Abstract
After the initial description of extrarenal synthesis of 1,25-dihydroxyvitamin D (1,25-(OH)2D) three decades ago, extensive progress has been made in unraveling the immunomodulatory roles of vitamin D in the pathogenesis of granulomatous disorders, including sarcoidosis. It has been shown that 1,25-(OH)2D has dual effects on the immune system, including upregulating innate immunity as well as downregulating the autoimmune response. The latter mechanism plays an important role in the pathogenesis and treatment of sarcoidosis. Vitamin D supplementation in patients with sarcoidosis has been hampered owing to concerns about the development of hypercalcemia and hypercalciuria given that extrarenal 1-α hydroxylase is substrate dependent. Recently, a few studies have cast doubt over the mechanisms underlying the development of hypercalcemia in this population. These studies demonstrated an inverse relationship between the level of vitamin D and severity of sarcoidosis. Consequently, clinical interest has been piqued in the use of vitamin D to attenuate the autoimmune response in this disorder. However, the development of hypercalcemia and the attendant detrimental effects are real possibilities. Although the average serum calcium concentration did not change following vitamin D supplementation, in two recent studies, hypercalciuria occurred in one out of 13 and two out of 16 patients. This review is a concise summary of the literature, outlining past work and newer developments in the use of vitamin D in sarcoidosis. We feel that larger-scale placebo-controlled randomized studies are needed in this population. Since the current first-line treatment of sarcoidosis is glucocorticoids, which confer many systemic adverse effects, and steroid-sparing immunosuppressant treatment options carry additional risks of adverse effects, adjunct management with vitamin D in combination with potent anti-osteoporotic medications could minimize the risk of glucocorticoid-induced osteoporosis and modulate the immune system to attenuate disease activity in sarcoidosis.
Keywords: Sarcoidosis, hypercalcemia, kidney stones, vitamin D, autoimmune
Introduction
Under normal circumstances, renal 1α-hydroxylase converts 25-hydroxyvitamin D (25(OH)D) to 1,25-dihydroxyvitamin D (1,25-(OH)2D). Evidence for increased circulating 1,25-(OH)2D and dysregulation of calcium metabolism in sarcoidosis was initially described in 19791,2. The underlying mechanisms for hypercalcemia were attributed to increased production and/or decreased catabolism of 1,25-(OH)2D. Several studies have indicated enhanced intestinal calcium absorption as the main mechanism for the development of hypercalcemia, hypercalciuria, nephrocalcinosis, and abnormal kidney function3–6. A case report of hypercalcemia in an anephric patient with sarcoidosis was the first evidence for extrarenal production of 1,25-(OH)2D7. Subsequent studies using cultured pulmonary alveolar macrophages and sarcoid lymph node homogenates explored the relationship between 25(OH)D and 1-α hydroxylase gene expression8–10. Positive findings in the alveolar macrophages support the extrarenal conversion of 25(OH)D to 1,25-(OH)2D in sarcoidosis patients8–10. These studies were pivotal in unraveling the immunomodulatory role of 1,25-(OH)2D in granulomatous diseases such as sarcoidosis and tuberculosis11. Here, we review evidence in two distinct ethnic populations with sarcoidosis; both sarcoidosis and vitamin D deficiency are more common in African Americans (AA) than in Caucasians12. Moreover, clinical, biochemical, and mineral metabolic perturbations of sarcoidosis in these two distinct ethnicities were similar. Repletion of vitamin D in a small subset of vitamin D-deficient patients with sarcoidosis was found to be safe, effective, and associated with a fall in surrogate markers of sarcoidosis activity and, interestingly, with a fall in serum 1,25-(OH)2D12. The underlying mechanism(s) of the observed response to vitamin D repletion in this population and the role of 24-hydroxylase in mediating this response deserve further investigation.
Epidemiology of sarcoidosis
Sarcoidosis is a multi-system inflammatory disorder associated with widespread granuloma deposition leading to multi-organ impairment. The complex causative factors dictate its heterogeneous presentation13,14. Geography, race, age, and gender modulate the presentation of sarcoidosis, and it is most prevalent in the Northern hemisphere and among AA individuals15–17. Studies in Northern Europe and Japan have described a bimodal age-specific incidence rate among women, with a first peak between 30 and 35 years of age and a second peak over 50 years of age18–20.
The incidence and prevalence of sarcoidosis was greater in females than in males in most but not all studies21,22. One study in Switzerland found no gender differences in incidence and prevalence of sarcoidosis23, while another study in Sweden found a higher incidence in men than in women15. The incidence of sarcoidosis in men peaks before 40 years of age; the incidence in women remains flat between the ages of 30 and 60 years24,25. The higher prevalence and severity of sarcoidosis among the AA population was purported to be associated with lower vitamin D stores in this group26–28. This notion was based on the immunomodulatory effects of vitamin D on the adaptive immune system, which is responsible for the suppression of granulomatous inflammation29. However, our study in Dallas12 comprising a total of 86 patients with sarcoidosis from two separate ethnic groups from the United States (93% AA) and Italy (95% Caucasian) showed no difference in the clinical, biochemical, vitamin D, and mineral profiles in these two distinct populations12.
Renal synthesis and classical action of 1,25-(OH)2D
Vitamin D3 (cholecalciferol) is synthesized from exposure to ultraviolet radiation transforming 7-dehydrocholestrol in the skin to vitamin D330–32. In addition, vitamin D3 is found in the circulation following the consumption of fortified dairy products, orange juice, and fish. Vitamin D2 (ergocalciferol) is also synthesized from ergosterol by exposure to ultraviolet radiation. Vitamin D2 is found naturally in mushrooms33, vitamin D supplements, and with food fortification. Nonetheless, both vitamin D2 and vitamin D3 undergo the same metabolic pathway.
Circulating vitamin D is initially hydroxylated by three different 25-hydroxylase enzymes (CYP27A1, CYP2R1, and CYP3A4)34 in the liver, producing 25(OH)D. 25(OH)D undergoes another hydroxylation by 25(OH)D-1-α hydroxylase (CYP27B1) in the kidney, resulting in the production of 1,25-(OH)2D, the most active vitamin D metabolite35. 25(OH)D and 1,25(OH)2D also undergo 24-hydroxylation by 24-hydroxylase (CYP24A1) to produce inactive metabolites35 24,25-(OH)2D and 1,24,25-trihydroxyvitamin D35, respectively. The activity of renal CYP27B1 is tightly regulated. Parathyroid hormone (PTH) and hypophosphatemia stimulate 25(OH)D-1-α hydroxylase (CYP27B1) activity36–39. Fibroblast growth factor 23 (FGF23) decreases CYP27B1 activity and stimulates CYP24A1 action40.
Similar to other steroidal hormones, 1,25-(OH)2D acts upon a specific intracellular vitamin D receptor (VDR) to fulfill major classical functions on the target organs41, including the enhancement of intestinal calcium, phosphate absorption, regulation of osteoclastic bone resorption, and osteoblastic bone formation42–44 (Figure 1).
Figure 1. Classical vitamin D metabolism and actions.
(A) 7-Dehydrocalciferol is transformed by UV light exposure to vitamin D in the skin. (B) In the liver, vitamin D is converted to 25-hydroxyvitamin-D (25-(OH)D) by three different 25-hydroxylase enzymes (CYP27A1, CYP2R1, and CYP3A4). (C) In the kidney, 25-(OH)D is converted by 25-(OH)D-1-α hydroxylase (CYP27B1) to 1,25-dihydroxyvitamin-D (1,25-(OH)2D); this conversion is stimulated by low serum phosphate and high parathyroid hormone (PTH) and inhibited by fibroblast growth factor 23 (FGF23). Both 25-(OH)D and 1,25-(OH)2D can be converted to the inactive metabolites 24,25-(OH)2D and 1,24,25-(OH)3D by the enzyme CYP24A1, which is stimulated by 1,25-(OH)2D and FGF23. (D) 1,25-(OH)2D stimulates both intestinal calcium (Ca) absorption (exceeding excretion) and osteoclastic bone resorption, leading to increased serum Ca concentration. Both 1,25-(OH)2D itself and the increased serum Ca inhibit PTH secretion; the lower PTH secretion in turn inhibits renal tubular Ca reabsorption. (E) A higher serum Ca increases renal filtered load of Ca and also reduces renal tubular Ca reabsorption. These combined actions result in a net increase in urinary Ca excretion.
