SUMMARY
Histone acetylation levels are regulated by histone acetyltransferases (HATs) and histone deacetylases (HDACs) that antagonistically control the overall balance of this post-translational modification. HDAC inhibitors (HDACi) are potent agents that disrupt this balance and are used clinically to treat diseases including cancer. Despite their use, little is known about their effects on chromatin regulators, particularly those that signal through lysine acetylation. We apply quantitative genomic and proteomic approaches to demonstrate that HDACi robustly increases a low-abundance histone 4 polyacetylation state, which serves as a preferred binding substrate for several bromodomain-containing proteins, including BRD4. Increased H4 polyacetylation occurs in transcribed genes and correlates with the targeting of BRD4. Collectively, these results suggest that HDAC inhibition functions, at least in part, through expansion of a rare histone acetylation state, which then retargets lysine-acetyl readers associated with changes in gene expression, partially mimicking the effect of bromodomain inhibition.
In Brief
Slaughter et al. use proteomic and genomic approaches to quantitatively assess the impact of histone deacetylase inhibitor (HDACi) treatment on histone acetylation and BRD4 binding genome-wide. Their studies show that HDACi treatment causes histone polyacetylation and recruitment of BRD4, particularly in the gene bodies of actively transcribed genes.
Graphical Abstract

INTRODUCTION
Acetylation of histone proteins has been associated with active transcription and weakens histone-DNA interaction by neutralizing lysine’s positive charge to enhance chromatin accessibility (Shahbazian and Grunstein, 2007). In addition to affecting chromatin accessibility, histone acetylation serves as a target of reader proteins and their associated complexes that carry out a wide variety of cellular functions. The bromodomain and extra-terminal (BET) family, which includes BRD2, BRD3, and BRD4, is an example of a class of acetyl reader-containing proteins that couples acetylation to transcription (Jang et al., 2005; LeRoy et al., 2008; Yang et al., 2005). Whereas the bromodomains in these proteins mediate interactions with lysine acetylation, the extra-terminal domains recruit various complexes that can regulate transcription. Specifically, BRD4 scaffolds the positive elongation factor b (P-TEFb) complex to chromatin and promotes transcription elongation at sites of histone acetylation (Jang et al., 2005; Kanno et al., 2014; Yang et al., 2005).
Histone acetylation is regulated by two opposing enzyme classes: histone acetyltransferases (HATs) and histone deacetylases (HDACs). The dynamic and reversible nature of acetylation makes it an ideal therapeutic target. To that end, long-standing interest in the development of HDAC inhibitors (HDACi) has resulted in the development of multiple inhibitors, including several that have been approved by the U.S. Food and Drug Administration (FDA) for oncological indications (West and Johnstone, 2014). HDACi have varying degrees of specificity for the 18 human HDACs, which are classified into class I (HDAC1, 2, 3, and 8), class IIA (HDAC4, 5, 7, and 9), class IIB (HDAC6 and 10), and class IV (HDAC11) (Dokmanovic et al., 2007). HDACi exhibit anti-proliferative effects in several cancer cell lines (Gui et al., 2004; Kwon et al., 2002) and mouse models (Lindemann et al., 2007). Although HDACi are used clinically, little is known about their genomic mode of action. A better understanding of their mechanism may offer insights leading to targeted applications.
HDACi treatment rapidly increases genome-wide histone acetylation, which is associated with both up- and downregulation of gene expression (Drogaris et al., 2012). The overall mechanism of HDACi-mediated gene regulation and the way in which specific subsets of genes are affected by HDACi remain poorly understood. Previous work demonstrated that treatment with the HDACi sodium butyrate (NaBut) led to decreased acetylation of select promoters that correlated with decreased expression of associated genes (Rada-Iglesias et al., 2007). Recent work suggests that HDACi increases chromatin accessibility (Qu et al., 2017) and affects enhancer status and chromatin conformation (Gryder et al., 2019; Sanchez et al., 2018) thereby affecting transcription elongation, particularly for highly expressed genes in cancer cells (Kim et al., 2013) In some cells, HDACi decreases MYC expression and alters the regulation of MYC target genes (Cheng et al., 2007).
Similar to HDACi, bromodomain inhibition by small molecules, such as JQ1, decreases the expression of MYC and its target genes (Bhadury et al., 2014; Mertz et al., 2011). HDACi and BRD4 inhibitors have similar anti-tumorigenic effects, and treatment with both HDACi and a BET inhibitor has a synergistic effect on tumor cell viability and xenografted mouse survival (Fiskus et al., 2014). The similarity in cellular effects of these inhibitors seems unexpected, as HDACi would be predicted to enhance BRD4 binding, whereas BET inhibitors prevent BRD4-chromatin interaction. It is possible that alterations in the histone acetylation landscape by HDACi may affect the chromatin targeting of BET proteins to elicit the observed changes in gene expression. Indeed, HDACi reduces the mobility of BRD4 on chromatin because of increased bromodomain-acetyl interaction (Dey et al., 2003). We hypothesized that HDACi disrupts the function of BRD4 and other BET proteins on chromatin, possibly through genomic redistribution of these proteins. Although previous studies have assessed the effect of HDACi on the acetyl landscape (Brocks et al., 2017; Greer et al., 2015; Gryder et al., 2019), and the effects of BRD4 inhibition (Delmore et al., 2011), studies have yet to integrate quantitative proteomic and genomic approaches to assess changes in specific histone acetylation marks and subsequent binding of acetyllysine reader proteins.
In this study, we investigated the proteomic and genomic consequences of treatment with the drug suberoylanilide hydroxamic acid (SAHA or vorinostat) on H4 acetylation and BRD4 targeting. We find that HDAC inhibition results in robust hyperacetylation of H4 at lysines 5, 8, 12, and 16, associated with altered transcription. SAHA both enhanced and diminished transcription of select genes, suggesting that HDACs can have opposing effects throughout the genome and do not function similarly genome-wide. SAHA preferentially enhances histone acetylation within gene bodies, and this increased H4 acetylation is associated with increased BRD4 binding and gene expression. As JQ1 inhibition similarly affected transcription of these genes, our findings suggest that the transcriptional effects of HDACi are mediated largely through altered targeting of BRD4 away from enhancers and toward gene bodies. Our findings suggest that HDAC inhibition potentially acts to re-shape the epigenome through increasing a rare polyacetylation chromatin signature that retargets chromatin readers that selectively recognize this modification.
RESULTS
HDAC inhibition results in H4 polyacetylation
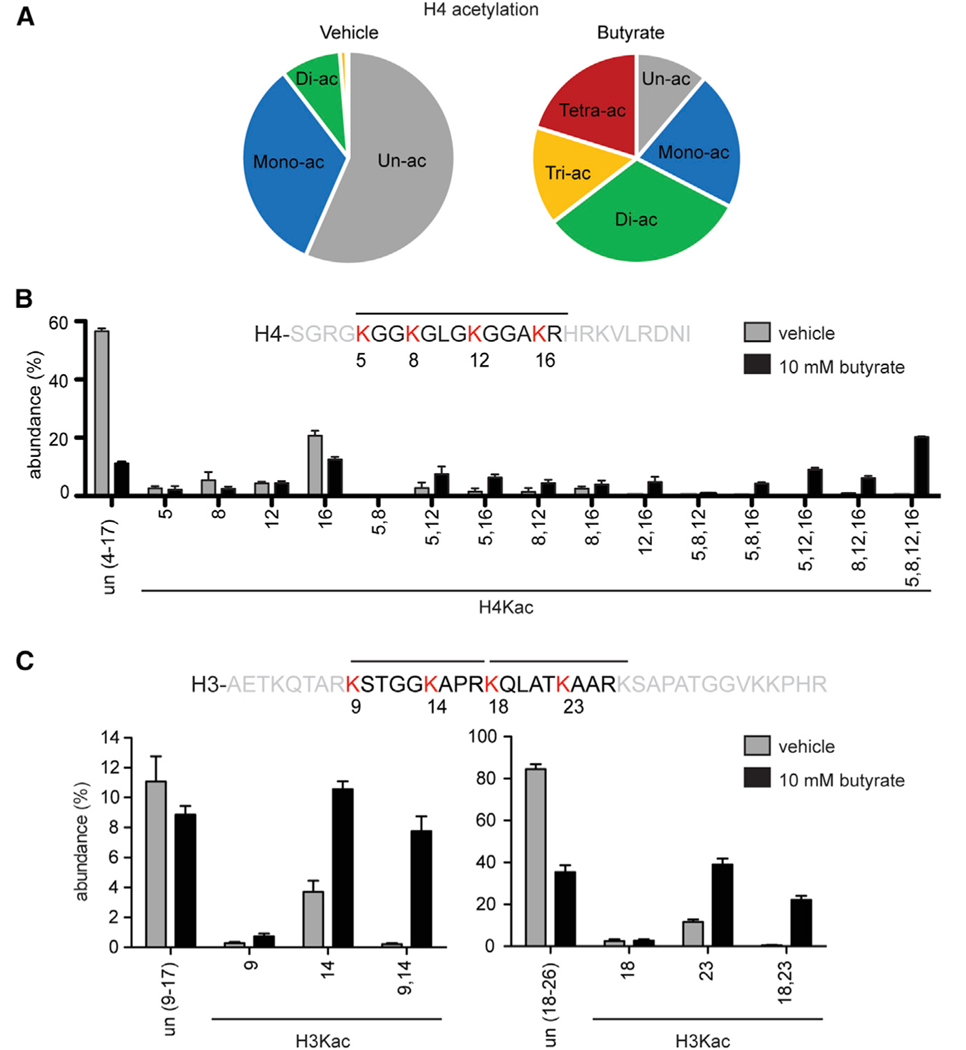
To identify the effects of HDAC inhibition on histone acetylation, we performed mass spectrometry on histone proteins extracted from HeLa cells with or without treatment with NaBut, a class I/II HDACi (Eckschlager et al., 2017) (Figure 1A). In the absence of HDAC inhibition, the majority of H4 histones were un- or mono-acetylated (89%). However, following treatment, histone H4 became hyperacetylated, with more than 65% of H4 demonstrating di-, tri-, or tetra-acetylation. To identify whether specific histone PTMs were preferentially affected by HDACi, we quantified the abundance of the H4 acetyl states before and after butyrate treatment (Figure 1B). The abundance of mono-acetylated histones decreased, regardless of histone post-translational modification, whereas di-, tri-, or tetra-acetylated histones increased. Histones with higher degrees of acetylation showed the greatest increase after butyrate treatment, with tetra-acetylated H4 histones accounting for 20% of histone H4 peptides following HDAC inhibition. We also assessed changes in the acetyl state of the H3 tail after butyrate treatment. Unlike H4ac, mono-acetylated and di-acetylated H3 histones peptides were increased, although peptide digestion limited the ability to assess higher order acetylation (Figure 1C; Figures S1A–S1D). Overall, HDAC inhibition greatly affected histone acetylation levels, with a striking increase in H4 polyacetylation (acetylation of two or more lysines).
