Abstract
Atherosclerotic cardiovascular disease remains a worldwide epidemic and one of the leading causes of death nowadays. Vessel wall imaging can be used to understand the etiology of atherosclerosis but it is rarely done due to the high cost. We recently identified the Osteoarthritis Initiative (OAI), a large prospective cohort study of knee osteoarthritis, which might serve as a valuable source for atherosclerosis research with its serial knee magnetic resonance imaging data. We have found that these images are suitable for vessel wall image analysis of the lower extremity arteries. Here, we will introduce the OAI dataset and explain why it could be used for cardiovascular research purposes. Also, we will briefly comment on peripheral artery atherosclerosis as it is covered in the OAI image dataset and review the use of vessel wall imaging for studying atherosclerosis. We believe data mining of imaging studies, not originally designed on cardiovascular research, can not only maximize the value of the imaging dataset, but also boost our understanding of atherosclerosis.
Subject Code: Atherosclerosis
The Vessels behind Knee: Opportunities from OAI
Cohort studies, as a principal component of study design in modern epidemiology, play a fundamental role in current medical research, including cardiovascular research. Although randomized controlled clinical trials convey the highest quality of evidence, it is not always conductible due to practical or ethical considerations. Additionally, clinical trials are always indicated by observational evidence, which strengthen the need for high quality cohort studies. Running a high-quality cohort study is challenging, even compared with a clinical trial. It usually needs a large sample size to provide adequate statistical power. A prospective design is generally favored over retrospective design to allow for more accurate measurement or determination of the “exposure”. An adequate follow-up time, which is usually very long for chronic diseases, is essential to reach an “outcome”. Moreover, a comprehensive phenotyping of the subjects is desired, despite the additional cost, as it can benefit further data mining as well as control for confounding effects. With these high-demanding requirements for qualified cohort studies, more efficient and effective use of existing data would be an attractive solution. Thus, we propose to use data from the Osteoarthritis Initiative (OAI)1, supported by the NIH, as it might provide unique insights into atherosclerotic cardiovascular disease (ACVD), beyond its original focus on knee osteoarthritis.
Osteoarthritis and ACVD are two of the most prevalent conditions affecting the US population2, 3. Both conditions are characterized by slow accumulation of pathological change leading, eventually, to adverse health outcomes. They share a number of risk factors including age and obesity. Both conditions develop as a result of a complex and not fully understood interplay between genetics, environment, lifestyle, as well as socioeconomic factors. Successful research addressing these conditions requires prospective studies using large cohorts of at-risk individuals, fully characterized in terms of genetics, demographics, clinical assessment and biomarkers. Interestingly, such studies benefit from intensive use of imaging to characterize the slow accumulation of pathologic change, though it is costly and rarely done4, 5.
The OAI (ClinicalTrials.gov Identifier: NCT00080171) is a multicenter, prospective cohort study of knee osteoarthritis, sponsored by the National Institute of Arthritis and Musculoskeletal and Skin Diseases with additional financial support from several pharmaceutical companies including GSK, Merck, Novartis and Pfizer. It was conceived in the late 1990’s, initiated in 2000, and the live phase ran from 2004 to 2014, with the specific aim to find biochemical, genetic and imaging biomarkers for development and progression of osteoarthritis. The sample size was 4796 and subjects underwent magnetic resonance imaging (MRI) of the knee at 7 timepoints using a rigorously standardized acquisition protocol on four identical Siemens 3T MRI scanners. Participants were recruited into one of three sub-cohorts: the progression cohort included participants with a diagnosis of symptomatic femorotibial osteoarthritis at baseline (N=1390), the incidence cohort included participants at risk of development of symptomatic femorotibial osteoarthritis (N=3284), and the reference cohort included individuals without osteoarthritis and with no relevant risk factors (N=122)6. Since its launch, the OAI has been fruitful in osteoarthritis research, with over 500 publications by June 2018, and has become a valuable resource with open access for researchers all around the world. The study design and its dataset lead us to believe that it also offers an exceptional opportunity for cardiovascular research.
OAI recruited a large number of predominantly African American and Caucasian subjects aged 45–79 years, an appropriate age group for studying subclinical and clinical atherosclerotic cardiovascular diseases. The basic demographic characteristics of the OAI dataset are briefly compared to several well-known cardiovascular cohorts in Table 1. The OAI also collected extensive clinical information providing opportunities to address diverse research questions regarding cardiovascular diseases. Cardiovascular risk factor data that have been collected in OAI include age, gender, ethnicity, smoking, body mass index, blood pressure, comorbidity, medications and abdominal circumference. Other data that might be of particular interest to cardiovascular researchers include performance measures (20- and 400-meter timed walk, chair stands timed), quality of life, depression (CES-D), physical activity and extensive evaluation of dietary nutrient intake (Block Brief 2000). More importantly, some of these measures have also been serially assessed during follow-up, enabling investigations into relevant temporal changes. Furthermore, biospecimens including blood (serum, plasma and buffy coat), urine and RNA have been collected at enrollment and consecutively at follow-up visits, serving as a valuable biobank for biomarker studies. Additionally, MRI data of the knee and thigh (both left and right) are available at baseline and follow-up for eligible participants. Because of the high spatial resolution and excellent image quality, it is possible to characterize the popliteal and mid- to distal superficial femoral arteries, and it may be possible to obtain quantitative measurements of the popliteal vessel wall (VW) morphology using methods previously developed and validated for atherosclerotic VW imaging in other vessels7.
Table 1.
Comparison of the basic characteristics of OAI to several well-known cohorts
| OAI | MESA | ARIC | CHS | |
|---|---|---|---|---|
| Number | 4796 | 6814 | 15792 | 5888 |
| Started | 2002 | 2000 | 1987 | 1989 |
| Length of Follow-up | 108 months | Ongoing with the 6th Exam (2016–2018) | Ongoing | Ongoing (Most recent available follow-up data through June 30th, 2015) |
| Age range | 45–79 | 45–84 | 45–64 | ≥65 |
| Male | 41.5% | 47.2% | 44.8% | 42.4% |
| Race | 79% White or Caucasian; 18% Black or African American | 39% White; 22% Hispanic; 28% African American; 12% Chinese-American | 73% White and 27% Non-White | 16% African American |
OAI: Osteoarthritis Initiative; MESA: The Multi-Ethnic Study of Atherosclerosis; ARIC: The Atherosclerosis Risk in Communities Study; CHS: Cardiovascular Health Study.
