Abstract
Background:
In individuals with chronic stroke, impairment of the paretic arm may be exacerbated by increased contralesional transcallosal inhibition (TCI). Continuous theta burst stimulation (cTBS) can decrease primary motor cortex (M1) excitability and TCI. However, contralesional cTBS shows inconsistent effects after stroke. Variable effects of cTBS could stem from failure to pair stimulation with skilled motor practice or a focus of applying cTBS over M1.
Objective:
Here, we investigated the effects of pairing cTBS with skilled practice on motor learning and arm function. We considered the differential effects of stimulation over two different brain regions: contralesional M1 (M1c) or contralesional primary somatosensory cortex (S1c).
Methods:
37 individuals with chronic stroke participated in five sessions of cTBS and paretic arm skilled practice of a serial targeting task (STT); participants received either cTBS over M1c or S1c or sham before STT practice. Changes in STT performance and Wolf Motor Function Test (WMFT) were assessed as primary outcomes. Assessment of bilateral corticospinal, intracortical excitability and TCI were secondary outcomes.
Results:
cTBS over sensorimotor cortex did not improve STT performance and paretic WMFT-rate beyond sham cTBS. TCI was reduced bi-directionally following the intervention, regardless of stimulation group. In addition, we observed an association between STT performance change and paretic WMFT-rate change in the M1c stimulation group only.
Conclusions:
Multiple sessions of STT practice can improve paretic arm function and decrease TCI bilaterally, with no additional benefit of prior cTBS. Our results suggest that improvement in STT practice following M1c cTBS scaled with change in paretic arm function in some individuals. Our results highlight the need for a better understanding of the mechanisms of cTBS to effectively identify who may benefit from this form of brain stimulation.
Keywords: Stroke, transcranial magnetic stimulation, skilled motor practice, motor function, transcallosal inhibition, continuous theta burst stimulation
1. Introduction
Upper-limb (UL) motor impairment and decreased motor function commonly persist into the chronic phase after stroke (Go et al., 2014). After stroke, increasing paretic UL use results in limited functional gains (Hendricks et al., 2002) and requires large numbers of repetitions (Birkenmeier et al., 2010). Skilled motor practice leads to neuroplasticity-like changes in the lesioned motor cortex and supports functional gains through re-learning (Boyd et al., 2007; Kleim et al., 2004; Liepert et al., 1998; Muratori et al., 2013). Past work demonstrates the capacity for motor learning even in the chronic phase after stroke (Boyd et al., 2009; Brodie et al., 2014; Meehan et al., 2011), yet this requires large amounts of practice (Meehan et al., 2011) that is often not feasible. Further, even with increased doses of practice after stroke, rates of change are diminished and overall gains decreased (Boyd et al., 2009). These data motivated interest in adjunct interventions, such as the use of repetitive transcranial magnetic stimulation (rTMS) (Auriat et al., 2015; Huang et al., 2005), to amplify the effects of skilled motor practice in an effort to positively impact functional outcomes in individuals with chronic stroke.
Altered brain excitability and connectivity is observed after stroke and can be indexed using single and paired-pulse transcranial magnetic stimulation (TMS) (Auriat et al., 2015; Di Pino et al., 2014). The ipsilesional primary motor cortex (M1i) typically shows less corticospinal excitability compared to contralesional M1 (M1c). However, M1c can demonstrate equal, or increased, corticospinal excitability (Liepert et al., 1998; Sawaki et al., 2008). Additionally, after stroke interhemispheric inhibition (IHI) from M1i is decreased (Boroojerdi et al., 1996; Shimizu et al., 2002) while M1c continues to show normal, or even increased interhemispheric inhibition (Duque et al., 2007, 2005; Harris-Love et al., 2016; Murase et al., 2004). After stroke, increased IHI from M1c to M1i has been indexed using either a dual-coil paired pulse method (Duque et al., 2007, 2005; Murase et al., 2004) or a single coil with ipsilateral isometric contraction via the ipsilateral silent period (iSP) (Harris-Love et al., 2016; Hayward et al., 2017). Together, these patterns of altered corticospinal excitability and interhemispheric communication after stroke may decrease excitability (Liepert et al., 1998), and interfere with corticospinal function and neuroplasticity, in M1i (Carey et al., 2006; Taub et al., 2006). Further, increased M1c to M1i IHI is associated with poorer motor function (Duque et al., 2005; Harris-Love et al., 2016) as described in the interhemispheric competition model (Auriat et al., 2015; Di Pino et al., 2014). In theory, excitatory imbalance between the hemispheres may contribute to diminished motor function of the paretic limb (Liepert et al., 1998; Sawaki et al., 2008).
Various forms of rTMS can be used to modulate corticospinal excitability and interhemispheric communication after stroke (Auriat et al., 2015; Di Pino et al., 2014). Typically, studies applied either excitatory rTMS directly over M1i to increase intrahemispheric excitability, or inhibitory rTMS over M1c to relieve inhibition on M1i (Auriat et al., 2015; Meehan et al., 2011). A previous meta-analysis showed that inhibitory rTMS over the M1c either in isolation or following therapy/motor training improved function and enhanced M1i excitability post-stroke (Hsu et al., 2012). However, response to rTMS protocols is highly variable in those with stroke (Auriat et al., 2015; Brodie et al., 2014; Carey et al., 2014), let alone in healthy individuals (Hamada et al., 2013). The vast majority of research employing rTMS delivered stimulation over M1 (Ameli et al., 2009; Auriat et al., 2015; Carey et al., 2014; Corti et al., 2012; Di Pino et al., 2014; Hsu et al., 2012; Khedr, 2005; Kim et al., 2006; Lee et al., 2015; Lüdemann-Podubecká et al., 2015; Malcolm et al., 2007; Takeuchi et al., 2005; Talelli et al., 2012; Tretriluxana et al., 2013), with a small number of studies considering the effects of applying rTMS over other cortical areas including the primary somatosensory cortex (S1) (Brodie et al., 2014; Meehan et al., 2011).
Previous work demonstrated the importance of somatosensory feedback during motor control and learning (Boyd et al., 2010; Debas et al., 2010; Pavlides et al., 1993; Vidoni et al., 2010). In humans, S1 is shown to be integral to the control of both unimanual and coordinated bimanual movements (Andres et al., 1999; Geffen et al., 1994; Sadato et al., 1996). Further, anatomical connections via the callosum are documented between the sensory cortices (Aboitiz, 1992; Fling et al., 2013; Hofer and Frahm, 2006), and there is evidence for S1 to S1 IHI (Brodie et al., 2014; Ragert et al., 2011). Therefore, altered patterns of excitability between the two S1 hemispheres may contribute to diminished paretic UL function after stroke. In fact, preliminary work from our group showed that both motor skill performance and paretic UL function are enhanced when continuous theta burst stimulation (cTBS) is delivered over contralesional S1 (S1c) and paired with skilled motor practice (Meehan et al., 2011). Thus, modulation of S1c excitability may be another approach to altering IHI between the sensorimotor cortices. In turn, this could enhance the response to skilled motor practice in individuals with chronic stroke.
Taken together, we hypothesized that variability in response to rTMS may be due to the region stimulated (e.g. M1, S1), and whether the aftereffects of rTMS are exploited by pairing skilled motor practice with stimulation (Brodie et al., 2014; Meehan et al., 2011). Our preliminary data for the current study demonstrated promising outcomes of cTBS over M1c and S1c followed by skilled motor practice compared to sham cTBS in individuals with chronic stroke (Meehan et al., 2011). Specifically, cTBS over M1c followed by skilled motor practice resulted in reductions in peak velocity and acceleration, whereas cTBS over S1c resulted in reductions in movement initiation time and motor function scores (Meehan et al., 2011a). In the current study we aimed to advance understanding of our previous work by: (1) investigating the effects of an intervention that delivered cTBS over M1c, S1c or sham cTBS, (2) pairing stimulation with skilled motor practice using the paretic UL, (3) increasing practice dose to five sessions, and (4) increasing the sample size. First, we hypothesized that cTBS over M1c or S1c followed by skilled motor practice would enhance motor learning and paretic UL function and that these improvements would be greater than those seen in the sham cTBS group. Second, based on our previous work (Brodie et al., 2014; Meehan et al., 2011), we hypothesized that cTBS facilitated motor learning would transfer to generalized improvements in paretic arm function, when compared with sham cTBS (Meehan et al., 2011). Third, we sought to understand potential changes in tactile somatosensory function, as well as corticospinal, intracortical and interhemispheric excitability associated with the intervention. We expected that the iSP would be decreased from the contralesional hemisphere after cTBS over M1c or S1c as compared to sham cTBS.
2. Methods
2.1. Participants
Thirty-seven individuals with first-time clinically diagnosed stroke in the chronic stage of recovery (≥6 months) participated (mean age = 65.7 ± 10.8 years; 14 females). These participants were enrolled in a registered clinical trial that can be found on clinicaltrials.gov (clinical registry trial number: NCT01371409 (https://register.clinicaltrials.gov/)). Fig. 1 displays the CONSORT participant flow chart for this clinical trial. The clinical research ethics board of the University of British Columbia approved this work. Participants provided written informed consent, in accordance with the Declaration of Helsinki. Participants were not enrolled if they: (1) had a Montreal Cognitive Assessment (MoCA) score ≤24, (2) any contraindications to TMS, or (3) were taking any GABAergic, NMDA-receptor agonist/antagonist or other drug known influence the neural receptors that modulate neuroplasticity.