Extra renal synthesis and non-classical action of 1,25-(OH)2D
It has been shown that monocytes, dendritic cells (DCs), macrophages, B-cells, and T-cells possess 25(OH)D-1-α hydroxylase activity locally to transform 25(OH)D into 1,25-(OH)2D45–52. Similar to its classical action, the immunomodulatory effect of vitamin D (non-classical action) is exerted via the VDR within the immunomodulatory cells53–59. This immunomodulatory function plays a key role in the pathogenesis of granulomatous disorders, specifically in sarcoidosis and tuberculosis11,58.
Unlike renal (CYP27B1) activity, extrarenal 25(OH)D-1-α hydroxylase CYP27B1 activity is highly substrate dependent, and its production is significantly affected by the prevailing serum concentration of 25(OH)D60. Therefore, the local production of 1,25-(OH)2D is deficient when serum concentration of 25(OH)D is low (below 25 ng/mL)60.
Vitamin D and innate immunity
The effect of vitamin D on the innate immune response is exerted via antigen presentation by macrophages or DCs, the principal cells which mediate the anti-bacterial effect against Mycobacterium tuberculosis (M.tb)61. The ability of monocytes and macrophages to attack M.tb was dependent not only on M.tb phagocytosis but also on sensing pathogen-associated molecular patterns (PAMPS) via specific pattern-recognition receptors (PRRs) such as Toll-like receptors (TLRs)62. TLRs are a family of noncatalytic transmembrane PRRs that interacts with specific PAMPS63,64. It was shown that intracrine induction of CYP27B1 and VDR by monocytes follows PAMP sensing by TLRs65. This interaction results in the production of cathelicidins, a class of host defense peptides that facilitate microbial killing61,66,67 (Figure 2). Granuloma formation may result from defects in innate immunity that impair the elimination of inciting antigens68. Since mycobacterial antigens have been implicated in the pathogenesis of sarcoidosis, several studies have suggested that the cathelicidins act as a bridge between sarcoidosis and tuberculosis69, and deficiency of cathelicidins in macrophages has been implicated in severe tuberculosis and sarcoidosis70. In this study, alveolar macrophage–cathelicidin mRNA expression, VDR, and the VDR coactivator steroid receptor coactivator-3 (SRC3) were measured by quantitative PCR in alveolar macrophages from bronchoalveolar lavage in patients with biopsy-proven sarcoidosis and healthy controls. Results showed reduced alveolar macrophage expression of cathelicidin and SRC3 in severe but not in non-severe sarcoidosis patients compared to controls70. Further in vitro studies showed that tumor necrosis factor (TNF)-α (a vitamin D3 antagonist) mediates the suppression of SRC3, leading to alveolar macrophage cathelicidin deficiency in severe sarcoidosis.
Figure 2. Activation and anti-microbial effects of vitamin D in macrophages.
1) Circulating 25-hydroxyvitamin-D (25-(OH)D) binds to plasma vitamin D-binding protein and enters macrophage. 2) 25-(OH)D is converted into 1,25-dihydroxyvitamin-D (1,25-(OH)2D) by mitochondrial CYP27B1. 3) 1,25-(OH)2D binds to vitamin D receptor and enters the nucleus. 4) The vitamin D receptor–1,25-(OH)2D complex acts as a transcriptional factor, resulting in the expression of cathelicidins. 5) Cathelicidins are incorporated into phagosomes containing internalized pathogens to function as a bactericidal agent.
In a recent prospective case-control study, serum 25(OH)D and cathelicidin levels were measured in 30 patients with active pulmonary tuberculosis, 30 patients with sarcoidosis, and 20 healthy control subjects. Results showed severe vitamin D deficiency in 47% of patients with sarcoidosis compared with 3% in those with tuberculosis69. Moreover, cathelicidin levels were significantly higher in control subjects than in sarcoidosis or tuberculosis patients; there was no significant difference in cathelicidin levels between tuberculosis and sarcoidosis patients69. An optimum cathelicidin cut-off value of 107.14 pg/mL, with a sensitivity of 81.5% and specificity of 71.2%, was found to differentiate sarcoidosis patients from healthy control subjects.
Vitamin D and adaptive immunity
Vitamin D action via adaptive immunity may attenuate overzealous inflammatory responses, thus protecting against tissue damage. This action has been demonstrated through downregulation of TLR2 and TLR4 expression in monocytes71 and attenuation of T-helper type 1 (Th1) lymphocytes known to increase autoimmune response64. The beneficial effects of 1,25-(OH)2D are exerted by balancing the proinflammatory cytokines by Th1 cells that produce pro-inflammatory cytokines (including IL-2, IFN-γ, and TNF-α) and Th2 cells that produce anti-inflammatory cytokines (including IL-3, Il-4, IL-5, and IL-10)61,72–75 (Figure 2). In addition, two other T-cell groups, Th17 and T-regulatory cells, play a role in the suppression of autoimmunity76–78 in response to 1,25-(OH)2D. Given the anti-microbial and anti-inflammatory properties of 1,25-(OH)2D, it has been suggested that extra renal hydroxylation of 25-OH-D and 1,25-(OH)2D represents an adaptive response aimed at minimizing inflammation, protecting against tissue destruction, and eliminating the inciting antigens that cause sarcoidosis79.
Vitamin D in sarcoidosis: a current paradigm
Sarcoidosis and vitamin D deficiency are more common and more severe in AA than in Caucasians in the US26–28. Previous studies have shown an inverse association between serum 25-(OH)D and severity of sarcoidosis activity80–82. Excess 1,25-(OH)2D has been perceived as detrimental, mainly because of concerns for the development of hypercalcemia. However, it is possible that excess production of 1,25-(OH)2D in sarcoidosis is an adaptive immune mechanism to mitigate granulomatous inflammation by promoting the removal of inciting antigen to protect tissue integrity. Concern over complications of vitamin D supplementation at the dosage normally insufficient to induce alteration in serum and urinary calcium levels in healthy subjects has hampered research into the role of vitamin D supplementation in sarcoidosis81,83,84. However, the incidence of hypercalcemia in sarcoidosis spans a wide range in different populations. In recent studies, the incidence of hypercalcemia ranges from 5.2–7.7% in sarcoidosis patients treated with calcium and vitamin D supplementation12,80,85.
Limited studies have addressed mineral status before and after treatment with calcium and vitamin D (Table 1). In 1979, Bell et al. implicated 1,25-(OH)2D in abnormal calcium metabolism in three patients with sarcoidosis1; treatment with prednisone corrected hypercalcemia and normalized serum 1,25-(OH)2D. To test whether the development of hypercalcemia is due to the increased sensitivity to vitamin D, seven normal subjects and seven sarcoidosis patients were challenged with oral vitamin D (10,000 IU daily) for 12 days. In this short-term study, vitamin D administration did not change serum calcium, 1,25-(OH)2D, or urinary calcium in normal subjects, while serum calcium, 1,25-(OH)2D, and urine calcium increased in sarcoidosis patients. The investigators concluded that abnormal calcium metabolism reflects impaired regulation of the synthesis or catabolism of 1,25-(OH)2D.