It should be acknowledged that there are also some limitations of the OAI dataset for cardiovascular research. First, although survival outcome data is available, cause of death was not recorded, and hard cardiovascular outcomes such as myocardial infarction, ischemic stroke, and revascularization were not rigorously collected. The lack of the cardiovascular event data from the study, such as myocardial infarction, stroke, etc., limits its value in establishing the association of potential risk factors with hard cardiovascular outcomes. However, it is possible to use the dataset to study the relationship between baseline atherosclerosis, rate of atherosclerosis progression, other biomarkers, demographic and clinical variables and other health outcomes, such as mental health, as there are data on depression at both baseline and follow-up visits. In principle, comparisons with cardiovascular biomarkers are possible as biospecimens had been collected, although very limited existing measurements are available as no funds were allocated for biospecimen analysis. This could be addressed in the future, cost-effectively, as just an incremental investment on biomarker measurement would be needed for the existing cohort. Second, although a major advantage of MR VW imaging is the identification of plaque components, it is not feasible to analyze the components other than calcification through the 3D double-echo steady state (DESS) sequence used in OAI.
Atherosclerosis in Peripheral Arteries
Atherosclerosis is a systemic pathological process affecting not only the coronary and carotid arteries, but also the peripheral arteries. As with the coronary circulation, atherosclerosis in the lower extremity may result in vascular stenosis with insufficient blood supply to the tissue leading to clinically overt peripheral arterial disease (PAD), presenting ischemic rest pain or intermittent claudication. Symptomatic and / or asymptomatic atherosclerosis is common in the lower limbs and has major prognostic significance8. In an international registry of over 50000 patients with established ACVD, one in four patients with coronary artery disease (CAD) also had cerebral vascular disease, PAD, or both9. Interestingly, there is a higher prevalence of multi-vascular disease in patients with PAD compared to those with CAD or cerebral vascular disease. In the general population, with aging being one of the most potent risk factors for atherosclerosis10, the prevalence of PAD was less than 5% among US adults aged 40 years and over, and increased sharply by 3-fold to nearly 15% among those aged over 70 in the National Health and Nutrition Examination Survey (NHANES) study11. Similar trends were also noted in other population-based studies and confirmed by systematic reviews12–16. Notably, most relevant studies used questionnaires on intermittent claudication or ankle-brachial index (ABI) to determine presence of PAD. However, questionnaires can only identify symptomatic patients and the sensitivity of ABI has been questioned in some cases. In a study using magnetic resonance angiography (MRA), the authors evaluated the relation of ABI to lower extremity artery stenosis, and found that the commonly used cut-off value of 0.9 has a sensitivity of only 15–20% for determining the presence of >=50% stenosis17. This result is supported by a subsequent systematic review18. The actual prevalence of peripheral artery atherosclerosis is, therefore, likely higher than reported. In the aforementioned study, the prevalence of high-grade (>=50%) stenosis among a group of subjects aged 70 years is 28% based on MRA17.
Patients with PAD, either symptomatic or asymptomatic, are at increased risk for future cardiovascular events19–26. In the REACH (Reduction of Atherothrombosis for Continued Health) registry, those with PAD had the highest cardiovascular mortality, compared to those with established CAD, or cerebral vascular disease or multiple atherothrombotic risk factors9. This high cardiovascular mortality in PAD patients may reflect the fact that nearly 60% PAD patients actually had multi-vascular disease in the study. In fact, given the systemic nature of atherosclerosis, presence of atherosclerotic injury at the peripheral vascular bed increases the frequency of disease at other vascular sites, including the coronary artery, the carotid artery and the cerebral arteries27–29. The prognostic value of PAD may, therefore, be based on its reflection of the underlying systemic atherosclerosis process. This is important since it suggests that studying the peripheral artery can provide reliable information on systemic atherosclerotic burden.
PAD and Osteoarthritis
There is limited information on the association between PAD and osteoarthritis, although there are existing data demonstrating that osteoarthritis is associated with cardiovascular risk. Veronese et al. studied 2158 elderly subjects without cardiovascular disease and found that osteoarthritis at baseline was associated with the risk of incident cardiovascular disease and the association was more prominent in women or when the knee was affected30. PAD was included in the composite cardiovascular endpoint (incident cardiovascular disease) but was not treated as a secondary independent outcome in the study. Gielis et al. found that incident osteoarthritis was independently and positively associated with incident artery calcification in women31. The association between osteoarthritis and cardiovascular disease has been confirmed in the OAI dataset32.
For PAD, Park et al. studied the prevalence of lower extremity peripheral vascular disease using ultrasonography in patients undergoing total knee arthroplasty due to osteoarthritis33. They found that 3.6% of the patients had atherosclerotic changes. This proportion is indeed extremely low especially given that the average age of the study population was 74.1 (range: 65–81) years33. However, the study excluded those who had vascular-related symptoms (intermittent pain, rest pain, or skin ulcers) and previous history of percutaneous transluminal angioplasty or bypass surgery, and may therefore significantly underestimate the true prevalence of PAD in the general osteoarthritis population.
The explanations for the association between PAD and osteoarthritis remain obscure. Both diseases share a number of common risk factors, including ageing, obesity and chronic inflammation34. These might predispose patients with osteoarthritis to increased risk of PAD, or vice versa. Noticeably, several consequences of osteoarthritis might increase the risk of atherosclerosis, including physical inactivity due to limited mobility, and the use of nonsteroidal anti-inflammatory drugs commonly prescribed for osteoarthritis. The possible role of physical inactivity in osteoarthritis-associated cardiovascular risk has been suggested in two independent observational cohort studies35, 36. A recent randomized controlled trial evaluated the effect of a dosed 12-week walking program on cardiovascular health in patients with severe knee osteoarthritis and found that it helped to achieve targeted blood pressures, as well as to reduce waist circumference37. There is also evidence suggesting that osteoarthritis-associated pain is related to incident CAD38. It is not clear whether this association is a reflection of the link between inflammation and CAD, or if it is due to the effect of stress induced by pain on cardiovascular health.
Vessel Wall MRI for Atherosclerosis Research
Traditionally, angiographic techniques, such as MRA, have been used to measure the degree of lumen stenosis as a proxy for the severity of atherosclerosis. However, atherosclerosis is a disease that resides within the vessel wall rather than in the lumen, with possible expansive, compensatory VW remodeling39. Thus lumen-based disease evaluation will inevitably be insensitive to early vascular insult and miss VW information40.
Over the past decade, high spatial resolution MRI has been proven as a reliable method in the study of atherosclerosis, providing information on VW morphology, plaque presence and size, as well as plaque composition41–50. Particularly, VW MRI has been considered as a cornerstone technique in atherosclerosis imaging research due to its major advantages including: 1. It can measure the thickness of the VW with submillimeter accuracy, enabling the detection of wall thickening before the development of luminal stenosis. This is of particular importance since atherosclerosis in its early phase progresses slowly and silently with wall thickening being the only imaging feature. MRI-derived wall thickness is therefore an attractive biomarker for early disease detection and progression monitoring51, 52. The direct visualization and measurement of VW is also important for detection of positive remodeling, in which the VW expands outwardly and the lumen diameter remains normal53. 2. It can also distinguish plaque components and, therefore, be used to classify the type of the plaque and identify those of high risk54. The work of our group has been focused on using VW imaging to determine high-risk plaques and has demonstrated that high-risk plaque components or features (i.e. intraplaque hemorrhage, thinned or ruptured fibrous caps, high lipid content) robustly predict cardiovascular risk compare to plaque burden (number and size) in different disease settings, including PAD55–59.