Fig. 1.
CONSORT Participant flow chart. Fifty-five individuals were initially contacted to participate in the study. Participants were community dwelling individuals with chronic stroke who had previously consented to be contracted for participation in research studies. Fourteen individuals were excluded once assessed for eligibility. Forty-one individuals were then pseudo-randomized (described in manuscript text) into the stimulation groups (cTBS over M1c, S1c or Sham). In the M1c cTBS group, one individual could not complete the intervention due to family issues. In the S1c cTBS group, one individual did not complete the serial tracking task (STT) due to a lack of endurance to complete all practice days. In the Sham cTBS group, one individual did not complete the STT due to a lack of endurance to complete all practice days, and one other individual did not complete the intervention due to technical issues. All remaining individuals completed all post-intervention measures, were analysed and included in the final data set (cTBS over M1c n = 12; cTBS over S1c n = 13; Sham cTBS n = 12).
2.2. Upper-limb motor and somatosensory assessments
Physical impairment was measured via the UL portion of the Fugl Meyer (FM) (Fugl-Meyer et al., 1975) assessment and motor function of the UL was tested using the Wolf Motor Function Test (WMFT)-rate. Both were administered by a physical therapist who was blind to group assignment (Hodics et al., 2012). The WMFT-rate is a valid and sensitive measure of paretic UL motor function in participants with stroke that is less likely to be affected by ‘ceiling’ effects (Hodics et al., 2012). To assess somatosensory function we employed the Semmes-Weinstein monofilament test, testing perceptual sensation thresholds summed at the dorsum, thenar and hypothenar regions of both hands (Novak et al., 1993).
2.3. Serial targeting task
Participants performed the serial targeting task (STT) (Brodie et al., 2014; Mang et al., 2016; Meehan et al., 2011) with the paretic hand (pronated) grasping a computer mouse (housed in a custom frame) to control the movements of an on-screen cursor (Fig. 2B,C). The custom frame was built to afford participants with more severe impairment (FM < 30) the ability to move the custom computer mouse with their paretic UL. Individuals were instructed to: “move the cursor as quickly and accurately as possible” to hit a series of sequentially appearing targets. Vision of the participant’s UL during practice was occluded to direct attention to the cursor representation of paretic hand position and to rely on somatosensory, rather than visual, feedback of arm position (Brodie et al., 2014; Meehan et al., 2011). Embedded within the series of targets was a repeated six-element sequence that was flanked by a seven-element random sequence. Each block contained nine random sequences, and eight repeated sequences. Participants performed: (1) one block of the STT during baseline testing (early practice); (2) 5 days of practice where cTBS was delivered according to group followed by 4 blocks of STT; and (3) one block of STT on a separate no-cTBS retention test day to index motor learning (Brodie et al., 2014; Mang et al., 2016; Meehan et al., 2011)
Fig. 2.
Experimental Procedure. A. Participants performed 9 experimental sessions on separate days over a 2-week period. Session 1: participants underwent a T1-weight anatomical MRI scan, clinical assessments of motor impairment (Fugl Meyer Upper Extremity (FM)), and early practice performance on the serial targeting task (STT). Session 2: participants completed pre-intervention assessments of somatosensory function using the Semmes-Weinstein monofilament test, motor function using the Wolf Motor Function Test (WMFT)-rate, pre-intervention bilateral (when possible) assessment of short-interval intracortical inhibition (SICI), intracortical facilitation (ICF) and transcallosal inhibition (TCI) and baseline STT motor task performance. Sessions 3–7: participants received continuous theta burst stimulation (cTBS) over the contralesional hemisphere according to group (M1/S1/Sham) immediately prior to STT practice. Session 8: STT retention test was employed with no cTBS to assess motor learning. Session 9: post-training assessments of WMFT-rate, monofilament test and AMT, RMT, SICI, ICF and TCI bilaterally. B. Schematic of the STT, a sample progression of targets, and illustration of a path of movements between 2 targets. C. Depiction of the adapted mouse used during STT practice.
2.4. Magnetic resonance imaging
Magnetic resonance images were collected at the UBC MRI Research Centre on a Philips Achieva 3.0 T whole-body MRI scanner (Phillips Health-care, Andover, MD, USA) using an eight-channel sensitivity encoding head coil (SENSE factor = 2.4) and parallel imaging. A high-resolution T1-weighted anatomical scan (TR = 7.47 ms, TE = 3.65 ms, flip angle θ = 6°, FOV = 256 × 256 mm, 160 slices, 1 mm3 isotropic voxel) was collected for use during TMS targeting during neurophysiological assessment and cTBS delivery.
2.5. Electromyography
Electromyography (EMG) was recorded bilaterally from participants’ extensor carpi radialis (ECR) muscle with 1 cm diameter circular surface recording electrodes (Covidien, Mansfield, MA). EMG data were collected using LabChart software (LabChart 7.0, AD Instruments, Colorado Springs, Colorado, USA). EMG signals were sampled at 2000 Hz, pre-amplified (1000×) and band-pass filtered at 10–1000 Hz using a Powerlab data acquisition system and two bioamplifiers (AD instruments, Colorado Springs, CO). Data were recorded in a 450 ms sweep from 100 ms before to 350 ms after TMS onset.
2.6. Experimental procedure
Participants were pseudo-randomly assigned to one of three groups while accounting for age, sex and FM score to ensure even distribution: (1) cTBS over M1c (n = 12), (2) cTBS over S1c (n = 13) or (3) Sham cTBS over M1c (n = 12). Figure 2A outlines the experimental procedure, which contained 9 sessions across a 3-week period. In Session 1, participants completed a T1-weight anatomical MRI scan, clinical assessments of motor impairment (FM), and early practice performance on the STT. During session 2, participants completed pre-intervention assessments of somatosensory function using the monofilament test (Novak et al., 1993), motor function (WMFT), pre-intervention bilateral assessment of short-interval intracortical inhibition (SICI), intracortical facilitation (ICF) and the iSP and baseline STT motor task performance. During sessions 3–7, participants received cTBS over the contralesional hemisphere according to group immediately prior to STT practice. Session 8 occurred 24-hrs following the last day of practice, and contained a no cTBS retention test of the STT to assess motor learning. Session 9 included post-training assessments of WMFT, monofilament test and neurophysiological assessments.
2.7. Transcranial magnetic stimulation and continuous theta burst stimulation
Single and paired-pulse TMS was delivered using a figure-of-eight shaped coil (Magstim 70 mm P/N 9790, Magstim Co., UK) connected to a Magstim 2002 stimulator (Magstim Co., UK) with participants sitting. Individual participant MRI scans were used for TMS targeting and position monitoring using Brainsight™ neuronavigation software package, except in 7 participants who had contraindications to MRI (e.g. surgical stent). A standard anatomical template brain was used for coil position monitoring (Rogue Research Inc., Montreal, QC, Canada) for these individuals. The ‘hotspot’ for eliciting motor evoked potentials (MEPs) in the contralateral ECR was found by positioning the coil over the scalp region overlying the hand/forearm M1 representation (Yousry et al., 1997) and standard procedures for determining resting motor threshold (RMT) (Rossini et al., 1999) and active motor threshold (AMT) (Rothwell et al., 1999) were performed. ECR was chosen as contractures after stroke make it difficult to localize a ‘hotspot’ for intrinsic hand muscles.
The paired-pulse paradigms, SICI and ICF, were performed as previously described (Kujirai et al., 1993). The interstimulus interval (ISI) for SICI and ICF was 2 and 12 ms to measure intracortical inhibitory and facilitatory circuits, respectively (Kujirai et al., 1993). Conditioning stimulus (CS) intensity was set at 90% of AMT for SICI and ICF. Test stimulus (TS) intensity was adjusted to consistently evoke MEPs in the contralateral ECR of 1 ~mV. SICI and ICF were expressed and analysed as a ratio of CT + TS over TS MEP amplitude (mV), where smaller values represent greater inhibition and larger values represent enhanced facilitation, respectively (Kujirai et al., 1993).
For iSP assessment, participants maintained a unilateral isometric hand-grip contraction of 50% maximum force output. Concurrently, twelve single TMS pulses were delivered at 150% RMT over the ECR hotspot ipsilateral to the muscle contraction. Where no ipsilesional MEP was identified, TMS pulses were delivered at 80% maximum stimulator output. The iSP was defined as the transient reduction in volitional EMG activity elicited by TMS applied over M1 ipsilateral to the active muscle (Mang et al., 2015; Neva et al., 2016). Calculation of the iSP was performed as described previously (Mang et al., 2015). Specifically, the iSPmean was expressed as a ratio of the mean pre-stimulus EMG (iSPmean/pre-stimmean), where a lower value indicates more inhibition. Custom MATLAB scripts (Math-works, Natick, MA) were used to analyse EMG data.
