Abstract
In over two decades since the discovery of phosphatase and tensin homologue deleted on chromosome 10 (PTEN), nearly 18,000 publications have attempted to elucidate its functions and roles in normal physiology and disease. The frequent disruption of PTEN in cancer cells was a strong indication that it had critical roles in tumour suppression. Germline PTEN mutations have been identified in patients with heterogeneous tumour syndromic diseases, known as PTEN hamartoma tumour syndrome (PHTS), and in some individuals with autism spectrum disorders (ASD). Today we know that by limiting oncogenic signalling through the phosphoinositide 3-kinase (PI3K) pathway, PTEN governs a number of processes including survival, proliferation, energy metabolism, and cellular architecture. Some of the most exciting recent advances in the understanding of PTEN biology and signalling have revisited its unappreciated roles as a protein phosphatase, identified non-enzymatic scaffold functions, and unravelled its nuclear function. These discoveries are certain to provide a new perspective on its full tumour suppressor potential, and knowledge from this work will lead to new anti-cancer strategies that exploit PTEN biology. In this review, we will highlight some outstanding questions and some of the very latest advances in the understanding of the tumour suppressor PTEN.
Keywords: PTEN signalling, nuclear PTEN, PI3K pathway, cancer, tumour suppression
Background
PTEN the lipid phosphatase
Best known as a critical tumour suppressor, phosphatase and tensin homologue deleted on chromosome 10 (PTEN) is a key member of a complex intracellular phosphoinositide signalling network. The canonical function of PTEN is as a lipid phosphatase that dephosphorylates the 3 position on the inositol ring of phosphatidylinositol-(3,4,5)-triphosphate (PIP3) to generate PI(4,5)P21 (Figure 1). By this mechanism, PTEN opposes signalling of the oncogenic phosphoinositide 3-kinase (PI3K) pathway by limiting the recruitment and activation of AKT at the cell membrane1,2 (Figure 2). Loss of PTEN function in cancer cells (through a diversity of mechanisms that are not discussed in this review because of space limitations) almost invariably leads to accumulation of PIP3 and associated activation of AKT signalling. Downstream activation of PI3K pathway effectors in cancer are the foremost hallmarks of PTEN loss; however, PTEN loss has also been demonstrated to activate a plethora of pathways including Ras–MAPK, Wnt/β-catenin, Notch, and Hippo pathways through PIP3-dependent signals3–7. Overall, many other thousands of publications have cemented PTEN as an essential tumour suppressive phosphoinositide phosphatase that controls crucial signalling events and processes including growth, proliferation, survival, and migration8–12.
Figure 1. Schematic of PTEN’s lipid phosphatase activity.
Briefly, PTEN dephosphorylates the 3 position on the inositol ring of phosphatidylinositol-(3,4,5)-triphosphate (PIP3) to generate PI(4,5)P2. PI3K, phosphoinositide 3-kinase; PTEN, phosphatase and tensin homologue deleted on chromosome 10.
Figure 2. PTEN opposes the PI3K pathway and downstream oncogenic signalling to AKT.
By dephosphorylating PIP3, PTEN prevents the activation of AKT via PDK1 and thus protects against tumorigenesis. mTORC2, mammalian target of rapamycin complex 2; PDK1, phosphoinositide-dependent protein kinase 1; PI3K, phosphoinositide 3-kinase; PIP3, phosphatidylinositol-(3,4,5)-triphosphate; PTEN, phosphatase and tensin homologue deleted on chromosome 10.
PTEN the protein phosphatase
Although critically debated since its discovery, PTEN’s protein phosphate activities have been shown to contribute to its tumour suppressive function by an increasing number of studies13–15. By using specific mutants of PTEN lacking lipid phosphatase function, an early study concluded that PTEN may block cell migration through a protein phosphatase-mediated function on focal adhesion kinase (FAK) protein14. PTEN-mediated G1 cell cycle arrest has also been linked to protein phosphatase-mediated downregulation of cyclin D116,17. Since these first studies, PTEN has been reported to directly dephosphorylate an array of proteins involved in cell motility and migration18. Convincing data also point to the PTEN protein as its own substrate in an auto-dephosphorylation mechanism at its C-terminal phosphorylation sites18.
The most compelling data on this topic come from the generation of mouse models expressing specific loss-of-function mutations of Pten19,20. By modelling two cancer-associated PTEN mutations at Cys124 and Gly129, Wang et al. and Papa et al., respectively, attempted to dissect the specific roles of Pten catalytic activities19,20. Pten G129E mutation renders the lipid phosphatase activity inactive whilst sparing protein phosphatase activity21, whereas the Pten C124S mutation eliminates the essential cysteine in the phosphatase consensus site and renders all Pten phosphatase activity dead22. In these studies, homozygosity of either of these mutant alleles was associated with embryonic lethality. In adulthood, heterozygosity of either allele was associated with tumour development similar to observations in Pten knockout mice23,24. Despite some measurable differences in the spectrum of tumours observed with each specific mutant allele, the results from these studies indicate that the lipid phosphatase function of PTEN is responsible for a majority of the PTEN loss-driven cancer phenotypes. Nevertheless, the abundance of in vitro data supporting a protein phosphatase role for PTEN remains compelling. Further cancer and non-cancer focussed studies on Pten G129E and C124S could shed light on protein phosphatase functions. Moreover, the characterisation of the cancer-associated PTEN Y138L mutation by Davidson et al., with specific loss of PTEN protein phosphatase activity and retention of lipid phosphatase activity, presents a new tool that should be investigated in vivo25. Investigation of Pten G129E, Pten C124S, and Pten Y138L mice, among others, will provide critical insight into both the physiological and the pathological roles of the lipid and protein phosphatase functions of PTEN. In addition to being a dual specificity phosphatase for lipid and protein substrates, PTEN can also be dephosphorylated at serine/threonine and tyrosine residues. In sum, the physiological relevance of the protein phosphatase and phosphatase-independent functions of PTEN have yet to be clearly elucidated. However, many excellent tools are available to resolve these questions.
PTEN the nuclear scaffold protein
A tumour suppressive role for nuclear PTEN has been supported by the discovery of a number of novel functions exerted in the nucleus, most of which are independent of its phosphatase activity. Indeed, a phosphatase-independent function of PTEN in the nucleus was observed to be crucial for chromosome stability26. This function was attributed to a role for PTEN in centromere organisation via direct physical association with centromere protein C (CENP-C)26. Additionally, nuclear regulation of the cell cycle was linked to direct binding of PTEN to the APC/C-E3-ligase27, which facilitated binding to APC, in turn facilitating APC/C and CDH1 interaction to promote the tumour-suppressive activity of the CDH1–APC/C complex27. PTEN was also found to physically associate with replication protein A1 (RPA1), which is a subunit of the RPA single-strand DNA-binding protein complex essential for maintaining genomic integrity, to thereby stabilise DNA replication forks and to protect against replication stress28. In another study, PTEN was observed to interact with histone H1 via the C-terminal domains of both proteins, leading to the maintenance of chromatin condensation and integrity29. PTEN can also physically associate with and dephosphorylate MCM2, a subunit of the MCM2–7 protein complex of the replisome30,31, to restrict replication fork progression under replicative stress conditions to prevent DNA strand breaks32. Moreover, PTEN has been found to associate with stalled replication forks and recruit Rad51, a protein involved in DNA double-strand break repair, to facilitate stalled replication fork restart33. Overall, nuclear functions of PTEN are emerging as critical determinants of the tumour suppressor function of PTEN in disease34.
Recent advances in PTEN regulatory mechanisms
While the PTEN–PI3K axis is well established, there are a plethora of regulatory mechanisms feeding into PTEN and an equal number of downstream mechanisms by which PTEN can function, contributing to the ever-growing complexity of PTEN signalling in cancer. Among these mechanisms, post-translational modifications (PTMs) and protein–protein interactions (PPIs) have been demonstrated to exquisitely control PTEN stability, activity, and localisation. We present a collection of PTEN studies that represent major current findings and highlight exciting directions for future PTEN research.
