Abstract
The contributors to persistent cognitive impairment and hippocampal atrophy in leucine‐rich glioma‐inactivated 1 antibody encephalitis (LGI1) patients are unknown. We evaluated whether tau neuropathology measured with [18F]flortaucipir PET neuroimaging associated with persistent cognitive impairment and hippocampal atrophy in four recovering LGI1 patients (3 men; median age, 67 [37–88] years). Imaging findings in cases were compared with those observed in age‐ and gender‐similar cognitively normal individuals (n = 124) and individuals with early‐symptomatic Alzheimer disease (n = 11). Elevated [18F]flortaucipir retention was observed in the two LGI1 patients with hippocampal atrophy and persistent cognitive impairment, including one with autopsy‐confirmed Alzheimer disease. Tau neuropathology may associate with cognitive complaints and hippocampal atrophy in recovering LGI1 patients.
Introduction
Autoimmune encephalitis associated with autoantibodies to the surface‐exposed leucine‐rich glioma‐inactivated 1 (LGI1) neuronal antigen most commonly affects individuals >60 years, 1 , 2 in whom it associates with persistent cognitive impairment and hippocampal atrophy. 1 , 3 , 4 , 5 It is unknown whether these changes result from classical immune‐mediated structural damage (i.e., neuronal loss secondary to complement‐ or other immune‐mediated damage), 6 , 7 the accelerated accrual of neuropathology in susceptible individuals, 8 , 9 or other causes.
The discovery of PET ligands capable of binding paired‐helical filaments that comprise neurofibrillary (tau) tangles provides a unique opportunity to evaluate the contribution of tau neuropathology to clinical phenotypes in living individuals. [18F]Flortaucipir (AV‐1451) exhibits reasonable specificity for tau neuropathology associated with Alzheimer disease (AD), 10 , 11 , 12 with tracer retention associated with clinical presentation, 13 , 14 disease severity and symptomatic decline. 15 , 16 , 17 We evaluated whether tau neuropathology measured with [18F]flortaucipir PET associated with persistent cognitive impairment and hippocampal atrophy in recovering LGI1 antibody encephalitis patients. One patient died 2.4 months following neuroimaging and underwent brain‐only autopsy, providing a unique opportunity to correlate neuroimaging and neuropathological findings.
Methods
Standard protocol approvals, registrations, and patient consents
Patients were enrolled from February 2016 to January 2017 within prospective studies permitting longitudinal collection and monitoring of clinical data and neuroimaging studies. Written informed consent was obtained from all individuals or their delegates. The Washington University School of Medicine Institutional Review Board (Saint Louis, Missouri, USA) approved all study procedures.
Participant selection and evaluation
Patients with LGI1 antibody encephalitis were evaluated and treated by experienced clinicians at Barnes Jewish Hospital (Saint Louis, Missouri, USA). All patients met criteria for definite autoimmune encephalitis. 18 LGI1 IgG autoantibodies were identified in the cerebrospinal fluid (CSF) or serum using cell‐based assays (Mayo Clinic Neuroimmunology Laboratory; Rochester, Minnesota). Community‐dwelling cognitively normal (CN) participants were enrolled in longitudinal studies of memory and aging at the Washington University Knight Alzheimer Disease Research Center (Saint Louis, Missouri). Cognitive impairment was graded using the Clinical Dementia Rating (CDR®), 19 and quantified longitudinally using the 18‐point CDR sum‐of‐boxes. 20 , 21 CN participants were devoid of cognitive symptoms (CDR 0), and of substantial [18F]florbetapir (AV‐45) retention (i.e., “amyloid” negative; mean SUVR < 1.27). Participants with early‐symptomatic AD had typical (amnestic) presentations with very mild impairment (CDR 0.5) and increased [18F]florbetapir retention consistent with AD (mean SUVR ≥ 1.27). 22 AD cohort characteristics were previously reported. 13