Delivery of cTBS was performed as described previously (Hamada et al., 2013; Huang et al., 2005) with a Magstim SuperRapid stimulator with a cooled 7 cm figure-of-eight coil (Magstim Co., UK), and applied at 80% AMT. S1c location was determined to be 2 cm posterior to the M1c hotspot and confirmed using Brainsight™ (Brodie et al., 2014). Sham cTBS was delivered over M1c with a dedicated coil that looked and sounded like active stimulation but did not induce any current. M1c, S1c and sham stimulation were localized and real-time position monitored using Brainsight™ software.
2.8. STT performance and exponential curve fitting
The primary dependent measure for motor learning of the STT was response time total (RTT). Response time for each trial was calculated from when the visual target appeared, to when the participant reached the visual target, which encompasses reaction time plus movement time. The sum of response times for all six movements within the repeated and random sequences was calculated to generate RTT. To assess motor learning, RTT for the baseline performance was compared to the retention-test performance for both sequences (random, repeated). For assessment of overall performance, the RTT for repeated and random sequences in each block across the seven sessions (baseline STT performance, Session 1; two to six practice sessions; and retention-test, Session 7) of task performance for each participant were subsequently fit to separate exponential function using the following equation (Wadden et al., 2017).
| Equation 1 : |
E(RTTN) is the expected value of RTT on practice trial N. A is the expected values of RTT after practice has been completed (asymptote parameter). B is the expected change in RTT from Session 1 to Session 7 (change score parameter). Alpha (α) is the exponential motor skill acquisition rate parameter (Wadden et al., 2017). Our dependent measure was the B-score derived from the exponential curve fitting, which demonstrates change in performance across all motor practice days (baseline STT performance, the 5 days of cTBS + STT practice, and retention). We use the exponential fitting curve as a method to incorporate all of the trials across STT practice, and to quantify how previous practice trials influence latter practice trials, providing an inclusive assessment of the motor learning processes (Wadden et al., 2017). Custom MATLAB (Version R2013b, Mathworks Inc., Natick, Massachusetts) scripts were used for all STT analyses.
2.9. Statistical analysis
Mixed model analysis of variance (ANOVA) was used for statistical analysis, unless otherwise indicated. Post hoc analyses were performed using Tukey’s HSD where appropriate. Statistical significance was set to p≤.05. Effect sizes were calculated and reported on the strength of effects were interpreted based on previously developed guidelines (Cohen, 2013). All statistical procedures were conducted using SPSS (SPSS 23.0). Statistical analyses of changes in STT performance and WMFT-rate scores were assessed as primary outcomes. Potential changes in bilateral corticospinal, intracortical excitability and TCI were tested as secondary outcomes. Statistical analyses for each dependent measure are described in more detail below.
2.10. STT performance
In order to assess baseline STT performance, a two-way mixed model ANOVA was performed with within-subjects factor SEQUENCE (repeated, random) and between-subjects factor STIMULATION-GROUP (M1c, S1c, Sham) with mean RTT as the dependent variable.
To assess differences between baseline STT performance with retention performance, a three-way mixed-model ANOVA was performed with within-subjects factors TIME (baseline STT performance, retention), SEQUENCE (repeated, random) and between-subjects factor STIMULATION GROUP (M1c, S1c, Sham) with mean RTT as the dependent variable.
To test performance improvement across all practice days (baseline STT performance, 5-days of cTBS + STT practice, and retention) using the exponential curve fitting function, a 2-way mixed model ANOVA was performed with within-subjects factors SEQUENCE (repeated, random) and between-subjects factor STIMULATION-GROUP (M1c, S1c, Sham) with the B-score as the dependent variable.
2.11. Motor function of the upper-limb
To assess baseline WMFT-rate to ensure there was no difference between groups, a one-way ANOVA was performed with between-subjects factor STIMULATION-GROUP (M1c, S1c, Sham) with mean WMFT-rate as the dependent variable.
To test whether motor function improved after multiple sessions of cTBS+STT, a two-way mixed-model ANOVA was performed with within-subjects factor TIME (pre-intervention, post-intervention) and between-subjects factor STIMULATION-GROUP (M1c, S1c, Sham) with mean WMFT-rate as the dependent variable.
2.12. Somatosensory function of the upper-limb
To ensure there was no difference between groups at baseline in perceptual sensation thresholds, a one-way ANOVA was performed with between-subjects factor STIMULATION-GROUP (M1c, S1c, Sham) with the sum of the monofilament recording scores at the three sites (thenar, hypothenar, dorsum of hand) as the dependent variable.
To assess whether perceptual sensation thresholds changed after multiple sessions of cTBS+STT, a two-way mixed-model ANOVA was performed with within-subjects factor TIME (pre-intervention, post-intervention) and between-subjects factor STIMULATION-GROUP (M1c, S1c, Sham) with monofilament recordings at the three sites (thenar, hypothenar, dorsum of hand) summed as the dependent variable.
2.13. Corticospinal and intracortical excitability, and transcallosal inhibition
To determine whether measures of corticospinal excitability or transcallosal inhibition were modulated after multiple sessions of cTBS+STT, three-way mixed-model ANOVAs were performed with within-subjects factors HEMISPHERE (ipsilesional, contralesional), TIME (pre-intervention, post-intervention) and between-subjects factor STIMULATION-GROUP (M1c, S1c, Sham) for each dependent variable of RMT, AMT, SICI, ICF and iSP. For RMT, AMT, SICI and ICF there were 7 individuals where a MEP could not be elicited over M1i, and were therefore not included in the statistical analysis. For iSP, 80% MSO was used on those without a M1i MEP, therefore, all individuals were included in the analysis.
2.14. Associations between motor learning, motor function, and transcallosal inhibition
Testing our secondary hypothesis that M1c and S1c cTBS facilitated motor learning would transfer to generalized improvements in paretic arm function, bivariate correlational analyses (Pearson’s rp for normally distributed data or Spearman’s rs for non-normally distributed data) were conducted. Correlational analyses were used to characterize relationships between change in motor learning performance (STT B-score) and change in motor function (WMFT-rate) for each intervention type (M1c, S1c, Sham paired with STT practice). Correlational analyses were also used to investigate whether modulation of iSP accounted for variance of change in motor learning performance (STT B-score) and motor function (WMFT-rate). Statistical significance was set to p≤.05. Based on our results in the M1c group, we performed a stepwise regression model to examine the contribution of our different dependent variables (age, post-stroke duration (PSD)), FM, repeated-sequence STT B-score, random-sequence STT B-score, change in contralesional-iSP, and change in ipsilesional-iSP) that accounted for unique variance in our primary outcome of improvement in paretic motor function (change in WMFT-rate). We did not correct for multiple comparisons on the principle that the restrictiveness of Bonferroni correction could hinder exploratory studies with low participant numbers (Pocock, 1997). The stepping criteria for regression analysis was p≤.05 to add and p>.1 to remove variables.
3. Results
3.1. Demographic information
Table 1 displays all the demographic information. Of note, there was a relationship between FM score and age for our sample of stroke participants (rs = 0.359, p = 0.029).
Table 1.
