Atherosclerosis, the leading cause of cardiovascular disease (CVD), is considered as both a disorder of lipid metabolism and a chronic inflammatory disease in the arterial wall 1. The trans-endothelial transport of pro-atherogenic lipoproteins [apo-B containing lipoproteins; low-density lipoproteins (LDL) and very-low density lipoproteins (VLDL)] from the circulation into the arterial wall and their accumulation in the inner lining of arteries initialize and promote the progression of atherosclerosis2. Unlike the classical cellular LDL uptake mediated by LDL receptor (LDLR)3, recent studies have identified caveolae/caveolin-1 (Cav-1), scavenger receptor B1 (SR-B1) and activin receptor-like kinase 1 (ALK1) as critical regulators of LDL transcytosis through the endothelium4–7. However, the mechanisms by which LDL transcytoses across the endothelial cell (EC) barrier and enters the sub-endothelial space is still not fully understood.
In this issue of Arterioscler Thromb Vasc Biol, Ghaffari and colleagues provide striking evidence that high mobility group box 1 (HMGB1) promotes the transcytosis of LDL in ECs.8 HMGB1 is a damage-associated molecular pattern (DAMP) protein secreted from ECs and leukocytes and acts as a critical pro-inflammatory mediator9–12. Previous reports have shown that HMGB1 is highly expressed in atherosclerotic lesions13 and levels of circulating HMGB1 positively correlated with the severity of atherosclerosis and coronary artery stenosis14. Notably, neutralization of HMGB1 significantly reduced the development of atherosclerosis14, 15, underscoring the biological relevance of HMGB1 in atherogenesis. In response to cellular stress, HMGB1 translocates from the nuclei to the cytosol and releases from cells, where exerts its biological functions by binding to several receptors including advanced glycation end products (RAGE) and Toll-like receptor 4 (TLR4)9. Looking for additional mechanisms that could mediate the pro-atherogenic effects of HMGB1, the authors found that suppression of HMGB1 by RNA interference (RNAi) reduced LDL transcytosis in human coronary artery EC, whereas overexpression of HMGB1 increased the transport of LDL across EC. Mechanistically, knockdown of HMGB1 in ECs resulted in a significant reduction in scavenger receptor B1 (SR-B1) expression, without affecting the levels of ALK1 and Cav-1 expression. Compared to knockdown either SR-B1 or HMGB1, combinatorial suppression of both SR-B1 and HMGB1 did not achieve any further reduction of LDL transcytosis, indicating that HMGB1 regulates LDL transcytosis through SR-B1-dependent mechanism. Interestingly, inhibition of HMGB1 release into the extracellular space by inhibitor glycyrrhizin and knockdown of RAGE, the main cell surface receptor for HMGB1, did not influence HMGB1-mediated LDL transcytosis, suggesting a intracellular role of HMGB1 in regulating LDL transcytosis in EC. However, the potential role of other HMGB1 receptors such as TLR4 in regulating LDL transcytosis was not evaluated this study and remains to be elucidated.
To glean mechanistic insights into how HMGB1 promotes SR-B1-dependent LDL transcytosis, Ghaffari and colleagues focused on the expression and activation of the sterol regulatory element-binding protein 2 (SREBP2), a transcription factor that regulates SR-B1 expression. The authors found that incubation of EC with LDL significantly increased total HMGB1 expression and its translocation from cytosol to nuclei, which was partially dependent on SR-B1 but not LDLR. Importantly, the redistribution of HMGB1 to nuclei stabilized SREBP2 and prolonged its half-life, leading to an increase expression of SR-B1. The relevance of SREBP2 in regulating HMGB1-mediated LDL transcytosis was further supported by the impaired ability of HMGB1 overexpression in promoting LDL transcytosis in ECs transfected with SREBP2 siRNA. In an effort to translate these in vitro studies to in vivo atherosclerosis models, the authors showed that the accumulation of LDL in the arterial wall of hyperlipidemic mice was significantly attenuated in endothelial HMGB1 deficient mice, which led to fewer fatty streaks and less atherosclerosis.
While the biological functions of extracellular HMGB1 in inflammation are well-known, the most relevant finding of Ghaffari’s study is the characterization of a novel intracellular role HMGB1 in regulating LDL transcytosis in ECs. Previous reports have shown the anti-inflammatory effects of intracellular HMGB1 involved in autophagy and inhibition of inflammatory nucleosomes to block inflammation.16, 17 In macrophages, intracellular HMGB1 interacts with Src Kinase to diminish the phagocytic ability.18 The regulation of intracellular HMGB1 in LDL transcytosis provides additional mechanism by which HMGB1 aggravates atherogenesis synergistically with pro-inflammatory activity of extracellular HMGB1. Another interesting finding from Ghaffari’s study is that HMGB1 specifically upregulates SR-B1, but not Cav-1 and ALK1, to promote the transcytosis of LDL across EC. SR-B1 is a receptor for both LDL and high-density lipoprotein (HDL). The major anti-atherosclerotic effect of HDL is the removal of cholesterol from foam cells in the atherosclerotic plaques and the transport of cholesterol to the liver, which is known as reverse cholesterol transport (RCT). Thus, It will be important to define whether HMGB1 promotes lipoproteins exit out of the arteries by regulating SR-B1 expression in ECs. Additionally, other beneficial effects in the vasculature such as the activation endothelial nitric oxide (eNOS) by HDL-SR-B1 pathway should be also assessed in mice lacking HMGB1 in the endothelium.
