Abstract
Colorectal cancers (CRCs) with deficient mismatch repair (dMMR) or microsatellite instability-high (MSI-H) often have sustained responses to immune checkpoint inhibitors (ICIs) including selective monoclonal antibodies against Program Death 1 (PD-1), Programmed Death Ligand 1(PD-L1), and cytotoxic T lymphocyte associated antigen 4 (CTLA-4). However, a substantial fraction of dMMR CRCs do not respond or ultimately develop resistance to immunotherapy. The majority (~85%) of CRCs are MMR proficient (pMMR) or microsatellite stable (MSS) and lack response to ICIs. Understanding the biology and mechanisms underlying dMMR-associated immunogenicity is urgently needed for improving the therapeutic efficacy of immunotherapy on CRC. Compared to pMMR/MSS CRCs, dMMR/MSI CRCs typically have increased tumor mutational burden (TMB), lower response rate to 5-fluorouracil-based chemotherapy, distinctive immunological features such as high tumor-infiltrating lymphocytes (TILs), and better prognosis. Here, we review the current understanding of the clinical relevance of dMMR/MSI in CRCs, the molecular basis and rationales for targeting dMMR CRC with immunotherapy, and clinical approaches using ICIs as single agents or in combination with other therapies for MSI-H CRCs. Furthermore, we address the potential strategies to sensitize pMMR/MSS CRC to immunotherapy by converting an immunologically “cold” microenvironment into a “hot” one.
Keywords: colorectal cancer, mismatch repair deficiency, microsatellite instability, immunotherapy, immune checkpoint inhibitors, combination therapy
1. Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed malignancy and the second leading cause of cancer deaths in the United States (US) [1]. The incidence rate of CRC among adults younger than age 55 has been steadily increasing in recent years [1]. Although advances in early detection and treatment have significantly improved the five-year survival rates for localized (90%) and regionalized CRCs (71%), survival rates for metastatic CRC (mCRC) remain grim at around 14% [1]. There is an urgent need to develop more effective treatment options.
As CRC is highly heterogeneous at both the genetic and molecular levels [2], treatment must be tailored to individual patients based on their distinct molecular profiles. Such profiles can predict response to specific systemic therapies. Surgery is currently the primary or first treatment for CRCs that have not spread to distant sites. Chemotherapy (i.e. 5-fluorouracil [5-FU] or other fluoropyrimidines) alone or in combination with radiation is the standard management for patients with advanced CRCs. For metastatic or refractory CRCs, targeted drugs, such as inhibitors of epidermal growth factor receptor (EGFR) and vascular endothelial growth factor (VEGF), are frequently used along with chemotherapy [3]. Immunotherapy, which uses and helps the body’s immune system fight tumors, is emerging as a powerful therapeutic option for many types of cancers. For example, melanoma and lung cancer generally respond well to immune checkpoint inhibitors (ICIs) [4, 5]. ICIs such as the anti-PD-1 antibodies pembrolizumab and nivolumab have shown efficacy in patients with mismatch repair deficient (dMMR) and microsatellite instability-high (MSI-H) CRCs [6–9]. In 2017, the US Food and Drug Administration (FDA) approved ICI therapy for the treatment of dMMR/MSI-H tumors including CRC, effectively becoming the first biomarker-based and tissue/site agnostic systemic therapy for cancer [10]. However, ICIs are generally ineffective for the majority (~85%) of CRCs that are mismatch repair proficient (pMMR) and microsatellite stable (MSS) or instability-low (MSI-L) [11]. In this review, we summarize the molecular and immunological features of dMMR and MSI-H in CRCs, discuss the rationale for targeting dMMR CRC and promising clinical advances, and also address the challenges and potential strategies in improving the efficacy of immunotherapy for pMMR CRC.
2. Biology of dMMR CRC
2.1. DNA mismatch repair system
Complex organisms have developed robust mechanisms to maintain genomic stability. DNA mismatch repair (MMR) is an evolutionarily conserved system for recognizing and repairing erroneous insertion, deletion, and misincorporation of bases that arise during DNA replication, DNA recombination, as well as some forms of DNA damage [12]. In mammalian cells, there are five key MMR proteins: MutS-Homologs (MSH) 2, 3, and 6, MutL-Homolog 1 (MLH1), and Post-Meiotic Segregation 2 (PMS2) (Fig. 1A). These proteins form heterodimers equivalent to bacterial MutS and MutL complexes, specifically, MutSα (MSH2-MSH6), MutSβ (MSH2-MSH3), and MutLα (MLH1-PMS2). MutSα repairs base-base mismatches and single nucleotide insertion-deletions (indels) while MutSβ repairs a range of larger indels [11, 12]. In addition to preventing the accumulation of mutations, MMR also plays a role in the cellular response to various DNA damaging agents by regulating cell cycle checkpoints or apoptosis induction (Fig. 1A) [13].
Fig. 1.
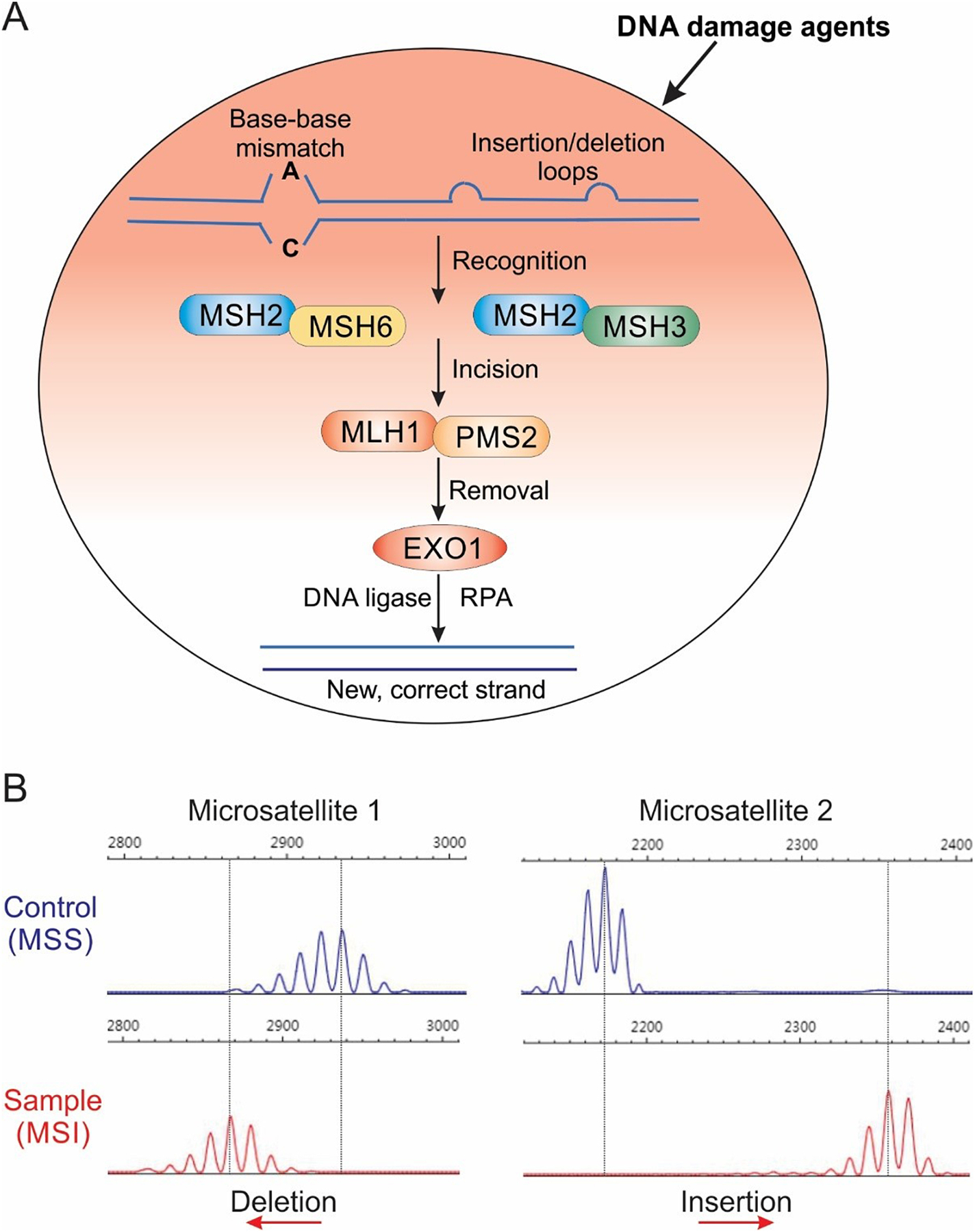
A. Mechanism of DNA mismatch repair (MMR). There are five key MMR proteins in mammalian cells including MSH2, MSH3, MSH6, MLH1, and PMS2. These proteins form heterodimer complexes to recognize and correct erroneous insertion, deletion, and misincorporation of bases that arise during DNA replication, DNA recombination, and some forms of DNA damage. B. Analysis of microstate instability (MSI). MSI can be assayed by PCR amplification of microsatellites followed by analysis of PCR product size. Due to DNA polymerase slippage in reading through repetitive sequences, PCR amplification of microsatellites typically generates multiple peaks on a sequencing gel. MSI is indicated by a shift in PCR produce size (blue peaks) relative to a microsatellite stable (MSS) sample (red peaks).
2.2. dMMR/MSI as CRC driver
CRC arises from normal colonic epithelium through the progressive accumulation of genetic and epigenetic alterations in oncogenes such as KRAS and tumor suppressor genes such as APC and p53 [2]. A key factor that drives colorectal tumorigenesis is genomic instability, which enables emerging tumor cells to acquire cancer hallmarks such as uncontrolled proliferation and evasion of cell death [14]. There are two types of genomic instability in CRC including chromosomal instability (CIN) and microsatellite instability (MSI) [2]. Most (~85%) CRCs are proficient in MMR (pMMR) and have CIN phenotypes such as abnormalities in chromosomal number and structure [15]. The remaining (~15%) CRCs have MSI, which is characterized by elevated rates of small indels and point mutations in short-tandem repeat sequences (1–6 nucleotide units) known as microsatellites (Fig. 1B) [16]. MSI was first identified in tumors from patients with Lynch syndrome, an inherited cancer syndrome associated with a genetic predisposition to different cancer types including CRC, as well as in a small subset of sporadic CRCs [17–19]. Germline mutations of MMR genes, most commonly MLH1 and MSH2, and less frequently PMS2 and MSH6, underlie MSI in the majority of Lynch syndrome cases [20–24]. In sporadic CRCs, MSI is predominantly caused by epigenetic silencing of MLH1 due to promoter hypermethylation [12]. Among ~15% CRCs with dMMR and MSI, ~3% are those associated with Lynch syndrome, and the other 12% are sporadic cases [11].
dMMR tumors are characterized by high tumor mutational burden (TMB), with 100- to 1,000-fold increased mutation rates compared to pMMR tumors [6]. MSI is believed to arise primarily as a result of polymerase slippage during DNA replication, creating indel loops that are stabilized by the repetitive sequence [25–27]. The degree of mutability of microsatellites depends on several factors such as genomic loci and number of repeats [25–27]. MSI status in patients is determined by five microsatellite markers known as the Bethesda panel, including two mononucleotides (Bat25 and Bat26) and three dinucleotides (D5s346, D2s123, and D17s250) repeats [28]. If instability is observed in two or more loci, the tumors are considered MSI-high (MSI-H). MSI-low (MSI-L) and microsatellite stable (MSS) tumors display instability at one and zero locus, respectively [29]. Typically, MSI is assayed by comparing the size of PCR products from tumors with matched normal samples from the same patient to determine the presence of a shift (Fig. 1B) [11]. Immunohistochemistry analysis of MMR protein loss and next-generation sequencing are also commonly used to assess MSI [30, 31].
