 cm) were cut from the adhesive transfer tape (≈110–130 μm thick) using a digital craft cutter (Silhouette Cameo, by Silhouette America, Lindon, UT). Four microfluidic channels were situated across the 2.5 cm × 7.5 cm substrate footprint.
Substrate-Induced Hemolysis:
The GO and control substrates were made to match the format of a glass side (7.5 cm × 2.5 cm). Due to the manufacturing process involved in the preparation of GO, the front and back sides of GO samples were not always identical. A custom-cut framework (300 μm thick, 5.8 cm × 1.8 cm in area, with 5 cm × 1 cm cutout in the middle) was created using restricted grade medical silicone to hold whole blood on top of the samples for incubation. The surface area of the silicone gasket (from the four walls) was ≈7% of the total surface area that would be exposed to blood. For control substrates such as the DEAE, RC, and PES membranes, 5 cm × 1 cm cutouts were placed on top of Teflon (7.5 cm × 2.5 cm) and held in place within the silicone frame.
Each sample was hosted within a petri dish to better ensure sterility and kept in an incubator (37 °C, 5% CO2, 80% relative humidity) for 2 h. Each petri dish also contained a wetted Kimwipe to help reduce blood evaporation. Minimal seepage of blood was observed onto Teflon after the 2 h incubation. The blood on top of the sample was gently mixed through titration and retrieved (700 μL) for hemoglobin (Hb) measurement. The 700 μL sample was centrifuged at 500 G for 20 min. The top 200 μL of the resulting plasma was then retrieved and centrifuged again at 3000 G for 10 min to further ensure the removal of RBCs. The top 100 μL of the resulting plasma was placed into a 96-well plate for hemolysis assessment.
Substrate-Induced Coagulation:
Coagulation was assessed based on thrombin time (TT). Thrombin (0.5 Unit mL−1) was added to fresh platelet poor plasma (PPP) to initiate the coagulation cascade and coagulation time was recorded. The setup of the platelet aggregometer (CHRONO-LOG Model 700) was used to conduct the coagulation testing. In this setup, the plasma was maintained at 37 °C in a narrow glass cuvette and well mixed with a siliconized magnetic stir bar. The time at which the stir bar stopped moving was recorded as the coagulation time.
Supplementary Material
Acknowledgements
Research reported in this paper was supported by the NIBIB of the National Institutes of Health under award number R21EB023527 to S.M. and T.R.G. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors thank B. Kwarta for creating the GO laminate illustration.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
Contributor Information
Richard P. Rode, Department of Mechanical Engineering, University of Florida, Gainesville, FL 32611, USA
Henry H. Chung, Biomedical Engineering Department, Rochester Institute of Technology, Rochester, NY 14623, USA
Hayley N. Miller, Biomedical Engineering Department, Rochester Institute of Technology, Rochester, NY 14623, USA
Thomas R. Gaborski, Biomedical Engineering Department, Rochester Institute of Technology, Rochester, NY 14623, USA
Saeed Moghaddam, Department of Mechanical Engineering, University of Florida, Gainesville, FL 32611, USA.
References
- [1].Zhu Y, Murali S, Cai W, Li X, Suk JW, Potts JR, Ruoff RS, Adv. Mater. 2010, 22, 3906. [DOI] [PubMed] [Google Scholar]
- [2].Lee C, Wei X, Kysar JW, Hone J, Science 2008, 321, 385. [DOI] [PubMed] [Google Scholar]
- [3].Cohen-Tanugi D, Grossman JC, Nano Lett. 2012, 12, 3602. [DOI] [PubMed] [Google Scholar]
- [4].Sint K, Wang B, Král P, J. Am. Chem. Soc. 2008, 130, 16448. [DOI] [PubMed] [Google Scholar]
- [5].Suk ME, Aluru NR, J. Phys. Chem. Lett. 2010, 1, 1590. [Google Scholar]
- [6].Lively RP, Sholl DS, Nat. Mater. 2017, 16, 276. [DOI] [PubMed] [Google Scholar]
- [7].O’Hern SC, Boutilier MSH, Idrobo J-C, Song Y, Kong J, Laoui T, Atieh M, Karnik R, Nano Lett. 2014, 14, 1234. [DOI] [PubMed] [Google Scholar]
- [8].Boutilier MSH, Jang D, Idrobo J-C, Kidambi PR, Hadjiconstantinou NG, Karnik R, ACS Nano 2017, 11, 5726. [DOI] [PubMed] [Google Scholar]
- [9].Morin A, Lucot D, Ouerghi A, Patriarche G, Bourhis E, Madouri A, Ulysse C, Pelta J, Auvray L, Jede R, Bruchhaus L, Gierak J, Microelectron. Eng. 2012, 97, 311. [Google Scholar]