PTEN phosphorylation
Novel signalling mechanisms by which inhibitory phosphorylation on the C-terminal tail of PTEN can regulate its tumour suppressive function have been recently uncovered35,36. Masson et al. previously identified six inhibitory phosphorylation sites in the PTEN C-terminal tail that effectively block both the phosphatase active site and the membrane-binding site of PTEN, where only the unphosphorylated state of PTEN was able to exert its phosphatase activity37. Phosphorylation status of T366 and S370 residues in PTEN were also found to influence its catalytic activity. When phosphorylated, these residues occluded the PTEN active site without affecting membrane binding. Notably, partial dephosphorylation at these sites allowed PTEN to act on only select substrates37. In supporting work, PDZ Domain Containing 1 (PDZK1) protein was shown to interact with and block phosphorylation of the C-terminal tail of PTEN to allow the PI3K pathway to remain suppressed38. Signalling through the PDZK1/PTEN/PI3K axis resulted in reduced growth and proliferation of gastric cancer (GC) cells38. Clinically, PDZK1 was low in GC patient specimens and was associated with poor disease prognosis38. In another study, the heat shock-like protein Clusterin was shown to increase AKT2 activity and promote the motility of both normal and malignant prostate cells via an inhibitory activity on PTEN-S380 phosphorylation and consequent inactivation of PTEN39. Clusterin was also found to specifically reduce the function of the AKT2-specific phosphatase PHLPP1 through miR‐19039. In sum, combined suppression of PTEN and PHLPP1 provides evidence for a Clusterin/PTEN/PHLPP1/AKT2 signalling axis involving regulation through miR-190 in prostate cells39. Altogether, these studies provide novel insights supporting the importance of C-terminal PTEN phosphorylation as a critical regulatory point of contact on the PTEN protein. Importantly, these findings demonstrate potential therapeutic targets that may mitigate cancer progression, at least in part through the regulation of PTEN phosphorylation at its C-terminus.
PTEN and ubiquitination
Second only to phosphorylation, PTEN ubiquitination is the most widely studied of all PTMs on PTEN. Indeed, intriguing insights into PTEN-associated cancers have been attributed to mechanisms associated with PTEN ubiquitination40–47, a recent example of which is a report demonstrating that the ubiquitin E3 ligase WWP1 can inhibit PTEN function by blocking its dimerisation and membrane recruitment48. This study proposed the existence of a putative MYC/WWP1/PTEN oncogenic axis, where WWP1 joins a list of thousands of genes transcriptionally regulated by the pleiotropic MYC oncoprotein48. Notably, the study of individuals with germline WWP1 variants identified gain-of-function effects that support a putative role for WWP1 as a cancer-susceptibility gene49. Finally, a natural compound called indole-3-carbinol was identified as a natural inhibitor of WWP1, thereby identifying a potential therapeutic strategy for cancer prevention and treatment through reactivation of PTEN function48.
In another study, the FOXO-regulated deubiquitinase (DUB) USP11 was identified to mediate a PTEN–PI3K autoregulatory loop50. This study uncovered that USP11/PTEN signalling integrates with PTEN/PI3K/AKT/FOXO signalling to generate a PTEN feedforward signalling network. Mechanistically, USP11 deubiquitinates PTEN to increase its stability, which promotes the inhibition of PI3K signalling50. Conversely, in cells where PI3K and AKT signalling is highly active, AKT-mediated phosphorylation promotes its cytoplasmic sequestration of FOXO. This event reduces USP11 expression and promotes ubiquitin-mediated PTEN degradation to sustain the feedforward PI3K activation that can drive malignant growth. The existence of the PTEN/PI3K/AKT/FOXO/USP11 axis confirms the importance of regulating PTEN stability in cancer.
Two new studies further highlight the importance of PTEN ubiquitination in cancer. First, RPN10, a ubiquitin receptor that is part of the 19S regulatory subunit of the 26S proteasome51, was found to promote PTEN ubiquitination and proteasomal degradation in hepatocellular carcinoma (HCC)52. Under hypoxic conditions, HIF1α translocation to the nucleus induced transcription of RPN10, leading to the increased degradation of PTEN, elevation of PI3K signalling, and accelerated growth and proliferation of HCC cells52. Second, LASP1, an actin-binding protein with roles in cytoskeletal organisation, was found to promote activation of the PI3K pathway and the progression of nasopharyngeal cancer (NPC) by promoting the ubiquitination-mediated degradation of PTEN53. The precise mechanism of how LASP1 promotes PTEN ubiquitination still remains elusive53. Both the LASP1/PTEN/PI3K/AKT/mTOR and the HIF1α/RPN10/PTEN/PI3K/AKT pathways represent new signalling axes that influence PTEN function and present novel therapeutic avenues.
In sum, these studies add to an increasing body of data demonstrating the diverse consequences of conjugation of monomeric ubiquitin or ubiquitin chains to PTEN including stability, cellular localisation, protein interactions, and catalytic activity54,55. The study by Lee et al. also exemplifies that agents with modulating effects on ubiquitin ligases and/or deubiquitinases may also be relevant targets for the development of therapies aiming to indirectly enhance PTEN expression48.
PTEN-interacting proteins
Advances in proteomic technologies and bioinformatic approaches for large-scale PPI mapping provide an attractive and emerging approach to identify novel therapeutics56. Through such work, novel insights into PTEN-associated PPIs and networks have been uncovered. As the complex interactome of PTEN is methodically unravelled, novel therapeutic approaches can be envisioned through the knowledge these studies provide. As such, PPIs represent potential therapeutic strategies to modulate endogenous levels of PTEN.
Novel studies have uncovered that DMBT1, a tumour suppressor in various cancers, can suppress PI3K pathway signalling through a stabilising interaction with PTEN57. In another study, FAM46C protein was demonstrated to inhibit prostate cancer (PCa) growth by promoting PTEN expression levels58. FAM46C stabilises PTEN protein by inhibiting ubiquitination to prevent its proteasomal degradation58. Sirtuin 6 (SIRT6) was recently reported to interact with PTEN, resulting in higher protein expression levels and lipid phosphatase activity in colon cancer cells59. The SIRT6–PTEN interaction was found to promote apoptosis and inhibit cell proliferation in vitro through inhibition of PI3K signalling, altogether revealing a novel SIRT6/PTEN/PI3K signalling axis with tumour suppressive capacity59.
An interesting comparative study found that the PTEN interactome shared a significant amount of overlap with the interactomes in autism spectrum disorders (ASD) and cancer, suggesting that PTEN is a crucial player in the biology of both diseases60. Moreover, this study identified that PTEN germline mutations leading to ASD induced a different conformation compared to germline mutations that led to cancer, which may perturb the PTEN interactome in different ways60. Given that both ASD and cancer are clinical manifestations of PTEN hamartoma tumour syndrome (PHTS)61–63, different germline mutations in PHTS individuals may govern which phenotype occurs by altering the PTEN interactome differently60. Overall, like PTMs, PTEN PPIs are emerging as important regulators of PTEN function.
Recent advances in PI3K-independent functions and beyond
An increasing amount of data suggests that both protein phosphatase activity and phosphatase-independent functions play roles in PTEN-mediated tumour suppression. Peculiarly, this is the case for most of the recently reported PTEN functions in the nucleus, where it has been characterised to have adaptor or scaffold functions. In sum, elucidating novel pathways that involve PTEN signalling will further our understanding and appreciation of PTEN’s role in protecting against tumorigenesis.
Nuclear PTEN
Nuclear transport of PTEN. A number of experimental and clinical observations have posited that nuclear localisation of PTEN is a contributor to its tumour suppressive functions. Indeed, PTEN is readily detectable in the nucleus of many healthy tissues, whereas nuclear exclusion of PTEN is frequently observed in advanced cancers64,65. A recent review on PTEN nuclear function by Ho and colleagues comprehensively described the current state of knowledge34. Studies examining PTEN in the nucleus have shed light on how it is transported, retained, or excluded from the nucleus. Mechanisms including monoubiquitination, sumoylation, and direct interactions have also been studied26,45,47,66–68. Many such studies utilise mutant PTEN species that harbour non-modifiable residues as clever molecular tools26,45,47,66–68. Data suggest that several lysine residues in PTEN have important roles in nuclear translocation mechanisms45,69.
In keeping with this theme, a new study has identified that the F-box only protein (FBXO22), a component of the SCF ubiquitin ligase complex, induces ubiquitylation at lysine 221 and degradation of nuclear but not cytoplasmic PTEN70. FBXO22 is overexpressed in various cancer types and contributes to the regulation of nuclear PTEN levels in colorectal cancer tissues70.
