Abstract

Ruthenium catalysts for olefin metathesis are widely viewed as water-tolerant. Evidence is presented, however, that even low concentrations of water cause catalyst decomposition, severely degrading yields. Of 11 catalysts studied, fast-initiating examples (e.g., the Grela catalyst RuCl2(H2IMes)(=CHC6H4-2-OiPr-5-NO2) were most affected. Maximum water tolerance was exhibited by slowly initiating iodide and cyclic (alkyl)(amino)carbene (CAAC) derivatives. Computational investigations indicated that hydrogen bonding of water to substrate can also play a role, by retarding cyclization relative to decomposition. These results have important implications for olefin metathesis in organic media, where water is a ubiquitous contaminant, and for aqueous metathesis, which currently requires superstoichiometric “catalyst” for demanding reactions.
Keywords: olefin metathesis, catalyst decomposition, macrocycle, conformation, water, aqueous metathesis, chemical biology, Z-selective
Olefin metathesis has been widely embraced for its versatility in the catalytic assembly of carbon–carbon bonds.1,2 The demand for catalysts that integrate high activity with robustness has intensified with a recent explosion in applications in chemical biology,3,4,5a materials science,6 and chemical manufacturing.7 Tolerance for water is critical in many contexts, most prominently olefin metathesis in water-rich environments. Successes in aqueous metathesis of model substrates with ruthenium catalysts5 (see, e.g., Chart 1) have been leveraged to advance metathetical modification of proteins,3 peptides,8 and DNA,4b,9 and to develop water-soluble materials for drug delivery and other applications.6a−6e,10,11
Chart 1. Olefin Metathesis Catalysts Discussed.
These applications place extreme demands on the water-tolerance of the catalysts. Decomposition by water is increasingly identified as an obstacle to olefin metathesis in chemical biology3,5a (where Isenegger and Davis describe bioconjugation as a race between metathesis and decomposition)3a and biomaterials applications.6a−6e,10−12 The catalyst loadings required are routinely orders of magnitude above those in organic media: in highly demanding contexts such as protein modification, the Ru reagent must be used in significant stoichiometric excess.13 An anticipated, undesirable consequence is accelerated bimolecular decomposition of the active Ru species14 and associated side-reactions. The decomposed catalyst is believed to trigger both DNA degradation9,15 and C=C migration.16
Given that the problems of water cosolvent are only beginning to be widely recognized,3,5a,17,18 it is unsurprising that challenges arising from low levels of water have not yet been considered. Here we demonstrate that even 0.1–1% v/v water19 can severely limit the productivity of leading N-heterocyclic carbene (NHC) and CAAC ruthenium catalysts. We also identify catalyst features that maximize water-tolerance, a finding that offers new opportunities in organic synthesis, and in broader contexts in which water is an essential cosolvent.
RCM macrocyclization (mRCM) represents a methodology of major current interest for the production of antiviral therapeutics.7,20 The first indication that even low concentrations of water might impede mRCM emerged in reactions involving the dianiline catalyst Ru-1 (Chart 1). In our hands, Ru-1 was exceptionally efficient,21 outperforming even the leading nitro-Grela catalyst Ru-2 in mRCM of challenging, highly flexible substrates bearing multiple polar sites. Synthetic collaborators, however, encountered variable performance. We speculated that the discrepancy might arise from the established21 hydrogen-bonding capacity of Ru-1. Sensitivity to water would have gone unobserved in our original work because rigorously dry22 solvents were used, a standard protocol in organometallic chemistry. In broader synthetic practice, water is a ubiquitous, little-regarded contaminant. It thus seemed plausible that water-induced decomposition might contribute to the inconsistent performance of Ru-1.
To probe this point, we examined the impact of water on mRCM of 1 (Figure 1a).23 This reaction affords the olfactory lactone 2 via a concentration-dependent ring–chain equilibrium.24 High dilutions are essential to favor the cyclic product, as with any conformationally flexible diene.24,25 For 1, in which the ester functionality confers the sole conformational bias toward cyclization,26 a diene concentration of ≤5 mM is required.24 At catalyst loadings of 0.05 mol %, this translates into 2.5 μM Ru: even low concentrations of water are thus stoichiometrically significant.
Figure 1.

Impact of water on mRCM. (a) For Ru-1 at 0.5 h. (b) For various catalysts at 2 h (except Ru-6: 24 h). *Ru-5, Ru-6: 60 °C, 0.01 atm.33 For tabulated data, see Table S1.
In dry toluene, mRCM of 1 reached 83% yield within 0.5 h at RT (Figure 1a). Addition of 0.01% water—that is, 100 ppm by volume—caused a ca. 60% drop in yield. At higher proportions of water (0.1 or 1% v/v), mRCM failed, signifying near-complete catalyst decomposition.
As shown in Figure 1b, the Grela catalyst Ru-2 is decomposed to a lesser extent, affording 30% mRCM in the presence of 0.1% v/v water (vs 87% mRCM in dry toluene).27 More robust is the iodide analogue Ru-3. Water—somewhat unexpectedly—is emerging as a much more aggressive agent than O2 in Ru-catalyzed olefin metathesis,28 and this higher water-tolerance is thus presumed to be key to the strong performance of Ru-3 in aerobic metathesis.29 One probable contributor to improved tolerance is the limited capacity of Ru-3 to enter into hydrogen-bonding interactions with water. ROH···Cl–Ru interactions have been reported for related metathesis catalysts,30,31 and the higher water-sensitivity of Ru-1 vs Ru-2 is consistent with stronger H-bonding to a dangling NH2 functionality.
To assess the potential impact of water on E/Z selectivity, two Z-selective catalysts (Ru-6, Ru-5) were also examined. Their lower reactivity necessitated use of elevated temperatures (60 °C) and higher catalyst loadings. For Ru-5, only 17% mRCM was observed even with 5 mol % catalyst: added water had no impact, probably because only a small proportion of catalyst had initiated.32Ru-6 afforded 64% mRCM in the anhydrous reaction (0.5 mol % Ru), and 25% in the presence of water. Of note, water had a negligible impact on Z-selectivity (Ru-6, 85%; Ru-5, 70%). We infer that decomposed catalyst does not promote E/Z isomerization, at least for Ru-6.