Structural MR (Biograph mMR) and [18F]flortaucipir PET neuroimaging (Biograph 40 PET/CT; Siemens Medical Solutions; Erlangern, Germany) were obtained in participants, as previously described. 12 , 13 [18F]Florbetapir PET images were obtained in CN participants and those with early‐symptomatic AD. Briefly, T1‐weighted images were acquired using magnetization‐prepared rapid‐acquisition gradient echo sequences, before cortical and subcortical parcellation was performed using FreeSurfer 5.3 (http://freesurfer.net), yielding 46 unique brain regions, corresponding to the Desikan atlas. Imaging was performed following injection of 6.8–10.9 mCi of flortaucipir or 7.4–11.3 mCi of florbetapir. Data from the 80‐ to 100‐min and 50‐ to 70‐min postinjection window were converted to SUVRs, respectively, using the cerebellar cortex as a reference; regional values were partial volume corrected using a geometric transfer matrix approach. 23 , 24 For visualization only, voxel‐wise PET were aligned to an individual patient’s T1 image and placed into a common anatomic space. [18F]flortaucipir retention was summarized using the average SUVR across all cortical regions and areas commonly affected in AD, including the left and right inferior temporal cortex, amygdala, entorhinal cortex, and lateral occipital cortices. 25
Brain‐only autopsy was performed in one patient who died following enrollment. Microscopic analyses included immunohistochemical staining with anti‐Aβ (1–42, clone H31L21) and antiphosphorylated tau (clone AT8) antibodies, performed by the Anatomic and Molecular Pathology Core Laboratory (Washington University School of Medicine), using a polymer‐based detection system and instrumentation by Ventana Medical Systems.
Statistical analyses
Clinical data were analyzed using SPSS Statistics (version 25.0; IBM Corp., Armonk, NY). On a region‐by‐region basis individual’s SUVR values were transformed into z‐scores relative to the mean and standard deviation of a cohort of 124 CN participants. P‐values for the z‐scores were inferred from the standard normal distribution. Correlations between neuroimaging findings and outcomes were evaluated using the nonparametric Spearman’s rank correlation coefficient (Spearman’s ρ).
Data availability statement
Anonymized study data will be shared pending review of a request from qualified individuals by the corresponding author.
Results
Table 1 documents the clinical course and longitudinal outcomes of LGI1 antibody encephalitis patients (75% male; median 67 years, range 37–88). Illness severity was similar across patients; all required inpatient assessment and treatment without cardiorespiratory support. [18F]Flortaucipir PET neuroimages were obtained a median of 27.4 months (range, 4.1–54.6) following symptom‐onset, and compared to findings in cohorts of amyloid‐negative CN individuals (n = 124; 58 men [47%]; median 67.7 years, range 46–91) and amyloid‐positive participants with early‐symptomatic AD (n = 11; 4 men [36%]; median 77.0 years, range 63–90). Visual inspection of images suggested prominent [18F]flortaucipir retention within the temporal lobes in Cases B and D. These findings were confirmed by quantitative analyses (Fig. 1). [18F]Flortaucipir retention within areas typically involved in AD were compared between LGI1 antibody encephalitis patients, and amyloid negative CN and amyloid positive participants with early‐symptomatic AD (Fig. 2A). In Case D, the greatest increases (vs. CN individuals) were noted in the amygdala (Z = 2.84, P = 0.005), and entorhinal (Z = 5.35, P < 0.001), inferior temporal (Z = 6.08, P < 0.001) and lateral occipital cortices (Z = 3.94, P < 0.001). Focused deposition within limbic structures was seen in Case B, including the parahippocampal gyri (Z = 2.61, P = 0.009), amygdala (Z = 2.17, P = 0.03), and posterior cingulate cortices (Z = 2.34, P = 0.02). When compared to CN individuals, both patients had smaller‐than‐expected hippocampal volumes (Fig. 2B; Case B: Z = −4.13, P < 0.001; Case D: Z = −1.64, P = 0.1), with persistent deficits in cognitive function observed at the last clinical evaluation (54.6 [Case D] and 76.0 months [Case B] from symptom onset). No differences were observed in [18F]flortaucipir retention or hippocampal volumes in Cases A and C (vs. CN participants); both patients were CN at the last clinical evaluation (35.4 [Case A] and 46.0 months [Case C] following symptom onset).