Demographic information for all participants
| ID | Stim. Group | Sex | Age (y) | PSD (mo) | MOCA | Lesion location (C/SC) | FM (/66) | Pre paretic WMFT-rate (#/60s) | Paretic Limb |
|---|---|---|---|---|---|---|---|---|---|
| S01 | M1c | M | 69 | 68 | 24 | - | 54 | 29.27 | R |
| S02 | M1c | M | 59 | 270 | 28 | C | 55 | 23.98 | L |
| S03 | M1c | M | 64 | 35 | 25 | - | 31 | 16.6 | R |
| S09 | M1c | F | 50 | 37 | 26 | SC | 63 | 58.2 | L |
| S14 | M1c | M | 65 | 67 | 30 | C | 62 | 44.4 | R |
| S18 | M1c | M | 64 | 94 | 26 | C | 56 | 46.9 | R |
| S19 | M1c | M | 82 | 12 | 26 | SC | 59 | 46.4 | R |
| S27 | M1c | M | 62 | 20 | 26 | SC | 62 | 58.7 | L |
| S28 | M1c | M | 57 | 22 | 26 | SC | 16 | 16.9 | L |
| S31 | M1c | F | 72 | 308 | 24 | Cerebellar | 44 | 20.2 | L |
| S33 | M1c | F | 57 | 160 | 25 | C | 30 | 11.9 | R |
| Mean | M1c | 3 F | 62.25 | 107.42 | 25.75 | 5 C | 47.75 | 33.5 | 6 L |
| SD | M1c | 9.65 | 102.2 | 1.86 | 15.34 | 16.6 | |||
| S04 | S1c | M | 67 | 82 | 25 | SC | 59 | 62.9 | R |
| S05 | S1c | M | 73 | 142 | 27 | SC | 60 | 57.6 | L |
| S06 | S1c | F | 71 | 83 | 27 | SC | 56 | 52.0 | R |
| S07 | S1c | M | 85 | 35 | 25 | SC | 60 | 34.5 | R |
| S11 | S1c | M | 76 | 81 | 29 | SC | 62 | 64.1 | R |
| S12 | S1c | M | 71 | 47 | 24 | - | 46 | 39.4 | L |
| S17 | S1c | M | 71 | 37 | 25 | - | 38 | 38.1 | R |
| S22 | S1c | M | 60 | 23 | 29 | SC | 54 | 39.9 | L |
| S23 | S1c | M | 57 | 94 | 24 | C | 7 | 9.2 | R |
| S25 | S1c | M | 55 | 24 | 21 | C | 62 | 59.2 | L |
| S32 | S1c | F | 33 | 33 | 29 | SC | 18 | 19.8 | L |
| S34 | S1c | M | 69 | 31 | 27 | SC | 62 | 55.6 | R |
| Mean | S1c | 2 F | 66.46 | 59.13 | 26.08 | 2 C | 49.23 | 44.86 | 6 L |
| SD | S1c | 12.97 | 35.23 | 2.36 | 17.86 | 16.77 | |||
| S08 | Sham | M | 63 | 41 | 28 | SC | 23 | 10.0 | R |
| S10 | Sham | F | 56 | 27 | 30 | SC | 35 | 38.4 | L |
| S13 | Sham | M | 71 | 20 | 28 | SC | 58 | 42.7 | R |
| S15 | Sham | M | 64 | 46 | 29 | - | 51 | 33.7 | R |
| S16 | Sham | M | 76 | 455 | 26 | SC | 49 | 34.0 | R |
| S20 | Sham | M | 69 | 15 | 28 | SC | 57 | 52.9 | R |
| S21 | Sham | M | 71 | 34 | 28 | - | 61 | 54.1 | R |
| S24 | Sham | M | 79 | 18 | 24 | SC | 61 | 45.0 | L |
| S29 | Sham | M | 68 | 15 | 29 | Cerebellar | 66 | 62.8 | L |
| S30 | S1c | M | 76 | 57 | 27 | - | 56 | 50.9 | L |
| S35 | Sham | F | 51 | 47 | 26 | SC | 29 | 18.8 | R |
| S36 | Sham | F | 67 | 200 | 29 | Cerebellar | 60 | 54.7 | L |
| S37 | Sham | M | 83 | 27 | 21 | SC | 57 | 55.0 | L |
| Mean | Sham | 3 F | 68.17 | 78.75 | 27.17 | 0 C | 50.58 | 41.84 | 5 L |
| SD | Sham | 9.06 | 128.77 | 2.55 | 13.99 | 15.8 | |||
| Mean | Total | 8 F | 65.6 | 81.2 | 26.3 | 7 C | 49.1 | 40.2 | 17 L |
| SD | Total | - | 10.8 | 95.3 | 2.3 | - | 15.5 | 16.7 | - |
Displayed are the participants, stimulation group assignment (contralesional motor cortex (M1c), contralesional somatosensory cortex (S1c) and Sham), sex (male (M) or female (F)), age (in years), post-stroke duration (PSD) in months (mo), Montreal Cognitive Assessment (MOCA) score out of 30, lesion location (cortical (C), subcortical (SC), or cerebellar), Fugl-Meyer (FM) out of 66, pre-intervention paretic Wolf Motor Function Test (WMFT)-rate (# of reps/60s) and paretic limb (left (L) or right (R)). Data shown are mean ± standard error of the mean (SEM).
3.2. Normality
Following Shapiro-Wilk testing performed with a significance level set at p < 0.001 (Gamst et al., 2008), all variables (pre and post-intervention) were found to be normally distributed (W(37) ≥0.936, p≥0.001), except for non-paretic limb WMFT-rate (W(37) ≥0.802, p < 0.001), FM (W(37) = 0.829, p < 0.001), post-stroke duration (W(37) = 0.756, p < 0.001), sensory perception threshold score for the paretic limb pre-intervention (W(38) = 0.764, p < 0.001), and SICI and ICF post-intervention (W(21) ≥0.551, p < 0.001). The non-normal data were log-transformed for statistical analysis.
3.3. STT performance
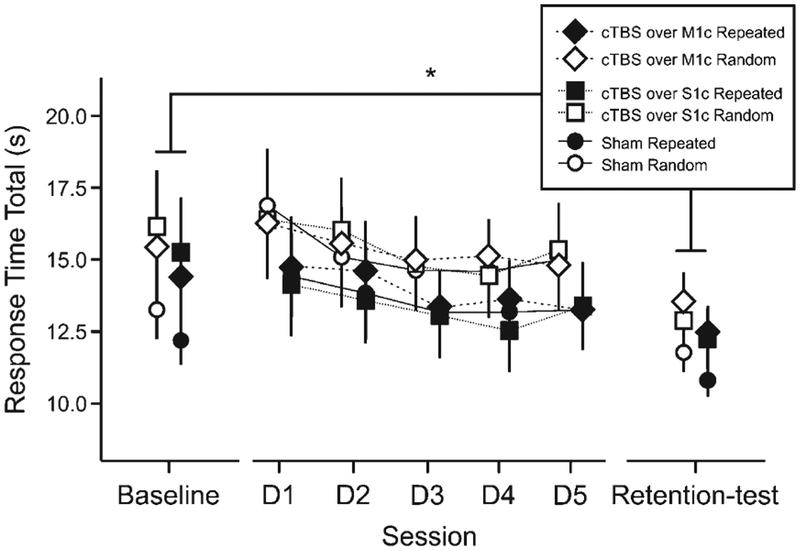
At baseline, two-way ANOVA revealed a main effect of SEQUENCE (F1,34 = 7.107, p = 0.012, η2partial = 0.173), and no effect of STIMULATION GROUP or interaction between factors. A three-way ANOVA assessed whether there were difference between baseline STT and retention performance and showed a main effect of TIME (F1,34) = 15.01, p = 0.0005, η2partial = 0.306) and a main effect of SEQUENCE (F1,34) = 19.96, p = 0.00008, η2partial = 0.370), but no effect of STIMULATION GROUP or an interaction between factors (Fig. 3).
Fig. 3.
Serial Tracking Task (STT) performance. All data points represent mean reaction total time (s) for each time-point (baseline STT performance, 5 practice days, and retention), sequence (repeated, random) and each stimulation group (M1c/S1c/Sham). D0 = baseline STT performance, D1–5: cTBS + STT practice days), D6 = retention test. Bars represent standard error, * p < 0.05.
Motor skill performance parameters across all motor practice sessions (including baseline STT performance, the 5 cTBS+STT practice days, and retention) were assessed using the B-score with a two-way ANOVA that revealed no main effect of SEQUENCE or STIMULATION GROUP, and no interaction.
3.4. Motor function of the upper-limb
One-way ANOVA showed no baseline differences in WMFT-rate among stimulation groups for both the paretic and non-paretic ULs. However, three-way ANOVA revealed a main effect of TIME (F1,34) = 26.754, p = 0.00001, η2partial = 0.35), where all individuals increased the WMFT-rate regardless of intervention type. Unsurprisingly, there was a main effect of LIMB (F1,2) = 18.281, p = 0.0001, η2partial = 0.44), such that the paretic limb had lower motor function (Fig. 4A).
Fig. 4.
Motor Function as assessed by the Wolf Motor Function Test (WMFT)-rate and Transcallosal Inhibition (TCI). Bar graphs represent average WMFT-rate across each stimulation group (M1c/S1c/Sham), time-point (pre and post intervention) and UL (paretic and nonparetic). Bar graphs represent average % pre-stimulus mean EMG across each stimulation group (M1c/S1c/Sham), time-point (pre and post intervention) and hemisphere (ipsilesional and contralesional). Bars represent standard error, * p < 0.05.
3.5. Somatosensory perception threshold
One-way ANOVA showed no baseline difference between stimulation groups in either the paretic or non-paretic ULs. Three-way ANOVA revealed a main effect of LIMB (F1,33) = 4.72, p = 0.037, η2partial = 0.125), demonstrating decreased somatosensory function in the paretic hand, with no other effects found.
3.6. Corticospinal, intracortical excitability, and transcallosal inhibition
For RMT, three-way ANOVA revealed no effect of TIME (F1,26) = 0.430, p = 0.518, η2partial = 0.016), HEMISPHERE (F1,26) = 2.833, p = 0.104, η2partial = 0.098), STIMULATION GROUP (F1,26) = 0.737, p = 0.488, η2partial = 0.054) or interaction between factors (all ps > 0.190). For AMT, three-way ANOVA revealed no effect of TIME (F1,27) = 0.052, p = 0.822, η2partial = 0.002), HEMISPHERE (F1,27) = 0.504, p = 0.484, η2partial = 0.018), STIMULATION GROUP (F1,27) = 2.963, p = 0.07, η2partial = 0.180) or interaction between factors (all ps > 0.1). There were no effects on SICI and ICF (see Table 2).
Table 2.