Strikingly, however, water significantly accelerated positional isomerization in the self-metathesis of allylbenzene 3 (Figure 2a). Terminal phenylpropenes are notoriously susceptible to isomerization to the conjugated β-methylstyrenes.16c For Ru-6, 8% isomerization was observed for the anhydrous reaction, vs 75% with 1% H2O present. In comparison, Ru-3 showed more isomerization in the anhydrous reaction, but added water affected primarily conversions.
Figure 2.
Impact of H2O on: (a) self-metathesis of allylbenzene. (b) Cross-metathesis of estragole and methyl acrylate.
A second, more demanding intermolecular metathesis reaction was also examined. In the cross-metathesis of anethole 6 with methyl acrylate 7 (Figure 2b), a ca. 30% drop in productivity was observed for Ru-3 in the presence of 1% H2O.
To assess whether the negative impact of water is limited to relatively challenging reactions, we turned to RCM of diethyl diallylmalonate 9 (Figure 3). Diene 9 sets a notoriously low bar for olefin metathesis activity: the extreme facility with which it undergoes RCM makes it a correspondingly aggressive test for the impact of water. Here, in addition to the catalysts examined above, we include benzylidene, indenylidene, NHC, and CAAC catalysts.29,34−36 Initial experiments were conducted at 0.005 mol % catalyst (50 ppm vs substrate), to enable “anhydrous” TONs in the thousands even for less active catalysts, without masking decomposition.37
Figure 3.
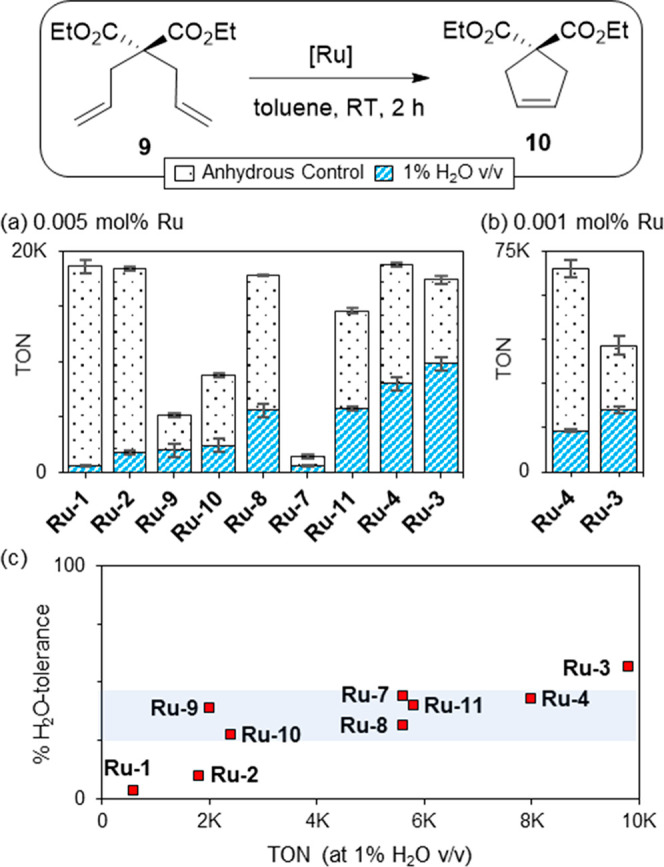
(a) Impact of H2O on RCM of 9. (b) Plot of catalyst water-tolerance, 100 – [(ΔTON/TONanhyd) × 100], vs TON (0.005 mol % Ru). The blue band signifies intermediate tolerance. For rate curves and tabulated data, see SI.
Shown in Figure 3a are TONs at 2 h, at which point conversions in the presence of water plateau (Figure S1) for all but Ru-7 and Ru-10. Notwithstanding the ease of this RCM reaction, yields decreased sharply in the presence of 1% H2O for all catalysts surveyed. TONs of only 600 or 1 800, respectively, were observed for Ru-1 and Ru-2 (vs ca. 18 000 in the anhydrous control reaction). Iodide complex Ru-3 gave maximum TONs (9800). For CAAC-Grela catalyst Ru-4, a top-performing catalyst under anhydrous conditions, TONs dropped by 60% (from nearly 19 000 to 8000).
Given the high susceptibility of Ru-4 to bimolecular decomposition,14b its performance relative to Ru-3 was re-evaluated at a catalyst loading 5-fold lower (Figure 3b). TONs in the anhydrous control reaction increased in both cases: by nearly 4× for Ru-4 and 2.5× for Ru-3. Clearly, bimolecular coupling occurs for both catalysts, even at 10 μM Ru. Higher water-sensitivity is evident at the lower catalyst loading: that is, decomposition by water competes more strongly with bimolecular decomposition as catalyst concentrations decline. Ru-3 remains most productive (TON 21 000 vs 14 000 for Ru-4).
In Figure 3c, we assess catalyst water tolerance independent of metathesis activity, by normalizing TONs in the presence of water to those under anhydrous conditions. For added context, water-tolerance is plotted against TONs in the presence of water: best performance is thus high on both axes. Least tolerant are the fast-initiating catalysts Ru-1 and Ru-2 (3% and 10%, respectively), suggesting that increased time in the active cycle increases vulnerability. Consistent with this analysis is the improved tolerance (27–44%) of the catalysts highlighted in the blue band in Figure 3b, none of which are fast-initating.38,39 Most robust is iodide catalyst Ru-3 (56% tolerance). Slow initiation40 is again a plausible contributor, in addition to the bulk and poor H-bonding capacity of the iodide ligands.29,34,35
The discussion above focuses on the impact of water on the catalyst. Given evidence for H-bonding of water to macrolactones, however,41 we queried whether H-bonding to the substrate might alter preferred diene conformations and hence the thermodynamics and/or kinetics of cyclization. To probe this point, we undertook a computational study of the impact of one H-bonded water molecule on the preferred conformations of prolactone 1 and diethyl diallylmalonate 9. The 1:1 ratio corresponds to 0.01% v/v H2O and 0.05 mol % Ru (Figure 1a). The highly precise ANAKIN-ME neural-network force field42,43 was used for extensive screening of possible geometries; calculated electronic energies for the most relevant conformers were refined using single-point energy calculations at the DLPNO–CCSD(T)/CBS level of theory.