Table 1. Studies of vitamin D and Ca supplementation in sarcoidosis.
| First author | Mark J. Bolland85 | Giovanna Capolongo12 | Lieke S. Kamphuis80 | Norman H. Bell1 | ||
| Year | 2013 | 2016 | 2014 | 1979 | ||
| Design | Randomized, placebo controlled |
Non-randomized | Retrospective | Non-randomized | ||
| Number of patients | 27 | 86 | 301 | 7 | ||
| Type of patients | Normocalcemic sarcoidosis with 25-(OH)D <50 nmol |
Sarcoidosis patients with serum 25-(OH)D <75 nmol/L |
Sarcoidosis patients | 4 normocalcemic patients and 3 patients with history of hypercalcemia |
||
| Age (years) | 57 | 51±6.7 | Unknown | 22–64 | ||
| Female number (%) | 19 (70%) | 15 (93.7%) | 174 (58%) | 7 (50%) | ||
| Race/ethnicity | European (77%) Indian (8%) Other (8%) |
African American (88%) Caucasian (12%) |
Unknown | |||
| Number of patients treated with vitamin D | 13 | 16 | 104 | 7 | ||
| Intervention | ||||||
|---|---|---|---|---|---|---|
| Type of vitamin D | Cholecalciferol (vitamin D3) | Ergocalciferol (vitamin D2) |
Vitamin D2 in propylene glycol was given daily as a single dose |
|||
| Dose/frequency/duration | 50,000 IU weekly for 4 weeks then monthly for 11 months |
50,000 IU once a week for 12 weeks |
10,000 IU daily for 12 days | |||
| Diet | Usual diet | Usual diet | Usual Diet | Constant metabolic diet | ||
| Glucocorticoid use | 54% past oral use 8% current oral use 46% current inhaled use |
48% US patients 47% Italian patients |
40% of hypercalcemic patients |
Unknown | ||
| Baseline laboratory parameters | Vitamin D (n = 13) |
Placebo (n = 14) |
Pre-vitamin D (n = 16) |
Ca and vitamin D supplementation (n = 104) |
Normal subjects (n = 7) |
Patients with normal Ca metabolism (n = 4) |
| 25-(OH)D (nmol/L) | 40±17 | 45±17 | 42±13 | 46 | 67.4±14.9 | 37.4±12.4 |
| 1,25-(OH)2D (pmol/L) | 109±34 | 116±25 | 94±30 | 114 | 72.±7.2 | 70±7.2 |
| Serum phosphorus (mmol/L) | 1.23±0.15 | 1.06±0.17 | 1.1 | |||
| Serum Ca (mmol/L) | 2.24±0.06 | 2.26±0.12 | 2.38±0.05 | 2.39 | 2.37±0.05 | 2.32±0.05 |
| Parathyroid hormone (pmol/L) | 4.0±1.6 | 4.9±2.0 | 5.8±3.1 | |||
| Urinary Ca (mmol/day) | 4.6±3.4 | 6.6±5.2 | 3.4±2.3 | 5.17±0.55 | 3.42±0.45 | |
| Outcome laboratory | Post-vitamin D repletion (n = 13) | Placebo (n = 14) |
Post-vitamin D repletion (n = 16) |
Ca and vitamin D supplementation (n = 104) |
Normal subjects (n = 7) |
Sarcoidosis patients with normal Ca metabolism (n = 4) |
| % hypercalcemia, n (%) | 1 (7.6%) | 0 | 1 (6.2%) | 5% (4% excluding 1 patient with primary hyperparathyroidism at baseline) |
0 | 0 |
| % hypercalciuria, n (%) | 1 (7.6%) | 0 | 2/16 (12.5%) | 0 | 0 | |
| 25-(OH)D (nmol/L) | 80 (68–93)a | 48 (34–62)a | 81±25 | 74 | 72.4±14.9 | 69.8±9.98 |
| 1,25-(OH)2D (pmol/L) | 141 (114–174)a | 127 (107–140)a |
49±21 | 74.4±4.8 | 79.2±4.8 | |
| Serum Ca (mmol/L) | 2.24 (2.19–2.30)a | 2.24 (2.18– 2.29)a |
2.40±0.15 | 4.8±0.05 | 4.8±0.2 | |
| Urinary Ca (mmol/day) | 7.3 (3.4–11.1)a | 5.3 (2.6–7.9)a |
4.2±3.3 | 4.9±0.45 | 4.15±0.62 | |
Results are expressed as mean ± SD. aData are extracted from figures in Boland et al. and expressed as mean (95% CI). 25-(OH)D, 25-hydroxyvitamin-D; 1,25-(OH)2D, 1,25-dihydroxyvitamin D; Ca, calcium
Bolland et al.85, in a randomized, placebo-controlled trial in New Zealand involving 27 normocalcemic patients with sarcoidosis and vitamin D insufficiency using 50,000 IU weekly cholecalciferol for 4 weeks followed by 50,000 IU monthly or placebo for 11 months, showed that vitamin D supplements did not change the mean serum calcium or urine calcium; one patient (7.7%) developed significant hypercalcemia at a cumulative dose of 250,000 IU of cholecalciferol over 6 weeks.
Kamphius et al.80, in a retrospective study of 301 sarcoidosis patients over 23 years, showed that supplementation of calcium (500 mg) and vitamin D (400 IU) daily was associated with a significant negative correlation between serum 25-(OH)D levels and disease activity assessed by somatostatin receptor scintigraphy; hypercalcemia developed in five out of 104 (4.8%) patients.
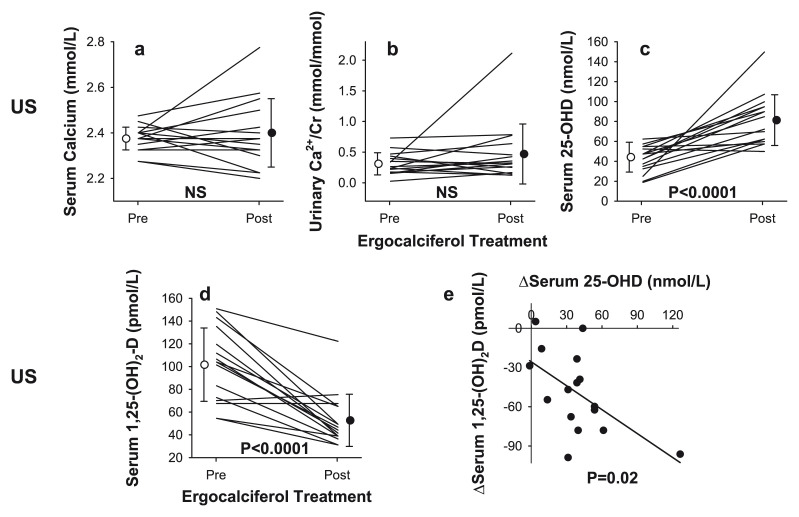
Capolongo et al.12 studied two sarcoidosis populations of distinct ethnic and lifestyle backgrounds from the US and Italy and showed largely similar baseline clinical, biochemical, and mineral metabolism parameters. The prevalence of vitamin D insufficiency in patients was not different from that in the correspondingly matched general population12. In 16 AA patients with sarcoidosis and vitamin D deficiency, oral ergocalciferol (50,000 IU) weekly for 12 weeks did not alter mean serum calcium level; one patient developed hypercalcemia (6.25%) (Figure 3), i.e. similar to the incidence in Bolland’s study (7.7%)85. In both prospective studies12,85, the lack of significant changes in bone mineral density or improvement in lung function12,85 may be due to the small sample size.
Figure 3. Serum and urine biochemical profiles in patients with sarcoidosis before and after vitamin D supplementation.
Changes in (a) serum calcium, (b) urine calcium, (c) serum 25-hydroxyvitamin-D (25-(OH)D), and (d) serum 1,25-dihydroxyvitamin-D (1,25-(OH)2D) and (e) relationship between changes in serum 1,25-(OH)2D and 25-(OH)D before and after vitamin D repletion in 16 patients with active sarcoidosis and vitamin D deficiency. This figure was reproduced from Vitamin-D status and mineral metabolism in two ethnic populations with sarcoidosis, Capolongo et al., 64, 1025–34, 2020 with permission from BMJ Publishing Group Ltd12.
