Abstract
Paraneoplastic autoimmune encephalitis (PAE) represents a group of rare neurological syndromes associated with neoplastic diseases. Here, we report a case that multiple anti‐neuronal antibodies were present in a patient with PAE who developed both small cell lung cancer and colorectal adenocarcinoma. Furthermore, the immunopathological investigation of the colorectal adenocarcinoma revealed the formation of abnormal neuronal antigens and a massive infiltration of plasma cells in the tumor tissue. These findings support the hypothesis that expression of neuronal antigens in neoplasm initiates autoimmune responses in PAE.
Introduction
Paraneoplastic autoimmune encephalitis (PAE) represents a group of rare neurological syndromes which is characterized by cognitive impairment, personality change, memory loss, depression, and seizures. PAE is often associated with various neoplasms of the lung, testis, ovary, and breast. 1 According to disease‐associated autoantibodies, PAE can be categorized into two groups. One group is featured by autoantibodies against neuronal intracellular antigens including Hu, Ma2, amphiphysin, and CV2/collapsin response mediator protein 5 (CRMP5), while the other is associated with autoantibodies to neuronal antigens on the extracellular surface antigens such as the voltage‐gated potassium channel (VGKC) complex, N‐methyl‐D‐aspartate receptor (NMDAR), alpha‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid receptor (AMPAR), and gamma aminobutyric acid B receptor (GABABR). 1 , 2 With regards to the pathomechanisms, it is believed that the disease in the former group is mediated mainly by autoreactive T cells and in the latter group is driven by autoantibodies. 1 , 3
PAE is typically associated with the expression of autoantibodies against neuronal antigens, 1 , 4 and a coexpression of multiple autoantibodies has been reported only in few cases with or without tumor. 5 , 6 , 7 However, the development of multiple antibodies in PAE patients with more than one tumor has not been reported so far. Here, we describe a case in which multiple autoantibodies (anti‐Hu, anti‐NMDAR, and anti‐GAD antibodies) were present in a PAE patient with two different cancers (small cell lung cancer (SCLC) and colorectal adenocarcinoma). Furthermore, we could demonstrate the presence of neuron cells and a massive infiltration of plasma cells in the colorectal adenocarcinoma of the patient.
Case Description and Results
A 57‐year‐old man was referred to our hospital on September 2, 2017, with a 20 days history of seizure, hallucination, inappropriate speech, and abnormal behaviors without preceding infections, fever, or vaccinations. Most laboratory tests were normal/negative, including serum lactate, copper, vitamins, thyroid function, Treponema pallidum antibody, human immunodeficiency virus antibody, and anti‐nuclear/neutrophil cytoplasmic/SSA/SSB antibodies. No abnormalities were observed in brain magnetic resonance imaging (MRI). Cerebrospinal fluid (CSF) analysis showed a normal level of white blood cell count (4 × 106/L) and level of total protein (350 mg/L). In addition, CSF culture was negative for bacterial and fungal cultures, and polymerase chain reaction (PCR) on CSF was negative for virus. Autoantibodies were determined by indirect immunostaining using a commercially available kit (EUROIMMUN Medizinische Labordiagnostika, Lübeck, Germany). Antibodies against N‐methyl‐D‐aspartate receptor (NMDAR) were positively detected in both serum (titer 1:32) and CSF (titer 1:10). Furthermore, the serum scored also positive for antibodies against glutamic acid decarboxylase (GAD) (titer 1:10), and anti‐Hu (titer 1:10) in indirect immunofluorescence test. In addition, the presence of serum anti‐Hu antibodies was confirmed by immunobloting assay using the Euroline Neuronal Antigens Profile 2 IgG kit (DL1111‐1601‐2 G; Euroimmun AG, Lübeck, Germany). However, the anti‐GAD and anti‐Hu antibodies were negative in CSF.
Positron Emission Tomography with Computed Tomography (PET/CT) scans revealed an abnormal increase in fluorodeoxy glucose (FDG) uptake in a small solid pulmonary nodule in the posterior segment of the right upper lobe with some lymph nodes in mediastinum and right supraclavicular fossa, and also in the sigmoid colon with single lymph node around the colon (Fig. 1). A biopsy of the lymph node in the right supraclavicular fossa revealed the metastasis of small cell lung cancer (SCLC) (Figure S1). Moreover, colonoscopy showed a moderately differentiated adenocarcinoma in junction of rectum and sigmoid (Fig. 2). For the management of his seizure and neuropsychiatric symptoms, the patient received oxcarbazepine, olanzapine, and haloperidol in our hospital. However, the family of the patient refused to immunotherapy such as treatment with methylprednisolone or intravenous immunoglobulin and other anti‐tumor therapies including tumor surgery or chemotherapy. Then, the patient was discharged and transferred to a local hospital and died 10 months later.