PTEN was also demonstrated to directly interact with the cytoplasmic protein myosin 1b (MYO1B)71, which is an actin-binding motor protein72. This interaction resulted in nuclear exclusion of PTEN, nuclear AKT activation, and suppression of cell apoptosis71. Furthermore, PHTS and ASD-associated germline PTEN Q17E mutant protein was reported to accumulate in the nucleus owing to changes in an N-terminal nuclear localisation sequence. The Q17E mutation and nuclear accumulation of PTEN were posited to have pathogenic effects73, illustrating that elevated levels of mutant Q17E PTEN are likely not well tolerated. Interestingly, a cytoplasmic localisation signal (CLS) was previously characterised to be adjacent to Q17 at the PTEN N-terminus, where mutations in this sequence induced PTEN nuclear localisation and subsequently impaired its tumour suppressive activity74. Given that Q17E resulted in the nuclear accumulation of PTEN73, this CLS could possibly include Q17. However, this study investigated the Q17A mutation and observed cytoplasmic localisation of PTEN74, which may suggest that only specific mutations at Q17 induce nuclear localisation.
Genome integrity and DNA damage. More than localisation, a clear understanding of the importance of PTEN nuclear function remains elusive. Roles for PTEN in DNA damage repair have gained momentum in recent years with studies showing the accumulation of DNA strand breaks in PTEN-deficient cells66. More recent contributions to this theme include the discovery that PTEN is a key scaffold protein in DNA repair complexes. One study showed that Nuclear Receptor Binding SET Domain Protein 2 (NSD2)-mediated dimethylation of PTEN promotes 53BP1 interactions and subsequent recruitment to sites of DNA-damage sites75. Another study demonstrated that phosphorylation of PTEN on tyrosine 240 by FGFR2 promotes chromatin binding through an interaction with Ki-67, which facilitates the recruitment of RAD51 to promote DNA repair76. Figure 3 summarises these novel functions and signalling axes of nuclear PTEN.
Figure 3. The complexity of PTEN signalling in the nucleus.
Schematic representation of the recent advances in PTEN nuclear biology. 53BP1, p53-binding protein 1; ARID4B, AT-rich interaction domain 4B; CENP-C, centromere protein C; FBXO22, F-box only protein; FGFR2, fibroblast growth factor receptor 2; MYO1B, myosin 1b; NSD2, nuclear receptor binding SET domain protein 2; PTEN, phosphatase and tensin homologue deleted on chromosome 10; RNAPII, RNA polymerase II.
PTEN-associated transcriptional signalling. As the repertoire of PTEN functions increases, a number of previously unappreciated roles for PTEN in the regulation of gene expression and processing of RNA transcripts have come to light in the last two years. It is known that AKT signaling plays a critical role in the regulation of pre-mRNA splicing77 and PTEN has been shown to modulate G6PD pre-mRNA splicing in an AKT-independent manner78. Newer studies add to this small body of data, including an intriguing study where a novel PTEN/ARID4B/PI3K pathway in which PTEN inhibits the expression of ARID4B was characterised. ARID4B is one of several members of the ARID gene family, which are chromatin remodelling factors. PTEN inhibits ARID4B expression and thus prevents the transcriptional activation of ARID4B transcriptional targets PIK3CA and PIK3R2 (PI3K subunits)79. This PTEN/ARID4B/PI3K signalling axis identifies a novel player in the PTEN-mediated suppression of the PI3K pathway and provides a new opportunity to design novel therapeutics to target this axis to promote the tumour suppressive functions of PTEN. Furthermore, nuclear PTEN directly interacted with and inhibited RNA polymerase II (RNAPII)-mediated transcription, where it was involved in direct downregulation of critical transcriptional control genes including AFF4 and POL2RA80. Similar findings were reported by Abbas et al., where PTEN was found to dephosphorylate the C-terminal domain of RNAPII, leading to its inhibition81. They also found that PTEN could modulate genome-wide transcription by redistributing RNAPII across the genome under conditions of metabolic stress82,83. Further roles for PTEN in transcriptional modulation were demonstrated in a report where nuclear PTEN interacted with spliceosomal proteins to promote pre-mRNA splicing in a phosphatase-independent manner84. PTEN was also found to be dimethylated at Arg159 by PRMT6; this methylation event was demonstrated to be involved in pre-mRNA alternative splicing85. Altogether, these studies identify roles for PTEN in global gene regulation and transcript processing that are consistent with previously reported changes in gene expression after loss of PTEN86,87. The extensive range of genes that are impacted by PTEN through these mechanisms provides further evidence of a complex role for nuclear PTEN.
PTEN and other oncogenic signalling pathways
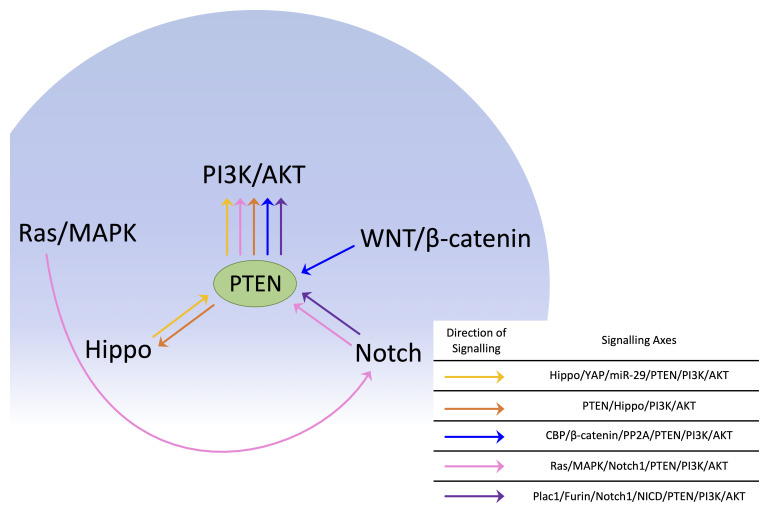
A large body of data demonstrates that PTEN signalling is involved in various cross-talks with other pathways88, including Hippo signalling, WNT/β-catenin signalling, and Notch pathways. A large majority of these cross-talk studies demonstrate an indirect association with PTEN through PI3K- and AKT-dependent mechanisms. In this section, we focus most of our discussions on those mechanisms where PTEN is directly linked to other pathways. For instance, the Hippo pathway was linked to the PI3K pathway through PTEN suppression via the induction of miR-29 by the Hippo pathway effector YAP6. A more recent study found that the inactivation of the lipid phosphatase activity of PTEN can inhibit the Hippo pathway by promoting the nuclear translocation of YAP and TAZ in GC. Hippo pathway inhibition allows oncogenic transcriptional programs to be induced89. These findings suggest that the tumorigenic effect of PTEN inactivation in GC is twofold, as Hippo inactivation is synergistic with the established derepression of PI3K signalling downstream of PTEN inactivation89.
Similarly, a large number of studies support that PI3K–AKT and WNT/β-catenin signalling pathways are highly connected. However, a new study highlights a direct interaction of PTEN with β-catenin and Wnt signalling90. This study investigated the role of CREB-binding protein (CBP)–β-catenin signalling on both the expression of the stem cell antigen CD133 and the PP2A–PTEN pathway in tumour-initiating cells (TICs) in liver cancer. CBP–β-catenin signalling regulated the levels of C-terminal PTEN phosphorylation in TICs and promoted stemness via CD133 induction. Overall, WNT/β-catenin was demonstrated to control PTEN phosphorylation via a PP2A-dependent mechanism90. This study provides a novel link between the two highly oncogenic PI3K and WNT/β-catenin pathways directly through PTEN in the form of a novel CBP/β-catenin/PP2A/PTEN/PI3K/AKT axis90.