MSI in coding sequences can lead to frameshift mutations with a direct role in tumor development. Several genes containing coding microsatellites are known to be frequently mutated in dMMR/MSI CRCs, such as transforming growth factor β receptor II (TGFβRII), CTNNB1 (encoding β-catenin), epidermal growth factor receptor (EGFR), and Bcl-2-associated X protein (BAX) [2]. Activating mutations in CTNNB1 can lead to stabilization and nuclear translocation of β-catenin resulting in hyperactivation of Wnt/Myc signaling [2]. TGFβRII is a tumor suppressor and a negative regulator of Wnt signaling [32]. Biallelic frameshift mutations in G8 tract in BAX were reported in ~50% of dMMR CRCs [33], and increases survival of CRC cells upon treatment with anticancer agents [34]. However, most mutations in dMMR CRCs are located in non-coding regions and likely represent passenger mutations [35].
2.3. dMMR CRC prognosis and chemotherapy response
Compared to MSS CRCs, MSI CRCs have distinct pathological features such as proximal colonic site, mucinous phenotype, poor differentiation, lower rates of KRAS and p53 mutations, increased immune cell infiltrates, and a lower rate of metastasis [11]. Early-stage CRC patients with dMMR/MSI-H CRCs have a significantly better prognosis and longer survival compared to those with pMMR/MSS [11, 36]. This better prognosis is believed to be due to enhanced antitumor immune response in dMMR/MSI-H CRCs [37]. However, MSI-H in stage II CRCs is associated with lack of benefit to adjuvant chemotherapy based on traditional cytotoxic agents such as 5-FU, oxaliplatin, or irinotecan. dMMR CRCs are resistant to 5-FU [38, 39], and associated with decreased overall survival following adjuvant 5-FU [40]. It was suggested that dMMR CRCs fail to trigger MMR-dependent recognition of 5-FU-modified DNA, which is required for the cytotoxic action [41]. The predictive value of MMR status is less clear in combination therapies. Some studies reported improved disease free survival (DFS) in dMMR CRCs treated with FOLFOX (5-FU/oxaliplatin/leucovorin) or FOLFIRI (5-FU/ irinotecan/leucovorin) regimen [42–44]. Stage III CRCs benefit from 5-FU-based adjuvant therapy regardless of MSI status [43]. In contrast to early-stage CRCs, MSI-H predicts significantly worse DFS and overall survival (OS) as compared to MSS in metastatic CRCs [45], while its predictive value to 5-FU-based chemotherapy is unclear [46] .
2.4. Immunogenic features of dMMR CRC
dMMR/MSI-H tumors often have a higher density of tumor-infiltrating lymphocytes (TILs) than MSS tumors, and display gene signatures related to cytotoxic T lymphocytes, suggesting an enhanced antitumor immune response [47, 48]. These immunogenic features in dMMR tumors are attributed to high mutational rates and increased levels of tumor-associated antigens (TAAs). TAAs are generated through several mechanisms, such as mutant peptides, aberrant gene expression, viral infection, and abnormal post-transcriptional modifications [49]. Frameshift mutations and indels in coding microsatellites can generate peptide neoantigens that activate immune surveillance [50] (Fig. 2). Presentation of these TAAs by major-histocompatibility-complex class I molecules facilitate T-cell-mediated tumor cell killing. A higher neoantigen load was associated with increases in lymphocytic infiltration, density of TILs, memory T cells, and CRC patient survival [51]. Recent studies have established a causative relationship between dMMR and antitumor immune response using syngeneic tumor models. Inactivation of MLH1 in mouse tumor cells led to increased TMB, accumulation of neoantigens, and improved immune surveillance [52]. Furthermore, genomic analysis suggested that immune invasion in dMMR and TIL-rich tumors can be caused by mutations in human leukocyte antigen (HLA) genes and other components of the antigen-processing machinery including beta-2-microglobulin (B2M) [51].
Fig. 2.
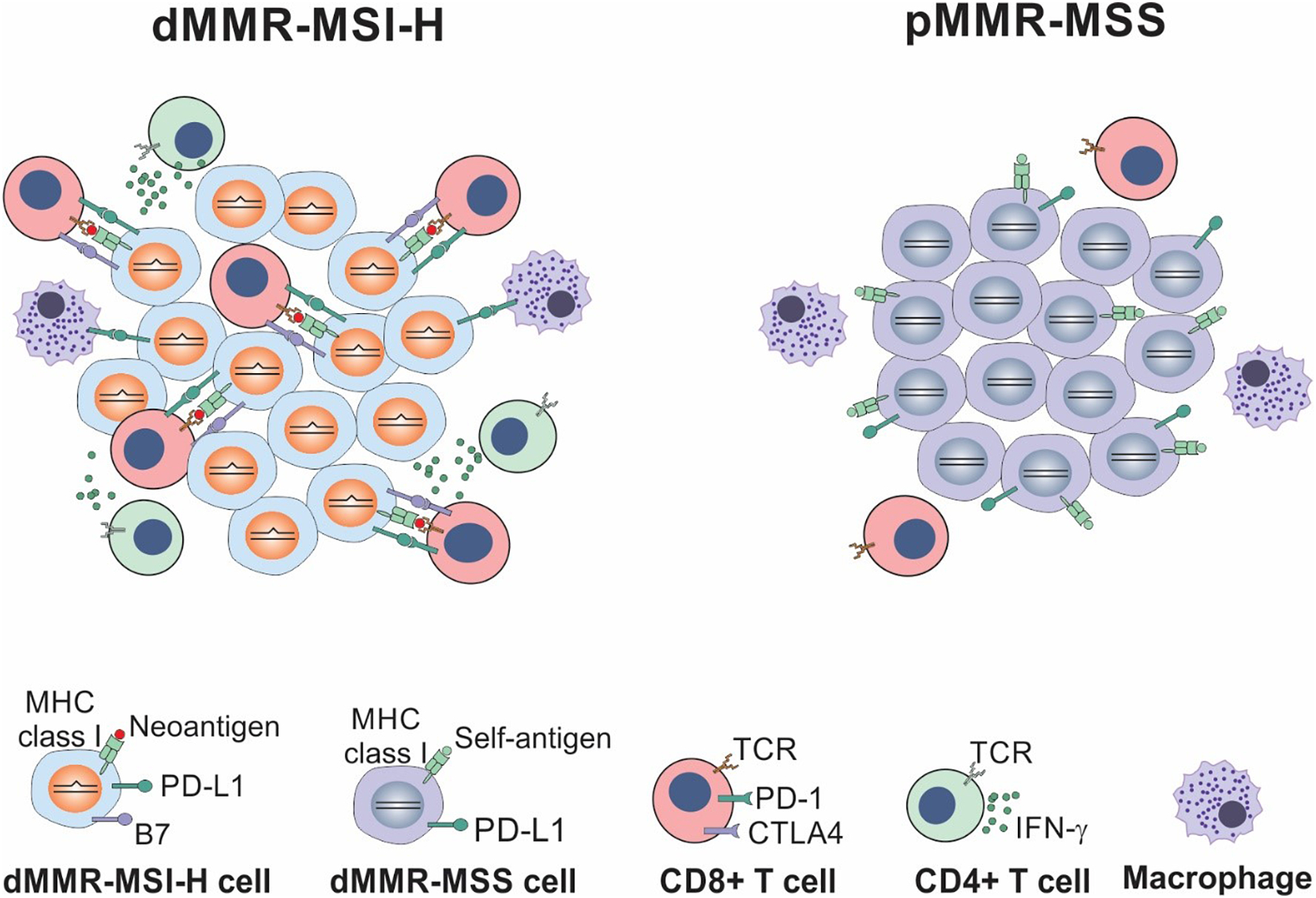
Comparison between dMMR/MSI-H and pMMR/MSS colorectal cancers. dMMR/MSI-H CRCs have a high mutational burden and persistent renewal of neoantigens, which is favorable for immune surveillance, while pMMR/MSS CRCs have a low mutational burden and lack immune surveillance.
3. Targeting dMMR/MSI CRC with immune checkpoint inhibitors
3.1. Efficacy of immune checkpoint inhibitors on dMMR/MSI CRC
The immunogenic microenvironment of dMMR/MSI-H CRCs prompted the testing of immunotherapy on these CRCs in the clinical setting. It is now well-established that dMMR/MSI-H CRCs tend to be more responsive to ICIs such as the anti-PD-1 antibodies nivolumab (OPDIVO) and pembrolizumab (KEYTRUDA), and the anti-CTLA-4 antibody ipilimumab (Yervoy). Initial analysis of dMMR/MSI-H CRC patients treated with pembrolizumab showed an objective response (OR) and progression-free survival (PFS) rates of 40% and 78%, respectively [6]. Based on this exciting result, the U.S. FDA granted accelerated approval to use pembrolizumab for dMMR/MSI-H solid tumors that are refractory to prior treatment, and dMMR/MSI-H CRCs that have progressed following chemotherapy [10]. This is the first-ever FDA approval for tissue/site agnostic indication. Sustained disease control was also observed in dMMR/MSI-H patients treated with nivolumab (one-year PFS rate over 50%) and in those with nivolumab plus ipilimumab (PFS rate over 70%) [8, 9]. A meta-analysis on 939 pretreated dMMR/MSI-H patients treated with ICIs from 14 studies indicates pooled overall response rate of 41.5%, disease control rate (DCR) of 62.8%, median PFS of 4.3 months, median OS of 24 months, and 1- and 2-year OS of 75.6% and 56.5%, respectively [53]. The KEYNOTE-177 trial (NCT02563002) is currently investigating the use of first-line pembrolizumab versus standard-of-care (SOC) chemotherapy in metastatic CRC. This clinical trial recently reported a remarkable improvement in median PFS of 16.5 months versus 8.2 months, and objective response rate (ORR) of 43.8% versus 33.1%, leading to FDA approval of pembrolizumab for first-line treatment of dMMR/MSI-H CRC [54]. Despite these findings, patients with dMMR tumors experience highly variable responses, and over 50% patients show no durable response to ICIs. Furthermore, the vast majority of pMMR/MMS CRCs are poorly immunogenic or “cold” tumors that are not responsive to ICIs alone.