Figure 1.
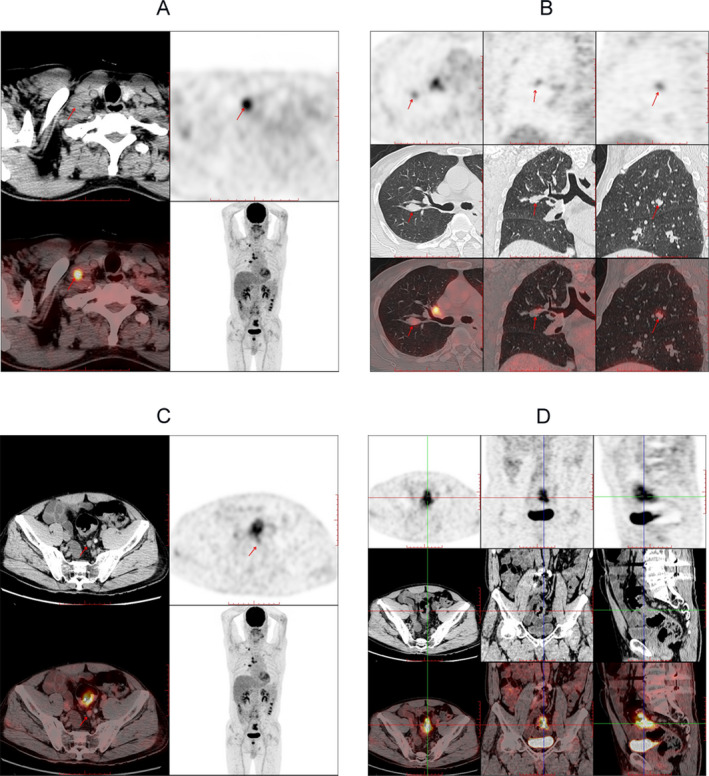
PET‐CT images from the current patient. PET‐CT images show an abnormal metabolism in the lymph node in the right supraclavicular fossa indicated by the arrow (A), small solid pulmonary nodule in the posterior segment of the right upper lobe (B, arrows), lymph node around the sigmoid colon (C, arrows), and the sigmoid colon (D, arrows).
Figure 2.
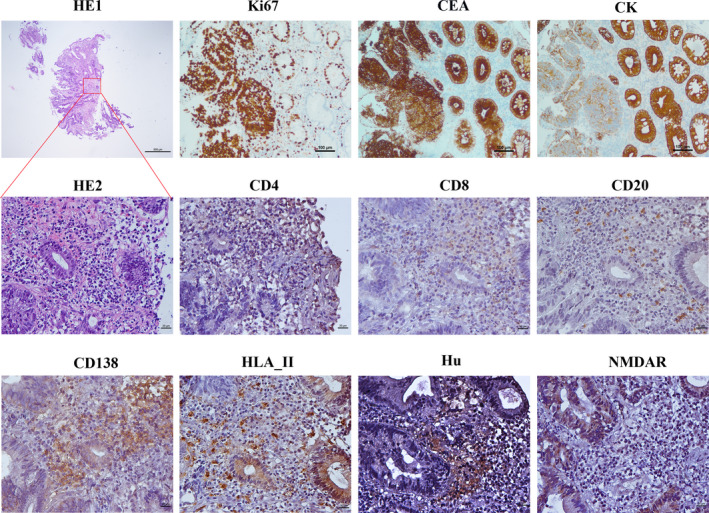
Histopathology of the colorectal adenocarcinoma of the patient. HE staining shows mucosal intrinsic gland with hyperplasia, densely arranged, as well as atypial cells with large nucleus, deep staining,, and mitotic images. In addition, inflammatory cells are accumulated in the same lesion (HE1 (Magnification × 40, bar = 500 μm); HE2 (Magnification × 400, bar = 20 μm)). The immunohistochemical staining shows the expression of Ki67 protein (80%) (Magnification × 100, bar = 100 μm), carcinoembryonic antigen (CEA) (Magnification × 100, bar = 100 μm), and cytokeratin (CK) (Magnification × 100, bar = 100 μm) as well as the presence of CD4+ T cells (Magnification × 400, bar = 20 μm), CD8+T‐cells (Magnification × 400, bar = 20 μm), some CD20+B cells (Magnification × 400, bar = 20 μm), abundant CD138 +plasma cells (Magnification × 400, bar = 20 μm), HLA II+ cells (Magnification × 400, bar = 20 μm), Hu‐positive cells (Magnification × 400, bar = 20μm), and NMDAR‐positive cells (Magnification × 400, bar = 20 μm) in the colon lesions of the patient.