Figure 1. Sodium butyrate leads to H4 hyperacetylation.
(A) Pie charts summarizing mass spectrometry analysis of H4 peptide acetyl states (un-, mono-, di-, tri-, or tetra-acetyl) identified in HeLa cells treated with vehicle or sodium butyrate.
(B and C) Bar graphs depicting percentage of individual peptides identified using mass spectrometry for (B) H4 or (C) H3 following treatment with 10 mM butyrate or vehicle control. Error bars represent standard deviation.
Polyacetylated H4 binds transcriptional machinery and BET proteins
Because HDAC inhibition greatly increased H4 polyacetylation, we identified proteins that preferentially bind tetra-acetylated H4. Using unmodified H4 or H4 tetra-acetylated peptides, we performed pull-downs with HeLa nuclear extracts and assessed the isolated proteins using mass spectrometry (Figure 2A). Proteins that demonstrated the greatest enrichment for H4 tetra-acetylated peptide binding included the BET family members BRD2, BRD3, and BRD4. Members of the HAT and the TFIID complex were also enriched (Figure 2B). A graphical representation of the interactions discovered using mass spectrometry analysis in relation to transcription is shown in Figure 2C. To confirm the direct and selective binding of bromodomains from proteins that bound the H4 tetra-acetylated peptide, recombinant bromodomains were hybridized to a peptide microarray composed of more than 250 histone tail peptides with single or multiple post-translational modifications, including mono- or polyacetylation (Figure 2D). Bromodomains from BRD2, BRD3, and BRD4, as well as 13 additional bromodomains, were tested. Results from the peptide microarray confirmed that the majority of the tested bromodomains favored binding to tri- or tetra-acetyl H4 peptides. Interestingly, H3 polyacetylation did not dramatically increase bromodomain binding. Rather, the majority of bromodomain interactions with H3 were with peptides containing H3K14 acetylation, a mark previously shown to target bromodomains found in a number of chromatin-remodeling complexes such as PBAF (Slaughter et al., 2018).
Figure 2. BRD4 recognizes polyacetylated H4.
(A) Scatterplot of proteins bound to H4 unmodified versus H4 tetra-acetylated peptides by peptide pull-down followed by mass spectrometry.
(B) Enrichment of complexes containing proteins preferentially bound to H4 tetra-acetylated peptides.
(C) Proteins associated with H4 tetra-acetylated peptide are colored on the basis of their abundance by mass spectrometry are depicted in a model in relation to their roles in transcriptional initiation or elongation.
(D) Peptide microarrays of select H3 and H4 acetyl peptides to the indicated bromodomains (BDs).
HDAC inhibition variably enhances H4 polyacetylation
We next sought to localize the increase in histone acetylation genome-wide. We explored the changes in histone acetylation following treatment with a clinically relevant pan-HDACi, SAHA (vorinostat), using a well-characterized human promyelocytic leukemia cell line (HL60). However, as we were most interested in the direct effects of HDAC inhibition, we first needed to select a relevant treatment length. We considered MYC levels as a relevant marker of HDACi-induced transcriptional change (Heller et al., 2008; Nebbioso et al., 2017). In addition, MYC can affect chromatin status (Frank et al., 2003). H3 and H4 acetylation was increased at 0.5 h, which preceded a decrease in MYC protein levels at 2 h (Figure 3A). Although MYC transcript levels began to diminish at 1 h, expression of the MYC target gene, CDKN1A (Wu et al., 2003), was not affected until 2 h after treatment (Figure 3B). From these data, we established 1 h of SAHA treatment as an appropriate interval to measure direct chromatin changes.
Figure 3. Kinetics of histone PTM and transcriptional changes following SAHA treatment.
HL60 cells treated with SAHA for 0, 0.5, 1, 2, or 6 h were assessed by (A) immunoblot for MYC and H3 and H4 acetylation and (B) quantitative PCR for MYC and CDKN1A transcript levels relative to untreated. Error bars represent standard deviation.
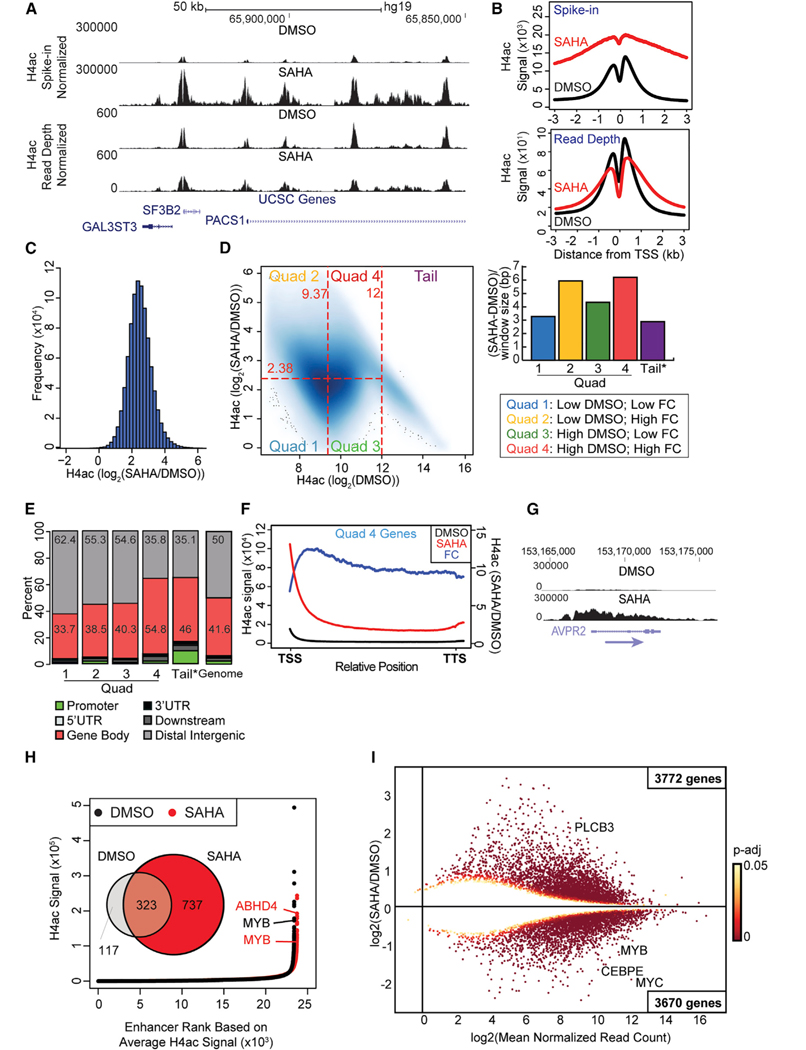
We performed quantitative chromatin immunoprecipitation (ChIP) for H4 polyacetylation (i.e., tetra-acetylation). We focused on H4 polyacetylation because it was robustly affected by HDAC inhibition and was a target for the bromodomains from multiple proteins. We performed quantitative ChIP sequencing (ChIP-seq) (Orlando et al., 2014), which incorporates Drosophila chromatin throughout sample processing as an internal reference to enable cross treatment comparisons (Figure S2A). Preferential binding of the antibody to polyacetylated H4 was demonstrated by peptide array analysis (Figure S2B). The antibody used for ChIP favored di-through tetra-acetyl H4. Following SAHA treatment, we observed a striking increase in histone acetylation. The signal increase associated with HDAC inhibition was not appreciated in the absence of spike-in normalization (Figures 4A and 4B), as has been previously noted for H3K27 acetylation (Gryder et al., 2019). The massive increase in H4 acetylation genome-wide was characterized by a broader distribution of H4 acetylation around transcription start sites (TSSs) (Figure 4B). The degree of polyacetylation varied across the genome, with a 4- to 8-fold increase in acetylation at most regions (Figure 4C). Select regions, however, demonstrated little change in acetylation (<4-fold change), whereas others exhibited up to a 60-fold change after treatment.
Figure 4. SAHA leads to increased acetylation in gene bodies in HL60 cells.
