Abstract
Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity and mortality. Early detection and appropriate treatment and management of COPD can lower morbidity and perhaps mortality. Clinicians in the primary care setting provide the majority of COPD care and are pivotal in the diagnosis and management of COPD. In this review, we provide an overview of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2020 report, with a focus on the management of COPD in the primary care setting. We discuss the pathophysiology of COPD; describe COPD risk factors, signs, and symptoms that may facilitate earlier diagnosis of COPD; and reinforce the importance of spirometry use in establishing the diagnosis of COPD. Disease monitoring, as well as a review of the 2020 GOLD treatment recommendations, is also discussed. Patients and families are important partners in COPD management; therefore, we outline simple steps that may assist them in caring for those affected by COPD. Finally, we discuss nonpharmacological treatment options for COPD, COPD monitoring tools that may aid in the evaluation of disease progression and response to therapy, and the importance of developing a COPD action plan on an individualized basis.
Keywords: COPD, LABA, LAMA, primary care
Introduction
Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity and mortality. COPD is the seventh leading cause of years of life lost globally, and lower respiratory disease is the fourth leading cause of death in the United States.1,2 In the United States, the age-standardized COPD-related death rate was 39.1 deaths per 100,000 in 2014 (44.3 in men and 35.6 in women per 100,000).3 COPD symptoms can be debilitating and prevent patients and their families from leading normal lives. Because of breathlessness, the level of daily physical activity is typically lower in patients with COPD than in healthy controls.4 Additionally, patients with COPD often have comorbidities such as anxiety, depression, osteoporosis, and cardiovascular disease,5 which further impact their overall health, quality of life (QoL), functional status, and clinical outcomes. COPD is also associated with substantial socioeconomic impact, with high direct and indirect costs—estimated at $36 billion annually—burdening the health care system.6 Direct costs predominantly result from exacerbations leading to emergency department (ED) visits, hospital admissions and readmissions, and unscheduled office visits.6,7 Clearly, COPD impacts not only patients but also the society as a whole and this impact can be reduced with early identification and appropriate treatment and management.8,9
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) report includes recommended strategies for the diagnosis, treatment, and management of COPD.5 The COPD Foundation (COPDF) also provides a brief, point-of-care pocket consultant guide that serves as a short summary to aid clinical decision-making during office, ED, or hospital visits.10 In this review, we provide an overview of the GOLD 2020 report, with a focus on recommendations for the treatment and management of COPD in the primary care setting.
Pathophysiology of COPD
COPD results from chronic inflammation of the airways, which leads to thickening of airway walls, increased mucus production, and eventually permanent changes in lung structure11 (Figure 1). Lung changes may include the destruction of lung parenchyma including air sac (alveolar) walls, resulting in fibrosis of the small airways (emphysema) and loss of elasticity.12 These structural changes may cause increased resistance to airflow, significant air trapping, and ultimately hyperinflation, all of which may manifest as breathlessness, cough, and increased phlegm production.11
Figure 1.
Pathophysiology of chronic obstructive pulmonary disease. During the time course of COPD, inflammation of the airways can lead to thickening of the airway walls, increased mucus production, and damage to alveoli and alveolar ducts that leads to enlargement of the air spaces/emphysema, and potentially to air-trapping.11
Chronic lung inflammation in COPD is generally characterized by increased neutrophils, activated macrophages, and activated CD8+ T lymphocytes.12 The predominant increase in types of inflammatory cells differs between COPD and asthma (Table 1).12 Macrophages facilitate the recruitment of other inflammatory cells (such as neutrophils) and the release of mediators and proteases, which may lead to emphysema.13 A subset of COPD patients have predominant eosinophilic inflammation even in the absence of exacerbations.14
Table 1.
Differences Between COPD and Asthma Pathophysiology and Symptoms
| COPD | Asthma | |
|---|---|---|
| Pathologic changes | ||
| Inflammatory cells | Neutrophils Eosinophils (mild elevation, not degranulated) CD8+, CD3+, CD68+, CD45+, VLA-1+, and HLA-DR+ T cells Macrophages |
Eosinophils (degranulated) CD4+, CD3+, CD25+, and CD45+ T cells Mast cells Macrophages |
| Bronchial smooth muscle | Enlarged mass in small airways | Enlarged mass in large airways |
| Mucus secretion | Present, heavy | Present |
| Clinical presentation | ||
| Symptoms | Progressive dyspnea | Variable dyspnea |
| Cough and sputum | Cough and/or wheeze | |
| Allergic etiology | None | Present in >50% of patients |
| Treatment response | ||
| Corticosteroids | Mildly positive/negative | Positive |
| Bronchodilators | Partially reversible | Reversible |
| Smoking status | Usually, history of heavy smoking | Nonsmokers affected |
| Airflow limitation (FEV1) | Cannot be normalized; is always reduced; deteriorates with advancing disease | Can be normalized after resolution of an episode |
Notes: Adapted from Am J Med, 117(Suppl12A). Doherty DE. The pathophysiology of airway dysfunction, 11–23, Copyright (2004), with permission from Elsevier.12
Abbreviations: COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; HLA-DR+, human leukocyte antigen-DR; VLA-1+, very late activation antigen-1.