3.2. Mechanisms of response and resistance
Intensive efforts have been devoted to understanding the mechanisms underlying successful immunotherapy. Tumors treated with ICIs likely undergo continuous alterations and evolution in their genome and microenvironment [52]. A recent study showed that the degree of MSI and resultant mutational load partially underlies the variable response to anti-PD-1 immunotherapy in dMMR human and mouse tumors [55]. The responsiveness to anti-PD-1 is particularly associated with the accumulation of indel mutational load, suggesting that genomic profiling of MSI and TMB may aid in the stratification of responders and non-responders. A combination of anti-PD-1 and anti-CTLA-4 antibodies caused the clonal deletion of tumor-specific T cells, which compromised antitumor response and resulted in resistance to this combination in mouse models and melanoma patients [56]. The combination treatment induced production of interferon-γ (IFN-γ) in the low tumor burden (LTB) state, which promoted the killing of tumor-specific T cells that express a high level of IFN-γ receptor. Another recent study analyzed dynamic changes in PD-1-/CD8+ TILs in response to ICI therapy in preclinical models, which identified three subsets that share features with naive, memory-precursor, and effector CD8+ T cells [57]. Different ICI therapies induced changes in their proportions across different tumor models. The increase of the memory precursor-like CD8+ T cells, likely important for the efficacy, is dependent on Tcf7/Tcf1-mediated transcription.
Several putative ICI resistance mechanisms in dMMR CRC patients have been described. A recent study revealed that dMMR CRC patients with BRAF V600E mutations had significantly worse outcomes compared to those with wild-type BRAF [58]. This finding suggests that oncogenic BRAF promotes immune escape and combinations of ICIs with a BRAF or MEK inhibitor may be beneficial for patients with this molecular subtype. This study also suggested differential effects of specific MMR gene losses, with loss of MSH2+MSH6 associated with better outcomes than the loss of MLH1+PMS2 [58]. As mentioned above, dMMR results in widespread indel mutations in the exonic microsatellites in genes such as B2M, whose product is critical to antigen presentation. B2M inactivation is thought to be a common ICI resistance mechanism in melanoma [59]. A recent study showed that B2M mutations were significantly enriched in MSI-H CRCs among all of 1,751 CRC cases analyzed (24% vs. 3.4%), and most (73%) of MSI-H CRCs with B2M mutations had a complete loss of B2M expression [60]. However, the majority (85%; 11/13) of the patient with B2M-mutant MSI-H CRC still benefited from ICI treatment, suggesting that B2M inactivation in dMMR/MSI-H CRCs may not have an important role in intrinsic resistance to ICIs as in melanoma. Further studies with more patients and longer follow-up will be required to assess if the loss of B2M is associated with shorter duration of response and could lead to resistance to ICIs.
3.3. Biomarkers of efficacy
Biomarkers are obviously needed to improve patient stratification and the efficacy of anti-PD-1 immunotherapy in dMMR/MSI-H tumors. Expression of the PD-1 ligand PD-L1 is a useful biomarker of response to anti-PD-1 therapy in some tumor types such as non-small-cell lung carcinoma (NSCLC) [61]. However, PD-L1 expression is generally low in dMMR/MSI-H CRCs and not predictive in response to ICIs [6, 62]. In a retrospective multi-center study, TMB, as a continuous variable, was found to have the strongest association with OR and might be a potential biomarker in predicting PFS in dMMR/MSI-H CRC treated with anti-PD-1 therapy [63]. The optimal predictive cut-point for TMB was ~37–41 mutations/Mb. A high degree of concordance (44%) was found between high TMB, not PD-L1 expression, and dMMR/MSI-H [30]. MSI and TMB in CRC patients can be detected by non-invasive methods such as analysis of liquid biopsy [64], which may facilitate their applications in the clinical setting. TMB was found to predict patient survival after immunotherapy across multiple cancer types and may be a more useful biomarker [65].
Immunoscore, which measures TILs in the core and invasive margin of a tumor, was shown to be prognostic independent of the dMMR/MSI-H status [66]. A lower Immunoscore is associated with worse survival and immunotherapy resistance of early-stage dMMR/MSI-H CRCs [67]. A recent case series examined the association of intratumoral CD3+ and CD8+ T-cell densities with ORR and duration of response in patients with dMMR metastatic CRCs who received pembrolizumab monotherapy after progressing on SOC chemotherapy [68]. Higher CD3+ and CD8+ T-cell densities were associated with higher ORR and duration of disease control. This, in turn, suggests that measuring these immune markers could help predict response to anti-PD-1 therapy in dMMR CRCs. Conversely, a low T-cell density may predict a lack of response to ICIs [68].
Comprehensive analysis of gene expression signatures has classified CRCs into 4 consensus molecular subtypes (CMS), among which CMS1 is mainly composed of dMMR/MSI-H CRC [69], likely respond well to immunotherapy. These findings support the notion that while MSI tumors are characterized by increased immune signaling, while substantial heterogeneity in the expression of other immune and checkpoint markers likely impact therapeutic response [37].
The gut microbiome has been shown to affect the outcome of cancer therapy by modulating the host immune response. Several recent studies suggest an important role for the gut microbiome in influencing the response to ICIs [70–72]. The use of antibiotics is associated with poor response to ICIs, and fecal microbial transplantation from responding patients to non-responders could improve tumor control by ICIs [70]. These findings were mostly made in melanoma patients, and the role of the gut microbiome in dMMR/MSI-H CRC remains to be further determined.
4. Ongoing clinical trials of ICIs on dMMR CRCs
Improving responses to immunotherapy in dMMR CRCs represents a high priority and an unmet need. Efforts are ongoing for testing different ICIs alone or in combination with other classes of anticancer agents (Fig. 3) [73].
Fig. 3.
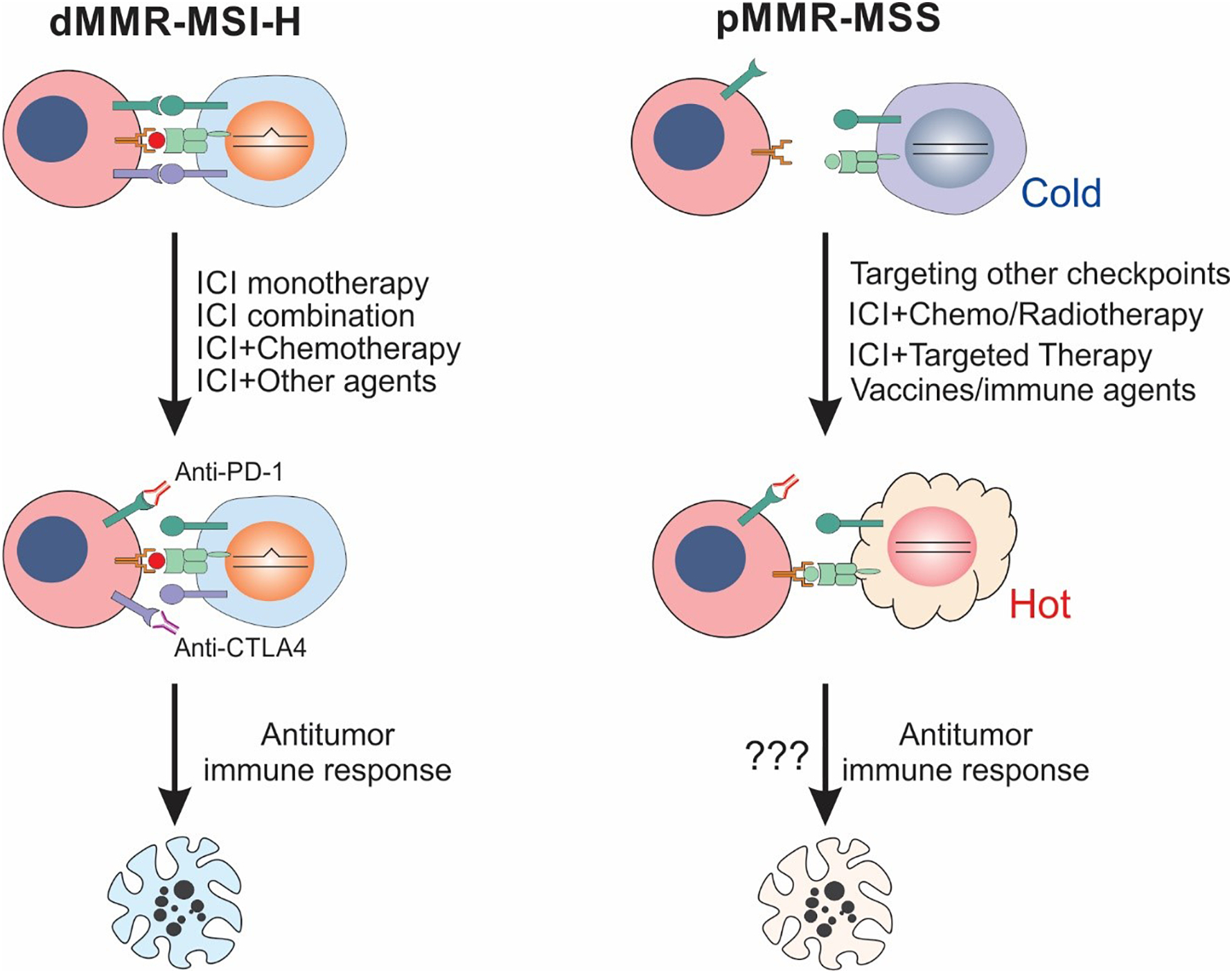
ICI therapy for targeting dMMR/MSI-H and pMMR/MSS CRCs. ICI therapy is effective for a subset of dMMR/MSI-H CRCs by triggering an antitumor immune response. Combinations of different ICIs and ICIs with chemotherapy, radiotherapy, targeted therapy, vaccines, or other immune agents may convert “cold” tumors into “hot” and are potentially effective for insensitive dMMR/MSI-H CRCs and most of pMMR/MSS CRCs.