PTEN and Notch have also been demonstrated to cross-talk extensively, mainly through PI3K- and AKT-dependent mechanisms. However, the evidence for direct interactions between PTEN and Notch signalling make up only a minority of those studies. In one of these studies, Baker et al. reported that Notch1 can mediate transcriptional suppression of PTEN, resulting in the derepression of PI3K signalling and development of trastuzumab resistance91. This study was the first to link the Ras–MAPK and PI3K pathways through Notch1 transcriptional suppression of PTEN91. Furthermore, the known cancer/testis antigen Plac1 was reported to interact with Furin, a proprotein processing enzyme92, to degrade Notch1 into Notch1 intracellular domain (NICD) fragments that undergo nuclear translocation to suppress PTEN transcription93, forming a Plac1/Furin/Notch1/NICD/PTEN signalling mechanism that results in transcriptional repression of PTEN and allows for the hyperactivation of AKT signalling in breast cancer (BC) cells93. Perhaps developing a small molecule to stabilise PPIs in the ASXL1–BAP1 complex could elevate the expression of PTEN and thereby tumour suppressive activity. Conversely, inhibiting the interaction of Plac1 with Furin could derepress PTEN expression. PTEN was also implicated in regulating epithelial–mesenchymal transition (EMT) and metastasis in tongue squamous cell carcinoma through a Numb/Notch1/RBP-Jκ/PTEN/p-FAK/EMT axis94. Numb inhibits Notch1, leading to the downregulation of RBP-Jκ94, which upregulates PTEN and anti-EMT effectors, leading to the downregulation of p-FAK and pro-EMT effectors94. However, the precise mechanisms remain elusive. Is the upregulation of PTEN due to increased transcription or reduced degradation? How does PTEN affect p-FAK levels? In spite of this, this report suggests yet another signalling axis in which PTEN is implicated.
Overall, each of these studies highlight the importance of PTEN signalling in protecting against tumorigenesis and build upon existing bodies of work on the complex crosstalk between PTEN signalling and other pathways. A further understanding of PTEN crosstalk with Hippo, WNT, and Notch signalling (Figure 4) and other signalling pathways in cancer will provide critical insights into an understanding of cancer development as well as novel therapeutic strategies and resistance pathways frequently observed in cancer relapse.
Figure 4. PTEN signalling in other major oncogenic pathways.
All of the major oncogenic pathways here signal through PTEN to affect the PI3K pathway. CBP, CREB-binding protein; MAPK, mitogen-activated protein kinase; miR-29, microRNA 29; NICD, Notch1 intracellular domain; PI3K, phosphoinositide 3-kinase; PTEN, phosphatase and tensin homologue deleted on chromosome 10; YAP, Yes-associated protein.
PTEN metabolic signalling
Metabolic reprogramming in cells is one of the hallmarks of cancer as described by Hanahan and Weinberg95. The Warburg effect is one of the most notable metabolic changes that takes place in cancerous cells, where cells become increasingly reliant on glycolysis compared to the more-efficient citric acid cycle96–98. In recent years, PTEN has been shown to be involved in the regulation of glycolysis in cancer cells; its loss or inactivation allows cells to become “Warburg-like” and become reliant on glycolysis, consequently making them more aggressive and resistant to chemotherapy. In a study by Qian et al., the protein phosphatase activity of PTEN was linked to metabolic changes that occur in tumorigenesis99. It was reported that PTEN could dephosphorylate PGK1, a glycolytic enzyme and protein kinase with a tumorigenic role in glioblastoma99. Dephosphorylation of PGK1 by PTEN was found to inhibit its activity, downstream glycolytic functions, and glioblastoma cell proliferation99, thereby presenting another mechanism in which PTEN functions as a tumour suppressor. Another role for PTEN in metabolic processes was reported in a study linking it to pyruvate dehydrogenase kinase 1 (PDHK1)100. In this study, PTEN was observed to dephosphorylate the NF-κB-activating protein (NKAP) and limit NF-κB activity and downstream transcriptional changes of target genes including PDHK1100. PTEN and PDHK1 were observed to have a synthetic-lethal relationship, as loss of PTEN and upregulation of PDHK1 in cells induced glycolysis and a dependency on PDHK1100. This was supported by observations that PTEN-deficient tumours have elevated PDHK1 levels, which is a biomarker for poor survival100. These data point to a potential PTEN/NKAP/NF-κB/PDHK1/glycolysis signalling axis that could potentially be targeted in PTEN-deficient cancers100.
In small-cell lung cancer (SCLC) cells, PTEN is targeted and suppressed by miR-214, which subsequently leaves the PI3K/AKT/mTOR pathway unopposed101. This was found to signal to hexokinase 2 (HK2) and pyruvate kinase isozyme 2 (PKM2), resulting in the upregulation of glycolysis and proliferation of SCLC cells101. Furthermore, inhibition of miR-214 resulted in the elevation of PTEN and downregulation of the PI3K/AKT/mTOR pathway and reversed the effects on glycolysis and proliferation101. This suggests that miR-214 and PTEN can signal onto HK2/PKM2 via the PI3K pathway in SCLC cells that regulates glycolysis and proliferation101. PTEN was also found to be involved in regulating glycolysis in refractory acute myeloid leukaemia (AML) cells, leading to the development of chemotherapy resistance102. In refractory AML cells, PTEN was depleted and phosphorylated AKT was increased compared to non-refractory cells102. Moreover, these changes in the PTEN/PI3K/AKT pathway were associated with increased glucose transporter 1 (GLUT1) and HK2 expression as well as lactate production102. Inhibition of AKT activity not only decreased proliferation and glycolysis in refractory AML cells but also sensitised these cells to chemotherapy102. The data from this study suggest that in refractory AML cells, depletion of PTEN and the unopposed hyperactivity of AKT result in the upregulation of glycolysis and subsequently confer resistance to chemotherapy102.
These studies provide more evidence that links PTEN to the regulation of glycolysis in cells. Indeed, suppressing glycolysis appears to be a major endpoint of PTEN tumour suppressive signalling. As the role of PTEN in glycolysis continues to expand, so will the number of possible signalling axes by which PTEN can regulate glycolysis. These new axes can then serve as potential targets in PTEN-deficient cancers that rely on glycolysis for tumorigenesis.
A major clinical challenge in PHTS is predicting which of these clinical manifestations individuals will develop63. Given that PTEN signalling has a role in metabolic reprogramming, particularly in glycolysis99,100 as we have described, it is intriguing that various tricarboxylic acid (TCA) cycle metabolites were found to be associated with various clinical manifestations of PHTS103. This metabolomic study identified that increased isocitrate and reduced citrate levels in PHTS individuals were associated with the development of BC103. Fumarate was also identified as a metabolite that was decreased in PHTS individuals who developed ASD compared to those who developed cancer103. The differential levels of these TCA metabolites and their association with clinical manifestations of PHTS103 could serve as a basis for the future development of prognostic metabolic biomarkers that could help predict the clinical progression of PHTS individuals.
PTEN isoforms
Several groups have identified alternative translational start sites upstream of the canonical PTEN start codon, resulting in the production of PTEN isoforms with an extended N-terminus104–106. To date, only two isoforms have been described: PTENα (or PTEN-Long)104,105 and PTENβ106. PTEN isoforms including PTENα and PTENβ have been reported to function both in and beyond the PI3K pathway, adding more complexity to the field of PTEN signalling biology (Figure 5).
Figure 5. PTEN isoform signalling.
Schematic representation of signalling axes involving the PTENα and PTENβ isoforms. Interestingly, PTENα/β appear to have tumour promoting functions that are in contrast to canonical PTEN. FBXW11, F-box/WD repeat-containing protein 11; PTEN, phosphatase and tensin homologue deleted on chromosome 10; PRKN, parkin RBR E3 ubiquitin protein ligase; USP9X, ubiquitin-specific peptidase 9, X-linked; WDR5, WD repeat-containing protein 5.
Initial characterisation of PTENα revealed that this isoform is membrane permeable, is secreted from cells, and can be taken up by neighbouring cells104. Indeed, exogenous PTENα was identified to oppose the PI3K pathway in the receiving cells and induced in vitro and in vivo cell death104. These data present a potential approach to restoring PTEN levels in deficient cells that could be explored in future studies104. The discovery of the PTENα isoform was subsequently confirmed by Liang et al. using mass spectrometry, where it was revealed to be co-localised with canonical PTEN at the mitochondria, suggesting a role in mitochondrial signalling105. Colocalisation of PTEN/PTENα promoted the function of PINK1, a mitochondrial-target kinase, and subsequently promoted energy production105. PTENα was also shown to play a role in regulating mitophagy through a direct interaction with the mitophagy initiator protein PRKN107. PTENα promotes PRKN self-association at the mitochondria in a PTENα phosphatase-independent manner107. PTENα/PRKN signalling in mitophagy was supported by evidence demonstrating that the PTENα–PRKN interaction was stronger when mitochondria were damaged and depolarised107. PTENα was also reported to regulate neutrophil morphology and chemotaxis through direct binding and dephosphorylation of Thr558 on moesin, a membrane cross-linking protein108. Moesin dephosphorylation disrupts actin filaments that are associated with the plasma membrane and results in morphologic changes in neutrophil pseudopodia that are required during chemotaxis108. This evidence suggests a role for PTENα, its protein phosphatase activity, and its signalling at the plasma membrane in the regulation of neutrophil morphology and chemotaxis.