Table 1.
Clinical course and longitudinal outcomes in LGI1 antibody encephalitis patients.
| Case, age/gender | Presenting features | Treatment | Flortaucipir PET | Final outcome | ||||
|---|---|---|---|---|---|---|---|---|
| Months from onset | Cognitive function | Global SUVR 1 | AD Summary SUVR 2 | Months from onset | Cognitive function | |||
| A, 35 y/M | Small fiber and autonomic neuropathy, followed by subacute‐onset memory loss, inattention and mental status change. | Steroids, IVIg, rituximab | 4.2 | CDR 0.5; CDRsb 2.0 | 1.12 | 1.05 | 36.0 | Cognitively normal; CDR 0; CDRsb 0 |
| B, 63 y/M | Subacute‐onset psychosis and FBDS. | Steroids, IVIg | 36.4 | CDR 0.5; CDRsb 3.0 | 1.21 | 1.32 | 48.0 | Very mild impairmentCDR 0.5; CDRsb 4.0 |
| C, 67 y/M | Subacute‐onset psychosis with FBDS. | Steroids, IVIg, rituximab | 18.4 | CDR 0; CDRsb 0 | 1.12 | 1.16 | 48.0 | Cognitively normal; CDR 0; CDRsb 0 |
| D, 83y/F | Subacute‐onset memory loss and psychoses with FBDS associated with altered consciousness. | Steroids, IVIg, rituximab | 54.6 | CDR 2; CDRsb 14.0 | 1.48 | 1.97 | 57.0 | Deceased |
AD, Alzheimer disease; CDR, Clinical Dementia Rating®; CDRsb, CDR sum‐of‐boxes; FBDS, faciobrachial dystonic seizures; IVIg, intravenous immunoglobulin (0.4 g/kg × 5 days); rituximab, 375 mg/m2 Q7 days × 4; steroids, methylprednisolone 1 g × 5 days, followed by prolonged oral taper); SUVR, standardized uptake value ratios.
Global SUVR reflects the average SUVR across all cortical regions. For comparison, mean Global SUVR was 1.11 ± 0.1 in cognitive normal individuals and 1.43 ± 0.25 in individuals with early‐symptomatic AD.
AD Summary SUVR reflects the average SUVR within regions commonly affected in AD, including the left and right inferior temporal cortex, amygdala, entorhinal cortex, and lateral occipital cortices. 25 For comparison, mean AD Summary SUVR was 1.16 ± 0.21 in cognitive normal individuals and 1.99 ± 0.64 in individuals with early‐symptomatic AD.
Figure 1.
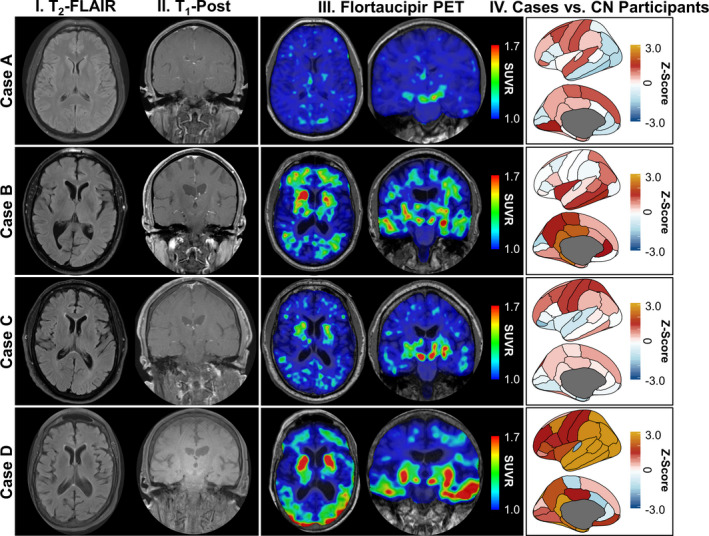
Selected neuroimaging in LGI1 antibody encephalitis patients. Structural magnetic resonance brain images (T2‐FLAIR [column I] and T1‐post contrast [column II]) and flortaucipir PET images (axial and coronal orientation, column III) are shown for each patient with LGI1 antibody encephalitis (cases A–D; presented in radiologic convention). Column IV shows [18F]flortaucipir uptake in LGI1 antibody encephalitis patients referenced to uptake in cognitively normal individuals (n = 124) across 46 regions of interest. FLAIR = fluid attenuated inversion recovery; PET = positron emission tomography; CN = cognitively normal; LGI1 = leucine‐rich glioma‐inactivated 1; SUVR = standardized uptake ratio value.