Measures of corticospinal excitability, intracortical excitability and transcallosal inhibition
| Stimulation Group Measure | Pre NL (mean ± SEM) |
Pre L (mean ± SEM) |
Post NL (mean ± SEM) |
Post L (mean ± SEM) |
|---|---|---|---|---|
| M1c | ||||
| RMT (%MSO) | 47.6±2.2 | 48.9±3.8 | 46.5±2.6 | 50.2±3.6 |
| AMT (%MSO) | 42±3.2 | 45±4.3 | 40.6±2.3 | 43.4±3.4 |
| SICI (CS+TS/TS) | 0.73±0.11 | 0.74±0.11 | 0.75±0.13 | 0.67±0.21 |
| ICF (CS+TS/TS) | 1.41±0.24 | 1.03±0.11 | 1.42±0.27 | 0.62±0.32 |
| TCI (iSPmean) | 0.67±0.04 | 0.69±0.03 | 0.71±0.04 | 0.72±0.04 |
| S1c | ||||
| RMT (%MSO) | 43.9±1.7 | 45.5±2.7 | 36.9±2.3 | 46.1±3.4 |
| AMT (%MSO) | 36.9±1.8 | 46.6±6.7 | 35.8±1.5 | 38.9±3.0 |
| SICI (CS+TS/TS) | 0.70±0.12 | 0.82±0.06 | 0.73±0.11 | 0.78±0.11 |
| ICF (CS+TS/TS) | 1.55±0.20 | 1.16±0.14 | 1.43±0.11 | 1.50±0.19 |
| TCI (iSPmean) | 0.70±0.03 | 0.70±0.02 | 0.77±0.04 | 0.74±0.03 |
| Sham | ||||
| RMT (%MSO) | 48.6±2.4 | 53.0±4.3 | 49.4±2.3 | 52.3±3.8 |
| AMT (%MSO) | 44.8±2.3 | 49.4±4.3 | 43.8±2.7 | 45.6±3.7 |
| SICI (CS+TS/TS) | 0.72±0.13 | 0.91±0.19 | 0.74±0.09 | 1.38±0.46 |
| ICF (CS+TS/TS) | 1.33±0.22 | 1.10±0.25 | 1.72±0.38 | 1.14±0.29 |
| TCI (iSPmean) | 0.69±0.04 | 0.64±0.05 | 0.76±0.03 | 0.72±0.04 |
Displayed are mean data across all stimulation groups pre and post intervention. NL = Non-lesioned hemisphere; L = Lesioned hemisphere; M1c = Contralesional primary motor cortex; S1c = Contralesional primary somatosensory cortex; Sham = Sham contralesional cTBS; RMT = Resting motor threshold; AMT = Active motor threshold; SICI = Short-interval intracortical inhibition; ICF = Intracortical facilitation; TCI = Transcallosal inhibition; %MSO = Percentage of maximum stimulator output; mV = millivolt; iSPmean = ipsilateral silent period as percentage of pre-stimulus EMG mean. Data shown are mean ± standard error of the mean (SEM).
For iSP, three-way ANOVA revealed a main effect of TIME (F1,32) = 7.049, p = 0.012, η2partial = 0.181), with no other effects. This suggests that there was a decrease in iSP bi-directionally after the STT practice regardless of stimulation type (Fig. 4B).
3.7. Associations between change in motor skill practice and paretic arm function
Change in STT performance (B score) for both the repeated (rp = 0.650, p = 0.022) and random (rp = 0.752, p = 0.005) sequences correlated with change in WMFT-rate of the paretic arm for the cTBS over M1c + STT group only (Fig. 5). Within the M1c stimulation group, the stepwise regression model identified change in STT performance in the random sequence (R2 change 0.566, p = 0.005) accounted for56.6% of variance in the change in paretic WMFT-rate.
Fig. 5.
Scatterplots of association between ΔWMFT-rate and B-score of STT performance. (A–C) Relationships between ΔWMFT-rate and B-score of STT repeated-sequence performance for cTBS over M1c (A), S1c (B) and Sham (C). (D–F) Relationships between ΔWMFT-rate and B-score of STT random-sequence performance for cTBS over M1c (D), S1c (E) and Sham (F). R2-linear is displayed for each relationship, * p < 0.05.
4. Discussion
We demonstrated cTBS over the contralesional sensorimotor cortex did not further enhance our primary outcome measures of motor function or skilled motor task performance, or reduce a secondary outcome measure of iSP beyond that of sham cTBS. Of note, we found enhanced paretic arm function (WMFT-rate), improved performance of a skilled motor learning task (STT), and decreased iSP bilaterally, regardless of stimulation group. Additionally, we found individuals who received M1c cTBS prior to motor practice showed an association between change in STT performance and altered paretic arm function (WMFT-rate). This finding suggests that change in performance of the STT scaled with WMFT-rate, and may indicate that improvement in motor skill contributed to motor function for some individuals following cTBS over M1c even in the chronic stage of stroke recovery. The current results should be taken into consideration when developing rTMS interventions in chronic stroke.
4.1. Improved STT performance regardless of prior cTBS over M1c, S1c or Sham
Across groups, individuals learned the STT; cTBS over M1c or S1c before practice did not enhance this effect. There are several possible explanations for these findings.
Mounting evidence demonstrates a high degree of variability in response to cTBS in healthy individuals (Hamada et al., 2013; Opie et al., 2017) and those with stroke (Talelli et al., 2007; Yamada et al., 2014). In healthy individuals, the expected response to cTBS occurs approximately 1/3 of the time (Hamada et al., 2013). Here, the primary purpose of contralesional sensorimotor cortex cTBS was to enhance ipsilesional cortical excitability prior to motor skill practice via the suppression of contralesional excitability. In theory, this approach reduces interhemispheric inhibition on the ipsilesional sensorimotor cortex (Auriat et al., 2015; Meehan et al., 2011). It is possible that the lack of a significant group effect of M1c or S1c cTBS on motor learning was due to variability in the response to cTBS (Hamada et al., 2013). Future work could comprehensively assess M1 neurophysiology (e.g. I-wave recruitment) of individuals with stroke in order to begin to predict individual response.
Though a single session cTBS over M1c can reduce MEP amplitude in the non-paretic hand, this does not necessarily alter motor behaviour or electrophysiological measures in the paretic hand (Talelli et al., 2007). It is possible that cTBS over the contralesional cortex did not alter behaviour beyond performance of the skilled task itself (sham). It is also possible that the dose of cTBS paired with skilled motor practice was either too low to produce significant changes in motor learning, or too high leading to ‘ceiling’ effects. Past work showed that three sessions of cTBS over M1c or S1c followed by skilled motor practice enhanced motor learning (Meehan et al., 2011). Therefore, it is possible that cTBS was effective for a shorter amount of training sessions, but with an increased dosage of motor practice there was no additional effect of stimulation. Finally, it may be that stronger effects would be present after a greater delay (1 week or 1 month) following the final practice session. Previous work showed that 5 sessions of rTMS applied over M1i produced greater improvement in motor function compared to sham stimulation at the 1 month to 1 year follow up measurements of motor function (Khedr et al., 2010). However, we did not measure these later time points post-intervention.
There is substantial evidence that the contralesional hemisphere may compensate for lost neurophysiological and motor function in individuals with severe impairment (Auriat et al., 2015; Hayward et al., 2017; Mang et al., 2015). In fact, some individuals with chronic stroke display increased TCI from the M1i to M1c (Mang et al., 2015), and this is associated with less impairment and better function (Mang et al., 2015). The current study corroborates these previous results, demonstrating that certain individuals show enhanced motor learning and function from M1c cTBS followed by motor skill practice, while others fail to benefit, or demonstrate a detriment. Future research should consider building personalized neurophysiological profiles to more effectively apply rTMS.
4.2. Improved motor function irrespective of prior cTBS over M1c, S1c or Sham
Our results suggest that with increased use of the paretic UL WMFT-rate scores improved regardless of stimulation. These findings agree with a meta-analysis showing that increasing skilled movement can improve UL function, even in the chronic stage after stroke (Lohse et al., 2014). Previous work focused on the effects of increased dosage of physical and occupational therapy in the chronic stage of stroke recovery, here we extend this finding to demonstrate that skilled motor practice even of well-controlled laboratory task can transfer to improved paretic UL function. These results demonstrate the potential usefulness of motor sequence tasks for increasing paretic UL use and perhaps improving UL function.
4.3. Decreased TCI bilaterally regardless of prior cTBS over M1c, S1c or sham
Our measure of interhemispheric inhibition (iSP) decreased bilaterally after the intervention regardless of stimulation type. Contrary to our hypothesis, we did not find a lasting decrease in iSP due to cTBS over M1c or S1c.
First, it is possible the lack of a greater decrease in TCI from M1c to M1i due to cTBS over M1c and S1c may relate to the inherent variability in response to cTBS (Hamada et al., 2013; Opie et al., 2017; Talelli et al., 2007; Yamada et al., 2014). If cTBS does not elicit the expected suppression of M1c corticospinal excitability, it follows that this may not translate to decreased TCI to M1i. Further, the neural pathways mediating corticospinal output and interhemispheric communication are likely distinct (Asanuma and Okuda, 1962; Chen et al., 2003; Ferbert et al., 1992; Meyer et al., 1998), and therefore cTBS over the corticospinal representation of contralateral UL may not necessarily translate to alterations in the ipsilateral UL cortical representation. Indeed, when cTBS is applied in healthy individuals there is variable modulation of contralateral M1 excitability (Neva et al., 2014; Stefan et al., 2008; Suppa et al., 2008). Future work could directly assess the acute effects of cTBS over M1c on TCI to M1i to characterize individual response after stroke.