Patients and families require accurate but simple explanations of COPD pathophysiology that can help them understand this chronic condition, its symptoms, and recommended pharmacological and nonpharmacological therapies. For example, when discussing dyspnea or shortness of breath, clinicians can explain that patients with even mild-to-moderate COPD can inhale a high volume of air (approaching total lung capacity). However, they may be able to exhale only a portion of the inhaled volume, resulting in air trapping and hyperinflation.15 As patients breathe more rapidly (eg, during exercise), they have even shorter time for exhalation and, less and less “room” to breathe in (ie progressively increased end-expiratory lung volume and decreased inspiratory capacity), both of which enhance dyspnea or shortness of breath because of inability to “get air in.” This phenomenon can be illustrated by asking the patient to take a deep breath in, exhale only a small amount of the breath (air trapping), and then attempt to take in three quick breaths. The patient will experience acute dyspnea and discomfort, as they will have more difficulty with inhalation because of residual air in the lungs (ie hyperinflation). This maneuver mimics what happens with hyperinflation during rest (static hyperinflation) and also during increased activity (dynamic hyperinflation) when the respiratory rate is faster.15 Exertion leaves less time to exhale and, consequently no room to let new air in. Common descriptors of air trapping, which are important to know to understand patients’ experiences and estimating disease severity, include “air hunger,” “unsatisfied” or “unrewarded” inhalation, “shallow breathing,” “suffocating,” and “cannot get a deep breath.”15,16
Patient Presentation
Patients with COPD commonly present with dyspnea, chronic cough, and/or sputum production, and occasionally wheezing (Table 1).5 Unfortunately, delayed diagnosis is common and many patients present only after they have experienced ≥1 exacerbation, often mistakenly labeled as recurrent bronchitis.17 Early diagnosis and treatment are essential to improve patients’ lung function, functional status, and QoL, and to reduce exacerbations.18 COPD can be detected early on if the clinicians consider COPD as part of their differential diagnosis. For example, when a patient is >40 years old and has recurrent acute bronchitis or bad colds that last for weeks, COPD should be suspected even if the patient is a nonsmoker. Of note, women are less likely to be suspected of having or diagnosed with COPD than men with similar symptoms,19 possibly because, COPD traditionally was considered a “male” disease. However, various factors, including smoking, have contributed to the rising prevalence of COPD in women.20 Clinicians can also increase the likelihood of detecting COPD by asking targeted questions focused on respiratory symptoms (eg, changes in the ability to do activities or changes in lifestyle because of shortness of breath). Patients often attribute these symptoms to being “old, overweight, or out of shape”. Questions such as “Do you get or have you ever gotten short of breath when you climb a flight of stairs or walk up a hill?” are more specific and easier for patients to answer than a vague question such as “Do you get short of breath?” Useful tools that are available to identify and assess baseline symptomatology can be used to monitor changes in severity over time. The modified Medical Research Council (mMRC) dyspnea scale comprises five statements that describe a range of dyspnea effects in increasing order of severity. Use of this questionnaire is recommended in the GOLD 2020 report5 (Supplementary Table 1). Although this questionnaire may be helpful for the initial identification of breathlessness and support evaluation for COPD,21 it is less useful for monitoring over time because moving from one grade to the next requires a very large change in functional abilities. Alternatively, the COPD Assessment Test (CAT), comprising eight items that are each scored using a 6-point scale (0–5), is useful in assessing the symptomatic impact of COPD. A higher CAT score indicates poorer health. CAT is more sensitive in detecting improvements with treatment or a decline of disease progression or exacerbations (Supplementary Figure 1).5,22 Supplementary Videos 1 and 2.