MRI has the additional advantage over ultrasound-based VW evaluation (i.e. intima-media thickness, IMT) of being less operator dependent. IMT suffers substantial inter-operator variability, while MRI VW measurements have been reported to have high test-to-test repeatability60.
Technically, VW identification on VW MRI relies primarily on fat-suppressed T1-weighted spin echo based black blood techniques. Fat suppression and black blood preparations are used for better delineation of the outer and inner boundaries of the arterial wall. Newer blood suppression methods using motion sensitized dephasing and water excitation, enable the VW to be visualized using gradient echo based DESS MRI in the peripheral arteries61–63. DESS MRI of the knee was acquired for all subjects in the OAI cohort. While the primary intention of DESS in the OAI study was for quantitative measurements of the knee and articular cartilage, the DESS images also provide an opportunity to measure the popliteal artery VW.
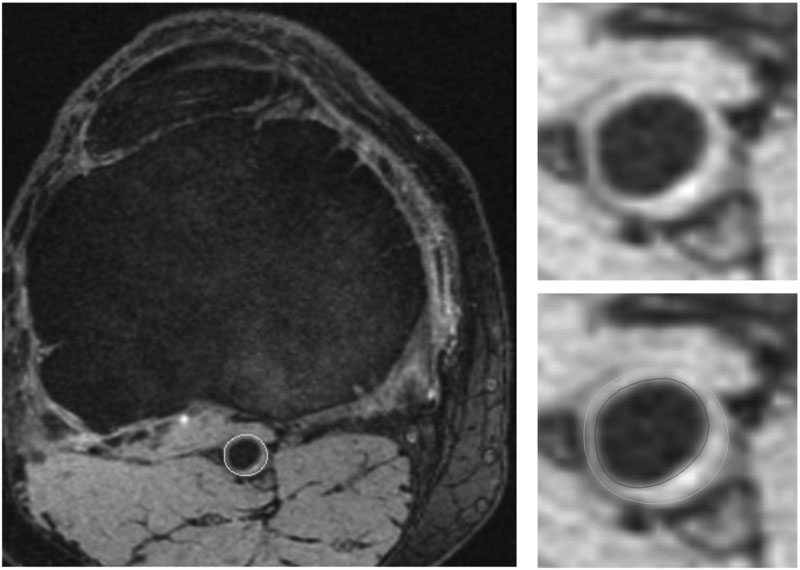
The OAI imaging protocol consists of the following sequences performed on both knees: Coronal and sagittal intermediate weighted 2D TSE and sagittal 3D DESS. Other sequences (coronal T1w 3D FLASH and 2D multi-echo spin echo) were only performed on one knee. The 3D DESS sequence is suitable for VW studies due to: 1) Blood is suppressed due to flow dephasing, 2) fat is suppressed due to water excitation and 3) although acquired sagittally, it can be reformatted to the axial plane with high resolution (acquired at 0.7mm slice thickness and 0.37mm × 0.46mm in-plane). Axial reformats of the sagittally acquired DESS show popliteal VW with good lumen and outer wall boundary contrast (Figure 1). However, it is to be noted that axial reformatting reduces the in-plane resolution to 0.7mm × 0.37mm while improving the slice resolution to 0.46mm. While artery segmentation is easier using axial reformats, there may be potential advantages in segmenting directly using the sagittal images due to higher in-plane resolution. The entire extent of the femoropopliteal artery in the large sagittal field-of-view (140mm), centered on the knee, can be targeted for VW measurements. The popliteal artery is conventionally divided into three sections P1, P2 and P364, of which the whole of P2 and parts of P1 and P3 including the bifurcation are covered by this field-of-view. Detailed imaging parameters for 3D DESS and other OAI protocol sequences can be found in a previous report65.
Figure 1:
Axial cross section through the popliteal artery showing wall thickening in one OAI subject. Right panel shows enlarged popliteal artery with and without contours (lumen contour in red, outer wall contour in blue).
As aforementioned, our initial inspection of the axial 3D DESS images suggests that it is feasible to obtain quantitative measurements of the popliteal VW using established methods. Using expert reviewer assisted segmentation of the lumen and outer wall in the CASCADE plaque analysis software (University of Washington, Seattle)7, measurements such as lumen area, percent stenosis, wall area and wall thickness can be obtained in addition to atherosclerotic plaque composition if present. It has to be noted that while calcification can be identified using 3D DESS, identification of other plaque components using 3D DESS have not yet been established. Therefore, it is unfortunately not feasible to depict detailed plaque component in the OAI dataset.
The image quality for applying established VW analysis methods also appears to be excellent. In a randomly selected set of 38 knees, we rated the image quality (scale of 1 to 3 with 3 being the best), presence of flow artifacts and presence of bifurcation/trifurcation of the popliteal artery. The mean image quality was 2.29±0.47. There were minor flow artifacts in 21/38 knees but did not affect the visualization of lumen boundaries.
A particular advantage of the OAI imaging data lies in its serial imaging, with annual imaging for 4 years after enrollment and biannual imaging from the 4th to the 8th year. A total of 7 time-point image sets, including baseline, were collected during that time-frame with identical imaging protocol, coverage and quality control. This compares favorably with most cardiovascular-specific cohorts, in which cardiovascular imaging was repeated less frequently or was just collected at one-time point. Serial measurement is particularly important in view of the fact that atherosclerosis is an evolving pathologic process. While atherosclerotic plaques remain asymptomatic for long periods, they are not static. Instead, lesions continually undergo a dynamic process involving both tissue injury and repair. Consequently, progression, as well as regression, of plaques has been observed in the patients66–69. In light of this, serial imaging over time is preferred due to the following advantages: 1) it aids in elucidating the temporal relationships of changes in risk factors and changes in atherosclerotic lesions, and 2) it allows for the evaluation of the potential effects of particular risk factors or interventions over time (e.g. yearly in OAI).
The highly detailed sequential image dataset in OAI will permit a comprehensive and highly quantitative assessment of the temporal evolution of atherosclerosis in vivo, including response to clinical or lifestyle changes. Given the high cost of MR imaging studies, it is unlikely to be superseded in the foreseeable future.
OAI for Cardiovascular Research
By the end of 2018, there have been over 500 publications based on OAI, with the MR imaging data contributing to a significant portion70, 71. Naturally most of those publications were focused directly on osteoarthritis, with little attention to cardiovascular risk or disease.