It is hypothesized that abnormal expression of neuronal antigens in neoplasm initiates autoimmune responses in PAE. 8 This hypothesis is supported by findings in teratoma‐associated anti‐NMDAR encephalitis, where ovarian teratoma is featured by ectopic expression of NMDAR and infiltration of immune cells. 9 , 10 However, it has not been investigated whether colorectal adenocarcinoma is featured by abnormal expression of neuronal antigens and infiltration of immune cells. To further explore the case of PAE associated with multiple autoantibodies, we determined the histopathology of the colorectal adenocarcinoma. After explantation, tumor material was fixed in 10% formalin and sections thereof were used for hematoxylin/eosin (HE) and immunohistochemical (IHC) staining. The respective primary antibodies used, their concentrations, and antigen retrieval procedures are summarized in Table S1.
HHistological analysis showed mucosal intrinsic gland with hyperplasia, densely arranged, as well as atypial cells with large nucleus, deep staining, and mitotic images. In addition, colon lesions scored positive for Ki67 (80%), carcinoembryonic antigen (CEA), and cytokeratin (CK) as shown by IHC staining (Fig. 2). Intriguingly, the tumor sample was positive for anti‐Hu and anti‐NMDAR staining, suggesting the presence of neuronal antigens (Fig. 2). To understand the immunological features of the colorectal adenocarcinoma, we performed IHC staining with antibodies against CD4, CD8, CD20, CD138, and HLA II. As typical for adenocarcinoma, we could detect CD4+ T cells, CD8+ T cells, CD20+ B cells, and HLA II‐positive cells in the tumor tissue. However, a tremendous high number of CD138+ cells indicate a massive infiltration of plasma cells in this tissue. (Fig. 2).
Discussion
In the current study, we report on a PAE patient characterized by the formation of two types of cancer and the development of autoantibodies against three neuronal antigens. Although coexpression of multiple anti‐neuronal antibodies has been reported in a few patients with PAE, 5 , 6 , 7 to the best of our knowledge, this is the first case of PAE patient associated with multiple cancer and diverse anti‐neuronal antibodies.
Although the etiology of PAE remains unclear, it is proposed that expression of neuronal antigens within neoplasms could initiate an autoimmune response which leads to the production of autoantibodies against neuronal antigens. 1 , 8 This hypothesis is supported by findings in anti‐NMDAR encephalitis where immune cells and neuronal antigens are found in the disease‐associated ovarian teratoma. 9 , 10 In the current case report, we demonstrate the presence of immune cells and neuronal antigens in the colon cancer of a patient, which further supports this hypothesis. Although the generation of autoantibodies is associated with neoplasm, it is difficult to figure out the exact relation of the three anti‐neuronal antibodies to the two cancers in the patient. Patients with anti‐Hu antibodies are usually associated with small cell lung cancer (SCLC), 11 anti‐NMDAR antibodies are often associated with ovarian teratoma, 12 while colorectal adenocarcinoma has been reported to be associated with anti‐Hu, 13 anti‐NMDAR, 14 and anti‐GAD65 15 autoantibodies. Since anti‐Hu and anti‐GAD65 were presented at low levels in sera and absent in CSF of the patient, we need to be careful in the interpretation of the clinical relevance of the two autoantibodies.
In the current case, three distinct neuronal antibodies, including anti‐Hu, anti‐NMDAR, and anti‐GAD antibodies, were found in the PAE patient with SCLC and sigmoid adenocarcinoma. Notably, although symptoms in PAE often overlap, each neuronal antibody is frequently associated with specific clinical phenotypes. For example, antibodies directed against Hu are associated with cerebellar and limbic dysfunction; anti‐NMDAR antibodies are associated with adventitious movements, acute psychosis, and mutism; and anti‐GAD‐65 antibodies are associated with stiff‐person syndrome, cerebellar atxia, seizures, and limbic encephalitis. 12 , 16 Given that the patient was characterized by seizure and psychiatric but not stiff‐person syndrome or cerebellar atxia, both anti‐NMDAR and anti‐GAD‐65 antibodies are likely to play an important role in the disease pathogenesis. However, it is unclear whether a single antibody was associated with all co‐existing tumors or whether this association includes a combination of two or all three antibodies.