COPD Risk Factors
In addition to understanding patients’ symptoms, knowledge of COPD risk factors is important. As major risk factors for COPD, current and past smoking history must be assessed.5 Nonsmokers exposed to second-hand smoke in childhood and adult years are also at an increased risk of developing COPD.23 Cigarette smoking was rated as the cause of COPD in 50%–70% of patients in developed countries.24 Genetics also contributes to the risk of developing COPD. In a meta-analysis of genome-wide association studies, several genetic loci were associated with COPD pathophysiology.25 Serine protease α1-antitrypsin deficiency, found in 1%–3% of patients with COPD, is the most widely reported genetic factor that increases COPD risk.26 Other COPD risk factors include occupational exposure (eg, to dust, vapors, organic materials, fumes, and chemicals), indoor and outdoor air pollutants (including biomass fuels), and aging.5
Diagnosis
Diagnosis begins with clinical suspicion, usually in patients who report shortness of breath with activity. Overall, symptomatic, at-risk individuals who require spirometry and evaluation for COPD include those with recurring respiratory events (eg, acute bronchitis, bad colds, chronic cough, and excess sputum production), history of risk factors, decrease in activities because of dyspnea, and/or a family history of COPD.5
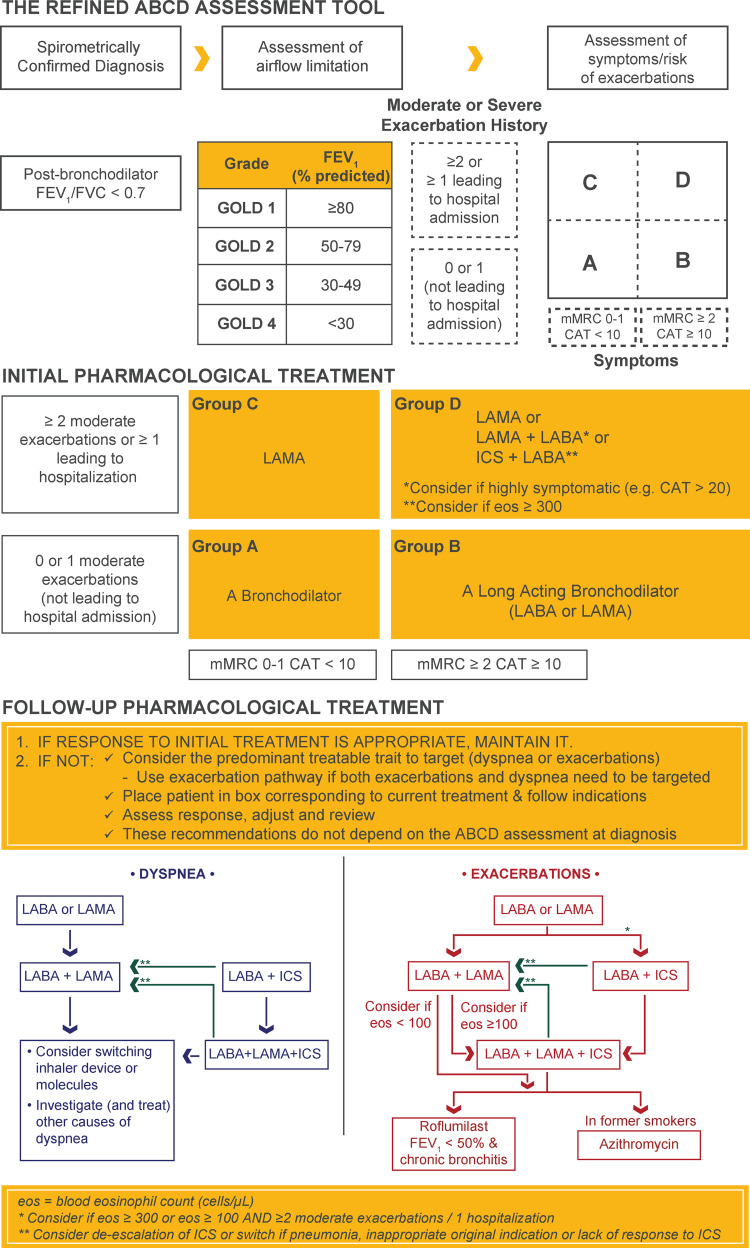
Spirometry is essential and required to confirm a COPD diagnosis.5 It also is useful for tracking treatment response, potentially adjusting medications, and monitoring disease progression. When rapid disease progression is identified, further evaluation and referral to a lung specialist are indicated.5 A postbronchodilator (10–15 minutes after 2–4 puffs of a short-acting bronchodilator) forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) ratio of <0.70 confirms the presence of persistent or fixed airflow limitation. FVC is the maximal volume of air that can be forcibly exhaled after taking in the deepest breath possible, and FEV1 is the maximal volume of air exhaled in the first second during an FVC maneuver (Figure 2).5
Figure 2.
Diagnosis, assessment, initial, and follow-up treatment of COPD.
Notes: Reproduced with permission from Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for Prevention, Diagnosis, and Management of Chronic Obstructive Pulmonary Disease. 2020. https://goldcopd.org.5
Abbreviations: CAT, COPD Assessment Test; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; GOLD, Global Initiative for Chronic Obstructive Lung Disease; ICS, inhaled corticosteroids; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; mMRC, modified Medical Research Council dyspnea scale.
Despite recommendations, spirometry is not regularly used in clinical practice.27 Underuse of spirometry in primary care settings is attributed to uncertainty about the benefit of COPD diagnosis, lack of time and resources, unfamiliarity with the technique, and/or difficulty in interpreting results.28,29 However, spirometry is required to confirm a COPD diagnosis, can be performed in primary care practice using an office-based system,30 and is a billable procedure reimbursed by payors. Although patients can be referred to specialists and hospitals for spirometry, follow-through may be limited; therefore, spirometry can and should be done in primary care offices.31
Assessment of Airflow Limitation Severity, Symptoms, and Exacerbation Frequency
Once a COPD diagnosis is confirmed, spirometry findings can also be used to determine the severity of airflow limitations (Figure 2), which is based on the patient’s FEV1 relative to normal values.5 However, treatment decisions are based on symptoms and history of exacerbations treated at home and in the hospital. Per the GOLD 2019 report, symptom burden and exacerbation frequency in the prior year are used to categorize patients into GOLD group A (few symptoms and 0–1 exacerbations not leading to hospitalization), group B (more symptoms and 0–1 exacerbations not leading to hospitalization), group C (few symptoms but ≥2 exacerbations or ≥1 exacerbation leading to hospitalization), or group D (more symptoms and ≥2 exacerbations or ≥1 exacerbation leading to hospitalization) to guide initial pharmacological therapy (Figure 2).5
Treatment of COPD
After categorizing a patient as belonging to GOLD group A, B, C, or D, the GOLD treatment algorithm (Figure 2) can be used to determine appropriate initial pharmacological treatment, which should be complemented with nonpharmacological approaches as appropriate.