PTENβ was more recently identified and has a longer N-terminus than both PTENα and canonical PTEN106. Liang et al. characterised the localisation of PTENβ at the nucleolus, where it interacts with and dephosphorylates Thr84 on nucleolin106. Interaction of PTENβ with nucleolin, a nucleolar protein that is essential in ribosomal biogenesis109,110, points to a role in ribosomes and translation106. Indeed, PTENβ overexpression was found to regulate rDNA transcription, and inhibiting PTENβ results in the promotion106 of ribosomal biogenesis. It was concluded that PTENβ regulates cell proliferation through regulating ribosomal biogenesis; however, an exact signalling mechanism has not been characterised and requires future study.
Given the renowned and classical role of canonical PTEN in tumour suppression, it is plausible to hypothesise that PTENα/β have similar tumour suppressive functions. However, Shen et al. demonstrated that PTENα/β isoforms may also be tumour promoting in specific contexts, in contrast to canonical PTEN111. Mechanistically, the isoforms were able to promote tumorigenesis by interacting with WDR5 and activating trimethylation of histone H3 lysine 4 (H3K4), which could maintain the expression of a tumour-promoting gene signature111. PTENα and PTENβ were also observed to be regulated by ubiquitin-specific peptidase 9, X-linked (USP9X), and F-box/WD repeat-containing protein 11 (FBXW11) through interactions with lysine residues on their extended N-terminal regions111. This study presents intriguing first evidence of a contrasting role for PTEN isoforms in the tumorigenic process111. Future studies are required to confirm these newly identified functions. Overall, the evidence presented from this study points to a more complex signalling network of PTEN and its isoforms than previously envisioned and raises questions about the established tumour suppressive role of PTEN.
Future directions and conclusion
It is evident that there is still much to learn about PTEN, as shown by the continuous high pace of discovery. As technological approaches continue to advance, the ability to measure, monitor, detect, visualise, and experimentally manipulate PTEN in vitro and in vivo brings forth the understanding of novel features of this extraordinary gene and protein. While this review mainly focused on PTEN signalling in cancer, PTEN signalling has been implicated in a variety of other diseases such as PHTS61–63, autoimmunity and immunological functions112, and other neurodevelopmental disorders113; future studies should be aimed at further understanding the role of PTEN signalling in these contexts and how it relates to its renowned function in cancer. Novel PTEN-linked signalling axes revealed by new studies present additional novel approaches for targeting the PTEN pathway for a wide range of diseases, both in and beyond cancer.
The peer reviewers who approve this article are:
Britta J. Eickholt, Department of Biochemistry, Charité-Universitätsmedizin Berlin, Charitéplatz 1, 10117 Berlin, Germany
Charis Eng, Genomic Medicine Institute, Lerner Research Institute, Cleveland Clinic, Cleveland, OH, USA; Taussig Cancer Institute, Cleveland Clinic, Cleveland, OH, USA; Department of Genetics and Genome Sciences, Case Western Reserve University School of Medicine, Cleveland, OH, USA; Germline High Risk Cancer Focus Group, Case Comprehensive Cancer Center, Case Western Reserve University, Cleveland, OH, USA
Nicholas R. Leslie, Institute of Biological Chemistry, Biophysics and Bioengineering, Heriot Watt University, Edinburgh, Scotland, UK
Yuxin Yin, Institute of Systems Biomedicine, Beijing Key Laboratory of Tumor Systems Biology, School of Basic Medical Sciences, Peking University Health Science Center, Beijing 100191, China; Peking-Tsinghua Center for Life Sciences, Peking University Health Science Center, Beijing 100191, China
Funding Statement
Leonardo Salmena is a recipient of a Tier II Canada Research Chair (950-232598). This work was supported by funds to LS from a Career Development Award (CDA00079/2011-C) from the Human Frontier Science Program and an Operating Grant from the Leukemia and Lymphoma Society of Canada (LLSC; 569015).
Jonathan Tak-Sum Chow is supported by an Ontario Graduate Scholarship (OGS).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Maehama T, Dixon JE: The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998; 273(22): 13375–8. 10.1074/jbc.273.22.13375 [DOI] [PubMed] [Google Scholar]
- 2. Ramaswamy S, Nakamura N, Vazquez F, et al. : Regulation of G1 progression by the PTEN tumor suppressor protein is linked to inhibition of the phosphatidylinositol 3-kinase/Akt pathway. Proc Natl Acad Sci U S A. 1999; 96(5): 2110–5. 10.1073/pnas.96.5.2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weng LP, Smith WM, Brown JL, et al. : PTEN inhibits insulin-stimulated MEK/MAPK activation and cell growth by blocking IRS-1 phosphorylation and IRS-1/Grb-2/Sos complex formation in a breast cancer model. Hum Mol Genet. 2001; 10(6): 605–16. 10.1093/hmg/10.6.605 [DOI] [PubMed] [Google Scholar]
- 4. Ong SH, Hadari YR, Gotoh N, et al. : Stimulation of phosphatidylinositol 3-kinase by fibroblast growth factor receptors is mediated by coordinated recruitment of multiple docking proteins. Proc Natl Acad Sci U S A. 2001; 98(11): 6074–9. 10.1073/pnas.111114298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yart A, Laffargue M, Mayeux P, et al. : A critical role for phosphoinositide 3-kinase upstream of Gab1 and SHP2 in the activation of ras and mitogen-activated protein kinases by epidermal growth factor. J Biol Chem. 2001; 276(12): 8856–64. 10.1074/jbc.M006966200 [DOI] [PubMed] [Google Scholar]
- 6. Tumaneng K, Schlegelmilch K, Russell RC, et al. : YAP mediates crosstalk between the Hippo and PI(3)K-TOR pathways by suppressing PTEN via miR-29. Nat Cell Biol. 2012; 14(12): 1322–9. 10.1038/ncb2615 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 7. Persad A, Venkateswaran G, Hao L, et al. : Active β-catenin is regulated by the PTEN/PI3 kinase pathway: A role for protein phosphatase PP2A. Genes Cancer. 2016; 7(11–12): 368–82. 10.18632/genesandcancer.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang S, Yu D: PI(3)king apart PTEN's role in cancer. Clin Cancer Res. 2010; 16(17): 4325–30. 10.1158/1078-0432.CCR-09-2990 [DOI] [PubMed] [Google Scholar]
- 9. Lee YR, Chen M, Pandolfi PP: The functions and regulation of the PTEN tumour suppressor: New modes and prospects. Nat Rev Mol Cell Biol. 2018; 19(9): 547–62. 10.1038/s41580-018-0015-0 [DOI] [PubMed] [Google Scholar]
- 10. Song MS, Salmena L, Pandolfi PP: The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol. 2012; 13(5): 283–96. 10.1038/nrm3330 [DOI] [PubMed] [Google Scholar]
- 11. Suzuki H, Freije D, Nusskern DR, et al. : Interfocal heterogeneity of PTEN/MMAC1 gene alterations in multiple metastatic prostate cancer tissues. Cancer Res. 1998; 58(2): 204–9. [PubMed] [Google Scholar]
- 12. Feilotter HE, Nagai MA, Boag AH, et al. : Analysis of PTEN and the 10q23 region in primary prostate carcinomas. Oncogene. 1998; 16(13): 1743–8. 10.1038/sj.onc.1200205 [DOI] [PubMed] [Google Scholar]
- 13. Myers MP, Stolarov JP, Eng C, et al. : P-TEN, the tumor suppressor from human chromosome 10q23, is a dual-specificity phosphatase. Proc Natl Acad Sci U S A. 1997; 94(17): 9052–7. 10.1073/pnas.94.17.9052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tamura M, Gu J, Matsumoto K, et al. : Inhibition of cell migration, spreading, and focal adhesions by tumor suppressor PTEN. Science. 1998; 280(5369): 1614–7. 10.1126/science.280.5369.1614 [DOI] [PubMed] [Google Scholar]