An unanticipated finding in the study by Capolongo et al.12 is a decline in serum 1,25-(OH)2D following vitamin D repletion12 (Figure 3). The mechanisms for the decline remain unclear; however, it is possible that, in the presence of vitamin D deficiency, extrarenal production of 1,25-(OH)2D in sarcoidosis by immune cells is accentuated as an adaptive response to mitigate antigen-stimulated granuloma formation. While there are no human studies addressing the regulation of serum 1,25-(OH)2D in sarcoidosis, a rodent study has shown that locally generated 1,25-(OH)2D upregulates 24-hydroxylase, which in turn stimulates the conversion of 25-(OH)D and/or 1,25-(OH)2D to the inactive 1,24,25(OH)D86,87. However, it has been shown that intracrine stimulation of 24-hydroxylase protein expresses a truncated splice variant which may be functionally inactive. This variant protein maintains its binding and may inhibit the effect of 25-(OH)D, limiting substrate provision for excess 1,25-(OH)2D production88 spilling over into extracellular space, causing hypercalcemia, but may also mitigate an overzealous autoimmune response89. If this hypothesis is validated in a controlled clinical trial, then vitamin D supplementation can be utilized in the treatment of sarcoidosis not only to downregulate the autoimmune response but also to spare steroid-related adverse effects.
Hypercalciuria and kidney stones in sarcoidosis
Hypercalciuria occurs in 50% of cases of sarcoidosis and increases the risk for calcium oxalate stone formation90–92. Approximately 10–13.8% of patients with chronic sarcoidosis suffer from at least one symptomatic stone93,94. In only 1% of patients with sarcoidosis, kidney stones are an initial manifestation of the disease95. However, in 2.7% of patients, asymptomatic stones are present when sarcoidosis is diagnosed otherwise96. In the study by Capolongo et al., the prevalence of kidney stones was 11% and 17%, respectively12. In this study, kidney stones were associated with a high urinary calcium excretion, but no association was found between serum vitamin D and urinary calcium levels (Figure 4), suggesting that hypercalciuria is independent of serum 25-(OH)D and 1,25-(OH)2D levels in this population and may be related to impairment in renal tubular calcium reabsorption as a result of interstitial renal tubule involvement by sarcoidosis97–100.
Figure 4. Urinary calcium excretion in non-stone formers and stone-forming subjects with sarcoidosis.
Lack of significant relationship of 24 hour urinary calcium with serum 25-hydroxyvitamin-D (25(OH)D) (left panel) and 1,25-dihydroxyvitamin-D (1,25-(OH)2D) (right panel) in patients with sarcoidosis. This figure was reproduced from Vitamin-D status and mineral metabolism in two ethnic populations with sarcoidosis, Capolongo et al., 64, 1025–34, 2020 with permission from BMJ Publishing Group Ltd12.
The development of hypercalciuria in sarcoidosis has been attributed to increased intestinal calcium absorption and bone resorption101,102. Excess 1,25-(OH)2D increases gut calcium absorption1,103–105, which increases renal filter load, while decreased serum PTH reduces renal tubular calcium reabsorption103. The mechanisms of kidney stone formation in sarcoidosis are similar to those in idiopathic absorptive hypercalciuria because of the increased serum 1,25-(OH)2D levels in the latter patients104,106. Glucocorticoid treatment produces a significant fall in serum 1,25-(OH)2D associated with decreased intestinal calcium absorption in sarcoidosis103 but not in absorptive hypercalciuria. Moreover, hypercalciuria in patients with sarcoidosis and high circulating 1,25-(OH)2D levels may be due to increased osteoclastic bone resorption102. A similar situation has been reported in normal subjects challenged with a large dose of 1,25-(OH)2D, suggesting that hypercalciuria originates in part from excessive calcium mobilization from bone107,108. In rare instances, nephrocalcinosis due to incomplete renal tubular acidosis may occur in sarcoidosis109.
Glucocorticoids are the first-line treatment to reduce endogenous 1,25-(OH)2D110 and serum calcium levels, often within days. Typically, urinary calcium falls significantly a few days after normalization of serum calcium and 1,25-(OH)2D levels in 7–10 days. A lack of response after 2 weeks of treatment should raise the possibility of primary hyperparathyroidism, malignancy, lymphoma, and multiple myeloma. It is customary to reduce glucocorticoid dose within 4–6 weeks of treatment. In case of glucocorticoid failure, hydroxychloroquine111–113 or anti-fungal agents such as ketoconazole114,115 that inhibit 1-α hydroxylase activity in granuloma may be used as steroid-sparing agents. Immunosuppressive agents such as methotrexate and azathioprine may also be considered21. In patients with recurrent kidney stones and persistent hypercalciuria, surgical intervention with shockwave lithotripsy is indicated116,117.
Treatment of abnormal vitamin D metabolism in sarcoidosis
Given that treatment with calcium and vitamin D supplements is required along with the first line of treatment in the management of steroid-induced osteoporosis, this approach was accepted into the 2017 American College of Rheumatology Guideline for the Prevention and Treatment of Glucocorticoid-Induced Osteoporosis118. However, the dosage of calcium and vitamin D must be carefully adjusted to avoid the development of hypercalcemia and hypercalciuria. If serum calcium or 24 hour urinary calcium rises above the normal upper limit (2.62 mmol/L) or urinary calcium above 400 mg/day (10 mmol/day) in males and >300 mg/day (7.5 mmol/day) in females, calcium supplements and dietary calcium should be adjusted and serum and urinary calcium levels monitored in 2 weeks. Withdrawal of supplements has been shown to reverse hypercalcemia in sarcoidosis12,80,81,85,101. If serum calcium is persistently elevated, then vitamin D dosage must be further adjusted with follow-up blood and urine chemistry. Bolland et al.85 proposed that given that the nature of hypercalcemia development in this population remains unknown, a smaller dose of vitamin D may avoid complications of hypercalcemia; this issue would need to be examined in future prospective controlled trials. Dose adjustment of vitamin D supplement may reduce its benefits on skeletal health; furthermore, the exact dosage of vitamin D influencing its immunomodulatory role at tissue level has not been tested.
Bolland et al., based on modelling, proposed that it is not ethically feasible to conduct a clinical trial aimed at improving skeletal health because the risks of developing hypercalcemia exceed the benefits to bone health. This modelling was based on 13 patients who received vitamin D, in which only one patient developed hypercalcemia. Gallagher et al.119, in a population of white postmenopausal women with vitamin D insufficiency, found that 8.8% of patients developed hypercalcemia (>2.55 mmol/L), i.e. a prevalence not different from that seen in the small studies of sarcoidosis patients with vitamin D insufficiency following vitamin D/calcium supplementation (4 to 7.6%, Table 1). Therefore, sarcoidosis patients with vitamin D insufficiency do not seem to be at a higher risk of developing hypercalcemia than do other patients commonly administered vitamin D. We believe the benefits of calcium and vitamin D supplementation in sarcoidosis have not been sufficiently examined to determine whether the risk of hypercalcemia outweighs the benefits.
Conclusion
Vitamin D and calcium disturbances clearly play a principal role in the pathophysiology of sarcoidosis, yet the practical management remains controversial. Because of the concerns of worsening abnormal calcium metabolism following vitamin D supplementation, the clinical community has been ambivalent on supplementation in vitamin D-deficient or -insufficient patients with sarcoidosis. This concern also limited the conduct of prospective clinical trials to address a novel but neglected aspect of vitamin D action in this population. A study in two distinct ethnic groups of patients with sarcoidosis has opened the door towards further unraveling the role of vitamin D12. The result of the study showing that repletion of 25-(OH)D may reverse some underlying pathophysiological abnormalities was compelling; the associated lowering of serum angiotensin-converting enzyme (ACE) and serum γ-globulin, both surrogate markers of active sarcoidosis, supports the suppression of granulomatous immune activity. These intervention studies were small in size and did not allow comprehensive investigation of the potential risk–benefit balance of vitamin D supplementation on different organ systems. Further prospective interventional investigation involving larger cohorts of patients is warranted to clarify the relationship between vitamin D repletion and inflammatory activity and outcome in sarcoidosis.
Acknowledgements
The authors thank Rubyth Aguirre and John Poindexter for their role in the preparation and submission of the manuscript and Xilong Li (Department of Population and Data Science) and Yulun Liu (Department of Population and Data Sciences) for their statistical support.