Pharmacological Interventions
Bronchodilators—the first-choice pharmacotherapy for COPD across all patient groups—increase airway diameter and decrease air trapping, thereby improving airflow and reducing dyspnea.5 GOLD group A patients should be offered a bronchodilator (short- or long-acting), if symptoms are present. A long-acting muscarinic antagonist (LAMA) or a long-acting β2-agonist (LABA) is suggested as initial treatment for GOLD group B patients, and—because of their complementary mechanisms of action—dual bronchodilator therapy with a LAMA and a LABA can be considered for highly symptomatic (CAT score ≥20) patients. LAMA monotherapy improves lung function and reduces exacerbations and is suggested for initial pharmacological treatment in GOLD group C (Figure 2). While initial therapy with LAMA is recommended for group GOLD group D patients, starting with a LAMA+LABA combination may be more appropriate because many of these patients are highly symptomatic (eg, CAT >20). Although commonly used as monotherapy for asthma control, inhaled corticosteroids (ICS) are not approved worldwide for use as monotherapy in COPD patients of any severity. Long-term ICS use is associated with safety concerns such as an increased risk of pneumonia, active tuberculosis, and osteoporosis.32 However, LABA+ICS may be the first-choice treatment in COPD GOLD group D patients with a history of asthma or blood eosinophil counts ≥300 cells/μL.5 Once recommended initial therapy is implemented, patients should be reassessed for treatment response. According to recommendations in the GOLD 2020 report, if response to initial therapy is not appropriate, follow-up treatment based on the patients’ symptoms and exacerbations—and not on their initial GOLD group classification—should be provided (Figure 2). Separate treatment algorithms are provided based on the need to treat dyspnea or prevent exacerbations.
For patients with persistent breathlessness or exercise limitation despite long-acting bronchodilator monotherapy,5 step-up to a LAMA+LABA is recommended. If dual bronchodilator therapy does not improve symptoms, step down to monotherapy, or switching inhalers or molecules are recommended. When patients experience persistent breathlessness or exercise limitation despite LABA+ICS therapy, triple therapy with a LAMA+LABA+ICS may be considered. However, if ICS was inappropriately indicated to treat patients without a history of exacerbations, caused side effects, or did not yield any response, switching to a LAMA+LABA is recommended.
For patients who continue to experience exacerbations despite long-acting bronchodilator monotherapy, step-up to a LAMA+LABA or LABA+ICS (in patients with a history of hospitalizations for COPD exacerbations, with ≥2 moderate COPD exacerbations per year, eosinophil counts >300 cells/μL, or a history of asthma) is recommended.5 For patients who continue to exacerbate despite maximal LAMA+LABA, triple therapy is recommended if eosinophil counts ≥100 cells/μL and roflumilast or azithromycin is recommended if eosinophil counts <100 cells/μL.
While ICS therapy has a role in COPD management, there may be a current over-use based on the GOLD 2020 treatment algorithms.33 In 2019, some of the recommendations surrounding the use of ICS in GOLD were changed due to concerns of over-use of ICS, but these recommendations were revised in 2020 to reflect the importance of ICS in certain circumstances, such as hospitalization for exacerbation. Whether, when, and how non-recommended ICS treatment can be withdrawn safely should be considered, particularly in patients who had ICS initiated despite no or infrequent exacerbations, especially if they also have low eosinophil counts. In the INSTEAD trial,34 non-exacerbating patients with moderate COPD were switched from salmeterol+fluticasone (a LABA+ICS) to indacaterol (a LABA) without a significant change in exacerbation rate. Patients in this trial were at low risk of exacerbations and should not have been prescribed ICS based on the GOLD 2020 treatment algorithm. Similarly, withdrawal of ICS did not increase exacerbation rates in the WISDOM35 and SUNSET36 trials, which included patients with severe/very severe and moderate-to-severe COPD, respectively.
To avoid potentially difficult decisions regarding stepping down treatment, ICS should be initiated only when recommended, and not in patients with no or infrequent exacerbations or in patients whose exacerbations can be controlled with dual bronchodilator therapy.
In addition to deciding on appropriate initial and maintenance medications, COPD clinicians should also consider which inhaler is optimal for each patient. Prescribing an inhaler based on patient characteristics and preferences, and training patients on correct inhaler use, will lead to better adherence.5 During follow-up, inhaler technique and adherence should be assessed. If not optimal, switching to a different inhaler device may be considered.5
Nonpharmacological Interventions
Pharmacological therapy for COPD should be complemented with nonpharmacological approaches, including behavioral therapies and pulmonary rehabilitation, as appropriate. Assessment of smoking history and initiation of a cessation program, if necessary, must be a part of all COPD patients’ treatment plans. Because relapses are common, smoking status and second-hand smoke exposure should be continually monitored over time. Reinforcement to remain a sustained quitter or encouragement to stop smoking should be given at each opportunity.