Data from the OAI has provided invaluable insight into the relationship between risk factors and specific osteoarthritis outcomes, as well as relationships between risk factors and morbidity and mortality outcomes in general. The length of the study and frequency of follow-up allowed some “natural experiments”, where the benefits of, for example, weight loss or changing levels of activity during the study, could be determined. Factors found to increase the risk of osteoarthritis incidence or progression included abnormal glycated serum protein, physical inactivity, slow gait, low dietary fibre intake, soft drink consumption, high saturated fat intake, high total fat intake, vitamin D deficiency, high baseline body mass index, and analgesic use72–83. Factors found to decrease the risk of osteoarthritis incidence or progression included weight loss during the study, high intake of mono- and poly-unsaturated fatty acids or adherence to a Mediterranean diet, and mitochondrial haplotypes T and J79, 84–88. Factors not found to increase the risk of osteoarthritis incidence or progression include depressive symptoms at baseline, a history of running, statin use, and smoking history89–92. More generally, the health benefit was observed to inactive older adults of increasing physical activity , while health risks were associated with sedentary behaviour, slow gait, obesity or central obesity93–98. Elevated mortality was associated with consumption of fried potato products99. Since many of these risk factors are also of interest in cardiovascular research, it may be illuminating to determine their effects on atherosclerosis initiation and progression, as quantified by vessel wall MRI.
One study specifically utilized the dataset for mining cardiovascular outcome (incident cardiovascular disease) and looked into the relationship between baseline osteoarthritis and future risk of cardiovascular disease32. The authors concluded that hand osteoarthritis predicts the risk of developing cardiovascular disease in women, but not in men. However, the lack of endpoint ascertainment for the self-reported onset of cardiovascular diseases and the large proportion of participants loss to follow-up posed significant limitations to such study. Another study group performed a cross-sectional analysis of the association between sedentary behavior and blood pressure control using the 48-month visit data, in which accelerometers were used to measure physical activity, and demonstrated a graded association between sedentary time and elevated blood pressure among participants who were not taking antihypertensive medication100.
Conclusions and Perspectives
Atherosclerosis remains the major leading public health burden globally. A thorough understanding of the disease’s natural history in vivo is essential for risk stratification and identification of novel targets for prevention and treatment. Serial atherosclerosis imaging study provides a critical tool for early detection and monitoring of disease development and progression in such studies. MR-based VW imaging is an ideal research tool for this purpose. The OAI is a large longitudinal study of knee osteoarthritis with annual MR imaging data of the knee and thigh for up to 8 years. We recently identified that these MR images could also be used for popliteal and superficial femoral arterial wall analysis. By leveraging its large sample size, available information on cardiovascular-related risk factors, and high-fidelity MR VW imaging data, the OAI could provide an unexpected and valuable opportunity for studying the natural history of atherosclerosis.
Supplementary Material
Highlights.
In this review, we propose to use a large, prospective, serial, bilateral knee MRI originally obtained to study osteoarthritis to now characterize lower extremity atherosclerosis by measuring the vessel wall of the femoral-popliteal artery.
We demonstrate the feasibility of such a study and discuss why this repurposing can enhance our understanding of atherosclerosis.
The leverage of the serial MR imaging of this osteoarthritis cohort will provide a unique opportunity to study the temporal resolution of atherosclerotic lesions in a large population.
Acknowledgements:
The authors thank Zach Miller for his assistance in preparing this paper.
Sources of Funding: This project is funded by a grant from American Heart Association to Dr. Chun Yuan (18AIML34280043). The OAI is a public-private partnership comprised of five contracts (N01-AR-2–2258; N01-AR-2–2259; N01-AR-2–2260; N01-AR-2–2261; N01-AR-2–2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript was prepared using an OAI public use data set and does not necessarily reflect the opinions or views of the OAI investigators, the NIH, or the private funding partners.
Abbreviations
- ABI
ankle-brachial index
- ACVD
atherosclerotic cardiovascular disease
- CAD
coronary artery disease
- DESS
double-echo steady state
- IMT
intima-media thickness
- MRA
magnetic resonance angiography
- MRI
magnetic resonance imaging
- OAI
Osteoarthritis Initiative
- PAD
peripheral arterial disease
- VW
vessel wall
Footnotes
Disclosures: None.
TOC category: Population study
TOC subcategory: Vascular Biology
References
- 1.Fawaz-Estrup F The osteoarthritis initiative: An overview. Medicine and health, Rhode Island. 2004;87:169–171 [PubMed] [Google Scholar]
- 2.Khera R, Valero-Elizondo J, Okunrintemi V, Saxena A, Das SR, de Lemos JA, Krumholz HM, Nasir K. Association of out-of-pocket annual health expenditures with financial hardship in low-income adults with atherosclerotic cardiovascular disease in the united states. JAMA cardiology. 2018;3:729–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, Gabriel S, Hirsch R, Hochberg MC, Hunder GG, Jordan JM, Katz JN, Kremers HM, Wolfe F. Estimates of the prevalence of arthritis and other rheumatic conditions in the united states. Part ii. Arthritis and rheumatism. 2008;58:26–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peterfy C, Kothari M. Imaging osteoarthritis: Magnetic resonance imaging versus x-ray. Current rheumatology reports. 2006;8:16–21 [DOI] [PubMed] [Google Scholar]
- 5.Tarkin JM, Dweck MR, Evans NR, Takx RA, Brown AJ, Tawakol A, Fayad ZA, Rudd JH. Imaging atherosclerosis. Circulation research. 2016;118:750–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eckstein F, Wirth W, Nevitt MC. Recent advances in osteoarthritis imaging--the osteoarthritis initiative. Nature reviews. Rheumatology 2012;8:622–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kerwin W, Xu D, Liu F, Saam T, Underhill H, Takaya N, Chu B, Hatsukami T, Yuan C. Magnetic resonance imaging of carotid atherosclerosis: Plaque analysis. Topics in magnetic resonance imaging : TMRI. 2007;18:371–378 [DOI] [PubMed] [Google Scholar]