- 15. Gu J, Tamura M, Yamada KM: Tumor suppressor PTEN inhibits integrin- and growth factor-mediated mitogen-activated protein (MAP) kinase signaling pathways. J Cell Biol. 1998; 143(5): 1375–83. 10.1083/jcb.143.5.1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Weng LP, Brown JL, Eng C: PTEN coordinates G(1) arrest by down-regulating cyclin D1 via its protein phosphatase activity and up-regulating p27 via its lipid phosphatase activity in a breast cancer model. Hum Mol Genet. 2001; 10(6): 599–604. 10.1093/hmg/10.6.599 [DOI] [PubMed] [Google Scholar]
- 17. Chu EC, Tarnawski AS: PTEN regulatory functions in tumor suppression and cell biology. Med Sci Monit. 2004; 10(10): RA235–41. [PubMed] [Google Scholar]
- 18. Leslie NR, Maccario H, Spinelli L, et al. : The significance of PTEN's protein phosphatase activity. Adv Enzyme Regul. 2009; 49(1): 190–6. 10.1016/j.advenzreg.2008.12.002 [DOI] [PubMed] [Google Scholar]
- 19. Wang H, Karikomi M, Naidu S, et al. : Allele-specific tumor spectrum in pten knockin mice. Proc Natl Acad Sci U S A. 2010; 107(11): 5142–7. 10.1073/pnas.0912524107 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 20. Papa A, Wan L, Bonora M, et al. : Cancer-associated PTEN mutants act in a dominant-negative manner to suppress PTEN protein function. Cell. 2014; 157(3): 595–610. 10.1016/j.cell.2014.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liaw D, Marsh DJ, Li J, et al. : Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat Genet. 1997; 16(1): 64–7. 10.1038/ng0597-64 [DOI] [PubMed] [Google Scholar]
- 22. Lee JO, Yang H, Georgescu MM, et al. : Crystal structure of the PTEN tumor suppressor: implications for its phosphoinositide phosphatase activity and membrane association. Cell. 1999; 99(3): 323–34. 10.1016/s0092-8674(00)81663-3 [DOI] [PubMed] [Google Scholar]
- 23. Di Cristofano A, Pesce B, Cordon-Cardo C, et al. : Pten is essential for embryonic development and tumour suppression. Nat Genet. 1998; 19(4): 348–55. 10.1038/1235 [DOI] [PubMed] [Google Scholar]
- 24. Suzuki A, de La Pompa JL, Stambolic V, et al. : High cancer susceptibility and embryonic lethality associated with mutation of the PTEN tumor suppressor gene in mice. Curr Biol. 1998; 8(21): 1169–78. 10.1016/s0960-9822(07)00488-5 [DOI] [PubMed] [Google Scholar]
- 25. Davidson L, Maccario H, Perera NM, et al. : Suppression of cellular proliferation and invasion by the concerted lipid and protein phosphatase activities of PTEN. Oncogene. 2010; 29(5): 687–97. 10.1038/onc.2009.384 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 26. Shen WH, Balajee AS, Wang J, et al. : Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell. 2007; 128(1): 157–70. 10.1016/j.cell.2006.11.042 [DOI] [PubMed] [Google Scholar]
- 27. Song MS, Carracedo A, Salmena L, et al. : Nuclear PTEN regulates the APC-CDH1 tumor-suppressive complex in a phosphatase-independent manner. Cell. 2011; 144(2): 187–99. 10.1016/j.cell.2010.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 28. Wang G, Li Y, Wang P, et al. : PTEN regulates RPA1 and protects DNA replication forks. Cell Res. 2015; 25(11): 1189–204. 10.1038/cr.2015.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen ZH, Zhu M, Yang J, et al. : PTEN interacts with histone H1 and controls chromatin condensation. Cell Rep. 2014; 8(6): 2003–14. 10.1016/j.celrep.2014.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Labib K, Tercero JA, Diffley JF: Uninterrupted MCM2-7 function required for DNA replication fork progression. Science. 2000; 288(5471): 1643–7. 10.1126/science.288.5471.1643 [DOI] [PubMed] [Google Scholar]
- 31. Bochman ML, Schwacha A: The Mcm2-7 complex has in vitro helicase activity. Mol Cell. 2008; 31(2): 287–93. 10.1016/j.molcel.2008.05.020 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 32. Feng J, Liang J, Li J, et al. : PTEN Controls the DNA Replication Process through MCM2 in Response to Replicative Stress. Cell Rep. 2015; 13(7): 1295–303. 10.1016/j.celrep.2015.10.016 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 33. He J, Kang X, Yin Y, et al. : PTEN regulates DNA replication progression and stalled fork recovery. Nat Commun. 2015; 6: 7620. 10.1038/ncomms8620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ho J, Cruise ES, Dowling RJO, et al. : PTEN Nuclear Functions. Cold Spring Harb Perspect Med. 2020; 10(5): a036079. 10.1101/cshperspect.a036079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vazquez F, Ramaswamy S, Nakamura N, et al. : Phosphorylation of the PTEN tail regulates protein stability and function. Mol Cell Biol. 2000; 20(14): 5010–8. 10.1128/mcb.20.14.5010-5018.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yang Z, Xie C, Xu W, et al. : Phosphorylation and inactivation of PTEN at residues Ser380/Thr382/383 induced by Helicobacter pylori promotes gastric epithelial cell survival through PI3K/Akt pathway. Oncotarget. 2015; 6(31): 31916–26. 10.18632/oncotarget.5577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Masson GR, Perisic O, Burke JE, et al. : The intrinsically disordered tails of PTEN and PTEN-L have distinct roles in regulating substrate specificity and membrane activity. Biochem J. 2016; 473(2): 135–44. 10.1042/BJ20150931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhao C, Tao T, Yang L, et al. : Loss of PDZK1 expression activates PI3K/AKT signaling via PTEN phosphorylation in gastric cancer. Cancer Lett. 2019; 453: 107–21. 10.1016/j.canlet.2019.03.043 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 39. Bertacchini J, Mediani L, Beretti F, et al. : Clusterin enhances AKT2-mediated motility of normal and cancer prostate cells through a PTEN and PHLPP1 circuit. J Cell Physiol. 2019; 234(7): 11188–99. 10.1002/jcp.27768 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 40. Yuan L, Lv Y, Li H, et al. : Deubiquitylase OTUD3 regulates PTEN stability and suppresses tumorigenesis. Nat Cell Biol. 2015; 17(9): 1169–81. 10.1038/ncb3218 [DOI] [PubMed] [Google Scholar]
- 41. Lee MS, Jeong MH, Lee HW, et al. : PI3K/AKT activation induces PTEN ubiquitination and destabilization accelerating tumourigenesis. Nat Commun. 2015; 6: 7769. 10.1038/ncomms8769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Maddika S, Kavela S, Rani N, et al. : WWP2 is an E3 ubiquitin ligase for PTEN. Nat Cell Biol. 2011; 13(6): 728–33. 10.1038/ncb2240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Maccario H, Perera NM, Gray A, et al. : Ubiquitination of PTEN (phosphatase and tensin homolog) inhibits phosphatase activity and is enhanced by membrane targeting and hyperosmotic stress. J Biol Chem. 2010; 285(17): 12620–8. 10.1074/jbc.M109.072280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang J, Zhang P, Wei Y, et al. : Deubiquitylation and stabilization of PTEN by USP13. Nat Cell Biol. 2013; 15(12): 1486–94. 10.1038/ncb2874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Trotman LC, Wang X, Alimonti A, et al. : Ubiquitination regulates PTEN nuclear import and tumor suppression. Cell. 2007; 128(1): 141–56. 10.1016/j.cell.2006.11.040 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 46. Wang X, Trotman LC, Koppie T, et al. : NEDD4-1 is a proto-oncogenic ubiquitin ligase for PTEN. Cell. 2007; 128(1): 129–39. 10.1016/j.cell.2006.11.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Song MS, Salmena L, Carracedo A, et al. : The deubiquitinylation and localization of PTEN are regulated by a HAUSP-PML network. Nature. 2008; 455(7214): 813–7. 10.1038/nature07290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lee Y-R, Chen M, Lee JD, et al. : Reactivation of PTEN tumor suppressor for cancer treatment through inhibition of a MYC-WWP1 inhibitory pathway. Science. 2019; 364(6441): eaau0159. 10.1126/science.aau0159 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 49. Lee Y-R, Yehia L, Kishikawa T, et al. : WWP1 Gain-of-Function Inactivation of PTEN in Cancer Predisposition. N Engl J Med. 2020; 382(22): 2103–16. 