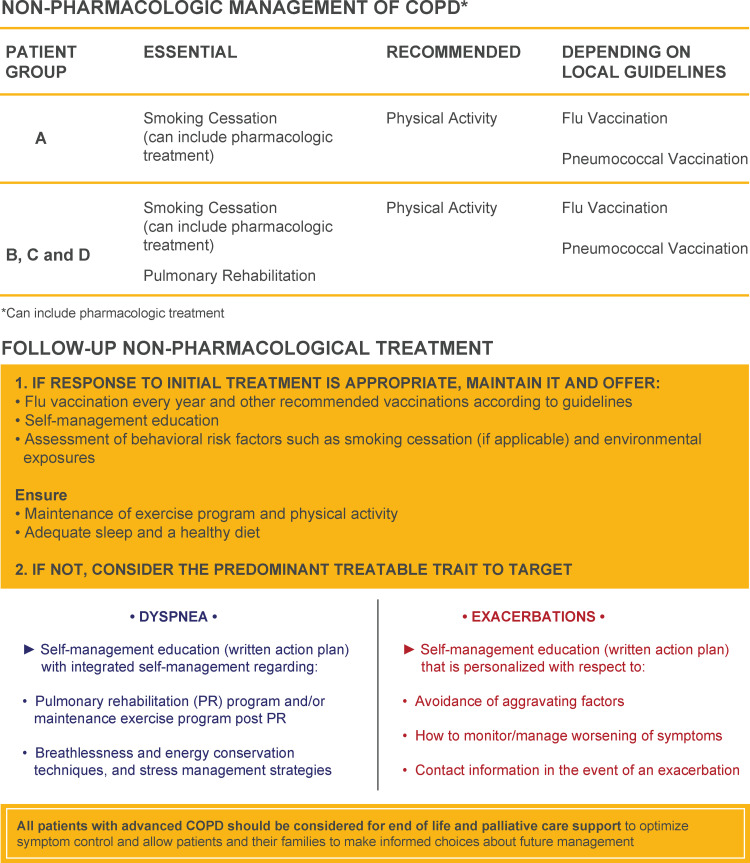
Other nonpharmacological approaches at diagnosis, based on GOLD 2020, include referring GOLD group A-D patients to pulmonary rehabilitation including exercise training, promoting physical activity, encouraging adherence to the prescribed medication, and prescribing vaccinations.5 Recommendations for nonpharmacological management of COPD at diagnosis and during follow-up are summarized in Figure 3. Teaching COPD patients breathing techniques aimed at improving respiratory muscle strength and decreasing air trapping in the lungs, which can reduce the sense of dyspnea is also beneficial.5 Pursed-lip breathing, which reduces heightened air trapping by a mechanical maneuver, is a practical and simple technique that can be taught quickly and can make a substantial difference to patients (Supplementary Figure 2). Pulmonary rehabilitation improves symptoms, reduces hospital readmissions, increases activity levels, and decreases levels of anxiety and depression.5,37 Although programs can be difficult to implement in some areas, largely because of low reimbursement rates, pulmonary rehabilitation is one of the single best treatments for patients with COPD.37 Exercise programs, disease education, and setting activity goals for patients can be helpful when pulmonary rehabilitation is not available.5 Finally, ensuring that patients with COPD receive all indicated immunizations (eg, influenza, pneumococcal pneumonia,5 Tdap, and Zoster) is important to overall patient care and may help to reduce exacerbations and other poor outcomes.
Figure 3.
Nonpharmacological management of COPD at diagnosis and follow-up.
Notes: Reproduced with permission from Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for Prevention, Diagnosis, and Management of Chronic Obstructive Pulmonary Disease. 2020. https://goldcopd.org.5
Abbreviation: Flu, influenza.
Referral to a pulmonologist may be considered at diagnosis, at discharge after hospitalization for an exacerbation, or when symptoms progressively deteriorate.5
Chronic Disease Management
Chronic disease management involves regular evaluation to monitor disease progression and treatment response. For COPD, important aspects include monitoring symptom burden and exacerbation frequency, reviewing and observing device/inhaler technique, reviewing medication adherence, and updating any action plans. But, patient evaluation and treatment are a continuous process and cannot be accomplished in a single visit. A COPD action plan should be developed and individualized for each patient; however, development is often not logical or feasible until the second or third visit. The COPDF action plan,38 which was designed to improve communication between clinicians and patients with COPD, and to encourage disease self-management, should be considered (Supplementary Figure 3). Further, while these regular evaluations are essential to achieve optimal treatment outcomes, evidence indicates clinicians and patients do not necessarily appreciate their importance. For example, according to results of quantitative, web-based, descriptive, cross-sectional surveys of clinicians and patients with COPD in the United States, both groups had limited concerns about proper device use.39 Less than half of the clinicians surveyed reported assessing device technique in every newly diagnosed patient, and many reported not routinely assessing and inconsistently educating about proper device use. Not surprisingly, incorrect inhaler use led to poor clinical outcomes.