The peer reviewers who approve this article are:
Martin Hewison, Institute of Metabolism and Systems Research, The University of Birmingham, Birmingham, UK
Mark Bolland, Department of Medicine, University of Auckland, Auckland, New Zealand
Funding Statement
The authors’ work was supported by the Julius and Louise Truelson Fellowship fund in Mineral Metabolism, Frederic C. Bartter Fund for Young Investigators, Charles Y.C. Pak and Donald W. Seldin Center for Metabolic Research, and Laura Kim Pak Professorship in Mineral Metabolism Research.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributions
Khashayar Sakhaee and Connie Hsia prepared, wrote, and revised the manuscript. Fabiola Gianella compiled the data.
References
- 1. Bell NH, Stern PH, Pantzer E, et al. : Evidence that increased circulating 1 alpha, 25-dihydroxyvitamin D is the probable cause for abnormal calcium metabolism in sarcoidosis. J Clin Invest. 1979; 64(1): 218–25. 10.1172/JCI109442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Papapoulos SE, Fraher LJ, Sandler LM, et al. : 1, 25-dihydroxycholecalciferol in the pathogenesis of the hypercalcaemia of sarcoidosis. Lancet. 1979; 1(8117): 627–30. 10.1016/s0140-6736(79)91076-6 [DOI] [PubMed] [Google Scholar]
- 3. Albright F, Carroll EL, Dempsey EF, et al. : The cause of hypercalcuria in sarcoid and its treatment with cortisone and sodium phytate. J Clin Invest. 1956; 35(11): 1229–42. 10.1172/JCI103378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bell NH, Bartter FC: Transient reversal of hyperabsorption of calcium and of abnormal sensitivity to vitamin D in a patient with sarcoidosis during episode of nephritis. Ann Intern Med. 1964; 61: 702–10. 10.7326/0003-4819-61-4-702 [DOI] [PubMed] [Google Scholar]
- 5. Bell NH, Gill JR, Bartter FC: On the abnormal calcium absorption in sarcoidosis. evidence for increased sensitivity to vitamin D. Am J Med. 1964; 36: 500–13. 10.1016/0002-9343(64)90099-3 [DOI] [PubMed] [Google Scholar]
- 6. Anderson J, Harper C, Dent CE, et al. : Effect of cortisone on calcium metabolism in sarcoidosis with hypercalcqmia possibly antagonistic actions of cortisone and vitamin D. Lancet. 1954; 267(6841): 720–4. 10.1016/s0140-6736(54)90492-4 [DOI] [PubMed] [Google Scholar]
- 7. Barbour GL, Coburn JW, Slatopolsky E, et al. : Hypercalcemia in an anephric patient with sarcoidosis: evidence for extrarenal generation of 1,25-dihydroxyvitamin D. N Engl J Med. 1981; 305(8): 440–3. 10.1056/NEJM198108203050807 [DOI] [PubMed] [Google Scholar]
- 8. Adams JS, Sharma OP, Gacad MA, et al. : Metabolism of 25-hydroxyvitamin D3 by cultured pulmonary alveolar macrophages in sarcoidosis. J Clin Invest. 1983; 72(5): 1856–60. 10.1172/JCI111147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mason RS, Frankel T, Chan YL, et al. : Vitamin D conversion by sarcoid lymph node homogenate. Ann Intern Med. 1984; 100(1): 59–61. 10.7326/0003-4819-100-1-59 [DOI] [PubMed] [Google Scholar]
- 10. Inui N, Murayama A, Sasaki S, et al. : Correlation between 25-hydroxyvitamin D3 1α-hydroxylase gene expression in alveolar macrophages and the activity of sarcoidosis. Am J Med. 2001; 110(9): 687–93. 10.1016/s0002-9343(01)00724-0 [DOI] [PubMed] [Google Scholar]
- 11. Fuss M, Pepersack T, Gillet C, et al. : Calcium and vitamin D metabolism in granulomatous diseases. Clin Rheumatol. 1992; 11(1): 28–36. 10.1007/BF02207080 [DOI] [PubMed] [Google Scholar]
- 12. Capolongo G, Xu LHR, Accardo M, et al. : Vitamin-D status and mineral metabolism in two ethnic populations with sarcoidosis. J Investig Med. 2016; 64(5): 1025–34. 10.1136/jim-2016-000101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Prasse A, Katic C, Germann M, et al. : Phenotyping sarcoidosis from a pulmonary perspective. Am J Respir Crit Care Med. 2008; 177(3): 330–6. 10.1164/rccm.200705-742OC [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 14. Du Bois RM, Goh N, McGrath D, et al. : Is there a role for microorganisms in the pathogenesis of sarcoidosis? J Intern Med. 2003; 253(1): 4–17. 10.1046/j.1365-2796.2003.01073.x [DOI] [PubMed] [Google Scholar]
- 15. Arkema EV, Grunewald J, Kullberg S, et al. : Sarcoidosis incidence and prevalence: a nationwide register-based assessment in Sweden. Eur Respir J. 2016; 48(1): 1690–9. 10.1183/13993003.00477-2016 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 16. Baughman RP, Field S, Costabel U, et al. : Sarcoidosis in America. Analysis Based on Health Care Use. Ann Am Thorac Soc. 2016; 13(8): 1244–52. 10.1513/AnnalsATS.201511-760OC [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 17. Dumas O, Abramovitz L, Wiley AS, et al. : Epidemiology of Sarcoidosis in a Prospective Cohort Study of U.S. Women. Ann Am Thorac Soc. 2016; 13(1): 67–71. 10.1513/AnnalsATS.201508-568BC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Byg KE, Milman N, Hansen S: Sarcoidosis in Denmark 1980-1994. A registry-based incidence study comprising 5536 patients. Sarcoidosis Vasc Diffuse Lung Dis. 2003; 20(1): 46–52. [PubMed] [Google Scholar]
- 19. Hillerdal G, Nöu E, Osterman K, et al. : Sarcoidosis: epidemiology and prognosis. A 15-year European study. Am Rev Respir Dis. 1984; 130(1): 29–32. [DOI] [PubMed] [Google Scholar]
- 20. Morimoto T, Azuma A, Abe S, et al. : Epidemiology of sarcoidosis in Japan. Eur Respir J. 2008; 31(2): 372–9. 10.1183/09031936.00075307 [DOI] [PubMed] [Google Scholar]
- 21. Culver DA, Judson MA: New advances in the management of pulmonary sarcoidosis. BMJ. 2019; 367: l5553. 10.1136/bmj.l5553 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 22. Brito-Zerón P, Sellarés J, Bosch X, et al. : Epidemiologic patterns of disease expression in sarcoidosis: age, gender and ethnicity-related differences. Clin Exp Rheumatol. 2016; 34(3): 380–8. [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 23. Deubelbeiss U, Gemperli A, Schindler C, et al. : Prevalence of sarcoidosis in Switzerland is associated with environmental factors. Eur Respir J. 2010; 35(5): 1088–97. 10.1183/09031936.00197808 [DOI] [PubMed] [Google Scholar]
- 24. Rybicki BA, Major M, Popovich J, et al. : Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol. 1997; 145(3): 234–41. 10.1093/oxfordjournals.aje.a009096 [DOI] [PubMed] [Google Scholar]
- 25. Kowalska M, Niewiadomska E, Zejda JE: Epidemiology of sarcoidosis recorded in 2006-2010 in the Silesian voivodeship on the basis of routine medical reporting. Ann Agric Environ Med. 2014; 21(1): 55–8. [PubMed] [Google Scholar]
- 26. Judson MA, Boan AD, Lackland DT: The clinical course of sarcoidosis: Presentation, diagnosis, and treatment in a large white and black cohort in the United States. Sarcoidosis Vasc Diffuse Lung Dis. 2012; 29(2): 119–27. [PubMed] [Google Scholar]
- 27. Zadshir A, Tareen N, Pan D, et al. : The prevalence of hypovitaminosis D among US adults: data from the NHANES III. Ethn Dis. 2005; 15(4 Suppl 5): S5–97–101. [PubMed] [Google Scholar]