In summary, the study by Ghaffari et al. provides the first evidence that intracellular HMGB1 in ECs promotes LDL transcytosis through SREBP2-SR-B1 axis (Figure) and raises the possibility to treat atherosclerosis by regulating LDL transcytosis though this novel mechanism. Nevertheless, there are some important questions yet to be explored. Hyperlipidemia induced the relocalization of HMGB1 from nuclear to cytosolic fraction and stimulated release of the HMGB1 protein in macrophages.19 Whether redistribution of HMGB1 to nuclei induced by LDL leads to impaired HMGB1 secretion in ECs should be studied. How does LDL regulate the redistribution of HMGB1 in a cell-specific manner?, and What are the mechanisms by which SR-B1 promotes the relocalization of HMGB1 from cytosol to nuclei under LDL treatment? are also important questions to be addressed. Lastly, Cav-1 and caveolae are critical regulators in LDL transcytosis4, 6 and their expression and cellular distribution in vitro is not altered in HMGB1 silencing cells. However, SR-B1 directly binds to LDL and locates in caveolae (Figure), thus further studies are required to investigate the involvement of Cav-1 and caveolae in HMGB1-mediated LDL transcytosis in an in vivo genetically engineered model.
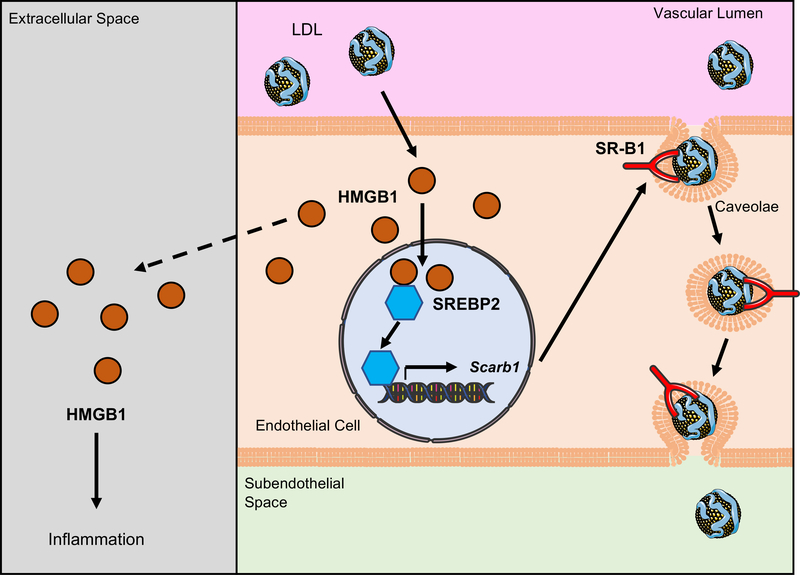
Figure. Regulation of endothelial high mobility group box 1 (HMGB1) in low density lipoprotein (LDL) transcytosis.
LDL induces increased HMGB1 expression and redistribution of HMGB1 from cytosol to nuclei in endothelial cells (ECs), where nuclear HMGB1 stabilizes SREBP2 and prolongs its half-life to upregulate scavenger receptor B1 (SR-B1) expression. SR-B1 locates in caveolae and mediates the transcytosis of LDL across EC layer and accumulation of LDL in subendothelial space. On the other side, extracellular HMGB1 released from cells under cellular stress triggers the inflammatory response acting as a damage-associated molecular pattern (DAMP). Intracellular and extracellular HMGB1 synergistically accelerate atherogenesis by facilitating LDL transcytosis and inflammation.
Acknowledgments
SOURCES OF FUNDING
This work was supported by grants from the National Institutes of Health (R35HL135820 to C. Fernández-Hernando), the American Heart Association (16EIA27550005 to C. Fernández-Hernando).