4.1. ICI monotherapy
As highlighted in Table 1, several ongoing studies are evaluating the efficacy of anti-PD-1 or anti-PD-L1 antibodies in patients with dMMR/MSI-H metastatic CRC. The above-mentioned KEYNOTE-177 trial is a phase 3 randomized trial investigating the efficacy of first-line pembrolizumab versus SOC chemotherapy in stage IV dMMR MSI-H (NCT02563002; Table 1). The use of anti-PD-L1 antibodies, including atezolizumab, avelumab, and durvalumab, in the first-line metastatic setting are also being investigated (NCT0318326, NCT02997228, NCT0291559; Table 1). Furthermore, the ongoing GARNET trial (NCT02715284) is evaluating dostarlimab (TSR-042), a novel anti-PD-1 antibody, in patients with advanced solid tumors. This trial recently showed a robust and durable antitumor activity of dostarlimab, as well as adverse events characteristic of anti-PD-1 therapies.
Table 1.
Clinical trials on MSI CRCs
| Identifier | Phase | Setting | Treatment | Participants | Outcome measures | Estimated Completion date |
|---|---|---|---|---|---|---|
| NCT04008030 | 3 | Advanced dMMR/MSI-H CRCs | Ipilimumab, nivolumab, oxaliplatin, leucovorin, fluorouracil, irinotecan, bevacizumab, cetuximab. | 494 | PFS | July 2025 |
| NCT04118933 | 2 | MSI-H advanced or recurrent CRCs | JS001 | 40 | RECIST v1.1 | July 2021 |
| NCT04001101 | 2 | dMMR/MSI-H metastatic solid tumors | PD-1 and limited metastatic site radiation therapy | 140 | ORR, PFS, OS | July 2021 |
| NCT03926338 | 1/2 | Resectable non-metastatic CRCs with MSI-H | Toripalimab +/− celecoxib | 20 | DFS, OS | May 2022 |
| NCT04304209 | 2/3 | dMMR/MSI-H locally advanced rectal cancer | Sintilimab ± chemoradiotherapy | 195 | CR, R0 resection rate | Oct 2026 |
| NCT04165772 | 2 | Locally advanced dMMR rectal adenocarcinoma | TSR-042, capecitabine or 5-FU, intensity modulated radiation therapy | 30 | pCR | Nov 2021 |
| NCT02715284 | 1 | Advanced solid tumors | TSR-042 | 740 | Safety, ORR | Nov 2023 |
| NCT03311334 | 1/2 | Advanced solid tumors | DSP-7888 dosing emulsion in combination with nivolumab or pembrolizumab | 84 | DLTs, ORR, PFS, OS, DOR, DCR | May 2022 |
| NCT03186326 | 2 | MSI CRCs | FOLFOX or FOLFIRI +/− avelumab | 132 | PFS | March 2025 |
| NCT03436563 | 1/2 | CMS4 mCRCs with MSI or CRC patients with detectable circulating tumor DNA | PD-L1/TGFbetaRII fusion protein M7824 | 74 | ORR, PFS, OS, DFS | Nov 2020 |
| NCT02997228 | 3 | dMMR mCRCs | mFOLFOX6/bevacizumab combination chemotherapy +/− atezolizumab or atezolizumab monotherapy | 347 | PFS, OS, ORR | April 2022 |
| NCT02983578 | 2 | Advanced pancreatic, NSCLC, and dMMR CRCs | AZD9150 (Antisense STAT3) with MEDI4736 | 75 | Disease control, OS, PFS | March 2021 |
| NCT02912559 | 2 | Stage III colon cancer and dMMR | Standard chemotherapy alone or combined with atezolizumab | 700 | DFS, OS | Dec 2020 |
| NCT03841110 | 1 | Advanced solid tumors | FT500, FT500 + nivolumab, pembrolizumab or atezolizumab | 76 | ORR | June 2022 |
| NCT03228667 | 2 | Disease progression following an initial response to PD-1/PD-L1 | ALT-803 + PD-1/PD-L1 checkpoint inhibitor | 611 | ORR, OS | Aug 2020 |
| NCT03667170 | 2 | Advanced dMMR or MSI- H solid tumors | KN035 | 110 | ORR, PFS, OS, DCR | July 2021 |
| NCT03126110 | 1/2 | Advanced or metastatic malignancies | INCAGN01876 + PD-1 or anti-CTLA4 or both | 285 | ORR, OS, PFS | Oct 2021 |
| NCT03538028 | 1 | Advanced malignancies | Anti-LAG-3 INCAGN02385 | 40 | ORR, DCR, DOR | Sep 2020 |
| NCT03607890 | 2 | Advanced dMMR cancers | Nivolumab + relatlimab | 21 | ORR | Oct 2022 |
| NCT03589339 | 1 | Advanced cancers | NBTXR3 + radiotherapy + PD-1 | 60 | anti-tumor response, safety and feasibility | March 2023 |
| NCT03836352 | 2 | Selected advanced & recurrent solid tumors | DPX-Survivac + cyclophosphamide + pembrolizumab | 184 | ORR, PFS, OS, DCR | Dec 2022 |
| NCT02460198 | 2 | Previously treated locally advanced unresectable or metastatic (stage IV) dMMR colorectal carcinoma | Pembrolizumab | 124 | ORR, PFS, OS, DCR, DOR | Aug 2020 |
| NCT02563002 | 3 | dMMR stage IV colorectal carcinoma | Pembrolizumab vs standard therapy | 308 | ORR, PFS, OS | Dec 2021 |
| NCT04301557 | 2 | dMMR/MSI-H locally advanced CRCs | Toripalimab and chemoradiotherapy | 25 | pCR, DFS | Dec 2024 |
| NCT04258111 | 2 | dMMR/MSI-H locally- advanced or metastatic CRCs | IBI310 + sintilimab | 68 | ORR, PFS, DCR | May 2023 |
| NCT03350126 | 2 | dMMR and/or MSI metastatic CRCs | Nivolumab and ipilimumab | 57 | ORR, PFS, OS, DCR | Dec 2020 |
| NCT01885702 | 1/2 | Lynch Syndrome or colorectal cancer with MSI | DC vaccination | 25 | Safety and feasibility, DFS | June 2020 |
Abbreviations: ORR: objective response rate; DCR: disease control rate; DOR: duration of response; PFS: progression-free survival; CR: complete response; PR: partial response; OS: overall survival; DLTs: dose-limiting toxicities; pCR: pathological complete response.
4.2. Combinations of ICIs
Given the clinical benefit observed with ICI monotherapy, it makes sense to test the combination of these agents. The phase 2 randomized trial (CCTG CO.26) was among the first studies to demonstrate that combined PD-L1 and CTLA-4 inhibition prolongs survival of dMMR/MSI-H patients [74]. It was reported that patients in the combination group had significantly prolonged overall survival compared to those receiving only SOC. As a first-line treatment, nivolumab plus low-dose ipilimumab demonstrated robust and durable clinical benefit (ORR 60%) and was well tolerated after a median follow-up of ~14 months [9, 75]. Among the 58 patients who responded, five had a complete response (CR) and 53 had a partial response (PR). Unfortunately, these combinatorial efforts resulted in more frequent adverse events. Lymphocyte activation gene-3 (LAG3), an immune checkpoint receptor protein, is upregulated in dMMR tumors and mediate T cell exhaustion [76]. Based on this finding, several studies are investigating the combination of the anti-LAG3 antibody relatlimab with nivolumab to potentially restore response in patients who previously progressed on ICI therapy (NCT03607890; Table 1). The safety, tolerability, and preliminary efficacy of INCAGN02385, an anti-LAG3 antibody, is also being tested in patients with advanced malignancies, including dMMR/MSI-H CRCs (NCT03538028; Table 1).
4.3. Combinations of ICIs and chemotherapy
Combinations of ICI with chemotherapy or targeted therapy are being activated tested in patients with dMMR/MSI-H mCRC (Table 1). Cytotoxic chemotherapy is the oldest class of systemic therapy for cancer, yet its utility has been revitalized through the lens of immuno-oncology. Recent studies have demonstrated that chemotherapeutic agents can induce immunogenic cell death (ICD), a cell death modality that stimulates an immune response against dead-cell antigens [77]. ICD induction may help boost immune response and enhance the therapeutic effects of ICIs. For example, a phase 3 randomized clinical trial is investigating whether the anti-PD-L1 avelumab, when given after adjuvant chemotherapy, can improve the survival of patients with dMMR or POLE-mutant CRCs (NCT03827044; Table 2). Multiple other studies are investigating the combination of ICIs with chemotherapy in metastatic dMMR CRC patients. The agents being investigated in these combinations include nivolumab, atezolizumab, ipilimumab, sintilimab, and toripalimab (NCT04008030, NCT04304209, NCT03186326, NCT02997228, NCT02912559, NCT04301557; Table 1).
Table 2.