Reaction free energies for RCM of 1 and 9 (see SI), showed no clear change arising from bound water, indicating that the negative effect is not thermodynamic in origin. The impact of water on preorganization was therefore examined. In the absence of water, the most stable conformer for diene 1 is essentially linear, with an end-to-end distance of >12 Å. In comparison, a distance of 3.72 Å is calculated for the most stable conformer of 9. Seen for the latter, but absent for 1, is a stabilizing π-stacking interaction between the two C=C bonds, a previously overlooked contributor to the facile RCM of 9. As expected, bringing the two C=C bonds of 9 into proximity incurs no penalty (ΔG = 0.0 kcal/mol: Figure 4a). For 1, the cost is higher (5.8 kcal/mol), consistent with the lower RCM reactivity of 1.
Figure 4.

(a) Impact of 1 H2O on the free energies required to adopt the incipient (“pre-cyclic”) conformation for 1 and 9. (b) Destabilization of precyclic conformations by H-bonded water. C···C distances: 3.72 Å (1); 4.21 Å (9).
The impact of H-bonded water on cyclization is examined in Figure 4b. For both 1 and 9, the diene conformation most favorable to cyclization is predicted to be less accessible in the presence of even one molecule of water, with a negative impact on the cyclization kinetics. Macrocyclization of 1 is impeded by location of the water molecule in the middle of the nascent cycle. For 9, the water molecule is outside the forming ring but nevertheless stabilizes the linear conformation. Indeed, water destabilizes the precyclic conformation even more strongly for 9 than for 1 (by 5.6 or 2.7 kcal/mol, respectively: Figure 4b),44 in part by disrupting the C=C π-stacking arrangement seen for anhydrous 9.
The unexpectedly greater negative impact of H2O on cyclization of 9 is supported by experiment. On repeating the RCM of 9 at the 5 mM diene concentration employed in mRCM, using the most robust catalyst Ru-3, we observe lower catalyst water-tolerance for 9 than 1 (30% vs 73%; Figure S3). Water thus has a greater negative impact on 9 than 1. By retarding the rate of metathesis relative to catalyst decomposition, H-bonded water can exacerbate catalyst decomposition, further limiting RCM performance in the presence of water.
The foregoing demonstrates that even low levels of water severely degrade the productivity of leading ruthenium catalysts in olefin metathesis. This is an important clue to the pathways by which water triggers decomposition: it points toward direct attack by water on the catalyst, independent of any effects arising from water as a medium. Fast-initiating catalysts prove particularly vulnerable. Maximum productivity is seen for slower-initiating, bulkier iodide and CAAC catalysts, which emerge as the systems of choice where drying is impractical, or where water is an essential cosolvent. These findings represent the first insights into structure–decomposition relationships for olefin metathesis in the presence of water. They are expected to aid in deconvoluting the mechanisms by which water causes decomposition and ultimately design of truly water-tolerant olefin metathesis catalysts.
Acknowledgments
This work was funded by the Natural Sciences and Engineering Research Council of Canada (NSERC), the Research Council of Norway (RCN, project 288135), and Centre National de la Recherche Scientifique: SYSPROD and AXELERA Pôle de Compétitivité (PSMN Data Center). The University of Ottawa, CNRS, and ENS Lyon are thanked for support via the International Associated Laboratory ‘Fundamental Catalysis for Green Chemistry’ (FUNCAT). Apeiron is thanked for gifts of CAAC catalysts.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscatal.0c04279.
The authors declare no competing financial interest.
Dedication
Dedicated to P. H. Dixneuf, in honor of his outstanding contributions to organometallic chemistry and catalysis.
Supplementary Material
References
- a Grela K.Olefin Metathesis-Theory and Practice; Wiley: Hoboken, NJ, 2014. [Google Scholar]; b Grubbs R. H.; Wenzel A. G.. Handbook of Metathesis, 2nd ed.; Wiley-VCH: Weinheim, 2015. [Google Scholar]
- a Cossy J.; Arseniyadis S.; Meyer C.. Metathesis in Natural Product Synthesis: Strategies, Substrates and Catalysts; Wiley-VCH: Weinheim, 2010. [Google Scholar]; b Furstner A. Metathesis in Total Synthesis. Chem. Commun. 2011, 47, 6505–6511. 10.1039/c1cc10464k. [DOI] [PubMed] [Google Scholar]; c Tyagi M.; Begnini F.; Poongavanam V.; Doak B. C.; Kihlberg J. Fabio; Poongavanam, Vasanthanathan, Drug Syntheses Beyond the Rule of 5. Chem. - Eur. J. 2020, 26, 49–88. 10.1002/chem.202080163. [DOI] [PubMed] [Google Scholar]; d Fürstner A. Catalysis for Total Synthesis: A Personal Account. Angew. Chem., Int. Ed. 2014, 53, 8587–8598. 10.1002/anie.201402719. [DOI] [PubMed] [Google Scholar]