(A) Representative Genome Browser tracks for H4ac ChIP in HL60 cells.
(B) H4ac signal at TSSs following SAHA treatment (or DMSO control) (n = 2) using spike-in (top) or read depth only (bottom) normalization.
(C) Histogram of log2 fold change of H4ac signal in SAHA and DMSO treatments in windows with differential H4ac signal.
(D) Density plot showing the log2 fold change of H4ac signal after SAHA treatment versus signal in DMSO sample in differential windows. Regions are divided into five classes on the basis of fold change and signal in DMSO treatment. Bar chart demonstrates the magnitude of acetyl difference (SAHA-DMSO) for the five classes of regions.
(E) Association of regions from (D) with genomic features compared with an overall genomic distribution. Promoters (−3 kb from TSS), gene bodies (TSS to transcription termination site [TTS]) and downstream (+3 kb from TTS).
(F) Metagene plot of H4ac signal in genes enriched for H4ac signal in genes in Quad 4 class. FC, fold change.
(G) Representative Genome Browser track depicting H4ac signal in a gene body after SAHA treatment.
(H) Venn diagram illustrates shared and unique genes associated with called super-enhancers using H4ac signal in DMSO and SAHA treatments. SEs were associated with the nearest gene within 50 kb. Scatterplot depicts distribution of H4ac ChIP-seq density and ranking across all enhancers. Replicates were averaged.
(I) MA plot comparing the log2 mean of normalized read counts with the log2 fold change between SAHA and DMSO treatments where the adjusted p value is ≤ 0.05. The color of each point represents the magnitude of adjusted p value from 0 to 0.05.
HDAC inhibition increases H4 polyacetylation in gene bodies
We then classified genomic intervals on the basis of the relative variation in acetylation following SAHA treatment and the absolute signal change. Overlapping 300 bp windows that demonstrated differential signal between control and SAHA treatment were merged into contiguous regions. These regions were initially divided into three classes on the basis of acetylation levels prior to HDACi treatment: low, high, and tail (Figure 4D; Figure S2C).
Regions of the genome that exhibited low levels of H4 acetylation prior to SAHA treatment (log2[H4ac] < 9.37) were further divided into two groups: those regions with less than (Quad 1) or greater than (Quad 2) the median fold change after SAHA treatment (log2[H4ac] = 2.38). The absolute change in acetyl signal was minimal for Quad 1 but more pronounced for Quad 2 regions. These data indicate that despite low levels of H4ac at baseline, there is variability in the response to HDAC inhibition, with some regions demonstrating limited changes (Quad 1) and others with pronounced H4ac changes (Quad 2).
Genomic intervals with H4 acetylation signal greater than median signal prior to SAHA treatment (log2[H4ac] > 9.37) were divided into classes on the basis of the relative magnitude of acetylation change: those regions with less than (Quad 3) or greater than (Quad 4) median fold change (log2[H4ac] = 2.38). Here, regions that showed a high fold change in H4 acetylation after SAHA treatment (Quad 4) also showed a large changein the absolute H4 acetylation levels, suggesting that these regions are targets of HATs and are maintained in a hypoacetylated state by ongoing HDAC activity. A fifth class was identified (Tail). These regions exhibited the greatest H4 acetylation at baseline (log2[H4ac] > 12) but a limited relative increase following SAHA treatment (Figure S2D). These regions are likely sites of robust HAT activity but with limited HDAC activity and may be at near maximal acetylation.
We then queried for enrichment of genomic features among regions in these classes (Shin et al., 2009) (Figure 4E). Compared with the overall genome-wide distribution of these features, segments of low acetylation that were least affected by SAHA (Quad 1) were enriched for distal intergenic regions. In contrast, regions most affected by SAHA (Quad 4) were within genes. Regions with very high acetylation that showed little relative change, but significant change in the absolute H4ac signal (Tail), were enriched for promoters. These data suggest that whereas HATs are active at both promoters and within genes, HDACs are present in the transcribed regions of genes and work to potently restrict gene body acetylation. However, whether bromodomain targeting might mediate or recruit additional HATs to gene bodies to increase acetylation is also plausible (Devaiah et al., 2016). We next examined H4ac signals in those genes that demonstrated increased H4ac within gene bodies (Figures 4F and 4G). Increased acetylation was found to extend into genes.
Because super-enhancers (SEs) have been defined as extended regions of histone hyperacetylation, a chromatin state that we noted was increased following HDACi treatment, we hypothesized that HDAC inhibition would in some way re-shape the overall enhancer landscape. We identified regions of extended hyperacetylation using a published algorithm based on H4ac signal, as H3K27ac and H4ac signal patterns were similar before and after SAHA treatment (Figure S2E) (ROSE; Lovén et al., 2013; Whyte et al., 2013). H4ac signal was quantified under H4ac ChIP-seq peaks, and peaks within 12.5 kb were merged. Regions were then ranked by overall signal (Figure 4H). Identified SEs were associated with the nearest gene within 50 kb (GREAT; McLean et al., 2010). Genes selectively associated with SEs in control and SAHA treatments were compared. These studies showed that SAHA treatment doubled the number of computationally defined SEs. However, the fraction of SEs associated with a given gene remained constant, suggesting that the increase in SEs may relate to even further extended SE regions at these genes. It was notable that SAHA treatment altered the relative ranking of SEs and that several top-ranking SEs were identified only following SAHA treatment.
We next tested for an association between transcription and the variation in identified enhancers following SAHA treatment. RNA sequencing (RNA-seq) was performed on HL60 cells following SAHA treatment (2 h) or control (DMSO) (Figure 4I). In total, 7,442 genes demonstrated altered mRNA levels (DESEQ2; p < 0.05) following 2 h of SAHA treatment. Of these, 51% (3,772 genes) were upregulated and 49% (3,670 genes) were downregulated. As expected, downregulated genes included the oncogenes MYC and MYB. Although upregulated genes were not enriched for specific cellular pathways, downregulated genes were significantly enriched for genes associated with regulation of metabolic processes (Figure S3). For genes associated with persistent SEs, the majority were downregulated (58% [107 of 185]). In contrast, genes associated with HDACi-induced enhancers were more likely to be upregulated (53% [220 of 419]). For example, the proto-oncogene MYB, which demonstrated significantly decreased RNA levels, was associated with a highly ranked SE under both treatment conditions. Together, these data suggest that SAHA does not uniformly influence acetylation genome-wide. Rather, HDACi preferentially affects regions with preexisting H4 acetylation, specifically in gene bodies. In addition, HDACi seems to have widespread consequences that alter the enhancer landscape. Interestingly, whereas new SEs are associated with increased transcription, preexisting SEs persist but are associated with decreased transcription.
HDAC inhibition increases BRD4 targeting to the bodies of upregulated genes
We then explored whether HDAC inhibition and histone polyacetylation affected bromodomain-containing protein targeting. We focused on BRD4, as it strongly recognized H4 polyacetylation (Figures 2A and 2D) and has been implicated in human cancers. We performed BRD4 ChIP-seq using HL60 chromatin isolated from cells with or without SAHA treatment. We observed changes in BRD4 targeting following SAHA treatment, although signal around TSSs was largely unchanged (Figures 5A and 5B). Although a small number of sites were lost following treatment, SAHA doubled the number of BRD4 binding sites (Figure 5C). As BRD4 protein levels were unaffected by SAHA treatment (Figure S4), we hypothesized that the additional BRD4 binding sites after SAHA treatment would lead to a decrease in BRD4 levels at preexisting binding sites. To test this, we compared the BRD4 signal at those sites that were detected in both control and SAHA-treated cells (Figure 5D). We found that for regions with differential signal, most sites had decreased signal following SAHA treatment (212 of 222). These data suggest that the increased number of BRD4 binding sites reflects a redistribution of BRD4. The absence of signal variation between DMSO and SAHA treatment at the vast majority of BRD4 sites argues against the possibility that this difference reflects an overall dilution of read counts. We then characterized the genomic features in BRD4 binding sites (Figure 5E). Similar to that observed for H4 acetylation, BRD4 binding sites gained with SAHA treatment were enriched at gene bodies and depleted at promoters.
Figure 5. SAHA treatment increases BRD4 binding in gene bodies.
(A) Representative genomic tracks for BRD4 ChIP-seq in HL60 cells with shared (green) and unique (blue) peaks.
(B) BRD4 signal at TSSs ± 3 kb.
(C) Venn diagram of unique and shared BRD4 peaks identified by ChIP-seq after SAHA treatment.
(D) Scatterplot demonstrating the fold change of the BRD4 ChIP signal at sites of BRD4 binding detected in both control and SAHA treatment. Red signifies p < 0.05.
(E) Genomic features enriched in shared and uniqueBRD4 binding sites, compared with the overall genome distribution.
(F) Number of genes differentially regulated in response to SAHA that were associated with BRD4 bound sites unique to DMSO or SAHA treatment or common to both.
To evaluate whether the BRD4 targeting was associated with transcription, we considered whether hyperacetylation and BRD4 binding following HDAC inhibition corresponded with gene expression changes. Ninety-one percent of genes that were associated with new BRD4 binding sites were upregulated in response to SAHA (Figure 5F). In contrast, greater than 65% of genes associated with decreased or unchanged BRD4 binding were downregulated in response to SAHA. Together, these data suggest that HDACi increase the number of BRD4 binding sites through BRD4 redistribution. New BRD4 target sites are preferentially detected in genes with increased transcription.