Anti‐NMDAR encephalitis is a PAE caused by autoantibodies. 8 It has been shown be associated with B‐cell scaffold protein with ankyrin repeats 1 (BANK1) 17 and HLA‐DRB1*1602 18 which is associated with many autoantibody‐mediated disorders. 19 Infiltration of B cells and plasma cells in disease‐associated tumor is a feature of anti‐NMDAR encephalitis. 9 , 20 However, in contrast to anti‐NMDAR encephalitis patients in previous studies 9 , 20 where only mild infiltration of plasma cells in ovarian teratoma is observed frequently, the patient of our current report showed an unusual massive infiltration of these cells in the colorectal adenocarcinoma. Infiltration of plasma cell in tumor is a normal phenomenon, which is believed to have some positive prognostic significance for the cancer. 21 Given the essential role of plasma cell in producing antibodies, it is conceivable that the very elevated infiltration of plasma cells is associated with the multiple anti‐neuronal antibodies. However, this notion needs to be validated in the future.
One major limitation of this study needs to be mentioned. Since the immunopathology of the lung biopsy was not investigated, the diagnosis of SCLC was only based on PET‐CT in lung and a biopsy of the lymph node in the right supraclavicular fossa. Therefore, the question whether neuronal antigens and infiltration of plasma cells were also present in the lung neoplasm has to be left open, and it remains unclear which cancer represent the dominant cause of encephalitis or if the two cancers have worked simultaneously.
In conclusion, the present study reported a case with PAE which was associated with several onconeural autoantibodies (anti‐Hu, NMDAR, GAD antibodies) and multiple cancers (SCLC and colorectal adenocarcinoma). The presence of neuronal antigens and plasma cells in colon cancer might be involved in the pathogenesis of PAE.
Author Contributions
Conception and design of the study: Wei Qiu, Xinhua Yu; Acquisition and analysis of data: Yaqing Shu, Dan He, Xiaoyu Ma, Jianfang Li, Haotian Wu, Chen Chen, Zhengqi Lu; Drafting of the text or preparing the figures: Yaqing Shu, Frank Petersen, Wei Qiu, Xinhua Yu.
Ethical Standards
This study was conducted in accordance with the Declaration of Helsinki Ethical Principles and was approved by the ethics committee of the Third Affiliated Hospital of Sun Yat‐sen University (2019‐637‐01). The family of the patient agreed to the study by written informed consent.
Conflicts of Interest
The authors declare that no competing interests exist.
Supporting information
Figure S1. Histopathology of lymph node in the right supraclavicular fossa of the patient.
Table S1. Antibodies and antigen retrieval procedures used in IHC staining in this study.
Funding Information
National Natural Science Foundation of China (NO.82071343), National Natural Science Foundation of China Youth Science Foundation (NO.81701188) and the First Major Talent Project of the Third Affiliated Hospital of Sun Yat‐sen University, the Deutsche Forschungsgemeinschaft via GRK1727 “Modulation of Autoimmunity” and the Bundesministerium für Bildung und Forschung (BMBF) via the German Center for Lung Research (DZL).
[Correction added on 11 Jan, 2020 after first online publication: funding information has been updated.]
Funding Statement
This work was funded by National Natural Science Foundation of China grant 81701188; Deutsche Forschungsgemeinschaft grant GRK1727.
Contributor Information
Wei Qiu, Email: qiuwei120@vip.163.com.
Xinhua Yu, Email: xinhuayu@fz-borstel.de.