- For recent reviews, see:; a Isenegger P. G.; Davis B. G. Concepts of Catalysis in Site-Selective Protein Modifications. J. Am. Chem. Soc. 2019, 141, 8005–8013. 10.1021/jacs.8b13187. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Vinogradova E. V. Organometallic Chemical Biology: An Organometallic Approach to Bioconjugation. Pure Appl. Chem. 2017, 89, 1619–1640. 10.1515/pac-2017-0207. [DOI] [Google Scholar]; c Messina M. S.; Maynard H. D. Modification of Proteins using Olefin Metathesis. Mater. Chem. Front. 2020, 4, 1040–1051. 10.1039/C9QM00494G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For selected recent examples of metathesis in chemical biology, see:; a Bhushan B.; Lin Y. A.; Bak M.; Phanumartwiwath A.; Yang N.; Bilyard M. K.; Tanaka T.; Hudson K. L.; Lercher L.; Stegmann M.; Mohammed S.; Davis B. G. Genetic Incorporation of Olefin Cross-Metathesis Reaction Tags for Protein Modification. J. Am. Chem. Soc. 2018, 140, 14599–14603. 10.1021/jacs.8b09433. [DOI] [PubMed] [Google Scholar]; b Lu X.; Fan L.; Phelps C. B.; Davie C. P.; Donahue C. P. Ruthenium Promoted On-DNA Ring-Closing Metathesis and Cross-Metathesis. Bioconjugate Chem. 2017, 28, 1625–1629. 10.1021/acs.bioconjchem.7b00292. [DOI] [PubMed] [Google Scholar]; c Grison C. M.; Burslem G. M.; Miles J. A.; Pilsl L. K. A.; Yeo D. J.; Imani Z.; Warriner S. L.; Webb M. E.; Wilson A. J. Double Quick, Double Click Reversible Peptide “Stapling. Chem. Sci. 2017, 8, 5166–5171. 10.1039/C7SC01342F. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Cromm P. M.; Spiegel J.; Kuchler P.; Dietrich L.; Kriegesmann J.; Wendt M.; Goody R. S.; Waldmann H.; Grossmann T. N. Protease-Resistant and Cell-Permeable Double-Stapled Peptides Targeting the Rab8a GTPase. ACS Chem. Biol. 2016, 11, 2375–2382. 10.1021/acschembio.6b00386. [DOI] [PubMed] [Google Scholar]
- a Sabatino V.; Ward T. R. Aqueous Olefin Metathesis: Recent Developments and Applications. Beilstein J. Org. Chem. 2019, 15, 445–468. 10.3762/bjoc.15.39. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Levin E.; Ivry E.; Diesendruck C. E.; Lemcoff N. G. Water in N-Heterocyclic Carbene-Assisted Catalysis. Chem. Rev. 2015, 115, 4607–4692. 10.1021/cr400640e. [DOI] [PubMed] [Google Scholar]; c Lipshutz B. H.; Ghorai S.. Olefin Metathesis in Water and Aqueous Media. In Olefin Metathesis - Theory and Practice; Grela K., Ed. 2014; pp 515–521. [Google Scholar]; d Grela K.; Gulajski L.; Skowerski K.. Alkene Metathesis in Water. In Metal-Catalyzed Reactions in Water; Dixneuf P. H., Cadierno V., Eds.; Wiley-VCH: Weinheim, 2013; pp 291–336. [Google Scholar]
- The last two years alone have seen spectacular advances in the applications of ring-opening metathesis polymerization (ROMP). Selected examples:; a Church D. C.; Pokorski J. K. Cell Engineering with Functional Poly(oxanorbornene) Block Copolymers. Angew. Chem., Int. Ed. 2020, 59, 11379–11383. 10.1002/anie.202005148. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Foster J. C.; Grocott M. C.; Arkinstall L. A.; Varlas S.; Redding M. J.; Grayson S. M.; O’Reilly R. K. It is Better with Salt: Aqueous Ring-Opening Metathesis Polymerization at Neutral pH. J. Am. Chem. Soc. 2020, 142, 13878–13885. 10.1021/jacs.0c05499. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Church D. C.; Takiguchi L.; Pokorski J. K. Optimization of Ring-Opening Metathesis Polymerization (ROMP) under Physiologically Relevant Conditions. Polym. Chem. 2020, 11, 4492–4499. 10.1039/D0PY00716A. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Varlas S.; Foster J. C.; O’Reilly R. K. Ring-opening metathesis polymerization-induced self-assembly (ROMPISA). Chem. Commun. 2019, 55, 9066–9071. 10.1039/C9CC04445K. [DOI] [PubMed] [Google Scholar]; e Varlas S.; Keogh R.; Xie Y.; Horswell S. L.; Foster J. C.; O’Reilly R. K. Polymerization-Induced Polymersome Fusion. J. Am. Chem. Soc. 2019, 141, 20234–20248. 10.1021/jacs.9b10152. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Feist J. D.; Xia Y. Enol Ethers Are Effective Monomers for Ring-Opening Metathesis Polymerization: Synthesis of Degradable and Depolymerizable Poly(2,3-dihydrofuran). J. Am. Chem. Soc. 2020, 142, 1186–1189. 10.1021/jacs.9b11834. [DOI] [PubMed] [Google Scholar]; g Sui X. L.; Zhang T. Q.; Pabarue A. B.; Fu L. B.; Gutekunst W. R. Alternating Cascade Metathesis Polymerization of Enynes and Cyclic Enol Ethers with Active Ruthenium Fischer Carbenes. J. Am. Chem. Soc. 2020, 142, 12942–12947. 10.1021/jacs.0c06045. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Song J. A.; Peterson G. I.; Bang K. T.; Ahmed T. S.; Sung J. C.; Grubbs R. H.; Choi T. L. Ru-Catalyzed, cis-Selective Living Ring-Opening Metathesis Polymerization of Various Monomers, Including a Dendronized Macromonomer, and Implications to Enhanced Shear Stability. J. Am. Chem. Soc. 2020, 142, 10438–10445. 10.1021/jacs.0c02785. [DOI] [PubMed] [Google Scholar]; i Hsu T. W.; Kim C.; Michaudel Q. Stereoretentive Ring-Opening Metathesis Polymerization to Access All-cis Poly(p-phenylenevinylene)s with Living Characteristics. J. Am. Chem. Soc. 2020, 142, 11983–11987. 10.1021/jacs.0c04068. [DOI] [PubMed] [Google Scholar]; j Yasir M.; Liu P.; Tennie I. K.; Kilbinger A. F. M. Catalytic living ring-opening metathesis polymerization with Grubbs’ second- and third-generation catalysts. Nat. Chem. 2019, 11, 488–494. 10.1038/s41557-019-0239-4. [DOI] [PubMed] [Google Scholar]; k Debsharma T.; Behrendt F. N.; Laschewsky A.; Schlaad H. Ring-Opening Metathesis Polymerization of Biomass-Derived Levoglucosenol. Angew. Chem., Int. Ed. 2019, 58, 6718–6721. 