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glass CK, Witztum JL. Atherosclerosis. The road ahead. Cell. 2001;104:503–516. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein JL, Brown MS. The ldl receptor. Arterioscler Thromb Vasc Biol. 2009;29:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramirez CM, Zhang X, Bandyopadhyay C, Rotllan N, Sugiyama MG, Aryal B, Liu X, He S, Kraehling JR, Ulrich V, Lin CS, Velazquez H, Lasuncion MA, Li G, Suarez Y, Tellides G, Swirski FK, Lee WL, Schwartz MA, Sessa WC, Fernandez-Hernando C. Caveolin-1 regulates atherogenesis by attenuating low-density lipoprotein transcytosis and vascular inflammation independently of endothelial nitric oxide synthase activation. Circulation. 2019;140:225–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kraehling JR, Chidlow JH, Rajagopal C, Sugiyama MG, Fowler JW, Lee MY, Zhang X, Ramirez CM, Park EJ, Tao B, Chen K, Kuruvilla L, Larrivee B, Folta-Stogniew E, Ola R, Rotllan N, Zhou W, Nagle MW, Herz J, Williams KJ, Eichmann A, Lee WL, Fernandez-Hernando C, Sessa WC. Genome-wide rnai screen reveals alk1 mediates ldl uptake and transcytosis in endothelial cells. Nat Commun. 2016;7:13516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandez-Hernando C, Yu J, Suarez Y, Rahner C, Davalos A, Lasuncion MA, Sessa WC. Genetic evidence supporting a critical role of endothelial caveolin-1 during the progression of atherosclerosis. Cell Metab. 2009;10:48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang L, Chambliss KL, Gao X, Yuhanna IS, Behling-Kelly E, Bergaya S, Ahmed M, Michaely P, Luby-Phelps K, Darehshouri A, Xu L, Fisher EA, Ge WP, Mineo C, Shaul PW. Sr-b1 drives endothelial cell ldl transcytosis via dock4 to promote atherosclerosis. Nature. 2019;569:565–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghaffari S, Jang E, Nabi FN, Sanwal R, Khosraviani N, Wang C, Steinberg BE, Goldenberg NM, Ikeda J, Lee WL. Endothelial hmgb1 is a critical regulator of ldl transcytosis via an srebp2-sr-bi axis. Arterioscler Thromb Vasc Biol. 2020:ATVBAHA120314557. [DOI] [PubMed] [Google Scholar]
- 9.Yang H, Wang H, Chavan SS, Andersson U. High mobility group box protein 1 (hmgb1): The prototypical endogenous danger molecule. Mol Med. 2015;21 Suppl 1:S6–S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang T, Cao G, Meng X, Ouyang C, Gao J, Sun Y, Wu J, Min Q, Zhang C, Zhang W. Lethal giant larvae 1 inhibits smooth muscle calcification via high mobility group box 1. J Mol Cell Cardiol. 2020;142:39–52. [DOI] [PubMed] [Google Scholar]
- 11.Yang J, Huang C, Yang J, Jiang H, Ding J. Statins attenuate high mobility group box-1 protein induced vascular endothelial activation : A key role for tlr4/nf-kappab signaling pathway. Mol Cell Biochem. 2010;345:189–195. [DOI] [PubMed] [Google Scholar]
- 12.Ding JW, Zhou T, Zheng XX, Wang XA, Tong XH, Luo CY, Zhang ZQ, Yu B. The effects of high mobility group box-1 protein on peripheral treg/th17 balance in patients with atherosclerosis. Acta Cardiol Sin. 2018;34:399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalinina N, Agrotis A, Antropova Y, DiVitto G, Kanellakis P, Kostolias G, Ilyinskaya O, Tararak E, Bobik A. Increased expression of the DNA-binding cytokine hmgb1 in human atherosclerotic lesions: Role of activated macrophages and cytokines. Arterioscler Thromb Vasc Biol. 2004;24:2320–2325. [DOI] [PubMed] [Google Scholar]
- 14.Hu X, Jiang H, Bai Q, Zhou X, Xu C, Lu Z, Cui B, Wen H. Increased serum hmgb1 is related to the severity of coronary artery stenosis. Clin Chim Acta. 2009;406:139–142. [DOI] [PubMed] [Google Scholar]
- 15.Kanellakis P, Agrotis A, Kyaw TS, Koulis C, Ahrens I, Mori S, Takahashi HK, Liu K, Peter K, Nishibori M, Bobik A. High-mobility group box protein 1 neutralization reduces development of diet-induced atherosclerosis in apolipoprotein e-deficient mice. Arterioscler Thromb Vasc Biol. 2011;31:313–319. [DOI] [PubMed] [Google Scholar]
- 16.Zhu X, Messer JS, Wang Y, Lin F, Cham CM, Chang J, Billiar TR, Lotze MT, Boone DL, Chang EB. Cytosolic hmgb1 controls the cellular autophagy/apoptosis checkpoint during inflammation. J Clin Invest. 2015;125:1098–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang R, Zhang Q, Hou W, Yan Z, Chen R, Bonaroti J, Bansal P, Billiar TR, Tsung A, Wang Q, Bartlett DL, Whitcomb DC, Chang EB, Zhu X, Wang H, Lu B, Tracey KJ, Cao L, Fan XG, Lotze MT, Zeh HJ 3rd, Tang D. Intracellular hmgb1 inhibits inflammatory nucleosome release and limits acute pancreatitis in mice. Gastroenterology. 2014;146:1097–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banerjee S, de Freitas A, Friggeri A, Zmijewski JW, Liu G, Abraham E. Intracellular hmgb1 negatively regulates efferocytosis. J Immunol. 2011;187:4686–4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haraba R, Suica VI, Uyy E, Ivan L, Antohe F. Hyperlipidemia stimulates the extracellular release of the nuclear high mobility group box 1 protein. Cell Tissue Res. 2011;346:361–368 [DOI] [PubMed] [Google Scholar]