Clinical trials on MSS CRCs
| Identifier | Phase | Setting | Treatment | Participants | Outcome measures | Completion date |
|---|---|---|---|---|---|---|
| NCT02693535 | 2 | Advanced solid sumors | Many, incl. pembrolizumab, ipilimumab + nivolumab | 660 | ORR | Dec 2021 |
| NCT03519412 | 2 | pMMR CRCs | Temazolamide (priming), pembrolizumab | 348 | ORR, PFS, OS | June 2022 |
| NCT04014530 | 1/2 | Metastatic pMMR and dMMR colorectal adenocarcinomas or metastatic dMMR endometrial carcinoma | Pembrolizumab + ataluren | 47 | ORR, PFS, OS | Aug 2023 |
| NCT03435107 | 2 | dMMR or POLE mutated mCRCs | Durvalumab | 33 | Objective response rates (RECIST 1.1) | May 2022 |
| NCT03827044 | 3 | MSI-H or POLE Exonuclease Domain mutant colon cancer | Avelumab plus 5-FU based chemotherapy | 402 | DFS, OS | July 2028 |
| NCT03104439 | 1 | MSI-H and MSS CRCs, pancreatic cancer | Nivolumab + ipilimumab + radiation therapy | 80 | PFS, OS | Oct 2024 |
| NCT03832621 | 2 | mCRCs | Temozolomide + nivolumab + ipilimumab | 100 | PFS, ORR, DOR | Feb 2022 |
| NCT03935893 | 2 | Advanced solid cancers | TIL, fludarabine + cyclophosphamide combination | 10 | ORR, CRR, DOR, DCR, PFS, OS | June 2030 |
| NCT03555149 | 1/2 | mCRCs excluding MSI-H | Atezolizumab + imprime PGG + bevacizumab, atezolizumab + isatuximab, atezolizumab + selicrelumab + bevacizumab, atezolizumab + idasanutlin, atezolizumab + regorafenib, atezolizumab + regorafenib + AB928 | 326 | ORR, DOR, DCR, PFS, OS | Jan 2022 |
| NCT03373188 | 1 | Resectable rancreatic and colorectal cancer | Anti-SEMA4D + surgery, anti-SEMA4D + ipilimumab or nivolumab + surgery | 32 | CD8+ T cell infiltration, AEs | Dec 2022 |
| NCT03250832 | 1 | Advanced solid tumors | TSR-033 + anti-PD-1 | 200 | AEs, CR, OS, PFS | May 2021 |
| NCT03642067 | 2 | MSS advanced colorectal cancer | Nivolumab + relatlimab | 64 | ORR, drug-related toxicities | Feb 2023 |
| NCT04117087 | 1 | Resected pMMR CRCs | KRAS peptide vaccine + nivolumab + ipilimumab | 30 | DFS, AEs, CD8 and CD4 T cells | June 2024 |
| NCT02851004 | 1/2 | mCRC | BBI608 + pembrolizumab | 94 | ORR, PFS, OS | Oct 2020 |
| NCT03711058 | 1/2 | Relapsed/Refractory solid tumors with expansions in MSS colorectal cancer | Copanlisib and nivolumab | 54 | MTD, ORR, DCR, PFS, OS | Jan 2022 |
| NCT04110093 | 1/2 | CRCs | Regorafenib + anti-PD-1 | 120 | ORR, PFS, OS | Aug 2021 |
| NCT04271813 | 2 | Advanced colorectal cancer | Anlotinib + sintilimab | 30 | PFS, OS | Dec 2021 |
| NCT03207867 | 2 | Solid tumors and non-Hodgkin lymphoma | NIR178 + PDR001 (mAb) | 310 | ORR, OS, PFS, DCR | June 2021 |
| NCT03549000 | 1 | Advanced malignancies | Anti-CD73 NZV930 + NIR178 or PDR001 (mAb) or both | 344 | ORR, PFS, CBR, safety and tolerability | Feb 2022 |
| NCT03150706 | 2 | dMMR or POLE mutated mCRCs | Avelumab | 33 | Serum CEA, TSH, T3, free T4, EKG | Dec 2021 |
| NCT04262687 | 2 | MSS metastatic colorectal cancer with high immune infiltrate | CAPEOX+ bevacizumab + pembrolizumab | 55 | OS, serum CEA and CA199 | Dec 2023 |
| NCT03189030 | 1 | Colorectal neoplasms | personalized live, attenuated, double-deleted Listeria monocytogenes (pLADD)-based immunotherapy | 28 | Safety and Tolerability | Dec 2020 |
| NCT04108481 | 1/2 | mCRCs | Y-90 glass microspheres + durvalumab | 18 | MTD, ORR, DCR, PFS, OS | Oct 2021 |
| NCT04109755 | 2 | Localised MSS rectal cancer | Pembrolizumab + radiotherapy | 25 | TRG, OS, DFS, DMFS | March 2028 |
| NCT03377361 | 1/2 | Previously treated metastatic colorectal cancers | Nivolumab + trametinib +/− ipilimumab | 345 | ORR, PFS, OS, AE | Nov 2022 |
| NCT03102047 | 2 | Stage II-IV rectal cancer | Chemotherapy + radiotherapy, durvalumab, and surgery | 47 | Pathologic response, ORR, AE | Jan 2021 |
| NCT03626922 | 1b | mCRC | Pemetrexed +/− oxaliplatin + pembrolizumab | 33 | Safety/tolerability, RP2D | Nov 2021 |
| NCT02671435 | 1/2 | MSS mCRC (cohort A) | Durvalumab + monalizumab + mFOLFOX +/− bevacizumab +/− cetuximab | 36 | Safety/tolerability, DOR, PFS/OS | Mar 2022 |
| NCT03174405 | 2 | MSS mCRC | FOLFOX + cetuximab + avelumab | 43 | PFS, ORR, safety | Aug 2021 |
| NCT02860546 | 2 | MSS mCRC | TAS-102 + nivolumab | 35 | ORR | Nov 2017 |
| EudraCT 2017-004392-32 | 2 | RAS wild type mCRC | Avelumab + cetuximab | 75 | OS, ORR, PFS, safety | N/A |
| NCT03391232 | 1 | MSS mCRC | PolyPEPI1018 + 5-FU + bevacizumab | 11 | Safety, immune response | July 2019 |
| NCT03256344 | 1b | mCRC | Talimogene laherparepvec + atezolizumab | 36 | Safety, ORR, PFS/OS | May 2022 |
| NCT03539822 | 1 | Advanced GI cancers | Cabozantinib + durvalumab | 30 | RP2D, AE, ORR, DCR, PFS/OS | Dec 2020 |
| NCT02837263 | 1b | MSS mCRC | SBRT + pembrolizumab + surgery | 15 | Recurrence rate, Time to recurrence, DFS, OS | Jun 2021 |
| NCT03657641 | 1/2 | mCRC | Regorafenib + pembrolizumab | 75 | Safety/tolerability, PFS/OS | Jun 2022 |
| NCT03865082 | 2 | MSS mCRC | Tilsotolimod (intratumoral) + nivolumab + ipilimumab | 77 | ORR, safety/tolerability | Apr 2022 |
| NCT03435640 | 1b/2 | MSI-H and MSS mCRC (phase 2 expansion cohort) | NKTR-262 + NKTR-214 + nivolumab | 383 (all cohorts) | Safety/tolerability, ORR | Dec 2022 |
| NCT03168139 | 1/2 | mCRC and pancreatic cancer | Olaptesed (NOX-A12) + pembrolizumab | 20 | Safety/tolerability, DCR | Mar 2020 |
| NCT02260440 | 2 | MSS mCRC | Azacitidine + pembrolizumab | 31 | ORR, PFS, OS, safety | Sept 2017 |
| NCT02512172 | 1 | MSS mCRC | Azacitidine + romidepsin + pembrolizumab | 30 | Safety/tolerability, ORR, immune correlatives | Dec 2020 |
| NCT02437136 | 1b/2 | MSS mCRC (phase 2 expansion cohort) | Entinostat + pembrolizumab | 202 (all cohorts) | Safety/tolerability, ORR, DCR, PFS/OS, DOR, TTR | N/A |
Abbreviations: ORR: Objective response rate; DCR: Disease control rate; DOR: Duration of response; PFS: Progression-free survival; CR: complete response; PR: Partial response; OS: Overall survival; DLTs: dose-limiting toxicities; pCR: pathological complete response; MTD: maximum tolerated dose; TRG: tumor regression grade; DMFS: distant metastasis-free survival; CBR: Clinical Benefit Rate; AE: Adverse Events.
4.4. Other approaches
Several ongoing trials are testing the combination of ICIs with molecules that target metabolic pathways. For instance, several preclinical studies reported cyclooxygenase-2 (COX-2) inhibitors improve antigen presentation and T-cell infiltration in tumors [78, 79]. The expression of COX-2 may induce Indoleamine 2,3-Dioxygenase 1 (IDO1), an intracellular enzyme that catalyzes tryptophan along the kynurenine pathway and induces depletion of tryptophan. IDO1-induced tryptophan depletion can lead to an immunosuppressive environment [80]. Based on these preclinical data, several ongoing clinical trials are investigating the combination of COX inhibitors or IDO1 inhibitors with ICIs in dMMR CRC patients [NCT03638297, NCT03926338; Table 1].
Approximately 98% of MSI CRCs harbor one or more mutations in AIM2, HT001, and TAF1B, which give rise to three commonly mutated frameshift peptide antigens [37]. These mutations may have a tumor-promoting driver function at early stages given their high prevalence in MSI cancers. These findings support these frameshift peptide mutations as specific targets to develop preventive vaccines [37]. Furthermore, adoptive cell-based immunotherapy with genetically modified T-cells, which can be engineered to express chimeric antigen receptors or selected for their ability to bind tumor antigen, is another emerging approach to treat MSI CRCs. Currently, EGFR-CAR-T or Mucin-1-CAR-T cells expressing anti-PD-1 or anti-PD-1 and anti-CTLA-4 are being investigated in the clinical setting.
5. Improving antitumor immune responses in pMMR CRCs
In contrast to dMMR/MSI-H CRCs, pMMR/MSS CRCs represent the majority of CRCs, generally are immunogenically “cold”, and lack a robust antitumor immune response (Fig. 2). This can be explained in part by their lack of high neoantigen burden and the expression of intrinsic and extrinsic immune suppressive factors [37]. Current efforts have been devoted to understanding the mechanisms of immune escape in pMMR CRCs, developing effective strategies to improve antitumor immunity in pMMR CRCs, and reversing immunogenically “cold” microenvironment into “hot” (Fig. 3).
5.1. Targeting alternate immune checkpoints
In addition to PD-L1 and CTLA-4, numerous other immune checkpoints have been identified, and some of them have been vigorously pursued as targets in cancer immunotherapy, such as TIM-3, LAG3, TIGIT, NKG2A, and OX40 [81]. One such checkpoint worth noting is natural killer group 2A (NKG2A), which is expressed in natural killer (NK) as well as T cells. NKG2A can be activated by HLA-E, and upon ligand binding, results in suppression of effector T and NK cell activity [82]. An ongoing clinical trial is investigating the safety and activity of the NKG2A inhibitor monalizumab, in combination with durvalumab, mFOLFOX6, and either bevacizumab or cetuximab, in first-line MSS mCRC [NCT02671435; Table 2]. Targeting LAG3 in addition to PD-l is also being intensely investigated in multiple solid tumors, including MSS mCRC.
5.2. Stratifying pMMR CRCs with features of dMMR CRCs
Although the vast majority of pMMR/MSS CRCs have relatively low TMB, a small fraction display unusually high TMB and may, therefore, capture the biology of MSI-H CRCs and potentially respond to ICIs. The TAPUR trial is a phase 2 clinical basket trial that will address this question by enrolling high TMB MSS CRC patients to treatment with either pembrolizumab or ipilimimab+nivolumab [NCT02693535; Table 2]. The POCHI trial is a phase 2 trial investigating the combination of chemotherapy and pembrolizumab in MSS mCRC patients with high TIL, which may eventually shed light on whether TIL burden is predictive to ICI response in MSS CRC [NCT04262687; Table 2]. Preclinical studies have suggested that MSS CRCs with MGMT promoter hypermethylation, which initially respond to temozolomide, eventually develop into MSI-H as an escape mechanism [52]. Based on these findings, the ARETHUSA and MAYA clinical trials will investigate the activity of ICIs in patients with MGMT-hypermethylated CRCs after receiving temozolomide [NCT03519412, NCT03832621; Table 2].