Patient education is important in ensuring successful disease management and optimal medication adherence and compliance. Regular demonstrations and direct observations of patients’ use of their medication delivery systems should be done at each visit to ensure proper use. In addition, patients should be reminded that although serious, COPD symptom burden and progression may be modifiable with treatment and behavioral changes.
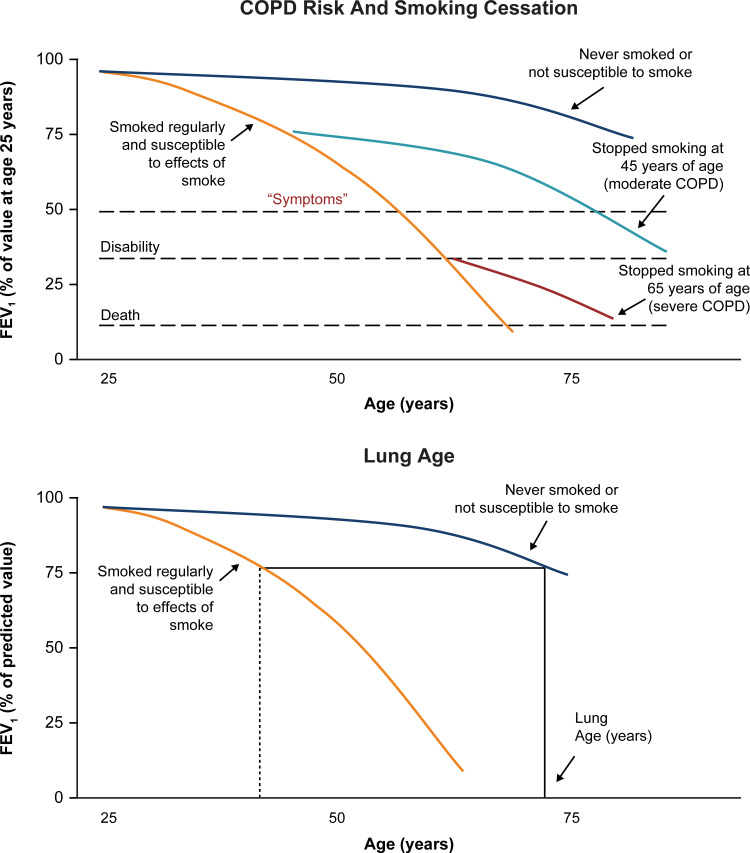
As mentioned, an important aspect of patient education is smoking cessation.40 Motivating current smokers with COPD to quit may be particularly challenging because they continue to smoke despite disease symptoms. “Lung age” may be a useful tool to demonstrate the effects of cigarette smoking and is known to increase smoking cessation rates.40 In addition, spirometry curves (Figure 4) are helpful in demonstrating that quitting smoking even at a late age can reduce morbidity and mortality.40–42
Figure 4.
Effects of smoking on COPD risk and lung age.
Notes: Adapted with permission from Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J. 1977;1(6077):1645–1648, Copyright© 1977 BMJ Publishing Group Ltd.41 COPD risk and smoking cessation:41,42 effects of smoking and smoking cessation on FEV1 in patients who are likely to develop COPD if they smoke are illustrated by solid lines. The graphs represent cross-sectional data from several individuals measured at different time points. Although the rate of loss of FEV1 for a susceptible smoker is shown, others may experience different rates of loss, and reach “disability” at different ages. While the underlying cause of “Death” is irreversible COPD, the immediate cause may be pneumonia, cor pulmonale, respiratory failure, or aggravation of heart disease by respiratory insufficiency. Adapted from Doherty DE. A review of the role of FEV1 in the COPD paradigm. COPD. 2008;5(5):310–318, Copyright© 2008. Taylor and Francis.42 Lung age:42 Nonsmokers or smokers who are not susceptible to the effects of tobacco smoke experience a normal decline in lung function (blue line). Susceptible smokers experience an accelerated loss of lung function over time (orange line). For example, if the FEV1 of a susceptible 40-year-old smoker (measured at 75% predicted; dotted vertical line) is extrapolated (horizontal line drawn to the curve of a nonsmoker/non-susceptible smoker), the “Lung Age” is approximately 74 years, suggesting that the lung function is equivalent to that of a nonsmoker/non-susceptible smoker at 74 years.
Abbreviations: COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second.
Ensuring continuity of care over time is central to chronic disease management programs, including those for COPD. During repeated visits, clinicians must confirm that patients are prescribed and are taking appropriate COPD maintenance therapy, addressing smoking cessation, offered support for an increased activity or pulmonary rehabilitation, and considered as candidates for palliative (not just end-of-life) care. All of these activities are especially important following any hospital admission for an exacerbation.
Conclusions
COPD is a leading cause of morbidity and mortality in the United States. Because most patients with COPD are managed in the primary care setting, primary care clinicians play a pivotal role in appropriately managing COPD. Following up-to-date treatment recommendations such as those provided in the GOLD 2020 strategy report, engaging in chronic disease management, and investing in patient education are important to achieve the greatest benefits of COPD treatment.