10.1056/NEJMoa1914919 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 50. Park MK, Yao Y, Xia W, et al. : PTEN self-regulates through USP11 via the PI3K-FOXO pathway to stabilize tumor suppression. Nat Commun. 2019; 10(1): 636. 10.1038/s41467-019-08481-x [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 51. Collins GA, Goldberg AL: The Logic of the 26S Proteasome. Cell. 2017; 169(5): 792–806. 10.1016/j.cell.2017.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jiang Z, Zhou Q, Ge C, et al. : Rpn10 promotes tumor progression by regulating hypoxia-inducible factor 1 alpha through the PTEN/Akt signaling pathway in hepatocellular carcinoma. Cancer Lett. 2019; 447: 1–11. 10.1016/j.canlet.2019.01.020 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 53. Gao Q, Tang L, Wu L, et al. : LASP1 promotes nasopharyngeal carcinoma progression through negatively regulation of the tumor suppressor PTEN. Cell Death Dis. 2018; 9(3): 393. 10.1038/s41419-018-0443-y [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 54. Xia Q, Ali S, Liu L, et al. : Role of Ubiquitination in PTEN Cellular Homeostasis and Its Implications in GB Drug Resistance. Front Oncol. 2020; 10: 1569. 10.3389/fonc.2020.01569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Deng L, Meng T, Chen L, et al. : The role of ubiquitination in tumorigenesis and targeted drug discovery. Signal Transduct Target Ther. 2020; 5(1): 11. 10.1038/s41392-020-0107-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mabonga L, Kappo AP: Protein-protein interaction modulators: Advances, successes and remaining challenges. Biophys Rev. 2019; 11(4): 559–81. 10.1007/s12551-019-00570-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sheng S, Jiwen W, Dexiang Z, et al. : DMBT1 suppresses progression of gallbladder carcinoma through PI3K/AKT signaling pathway by targeting PTEN. Biosci Biotechnol Biochem. 2019; 83(12): 2257–64. 10.1080/09168451.2019.1654361 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 58. Ma L, He H, Jiang K, et al. : FAM46C inhibits cell proliferation and cell cycle progression and promotes apoptosis through PTEN/AKT signaling pathway and is associated with chemosensitivity in prostate cancer. Aging (Albany NY). 2020; 12(7): 6352–69. 10.18632/aging.103030 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 59. Tian J, Yuan L: Sirtuin 6 inhibits colon cancer progression by modulating PTEN/AKT signaling. Biomed Pharmacother. 2018; 106: 109–16. 10.1016/j.biopha.2018.06.070 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 60. Smith IN, Thacker S, Seyfi M: Conformational Dynamics and Allosteric Regulation Landscapes of Germline PTEN Mutations Associated with Autism Compared to Those Associated with Cancer. Am J Hum Genet. 2019; 104(5): 861–78. 10.1016/j.ajhg.2019.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 61. Mester J, Charis E: PTEN hamartoma tumor syndrome. Handb Clin Neurol. 2015; 132: 129–37. 10.1016/B978-0-444-62702-5.00009-3 [DOI] [PubMed] [Google Scholar]
- 62. Yehia L, Keel E, Eng C: The Clinical Spectrum of PTEN Mutations. Annu Rev Med. 2020; 71: 103–16. 10.1146/annurev-med-052218-125823 [DOI] [PubMed] [Google Scholar]
- 63. Yehia L, Ngeow J, Eng C: PTEN-opathies: From biological insights to evidence-based precision medicine. J Clin Invest. 2019; 129(2): 452–64. 10.1172/JCI121277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Whiteman DC, Zhou XP, Cummings MC, et al. : Nuclear PTEN expression and clinicopathologic features in a population-based series of primary cutaneous melanoma. Int J Cancer. 2002; 99(1): 63–7. [DOI] [PubMed] [Google Scholar]
- 65. Gimm O, Perren A, Weng LP, et al. : Differential Nuclear and Cytoplasmic Expression of PTEN in Normal Thyroid Tissue, and Benign and Malignant Epithelial Thyroid Tumors. Am J Pathol. 2000; 156(5): 1693–700. 10.1016/S0002-9440(10)65040-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bassi C, Ho J, Srikumar T, et al. : Nuclear PTEN controls DNA repair and sensitivity to genotoxic stress. Science. 2013; 341(6144): 395–9. 10.1126/science.1236188 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 67. He X, Ni Y, Wang Y, et al. : Naturally occurring germline and tumor-associated mutations within the ATP-binding motifs of PTEN lead to oxidative damage of DNA associated with decreased nuclear p53. Hum Mol Genet. 2011; 20(1): 80–9. 10.1093/hmg/ddq434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lobo GP, Waite KA, Planchon SM, et al. : Germline and somatic cancer-associated mutations in the ATP-binding motifs of PTEN influence its subcellular localization and tumor suppressive function. Hum Mol Genet. 2009; 18(15): 2851–62. 10.1093/hmg/ddp220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Salmena L, Pandolfi PP: Changing venues for tumour suppression: Balancing destruction and localization by monoubiquitylation. Nat Rev Cancer. 2007; 7(6): 409–13. 10.1038/nrc2145 [DOI] [PubMed] [Google Scholar]
- 70. Ge MK, Zhang N, Xia L, et al. : FBXO22 degrades nuclear PTEN to promote tumorigenesis. Nat Commun. 2020; 11(1): 1720. 10.1038/s41467-020-15578-1 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 71. Yu Y, Xiong Y, Ladeiras D, et al. : Myosin 1b Regulates Nuclear AKT Activation by Preventing Localization of PTEN in the Nucleus. iScience. 2019; 19: 39–53. 10.1016/j.isci.2019.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 72. Komaba S, Coluccio LM: Localization of myosin 1b to actin protrusions requires phosphoinositide binding. J Biol Chem. 2010; 285(36): 27686–93. 10.1074/jbc.M109.087270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mingo J, Rodríguez-Escudero I, Luna S, et al. : A pathogenic role for germline PTEN variants which accumulate into the nucleus. Eur J Hum Genet. 2018; 26(8): 1180–7. 10.1038/s41431-018-0155-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Denning G, Jean-Joseph B, Prince C, et al. : A short N-terminal sequence of PTEN controls cytoplasmic localization and is required for suppression of cell growth. Oncogene. 2007; 26(27): 3930–40. 10.1038/sj.onc.1210175 [DOI] [PubMed] [Google Scholar]
- 75. Zhang J, Lee YR, Dang F, et al. : PTEN Methylation by NSD2 Controls Cellular Sensitivity to DNA Damage. Cancer Discov. 2019; 9(9): 1306–23. 10.1158/2159-8290.CD-18-0083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ma J, Benitez JA, Li J, et al. : Inhibition of Nuclear PTEN Tyrosine Phosphorylation Enhances Glioma Radiation Sensitivity through Attenuated DNA Repair. Cancer Cell. 2019; 35(3): 504–518.e7. 10.1016/j.ccell.2019.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 77. Lee G, Blenis J: Akt-ivation of RNA splicing. Mol Cell. 2014; 53(4): 519–20. 10.1016/j.molcel.2014.02.010 [DOI] [PubMed] [Google Scholar]
- 78. Hong X, Song R, Song H, et al. : PTEN antagonises Tcl1/hnRNPK-mediated G6PD pre-mRNA splicing which contributes to hepatocarcinogenesis. Gut. 2014; 63(10): 1635–47. 10.1136/gutjnl-2013-305302 [DOI] [PubMed] [Google Scholar]
- 79. Wu RC, Young IC, Chen YF, et al. : Identification of the PTEN-ARID4B-PI3K pathway reveals the dependency on ARID4B by PTEN-deficient prostate cancer. Nat Commun. 2019; 10(1): 4332. 10.1038/s41467-019-12184-8 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 80. Steinbach N, Hasson D, Mathur D, et al. : PTEN interacts with the transcription machinery on chromatin and regulates RNA polymerase II-mediated transcription. Nucleic Acids Res. 2019; 47(11): 5573–86. 10.1093/nar/gkz272 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 81. Abbas A, Romigh T, Eng C: PTEN interacts with RNA polymerase II to dephosphorylate polymerase II C-terminal domain. Oncotarget. 2019; 10(48): 4951–9. 10.18632/oncotarget.27128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Abbas A, Padmanabhan R, Romigh T, et al. : PTEN modulates gene transcription by redistributing genome-wide RNA polymerase II occupancy. Hum Mol Genet. 2019; 28(17): 2826–34. 10.1093/hmg/ddz112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Abbas A, Padmanabhan R, Eng C: Metabolic stress regulates genome-wide transcription in a PTEN-dependent manner. Hum Mol Genet. 2020; 29(16): 2736–45. 10.1093/hmg/ddaa168 [DOI] [PubMed] [Google Scholar]