Gained BRD4 binding sites are enriched for H4 acetylation in introns of genes exhibiting increased transcription
We then asked whether genes with increased BRD4 signal in gene bodies after SAHA treatment also exhibited increased H4 polyacetylation. We compared the genes with increased BRD4 in gene bodies after SAHA treatment (BRD4 SAHA Unique; Figure 5C) with the genes associated with H4ac in gene bodies after treatment (Quad 4 windows; Figure 4D) (Figure 6A). Genes with increased BRD4 signal in gene bodies were greatly enriched for those with increased H4 polyacetylation in the gene body (78%, p = 0 for random permuted control) (Figure 6B). H4ac and BRD4 signal patterns in genes that demonstrated shared increased signal (Figure 6A) were similar (Figure 6C). Greater than 70% of these genes showed increased expression (Figure 6D). Together, these data suggest that treatment with HDACi increases the number of BRD4 binding sites within gene bodies through increased H4 polyacetylation. Genes associated with elevated BRD4 and H4ac signal within gene bodies are upregulated in expression in response to HDACi.
Figure 6. BRD4 is targeted to regions of hyperacetylation in gene bodies and is associated with upregulated gene expression.
(A) Venn diagram comparing genes that demonstrated increased H4 acetylation in the gene bodies (from Quad 4; Figure 4) compared with those with increased BRD4 signal following SAHA treatment.
(B) Fraction of shared BRD4 marked genes and Quad 4 genes compared with permuted control.
(C) Representative signal tracks for a gene exhibiting increased H4ac and BRD4 in the gene body after SAHA treatment.
(D) Direction of regulation of genes with increasedH4ac and BRD4 in gene bodies.
(E) Heatmap demonstrating differentially regulated genes following treatment with SAHA, JQ1, or both. Venn diagram demonstrating relationship of differentially regulated genes.
(F) Heatmap of RNA abundance for genes regulated by SAHA, JQ1 and both compounds. Boxplot of variation in RNA abundance following treatment with either or both compounds.
(G) Western blot analyses of BRD2, BRD3, BRD4 (long isoform [L]), and CDK9 in the soluble or chromatin-associated fraction following treatment with SAHA or JQ1 or both. Tubulin and H3K14ac serve as controls for fractionation and demonstrate the effect of SAHA on histone acetylation. Cross-reactive bands are marked with asterisk.
Our study was built upon the observation that HDACi and BET inhibitors show similar cellular effects despite the expectation of opposing effects of these agents: that HDACi would increase, while BET inhibitors decrease, the number of BET binding sites. Our data suggest that HDAC inhibition decreases the binding of BRD4 to intergenic sites through apparent redistribution of BRD4 to gene bodies. We therefore assessed whether treatment with an HDACi (SAHA) and a BET inhibitor (JQ1) would demonstrate a similar additive or synergistic effect on transcription. RNA-seq was performed on HL60 cells treated with SAHA, JQ1, or both. Greater than 48% of genes regulated by either SAHA or JQ1 were also co-regulated when treated in combination (SAHA + JQ1) (Figure 6E). Intriguingly, both SAHA and JQ1 affected similar sets of genes and resulted in mostly an additive effect on transcription of those genes. Focusing exclusively on those genes regulated by SAHA and JQ1, treatment with the combination of both inhibitors resulted in similar directions and magnitude of gene expression changes (Figure 6F). These results suggest that the overall influence of JQ1 and SAHA on transcriptional regulation is similar despite their distinct mechanisms of action.
As the impact of JQ1 and SAHA would be expected to displace and/or relocalize BET family proteins, we next examined the effects of individual or combinatorial treatment of SAHA and JQ1 on the association of BRD2, BRD3, and BRD4 with chromatin using a biochemical fractionation approach. Expectedly, JQ1 treatment resulted in a shift of BRD2, BRD3, and BRD4 from chromatin to soluble fractions, and SAHA treatment resulted in a decrease in soluble proteins and increased BRD reader association with chromatin (Figure 6G). Interestingly, the combined treatment of SAHA and JQ1 resulted in a JQ1 dose-dependent decrease of BRD proteins on chromatin. As a control, CDK9 levels remained unchanged in soluble and chromatin fractions upon the treatments. Taken together, these data indicate that the chromatin association of BRD proteins can be dramatically increased following HDAC inhibition. However, even in this context, JQ1 can overcome the effects of HDACi and displace BRD4 from chromatin.
Taken together, polyacetylated H4 appears to be sufficient for the assembly of transcriptional machinery, including members of the pre-initiation complex (TFIID and TBP) and members that facilitate elongation (BRD4 and P-TEFb), as determined using peptide pull-downs followed by mass spectrometry (Figure 2). Altered acetylation due to HDACi may therefore have considerable potential to dysregulate transcription via altered targeting of the transcriptional machinery. We suggest a model in which actively transcribed genes have high levels of H4ac at promoters and low levels of acetylation within gene bodies, a state maintained by HDACs. Under normal conditions, members of the pre-initiation complex bind to polyacetylated promoters, fostering normal transcription. HDAC inhibition, however, leads to elevated H4ac preferentially within gene bodies of these actively transcribed genes, leading to the enhanced binding of factors, including BRD4.
DISCUSSION
As readers of histone acetylation, bromodomain-containing proteins play a critical role in linking histone acetylation with transcription. Although HDACi increase histone acetylation and alter gene expression, the precise effects of these compounds on H4 hyperacetylation and bromodomain targeting to this preferred binding signature has not been quantitatively examined at a genome-wide level. The two HDACi used in this study, SAHA and NaBut, similarly inhibit class I and class IIA HDACs, with SAHA exhibiting additional inhibition of class IIB HDACs. Using a combined proteomic and genomic approach, we demonstrate that polyacetylated histone H4 is a preferred target for BRD4 and other bromodomains, which is supported by previous studies that have demonstrated many BET proteins preferentially interact with H4ac (Filippakopoulos et al., 2012). Using mass spectrometry, we demonstrate that H4ac, most notably polyacetylated H4, was more greatly affected by HDAC inhibition than H3ac. Thus, we quantitatively measured genome-wide changes in H4ac and found that H4ac is increased by class I and class II HDACi at specific genomic locations, particularly in the bodies of upregulated genes and that BRD4 is targeted to these regions.
Our study demonstrates that HDACs have variable activity across the genome. Our data suggest that HDACs have limited activity at some highly acetylated regions (enriched for promoters), as these regions showed little change in acetylation following HDACi treatment (Figures 4D and 4E, Tail). Gene bodies of highly transcribed genes, however, showed a robust change in acetylation in response to HDACi treatment. These data suggest that under normal conditions, HDACs act in gene bodies to maintain low levels of acetylation, whereas HDACs are less active at promoters, and HAT activity maintains a hyperacetylated state. This highlights the tightly regulated balance between HATs and HDACs within genes to maintain proper chromatin states. Previous studies have demonstrated that various HATs and HDACs localize to different genomic regions. Specifically, HDAC1 and HDAC3 localize to promoters, while HDAC2 and HDAC6 bind promoters and gene bodies of active genes (Ram et al., 2011; Wang et al., 2009). HATs have also been shown to most prominently localize to promoters but also within gene bodies (Wang et al., 2009). Tip60, a member of the MYST family of HATs, is found at high levels at promoter regions but was also detected in active genes (Wang et al., 2009). Therefore, a balance exists between HATs and HDACs at promoters and gene bodies to properly regulate gene transcription.
In addition to regulating acetylation levels within genes, our findings suggest that HDACs regulate enhancer development, as SAHA treatment altered the formation of enhancers, consistent with other studies (Gryder et al., 2019). BRD4 may facilitate transcription through a looping mechanism between promoters and enhancers (Liu et al., 2013) and has been shown to track closely with Med1 at enhancers and TSSs (Lové n et al., 2013). Here, we demonstrate that multiple components of the transcriptional machinery can bind H4 tetra-acetylation (Figure 2). These interactions may be indirect, mediated through interaction with BRD4 or other bromodomain-containing proteins. HDACi may disrupt the association between enhancers and promoters, thereby disrupting transcription. Future studies are necessary to fully elucidate the effect of HDACi on chromatin conformation.
BRD4 targeting in gene bodies reflected that of H4 acetylation. Nearly all genes with increased H4ac following SAHA treatment also exhibited elevated BRD4 signal. These genes were also associated with elevated gene expression in response to SAHA. It is likely that BRD4 associates with genes that are actively transcribed and that these genes are targets for HDAC activity. However, it is possible that BRD4 binding enhances transcription. In support of this model, it has been shown that HDACs bind promoters and/or gene bodies of actively transcribed genes (Wang et al., 2009). In addition, BRD4 has been shown to function as a HAT to acetylate both H3 and H4 residues (Devaiah et al., 2016). BRD4 acetylates H3K122, a mark crucial for nucleosome stability. Consequently, increased BRD4 within gene bodies may further acetylate gene bodies and decompact chromatin, thereby facilitating the disruption of nucleosomes by RNA Polymerase II. Additionally, as HDAC inhibition expands BRD4 binding in the context of unchanged protein levels, we speculate that BRD4 binding is decreased at interacting sites prior to treatment. In support of this concept, previous work has shown that increased chromatin acetylation decreases BRD4 mobility (Dey et al., 2003). Together, our data suggest that BRD4 is targeted to the bodies of actively transcribed genes in response to SAHA, potentially acting as a sink that draws away BRD4 from some promoters and enhancers. Although it is an apparent contradiction that HDACi and BET inhibitors would elicit similar cellular effects, we suggest that the two inhibitors may ultimately act similarly by decreasing BRD4 binding at critical sites: bromodomain inhibitors through inhibition of chromatin interaction and HDACi by redistributing BRD4 throughout the genome.