Figure 2.
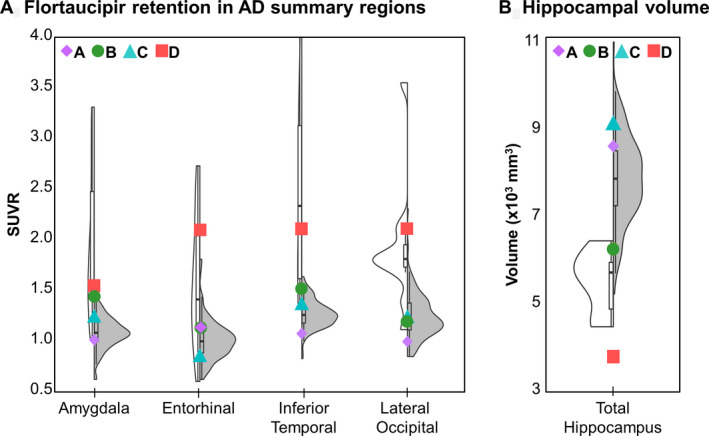
[18F]Flortaucipir retention and hippocampal volume in LGI1 antibody encephalitis patients versus cognitively normal and early‐symptomatic Alzheimer disease participants. Plots showing [18F]flortaucipir PET standardized uptake value ratios (SUVRs; A) within areas commonly implicated in AD 17 , 25 (A), and hippocampal volumes measured on structural MR (B) for LGI1 antibody encephalitis patients (symbols), cognitively normal participants (gray violin plots) and participants with early‐symptomatic Alzheimer disease (white violin plot).
In LGI1 encephalitis patients, global and AD summary SUVRs were closely associated with the rank‐order of CDR sum‐of‐boxes at last clinical follow‐up (Spearman’s ρ = 0.95; P = 0.051), but not with CDR sum‐of‐boxes at the time of imaging (Spearman’s ρ = 0.80; P = 0.20). Hippocampal volumes closely tracked CDR sum‐of‐boxes, with smaller volumes associated with greater impairment at the time of imaging (Spearman’s ρ < −0.99; P < 0.01) and last follow‐up (Spearman’s ρ = −0.95; P = 0.051).
Case D initially responded to immunotherapy, but presented 3 years later with worsening cognition, paranoid delusions and visual hallucinations. Autoantibodies were not detected in serum or CSF (WBC = 0 cells/µL, 0 CSF‐specific oligoclonal bands). Deficits persisted despite treatment with steroids (1g IV × 5 days followed by prolonged oral taper), intravenous immunoglobulin (2 g/kg over 5 days) and rituximab (375 mg/m2 IV weekly × 4). She died due to complications of dementia 57.0 months after the symptomatic‐onset of LGI1 antibody encephalitis (2.4 months following [18F]flortaucipir PET). Brain‐only autopsy confirmed “intermediate” AD neuropathologic change (A2, B2, C2) with Braak neurofibrillary tangle stage IV, 26 without inflammation or sequelae of encephalitis.