- 28. Gallagher JC, Sai AJ: Vitamin D insufficiency, deficiency, and bone health. J Clin Endocrinol Metab. 2010; 95(6): 2630–3. 10.1210/jc.2010-0918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lemire JM: Immunomodulatory role of 1,25-dihydroxyvitamin D3. J Cell Biochem. 1992; 49(1): 26–31. 10.1002/jcb.240490106 [DOI] [PubMed] [Google Scholar]
- 30. Holick MF, Richtand NM, McNeill SC, et al. : Isolation and identification of previtamin D3 from the skin of rats exposed to ultraviolet irradiation. Biochemistry. 1979; 18(6): 1003–8. 10.1021/bi00573a011 [DOI] [PubMed] [Google Scholar]
- 31. Holick MF, MacLaughlin JA, Clark MB, et al. : Photosynthesis of previtamin D3 in human skin and the physiologic consequences. Science. 1980; 210(4466): 203–5. 10.1126/science.6251551 [DOI] [PubMed] [Google Scholar]
- 32. Holick MF, MacLaughlin JA, Doppelt SH: Regulation of cutaneous previtamin D3 photosynthesis in man: skin pigment is not an essential regulator. Science. 1981; 211(4482): 590–3. 10.1126/science.6256855 [DOI] [PubMed] [Google Scholar]
- 33. Chen TC, Chimeh F, Lu Z, et al. : Factors that influence the cutaneous synthesis and dietary sources of vitamin D. Arch Biochem Biophys. 2007; 460(2): 213–7. 10.1016/j.abb.2006.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cheng JB, Levine MA, Bell NH, et al. : Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylase. Proc Natl Acad Sci U S A. 2004; 101(20): 7711–5. 10.1073/pnas.0402490101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jones G, Prosser DE, Kaufmann M: 25-Hydroxyvitamin D-24-hydroxylase (CYP24A1): its important role in the degradation of vitamin D. Arch Biochem Biophys. 2012; 523(1): 9–18. 10.1016/j.abb.2011.11.003 [DOI] [PubMed] [Google Scholar]
- 36. Fraser DR: Regulation of the metabolism of vitamin D. Physiol Rev. 1980; 60(2): 551–613. 10.1152/physrev.1980.60.2.551 [DOI] [PubMed] [Google Scholar]
- 37. Gray RW, Wilz DR, Caldas AE, et al. : The importance of phosphate in regulating plasma 1,25-(OH)2-vitamin D levels in humans: studies in healthy subjects in calcium-stone formers and in patients with primary hyperparathyroidism. J Clin Endocrinol Metab. 1977; 45(2): 299–306. 10.1210/jcem-45-2-299 [DOI] [PubMed] [Google Scholar]
- 38. Kremer R, Goltzman D: Parathyroid hormone stimulates mammalian renal 25-hydroxyvitamin D3-1 alpha-hydroxylase in vitro. Endocrinology. 1982; 110(1): 294–6. 10.1210/endo-110-1-294 [DOI] [PubMed] [Google Scholar]
- 39. Murayama A, Takeyama K, Kitanaka S, et al. : The promoter of the human 25-hydroxyvitamin D3 1 alpha-hydroxylase gene confers positive and negative responsiveness to PTH, calcitonin, and 1 alpha,25(OH)2D3. Biochem Biophys Res Commun. 1998; 249(1): 11–6. 10.1006/bbrc.1998.9098 [DOI] [PubMed] [Google Scholar]
- 40. Shimada T, Kakitani M, Yamazaki Y, et al. : Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004; 113(4): 561–8. 10.1172/JCI19081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Evans RM: The steroid and thyroid hormone receptor superfamily. Science. 1988; 240(4854): 889–95. 10.1126/science.3283939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Adams JS: Vitamin D metabolite-mediated hypercalcemia. Endocrinol Metab Clin North Am. 1989; 18(3): 765–78. 10.1016/S0889-8529(18)30365-7 [DOI] [PubMed] [Google Scholar]
- 43. Raisz LG, Kream BE: Regulation of bone formation. N Engl J Med. 1983; 309(1): 29–35. 10.1056/NEJM198307073090107 [DOI] [PubMed] [Google Scholar]
- 44. Tanaka Y, DeLuca HF: Bone mineral mobilization activity of 1,25-dihydroxycholecalciferol, a metabolite of vitamin D. Arch Biochem Biophys. 1971; 146(2): 574–8. 10.1016/0003-9861(71)90163-9 [DOI] [PubMed] [Google Scholar]
- 45. Kongsbak M, von Essen MR, Levring TB, et al. : Vitamin D-binding protein controls T cell responses to vitamin D. BMC Immunol. 2014; 15: 35. 10.1186/s12865-014-0035-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Morgan JW, Reddy GS, Uskokovic MR, et al. : Functional block for 1 alpha,25-dihydroxyvitamin D3-mediated gene regulation in human B lymphocytes. J Biol Chem. 1994; 269(18): 13437–43. [PubMed] [Google Scholar]
- 47. Sigmundsdottir H, Pan J, Debes GF, et al. : DCs metabolize sunlight-induced vitamin D3 to 'program' T cell attraction to the epidermal chemokine CCL27. Nat Immunol. 2007; 8(3): 285–93. 10.1038/ni1433 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 48. Jeffery LE, Wood AM, Qureshi OS, et al. : Availability of 25-hydroxyvitamin D(3) to APCs controls the balance between regulatory and inflammatory T cell responses. J Immunol. 2012; 189(11): 5155–64. 10.4049/jimmunol.1200786 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 49. Heine G, Niesner U, Chang HD, et al. : 1,25-dihydroxyvitamin D(3) promotes IL-10 production in human B cells. Eur J Immunol. 2008; 38(8): 2210–8. 10.1002/eji.200838216 [DOI] [PubMed] [Google Scholar]
- 50. Correale J, Ysrraelit MC, Gaitán MI: Immunomodulatory effects of Vitamin D in multiple sclerosis. Brain. 2009; 132(Pt 5): 1146–60. 10.1093/brain/awp033 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 51. von Essen MR, Kongsbak M, Schjerling P, et al. : Vitamin D controls T cell antigen receptor signaling and activation of human T cells. Nat Immunol. 2010; 11(4): 344–9. 10.1038/ni.1851 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 52. Bakdash G, van Capel TMM, Mason LMK, et al. : Vitamin D3 metabolite calcidiol primes human dendritic cells to promote the development of immunomodulatory IL-10-producing T cells. Vaccine. 2014; 32(47): 6294–302. 10.1016/j.vaccine.2014.08.075 [DOI] [PubMed] [Google Scholar]
- 53. Bhalla AK, Amento EP, Clemens TL, et al. : Specific high-affinity receptors for 1,25-dihydroxyvitamin D3 in human peripheral blood mononuclear cells: presence in monocytes and induction in T lymphocytes following activation. J Clin Endocrinol Metab. 1983; 57(6): 1308–10. 10.1210/jcem-57-6-1308 [DOI] [PubMed] [Google Scholar]
- 54. Provvedini DM, Tsoukas CD, Deftos LJ, et al. : 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983; 221(4616): 1181–3. 10.1126/science.6310748 [DOI] [PubMed] [Google Scholar]
- 55. Brennan A, Katz DR, Nunn JD, et al. : Dendritic cells from human tissues express receptors for the immunoregulatory vitamin D3 metabolite, dihydroxycholecalciferol. Immunology. 1987; 61(4): 457–61. [PMC free article] [PubMed] [Google Scholar]
- 56. Morgan JW, Kouttab N, Ford D, et al. : Vitamin D-mediated gene regulation in phenotypically defined human B cell subpopulations. Endocrinology. 2000; 141(9): 3225–34. 10.1210/endo.141.9.7666 [DOI] [PubMed] [Google Scholar]