References
- 1. Dalmau J, Rosenfeld MR. Autoimmune encephalitis update. Neuro Oncol. 2014;16:771–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Geng G, Yu X, Jiang J, et al. Aetiology and pathogenesis of paraneoplastic autoimmune disorders. Autoimmun Rev. 2020;19:102422. [DOI] [PubMed] [Google Scholar]
- 3. Rosenfeld MR, Dalmau J. Paraneoplastic neurologic syndromes. Neurol Clin. 2018;36:675–685. [DOI] [PubMed] [Google Scholar]
- 4. Zekeridou A, McKeon A, Lennon VA. Frequency of synaptic autoantibody accompaniments and neurological manifestations of thymoma. JAMA Neurol. 2016;73:853–859. [DOI] [PubMed] [Google Scholar]
- 5. Fukuda TG, do Rosario MS, Branco RCC, et al. Multiple paraneoplastic antibodies (anti‐SOX1, anti‐Hu, and anti‐Amphiphysin) detected in a patient with limbic encephalitis and small cell lung cancer. Neurol India. 2017;65:1127–1128. [DOI] [PubMed] [Google Scholar]
- 6. Kammeyer R, Piquet AL. Multiple co‐existing antibodies in autoimmune encephalitis: a case and review of the literature. J Neuroimmunol. 2019;337:577084. [DOI] [PubMed] [Google Scholar]
- 7. Kim AE, Kang P, Bucelli RC, et al. Autoimmune encephalitis with multiple autoantibodies: a diagnostic and therapeutic challenge. Neurologist. 2018;23:55–59. [DOI] [PubMed] [Google Scholar]
- 8. Dalmau J, Graus F. Antibody‐mediated encephalitis. N Engl J Med. 2018;378:840–851. [DOI] [PubMed] [Google Scholar]
- 9. Chefdeville A, Treilleux I, Mayeur ME, et al. Immunopathological characterization of ovarian teratomas associated with anti‐N‐methyl‐D‐aspartate receptor encephalitis. Acta Neuropathol Commun. 2019;7:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tuzun E, Zhou L, Baehring JM, et al. Evidence for antibody‐mediated pathogenesis in anti‐NMDAR encephalitis associated with ovarian teratoma. Acta Neuropathol. 2009;118:737–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gultekin SH, Rosenfeld MR, Voltz R, et al. Paraneoplastic limbic encephalitis: neurological symptoms, immunological findings and tumour association in 50 patients. Brain 2000;123(Pt 7):1481–1494. [DOI] [PubMed] [Google Scholar]
- 12. Graus F, Saiz A, Dalmau J. Antibodies and neuronal autoimmune disorders of the CNS. J Neurol. 2010;257:509–517. [DOI] [PubMed] [Google Scholar]
- 13. Tsukamoto T, Mochizuki R, Mochizuki H, et al. Paraneoplastic cerebellar degeneration and limbic encephalitis in a patient with adenocarcinoma of the colon. J Neurol Neurosurg Psychiatry. 1993;56:713–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Park BS, Son GM, Kim HS, et al. Anti‐N‐methyl‐d‐aspartate receptor encephalitis in a patient with colon cancer. Clin Neurol Neurosurg. 2019;177:114–116. [DOI] [PubMed] [Google Scholar]
- 15. Kojima G, Inaba M, Bruno MK. PET‐positive extralimbic presentation of anti‐glutamic acid decarboxylase antibody‐associated encephalitis. Epileptic Disord. 2014;16:358–361. [DOI] [PubMed] [Google Scholar]
- 16. Korff CM, Parvex P, Cimasoni L, et al. Encephalitis associated with glutamic acid decarboxylase autoantibodies in a child: a treatable condition? Arch Neurol. 2011;68:1065–1068. [DOI] [PubMed] [Google Scholar]
- 17. Shu Y, Guo J, Ma X, et al. Anti‐NMDAR encephalitis is associated with IRF7, BANK1 and TBX21 polymorphisms in two populations. Eur J Neurol 2020. [DOI] [PubMed] [Google Scholar]
- 18. Shu Y, Qiu W, Zheng J, et al. HLA class II allele DRB1*16:02 is associated with anti‐NMDAR encephalitis. J Neurol Neurosurg Psychiatry. 2019. [DOI] [PubMed] [Google Scholar]
- 19. Chen Y, Li S, Huang R, et al. Comprehensive meta‐analysis reveals an association of the HLA‐DRB1*1602 allele with autoimmune diseases mediated predominantly by autoantibodies. Autoimmun Rev. 2020;19:102532. [DOI] [PubMed] [Google Scholar]
- 20. Makuch M, Wilson R, Al‐Diwani A, et al. N‐methyl‐D‐aspartate receptor antibody production from germinal center reactions: Therapeutic implications. Ann Neurol. 2018;83:553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wouters MCA, Nelson BH. Prognostic significance of tumor‐infiltrating B cells and plasma cells in human cancer. Clin Cancer Res. 2018;24:6125–6135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Histopathology of lymph node in the right supraclavicular fossa of the patient.
Table S1. Antibodies and antigen retrieval procedures used in IHC staining in this study.