Second, it is possible that skilled motor practice of the STT influenced the iSP bi-directionally. The iSP measures interhemispheric inhibition on the contralateral homologous M1 representation which is likely mediated by transcallosal pathways (Boroojerdi et al., 1996; Karni et al., 1998). In young healthy individuals, interhemispheric inhibition is modulated with mirror-visual feedback training with a unilateral limb (Avanzino et al., 2014) and UL bimanual movement training (Neva et al., 2015). Little work demonstrates changes in TCI after unilateral UL motor practice in healthy individuals or those with stroke. However, functional MRI research demonstrates enhanced bilateral activation in M1 during unilateral tasks that require a higher level of precision in healthy older individuals (Buetefisch et al., 2014). Further, there is evidence that performing complex unilateral tasks decreases interhemispheric inhibition during the planning stages of movements in healthy individuals (Wischnewski et al., 2016). Therefore, it is possible that unilateral paretic arm motor practice of a more complex task (the STT) may have altered interhemispheric communication between the motor cortices in our group of chronic stroke participants. Future work could investigate the modulatory effects of interhemispheric inhibition during more complex and simple motor tasks in those with acute and chronic stroke to advance our findings.
4.4. Association between change in STT performance and WMFT-rate
We found an association between STT performance of the repeated and random sequences and change in WMFT-rate only within the M1c cTBS group. Changes in random sequence performance accounted for greatest amount of unique variance in WMFT-rate change. This suggests that within the M1c cTBS group, change in performance of the STT scaled with WMFT-rate. Additionally, our data suggests that change in motor control, shown by performance of random sequences, may translate to change in motor function. Previous work demonstrates that those with chronic stroke consistently activate prefrontal cortices after practicing a motor sequence learning task. In contrast, healthy controls shift from prefrontal to premotor activation once the task is learned (Meehan et al., 2011). In the current study, it is possible that prefrontal regions were similarly engaged by random sequence performance, where target locations and sequences that are changing and challenging. However, as we did not test activity of these regions this interpretation is speculative.
4.5. Limitations
There are several limitations to this study. The sample of participants was heterogeneous. Participants demonstrated a large range of paretic UL impairment level (FM score of 7 to 66). While, heterogeneity in the sample could increase the generalizability of the results to a larger stroke population, it created challenges in delineating the relationships between behaviour and neurophysiology. We did not measure paretic UL impairment level (FM score) following the intervention, as skilled motor performance (STT) and motor function (WMFT-rate) were our primary outcome measures of interest, and individuals with chronic stroke generally show stable levels of impairment (Duncan et al., 1983; Xu et al., 2019). Future work could also measure paretic UL impairment following an intervention to provide insight as to whether change in motor skill or motor function coincided with reduced motor impairment. It is possible that the increase in WMFT-rate across all participants regardless of stimulation time was influenced by proximity of the pre- and post-intervention tests (3 weeks apart). Although previous work showed no significant increase in WMFT-performance with the paretic and non-paretic limbs when tested 12 to 16 days apart (Wolf et al., 2001), we cannot discount this possibility. We included 7 individuals without an ipsilesional MEP in our analysis. The lack of an ipsilesional MEP indicates diminished corticospinal functioning, which may have influenced the response to the cTBS over contralesional sensorimotor cortex. We elected to include these individuals in the study since they were able to perform the STT and therefore may have benefitted from our intervention; their inclusion also increase the potential generalizability of our study. Importantly, we had approximately even numbers of individuals without a M1i MEP in each of our stimulation groups (MEP absent by stimulation location: cTBS over M1c n = 2, cTBS over S1c n = 2, sham cTBS n = 3), thereby mitigating this potential confound. Our M1c cTBS group had a larger proportion of individuals with cortical stroke. It is possible this influenced our finding that there was not an overall benefit of cTBS delivered prior to skilled motor practice as previous work showed individuals with subcortical stroke may benefit more from rTMS (Ameli et al., 2009; Lee et al., 2015). Since our pseudo-randomization process did not control for the presence of cortical or subcortical stroke and due to participant drop-out, we were unable to avoid this confound. Future work could consider stratifying groups of participants with cortical and subcortical stroke to further investigate this issue. It is also possible that we were underpowered given the heterogeneity of our sample. Though we met the recruitment goals the numbers of participants was determined a priori using pilot data that could have contained less variability as compared to our actual sample. As such future work could contain larger numbers or subgroups of patients identified by biomarkers who are more likely to respond to noninvasive brain stimulation interventions (Boyd et al., 2017).
5. Conclusions
In summary, cTBS over M1c/S1c did not significantly enhance the effects of skilled motor practice and motor function beyond that of sham cTBS in individuals with chronic stroke. However, multiple sessions of skilled motor practice did benefit paretic UL function and modulate TCI bilaterally, regardless of stimulation group. There was an association between change in skilled motor performance and paretic UL function in the M1c cTBS group. This suggests that some individuals demonstrated a scaled improvement of skilled motor practice and motor function with inhibitory stimulation over M1c paired with skilled motor practice. Our results demonstrate the need to better understand individualized responses to rTMS and should to be considered when developing rTMS protocols in chronic stroke.
Acknowledgments
This work was funded by the Canadian Institutes of Health Research (CIHR; MOP – 106651 to LAB). JLN receives funding support from the Michael Smith Foundation for Health Research (MSFHR) and CIHR. KEB, CSM and KPW were supported by NSERC and CSM also received support from the Imperial Order Daughters of the Empire War Memorial Scholarship and the University of British Columbia (UBC) Four Year Fellowship program. MRB is supported, in part, by the NIH National Center for Medical Rehabilitation Research (K12 HD055931). SKM is partially supported by a KL2 scholar award from the Claude D. Pepper Older Americans Independence Center at the University of Michigan (National Institutes on Aging, P30AG024824). LAB received salary support from the Canada Research Chairs and MSFHR.
References
- Aboitiz F (1992). Brain connections: Interhemispheric fiber systems and anatomical brain asymmetries in humans. Biological Research, 25, 51–61. [PubMed] [Google Scholar]
- Ameli M, Grefkes C, Kemper F, Riegg FP, Rehme AK, Karbe H, … Nowak DA (2009). Differential effects of high-frequency repetitive transcranial magnetic stimulation over ipsilesional primary motor cortex in cortical and sub-cortical middle cerebral artery stroke. Annals of Neurology, 66(3), 298–309. [DOI] [PubMed] [Google Scholar]
- Asanuma H, & Okuda O (1962). Effects of transcallosal volleys on pyramidal tract cell activity of cat. Journal of Neurophysiology, 25, 198–208. [DOI] [PubMed] [Google Scholar]
- Andres FG, Mima T, Schulman AE, Dichgans J, Hallett M, & Gerloff C (1999). Functional coupling of human cortical sensorimotor areas during bimanual skill acquisition. Brain, 122(5), 855–70. [DOI] [PubMed] [Google Scholar]
- Auriat AM, Borich MR, Snow NJ, Wadden KP, & Boyd LA (2015). Comparing a diffusion tensor and non-tensor approach to white matter fiber tractography in chronic stroke. NeuroImage: Clinical, 7, 771–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auriat AM, Neva JL, Peters S, Ferris JK, & Boyd LA (2015). A Review of Transcranial Magnetic Stimulation and Multimodal Neuroimaging to Characterize Post-Stroke Neuroplasticity. Frontiers in Neurology, 6, 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avanzino L, Raffo A, Pelosin E, Ogliastro C, Marchese R, Ruggeri P, & Abbruzzese G (2014). Training based on mirror visual feedback influences transcallosal communication. European Journal of Neuroscience, 40(3), 2581–2588. [DOI] [PubMed] [Google Scholar]