Discussion
[18F]Flortaucipir PET retention was observed in brain areas associated with AD 17 , 25 in two of four recovering LGI1 antibody encephalitis patients. These findings suggest that inflammation associated with autoimmune encephalitis may accelerate the accrual of neurofibrillary pathology in susceptible individuals. Interestingly, elevations in tau were associated with hippocampal atrophy and persistent cognitive impairment, suggesting that tau neuropathology may contribute to cognitive complaints and structural and functional neuroimaging changes reported in larger cohorts of LGI1 antibody encephalitis patients. 1 , 3 , 4 , 5 The discovery of AD neuropathologic change without sequelae of encephalitis in one patient further emphasizes the potential that biomarkers with reasonable sensitivity and specificity for specific neuropathology may be leveraged to inform the contributors to resurgent or treatment‐refractory symptoms and signs in patients with autoimmune encephalitis.
Confluent lines of research implicate inflammation in the formation and spread of neurofibrillary neuropathology in AD 8 , 9 and autoimmune encephalitis. 27 , 28 It is possible that tau neuropathology may accumulate in response to inflammation or immune‐mediated injury in susceptible individuals, or that that immune‐mediated injury may unmask symptoms in patients with underlying neuropathology, including patients with co‐occurring preclinical Alzheimer disease. 29 It is also plausible that LGI1 antibody encephalitis and symptomatic Alzheimer disease may present in the same patient, representing two independent and unrelated disease processes. Systematic evaluation of patients recovering with LGI1 antibody encephalitis may inform the dynamic relationship between inflammation (severity, duration, etc.) and relevant neuropathology. Future studies integrating emerging markers of synaptic function and neuroinflammation (e.g., PET markers of synaptic vesicle and translocator proteins 30 ), as well as neuropathological assessment are key to this goal. Studies in LGI1 antibody encephalitis patients may be particularly insightful as the LGI1 protein is most strongly expressed in the hippocampus, 31 and LGI1 antibody encephalitis preferentially affects older individuals, 1 , 2 who are most susceptible to AD neuropathology.
Caution is advised when interpreting these results in four patients with LGI1 antibody encephalitis. Longitudinal biomarker measures are needed in greater numbers of recovering patients to circumvent limitations inherent with cross‐sectional [18F]flortaucipir PET neuroimaging performed at variable intervals following symptom onset. These same studies should also consider the relationship between known AD risk factors, including APOE allele status (not measured here), and imaging changes observed following LGI1 antibody encephalitis. Such efforts may aid in deciphering the contributors to cognitive impairment and structural changes in patients with LGI1 antibody encephalitis, while informing the roles of inflammation in promoting the accrual and spread of neuropathology in individuals with common age‐associated neurodegenerative diseases.
Conclusion
Increased [18F]flortaucipir PET retention was observed in the two LGI1 antibody encephalitis patients with hippocampal atrophy and persistent cognitive impairment, including one patient with autopsy‐proven AD and moderate‐stage neurofibrillary pathology. Inflammation associated with autoimmune encephalitis may accelerate the accrual of tau neuropathology in susceptible individuals, and may associate with hippocampal atrophy and persistent cognitive complaints.
Conflict of Interest
GS Day is supported by a career development grant from the NIH (K23AG064029). He owns stock (>$10,000) in ANI Pharmaceuticals (a generic pharmaceutical company). He serves as a topic editor for DynaMed (EBSCO), overseeing development of evidence‐based educational content; consultant for Parabon Nanolabs (Inc), and as the Clinical Director of the Anti‐NMDA Receptor Encephalitis Foundation (Inc, Canada; uncompensated). BA Gordon takes part in clinical trials sponsored by Roche and Eli Lilly (via DIAN‐TU) outside of the reported work. A McCullough reports no disclosures. RC Bucelli has served on an advisory board for MT Pharma, has a consulting role with Biogen, has Equity in Neuroquestions.LLC and receives a recurring annual gift from a patient’s family for research on neuralgic amyotrophy. RJ Perrin takes part in clinical trials sponsored by Roche and Eli Lilly (via DIAN‐TU) outside of the reported work. TLS Benzinger takes part in clinical trials sponsored by Roche and Eli Lilly (via DIAN‐TU) outside of the reported work. BM Ances reports no disclosures.