Acknowledgments
The authors meet the criteria for authorship as recommended by the International Committee of Medical Journal Editors. The authors received no direct compensation related to the development of the manuscript. Writing, editorial support, and formatting assistance were provided by Suchita Nath-Sain, PhD, Michelle Rebello, PhD, and Maribeth Bogush, PhD, of Cactus Life Sciences (part of Cactus Communications), which was contracted and compensated by Boehringer Ingelheim Pharmaceuticals, Inc. (BIPI) for these services. BIPI was given the opportunity to review the manuscript for medical and scientific accuracy, as well as intellectual property considerations.
Funding Statement
Writing, editorial support, and formatting service for this review was funded by Boehringer Ingelheim Pharmaceuticals, Inc.
Abbreviations
CAT, COPD Assessment Test; CD, Cluster differentiation; COPD, Chronic Obstructive Pulmonary Disease; COPDF, Chronic Obstructive Pulmonary Disease foundation; ED, Emergency department; FEV1, Forced expiratory volume in 1 second; FVC, Forced vital capacity; GOLD, Global Initiative for Chronic Obstructive Lung Disease; ICS, Inhaled corticosteroids; LABA, Long-acting β2-agonist; LAMA, Long-acting muscarinic antagonist; mMRC, modified Medical Research Council; QoL, Quality of life; Tdap, Tetanus, diphtheria, and pertussis combination vaccine.
Author Contributions
All authors made a significant contribution to the conception and interpretation of the article; took part in critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
BPY served on advisory boards for Boehringer Ingelheim, AstraZeneca, TEVA, and GlaxoSmithKline (GSK) and received consulting fees from GSK related to COPD; and received grants from the COPD Foundation, Boehringer Ingelheim, and National Heart, Lung, and Blood Institute (NHLBI), outside the submitted work. MLM received speaking and consulting fees from GSK, Mylan, and Boehringer Ingelheim, outside the submitted work. DED served on advisory boards and received speaker fees from AstraZeneca and Boehringer Ingelheim and received grants from Boehringer Ingelheim and NHLBI outside of the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Viegi G, Maio S, Fasola S, Baldacci S. Global burden of chronic respiratory diseases. J Aerosol Med Pulm Drug Deliv. 2020;33(4):171–177. doi: 10.1089/jamp.2019.1576 [DOI] [PubMed] [Google Scholar]
- 2.Murphy SL, Xu J, Kochanek KD, Arias E. Mortality in the United States, 2017. NCHS Data Brief. 2018;(328):1–8. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. COPD death rates in the United States. Available from: https://www.cdc.gov/copd/data.html. Accessed October26, 2020.
- 4.Vorrink SN, Kort HS, Troosters T, Lammers JW. Level of daily physical activity in individuals with COPD compared with healthy controls. Respir Res. 2011;12(1):33. doi: 10.1186/1465-9921-12-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Global Initiative for Chronic Obstructive Lung Disease. Global strategy for prevention, diagnosis, and management of chronic obstructive pulmonary disease; 2020. Available from: https://goldcopd.org. Accessed October26, 2020.
- 6.Ford ES, Murphy LB, Khavjou O, Giles WH, Holt JB, Croft JB. Total and state-specific medical and absenteeism costs of COPD among adults aged ≥18 years in the United States for 2010 and projections through 2020. Chest. 2015;147(1):31–45. doi: 10.1378/chest.14-0972 [DOI] [PubMed] [Google Scholar]
- 7.Bartels W, Adamson S, Leung L, Sin DD, van Eeden SF. Emergency department management of acute exacerbations of chronic obstructive pulmonary disease: factors predicting readmission. Int J Chron Obstruct Pulmon Dis. 2018;13:1647–1654. doi: 10.2147/COPD.S163250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Csikesz NG, Gartman EJ. New developments in the assessment of COPD: early diagnosis is key. Int J Chronic Obstr Pulm Dis. 2014;9:277–286. doi: 10.2147/COPD.S46198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soriano JB, Polverino F, Cosio BG. What is early COPD and why is it important? Eur Respir J. 2018;52(6). doi: 10.1183/13993003.01448-2018 [DOI] [PubMed] [Google Scholar]
- 10.Yawn BB, Thomashaw B, Mannino DM, et al. The 2017 update to the COPD Foundation COPD Pocket Consultant Guide. Chronic Obstr Pulm Dis. 2017;4(3):177–185. doi: 10.15326/jcopdf.4.3.2017.0136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodríguez-Roisin R. The airway pathophysiology of COPD: implications for treatment. COPD. 2005;2(2):253–262. doi: 10.1081/COPD-57598 [DOI] [PubMed] [Google Scholar]
- 12.Doherty DE. The pathophysiology of airway dysfunction. Am J Med. 2004;117(Suppl12A):11–23. doi: 10.1016/j.amjmed.2004.10.017 [DOI] [PubMed] [Google Scholar]