- Birkenmeier RL, Prager EM, & Lang CE (2010). Translating animal doses of task-specific training to people with chronic stroke in 1-hour therapy sessions: A proof-of-concept study. Neurorehabilitation and Neural Repair, 24(7), 620–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boroojerdi B, Diefenbach K, & Ferbert A (1996). Transcallosal inhibition in cortical and subcortical cerebral vascular lesions. Journal of the Neurological Sciences, 144(1–2), 160–170. [DOI] [PubMed] [Google Scholar]
- Boyd LA, Hayward KS, Ward NS, Stinear CM, Rosso C, Fisher RJ, … Cramer SC (2017). Biomarkers of Stroke Recovery: Consensus-Based Core Recommendations from the Stroke Recovery and Rehabilitation Roundtable. Neurorehabilitation and Neural Repair, 31(10–11), 864–876. [DOI] [PubMed] [Google Scholar]
- Boyd LA, Vidoni ED, & Wessel BD (2010). Motor learning after stroke: Is skill acquisition a prerequisite for contralesional neuroplastic change? Neuroscience Letters, 482(1), 21–5. [DOI] [PubMed] [Google Scholar]
- Boyd L, Edwards JD, Siengsukon CF, Vidoni ED, Wessel BD, & Linsdell MA (2009). Motor sequence chunking is impaired by basal gnaglia stroke. Neurobiology of Learning and Memory, 92(1), 35–44. [DOI] [PubMed] [Google Scholar]
- Boyd L, Quaney B, Pohl P, & Winstein C (2007). Learning implicitly: Effects of task and severity after stroke. Neurorehabilitation and Neural Repair, 21(5), 444–54. [DOI] [PubMed] [Google Scholar]
- Brodie S, Borich M, & Boyd L (2014). Impact of 5-Hz rTMS over the primary sensory cortex is related to white matter volume in individuals with chronic stroke. European Journal of Neuroscience, 40(9), 3405–12. [DOI] [PubMed] [Google Scholar]
- Brodie S, Meehan S, Borich M, & Boyd L (2014). 5 Hz repetitive transcranial magnetic stimulation over the ipsilesional sensory cortex enhances motor learning after stroke. Frontiers in Human Neuroscience, 8, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie S, Villamayor A, Borich M, & Boyd L (2014). Exploring the specific time course of interhemispheric inhibition between the human primary sensory cortices. Journal of Neurophysiology, 112(6), 1470–1476. [DOI] [PubMed] [Google Scholar]
- Buetefisch CM, Revill KP, Shuster L, Hines B, & Parsons M (2014). Motor demand-dependent activation of ipsilateral motor cortex. Journal of Neurophysiology, 112(4), 999–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey JR, Deng H, Gillick BT, Cassidy JM, Anderson DC, Zhang L, & Thomas W (2014). Serial treatments of primed low-frequency rTMS in stroke: Characteristics of responders vs. nonresponders. Restorative Neurology and Neuroscience, 32(2), 323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey JR, Fregni F, & Pascual-Leone A (2006). rTMS combined with motor learning training in healthy subjects. Restorative Neurology and Neuroscience, 24(3), 191–199. [PubMed] [Google Scholar]
- Chen R, Yung D, & Li J (2003). Organization of ipsilateral excitatory and inhibitory pathways in the human motor cortex. Journal of Neurophysiology, 89(89), 1256–1264. [DOI] [PubMed] [Google Scholar]
- Cohen J (2013). Statistical Power Analysis for the Behavioral Sciences. Academic Press. [Google Scholar]
- Corti M, Patten C, & Triggs W (2012). Repetitive Transcranial Magnetic Stimulation of Motor Cortex after Stroke. American Journal of Physical Medicine & Rehabilitation, 91(3), 254–270. [DOI] [PubMed] [Google Scholar]
- Debas K, Carrier J, Orban P, Barakat M, Lungu O, Vandewalle G, … Doyon J (2010). Brain plasticity related to the consolidation of motor sequence learning and motor adaptation. Proceedings of the National Academy of Sciences, 107(41), 17839–17844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pino G, Pellegrino G, Assenza G, Capone F, Ferreri F, Formica D, … Di Lazzaro V (2014). Modulation of brain plasticity in stroke: A novel model for neurorehabilitation. Nature Reviews. Neurology, 10(10), 597–608. [DOI] [PubMed] [Google Scholar]
- Duque J, Hummel F, Celnik P, Murase N, Mazzocchio R, & Cohen LG (2005). Transcallosal inhibition in chronic subcortical stroke. NeuroImage, 28(4), 940–6. [DOI] [PubMed] [Google Scholar]
- Duque J, Murase N, Celnik P, Hummel F, Harris-Love M, Mazzocchio R, … Cohen LG (2007). Intermanual differences in movement-related interhemispheric inhibition. Journal of Cognitive Neuroscience, 19(2), 204–213. [DOI] [PubMed] [Google Scholar]
- Fling BW, Benson BL, & Seidler RD (2013). Transcallosal sensorimotor fiber tract structure-function relationships. Human Brain Mapping, 34(2), 384–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fugl-Meyer A, Jaasko L, Leyman I, Olsson S, & Steglind S (1975). The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand J Rehabil Med, 7, 13–31. [PubMed] [Google Scholar]
- Gamst G, Meyers L, & Guarino A (2008). ANOVA assumptions. Analysis of Variance Designs. New York, NY: Cambridge University Press. [Google Scholar]
- Geffen G, Jones D, & Geffen L (1994). Interhemispheric control of manual motor activity. Behavioural Brain Research, 64(1–2), 131–40. [DOI] [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, … Turner MB (2014). Heart disease and stroke statistics–2014 update: A report from the American Heart Association. Circulation (Vol. 129). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada M, Murase N, Hasan A, Balaratnam M, & Rothwell JC (2013). The role of interneuron networks in driving human motor cortical plasticity. Cerebral Cortex, 23(7), 1593–605. [DOI] [PubMed] [Google Scholar]
- Harris-Love ML, Chan E, Dromerick AW, & Cohen LG (2016). Neural Substrates of Motor Recovery in Severely Impaired Stroke Patients With Hand Paralysis. Neurorehabilitation and Neural Repair, 30(4), 328–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward K, Neva J, Mang C, Peters S, Wadden K, Ferris J, & Boyd L (2017). Interhemispheric Pathways Are Important for Motor Outcome in Individuals with Chronic and Severe Upper Limb Impairment Post Stroke. Neural Plasticity, 2017, 4281532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks HT, van Limbeek J, Geurts AC, & Zwarts MJ (2002). Motor recovery after stroke: A systematic review of the literature. Archives of Physical Medicine and Rehabilitation, 83(11), 1629–1637. [DOI] [PubMed] [Google Scholar]
- Hodics TM, Nakatsuka K, Upreti B, Alex A, Smith PS, & Pezzullo JC (2012). Wolf Motor Function Test for characterizing moderate to severe hemiparesis in stroke patients. Archives of Physical Medicine and Rehabilitation, 93(11), 1963–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer S, & Frahm J (2006). Topography of the human corpus callosum revisited—comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. NeuroImage, 32(3), 989–94. [DOI] [PubMed] [Google Scholar]
- Hsu WY, Cheng CH, Liao KK, Lee IH, & Lin YY (2012). Effects of repetitive transcranial magnetic stimulation on motor functions in patients with stroke: A meta-analysis. Stroke, 43(7), 1849–1857. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, & Roth-well JC (2005). Theta burst stimulation of the human motor cortex. Neuron, 45(2), 201–206. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Rey-Hipolito C, Jezzard P, Adams M, Turner R, & Ungerleider L (1998). The acquisition of skilled motor performance: Fast and slow experience-driven changes in primary motor cortex. Proceedings of the National Academy of Sciences of the United States of America, 95, 861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khedr E, Etraby A, Memeda M, Nasef A, & Razek A (2010). Long-term effect of repetitive transcranial magnetic stimulation on motor function recovery after acute ischemic stroke. Acta Neurologica Scandinavica, 121(1), 30–7. [DOI] [PubMed] [Google Scholar]
- Khedr EM (2005). Therapeutic trial of repetitive transcranial magnetic stimulation after acute ischemic stroke. Neurology, 466–468. [DOI] [PubMed] [Google Scholar]
- Kim Y-H, You SH, Ko M-H, Park J-W, Lee KH, Jang SH, … Hallett M (2006). Repetitive transcranial magnetic stimulation-induced corticomotor excitability and associated motor skill acquisition in chronic stroke. Stroke; a Journal of Cerebral Circulation, 37(6), 1471–6. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Hogg TM, VandenBerg PM, Cooper NR, Bruneau R, & Remple M (2004). Cortical synaptogenesis and motor map reorganization occur during late, but not early, phase of motor skill learning. The Journal of Neuroscience, 24(3), 628–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, … Marsden CD (1993). Corticocortical inhibition in human motor cortex. The Journal of Physiology, 471, 501–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Kim SB, Lee KW, Kim MA, Lee SJ, & Choi SJ (2015). Factors Associated With Upper Extremity Motor Recovery After Repetitive Transcranial Magnetic Stimulation in Stroke Patients. Annals of Rehabilitation Medicine, 39(2), 268–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepert J, Miltner WH, Bauder H, Sommer M, Dettmers C, Taub E, & Weiller C (1998). Motor cortex plasticity during constraint-induced movement therapy in stroke patients. Neuroscience Letters, 250(1), 5–8. [DOI] [PubMed] [Google Scholar]