- 57. Dankers W, Colin EM, van Hamburg JP, et al. : Vitamin D in Autoimmunity: Molecular Mechanisms and Therapeutic Potential. Front Immunol. 2016; 7: 697. 10.3389/fimmu.2016.00697 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 58. Chun RF, Shieh A, Gottlieb C, et al. : Vitamin D Binding Protein and the Biological Activity of Vitamin D. Front Endocrinol (Lausanne). 2019; 10: 718. 10.3389/fendo.2019.00718 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 59. Chun RF, Liu PT, Modlin RL, et al. : Impact of vitamin D on immune function: lessons learned from genome-wide analysis. Front Physiol. 2014; 5: 151. 10.3389/fphys.2014.00151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Adams JS, Hewison M: Extrarenal expression of the 25-hydroxyvitamin D-1-hydroxylase. Arch Biochem Biophys. 2012; 523(1): 95–102. 10.1016/j.abb.2012.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 61. Hewison M: An update on vitamin D and human immunity. Clin Endocrinol (Oxf). 2012; 76(3): 315–25. 10.1111/j.1365-2265.2011.04261.x [DOI] [PubMed] [Google Scholar]
- 62. Takeda K, Akira S: Toll-like receptors in innate immunity. Int Immunol. 2005; 17(1): 1–14. 10.1093/intimm/dxh186 [DOI] [PubMed] [Google Scholar]
- 63. Trinchieri G, Sher A: Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007; 7(3): 179–90. 10.1038/nri2038 [DOI] [PubMed] [Google Scholar]
- 64. Adams JS, Hewison M: Unexpected actions of vitamin D: New perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metab. 2008; 4(2): 80–90. 10.1038/ncpendmet0716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Liu PT, Stenger S, Li H, et al. : Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006; 311(5768): 1770–3. 10.1126/science.1123933 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 66. Wang TT, Nestel FP, Bourdeau V, et al. : Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004; 173(5): 2909–12. 10.4049/jimmunol.173.5.2909 [DOI] [PubMed] [Google Scholar]
- 67. Gombart AF, Borregaard N, Koeffler HP: Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005; 19(9): 1067–77. 10.1096/fj.04-3284com [DOI] [PubMed] [Google Scholar]
- 68. Richmond BW, Drake WP: Vitamin D, innate immunity, and sarcoidosis granulomatous inflammation: Insights from mycobacterial research. Curr Opin Pulm Med. 2010; 16(5): 461–4. 10.1097/MCP.0b013e32833af7e8 [DOI] [PubMed] [Google Scholar]
- 69. Korucu E, Pur Ozyigit L, Ortakoylu MG, et al. : Cathelicidin as a link between sarcoidosis and tuberculosis. Sarcoidosis Vasc Diffuse Lung Dis. 2015; 32(3): 222–7. [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 70. Barna BP, Culver DA, Kanchwala A, et al. : Alveolar macrophage cathelicidin deficiency in severe sarcoidosis. J Innate Immun. 2012; 4(5–6): 569–78. 10.1159/000339149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sadeghi K, Wessner B, Laggner U, et al. : Vitamin D3 down-regulates monocyte TLR expression and triggers hyporesponsiveness to pathogen-associated molecular patterns. Eur J Immunol. 2006; 36(2): 361–70. 10.1002/eji.200425995 [DOI] [PubMed] [Google Scholar]
- 72. Abbas AK, Murphy KM, Sher A: Functional diversity of helper T lymphocytes. Nature. 1996; 383(6603): 787–93. 10.1038/383787a0 [DOI] [PubMed] [Google Scholar]
- 73. Romagnani S: Regulation of the T cell response. Clin Exp Allergy. 2006; 36(11): 1357–66. 10.1111/j.1365-2222.2006.02606.x [DOI] [PubMed] [Google Scholar]
- 74. Lemire JM, Archer DC, Beck L, et al. : Immunosuppressive actions of 1,25-dihydroxyvitamin D3: Preferential inhibition of Th1 functions. J Nutr. 1995; 125(6 Suppl): 1704S–1708S. [DOI] [PubMed] [Google Scholar]
- 75. Boonstra A, Barrat FJ, Crain C, et al. : 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4+ T cells to enhance the development of Th2 cells. J Immunol. 2001; 167(9): 4974–80. 10.4049/jimmunol.167.9.4974 [DOI] [PubMed] [Google Scholar]
- 76. Penna G, Amuchastegui S, Cossetti C, et al. : Treatment of experimental autoimmune prostatitis in nonobese diabetic mice by the vitamin D receptor agonist elocalcitol. J Immunol. 2006; 177(12): 8504–11. 10.4049/jimmunol.177.12.8504 [DOI] [PubMed] [Google Scholar]
- 77. Tang J, Zhou R, Luger D, et al. : Calcitriol suppresses antiretinal autoimmunity through inhibitory effects on the Th17 effector response. J Immunol. 2009; 182(8): 4624–32. 10.4049/jimmunol.0801543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Barrat FJ, Cua DJ, Boonstra A, et al. : In vitro generation of interleukin 10-producing regulatory CD4+ T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med. 2002; 195(5): 603–16. 10.1084/jem.20011629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Conron M, Young C, Beynon HL: Calcium metabolism in sarcoidosis and its clinical implications. Rheumatology (Oxford). 2000; 39(7): 707–13. 10.1093/rheumatology/39.7.707 [DOI] [PubMed] [Google Scholar]
- 80. Kamphuis LS, Bonte-Mineur F, van Laar JA, et al. : Calcium and vitamin D in sarcoidosis: Is supplementation safe? J Bone Miner Res. 2014; 29(11): 2498–503. 10.1002/jbmr.2262 [DOI] [PubMed] [Google Scholar]
- 81. Baughman RP, Janovcik J, Ray M, et al. : Calcium and vitamin D metabolism in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2013; 30(2): 113–20. [PubMed] [Google Scholar]
- 82. Burke RR, Rybicki BA, Rao DS: Calcium and vitamin D in sarcoidosis: How to assess and manage. Semin Respir Crit Care Med. 2010; 31(4): 474–84. 10.1055/s-0030-1262215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Sodhi A, Aldrich T: Vitamin D Supplementation: Not So Simple in Sarcoidosis. Am J Med Sci. 2016; 352(3): 252–7. 10.1016/j.amjms.2016.05.027 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 84. Saidenberg-Kermanac'h N, Semerano L, Nunes H, et al. : Bone fragility in sarcoidosis and relationships with calcium metabolism disorders: A cross sectional study on 142 patients. Arthritis Res Ther. 2014; 16(2): R78. 10.1186/ar4519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Bolland MJ, Wilsher ML, Grey A, et al. : Randomised controlled trial of vitamin D supplementation in sarcoidosis. BMJ Open. 2013; 3(10): e003562. 10.1136/bmjopen-2013-003562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. O’Kelly J, Hisatake J, Hisatake Y, et al. : Normal myelopoiesis but abnormal T lymphocyte responses in vitamin D receptor knockout mice. J Clin Invest. 2002; 109(8): 1091–9. 10.1172/JCI12392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hewison M: Vitamin D and the intracrinology of innate immunity. Mol Cell Endocrinol. 2010; 321(2): 103–11. 10.1016/j.mce.2010.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sharma OP, Trowell JNC: Abnormal calcium metabolism in sarcoidosis. In: Turiaf JCJ eds, ed. La sarcoidose: Rapp IV Conf Intern. Paris: Maison et Cie; 1967. [Google Scholar]
- 89. Jeffery LE, Burke F, Mura M, et al. : 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J Immunol. 2009; 183(9): 5458–67. 10.4049/jimmunol.0803217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Berliner AR, Haas M, Choi MJ: Sarcoidosis: The nephrologist's perspective. Am J Kidney Dis. 2006; 48(5): 856–70. 10.1053/j.ajkd.2006.07.022 [DOI] [PubMed] [Google Scholar]