5.3. Combination with chemotherapy or radiation therapy
Multiple chemotherapy agents such as doxorubicin, oxaliplatin, and cyclophosphamide have been shown to induce antitumor immunity through induction of ICD or other mechanisms [77]. This premise is being tested clinically in CRC through multiple ongoing trials of ICIs + chemotherapy, including agents such as 5-FU, capecitabine, TAS-102, oxaliplatin, or pemetrexed [NCT03174405, NCT02860546, NCT03626922; Table 2].
Radiation damage has been broadly demonstrated to induce ICD [83]. The combination of various radiation therapy modalities with immunotherapy is being studied in almost all immunotherapy-resistant solid tumors, including MSS CRCs. Interim results of a phase 2 trial of ipilimumab and nivolumab + radiation in MSS mCRC were recently presented. This treatment combination yielded an ORR of 12.5% (3/24) and DCR of 29.2% (7/24). Of note, 33% of enrolled patients never received radiation therapy, due to significant side effects from the dual checkpoint blockade [NCT03104439; Table 2] [84]. Other ongoing clinical trials are investigating stereotactic body radiotherapy or Yttrium-90 radioembolization in combination with ICIs for MSS mCRC [NCT03102047, NCT04108481, NCT04109755; Table 2]. The correlative biomarker analysis of these studies may provide more definitive clinical evidence for chemo- or radiation-induced ICD.
5.4. Inhibition of oncogene-mediated immune escape
A large body of literature has now been built around the premise that traditional oncogenic pathways many activate immunosuppression in addition to many tumor cell-intrinsic hallmarks [85]. The JAK/STAT, Wnt/APC/Myc, TGF-β, and PIK3CA signaling pathways have all been demonstrated preclinically to drive tumor immune evasion [86, 87]. Accordingly, combinations of ICIs and inhibitors of STAT3 (BBI608) or PIK3CA (copanlisib) are being investigated in ongoing clinical trials [NCT02851004, NCT03711058; Table 2] [87].
5.5. Heating an immunogenic “cold” tumor microenvironment
The tumor immune microenvironment is extremely complex and heterogeneous and many immune-suppressive pathways have been identified in MSS CRCs [88]. Tyrosine kinase inhibitors (TKIs) have been widely studied due to their activities on multiple signaling molecules known to drive tumor growth. The TKI regorafenib is one of the only two drugs approved for chemotherapy-refractory MSS mCRC [89]. Since the initial development of regorafenib, multiple immune-related mechanisms have been discovered in various gastrointestinal (GI) cancers including CRC [90]. It was shown that the CT26 syngeneic CRC mouse model treated with regorafenib had significantly decreased tumor-associated macrophages as compared to controls [91]. The phase 1 REGONIVO study recently demonstrated an impressive 36% (9/24) response rate in metastatic MSS CRCs [92]. In addition to regorafenib, the TKI cabozantinib is also under investigation in combination with durvalumab in GI cancers including mCRC [NCT03539822; Table 2]. Toll-like receptor (TLR) modifiers are a newer class of agents being studied for their immune-modulatory mechanisms [93]. The REVEAL study is an ongoing phase 1b/2 trial investigating the combination of the TLR7/8 agonist NKTR-262, in combination with the CD122-biased IL-2 mimetic NKTR-214, and nivolumab [NCT03435640; Table 2]. Intra-tumoral NKTR-262 is thought to promote dendritic cell activation and antigen presentation in tumors. NKTR-214 is believed to promote both extra-tumoral CD8+ TIL expansion, as well as tumor infiltration of TILs. The ongoing Keynote-559 study is a Phase 1/2 trial on the CXCL12 antagonist olaptesed (NOX-A12) in combination with pembrolizumab in both mCRC and metastatic pancreatic cancer [NCT03168139; Table 2]. The chemokine CXCL12 signals through the receptors CXCR4 and CXCR7 to promote tumor proliferation, survival, metastasis, and angiogenesis. CXCL12 has also be found to recruit B cells, plasmacytoid DCs, and regulatory T cells, suggesting that it likely induces an immune-suppressive environment [94].
5.6. Modifying epigenetic dysregulations
Immune modulation using epigenetic therapies continues to garner substantial interest in translational oncology. Several mechanisms of antitumor immune activation have been proposed for agents that target DNA methylation and histone modifications, such as augmenting TME and restoring immune recognition and immunogenicity [95–97]. Among different classes of epigenetic agents, DNA methyltransferase inhibitors (DNMTi) have been studied most extensively. A recent single-arm phase 2 clinical trial investigating the DNMTi azacitidine in combination with pembrolizumab in MSS mCRC lead to an ORR of 3% (1/29) and stable disease rate of 3% (1/29) [NCT02260440; Table 2]. Correlative biomarkers from this study demonstrated a decrease in global DNA methylation, suggesting on-target effect of DNMTi. The ENCORE 601 study is a Phase 1b/2 single-arm clinical trial investigating the combination of pembrolizumab and the histone deacetylation inhibitor (HDACi) entinostat in MSS mCRC [NCT02437136; Table 2]. Interim results from this study demonstrated that 38% (6/16) patients enrolled in the phase 1 portion experienced disease control. There is also an ongoing study investigating the combination of azacitidine, the HDACi romidepsin, and pembrolizumab in MSS mCRC [NCT02437136; Table 2]. This particular clinical trial employs an immune priming period in which patients receive the epigenetic agents first, before proceeding to receive pembrolizumab. The full analysis of these trials will likely help guide the design of future studies investigating tumor immune priming by epigenetic therapies.
5.7. Vaccine therapy
Cancer vaccines hold tremendous therapeutic potential which has yet to be realized. Vaccine therapies can be designed to target different aspects of antitumor immunity, with the end goal of promoting or re-invigorating the antitumor adaptive immune response [98]. Although the granulocyte-macrophage colony-stimulating factor secreting GVAX vaccine failed to meet its clinical endpoint in a recent phase 1 trial on MSS mCRC patients, the study did demonstrate increased PD-L1 expression and tumor necrosis in post-treatment biopsies [99]. These biomarker indications suggest modulation of the antitumor immune response by the GVAX vaccine. Immune correlative biomarkers were recently reported from a phase 1 clinical trial investigating the peptide vaccine PolyPEPI1018, which was designed to mimic a pool of highly conserved cancer-testis antigens in mCRC [NCT03391232; Table 2]. The PolyPEPI1018 vaccine was found to be safe when given to MSS mCRC patients in combination with maintenance chemotherapy, and encouraging immune responses were seen after a single dose of the vaccine. Additional ongoing vaccine clinical trials include the oncolytic virus Pexa-Vec vaccine, a KRAS-mutant peptide vaccine, and a personalized live, attenuated Listeria vaccine [NCT03256344, NCT03189030, NCT04117087; Table 2]. These vaccine studies are still in early stages, while the results can help use better understand and target MSS CRCs immunologically.
6. Conclusions and future directions
The status of MMR/MSI has recently emerged as an effective and actionable biomarker for ICI therapy, stimulating wide interest and intensive efforts for understanding and targeting the immuno-oncology of dMMR cancers. Despite the clinical success of ICI therapy in dMMR/MSI-H CRCs, several challenges remain, in particular, intrinsic and acquired resistance to ICIs. More importantly, the vast majority of pMMR/MSS mCRCs do not respond to ICIs. A major challenge is to convert immunologically “cold” pMMR tumors into “hot”. Several future directions may hold the key for improving ICI therapy in dMMR CRCs, as well as for resensitizing dMMR CRCs. These directions include elucidating the complex interactions between tumors and immune-suppressive microenvironment, understanding the molecular mechanisms of immune escape, identifying additional immune checkpoints, and developing more effective targeting agents, combination regimens, and biomarker-based patient stratification strategies.
Acknowledgments:
We apologize for not being able to cite many excellent original articles by our colleagues due to space limitation. We thank our lab members for critical reading. Work in authors’ laboratories is supported by the U.S. National Institute of Health grants (R01CA203028, R01CA217141, R01CA236271, and R01CA247231 to LZ; U19AI068021 and R01CA215481 to JY; T32CA193205 to CK; and P30CA047904 to UPMC Hillman Cancer Center).
Abbreviations
- 5-FU
5-fluorouracil
- BAX
Bcl-2-associated X protein
- B2M
beta-2-microglobulin
- CAPEOX
capecitabine/oxaliplatin
- CIN
chromosome instability
- CMS
consensus molecular subtype
- COX2
cyclooxygenase 2
- CR
complete response
- CRC
colorectal cancer
- CTLA-4
cytotoxic T-lymphocyte-associated antigen 4
- DCR
disease control rate
- DFS
disease free survival
- dMMR
mismatch repair deficient
- DNMTi
DNA methyltransferase inhibitor
- EGFR
epithelial growth factor receptor
- FDA
Food and Drug Administration
- FOLFIRI
5-FU/leucovorin/irinotecan
- FOLFOX
5-FU/leucovorin/oxaliplatin
- HDACi
histone deacetylase inhibitor
- HLA
Human Leukocyte Antigen
- ICD
immunogenic cell death
- ICI
immune checkpoint inhibitor
- IDO
indolamine-2,3-dioxygenase
- indel
insertion-deletion
- IFN-γ
interferon-γ
- GI
gastrointestinal
- LTB
low tumor burden
- mCRC
metastatic colorectal cancer
- MLH1
mutL-homolog 1
- MMR
mismatch repair
- MSH
MutS-Homologs
- MSI
microsatellite instability
- MSI-H
microsatellite instability-high
- MSI-L
microsatellite instability-low
- MSS
microsatellite stable
- NK
natural killer
- NKG2A
natural killer group 2A
- ORR
objective response rate
- OS
overall survival
- PD-1
Program Death 1
- PD-L1
Programmed Death Ligand 1
- PFS
progression-free survival
- pMMR
mismatch repair proficient
- PMS2
post-meiotic segregation 2
- PR
partial response
- SBRT
stereotactic body radiation therapy
- SOC
standard of care
- TAA
tumor associated antigen
- TGFBR2
Transforming Growth Factor β Receptor II
- TKI
tyrosine kinase inhibitor
- TMB
tumor mutation burden
- TILs
tumor infiltrating lymphocytes
- TLR
Toll-like receptor
- US
united states
- VEGF
vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: There are no conflicts of interest.