Author Contributions
GS Day participated in the conception and design of the study; patient recruitment; acquisition, statistical analysis and interpretation of clinical and neuroimaging data; and drafting, revision and finalization of the manuscript. He had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. BA Gordon participated in the analysis and interpretation of neuroimaging data; and finalization of the manuscript. A McCullough participated in the analysis and interpretation of neuroimaging data; and finalization of the manuscript. RC Bucelli participated in patient recruitment; acquisition and interpretation of clinical data; and revision and finalization of the manuscript. RJ Perrin participated in the acquisition and interpretation of neuropathological data, and revision and finalization of the manuscript. TLS Benzinger participated in the acquisition and interpretation of neuroimaging data, and revision and finalization of the manuscript. BM Ances participated in the conception and design of the study; acquisition and interpretation of clinical and neuroimaging data; and revision and finalization of the manuscript.
Acknowledgments
This study was supported by grants from the National Institutes of Health (K23AG064029, K01AG053474, P50AG005681, P01AG003991, P01AG026276, R01AG057680, R01AG052550, the Paula and Roger O. Riney Fund, the Daniel J Brennan Fund (BMA), and patient‐directed donations (GSD). [18F]Flortaucipir precursor and technology were supported by AVID Radiopharmaceuticals. The funding organization did not contribute to the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Funding Information
This study was supported by grants from the National Institutes of Health (K23AG064029, K01AG053474, P50AG005681, P01AG003991, P01AG026276, R01AG057680, R01AG052550, the Paula and Roger O. Riney Fund, the Daniel J Brennan Fund (BMA), and patient‐directed donations (GSD).
Funding Statement
This work was funded by National Institutes of Health grants K23AG064029, K01AG053474, P50AG005681, P01AG003991, P01AG026276, R01AG057680, and R01AG052550; the Paula and Roger O. Riney Fund grant ; the Daniel J Brennan Fund grant ; patient‐directed donations grant .
References
- 1. Gadoth A, Pittock SJ, Dubey D, et al. Expanded phenotypes and outcomes among 256 LGI1/CASPR2‐IgG‐positive patients. Ann Neurol 2017;82:79–92. [DOI] [PubMed] [Google Scholar]
- 2. Dalmau J, Graus F. Antibody‐mediated encephalitis. N Engl J Med 2018;378:840–851. [DOI] [PubMed] [Google Scholar]
- 3. Finke C, Pruss H, Heine J, et al. Evaluation of cognitive deficits and structural hippocampal damage in encephalitis with leucine‐rich, glioma‐inactivated 1 antibodies. JAMA Neurol 2017;74:50–59. [DOI] [PubMed] [Google Scholar]
- 4. Loane C, Argyropoulos GPD, Roca‐Fernandez A, et al. Hippocampal network abnormalities explain amnesia after VGKCC‐Ab related autoimmune limbic encephalitis. J Neurol Neurosurg Psychiatry 2019;90:965–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Irani SR, Stagg CJ, Schott JM, et al. Faciobrachial dystonic seizures: the influence of immunotherapy on seizure control and prevention of cognitive impairment in a broadening phenotype. Brain 2013;136(Pt 10):3151–3162. [DOI] [PubMed] [Google Scholar]
- 6. Bien CG, Vincent A, Barnett MH, et al. Immunopathology of autoantibody‐associated encephalitides: clues for pathogenesis. Brain 2012;135(Pt 5):1622–1638. [DOI] [PubMed] [Google Scholar]
- 7. Thompson J, Bi M, Murchison AG, et al. The importance of early immunotherapy in patients with faciobrachial dystonic seizures. Brain 2018;141:348–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pillai JA, Maxwell S, Bena J, et al. Key inflammatory pathway activations in the MCI stage of Alzheimer's disease. Ann Clin Transl Neurol 2019;6:1248–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Heneka MT, Carson MJ, El Khoury J, et al. Neuroinflammation in Alzheimer's disease. Lancet Neurol 2015;14:388–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marquie M, Normandin MD, Vanderburg CR, et al. Validating novel tau positron emission tomography tracer [F‐18]‐AV‐1451 (T807) on postmortem brain tissue. Ann Neurol 2015;78:787–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fleisher AS, Pontecorvo MJ, Devous MD Sr, et al. Positron emission tomography imaging with [18F]flortaucipir and postmortem assessment of alzheimer disease neuropathologic changes. JAMA Neurol 2020;77:829–839. 