10.1002/anie.201814501. [DOI] [PubMed] [Google Scholar]; l Jung K.; Ahmed T. S.; Lee J.; Sung J. C.; Keum H.; Grubbs R. H.; Choi T. L. Living beta-selective cyclopolymerization using Ru dithiolate catalysts. Chem. Sci. 2019, 10, 8955–8963. 10.1039/C9SC01326A. [DOI] [PMC free article] [PubMed] [Google Scholar]; m Theunissen C.; Ashley M. A.; Rovis T. Visible-Light-Controlled Ruthenium-Catalyzed Olefin Metathesis. J. Am. Chem. Soc. 2019, 141, 6791–6796. 10.1021/jacs.8b13663. [DOI] [PMC free article] [PubMed] [Google Scholar]; n Song K.; Kim K.; Hong D.; Kim J.; Heo C. E.; Kim H. I.; Hong S. H. Highly Active Ruthenium Metathesis Catalysts Enabling Ring-Opening Metathesis Polymerization of Cyclopentadiene at Low Temperatures. Nat. Commun. 2019, 10, 3860. 10.1038/s41467-019-11806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Higman C. S.; Lummiss J. A. M.; Fogg D. E. Olefin Metathesis at the Dawn of Uptake in Pharmaceutical and Specialty Chemicals Manufacturing. Angew. Chem., Int. Ed. 2016, 55, 3552–3565. 10.1002/anie.201506846. [DOI] [PubMed] [Google Scholar]; b Yu M.; Lou S.; Gonzalez-Bobes F. Ring-Closing Metathesis in Pharmaceutical Development: Fundamentals, Applications, and Future Directions. Org. Process Res. Dev. 2018, 22, 918–946. 10.1021/acs.oprd.8b00093. [DOI] [Google Scholar]; c Farina V.; Horváth A.. Ring-Closing Metathesis in the Large-Scale Synthesis of Pharmaceuticals. In Handbook of Metathesis; Grubbs R. H., Wenzel A. G., Eds.; Wiley-VCH: Weinheim, 2015; Vol. 2, pp 633–658. [Google Scholar]; d Fandrick K. R.; Savoie J.; Jinhua N. Y.; Song J. J.; Senanayake C. H.. Challenges and Opportunities for Scaling the Ring-Closing Metathesis Reaction in the Pharmaceutical Industry. In Olefin Metathesis - Theory and Practice; Grela K., Ed.; Wiley: Hoboken, 2014; pp 349–366. [Google Scholar]; e For a recent example of an oncology drug candidate, see: St-Pierre G.; Cherney A. H.; Chen W.; Dong X.; Dornan P. K.; Griffin D. J.; Houk K. N.; Lin J. B.; Osgood S.; Elipe M. V. S.; Timmons H. C.; Xie Y.; Tedrow J. S.; Thiel O. R.; Smith A. G., Accelerated Development of a Scalable Ring-Closing Metathesis to Manufacture AMG 176 Using a Combined High-Throughput Experimentation and Computational Modeling Approach. Org. Process Res. Dev. 2020, ASAP article. 10.1021/acs.oprd.0c00416 [DOI] [Google Scholar]
- Masuda S.; Tsuda S.; Yoshiya T. Ring-closing metathesis of unprotected peptides in water. Org. Biomol. Chem. 2018, 16, 9364–9367. 10.1039/C8OB02778A. [DOI] [PubMed] [Google Scholar]
- Monty O. B. C.; Nyshadham P.; Bohren K. M.; Palaniappan M.; Matzuk M. M.; Young D. W.; Simmons N. Homogeneous and Functional Group Tolerant Ring-Closing Metathesis for DNA-Encoded Chemical Libraries. ACS Comb. Sci. 2020, 22, 80–88. 10.1021/acscombsci.9b00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlas S.; Lawrenson S. B.; Arkinstall L. A.; O’Reilly R. K.; Foster J. C. Self-assembled nanostructures from amphiphilic block copolymers prepared via ring-opening metathesis polymerization (ROMP). Prog. Polym. Sci. 2020, 107, 101278–101318. 10.1016/j.progpolymsci.2020.101278. [DOI] [Google Scholar]
- Foster J. C.; Varlas S.; Blackman L. D.; Arkinstall L. A.; O’Reilly R. K. Ring-Opening Metathesis Polymerization in Aqueous Media Using a Macroinitiator Approach. Angew. Chem., Int. Ed. 2018, 57, 10672–10676. 10.1002/anie.201806719. [DOI] [PubMed] [Google Scholar]
- a For a similar perspective in the context of ROMP, and the consequent advantages associated with high-strain monomers, see:Camm K. D.; Fogg D. E.. From Drug Cocktails to Tissue Engineering: Synthesis of ROMP Polymers for Biological Applications. In NATO Sci. Ser. II, Imamoglu Y.; Dragutan V., Eds. Springer Verlag: Berlin, 2007; Vol. 243, pp 285–303. [Google Scholar]
- The breakthrough discovery that a chalcogen relay (S-allyl or Se-allyl cysteine) can greatly accelerate metathesis relative to decomposition opened the door to site-selective protein modification. See ref (3a) and:; a Lin Y. A.; Chalker J. M.; Floyd N.; Bernardes G. J. L.; Davis B. G. Allyl Sulfides Are Privileged Substrates in Aqueous Cross-Metathesis: Application to Site-Selective Protein Modification. J. Am. Chem. Soc. 2008, 130, 9642–9643. 10.1021/ja8026168. [DOI] [PubMed] [Google Scholar]; b Lin Y. A.; Davis B. G. The Allylic Chalcogen Effect in Olefin Metathesis. Beilstein J. Org. Chem. 2010, 6, 1219–1228. Author: Superstoichiometric Ru is essential, however (for example, 200 equiv relative to the S-allylcysteine-tagged serine protease subtilisin Bacillus lentus in ref (13a)). Davis makes cogent arguments for this sacrifice in light of the strategic importance of the modified protein target (ref (3a)). Nevertheless, Vinogravoda notes that protected peptides and solid-phase synthesis are widely employed in olefin metathesis of peptidic substrates, to circumvent decomposition. See: ref (3b). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bimolecular decomposition of the four-coordinate intermediates RuCl2(L)(=CH2) has now been established for phosphine, NHC, and CAAC derivatives. See:; a Bailey G. A.; Foscato M.; Higman C. S.; Day C. S.; Jensen V. R.; Fogg D. E. Bimolecular Coupling as a Vector for Decomposition of Fast-Initiating Olefin Metathesis Catalysts. J. Am. Chem. Soc. 2018, 140, 6931–6944. 