- 8.Gallino A, Aboyans V, Diehm C, Cosentino F, Stricker H, Falk E, Schouten O, Lekakis J, Amann-Vesti B, Siclari F, Poredos P, Novo S, Brodmann M, Schulte KL, Vlachopoulos C, De Caterina R, Libby P, Baumgartner I, European Society of Cardiology Working Group on Peripheral C. Non-coronary atherosclerosis. European heart journal. 2014;35:1112–1119 [DOI] [PubMed] [Google Scholar]
- 9.Steg PG, Bhatt DL, Wilson PW, D’Agostino R, Sr., Ohman EM, Rother J, Liau CS, Hirsch AT, Mas JL, Ikeda Y, Pencina MJ, Goto S, Investigators RR. One-year cardiovascular event rates in outpatients with atherothrombosis. Jama. 2007;297:1197–1206 [DOI] [PubMed] [Google Scholar]
- 10.Wang JC, Bennett M. Aging and atherosclerosis: Mechanisms, functional consequences, and potential therapeutics for cellular senescence. Circulation research. 2012;111:245–259 [DOI] [PubMed] [Google Scholar]
- 11.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the united states: Results from the national health and nutrition examination survey, 1999–2000. Circulation. 2004;110:738–743 [DOI] [PubMed] [Google Scholar]
- 12.Fowkes FG, Housley E, Cawood EH, Macintyre CC, Ruckley CV, Prescott RJ. Edinburgh artery study: Prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. International journal of epidemiology. 1991;20:384–392 [DOI] [PubMed] [Google Scholar]
- 13.Stoffers HE, Rinkens PE, Kester AD, Kaiser V, Knottnerus JA. The prevalence of asymptomatic and unrecognized peripheral arterial occlusive disease. International journal of epidemiology. 1996;25:282–290 [DOI] [PubMed] [Google Scholar]
- 14.Meijer WT, Hoes AW, Rutgers D, Bots ML, Hofman A, Grobbee DE. Peripheral arterial disease in the elderly: The rotterdam study. Arteriosclerosis, thrombosis, and vascular biology. 1998;18:185–192 [DOI] [PubMed] [Google Scholar]
- 15.Allison MA, Ho E, Denenberg JO, Langer RD, Newman AB, Fabsitz RR, Criqui MH. Ethnic-specific prevalence of peripheral arterial disease in the united states. American journal of preventive medicine. 2007;32:328–333 [DOI] [PubMed] [Google Scholar]
- 16.Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ, Mensah GA, Criqui MH. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: A systematic review and analysis. Lancet. 2013;382:1329–1340 [DOI] [PubMed] [Google Scholar]
- 17.Wikstrom J, Hansen T, Johansson L, Lind L, Ahlstrom H. Ankle brachial index <0.9 underestimates the prevalence of peripheral artery occlusive disease assessed with whole-body magnetic resonance angiography in the elderly. Acta radiologica. 2008;49:143–149 [DOI] [PubMed] [Google Scholar]
- 18.Dachun X, Jue L, Liling Z, Yawei X, Dayi H, Pagoto SL, Yunsheng M. Sensitivity and specificity of the ankle--brachial index to diagnose peripheral artery disease: A structured review. Vascular medicine. 2010;15:361–369 [DOI] [PubMed] [Google Scholar]
- 19.Criqui MH, Langer RD, Fronek A, Feigelson HS, Klauber MR, McCann TJ, Browner D. Mortality over a period of 10 years in patients with peripheral arterial disease. The New England journal of medicine. 1992;326:381–386 [DOI] [PubMed] [Google Scholar]
- 20.Newman AB, Shemanski L, Manolio TA, Cushman M, Mittelmark M, Polak JF, Powe NR, Siscovick D. Ankle-arm index as a predictor of cardiovascular disease and mortality in the cardiovascular health study. The cardiovascular health study group. Arteriosclerosis, thrombosis, and vascular biology. 1999;19:538–545 [DOI] [PubMed] [Google Scholar]
- 21.Kannel WB, McGee DL. Update on some epidemiologic features of intermittent claudication: The framingham study. Journal of the American Geriatrics Society. 1985;33:13–18 [DOI] [PubMed] [Google Scholar]
- 22.Dagenais GR, Maurice S, Robitaille NM, Gingras S, Lupien PJ. Intermittent claudication in quebec men from 1974–1986: The quebec cardiovascular study. Clinical and investigative medicine. Medecine clinique et experimentale. 1991;14:93–100 [PubMed] [Google Scholar]
- 23.Jager A, Kostense PJ, Ruhe HG, Heine RJ, Nijpels G, Dekker JM, Bouter LM, Stehouwer CD. Microalbuminuria and peripheral arterial disease are independent predictors of cardiovascular and all-cause mortality, especially among hypertensive subjects: Five-year follow-up of the hoorn study. Arteriosclerosis, thrombosis, and vascular biology. 1999;19:617–624 [DOI] [PubMed] [Google Scholar]
- 24.Hooi JD, Stoffers HE, Kester AD, van RJ, Knottnerus JA. Peripheral arterial occlusive disease: Prognostic value of signs, symptoms, and the ankle-brachial pressure index. Medical decision making : an international journal of the Society for Medical Decision Making. 2002;22:99–107 [DOI] [PubMed] [Google Scholar]
- 25.Lee AJ, Price JF, Russell MJ, Smith FB, van Wijk MC, Fowkes FG. Improved prediction of fatal myocardial infarction using the ankle brachial index in addition to conventional risk factors: The edinburgh artery study. Circulation. 2004;110:3075–3080 [DOI] [PubMed] [Google Scholar]
- 26.Criqui MH, McClelland RL, McDermott MM, Allison MA, Blumenthal RS, Aboyans V, Ix JH, Burke GL, Liu K, Shea S. The ankle-brachial index and incident cardiovascular events in the mesa (multi-ethnic study of atherosclerosis). Journal of the American College of Cardiology. 2010;56:1506–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Committee CS. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (caprie). Caprie steering committee. Lancet. 1996;348:1329–1339 [DOI] [PubMed] [Google Scholar]
- 28.Ouriel K Peripheral arterial disease. Lancet. 2001;358:1257–1264 [DOI] [PubMed] [Google Scholar]
- 29.Hertzer NR, Beven EG, Young JR, O’Hara PJ, Ruschhaupt WF 3rd, Graor RA, Dewolfe VG, Maljovec LC. Coronary artery disease in peripheral vascular patients. A classification of 1000 coronary angiograms and results of surgical management. Annals of surgery. 1984;199:223–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Veronese N, Trevisan C, De Rui M, Bolzetta F, Maggi S, Zambon S, Musacchio E, Sartori L, Perissinotto E, Crepaldi G, Manzato E, Sergi G. Association of osteoarthritis with increased risk of cardiovascular diseases in the elderly: Findings from the progetto veneto anziano study cohort. Arthritis & rheumatology. 2016;68:1136–1144 [DOI] [PubMed] [Google Scholar]
- 31.Gielis WP, Welsing PMJ, van Spil WE, Runhaar J, Weinans H, de Jong PA. A sex-specific association between incident radiographic osteoarthritis of hip or knee and incident peripheral arterial calcifications: 8-year prospective data from cohort hip and cohort knee (check). Osteoarthritis and cartilage. 2017;25:1814–1821 [DOI] [PubMed] [Google Scholar]
- 32.Veronese N, Stubbs B, Solmi M, Smith TO, Reginster JY, Maggi S. Osteoarthristis increases the risk of cardiovascular disease: Data from the osteoarthritis initiative. The journal of nutrition, health & aging. 2018;22:371–376 [DOI] [PubMed] [Google Scholar]