STAR★METHODS
RESOURCE AVAILABILITY
Lead Contact
Further information and requests for resources and reagents should be directed and will be fulfilled by the Lead Contact, Ian Davis (ian_davis@med.unc.edu), UNC Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, NC 27599.
Materials Availability
Reagents generated in this study are available from the Lead Contact without restriction.
Data and Code Availability
Sequencing data generated as part of this study have been submitted to GEO under accession number GSE159620. Western blot data are available at Mendeley Data at https://doi.org/10.17632/6bhbxbcyd3.1
EXPERIMENTAL MODEL AND SUBJECT DETAILS
HL60 cells were cultured in RPMI-1640 supplemented with 20% fetal bovine serum. HeLa cells were cultured in DMEM supplemented with 10% fetal bovine serum.
METHOD DETAILS
Histone Preparation and Mass Spectrometry Analysis
Extracted histones from sodium butyrate-treated HeLa cells (16 hours) were chemically derivatized and digested to tryptic peptides to make them amenable for bottom up mass spectrometry as previously described (Bhanu et al., 2016). The derivatized samples were desalted using C18 Stage-tips prior LC-MS analysis. For mass spectrometry, the peptides were separated using a 75 μm ID × 17 cm Reprosil-Pur C18-AQ (3 μm; Dr. Maisch GmbH, Germany) nano-column fitted on an EASY-nLC nanoHPLC (Thermo Scientific, San Jose, Ca, USA). An HPLC gradient comprising 0%–35% solvent B (A = 0.1% formic acid; B = 95% acetonitrile, 0.1% formic acid) over 40 min and from 34% to 100% solvent B in 7 minutes at a flow-rate of 250 nL/min was used. The nano LC was coupled with an Orbitrap Fusion mass spectrometer (Thermo Fisher Scientific, San Jose, CA, USA) with a spray voltage of 2.3 kV and capillary temperature of 275°C. Full scan MS spectrum (m/z 300–1200) was acquired in the Orbitrap with a resolution of 60,000 (at 200 m/z) with an AGC target of 5×105. By using the Top Speed MS/MS option set to 2 s, the most intense ions above a threshold of 2000 counts were selected for fragmentation. Fragmentation was performed with higher-energy collisional dissociation (HCD) with normalized collision energy of 29, an AGC target of 1×104 and a maximum injection time of 200 msec. MS/MS data were collected in centroid mode in the ion trap mass analyzer (normal scan rate). Only charge states 2–4 were included. The dynamic exclusion was set at 30 s. Peak area was extracted from raw files by using our in-house software EpiProfile (Yuan et al., 2015). The relative abundance of a given PTM was calculated by dividing its intensity by the sum of all modified and unmodified peptides sharing the same sequence, using the total area under the extracted ion chromatograms.
Peptide Pulldown and Mass Spectrometry
Nuclear extracts were prepared from HeLa cells as previously described (Carey et al., 2009). Peptides were immobilized on High Capacity Streptavidin Agarose Resin (Thermo Scientific) and rinsed twice with 0.01% NP-40 in PBS. Nuclear extracts were pre-cleared with resin for 45 min at 4°C prior to incubation with peptide-conjugated resin for 4 h at 4°C. After washing four times with 150 mM KCl and three times with 250 mM KCl, bound proteins were eluted using 50 mM glycine, 150 mM NaCl. A fraction of the final elutions were resolved by SDS-PAGE and gels were stained with SilverQuest Staining Kit (Invitrogen) to confirm pulldowns. The remaining elutions were precipitated with 6X ice-cold acetone overnight at −20°C. After centrifugation, the pellet was solubilized in 8 M urea and 0.1 M ammonium bicarbonate.
Chromatin Association Assay
HL60 cells were treated with SAHA (1μM) and/or JQ1 at the indicated concentrations for 2 hr. Approximately 1×106 cells were harvested by pelleting and washing with PBS once. Following the PBS wash, cells were resuspended in 200 μl CSK buffer (10 mM PIPES pH 7.0, 100 mM NaCl, 3 mM MgCl2, 300 mM sucrose, 0.1% Triton X-100) supplemented with protease and phosphatase inhibitors for 10 min on ice. Nuclei were pelleted at 1300 g for 5 min. The supernatant was collected as the ‘soluble’ fraction. Nuclei were washed once with CSK buffer and supernatant was discarded. For ‘chromatin’ fraction, nuclei were then resuspended in 200 μl CSK buffer supplemented with Universal Nuclease (Thermo Scientific). After incubation on ice for 30 min, lysates were briefly sonicated to solubilize the chromatin. Lysates were boiled with SDS loading buffer; equal volumes of ‘soluble’ and ‘chromatin’ fractions were loaded onto an SDS-PAGE gel and western blot analyses were performed using antibodies against BRD2 (Bethyl A302–583A), BRD3 (Bethyl A302–368A), BRD4 (Bethyl A301–985A100), CDK9 (ThermoFisher 703404).
Western Blot
Cells were treated with 1 μM SAHA (Sigma), 500 nM JQ1 (Sigma) or 0.1% DMSO for the indicated time points and collected by centrifugation. After washing once with cold PBS, cells were flash frozen and stored at −80°C. For lysis, cells were resuspended in lysis buffer (10 mM PIPES pH 7.0, 100 mM NaCl, 3 mM MgCl2, 300 mM sucrose, 0.1% Triton X-100) supplemented with protease inhibitors (Roche) and universal nuclease (Thermo Scientific). After 20 min incubation on ice, the lysate was centrifuged and quantified by Bradford assay (Biorad). For histone blots, 1 μg of total protein was resolved on a 12% SDS-PAGE gel and transferred to PVDF membrane. The following antibodies were used to detect histones: total H4 (Abcam ab10158), H4 acetyl (Millipore 06–866), H4K16ac (Active Motif 39167), total H3 (Epicypher 13–0001), H3K14ac (Millipore 03–353 Lot 2387522), and H3K4me3 (Epicypher 13–004). MYC and tubulin were detected by resolving 20 μg of total protein was resolved on an 8% SDS-PAGE gel and blotting with MYC (Abcam ab32072) or beta-tubulin (Millipore 05–661) antibodies.
RNA Sequencing and Analysis
Total RNA was isolated using TRIzol reagent (Invitrogen 5596026) according to manufacturer’s protocol. 750 ng of DNase-treated total RNA was subjected to rRNA depletion (RiboZero Gold, Illumina MRZG12324). rRNA-depleted total RNA was fragmented and reverse transcribed (SuperScript II, ThermoFisher 18064014). Sequencing libraries were generated using Illumina TruSeq Stranded Total RNA Library Prep Kit according to manufacturer’s protocol and 76 bp single-end sequencing was performed (Illumina, NextSeq500). Sequencing reads were aligned to hg19 (STAR v2.5.2b, Dobin et al., 2013) using the following options:–genome-Dir < hg19 > –runThreadN 12–quantMode TranscriptomeSAM–sjdbGTFfile < hg19 > .gtf–outFilterMismatchNmax 2–alignIntronMax 1000000–alignIntronMin 20–chimSegmentMin 15–chimJunctionOverhangMin 15–outSAMtype BAM Unsorted–outFilterType By-SJout–outFilterScoreMin 1–outFilterMultimapNmax 1–outFileNamePrefix < /out/dir/samplename > –readFilesIn < /path/to/dir >. Differentially expressed genes were identified by DESEQ2 (Love et al., 2014), and count data was used to generate the MAplot using RStudio. Gene ontology analysis was performed using DAVID (Dennis et al., 2003).
RT-qPCR
For quantitative PCR analysis, 1.5×106 HL60 cells were treated with 1 μM SAHA, 500 nM JQ1 or 0.1% DMSO for the indicated time points. Cells were collected, washed once with cold PBS, and flash frozen. RNA was isolated (RNeasy, QIAGEN). Two micrograms of RNA were reverse transcribed (SuperScript III, Thermo Fisher) and random hexamer primers. cDNA was diluted 1:20 and target genes were amplified using SYBR Green Master Mix (Biorad) and the following primers: MYC (5′-CCTGGTGCTCCATGAGGA GAC-3′, 5′-CAGACTCTGACCTTTTGCCAGG-3′), CDKN1A (5′-AGGTGGACCTGGAGACTCTCAG-3′, 5′-TCCTCTTGGAGAAGATCA GCCG-3′), GAPDH (5′-GTCTCCTCTGACTTCAACAGCG-3′, 5′-ACCACCCTGTTGCTGTAGCCAA-3′). Experiments were performed in triplicate and data were collected and analyzed using an ABI 7900HT by normalizing to GAPDH expression and calculating fold change relative to DMSO samples.
Chromatin immunoprecipitation, Library Preparation and Sequencing
ChIP with a spike in Drosophila reference chromatin was performed as previously described with minor modifications (Orlando et al., 2014). Drosophila S2 cells were cultured to a density of 1×106 cell/mL. Cells were harvested and fixed for 10 min at room temperature using 1/10 volume of fresh formaldehyde fixation solution (11% formaldehyde, 100 mM NaCl, 1 mM EDTA, 0.5 mM EGTA, 50 mM HEPES). Crosslinking was quenched with 0.125 M glycine for 5 min. Cells were washed two times with cold PBS, flash frozen and stored at −80°C. HL60 cells were cultured to a density of 7×105 cell/mL and treated with 1 μM SAHA or 0.01% DMSO for 1 h. After treatment, cells were treated with 1/10 volume of fresh formaldehyde fixation solution for 10 minutes at room temperature and quenched for 5 min. Cells were washed two times with cold PBS, flash frozen and stored at −80°C.