10.1021/jacs.8b02709. [DOI] [PubMed] [Google Scholar]; b Nascimento D. L.; Fogg D. E. Origin of the Breakthrough Productivity of Ruthenium-CAAC Catalysts in Olefin Metathesis (CAAC = Cyclic Alkyl Amino Carbene). J. Am. Chem. Soc. 2019, 141, 19236–19240. 10.1021/jacs.9b10750. [DOI] [PubMed] [Google Scholar]; c Nascimento D. L.; Gawin A.; Gawin R.; Guńka P. A.; Zachara J.; Skowerski K.; Fogg D. E. Integrating Activity with Accessibility in Olefin Metathesis: An Unprecedentedly Reactive Ruthenium-Indenylidene Catalyst Bearing a Cyclic Alkyl Amino Carbene. J. Am. Chem. Soc. 2019, 141, 10626–10631. 10.1021/jacs.9b05362. [DOI] [PubMed] [Google Scholar]; d Thiel V.; Wannowius K.-J.; Wolff C.; Thiele C. M.; Plenio H. Ring-Closing Metathesis Reactions: Interpretation of Conversion-Time Data. Chem. - Eur. J. 2013, 19, 16403–16414. 10.1002/chem.201204150. [DOI] [PubMed] [Google Scholar]; e Schrodi Y.Mechanisms of Olefin Metathesis Catalyst Decomposition and Methods of Catalyst Reactivation. In Handbook of Metathesis; Grubbs R. H., Wenzel A. G., Eds.; Wiley-VCH: Weinheim, 2015; pp 323–342. Of keen relevance to metathesis in basic water, bimolecular coupling contributes to complete inactivation of hydroxide derivative Ru(OH)2(L)(=CH2). [Google Scholar]; f See:Goudreault A. Y.; Walden D. M.; Nascimento D. L.; Botti A. G.; Steinmann S. N.; Michel C.; Fogg D. E. Hydroxide-Induced Degradation of Olefin Metathesis Catalysts: A Challenge for Metathesis in Alkaline Media. ACS Catal. 2020, 10, 3838–3843. 10.1021/acscatal.9b05163. [DOI] [Google Scholar]
- Lu X.; Fan L.; Phelps C. B.; Davie C. P.; Donahue C. P. Ruthenium Promoted On-DNA Ring-Closing Metathesis and Cross-Metathesis. Bioconjugate Chem. 2017, 28, 1625–1629. 10.1021/acs.bioconjchem.7b00292. [DOI] [PubMed] [Google Scholar]
- a For an overview of these challenges, and examples of unintended C=C positional isomerism, see:van Lierop B. J.; Lummiss J. A. M.; Fogg D. E.. Ring-Closing Metathesis. In Olefin Metathesis-Theory and Practice; Grela K., Ed.; Wiley: Hoboken, NJ, 2014; pp 85–152. [Google Scholar]; b For studies identifying some of the Ru species responsible, and excluding some commonly-proposed candidates, see:Higman C. S.; Lanterna A. E.; Marin M. L.; Scaiano J. C.; Fogg D. E. Catalyst Decomposition During Olefin Metathesis Yields Isomerization-Active Ru Nanoparticles. ChemCatChem 2016, 8, 2446–2449. 10.1002/cctc.201600738. [DOI] [Google Scholar]; c Higman C. S.; Plais L.; Fogg D. E. Isomerization During Olefin Metathesis: An Assessment of Potential Catalyst Culprits. ChemCatChem 2013, 5, 3548–3551. 10.1002/cctc.201300886. [DOI] [Google Scholar]
- For a recent study of the negative impact of water on Hoveyda-class catalysts (including Ru-8 and water-soluble variants), and a potential solution, see:; a Jongkind L. J.; Rahimi M.; Poole D.; Ton S. J.; Fogg D. E.; Reek J. N. H. Protection of Ruthenium Olefin Metathesis Catalysts by Encapsulation in a Self-assembled Resorcinarene Capsule. ChemCatChem 2020, 12, 4019–4023. 10.1002/cctc.202000111. [DOI] [Google Scholar]; b For the mechanism by which water decomposes the second-generation Grubbs catalyst Ru-11 and other PCy3-stabilized catalysts, see:McClennan W. L.; Rufh S. A.; Lummiss J. A. M.; Fogg D. E. A General Decomposition Pathway for Phosphine-Stabilized Metathesis Catalysts: Lewis Donors Accelerate Methylidene Abstraction. J. Am. Chem. Soc. 2016, 138, 14668–14677. 10.1021/jacs.6b08372. [DOI] [PubMed] [Google Scholar]; c For the slower decomposition of the first-generation Grubbs catalyst by this pathway, see:Lummiss J. A. M.; McClennan W. L.; McDonald R.; Fogg D. E. Donor-Induced Decomposition of the Grubbs Catalysts: An Intercepted Intermediate. Organometallics 2014, 33, 6738–6741. 10.1021/om501011y. [DOI] [Google Scholar]; d For studies showing quenching of metathesis by hydroxide ion via formation of inactive Ru-OH species, see ref (14f) and:Santos A. G.; Bailey G. A.; dos Santos E. N.; Fogg D. E. Overcoming Catalyst Decomposition in Acrylate Metathesis: Poly-phenol Resins as Enabling Agents for Phosphine-Stabilized Metathesis Catalysts. ACS Catal. 2017, 7, 3181–3189. Recent work reveals that added HCl improves the performance of related Ru catalysts even at neutral pH: reversal of Ru-OH formation was proposed. See: ref (6b). [Google Scholar]
- For an early review noting that water is not an innocuous medium for Ru-promoted ROMP chemistry, and that success is determined by the kinetics of polymerization vs catalyst decomposition, see ref (12). For other early reports of the negative effect of water in Ru-catalyzed olefin metathesis, see:; a Kim M.; Eum M. S.; Jin M. Y.; Jun K. W.; Lee C. W.; Kuen K. A.; Kim C. H.; Chin C. S. Reactions of Ruthenium Benzylidenes with H2O to give Benzaldehyde and (Aqua)ruthenium complex. J. Organomet. Chem. 2004, 689, 3535–3540. 10.1016/j.jorganchem.2004.08.017. [DOI] [Google Scholar]; b Dinger M. B.; Mol J. C. Degradation of the First-Generation Grubbs Metathesis Catalyst with Primary Alcohols, Water, and Oxygen. Formation and Catalytic Activity of Ruthenium(II) Monocarbonyl Species. Organometallics 2003, 22, 1089–1095. 10.1021/om0208218. [DOI] [Google Scholar]
- The limiting solubility of water in toluene is reported as 0.05% v/v. See:; Kirchnerová J.; Cave C. B. G. The Solubility of Water in Low-Dielectric Solvents. Can. J. Chem. 1976, 54, 3909–3916. 10.1139/v76-562. [DOI] [Google Scholar]