References
- [1].Siegel RL, Miller KD, Jemal A, Cancer statistics, 2019, CA Cancer J Clin 69 (2019) 7–34. [DOI] [PubMed] [Google Scholar]
- [2].Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA Jr., Kinzler KW, Cancer genome landscapes, Science 339 (2013) 1546–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chu E, An update on the current and emerging targeted agents in metastatic colorectal cancer, Clinical colorectal cancer 11 (2012) 1–13. [DOI] [PubMed] [Google Scholar]
- [4].Schachter J, Ribas A, Long GV, et al. , Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006), Lancet 390 (2017) 1853–1862. [DOI] [PubMed] [Google Scholar]
- [5].Eggermont AMM, Blank CU, Mandala M, et al. , Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma, N Engl J Med 378 (2018) 1789–1801. [DOI] [PubMed] [Google Scholar]
- [6].Le DT, Uram JN, Wang H, et al. , PD-1 Blockade in Tumors with Mismatch-Repair Deficiency, N Engl J Med 372 (2015) 2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Le DT, Durham JN, Smith KN, et al. , Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade, Science 357 (2017) 409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Overman MJ, McDermott R, Leach JL, et al. , Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study, Lancet Oncol 18 (2017) 1182–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Overman MJ, Lonardi S, Wong KYM, et al. , Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer, J Clin Oncol 36 (2018) 773–779. [DOI] [PubMed] [Google Scholar]
- [10]. http://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-first-tissuesite-agnostic-indication.
- [11].Boland CR, Goel A, Microsatellite instability in colorectal cancer, Gastroenterology 138 (2010) 2073–2087 e2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jiricny J, The multifaceted mismatch-repair system, Nat Rev Mol Cell Biol 7 (2006) 335–346. [DOI] [PubMed] [Google Scholar]
- [13].Li Z, Pearlman AH, Hsieh P, DNA mismatch repair and the DNA damage response, DNA Repair (Amst) 38 (2016) 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hanahan D, Weinberg RA, Hallmarks of cancer: the next generation, Cell 144 (2011) 646–674. [DOI] [PubMed] [Google Scholar]
- [15].Lengauer C, Kinzler KW, Vogelstein B, Genetic instabilities in human cancers, Nature 396 (1998) 643–649. [DOI] [PubMed] [Google Scholar]
- [16].Richard GF, Kerrest A, Dujon B, Comparative genomics and molecular dynamics of DNA repeats in eukaryotes, Microbiology and molecular biology reviews : MMBR 72 (2008) 686–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ionov Y, Peinado MA, Malkhosyan S, Shibata D, Perucho M, Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis, Nature 363 (1993) 558–561. [DOI] [PubMed] [Google Scholar]
- [18].Thibodeau SN, Bren G, Schaid D, Microsatellite instability in cancer of the proximal colon, Science 260 (1993) 816–819. [DOI] [PubMed] [Google Scholar]
- [19].Aaltonen LA, Peltomaki P, Leach FS, et al. , Clues to the pathogenesis of familial colorectal cancer, Science 260 (1993) 812–816. [DOI] [PubMed] [Google Scholar]
- [20].Fishel R, Lescoe MK, Rao MR, et al. , The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer, Cell 75 (1993) 1027–1038. [DOI] [PubMed] [Google Scholar]
- [21].Leach FS, Nicolaides NC, Papadopoulos N, et al. , Mutations of a mutS homolog in hereditary nonpolyposis colorectal cancer, Cell 75 (1993) 1215–1225. [DOI] [PubMed] [Google Scholar]
- [22].Nicolaides NC, Papadopoulos N, Liu B, et al. , Mutations of two PMS homologues in hereditary nonpolyposis colon cancer, Nature 371 (1994) 75–80. [DOI] [PubMed] [Google Scholar]
- [23].Papadopoulos N, Nicolaides NC, Wei YF, et al. , Mutation of a mutL homolog in hereditary colon cancer, Science 263 (1994) 1625–1629. [DOI] [PubMed] [Google Scholar]
- [24].Miyaki M, Konishi M, Tanaka K, et al. , Germline mutation of MSH6 as the cause of hereditary nonpolyposis colorectal cancer, Nat Genet 17 (1997) 271–272. [DOI] [PubMed] [Google Scholar]
- [25].Kelkar YD, Tyekucheva S, Chiaromonte F, Makova KD, The genome-wide determinants of human and chimpanzee microsatellite evolution, Genome Res 18 (2008) 30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bacolla A, Larson JE, Collins JR, et al. , Abundance and length of simple repeats in vertebrate genomes are determined by their structural properties, Genome Res 18 (2008) 1545–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Fan H, Chu JY, A brief review of short tandem repeat mutation, Genomics Proteomics Bioinformatics 5 (2007) 7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Boland CR, Thibodeau SN, Hamilton SR, et al. , A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer, Cancer Res 58 (1998) 5248–5257. [PubMed] [Google Scholar]
- [29].Vilar E, Gruber SB, Microsatellite instability in colorectal cancer-the stable evidence, Nat Rev Clin Oncol 7 (2010) 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Luchini C, Bibeau F, Ligtenberg MJL, et al. , ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: a systematic review-based approach, Ann Oncol 30 (2019) 1232–1243. [DOI] [PubMed] [Google Scholar]
- [31].Stadler ZK, Battaglin F, Middha S, et al. , Reliable Detection of Mismatch Repair Deficiency in Colorectal Cancers Using Mutational Load in Next-Generation Sequencing Panels, J Clin Oncol 34 (2016) 2141–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Markowitz S, Wang J, Myeroff L, et al. , Inactivation of the type II TGF-beta receptor in colon cancer cells with microsatellite instability, Science 268 (1995) 1336–1338. [DOI] [PubMed] [Google Scholar]
- [33].Rampino N, Yamamoto H, Ionov Y, et al. , Somatic frameshift mutations in the BAX gene in colon cancers of the microsatellite mutator phenotype, Science 275 (1997) 967–969. [DOI] [PubMed] [Google Scholar]
- [34].Zhang L, Yu J, Park BH, Kinzler KW, Vogelstein B, Role of BAX in the apoptotic response to anticancer agents, Science 290 (2000) 989–992. [DOI] [PubMed] [Google Scholar]
- [35].Zhang L, Yu J, Willson JK, Markowitz SD, Kinzler KW, Vogelstein B, Short mononucleotide repeat sequence variability in mismatch repair-deficient cancers, Cancer Res 61 (2001) 3801–3805. [PubMed] [Google Scholar]
- [36].Popat S, Hubner R, Houlston RS, Systematic review of microsatellite instability and colorectal cancer prognosis, J Clin Oncol 23 (2005) 609–618. [DOI] [PubMed] [Google Scholar]
- [37].Kloor M, von Knebel Doeberitz M, The Immune Biology of Microsatellite-Unstable Cancer, Trends Cancer 2 (2016) 121–133. [DOI] [PubMed] [Google Scholar]
- [38].Ribic CM, Sargent DJ, Moore MJ, et al. , Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer, N Engl J Med 349 (2003) 247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Jover R, Zapater P, Castells A, et al. , Mismatch repair status in the prediction of benefit from adjuvant fluorouracil chemotherapy in colorectal cancer, Gut 55 (2006) 848–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Sargent DJ, Marsoni S, Monges G, et al. , Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer, J Clin Oncol 28 (2010) 3219–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Tajima A, Hess MT, Cabrera BL, Kolodner RD, Carethers JM, The mismatch repair complex hMutS alpha recognizes 5-fluorouracil-modified DNA: implications for chemosensitivity and resistance, Gastroenterology 127 (2004) 1678–1684. [DOI] [PubMed] [Google Scholar]
- [42].Bertagnolli MM, Niedzwiecki D, Compton CC, et al. , Microsatellite instability predicts improved response to adjuvant therapy with irinotecan, fluorouracil, and leucovorin in stage III colon cancer: Cancer and Leukemia Group B Protocol 89803, J Clin Oncol 27 (2009) 1814–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Andre T, de Gramont A, Vernerey D, et al. , Adjuvant Fluorouracil, Leucovorin, and Oxaliplatin in Stage II to III Colon Cancer: Updated 10-Year Survival and Outcomes According to BRAF Mutation and Mismatch Repair Status of the MOSAIC Study, J Clin Oncol 33 (2015) 4176–4187. [DOI] [PubMed] [Google Scholar]
- [44].Klingbiel D, Saridaki Z, Roth AD, Bosman FT, Delorenzi M, Tejpar S, Prognosis of stage II and III colon cancer treated with adjuvant 5-fluorouracil or FOLFIRI in relation to microsatellite status: results of the PETACC-3 trial, Ann Oncol 26 (2015) 126–132. [DOI] [PubMed] [Google Scholar]
- [45].Venderbosch S, Nagtegaal ID, Maughan TS, et al. , Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: a pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies, Clin Cancer Res 20 (2014) 5322–5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Webber EM, Kauffman TL, O’Connor E, Goddard KA, Systematic review of the predictive effect of MSI status in colorectal cancer patients undergoing 5FU-based chemotherapy, BMC cancer 15 (2015) 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Turajlic S, Litchfield K, Xu H, et al. , Insertion-and-deletion-derived tumour-specific neoantigens and the immunogenic phenotype: a pan-cancer analysis, Lancet Oncol 18 (2017) 1009–1021. [DOI] [PubMed] [Google Scholar]
- [48].Willis JA, Reyes-Uribe L, Chang K, Lipkin SM, Vilar E, Immune Activation in Mismatch Repair-Deficient Carcinogenesis: More Than Just Mutational Rate, Clin Cancer Res 26 (2020) 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Schumacher TN, Scheper W, Kvistborg P, Cancer Neoantigens, Annu Rev Immunol 37 (2019) 173–200. [DOI] [PubMed] [Google Scholar]
- [50].Maby P, Tougeron D, Hamieh M, et al. , Correlation between Density of CD8+ T-cell Infiltrate in Microsatellite Unstable Colorectal Cancers and Frameshift Mutations: A Rationale for Personalized Immunotherapy, Cancer Res 75 (2015) 3446–3455. [DOI] [PubMed] [Google Scholar]
- [51].Giannakis M, Mu XJ, Shukla SA, et al. , Genomic Correlates of Immune-Cell Infiltrates in Colorectal Carcinoma, Cell Rep 15 (2016) 857–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Germano G, Lamba S, Rospo G, et al. , Inactivation of DNA repair triggers neoantigen generation and impairs tumour growth, Nature 552 (2017) 116–120. [DOI] [PubMed] [Google Scholar]
- [53].Petrelli F, Ghidini M, Ghidini A, Tomasello G, Outcomes Following Immune Checkpoint Inhibitor Treatment of Patients With Microsatellite Instability-High Cancers: A Systematic Review and Meta-analysis, JAMA oncology 7 (2020) 1068–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54]. http://www.fda.gov/news-events/press-announcements/fda-approves-first-line-immunotherapy-patients-msi-hdmmr-metastatic-colorectal-cancer.