- 33.Park IH, Lee SC, Park IS, Nam CH, Ahn HS, Park HY, Gondalia VH, Jung KA. Asymptomatic peripheral vascular disease in total knee arthroplasty: Preoperative prevalence and risk factors. Journal of orthopaedics and traumatology : official journal of the Italian Society of Orthopaedics and Traumatology. 2015;16:23–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernandes GS, Valdes AM. Cardiovascular disease and osteoarthritis: Common pathways and patient outcomes. European journal of clinical investigation. 2015;45:405–414 [DOI] [PubMed] [Google Scholar]
- 35.Hoeven TA, Leening MJ, Bindels PJ, Castano-Betancourt M, van Meurs JB, Franco OH, Kavousi M, Hofman A, Ikram MA, Witteman JC, Bierma-Zeinstra SM. Disability and not osteoarthritis predicts cardiovascular disease: A prospective population-based cohort study. Annals of the rheumatic diseases. 2015;74:752–756 [DOI] [PubMed] [Google Scholar]
- 36.Kendzerska T, Juni P, King LK, Croxford R, Stanaitis I, Hawker GA. The longitudinal relationship between hand, hip and knee osteoarthritis and cardiovascular events: A population-based cohort study. Osteoarthritis and cartilage. 2017;25:1771–1780 [DOI] [PubMed] [Google Scholar]
- 37.Wallis JA, Webster KE, Levinger P, Singh PJ, Fong C, Taylor NF. A walking program for people with severe knee osteoarthritis did not reduce pain but may have benefits for cardiovascular health: A phase ii randomised controlled trial. Osteoarthritis and cartilage. 2017;25:1969–1979 [DOI] [PubMed] [Google Scholar]
- 38.Haugen IK, Ramachandran VS, Misra D, Neogi T, Niu J, Yang T, Zhang Y, Felson DT. Hand osteoarthritis in relation to mortality and incidence of cardiovascular disease: Data from the framingham heart study. Annals of the rheumatic diseases. 2015;74:74–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gibbons GH, Dzau VJ. The emerging concept of vascular remodeling. The New England journal of medicine. 1994;330:1431–1438 [DOI] [PubMed] [Google Scholar]
- 40.Kim YS, Lim SH, Oh KW, Kim JY, Koh SH, Kim J, Heo SH, Chang DI, Lee YJ, Kim HY. The advantage of high-resolution mri in evaluating basilar plaques: A comparison study with mra. Atherosclerosis. 2012;224:411–416 [DOI] [PubMed] [Google Scholar]
- 41.Zhou C, Qiao H, He L, Yuan C, Chen H, Zhang Q, Li R, Wang W, Du F, Li C, Zhao X. Characterization of atherosclerotic disease in thoracic aorta: A 3d, multicontrast vessel wall imaging study. European journal of radiology. 2016;85:2030–2035 [DOI] [PubMed] [Google Scholar]
- 42.Liu CY, Chen D, Bluemke DA, Wu CO, Teixido-Tura G, Chugh A, Vasu S, Lima JA, Hundley WG. Evolution of aortic wall thickness and stiffness with atherosclerosis: Long-term follow up from the multi-ethnic study of atherosclerosis. Hypertension. 2015;65:1015–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qiao Y, Guallar E, Suri FK, Liu L, Zhang Y, Anwar Z, Mirbagheri S, Xie YJ, Nezami N, Intrapiromkul J, Zhang S, Alonso A, Chu H, Couper D, Wasserman BA. Mr imaging measures of intracranial atherosclerosis in a population-based study. Radiology. 2016;280:860–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khera A, de Lemos JA, Peshock RM, Lo HS, Stanek HG, Murphy SA, Wians FH, Jr., Grundy SM, McGuire DK. Relationship between c-reactive protein and subclinical atherosclerosis: The dallas heart study. Circulation. 2006;113:38–43 [DOI] [PubMed] [Google Scholar]
- 45.Chu B, Kampschulte A, Ferguson MS, Kerwin WS, Yarnykh VL, O’Brien KD, Polissar NL, Hatsukami TS, Yuan C. Hemorrhage in the atherosclerotic carotid plaque: A high-resolution mri study. Stroke. 2004;35:1079–1084 [DOI] [PubMed] [Google Scholar]
- 46.Chu B, Ferguson MS, Underhill H, Takaya N, Cai J, Kliot M, Yuan C, Hatsukami TS. Images in cardiovascular medicine. Detection of carotid atherosclerotic plaque ulceration, calcification, and thrombosis by multicontrast weighted magnetic resonance imaging. Circulation. 2005;112:e3–4 [DOI] [PubMed] [Google Scholar]
- 47.Mitsumori LM, Hatsukami TS, Ferguson MS, Kerwin WS, Cai J, Yuan C. In vivo accuracy of multisequence mr imaging for identifying unstable fibrous caps in advanced human carotid plaques. Journal of magnetic resonance imaging : JMRI. 2003;17:410–420 [DOI] [PubMed] [Google Scholar]
- 48.Zhao X, Zhao Q, Chu B, Yang Y, Li F, Zhou XH, Cai J, Cai Z, Yuan C. Prevalence of compositional features in subclinical carotid atherosclerosis determined by high-resolution magnetic resonance imaging in chinese patients with coronary artery disease. Stroke. 2010;41:1157–1162 [DOI] [PubMed] [Google Scholar]
- 49.Cai J, Hatsukami TS, Ferguson MS, Kerwin WS, Saam T, Chu B, Takaya N, Polissar NL, Yuan C. In vivo quantitative measurement of intact fibrous cap and lipid-rich necrotic core size in atherosclerotic carotid plaque: Comparison of high-resolution, contrast-enhanced magnetic resonance imaging and histology. Circulation. 2005;112:3437–3444 [DOI] [PubMed] [Google Scholar]
- 50.Chu B, Hatsukami TS, Polissar NL, Zhao XQ, Kraiss LW, Parker DL, Waterton JC, Raichlen JS, Hamar W, Yuan C. Determination of carotid artery atherosclerotic lesion type and distribution in hypercholesterolemic patients with moderate carotid stenosis using noninvasive magnetic resonance imaging. Stroke. 2004;35:2444–2448 [DOI] [PubMed] [Google Scholar]
- 51.Macedo R, Chen S, Lai S, Shea S, Malayeri AA, Szklo M, Lima JA, Bluemke DA. Mri detects increased coronary wall thickness in asymptomatic individuals: The multi-ethnic study of atherosclerosis (mesa). Journal of magnetic resonance imaging : JMRI. 2008;28:1108–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gerretsen SC, Kooi ME, Kessels AG, Schalla S, Katoh M, van der Geest RJ, Manning WJ, Waltenberger J, van Engelshoven JM, Botnar RM, Leiner T. Visualization of coronary wall atherosclerosis in asymptomatic subjects and patients with coronary artery disease using magnetic resonance imaging. PloS one. 2010;5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. The New England journal of medicine. 1987;316:1371–1375 [DOI] [PubMed] [Google Scholar]
- 54.Zhao X, Hippe DS, Li R, Canton GM, Sui B, Song Y, Li F, Xue Y, Sun J, Yamada K, Hatsukami TS, Xu D, Wang M, Yuan C, Collaborators C-IS. Prevalence and characteristics of carotid artery high-risk atherosclerotic plaques in chinese patients with cerebrovascular symptoms: A chinese atherosclerosis risk evaluation ii study. Journal of the American Heart Association. 2017;6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yuan C, Zhang SX, Polissar NL, Echelard D, Ortiz G, Davis JW, Ellington E, Ferguson MS, Hatsukami TS. Identification of fibrous cap rupture with magnetic resonance imaging is highly associated with recent transient ischemic attack or stroke. Circulation. 2002;105:181–185 [DOI] [PubMed] [Google Scholar]