- [10].Xu Q, Wu MY, Schneider GF, Houben L, Malladi SK, Dekker C, Yucelen E, Dunin-Borkowski RE, Zandbergen HW, ACS Nano 2013, 7, 1566. [DOI] [PubMed] [Google Scholar]
- [11].Sommer B, Sonntag J, Ganczarczyk A, Braam D, Prinz G, Lorke A, Geller M, Sci. Rep. 2015, 5, 7781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kim KS, Zhao Y, Jang H, Lee SY, Kim JM, Kim KS, Ahn JH, Kim P, Choi JY, Hong BH, Nature 2009, 457, 706. [DOI] [PubMed] [Google Scholar]
- [13].Kumar S, Peltekis N, Lee K, Kim HY, Duesberg GS, Nanoscale Res. Lett. 2011, 6, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].O’Hern SC, Stewart CA, Boutilier MSH, Idrobo JC, Bhaviripudi S, Das SK, Kong J, Laoui T, Atieh M, Karnik R, ACS Nano 2012, 6, 10130. [DOI] [PubMed] [Google Scholar]
- [15].Dikin DA, Stankovich S, Zimney EJ, Piner RD, Dommett GHB, Evmenenko G, Nguyen ST, Ruoff RS, Nature 2007, 448, 457. [DOI] [PubMed] [Google Scholar]
- [16].Yeh C-N, Raidongia K, Shao J, Yang Q-H, Huang J, Nat. Chem. 2015, 7, 166. [DOI] [PubMed] [Google Scholar]
- [17].Choi W, Choi J, Bang J, Lee JH, ACS Appl. Mater. Interfaces 2013, 5, 12510. [DOI] [PubMed] [Google Scholar]
- [18].Zhang Y, Zhang S, Gao J, Chung TS, J. Membr. Sci. 2016, 515, 230. [Google Scholar]
- [19].Hu M, Mi B, Environ. Sci. Technol. 2013, 47, 3715. [DOI] [PubMed] [Google Scholar]
- [20].Wang T, Lu J, Mao L, Wang Z, J. Membr. Sci. 2016, 515, 125. [Google Scholar]
- [21].Yuan Y, Gao X, Wei Y, Wang X, Wang J, Zhang Y, Gao C, Desalination 2017, 405, 29. [Google Scholar]
- [22].Nan Q, Li P, Cao B, Appl. Surf. Sci. 2016, 387, 521. [Google Scholar]
- [23].Wang L, Wang N, Li J, Li J, Bian W, Ji S, Sep. Purif. Technol. 2016, 160, 123. [Google Scholar]
- [24].Peng C, Lai H, Orazem ME, Moghaddam S, Appl. Clay Sci. 2018, 158, 94. [Google Scholar]
- [25].Zhao X, Zhang Q, Hao Y, Li Y, Fang Y, Chen D, Macromolecules 2010, 43, 9411. [Google Scholar]
- [26].Han Y, Xu Z, Gao C, Adv. Funct. Mater. 2013, 23, 3693. [Google Scholar]
- [27].Abraham J, Vasu KS, Williams CD, Gopinadhan K, Su Y, Cherian CT, Dix J, Prestat E, Haigh SJ, Grigorieva IV, Carbone P, Geim AK, Nair RR, Nat. Nanotechnol. 2017, 12, 546. [DOI] [PubMed] [Google Scholar]
- [28].Li X, Zhu B, Zhu J, Carbon 2019, 146, 320. [Google Scholar]
- [29].Paneri A, Moghaddam S, Carbon 2015, 86, 245. [Google Scholar]
- [30].Barzin J, Feng C, Khulbe KC, Matsuura T, Madaeni SS, Mirzadeh H, J. Membr. Sci. 2004, 237, 77. [Google Scholar]
- [31].Peyravi M, Rahimpour A, Jahanshahi M, Javadi A, Shockravi A, Microporous Mesoporous Mater. 2012, 160, 114. [Google Scholar]
- [32].Suh JH, Miner JH, Nat. Rev. Nephrol. 2013, 9, 470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Huff C, Nature 2020, 579, 186. [DOI] [PubMed] [Google Scholar]
- [34].Hummers W, Offeman R, Water 1957, 208, 1937. [Google Scholar]
- [35].Marcano D, Kosynkin D, Berlin J, ACS Nano 2010, 4, 4806. [DOI] [PubMed] [Google Scholar]
- [36].Han JT, Jang JI, Kim H, Hwang JY, Yoo HK, Woo JS, Choi S, Kim HY, Jeong HJ, Jeong SY, Baeg K-J, Cho K, Lee G-W, Sci. Rep. 2015, 4, 5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Coleman BR, Knight T, Gies V, Jakubek ZJ, Zou S, ACS Appl. Mater. Interfaces 2017, 9, 28911. [DOI] [PubMed] [Google Scholar]
- [38].ASTM, ASTM F756–13, Standard Practice for Assessment of Hemolytic Properties of Materials, ASTM International, West Conshohocken, PA: 2013. [Google Scholar]
- [39].Durán M, Andrade PF, Durán N, Luzo ACM, Fávaro WJ, J. Phys.: Conf. Ser 2015, 617, 012021. [Google Scholar]
- [40].Zare-Zardini H, Taheri-Kafrani A, Amiri A, Bordbar A-K, Sci. Rep. 2018, 8, 586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Duan G, Zhang Y, Luan B, Weber JK, Zhou RW, Yang Z, Zhao L, Xu J, Luo J, Zhou R, Sci. Rep. 2017, 7, 42767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Liu X, Chen KL, Langmuir 2015, 31, 12076. [DOI] [PubMed] [Google Scholar]
- [43].Li R, Guiney LM, Chang CH, Mansukhani ND, Ji Z, Wang X, Liao Y-P, Jiang W, Sun B, Hersam MC, Nel AE, Xia T, ACS Nano 2018, 12, 1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Doll PW, Al-Ahmad A, Bacher A, Muslija A, Thelen R, Hahn L, Ahrens R, Spindler B, Guber AE, Mater. Res. Express 2019, 6, 065402. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.