- 91. James DG: Sarcoidosis. Postgrad Med J. 1984; 60(701): 234–41. 10.1136/pgmj.60.701.234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Mayock RL, Bertrand P, Morrison CE, et al. : Manifestations of sarcoidosis. Analysis of 145 patients, with a review of nine series selected from the literature. Am J Med. 1963; 35(1): 67–89. 10.1016/0002-9343(63)90165-7 [DOI] [PubMed] [Google Scholar]
- 93. Lebacq E, Desmet V, Verhaegen H: Renal involvement in sarcoidosis. Postgrad Med J. 1970; 46(538): 526–9. 10.1136/pgmj.46.538.526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Rodman JS, Mahler RJ: Kidney stones as a manifestation of hypercalcemic disorders. Hyperparathyroidism and sarcoidosis. Urol Clin North Am. 2000; 27(2): 275–85, viii. 10.1016/s0094-0143(05)70257-3 [DOI] [PubMed] [Google Scholar]
- 95. Rizzato G, Fraioli P, Montemurro L: Nephrolithiasis as a presenting feature of chronic sarcoidosis. Thorax. 1995; 50(5): 555–9. 10.1136/thx.50.5.555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Rizzato G, Colombo P: Nephrolithiasis as a presenting feature of chronic sarcoidosis: A prospective study. Sarcoidosis Vasc Diffuse Lung Dis. 1996; 13(2): 167–72. [PubMed] [Google Scholar]
- 97. Gupta R, Beaudet L, Moore J, et al. : Treatment of sarcoid granulomatous interstitial nephritis with adalimumab. NDT plus. 2009; 2(2): 139–42. 10.1093/ndtplus/sfn200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Rajakariar R, Sharples EJ, Raftery MJ, et al. : Sarcoid tubulo-interstitial nephritis: Long-term outcome and response to corticosteroid therapy. Kidney Int. 2006; 70(1): 165–9. 10.1038/sj.ki.5001512 [DOI] [PubMed] [Google Scholar]
- 99. Robson MG, Banerjee D, Hopster D, et al. : Seven cases of granulomatous interstitial nephritis in the absence of extrarenal sarcoid. Nephrol Dial Transplant. 2003; 18(2): 280–4. 10.1093/ndt/18.2.280 [DOI] [PubMed] [Google Scholar]
- 100. Joss N, Morris S, Young B, et al. : Granulomatous interstitial nephritis. Clin J Am Soc Nephrol. 2007; 2(2): 222–30. 10.2215/CJN.01790506 [DOI] [PubMed] [Google Scholar]
- 101. Crouser ED, Maier LA, Wilson KC, et al. : Diagnosis and Detection of Sarcoidosis. An Official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020; 201(8): e26–e51. 10.1164/rccm.202002-0251ST [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 102. Meyrier A, Valeyre D, Bouillon R, et al. : Resorptive versus absorptive hypercalciuria in sarcoidosis: correlations with 25-hydroxy vitamin D3 and 1,25-dihydroxy vitamin D3 and parameters of disease activity. Q J Med. 1985; 54(215): 269–81. 10.1093/oxfordjournals.qjmed.a067848 [DOI] [PubMed] [Google Scholar]
- 103. Zerwekh JE, Pak CY, Kaplan RA, et al. : Pathogenetic role of 1 alpha,25-dihydroxyvitamin D in sarcoidosis and absorptive hypercalciuria: different response to prednisolone therapy. J Clin Endocrinol Metab. 1980; 51(2): 381–6. 10.1210/jcem-51-2-381 [DOI] [PubMed] [Google Scholar]
- 104. Haussler MR, Boyce DW, Littledike ET, et al. : A rapidly acting metabolite of vitamin D3. Proc Natl Acad Sci U S A. 1971; 68(1): 177–81. 10.1073/pnas.68.1.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Holick MF, Schnoes HK, DeLuca HF, et al. : Isolation and identification of 1,25-dihydroxycholecalciferol. A metabolite of vitamin D active in intestine. Biochemistry. 1971; 10(14): 2799–804. 10.1021/bi00790a023 [DOI] [PubMed] [Google Scholar]
- 106. Pak CY, Oata M, Lawrence EC, et al. : The hypercalciurias. Causes, parathyroid functions, and diagnostic criteria. J Clin Invest. 1974; 54(2): 387–400. 10.1172/JCI107774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Broadus AE, Insogna KL, Lang R, et al. : A consideration of the hormonal basis and phosphate leak hypothesis of absorptive hypercalciuria. J Clin Endocrinol Metab. 1984; 58(1): 161–9. 10.1210/jcem-58-1-161 [DOI] [PubMed] [Google Scholar]
- 108. Maierhofer WJ, Gray RW, Cheung HS, et al. : Bone resorption stimulated by elevated serum 1,25-(OH)2-vitamin D concentrations in healthy men. Kidney Int. 1983; 24(4): 555–60. 10.1038/ki.1983.193 [DOI] [PubMed] [Google Scholar]
- 109. AIon US: Nephrocalcinosis. Curr Opin Pediatr. 1997; 9: 160–5. 10.1097/00008480-199704000-00008 [DOI] [PubMed] [Google Scholar]
- 110. Sharma OP: Vitamin D, calcium, and sarcoidosis. Chest. 1996; 109(2): 535–9. 10.1378/chest.109.2.535 [DOI] [PubMed] [Google Scholar]
- 111. Adams JS, Diz MM, Sharma OP: Effective reduction in the serum 1,25-dihydroxyvitamin D and calcium concentration in sarcoidosis-associated hypercalcemia with short-course chloroquine therapy. Ann Intern Med. 1989; 111(5): 437–8. 10.7326/0003-4819-111-5-437 [DOI] [PubMed] [Google Scholar]
- 112. Barré PE, Gascon-Barré M, Meakins JL, et al. : Hydroxychloroquine treatment of hypercalcemia in a patient with sarcoidosis undergoing hemodialysis. Am J Med. 1987; 82(6): 1259–62. 10.1016/0002-9343(87)90237-3 [DOI] [PubMed] [Google Scholar]
- 113. O'Leary TJ, Jones G, Yip A, et al. : The effects of chloroquine on serum 1,25-dihydroxyvitamin D and calcium metabolism in sarcoidosis. N Engl J Med. 1986; 315(12): 727–30. 10.1056/NEJM198609183151203 [DOI] [PubMed] [Google Scholar]
- 114. Adams JS, Sharma OP, Diz MM, et al. : Ketoconazole decreases the serum 1,25-dihydroxyvitamin D and calcium concentration in sarcoidosis-associated hypercalcemia. J Clin Endocrinol Metab. 1990; 70(4): 1090–5. 10.1210/jcem-70-4-1090 [DOI] [PubMed] [Google Scholar]
- 115. Ejaz AA, Zabaneh RI, Tiwari P, et al. : Ketoconazole in the treatment of recurrent nephrolithiasis associated with sarcoidosis. Nephrol Dial Transplant. 1994; 9(10): 1492–4. 10.1093/ndt/9.10.1492 [DOI] [PubMed] [Google Scholar]
- 116. Kruithoff KL, Gyetko MR, Scheiman JM: Giant splenomegaly and refractory hypercalcemia due to extrapulmonary sarcoidosis. Successful treatment by splenectomy. Arch Intern Med. 1993; 153(24): 2793–6. 10.1001/archinte.1993.00410240105013 [DOI] [PubMed] [Google Scholar]
- 117. Sharma OP, Alfaro C: Hypercalciuria and renal stones in a sarcoidosis patient treated by extracorporeal shockwave lithotripsy. Sarcoidosis. 1986; 3(1): 7–9. [PubMed] [Google Scholar]
- 118. Buckley L, Guyatt G, Fink HA, et al. : 2017 American College of Rheumatology Guideline for the Prevention and Treatment of Glucocorticoid-Induced Osteoporosis. Arthritis Rheumatol. 2017; 69(8): 1521–37. 10.1002/art.40137 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 119. Gallagher JC, Smith LM, Yalamanchili V: Incidence of hypercalciuria and hypercalcemia during vitamin D and calcium supplementation in older women. Menopause. 2014; 21(11): 1173–80. 10.1097/GME.0000000000000270 [DOI] [PMC free article] [PubMed] [Google Scholar]