- 56.Takaya N, Yuan C, Chu B, Saam T, Polissar NL, Jarvik GP, Isaac C, McDonough J, Natiello C, Small R, Ferguson MS, Hatsukami TS. Presence of intraplaque hemorrhage stimulates progression of carotid atherosclerotic plaques: A high-resolution magnetic resonance imaging study. Circulation. 2005;111:2768–2775 [DOI] [PubMed] [Google Scholar]
- 57.Takaya N, Yuan C, Chu B, Saam T, Underhill H, Cai J, Tran N, Polissar NL, Isaac C, Ferguson MS, Garden GA, Cramer SC, Maravilla KR, Hashimoto B, Hatsukami TS. Association between carotid plaque characteristics and subsequent ischemic cerebrovascular events: A prospective assessment with mri--initial results. Stroke. 2006;37:818–823 [DOI] [PubMed] [Google Scholar]
- 58.McDermott MM, Kramer CM, Tian L, Carr J, Guralnik JM, Polonsky T, Carroll T, Kibbe M, Criqui MH, Ferrucci L, Zhao L, Hippe DS, Wilkins J, Xu D, Liao Y, McCarthy W, Yuan C. Plaque composition in the proximal superficial femoral artery and peripheral artery disease events. JACC. Cardiovascular imaging. 2017;10:1003–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun J, Zhao XQ, Balu N, Neradilek MB, Isquith DA, Yamada K, Canton G, Crouse JR, Anderson TJ 3rd, Huston J 3rd, O’Brien K, Hippe DS, Polissar NL, Yuan C, Hatsukami TS. Carotid plaque lipid content and fibrous cap status predict systemic cv outcomes: The mri substudy in aim-high. JACC. Cardiovascular imaging. 2017;10:241–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luo Y, Polissar N, Han C, Yarnykh V, Kerwin WS, Hatsukami TS, Yuan C. Accuracy and uniqueness of three in vivo measurements of atherosclerotic carotid plaque morphology with black blood mri. Magnetic resonance in medicine. 2003;50:75–82 [DOI] [PubMed] [Google Scholar]
- 61.Wang J, Yarnykh VL, Hatsukami T, Chu B, Balu N, Yuan C. Improved suppression of plaque-mimicking artifacts in black-blood carotid atherosclerosis imaging using a multislice motion-sensitized driven-equilibrium (msde) turbo spin-echo (tse) sequence. Magnetic resonance in medicine. 2007;58:973–981 [DOI] [PubMed] [Google Scholar]
- 62.Koktzoglou I, Li D. Diffusion-prepared segmented steady-state free precession: Application to 3d black-blood cardiovascular magnetic resonance of the thoracic aorta and carotid artery walls. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2007;9:33–42 [DOI] [PubMed] [Google Scholar]
- 63.Langham MC, Desjardins B, Englund EK, Mohler ER 3rd, Floyd TF, Wehrli FW. Rapid high-resolution, self-registered, dual lumen-contrast mri method for vessel-wall assessment in peripheral artery disease:: A preliminary investigation. Academic radiology. 2016;23:457–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kruse RR, Doomernik DE, Maltha KV, Kooloos JGM, Kozicz TL, Reijnen M. Collateral artery pathways of the femoral and popliteal artery. The Journal of surgical research. 2017;211:45–52 [DOI] [PubMed] [Google Scholar]
- 65.Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: Report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis and cartilage. 2008;16:1433–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chambless LE, Folsom AR, Davis V, Sharrett R, Heiss G, Sorlie P, Szklo M, Howard G, Evans GW. Risk factors for progression of common carotid atherosclerosis: The atherosclerosis risk in communities study, 1987–1998. American journal of epidemiology. 2002;155:38–47 [DOI] [PubMed] [Google Scholar]
- 67.Elias-Smale SE, Kardys I, Oudkerk M, Hofman A, Witteman JC. C-reactive protein is related to extent and progression of coronary and extra-coronary atherosclerosis; results from the rotterdam study. Atherosclerosis. 2007;195:e195–202 [DOI] [PubMed] [Google Scholar]
- 68.Esposito K, Giugliano D, Nappo F, Marfella R, Campanian Postprandial Hyperglycemia Study G. Regression of carotid atherosclerosis by control of postprandial hyperglycemia in type 2 diabetes mellitus. Circulation. 2004;110:214–219 [DOI] [PubMed] [Google Scholar]
- 69.Nicholls SJ, Tuzcu EM, Sipahi I, Grasso AW, Schoenhagen P, Hu T, Wolski K, Crowe T, Desai MY, Hazen SL, Kapadia SR, Nissen SE. Statins, high-density lipoprotein cholesterol, and regression of coronary atherosclerosis. Jama. 2007;297:499–508 [DOI] [PubMed] [Google Scholar]
- 70.Oai publications listing.2018
- 71.Eckstein F, Kwoh CK, Link TM, investigators OAI. Imaging research results from the osteoarthritis initiative (oai): A review and lessons learned 10 years after start of enrolment. Annals of the rheumatic diseases. 2014;73:1289–1300 [DOI] [PubMed] [Google Scholar]
- 72.Driban JB, Eaton CB, Amin M, Stout AC, Price LL, Lu B, Lo GH, McAlindon TE, Barbe MF. Glucose homeostasis influences the risk of incident knee osteoarthritis: Data from the osteoarthritis initiative. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2017;35:2282–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dunlop DD, Song J, Semanik PA, Sharma L, Bathon JM, Eaton CB, Hochberg MC, Jackson RD, Kwoh CK, Mysiw WJ, Nevitt MC, Chang RW. Relation of physical activity time to incident disability in community dwelling adults with or at risk of knee arthritis: Prospective cohort study. Bmj. 2014;348:g2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu SH, Driban JB, Eaton CB, McAlindon TE, Harrold LR, Lapane KL. Objectively measured physical activity and symptoms change in knee osteoarthritis. The American journal of medicine. 2016;129:497–505 e491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dunlop DD, Song J, Lee J, Gilbert AL, Semanik PA, Ehrlich-Jones L, Pellegrini CA, Pinto D, Ainsworth B, Chang RW. Physical activity minimum threshold predicting improved function in adults with lower-extremity symptoms. Arthritis care & research. 2017;69:475–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bindawas SM. Relationship between frequent knee pain, obesity, and gait speed in older adults: Data from the osteoarthritis initiative. Clinical interventions in aging. 2016;11:237–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dai Z, Lu N, Niu J, Felson DT, Zhang Y. Dietary fiber intake in relation to knee pain trajectory. Arthritis care & research. 2017;69:1331–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lu B, Ahmad O, Zhang FF, Driban JB, Duryea J, Lapane KL, McAlindon T, Eaton CB. Soft drink intake and progression of radiographic knee osteoarthritis: Data from the osteoarthritis initiative. BMJ open. 2013;3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lu B, Driban JB, Xu C, Lapane KL, McAlindon TE, Eaton CB. Dietary fat intake and radiographic progression of knee osteoarthritis: Data from the osteoarthritis initiative. Arthritis care & research. 