- See ref (7a) and:; a Morse J. S.; Lalonde T.; Xu S.; Liu W. R. Learning from the Past: Possible Urgent Prevention and Treatment Options for Severe Acute Respiratory Infections Caused by 2019-nCoV. ChemBioChem 2020, 21, 730–738. 10.1002/cbic.202000047. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Schwartz A. D.; Graham L. A. Potential Maternal and Infant Outcomes from Coronavirus 2019-nCoV (SARS-CoV-2) Infecting Pregnant Women: Lessons from SARS, MERS, and Other Human Coronavirus Infections. Viruses 2020, 12, 194. 10.3390/v12020194. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Cink R. D.; Lukin K. A.; Bishop R. D.; Zhao G.; Pelc M. J.; Towne T. B.; Gates B. D.; Ravn M. M.; Hill D. R.; Ding C.; Cullen S. C.; Mei J. Z.; Leanna M. R.; Henle J.; Napolitano J. G.; Nere N. K.; Chen S.; Sheikh A.; Kallemeyn J. M. Development of the Enabling Route for Glecaprevir via Ring-Closing Metathesis. Org. Process Res. Dev. 2020, 24, 183–200. 10.1021/acs.oprd.9b00469. [DOI] [Google Scholar]; d Caspi D. D.; Cink R. D.; Clyne D.; Diwan M.; Engstrom K. M.; Grieme T.; Mei J. Z.; Miller R. W.; Mitchell C.; Napolitano J. G.; Nere N.; Ravn M. M.; Sheikh A.; Wagaw S.; Zhang H. Q. Process Development of ABT-450. A First Generation NS3/4A Protease Inhibitor for HC. Tetrahedron 2019, 75, 4271–4286. 10.1016/j.tet.2019.05.064. [DOI] [Google Scholar]; e Horvath A.; Depre D.; Vermeulen W. A. A.; Wuyts S. L.; Harutyunyan S. R.; Binot G.; Cuypers J.; Couck W.; Van den Heuvel D. Ring-Closing Metathesis on Commercial Scale: Synthesis of HCV Protease Inhibitor Simeprevir. J. Org. Chem. 2019, 84, 4932–4939. 10.1021/acs.joc.8b03124. [DOI] [PubMed] [Google Scholar]
- Higman C. S.; Nascimento D. L.; Ireland B. J.; Audorsch S.; Bailey G. A.; McDonald R.; Fogg D. E. Chelate-Assisted Ring-Closing Metathesis: A Strategy for Accelerating Macrocyclization at Ambient Temperatures. J. Am. Chem. Soc. 2018, 140, 1604–1607. 10.1021/jacs.7b13257. [DOI] [PubMed] [Google Scholar]
- Water content is typically < 5 ppm, as indicated by regular Karl Fischer titration.
- Kraft P.Aroma Chemicals IV: Musks. In Chemistry and Technology of Flavors and Fragrances; Rowe D. J., Ed.; CRC Press: Boca Raton, 2005; pp 143–168. [Google Scholar]
- a Conrad J. C.; Eelman M. D.; Duarte Silva J. A.; Monfette S.; Parnas H. H.; Snelgrove J. L.; Fogg D. E. Oligomers as Intermediates in Ring-Closing Metathesis. J. Am. Chem. Soc. 2007, 129, 1024–1025. 10.1021/ja067531t. [DOI] [PubMed] [Google Scholar]; b Monfette S.; Fogg D. E. Equilibrium Ring-Closing Metathesis. Chem. Rev. 2009, 109, 3783–3816. 10.1021/cr800541y. [DOI] [PubMed] [Google Scholar]
- Higher concentrations can be tolerated where partial rigidity creates a conformational bias toward cyclization. In the production of peptidomimetic macrocycles as drug candidates for the treatment of Hepatitis C, the substrate concentrations at which mRCM could be achieved ranged from 2,4 mM for Idenex’s IDX320, to a remarkable 0.2 M (for Boehringer-Ingelheim’s Ciluprevir). See: ref (7a). The latter was due to a conformational bias toward cyclization, reinforced by the serendipitous sensitivity of the effective molarity to protection of a key amide site.
- a Fürstner A.; Langemann K. Conformationally Unbiased Macrocyclization Reactions by Ring Closing Metathesis. J. Org. Chem. 1996, 61, 3942–3943. 10.1021/jo960733v. [DOI] [PubMed] [Google Scholar]; b Fürstner A.; Langemann K. Macrocycles by Ring-Closing Metathesis. Synthesis 1997, 1997, 792–803. 10.1055/s-1997-4472. [DOI] [Google Scholar]
- Even at 1 mol% Ru-2, however, mRCM yields remained well below those of the anhydrous reaction: 73%, vs 92%. See Figure S1.
- For studies demonstrating that O2 is less detrimental than air, see:; a Guidone S.; Songis O.; Nahra F.; Cazin C. S. J. Conducting Olefin Metathesis Reactions in Air: Breaking the Paradigm. ACS Catal. 2015, 5, 2697–2701. 10.1021/acscatal.5b00197. [DOI] [Google Scholar]; b Ton S. J.; Fogg D. E. The Impact of Oxygen on Leading and Emerging Ru-Carbene Catalysts for Olefin Metathesis: An Unanticipated Correlation Between Robustness and Metathesis Activity ACS Catal. ACS Catal. 2019, 9, 11329–11334. 10.1021/acscatal.9b03285. [DOI] [Google Scholar]
- Tracz A.; Matczak M.; Urbaniak K.; Skowerski K. Nitro-Grela-Type Complexes Containing Iodides - Robust and Selective Catalysts for Olefin Metathesis Under Challenging Conditions. Beilstein J. Org. Chem. 2015, 11, 1823–1832. 10.3762/bjoc.11.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoveyda A. H.; Lombardi P. J.; O’Brien R. V.; Zhugralin A. R. H-Bonding as a Control Element in Stereoselective Ru-Catalyzed Olefin Metathesis. J. Am. Chem. Soc. 2009, 131, 8378–8379. 10.1021/ja9030903. [DOI] [PubMed] [Google Scholar]
- Torker S.; Khan R. K. M.; Hoveyda A. H. The Influence of Anionic Ligands on Stereoisomerism of Ru Carbenes and Their Importance to Efficiency and Selectivity of Catalytic Olefin Metathesis Reactions. J. Am. Chem. Soc. 2014, 136, 3439–3455. 10.1021/ja410606b. [DOI] [PubMed] [Google Scholar]
- For the slow initiation of Ru metathesis catalysts containing cis-chelating pseudohalide ligands, see:; Monfette S.; Camm K. D.; Gorelsky S. I.; Fogg D. E. Electronic Effects of the Anionic Ligand in Ruthenium-Catalyzed Olefin Metathesis. Organometallics 2009, 28, 944–946. 10.1021/om900006f. [DOI] [Google Scholar]