Chromatin was prepared by resuspending HL60 and S2 cell pellets in parallel in cold Lysis Buffer 1 (107 cell/mL; 140 mM NaCl, 50 mM HEPES pH 7.5, 1 mM EDTA, 10% glycerol, 0.5% NP-40, 0.25% Triton X-100, protease inhibitors) followed by rotation at 4°C for 15 min. Cells were spun at 1350 × g for 5 min, and resuspended in cold Lysis Buffer 2 (107 cell/mL; 200 mM NaCl, 10 mM Tris-HCl pH 8.0, 1 mM EDTA, 0.5 mM EGTA, protease inhibitors) followed by rotation at room temperature for 10 min. Cells were spun at 1350 × g for 5 min, and resuspended in sonication buffer (5×107 cell/mL; 10 mM Tris-HCl pH 8.0, 1 mM EDTA, 0.1% SDS, protease inhibitors). The suspensions were passed through an 18G needle to break up clumps, and the samples were then combined to a ratio of 2:1 HL60:S2 cells.
The combined samples were sonicated in a Bioruptor (Diagenode) for 15 cycles of 30 s on, 30 s ice. The chromatin was centrifuged at 14,000 × g for 12 min and the supernatant was diluted 1:1 with dilution buffer (300 mM NaCl, 2 mM EDTA, 50 mM Tris-HCl pH 8.0, 0.75% Triton X-100, 0.1% SDS). Immunoprecipitation was performed using Protein A Dynabeads (Thermo Fisher) conjugated with rabbit IgG (Santa Cruz, sc-2027) or H4 acetyl (Millipore 06–866, Lot 2491213). After overnight incubation at 4°C with rotation, the beads were washed twice with low salt wash buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 150 mM NaCl, 20 mM Tris-HCl pH 8.1), twice with high salt wash buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 500 mM NaCl, 20 mM Tris-HCl pH 8.1), once with LiCl wash buffer (0.25 M LiCl, 1% NP-40, 1% deoxycholate, 1 mM EDTA, 10 mM Tris-HCl pH 8.1), and twice with TE buffer (10 mM Tris-HCl pH 8.1, 1 mM EDTA). Samples were eluted from beads by incubating in elution buffer (50 mM Tris-HCl pH 8.0, 10 mM EDTA, 1% SDS) at 65°C for 30 min. Crosslinks were reversed by incubating supernatant and input samples at 65°C for 16 h. Samples were treated with 0.2 mg/mL RNase A for 1 h at 37°C, and 0.2 mg/mL Proteinase K for 2 h at 42°C. DNA was isolated using MinElute DNA purification columns (QIAGEN). BRD4 ChIP was performed using 4.5×108 HL60 cells treated with 1 μM SAHA or 0.01% DMSO for 1 h as previously described except that formaldehyde crosslinking was for 15 minutes. Immunoprecipitation was performed using Protein A Dynabeads conjugated to rabbit IgG or BRD4 antibody (Bethyl Labs A301–985A100, Lot 4). After overnight incubation at 4°C with rotation, beads were washed, crosslinks were reversed, samples were treated with RNaseA and Proteinase K and eluted as described above. DNA was isolated using MinElute DNA purification columns. Sequencing libraries were generated from DNA enriched by ChIP following the manufacturer’s specification for the Tru-Seq library preparation kit (Illumina). Following library generation, single end sequencing was performed using Illumina HiSeq2000 (UNC Chapel Hill High Throughput Sequencing Facility).
Peptide microarray
Peptide arrays were synthesized as previously described (Fuchs and Strahl, 2011; Rothbart et al., 2012). Bromodomains were cloned into pGEX-6P1 vectors, expressed in BL21 E. coli, and purified using batch purification with glutathione resin (Pierce). Proteins were eluted and dialyzed in PBS. Domains were arrayed as previously described using GST antibody (Sigma) to detect the bound protein (Rothbart et al., 2012). H4 acetyl antibody (Millipore 06–866, Lot 2491213) specificity was determined as previously described (Rothbart et al., 2015) using 1:2000 dilution in PBS with 0.1% Tween-20 and incubation for 1 h at room temperature. After incubation with domains or antibody, arrays were washed in cold PBS, incubated in secondary antibody and scanned (Typhoon, GE). After normalizing the average signal of triplicate spots to the peptide with the highest signal, heatmaps were generated with Java TreeView with a range of intensity from 0 to 1 (Saldanha, 2004).
H4ac ChIP
Single end 50-bp reads were filtered using TagDust (Lassmann et al., 2009) using the following options: -f 0.001 -a < /path/to/out/artifact.fastq > -s < /path/to/adapter_seq.txt > -o < /path/to/filtered/file.fastq > < /path/to/input.fastq >. ChIP-Rx normalization was performed as described (Orlando et al., 2014). Reads were independently aligned to the human hg19 genome and the Drosophila dm3 genome using Bowtie (Langmead et al., 2009) using the following options: -m 1–best–seed = 123 -p 8–nomaqround < /path/to/bowtie/hg19_index_prefix > < /path/to/input.fastq > < /path/to/alignment.hits >. For Drosophila S2 alignment hg19 was replaced with dm3. Unmapped reads were then aligned to the human hg19 genome using Bowtie and reads were normalized to Drosophila mapped read counts (Figure S2A). Correlation between replicates was assessed by Pearson correlation using wig correlate (UCSC).
To generate differential windows, input signal was subtracted from ChIP signal. Signal was then averaged across 300bp sliding windows offset by 100 bp. Differential windows were identified using DESEQ2 (Love et al., 2014). Windows with significant enrichment were merged and identified as a peak. H4ac signal in differential windows was then plotted as a fraction of SAHA/DMSO to generate histogram of fold-change after SAHA treatment. Smoothed scatterplots of H4ac signal in differential windows were generated using R as average signal over window size. Enrichment for genomic features in differential windows was analyzed by CEAS (Shin et al., 2009). Genes with acetyl signal within the gene body were identified by having signal greater than 0 within the gene body. Any gene that was found within multiple categories (Quad 1–4) was discarded.
BRD4 ChIP
Single end 50-bp reads were filtered using TagDust (Lassmann et al., 2009) as above and aligned to hg19 using Bowtie (Langmead et al., 2009) as described for H4a ChIP. Peaks were called with MACS2 callpeak (Zhang et al., 2008) using default parameters. BED-Tools (Quinlan and Hall, 2010) was used to merge overlapping peaks between replicates and across treatments to identify shared and unique peaks. Enrichment for genomic features was analyzed using CEAS (Shin et al., 2009). Each peak was matched with its closest genes within 1000 kb using the Genomic Regions Enrichment of Annotations Tool (GREAT) (McLean et al., 2010). Genes were matched to differentially expressed genes from RNA-seq. Genes with BRD4 signal within gene bodies were identified as previously described for H4ac.