2017;69:368–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang FF, Driban JB, Lo GH, Price LL, Booth S, Eaton CB, Lu B, Nevitt M, Jackson B, Garganta C, Hochberg MC, Kwoh K, McAlindon TE. Vitamin d deficiency is associated with progression of knee osteoarthritis. The Journal of nutrition. 2014;144:2002–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Driban JB, Eaton CB, Lo GH, Price LL, Lu B, Barbe MF, McAlindon TE. Overweight older adults, particularly after an injury, are at high risk for accelerated knee osteoarthritis: Data from the osteoarthritis initiative. Clinical rheumatology. 2016;35:1071–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Laberge MA, Baum T, Virayavanich W, Nardo L, Nevitt MC, Lynch J, McCulloch CE, Link TM. Obesity increases the prevalence and severity of focal knee abnormalities diagnosed using 3t mri in middle-aged subjects--data from the osteoarthritis initiative. Skeletal radiology. 2012;41:633–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hafezi-Nejad N, Guermazi A, Roemer FW, Eng J, Zikria B, Demehri S. Long term use of analgesics and risk of osteoarthritis progressions and knee replacement: Propensity score matched cohort analysis of data from the osteoarthritis initiative. Osteoarthritis and cartilage. 2016;24:597–604 [DOI] [PubMed] [Google Scholar]
- 84.Gersing AS, Solka M, Joseph GB, Schwaiger BJ, Heilmeier U, Feuerriegel G, Nevitt MC, McCulloch CE, Link TM. Progression of cartilage degeneration and clinical symptoms in obese and overweight individuals is dependent on the amount of weight loss: 48-month data from the osteoarthritis initiative. Osteoarthritis and cartilage. 2016;24:1126–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Veronese N, Stubbs B, Noale M, Solmi M, Luchini C, Smith TO, Cooper C, Guglielmi G, Reginster JY, Rizzoli R, Maggi S. Adherence to a mediterranean diet is associated with lower prevalence of osteoarthritis: Data from the osteoarthritis initiative. Clinical nutrition. 2017;36:1609–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Veronese N, Stubbs B, Noale M, Solmi M, Luchini C, Maggi S. Adherence to the mediterranean diet is associated with better quality of life: Data from the osteoarthritis initiative. The American journal of clinical nutrition. 2016;104:1403–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fernandez-Moreno M, Soto-Hermida A, Vazquez-Mosquera ME, Cortes-Pereira E, Relano S, Hermida-Gomez T, Pertega S, Oreiro-Villar N, Fernandez-Lopez C, Garesse R, Blanco FJ, Rego-Perez I. Mitochondrial DNA haplogroups influence the risk of incident knee osteoarthritis in oai and check cohorts. A meta-analysis and functional study. Annals of the rheumatic diseases. 2017;76:1114–1122 [DOI] [PubMed] [Google Scholar]
- 88.Soto-Hermida A, Fernandez-Moreno M, Oreiro N, Fernandez-Lopez C, Pertega S, Cortes-Pereira E, Rego-Perez I, Blanco FJ. Mitochondrial DNA (mtdna) haplogroups influence the progression of knee osteoarthritis. Data from the osteoarthritis initiative (oai). PloS one. 2014;9:e112735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rathbun AM, Yau MS, Shardell M, Stuart EA, Hochberg MC. Depressive symptoms and structural disease progression in knee osteoarthritis: Data from the osteoarthritis initiative. Clinical rheumatology. 2017;36:155–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lo GH, Driban JB, Kriska AM, McAlindon TE, Souza RB, Petersen NJ, Storti KL, Eaton CB, Hochberg MC, Jackson RD, Kent Kwoh C, Nevitt MC, Suarez-Almazor ME. Is there an association between a history of running and symptomatic knee osteoarthritis? A cross-sectional study from the osteoarthritis initiative. Arthritis care & research. 2017;69:183–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Riddle DL, Moxley G, Dumenci L. Associations between statin use and changes in pain, function and structural progression: A longitudinal study of persons with knee osteoarthritis. Annals of the rheumatic diseases. 2013;72:196–203 [DOI] [PubMed] [Google Scholar]
- 92.Dube CE, Liu SH, Driban JB, McAlindon TE, Eaton CB, Lapane KL. The relationship between smoking and knee osteoarthritis in the osteoarthritis initiative. Osteoarthritis and cartilage. 2016;24:465–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Song J, Gilbert AL, Chang RW, Pellegrini CA, Ehrlich-Jones LS, Lee J, Pinto D, Semanik PA, Sharma L, Kwoh CK, Jackson RD, Dunlop DD. Do inactive older adults who increase physical activity experience less disability: Evidence from the osteoarthritis initiative. Journal of clinical rheumatology : practical reports on rheumatic & musculoskeletal diseases. 2017;23:26–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Song J, Lindquist LA, Chang RW, Semanik PA, Ehrlich-Jones LS, Lee J, Sohn MW, Dunlop DD. Sedentary behavior as a risk factor for physical frailty independent of moderate activity: Results from the osteoarthritis initiative. American journal of public health. 2015;105:1439–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.White DK, Neogi T, Zhang Y, Niu J, Katz PP. Association of slow gait speed with trajectories of orsening depressive symptoms in knee osteoarthritis: An observational study. Arthritis care & research. 2017;69:209–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Batsis JA, Zbehlik AJ, Barre LK, Bynum JP, Pidgeon D, Bartels SJ. Impact of obesity on disability, function, and physical activity: Data from the osteoarthritis initiative. Scandinavian journal of rheumatology. 2015;44:495–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Batsis JA, Zbehlik AJ, Scherer EA, Barre LK, Bartels SJ. Normal weight with central obesity, physical activity, and functional decline: Data from the osteoarthritis initiative. Journal of the American Geriatrics Society. 2015;63:1552–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Batsis JA, Zbehlik AJ, Barre LK, Mackenzie TA, Bartels SJ. The impact of waist circumference on function and physical activity in older adults: Longitudinal observational data from the osteoarthritis initiative. Nutrition journal. 2014;13:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Veronese N, Stubbs B, Noale M, Solmi M, Vaona A, Demurtas J, Nicetto D, Crepaldi G, Schofield P, Koyanagi A, Maggi S, Fontana L. Fried potato consumption is associated with elevated mortality: An 8-y longitudinal cohort study. The American journal of clinical nutrition. 2017;106:162–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sohn MW, Manheim LM, Chang RW, Greenland P, Hochberg MC, Nevitt MC, Semanik PA, Dunlop DD. Sedentary behavior and blood pressure control among osteoarthritis initiative participants. Osteoarthritis and cartilage. 2014;22:1234–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.