- A slight vacuum was introduced to protect these lower-productivity catalysts against ethylene-induced decomposition. For a recent overview of the problem, see:; a Hoveyda A. H.; Liu Z.; Qin C.; Koengeter T.; Mu Y. Impact of Ethylene on Efficiency and Stereocontrol in Olefin Metathesis: When to Add It, When to Remove It, and When to Avoid It. Angew. Chem., Int. Ed. 2020, 59, 22324. 10.1002/anie.202010205. [DOI] [PubMed] [Google Scholar]; b For the detrimental effect of ethylene on mRCM of 1, see:Monfette S.; Eyholzer M.; Roberge D. M.; Fogg D. E. Getting RCM Off the Bench: Reaction-Reactor Matching Transforms Metathesis Efficiency in the Assembly of Large Rings. Chem. - Eur. J. 2010, 16, 11720–11725. 10.1002/chem.201001210. [DOI] [PubMed] [Google Scholar]
- Abbas M.; Slugovc C. As Low as Reasonably Achievable Catalyst Loadings in the Cross Metathesis of Olefins with Ethyl Acrylate. Tetrahedron Lett. 2011, 52, 2560–2562. 10.1016/j.tetlet.2011.03.038. [DOI] [Google Scholar]
- Nechmad N. B.; Phatake R.; Ivry E.; Poater A.; Lemcoff N. G. Unprecedented Selectivity of Ruthenium Iodide Benzylidenes in Olefin Metathesis Reactions. Angew. Chem., Int. Ed. 2020, 59, 3539–3543. 10.1002/anie.201914667. [DOI] [PubMed] [Google Scholar]
- Neither Z-selective catalyst reacted with 2, presumably owing to steric clashes with the homoallylic ester groups, as they react with allylbenzene and the prolactone 1. They are therefore omitted from Figure 3.
- Least active is the first-generation Hoveyda catalyst Ru-7, for which TON at 2 h = 600 when water is present. The water-tolerance of Ru-7 was therefore reassayed at 24 h (TON 13 300). Its water-tolerance was maintained: 44%, vs 42% at 2 h.
- Literature evidence for slow initiation is given in Table S3. While direct comparisons are limited, the Grubbs group established that the observed rate constants for initiation of a series of CAAC catalysts was on the order of 10–3 s–1 and below, even at 60 °C. See; a Engle K. M.; Lu G.; Luo S.-X.; Henling L. M.; Takase M. K.; Liu P.; Houk K. N.; Grubbs R. H. Origins of Initiation Rate Differences in Ruthenium Olefin Metathesis Catalysts Containing Chelating Benzylidenes. J. Am. Chem. Soc. 2015, 137, 5782–5792. 10.1021/jacs.5b01144. [DOI] [PubMed] [Google Scholar]; b Marx V. M.; Sullivan A. H.; Melaimi M.; Virgil S. C.; Keitz B. K.; Weinberger D. S.; Bertrand G.; Grubbs R. H. Cyclic Alkyl Amino Carbene (CAAC) Ruthenium Complexes as Remarkably Active Catalysts for Ethenolysis. Angew. Chem., Int. Ed. 2015, 54, 1919–1923. For context, initiation of Ru-10 and Ru-9 was slower than Ru-4 by 225× and 900×, respectively, at RT. See: ref (14c). [DOI] [PMC free article] [PubMed] [Google Scholar]
- For the slow initiation of Ru-11 even at 80 °C, see:; a Sanford M. S.; Love J. A.; Grubbs R. H. Mechanism and Activity of Ruthenium Olefin Metathesis Catalysts. J. Am. Chem. Soc. 2001, 123, 6543–6554. 10.1021/ja010624k. [DOI] [PubMed] [Google Scholar]; b Re-entry into catalysis is much slower for the resting-state methylidene species formed by Ru-11 and the indenylidene catalyst Ru-9, by 275× in the case of Ru-11. SeeLummiss J. A. M.; Higman C. S.; Fyson D. L.; McDonald R.; Fogg D. E. The Divergent Effects of Strong NHC Donation in Catalysis. Chem. Sci. 2015, 6, 6739–6746. 10.1039/C5SC02592C. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Lummiss J. A. M.; Perras F. A.; McDonald R.; Bryce D. L.; Fogg D. E. Sterically-Driven Metathesis: The Impact of Alkylidene Substitution on the Reactivity of the Grubbs Catalysts. Organometallics 2016, 35, 691–698. 10.1021/acs.organomet.5b00984. [DOI] [Google Scholar]
- Wappel J.; Urbina-Blanco C. A.; Abbas M.; Albering J. H.; Saf R.; Nolan S. P.; Slugovc C. Halide Exchanged Hoveyda-Type Complexes in Olefin Metathesis. Beilstein J. Org. Chem. 2010, 6, 1091–1098. 10.3762/bjoc.6.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. G. M.; Hamprecht D.; James R. A.; Ohkubo M.; Procopiou P. A.; Toledo M. A.; White A. J. P.; Williams D. J. Synthesis and Characterization of Coronanes: Multicyclopropane-Fused Macrocyclic Arrays. J. Org. Chem. 2001, 66, 2187–2196. 10.1021/jo001167d. [DOI] [PubMed] [Google Scholar]
- Smith J. S.; Isayev O.; Roitberg A. E. ANI-1: an extensible neural network potential with DFT accuracy at force field computational cost. Chem. Sci. 2017, 8, 3192–3203. 10.1039/C6SC05720A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The new neural network force field ANAKIN (ref (42)) affords the best accuracy-to-cost ratio for organic molecules, with ab initio-quality energies and geometries, for the reduced computational cost of classic force-field simulations.
- In the equilibrium between the extended conformer and its precyclic form, the added water molecule favors the extended conformer by 100-fold for 1, vs 5 orders of magnitude for diethyl diallylmalonate 9. For anhydrous 1: ΔG = 5.8 kcal/mol (i.e., K = 5.5 × 10–5). With 1 H2O: ΔG = 8.5 kcal/mol (i.e., K = 5.7 × 10–7). For anhydrous 9: ΔG = 0 kcal/mol (K = 1). With 1 H2O: ΔG = 5.6 kcal/mol (K = 8.10–5).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