- Lohse KR, Lang CE, & Boyd LA (2014). Is more better? Using metadata to explore dose-response relationships in stroke rehabilitation. Stroke, 45(7), 2053–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüdemann-Podubecká J, Bösl K, Theilig S, Wiederer R, & Nowak DA (2015). The effectiveness of 1Hz rTMS over the primary motor area of the unaffected hemisphere to improve hand function after stroke depends on hemispheric dominance. Brain Stimulation, 8(4), 823–30. [DOI] [PubMed] [Google Scholar]
- Malcolm MP, Triggs WJ, Light KE, Gonzalez LJ, Wu S, Reid K, & Nadeau SE (2007). Repetitive Transcranial Magnetic Stimulation as an Adjunct to Constraint-Induced Therapy: An Exploratory Randomized Controlled Trial. American Journal of Physical Medicine & Rehabilitation, 86(9), 707–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mang CS, Borich MR, Brodie SM, & Boyd LA (2015). Diffusion imaging and transcranial magnetic stimulation assessment of transcallosal pathways in chronic stroke. Clinical Neurophysiology, 126(10), 1959–71. [DOI] [PubMed] [Google Scholar]
- Mang C, Snow N, Wadden K, Campbell K, & Boyd L (2016). High-Intensity Aerobic Exercise Enhances Motor Memory Retrieval. Medicine & Science in Sports & Exercise, 48(12), 2477–2486. [DOI] [PubMed] [Google Scholar]
- Meehan S, Dao E, Linsdell M, & Boyd L (2011). Continuous theta burst stimulation over the contralesional sensory and motor cortex enhances motor learning post-stroke. Neuroscience Letters, 500(1), 26–30. [DOI] [PubMed] [Google Scholar]
- Meehan S, Randhawa B, Wessel B, & Boyd L (2011). Implicit sequence-specific motor learning after subcortical stroke is associated with increased prefrontal brain activations: An fMRI study. Human Brain Mapping, 32(2), 290–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer BU, Röricht S, & Woiciechowsky C (1998). Topography of fibers in the human corpus callosum mediating interhemispheric inhibition between the motor cortices. Annals of Neurology, 43(3), 360–369.9506553 [Google Scholar]
- Murase N, Duque J, Mazzocchio R, & Cohen LG (2004). Influence of Interhemispheric Interactions on Motor Function in Chronic Stroke. Annals of Neurology, 55(3), 400–409. [DOI] [PubMed] [Google Scholar]
- Muratori LM, Lamberg EM, Quinn L, & Duff SV (2013). Applying principles of motor learning and control to upper extremity rehabilitation. Journal of Hand Therapy, 26(2), 94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neva JL, Lakhani B, Brown KE, Wadden KP, Mang CS, Ledwell NHM, … Boyd LA (2016). Multiple measures of corticospinal excitability are associated with clinical features of multiple sclerosis. Behavioural Brain Research, 297, 187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neva JL, Singh AM, Vesia M, & Staines WR (2014). Selective modulation of left primary motor cortex excitability after continuous theta burst stimulation to right primary motor cortex and bimanual training. Behavioural Brain Research, 269, 139–46. [DOI] [PubMed] [Google Scholar]
- Neva JL, Vesia M, Singh AM, & Staines WR (2015). Bilateral primary motor cortex circuitry is modulated due to theta burst stimulation to left dorsal premotor cortex and bimanual training. Brain Research, 1618, 61–74. [DOI] [PubMed] [Google Scholar]
- Novak CB, Mackinnon SE, Williams JI, & Kelly L (1993). Establishment of reliability in the evaluation of hand sensibility. Plastic and Reconstructive Surgery, 92(2), 311–22. [DOI] [PubMed] [Google Scholar]
- Opie GM, Vosnakis E, Ridding MC, Ziemann U, & Semmler JG (2017). Priming theta burst stimulation enhances motor cortex plasticity in young but not old adults. Brain Stimulation, 10(2), 298–304. [DOI] [PubMed] [Google Scholar]
- Pavlides C, Miyashita E, & Asanuma H (1993). Projection from the sensory to the motor cortex is important in learning motor skills in the monkey. Journal of Neurophysiology, 70(2), 733–741. [DOI] [PubMed] [Google Scholar]
- Pocock S (1997). Clinical trials with multiple outcomes: A statistical perspective on their design, analysis, and interpretation. Controlled Clinical Trials, 18, 530–545. [DOI] [PubMed] [Google Scholar]
- Ragert P, Nierhaus T, Cohen LG, & Villringer A (2011). Interhemispheric Interactions between the Human Primary Somatosensory Cortices. Plos One, 6(2), 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini P, Berardelli A, Deuschl G, Hallett M, Maertens de Noordhout A, Paulus W, & Pauri F (1999). Applications of magnetic cortical stimulation. The International Federation of Clinical Neurophysiology. Electroencephalography and Clinical Neurophysiology, 52, 171–85. [PubMed] [Google Scholar]
- Rothwell JC, Hallett M, Berardelli A, Eisen A, Rossini P, & Paulus W (1999). Magnetic stimulation: Motor evoked potentials. The International Federation of Clinical Neurophysiology. Electroencephalography and Clinical Neurophysiology. Supplement, 52, 97–103. [PubMed] [Google Scholar]
- Sadato N, Ibanez V, Deiber M, Campbell G, Leonardo M, & Hallett M (1996). Frequency-Dependent Changes of Regional Cerebral Blood Flow During Finger Movements. Journal of Cerebral Blood Flow and Metabolism, (16), 23–33. [DOI] [PubMed] [Google Scholar]
- Sawaki L, Butler AJ, Leng X, Wassenaar P. a, Mohammad YM, Blanton S, … Wittenberg GF (2008). Constraint-induced movement therapy results in increased motor map area in subjects 3 to 9 months after stroke. Neurorehabilitation and Neural Repair, 22(5), 505–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Hosaki A, Hino T, Sato M, Komori T, Hirai S, & Rossini PM (2002). Motor cortical disinhibition in the unaffected hemisphere after unilateral cortical stroke. Brain, 125(8), 1896–907. [DOI] [PubMed] [Google Scholar]
- Stefan K, Gentner R, Zeller D, Dang S, & Classen J (2008). Theta-burst stimulation: Remote physiological and local behavioral after-effects. Neuroimage, 40(1), 265–274. [DOI] [PubMed] [Google Scholar]
- Suppa A, Ortu E, Zafar N, Deriu F, Paulus W, Berardelli A, & Rothwell JC (2008). Theta burst stimulation induces after-łeffects on contralateral primary motor cortex excitability in humans. The Journal of Physiology, 18(Pt 18), 4489–4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi N, Chuma T, Matsuo Y, Watanabe I, & Ikoma K (2005). Repetitive transcranial magnetic stimulation of contralesional primary motor cortex improves hand function after stroke. Stroke, 36(12), 2681–2686. [DOI] [PubMed] [Google Scholar]
- Talelli P, Greenwood RJ, & Rothwell JC (2007). Exploring Theta Burst Stimulation as an intervention to improve motor recovery in chronic stroke. Clinical Neurophysiology, 118(2), 333–342. [DOI] [PubMed] [Google Scholar]
- Talelli P, Wallace A, Dileone M, Hoad D, Cheeran B, Oliver R, … Rothwell J (2012). Theta Burst Stimulation in the Rehabilitation of the Upper Limb: A Semirandomized, Placebo-Controlled Trial in Chronic Stroke Patients. Neurorehabilitation and Neural Repair, 26(8), 976–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub E, Uswatte G, King DK, Morris D, Crago JE, & Chatterjee A (2006). A placebo-controlled trial of constraint-induced movement therapy for upper extremity after stroke. Stroke, 37(4), 1045–1049. [DOI] [PubMed] [Google Scholar]
- Tretriluxana J, Kantak S, Tretriluxana S, Wu AD, & Fisher BE (2013). Low frequency repetitive transcranial magnetic stimulation to the non-lesioned hemisphere improves paretic arm reach-to-grasp performance after chronic stroke. Disability and Rehabilitation. Assistive Technology, 8(2), 121–4. [DOI] [PubMed] [Google Scholar]
- Vidoni ED, Acerra NE, Dao E, Meehan SK, & Boyd LA (2010). Role of the primary somatosensory cortex in motor learning: An rTMS study. Neurobiology of Learning and Memory, 93(4), 532–539. [DOI] [PubMed] [Google Scholar]
- Wadden K, Asis K, Mang C, Neva J, Peters S, Lakhani B, & Boyd L (2017). Predicting Motor Sequence Learning in Individuals With Chronic Stroke. Neurorehabilitation and Neural Repair, 31(1), 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wischnewski M, Kowalski GM, Rink F, Belagaje SR, Haut MW, Hobbs G, & Buetefisch CM (2016). Demand on skillfulness modulates interhemispheric inhibition of motor cortices. Journal of Neurophysiology, 115(6), 2803–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf S, Catlin P, Ellis M, Archer A, Morgan B, & Piacentino A (2001). Assessing Wolf motor function test as outcome measure for research in patients after stroke. Stroke, 32(7), 1635–9. [DOI] [PubMed] [Google Scholar]
- Xu J, Branscheidt M, Schambra H, Steiner L, Widmer M, Diedrichsen J, … Celnik P (2019). Rethinking interhemispheric imbalance as a target for stroke neurorehabilitation. Annals of Neurology, Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada N, Kakuda W, Kondo T, Shimizu M, Sageshima M, Mitani S, & Abo M (2014). Continuous theta-burst stimulation combined with occupational therapy for upper limb hemiparesis after stroke: A preliminary study. Acta Neurologica Belgica, 114(4), 279–284. [DOI] [PubMed] [Google Scholar]
- Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, & Winkler P (1997). Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain, 120(1), 141–57. [DOI] [PubMed] [Google Scholar]