QUANTIFICATION AND STATISTICAL ANALYSIS
Variation quantified immunoblot data represent standard deviation. Genomic and proteomic statistical significance testing was derived from algorithms used to analyze the respective data types (3B, 4I, 5D, S3). Statistical significance of genomic overlaps (Figure 6B) was determined by permutation testing by scrambling the genomic locations of identically sized regions (n = 10000). Variance in proteomic and western blot studies represents mean with one standard deviation depicted by error bars.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| BRD2 | Bethyl | Cat#A302-583A; RRID:AB_2034829 |
| BRD3 | Bethyl | Cat#A302-368A; RRID:AB_1907251 |
| BRD4 | Bethyl | Cat#A301-985A100; Lot#4; RRID:AB_2620184 |
| CDK9 | Thermo Fisher | Cat#703404: RRID:AB_2784585 |
| H4 | Abcam | Cat#ab10158; RRID:AB_296888 |
| H4-acetyl | Millipore | Cat#06-866; Lot#2491213; RRID:AB_310270 |
| H4K16ac | Active Motif | Cat#39167; RRID:AB_2636968 |
| H3 | Epicypher | Cat#13-0001 |
| H3K14ac | Millipore | Cat#07-353, Lot#2387522; RRID:AB_310545 |
| H3K4me3 | Epicypher | Cat#13-004 |
| MYC | Abcam | Cat#ab32072; RRID:AB_731658 |
| Beta-Tubulin | Millipore | Cat#05-661; RRID:AB_309885 |
| Rabbit IgG | Santa Cruz | Cat#sc-2027, Lot#D1415; RRID:AB_737197 |
| GST | Sigma | Cat#G7781; RRID:AB_259965 |
| Bacterial and Virus Strains | ||
| BL21 E. coli | ThermoFisher | Cat# EC0114 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| SAHA | Sigma | Cat# SML0061 |
| JQ1 | Sigma | Cat# SML1524 |
| DMSO | Sigma | Cat# D2438 |
| Sodium Butyrate | Sigma | Cat# 303410 |
| RPMI-1640 | GIBCO | Cat# 11875101 |
| DMEM | GIBCO | Cat# 11995040 |
| FBS | Sigma | Cat# F4135 |
| Schneider’s Media | GIBCO | Cat# 21720024 |
| Peptides | This study | N/A |
| High Capacity Streptavidin Agarose Resin | Thermo Scientific | Cat# 20357 |
| SilverQuest Staining Kit | Invitrogen | Cat# LC6070 |
| TRIzol reagent | Invitrogen | Cat# 5596026 |
| Universal Nuclease | Thermo Scientific | Cat# 88700 |
| Protease Inhibitor Cocktail | Roche | Cat# 11836170001 |
| Superscript III | Thermo Fisher | Cat# 18080044 |
| SYBR Green Master Mix | BioRad | Cat# 1725270 |
| Protein A Dynabeads | Thermo Fisher | Cat#10001D |
| RNase A | Roche | Cat# 10109142001 |
| DNase | Thermo Fisher | Cat# AM2222 |
| Poly-AN Streptavidin Coated Slides | Grainger | Cat# 439003-25 |
| Critical Commercial Assays | ||
| RiboZero Gold Prep Kit | Illumina | Cat# MRZG12324 |
| RNeasy Mini Kit | QIAGEN | Cat# 74106 |
| TruSeq Stranded Total RNA Library Prep Kit Gold | Illumina | Cat# 20020599 |
| MinElute DNA purification columns | QIAGEN | Cat# 28204 |
| TruSeq ChIP Library Prep Kit | QIAGEN | Cat# IP-202-1012 |
| Deposited Data | ||
| RNA-seq Data and ChIP-seq Data | This study | GEO: GSE159620 |
| Raw data for western blots and spotting assays | This study | Mendeley: https://doi.org/10.17632/6bhbxbcyd3.1 |
| Experimental Models: Cell Lines | ||
| HeLa | ATCC | Cat# CCL-2 |
| Drosophila S2 cell line | ATCC | Cat# CRL-1963 |
| HL60 | ATCC | Cat# CCL-240 |
| Oligonucleotides | ||
| 5′-CCTGGTGCTCCATGAGGAGAC-3′ | IDT | MYC Forward |
| 5′-CAGACTCTGACCTTTTGCCAGG-3′ | IDT | MYC Reverse |
| 5′-AGGTGGACCTGGAGACTCTCAG-3′ | IDT | CDKN1A Forward |
| 5′-TCCTCTTGGAGAAGATCAGCCG-3′ | IDT | CDKN1A Reverse |
| 5′-GTCTCCTCTGACTTCAACAGCG-3′ | IDT | GAPDH Forward |
| 5′-ACCACCCTGTTGCTGTAGCCAA-3′ | IDT | GAPDH Reverse |
| Recombinant DNA | ||
| pGEX-6P1 | Cytiva | Cat# 28954648 |
| pGEX-6P1-BRD2-BD1 | This study | N/A |
| pGEX-6P1-BRD2-BD2 | This study | N/A |
| pGEX-6P1-BRD3-BD1 | This study | N/A |
| pGEX-6P1-BRD4-BD1 | This study | N/A |
| pGEX-6P1-BRD4-BD2 | This study | N/A |
| pGEX-6P1-BRDT-BD1 | This study | N/A |
| pGEX-6P1-BRDT-BD2 | This study | N/A |
| pGEX-6P1-BRD9 | This study | N/A |
| pGEX-6P1-BPTF | This study | N/A |
| pGEX-6P1-BRPF3 | This study | N/A |
| pGEX-6P1-CECR2 | This study | N/A |
| pGEX-6P1-Bdf1-BD1-2 | This study | N/A |
| pGEX-6P1-Nrc1-BD1-2 | This study | N/A |
| pGEX-6P1-p300 | This study | N/A |
| pGEX-6P1-BRPF1 | This study | N/A |
| pGEX-6P1-TAF1-BD1 | This study | N/A |
| pGEX-6P1-TAF1-BD2 | This study | N/A |
| pGEX-6P1-PHIP-BD2 | This study | N/A |
| Software and Algorithms | ||
| TagDust v1.12 | Lassmann et al., 2009 | http://tagdust.sourceforge.net/ |
| Bowtie v1.1.0 | Langmead et al., 2009 | https://sourceforge.net/projects/bowtie-bio/files/bowtie/1.1.0/ |
| DESEQ2 v1.63 | Love et al., 2014 | https://bioconductor.org/packages/release/bioc/html/DESeq2.html |
| CEAS v0.9.9.7 | Shin et al., 2009 | https://liulab-dfci.github.io/software/ |
| MACS2 v2.1.2 | Zhang et al., 2008 | https://github.com/macs3-project/MACS |
| GREAT v3.0.0 | McLean et al., 2010 | http://great.stanford.edu/great/public-3.0.0/html/ |
| STAR v2.5.2b | Dobin et al., 2013 | https://github.com/alexdobin/STAR |
Highlights.
HDAC inhibition results histone 4 polyacetylation
H4 polyacetylation serves as a preferred target for bromodomain interactions
Hyperacetylation in actively transcribed genes corresponds to increased BRD4 binding
HDAC and bromodomain inhibition have a similar effect transcription
ACKNOWLEDGMENTS
This work was funded, in part, through a University Cancer Research Fund pilot grant from the Lineberger Comprehensive Cancer Center to B.D.S. and I.J.D. and from grants from the NIH to B.D.S. (GM12690), I.J.D (CA198482), and S.B.R. (GM124736). We also thank Alexey Soshnev for assistance in creating the schematic in the Graphical Abstract and Figure 2C.
Footnotes
DECLARATION OF INTERESTS
B.D.S. is a cofounder and scientific advisory board member of EpiCypher, Inc., and a scientific advisory board member of Triangle Biotechnology, Inc. I.J.D. is a scientific advisory board member of Triangle Biotechnology, Inc.
SUPPLEMENTAL INFORMATION
Supplemental information can be found online at https://doi.org/10.1016/j.celrep.2020.108638.
REFERENCES
- Bhadury J, Nilsson LM, Muralidharan SV, Green LC, Li Z, Gesner EM, Hansen HC, Keller UB, McLure KG, and Nilsson JA (2014). BET and HDAC inhibitors induce similar genes and biological effects and synergize to kill in Myc-induced murine lymphoma. Proc. Natl. Acad. Sci. U S A 111, E2721–E2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhanu NV, Sidoli S, and Garcia BA (2016). Histone modification profiling reveals differential signatures associated with human embryonic stem cell self-renewal and differentiation. Proteomics 16, 448–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocks D, Schmidt CR, Daskalakis M, Jang HS, Shah NM, Li D, Li J, Zhang B, Hou Y, Laudato S, et al. (2017). DNMT and HDAC inhibitors induce cryptic transcription start sites encoded in long terminal repeats. Nat. Genet. 49, 1052–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey MF, Peterson CL, and Smale ST (2009). Dignam and Roeder nuclear extract preparation. Cold Spring Harb. Protoc. 2009, pdb.prot5330. [DOI] [PubMed] [Google Scholar]
- Cheng YC, Lin H, Huang MJ, Chow JM, Lin S, and Liu HE (2007). Downregulation of c-Myc is critical for valproic acid-induced growth arrest and myeloid differentiation of acute myeloid leukemia. Leuk. Res. 31, 1403–1411. [DOI] [PubMed] [Google Scholar]
- Delmore JE, Issa GC, Lemieux ME, Rahl PB, Shi J, Jacobs HM, Kastritis E, Gilpatrick T, Paranal RM, Qi J, et al. (2011). BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell 146, 904–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis G Jr., Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, and Lempicki RA (2003). DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 4, 3. [PubMed] [Google Scholar]
- Devaiah BN, Case-Borden C, Gegonne A, Hsu CH, Chen Q, Meerzaman D, Dey A, Ozato K, and Singer DS (2016). BRD4 is a histone acetyltransferase that evicts nucleosomes from chromatin. Nat. Struct. Mol. Biol. 23, 540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey A, Chitsaz F, Abbasi A, Misteli T, and Ozato K. (2003). The double bromodomain protein Brd4 binds to acetylated chromatin during interphase and mitosis. Proc. Natl. Acad. Sci. U S A 100, 8758–8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, and Gingeras TR (2013). STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokmanovic M, Clarke C, and Marks PA (2007). Histone deacetylase inhibitors: overview and perspectives. Mol. Cancer Res. 5, 981–989. [DOI] [PubMed] [Google Scholar]
- Drogaris P, Villeneuve V, Pomie s, C., Lee EH, Bourdeau V, Bonneil E, Ferbeyre G, Verreault A, and Thibault P. (2012). Histone deacetylase inhibitors globally enhance h3/h4 tail acetylation without affecting h3 lysine 56 acetylation. Sci. Rep. 2, 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckschlager T, Plch J, Stiborova M, and Hrabeta J. (2017). Histone deacetylase inhibitors as anticancer drugs. Int. J. Mol. Sci. 18, 1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippakopoulos P, Picaud S, Mangos M, Keates T, Lambert JP, Barsyte-Lovejoy D, Felletar I, Volkmer R, Müller S, Pawson T, et al. (2012). Histone recognition and large-scale structural analysis of the human bromodomain family. Cell 149, 214–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiskus W, Sharma S, Qi J, Valenta JA, Schaub LJ, Shah B, Peth K, Portier BP, Rodriguez M, Devaraj SG, et al. (2014). Highly active combination of BRD4 antagonist and histone deacetylase inhibitor against human acute myelogenous leukemia cells. Mol. Cancer Ther. 13, 1142–1154. [DOI] [PubMed] [Google Scholar]