10.1001/jamaneurol.2020.0528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Day GS, Gordon BA, Perrin RJ, et al. In vivo [(18)F]‐AV‐1451 tau‐PET imaging in sporadic Creutzfeldt‐Jakob disease. Neurology 2018;90(10):e896–e906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Day GS, Gordon BA, Jackson K, et al. Tau‐PET binding distinguishes patients with early‐stage posterior cortical atrophy from amnestic Alzheimer Disease Dementia. Alzheimer Dis Associ Disord 2017;31:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ossenkoppele R, Schonhaut DR, Scholl M, et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer's disease. Brain 2016;139(Pt 5):1551–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang L, Benzinger TL, Su Y, et al. Evaluation of tau imaging in staging Alzheimer disease and revealing interactions between beta‐amyloid and tauopathy. JAMA Neurol 2016;73:1070–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jack CR Jr, Wiste HJ, Schwarz CG, et al. Longitudinal tau PET in ageing and Alzheimer's disease. Brain 2018;141:1517–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brier MR, Gordon B, Friedrichsen K, et al. Tau and Abeta imaging, CSF measures, and cognition in Alzheimer's disease. Sci Transl Med 2016;8:338ra66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15:391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 20. Williams MM, Storandt M, Roe CM, Morris JC. Progression of Alzheimer's disease as measured by Clinical Dementia Rating Sum of Boxes scores. Alzheimers Dement 2013;9(1 Suppl):S39–S44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cedarbaum JM, Jaros M, Hernandez C, et al. Rationale for use of the Clinical Dementia Rating Sum of Boxes as a primary outcome measure for Alzheimer's disease clinical trials. Alzheimers Dement 2013;9(1 Suppl):S45–S55. [DOI] [PubMed] [Google Scholar]
- 22. Gordon BA, Friedrichsen K, Brier M, et al. The relationship between cerebrospinal fluid markers of Alzheimer pathology and positron emission tomography tau imaging. Brain 2016;139(Pt 8):2249–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Su Y, Blazey TM, Snyder AZ, et al. Partial volume correction in quantitative amyloid imaging. NeuroImage 2015;15:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Su Y, D'Angelo GM, Vlassenko AG, et al. Quantitative analysis of PiB‐PET with FreeSurfer ROIs. PLoS One 2013;8:e73377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mishra S, Gordon BA, Su Y, et al. AV‐1451 PET imaging of tau pathology in preclinical Alzheimer disease: defining a summary measure. NeuroImage 2017;1:171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging‐Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol 2012;123:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gelpi E, Hoftberger R, Graus F, et al. Neuropathological criteria of anti‐IgLON5‐related tauopathy. Acta Neuropathol 2016;132:531–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gamre M, Ryding M, Nissen MS, et al. Investigation of anti‐IgLON5‐induced neurodegenerative changes in human neurons. Manuscript BioRxiv 2020;28:2020. [Google Scholar]
- 29. Jack CR Jr, Bennett DA, Blennow K, et al. NIA‐AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement 2018;14:535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jain P, Chaney AM, Carlson ML, et al. Neuroinflammation PET imaging: current opinion and future directions. J Nucl Med 2020;61:1107–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Irani SR, Alexander S, Waters P, et al. Antibodies to Kv1 potassium channel‐complex proteins leucine‐rich, glioma inactivated 1 protein and contactin‐associated protein‐2 in limbic encephalitis. Morvan's syndrome and acquired neuromyotonia. Brain 2010;133:2734–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized study data will be shared pending review of a request from qualified individuals by the corresponding author.