- 84. Shen SM, Ji Y, Zhang C, et al. : Nuclear PTEN safeguards pre-mRNA splicing to link Golgi apparatus for its tumor suppressive role. Nat Commun. 2018; 9(1): 2392. 10.1038/s41467-018-04760-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Feng J, Dang Y, Zhang W, et al. : PTEN arginine methylation by PRMT6 suppresses PI3K-AKT signaling and modulates pre-mRNA splicing. Proc Natl Acad Sci U S A. 2019; 116(14): 6868–77. 10.1073/pnas.1811028116 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 86. Tibarewal P, Zilidis G, Spinelli L, et al. : PTEN protein phosphatase activity correlates with control of gene expression and invasion, a tumor-suppressing phenotype, but not with AKT activity. Sci Signal. 2012; 5(213): ra18. 10.1126/scisignal.2002138 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 87. Saal LH, Johansson P, Holm K, et al. : Poor prognosis in carcinoma is associated with a gene expression signature of aberrant PTEN tumor suppressor pathway activity. Proc Natl Acad Sci U S A. 2007; 104(18): 7564–9. 10.1073/pnas.0702507104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Carracedo A, Pandolfi PP: The PTEN-PI3K pathway: of feedbacks and cross-talks. Oncogene. 2008; 27(41): 5527–41. 10.1038/onc.2008.247 [DOI] [PubMed] [Google Scholar]
- 89. Xu W, Yang Z, Xie C, et al. : PTEN lipid phosphatase inactivation links the hippo and PI3K/Akt pathways to induce gastric tumorigenesis. J Exp Clin Cancer Res. 2018; 37(1): 198. 10.1186/s13046-018-0795-2 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 90. Tang Y, Berlind J, Mavila N: Inhibition of CREB binding protein-beta-catenin signaling down regulates CD133 expression and activates PP2A-PTEN signaling in tumor initiating liver cancer cells. Cell Commun Signal. 2018; 16(1): 9. 10.1186/s12964-018-0222-5 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 91. Baker A, Wyatt D, Bocchetta M, et al. : Notch-1-PTEN-ERK1/2 signaling axis promotes HER2+ breast cancer cell proliferation and stem cell survival. Oncogene. 2018; 37(33): 4489–504. 10.1038/s41388-018-0251-y [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 92. Wise RJ, Barr PJ, Wong PA, et al. : Expression of a human proprotein processing enzyme: correct cleavage of the von Willebrand factor precursor at a paired basic amino acid site. Proc Natl Acad Sci U S A. 1990; 87(23): 9378–82. 10.1073/pnas.87.23.9378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Li Y, Chu J, Li J, et al. : Cancer/testis antigen-Plac1 promotes invasion and metastasis of breast cancer through Furin/NICD/PTEN signaling pathway. Mol Oncol. 2018; 12(8): 1233–48. 10.1002/1878-0261.12311 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 94. Li JY, Huang WX, Zhou X, et al. : Numb inhibits epithelial-mesenchymal transition via RBP-Jκ-dependent Notch1/PTEN/FAK signaling pathway in tongue cancer. BMC Cancer. 2019; 19(1): 391. 10.1186/s12885-019-5605-5 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]; Faculty Opinions Recommendation
- 95. Hanahan D, Weinberg RA: Hallmarks of cancer: the next generation. Cell. 2011; 144(5): 646–74. 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 96. Warburg O: On the origin of cancer cells. Science. 1956; 123(3191): 309–14. 10.1126/science.123.3191.309 [DOI] [PubMed] [Google Scholar]
- 97. Warburg O: On respiratory impairment in cancer cells. Science. 1956; 124(3215): 269–70. [PubMed] [Google Scholar]
- 98. Vander Heiden MG, Cantley LC, Thompson CB: Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009; 324(5930): 1029–33. 10.1126/science.1160809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Qian X, Li X, Shi Z, et al. : PTEN Suppresses Glycolysis by Dephosphorylating and Inhibiting Autophosphorylated PGK1. Mol Cell. 2019; 76(3): 516–527.e7. 10.1016/j.molcel.2019.08.006 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 100. Chatterjee N, Pazarentzos E, Mayekar MK, et al. : Synthetic Essentiality of Metabolic Regulator PDHK1 in PTEN-Deficient Cells and Cancers. Cell Rep. 2019; 28(9): 2317–2330.e8. 10.1016/j.celrep.2019.07.063 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 101. Zhang K, Zhang M, Jiang H, et al. : Down-regulation of miR-214 inhibits proliferation and glycolysis in non-small-cell lung cancer cells via down-regulating the expression of hexokinase 2 and pyruvate kinase isozyme M2. Biomed Pharmacother. 2018; 105: 545–52. 10.1016/j.biopha.2018.06.009 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 102. Ryu MJ, Han J, Kim SJ, et al. : PTEN/AKT signaling mediates chemoresistance in refractory acute myeloid leukemia through enhanced glycolysis. Oncol Rep. 2019; 42(5): 2149–58. 10.3892/or.2019.7308 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 103. Yehia L, Ni Y, Feng F, et al. : Distinct Alterations in Tricarboxylic Acid Cycle Metabolites Associate with Cancer and Autism Phenotypes in Cowden Syndrome and Bannayan-Riley-Ruvalcaba Syndrome. Am J Hum Genet. 2019; 105(4): 813–21. 10.1016/j.ajhg.2019.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 104. Hopkins BD, Fine B, Steinbach N, et al. : A secreted PTEN phosphatase that enters cells to alter signaling and survival. Science. 2013; 341(6144): 399–402. 10.1126/science.1234907 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 105. Liang H, He S, Yang J, et al. : PTENα, a PTEN isoform translated through alternative initiation, regulates mitochondrial function and energy metabolism. Cell Metab. 2014; 19(5): 836–48. 10.1016/j.cmet.2014.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Liang H, Chen X, Yin Q, et al. : PTENβ is an alternatively translated isoform of PTEN that regulates rDNA transcription. Nat Commun. 2017; 8: 14771. 10.1038/ncomms14771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Li G, Yang J, Yang C, et al. : PTENα regulates mitophagy and maintains mitochondrial quality control. Autophagy. 2018; 14(10): 1742–60. 10.1080/15548627.2018.1489477 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 108. Li Y, Jin Y, Liu B, et al. : PTENα promotes neutrophil chemotaxis through regulation of cell deformability. Blood. 2019; 133(19): 2079–89. 10.1182/blood-2019-01-899864 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 109. Ginisty H, Amalric F, Bouvet P: Nucleolin functions in the first step of ribosomal RNA processing. EMBO J. 1998; 17(5): 1476–86. 10.1093/emboj/17.5.1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Tuteja R, Tuteja N: Nucleolin: A multifunctional major nucleolar phosphoprotein. Crit Rev Biochem Mol Biol. 1998; 33(6): 407–36. 10.1080/10409239891204260 [DOI] [PubMed] [Google Scholar]
- 111. Shen SM, Zhang SM, Ge MK, et al. : PTENα and PTENβ promote carcinogenesis through WDR5 and H3K4 trimethylation. Nat Cell Biol. 2019; 21(11): 1436–48. 10.1038/s41556-019-0409-z [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 112. Taylor H, Laurence ADJ, Uhlig HH: The Role of PTEN in Innate and Adaptive Immunity. Cold Spring Harb Perspect Med. 2019; 9(12): a036996. 10.1101/cshperspect.a036996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Rademacher S, Eickholt BJ: PTEN in Autism and Neurodevelopmental Disorders. Cold Spring Harb Perspect Med. 2019; 9(11): a036780. 10.1101/cshperspect.a036780 [DOI] [PMC free article] [PubMed] [Google Scholar]