- Frank SR, Parisi T, Taubert S, Fernandez P, Fuchs M, Chan HM, Livingston DM, and Amati B. (2003). MYC recruits the TIP60 histone acetyltransferase complex to chromatin. EMBO Rep. 4, 575–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs SM, and Strahl BD (2011). Antibody recognition of histone post-translational modifications: emerging issues and future prospects. Epigenomics 3, 247–249. [DOI] [PubMed] [Google Scholar]
- Greer CB, Tanaka Y, Kim YJ, Xie P, Zhang MQ, Park IH, and Kim TH (2015). Histone deacetylases positively regulate transcription through the elongation machinery. Cell Rep. 13, 1444–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryder BE, Pomella S, Sayers C, Wu XS, Song Y, Chiarella AM, Bagchi S, Chou HC, Sinniah RS, Walton A, et al. (2019). Histone hyperacetylation disrupts core gene regulatory architecture in rhabdomyosarcoma. Nat. Genet. 51, 1714–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui CY, Ngo L, Xu WS, Richon VM, and Marks PA (2004). Histone deacetylase (HDAC) inhibitor activation of p21WAF1 involves changes in promoter-associated proteins, including HDAC1. Proc. Natl. Acad. Sci. U S A 101, 1241–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller G, Schmidt WM, Ziegler B, Holzer S, Müllauer L, Bilban M, Zielinski CC, Drach J, and Zöchbauer-Müller S. (2008). Genome-wide transcriptional response to 5-aza-2 ′-deoxycytidine and trichostatin a in multiple myeloma cells. Cancer Res. 68, 44–54. [DOI] [PubMed] [Google Scholar]
- Jang MK, Mochizuki K, Zhou M, Jeong HS, Brady JN, and Ozato K. (2005). The bromodomain protein Brd4 is a positive regulatory component of P-TEFb and stimulates RNA polymerase II-dependent transcription. Mol. Cell 19, 523–534. [DOI] [PubMed] [Google Scholar]
- Kanno T, Kanno Y, LeRoy G, Campos E, Sun HW, Brooks SR, Vahedi G, Heightman TD, Garcia BA, Reinberg D, et al. (2014). BRD4 assists elongation of both coding and enhancer RNAs by interacting with acetylated histones. Nat. Struct. Mol. Biol. 21, 1047–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Greer CB, Cecchini KR, Harris LN, Tuck DP, and Kim TH (2013). HDAC inhibitors induce transcriptional repression of high copy number genes in breast cancer through elongation blockade. Oncogene 32, 2828–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon SH, Ahn SH, Kim YK, Bae GU, Yoon JW, Hong S, Lee HY, Lee YW, Lee HW, and Han JW (2002). Apicidin, a histone deacetylase inhibitor, induces apoptosis and Fas/Fas ligand expression in human acute promyelocytic leukemia cells. J. Biol. Chem. 277, 2073–2080. [DOI] [PubMed] [Google Scholar]
- Langmead B, Trapnell C, Pop M, and Salzberg SL (2009). Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassmann T, Hayashizaki Y, and Daub CO (2009). TagDust—a program to eliminate artifacts from next generation sequencing data. Bioinformatics 25, 2839–2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeRoy G, Rickards B, and Flint SJ (2008). The double bromodomain proteins Brd2 and Brd3 couple histone acetylation to transcription. Mol. Cell 30, 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann RK, Newbold A, Whitecross KF, Cluse LA, Frew AJ, Ellis L, Williams S, Wiegmans AP, Dear AE, Scott CL, et al. (2007). Analysis of the apoptotic and therapeutic activities of histone deacetylase inhibitors by using a mouse model of B cell lymphoma. Proc. Natl. Acad. Sci. U S A 104, 8071–8076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Ma Q, Wong K, Li W, Ohgi K, Zhang J, Aggarwal A, and Rosenfeld MG (2013). Brd4 and JMJD6-associated anti-pause enhancers in regulation of transcriptional pause release. Cell 155, 1581–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, and Anders S. (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovén J, Hoke HA, Lin CY, Lau A, Orlando DA, Vakoc CR, Bradner JE, Lee TI, and Young RA (2013). Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell 153, 320–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean CY, Bristor D, Hiller M, Clarke SL, Schaar BT, Lowe CB, Wenger AM, and Bejerano G. (2010). GREAT improves functional interpretation of cis-regulatory regions. Nat. Biotechnol. 28, 495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertz JA, Conery AR, Bryant BM, Sandy P, Balasubramanian S, Mele DA, Bergeron L, and Sims RJ 3rd. (2011). Targeting MYC dependence in cancer by inhibiting BET bromodomains. Proc. Natl. Acad. Sci. U S A 108, 16669–16674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebbioso A, Carafa V, Conte M, Tambaro FP, Abbondanza C, Martens J, Nees M, Benedetti R, Pallavicini I, Minucci S, et al. (2017). c-Myc modulation and acetylation is a key HDAC inhibitor target in cancer. Clin. Cancer Res. 23, 2542–2555. [DOI] [PubMed] [Google Scholar]
- Orlando DA, Chen MW, Brown VE, Solanki S, Choi YJ, Olson ER, Fritz CC, Bradner JE, and Guenther MG (2014). Quantitative ChIP-Seq normalization reveals global modulation of the epigenome. Cell Rep. 9, 1163–1170. [DOI] [PubMed] [Google Scholar]
- Qu K, Zaba LC, Satpathy AT, Giresi PG, Li R, Jin Y, Armstrong R, Jin C, Schmitt N, Rahbar Z, et al. (2017). Chromatin accessibility landscape of cutaneous T cell lymphoma and dynamic response to HDAC inhibitors. Cancer Cell 32, 27–41.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan AR, and Hall IM (2010). BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada-Iglesias A, Enroth S, Ameur A, Koch CM, Clelland GK, RespuelaAlonso P, Wilcox S, Dovey OM, Ellis PD, Langford CF, et al. (2007). Butyrate mediates decrease of histone acetylation centered on transcription start sites and down-regulation of associated genes. Genome Res. 17, 708–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ram O, Goren A, Amit I, Shoresh N, Yosef N, Ernst J, Kellis M, Gymrek M, Issner R, Coyne M, et al. (2011). Combinatorial patterning of chromatin regulators uncovered by genome-wide location analysis in human cells. Cell 147, 1628–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbart SB, Krajewski K, Strahl BD, and Fuchs SM (2012). Peptide microarrays to interrogate the “histone code”. Methods Enzymol. 512, 107–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbart SB, Dickson BM, Raab JR, Grzybowski AT, Krajewski K, Guo AH, Shanle EK, Josefowicz SZ, Fuchs SM, Allis CD, et al. (2015). An interactive database for the assessment of histone antibody specificity. Mol. Cell 59, 502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha AJ (2004). Java Treeview—extensible visualization of microarray data. Bioinformatics 20, 3246–3248. [DOI] [PubMed] [Google Scholar]
- Sanchez GJ, Richmond PA, Bunker EN, Karman SS, Azofeifa J, Garnett AT, Xu Q, Wheeler GE, Toomey CM, Zhang Q, et al. (2018). Genome-wide dose-dependent inhibition of histone deacetylases studies reveal their roles in enhancer remodeling and suppression of oncogenic super-enhancers. Nucleic Acids Res. 46, 1756–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbazian MD, and Grunstein M. (2007). Functions of site-specific histone acetylation and deacetylation. Annu. Rev. Biochem. 76, 75–100. [DOI] [PubMed] [Google Scholar]
- Shin H, Liu T, Manrai AK, and Liu XS (2009). CEAS: cis-regulatory element annotation system. Bioinformatics 25, 2605–2606. [DOI] [PubMed] [Google Scholar]
- Slaughter MJ, Shanle EK, McFadden AW, Hollis ES, Suttle LE, Strahl BD, and Davis IJ (2018). PBRM1 bromodomains variably influence nucleosome interactions and cellular function. J. Biol. Chem. 293, 13592–13603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Zang C, Cui K, Schones DE, Barski A, Peng W, and Zhao K. (2009). Genome-wide mapping of HATs and HDACs reveals distinct functions in active and inactive genes. Cell 138, 1019–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AC, and Johnstone RW (2014). New and emerging HDAC inhibitors for cancer treatment. J. Clin. Invest. 124, 30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte WA, Orlando DA, Hnisz D, Abraham BJ, Lin CY, Kagey MH, Rahl PB, Lee TI, and Young RA (2013). Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell 153, 307–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Cetinkaya C, Munoz-Alonso MJ, von der Lehr N, Bahram F, Beuger V, Eilers M, Leon J, and Larsson LG (2003). Myc represses differentiation-induced p21CIP1 expression via Miz-1-dependent interaction with the p21 core promoter. Oncogene 22, 351–360. [DOI] [PubMed] [Google Scholar]
- Yang Z, Yik JH, Chen R, He N, Jang MK, Ozato K, and Zhou Q. (2005). Recruitment of P-TEFb for stimulation of transcriptional elongation by the bromodomain protein Brd4. Mol. Cell 19, 535–545. [DOI] [PubMed] [Google Scholar]
- Yuan ZF, Lin S, Molden RC, Cao XJ, Bhanu NV, Wang X, Sidoli S, Liu S, and Garcia BA (2015). EpiProfile quantifies histone peptides with modifications by extracting retention time and intensity in high-resolution mass spectra. Mol. Cell. Proteomics 14, 1696–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, Nusbaum C, Myers RM, Brown M, Li W, and Liu XS (2008). Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9, R137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequencing data generated as part of this study have been submitted to GEO under accession number GSE159620. Western blot data are available at Mendeley Data at https://doi.org/10.17632/6bhbxbcyd3.1