- [55].Mandal R, Samstein RM, Lee KW, et al. , Genetic diversity of tumors with mismatch repair deficiency influences anti-PD-1 immunotherapy response, Science 364 (2019) 485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Pai CS, Huang JT, Lu X, et al. , Clonal Deletion of Tumor-Specific T Cells by Interferon-gamma Confers Therapeutic Resistance to Combination Immune Checkpoint Blockade, Immunity 50 (2019) 477–492 e478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kurtulus S, Madi A, Escobar G, et al. , Checkpoint Blockade Immunotherapy Induces Dynamic Changes in PD-1(−)CD8(+) Tumor-Infiltrating T Cells, Immunity 50 (2019) 181–194 e186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Sahin IH, Goyal S, Pumpalova YS, et al. , Clinical and molecular markers of immune checkpoint inhibitor (ICI) response in dMMR colorectal cancer (CRC) patients (pts), Journal of Clinical Oncology 38 (2020) 225–225. [Google Scholar]
- [59].Sade-Feldman M, Jiao YJ, Chen JH, et al. , Resistance to checkpoint blockade therapy through inactivation of antigen presentation, Nat Commun 8 (2017) 1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Middha S, Yaeger R, Shia J, et al. , Majority of B2M-Mutant and -Deficient Colorectal Carcinomas Achieve Clinical Benefit From Immune Checkpoint Inhibitor Therapy and Are Microsatellite Instability-High, JCO precision oncology 3 (2019) 10.1200/PO.1218.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Garon EB, Rizvi NA, Hui R, et al. , Pembrolizumab for the treatment of non-small-cell lung cancer, N Engl J Med 372 (2015) 2018–2028. [DOI] [PubMed] [Google Scholar]
- [62].Lee JJ, Chu E, Recent Advances in the Clinical Development of Immune Checkpoint Blockade Therapy for Mismatch Repair Proficient (pMMR)/non-MSI-H Metastatic Colorectal Cancer, Clinical colorectal cancer 17 (2018) 258–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Schrock AB, Ouyang C, Sandhu J, et al. , Tumor mutational burden is predictive of response to immune checkpoint inhibitors in MSI-high metastatic colorectal cancer, Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 30 (2019) 1096–1103. [DOI] [PubMed] [Google Scholar]
- [64].Georgiadis A, Durham JN, Keefer LA, et al. , Noninvasive Detection of Microsatellite Instability and High Tumor Mutation Burden in Cancer Patients Treated with PD-1 Blockade, Clin Cancer Res 25 (2019) 7024–7034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Samstein RM, Lee CH, Shoushtari AN, et al. , Tumor mutational load predicts survival after immunotherapy across multiple cancer types, Nat Genet 51 (2019) 202–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Pages F, Mlecnik B, Marliot F, et al. , International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study, Lancet 391 (2018) 2128–2139. [DOI] [PubMed] [Google Scholar]
- [67].Yoon HH, Shi Q, Heying EN, et al. , Intertumoral Heterogeneity of CD3(+) and CD8(+) T-Cell Densities in the Microenvironment of DNA Mismatch-Repair-Deficient Colon Cancers: Implications for Prognosis, Clin Cancer Res 25 (2019) 125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Chakrabarti S, Huebner LJ, Finnes HD, et al. , Intratumoral CD3+ and CD8+ T-cell densities in patients with deficient DNA mismatch repair (dMMR) metastatic colorectal cancer (mCRC) receiving programmed death-1 (PD-1) blockade, Journal of Clinical Oncology 37 (2019) 3532–3532. [DOI] [PubMed] [Google Scholar]
- [69].Guinney J, Dienstmann R, Wang X, et al. , The consensus molecular subtypes of colorectal cancer, Nat Med 21 (2015) 1350–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Vetizou M, Pitt JM, Daillere R, et al. , Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota, Science 350 (2015) 1079–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Routy B, Le Chatelier E, Derosa L, et al. , Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors, Science 359 (2018) 91–97. [DOI] [PubMed] [Google Scholar]
- [72].Matson V, Fessler J, Bao R, et al. , The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients, Science 359 (2018) 104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Huyghe N, Baldin P, Van den Eynde M, Immunotherapy with immune checkpoint inhibitors in colorectal cancer: what is the future beyond deficient mismatch-repair tumours?, Gastroenterol Rep (Oxf) 8 (2020) 11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Chen EX, Jonker DJ, Kennecke HF, et al. , CCTG CO.26 trial: A phase II randomized study of durvalumab (D) plus tremelimumab (T) and best supportive care (BSC) versus BSC alone in patients (pts) with advanced refractory colorectal carcinoma (rCRC), Journal of Clinical Oncology 37 (2019) 481–481.30620669 [Google Scholar]
- [75].Andre T, Lonardi S, Wong M, et al. , Nivolumab + ipilimumab combination in patients with DNA mismatch repair-deficient/microsatellite instability-high (dMMR/MSI-H) metastatic colorectal cancer (mCRC): First report of the full cohort from CheckMate-142, Journal of Clinical Oncology 36 (2018) 553–553. [Google Scholar]
- [76].Andrews LP, Yano H, Vignali DAA, Inhibitory receptors and ligands beyond PD-1, PD-L1 and CTLA-4: breakthroughs or backups, Nat Immunol 20 (2019) 1425–1434. [DOI] [PubMed] [Google Scholar]
- [77].Wang YJ, Fletcher R, Yu J, Zhang L, Immunogenic effects of chemotherapy-induced tumor cell death, Genes Dis 5 (2018) 194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Zelenay S, van der Veen AG, Bottcher JP, et al. , Cyclooxygenase-Dependent Tumor Growth through Evasion of Immunity, Cell 162 (2015) 1257–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Gobel C, Breitenbuecher F, Kalkavan H, et al. , Functional expression cloning identifies COX-2 as a suppressor of antigen-specific cancer immunity, Cell death & disease 5 (2014) e1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Fallarino F, Grohmann U, You S, et al. , The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells, Journal of immunology 176 (2006) 6752–6761. [DOI] [PubMed] [Google Scholar]
- [81].Qin S, Xu L, Yi M, Yu S, Wu K, Luo S, Novel immune checkpoint targets: moving beyond PD-1 and CTLA-4, Mol Cancer 18 (2019) 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Bi J, Tian Z, NK Cell Dysfunction and Checkpoint Immunotherapy, Front Immunol 10 (2019) 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Kroemer G, Galluzzi L, Kepp O, Zitvogel L, Immunogenic cell death in cancer therapy, Annu Rev Immunol 31 (2013) 51–72. [DOI] [PubMed] [Google Scholar]
- [84].Parikh AR, Clark JW, Wo JY-L, et al. , A phase II study of ipilimumab and nivolumab with radiation in microsatellite stable (MSS) metastatic colorectal adenocarcinoma (mCRC), Journal of Clinical Oncology 37 (2019) 3514–3514. [Google Scholar]
- [85].Gajewski TF, Corrales L, Williams J, Horton B, Sivan A, Spranger S, Cancer Immunotherapy Targets Based on Understanding the T Cell-Inflamed Versus Non-T Cell-Inflamed Tumor Microenvironment, Adv Exp Med Biol 1036 (2017) 19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Rebe C, Ghiringhelli F, STAT3, a Master Regulator of Anti-Tumor Immune Response, Cancers (Basel) 11 (2019) 1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Pai SG, Carneiro BA, Mota JM, et al. , Wnt/beta-catenin pathway: modulating anticancer immune response, J Hematol Oncol 10 (2017) 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Lazarus J, Maj T, Smith JJ, et al. , Spatial and phenotypic immune profiling of metastatic colon cancer, JCI insight 3 (2018) e121932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Grothey A, Van Cutsem E, Sobrero A, et al. , Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial, Lancet 381 (2013) 303–312. [DOI] [PubMed] [Google Scholar]
- [90].Fondevila F, Mendez-Blanco C, Fernandez-Palanca P, Gonzalez-Gallego J, Mauriz JL, Anti-tumoral activity of single and combined regorafenib treatments in preclinical models of liver and gastrointestinal cancers, Exp Mol Med 51 (2019) 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Abou-Elkacem L, Arns S, Brix G, et al. , Regorafenib inhibits growth, angiogenesis, and metastasis in a highly aggressive, orthotopic colon cancer model, Mol Cancer Ther 12 (2013) 1322–1331. [DOI] [PubMed] [Google Scholar]
- [92].Fukuoka S, Hara H, Takahashi N, et al. , Regorafenib Plus Nivolumab in Patients With Advanced Gastric or Colorectal Cancer: An Open-Label, Dose-Escalation, and Dose-Expansion Phase Ib Trial (REGONIVO, EPOC1603), J Clin Oncol 38 (2020) 2053–2061. [DOI] [PubMed] [Google Scholar]
- [93].Urban-Wojciuk Z, Khan MM, Oyler BL, et al. , The Role of TLRs in Anti-cancer Immunity and Tumor Rejection, Front Immunol 10 (2019) 2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Nagarsheth N, Wicha MS, Zou W, Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy, Nat Rev Immunol 17 (2017) 559–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Jones PA, Ohtani H, Chakravarthy A, De Carvalho DD, Epigenetic therapy in immune-oncology, Nat Rev Cancer 19 (2019) 151–161. [DOI] [PubMed] [Google Scholar]
- [96].Cao J, Yan Q, Cancer Epigenetics, Tumor Immunity, and Immunotherapy, Trends in cancer 6 (2020) 580–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Mohammad HP, Barbash O, Creasy CL, Targeting epigenetic modifications in cancer therapy: erasing the roadmap to cancer, Nat Med 25 (2019) 403–418. [DOI] [PubMed] [Google Scholar]
- [98].Hu Z, Ott PA, Wu CJ, Towards personalized, tumour-specific, therapeutic vaccines for cancer, Nat Rev Immunol 18 (2018) 168–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Yarchoan M, Huang CY, Zhu Q, et al. , A phase 2 study of GVAX colon vaccine with cyclophosphamide and pembrolizumab in patients with mismatch repair proficient advanced colorectal cancer, Cancer medicine 9 (2020) 1485–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]