- 13.Stockley RA, Mannino D, Barnes PJ. Burden and pathogenesis of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2009;6(6):524–526. doi: 10.1513/pats.200904-016DS [DOI] [PubMed] [Google Scholar]
- 14.Singh D, Kolsum U, Brightling CE, Locantore N, Agusti A, Tal-Singer R. Eosinophilic inflammation in COPD: prevalence and clinical characteristics. Eur Respir J. 2014;44(6):1697–1700. doi: 10.1183/09031936.00162414 [DOI] [PubMed] [Google Scholar]
- 15.Marchetti N, Kaplan A. Dyspnea and hyperinflation in chronic obstructive pulmonary disease: impact on physical activity. J Fam Pract. 2018;67(2):S3–S10. doi: 10.3949/ccjm.85.s1.02 [DOI] [PubMed] [Google Scholar]
- 16.Chowienczyk S, Javadzadeh S, Booth S, Farquhar M. Association of descriptors of breathlessness with diagnosis and self-reported severity of breathlessness in patients with advanced chronic obstructive pulmonary disease or cancer. J Pain Symptom Manage. 2016;52(2):259–264. doi: 10.1016/j.jpainsymman.2016.01.014 [DOI] [PubMed] [Google Scholar]
- 17.Jagana R, Bartter T, Joshi M. Delay in diagnosis of chronic obstructive pulmonary disease: reasons and solutions. Curr Opin Pulm Med. 2015;21(2):121–126. doi: 10.1097/MCP.0000000000000133 [DOI] [PubMed] [Google Scholar]
- 18.Decramer M, Miravitlles M, Price D, et al. New horizons in early stage COPD–improving knowledge, detection and treatment. Respir Med. 2011;105(11):1576–1587. doi: 10.1016/j.rmed.2010.12.015 [DOI] [PubMed] [Google Scholar]
- 19.Chapman KR, Tashkin DP, Pye DJ. Gender bias in the diagnosis of COPD. Chest. 2001;119(6):1691–1695. doi: 10.1378/chest.119.6.1691 [DOI] [PubMed] [Google Scholar]
- 20.Ntritsos G, Franek J, Belbasis L, et al. Gender-specific estimates of COPD prevalence: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2018;13:1507–1514. doi: 10.2147/COPD.S146390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang W-C, Wu M-F, Chen H-C, Hsu J-Y. Features of COPD patients by comparing CAT with mMRC: a retrospective, cross-sectional study. NPJ Prim Care Respir Med. 2015;25:15063. doi: 10.1038/npjpcrm.2015.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holt S, Sheahan D, Helm C, Tofield C, Corin A, Kocks JWH. Little agreement in GOLD category using CAT and mMRC in 450 primary care COPD patients in New Zealand. NPJ Prim Care Respir Med. 2014;24(1):14025. doi: 10.1038/npjpcrm.2014.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Postma DS, Bush A, van den Berge M. Risk factors and early origins of chronic obstructive pulmonary disease. Lancet. 2015;385(9971):899–909. doi: 10.1016/S0140-6736(14)60446-3 [DOI] [PubMed] [Google Scholar]
- 24.Chilvers ER, Lomas DA. Diagnosing COPD in non-smokers: splitting not lumping. Thorax. 2010;65(6):465–466. doi: 10.1136/thx.2009.128421 [DOI] [PubMed] [Google Scholar]
- 25.Busch R, Hobbs B, Zhou J, et al. Genetic association and risk scores in a chronic obstructive pulmonary disease meta-analysis of 16,707 subjects. Am J Respir Cell Mol Biol. 2017;57(1):35–46. doi: 10.1165/rcmb.2016-0331OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet. 2007;370(9589):765–773. doi: 10.1016/S0140-6736(07)61380-4 [DOI] [PubMed] [Google Scholar]
- 27.Han MK, Kim MG, Mardon R, et al. Spirometry utilization for COPD: how do we measure up? Chest. 2007;132(2):403–409. doi: 10.1378/chest.06-2846 [DOI] [PubMed] [Google Scholar]
- 28.Joo MJ, Sharp LK, Au DH, Lee TA, Fitzgibbon ML. Use of spirometry in the diagnosis of COPD: a qualitative study in primary care. COPD. 2013;10(4):444–449. doi: 10.3109/15412555.2013.766683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saad N, Sedeno M, Metz K, Bourbeau J. Early COPD diagnosis in family medicine practice: how to implement spirometry? Int J Family Med. 2014;2014:962901. doi: 10.1155/2014/962901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruppel GL, Carlin BW, Hart M, Doherty DE. Office spirometry in primary care for the diagnosis and management of COPD: National Lung Health Education Program update. Respir Care. 2018;63(2):242–252. doi: 10.4187/respcare.05710 [DOI] [PubMed] [Google Scholar]
- 31.Yawn BP, Enright PL, Lemanske RF Jr, et al. Spirometry can be done in family physicians’ offices and alters clinical decisions in management of asthma and COPD. Chest. 2007;132(4):1162–1168. doi: 10.1378/chest.06-2722 [DOI] [PubMed] [Google Scholar]
- 32.Ernst P, Saad N, Suissa S. Inhaled corticosteroids in COPD: the clinical evidence. Eur Respir J. 2015;45(2):525–537. doi: 10.1183/09031936.00128914 [DOI] [PubMed] [Google Scholar]
- 33.Price D, West D, Brusselle G, et al. Management of COPD in the UK primary-care setting: an analysis of real-life prescribing patterns. Int J Chron Obstruct Pulmon Dis. 2014;9:889–904. doi: 10.2147/COPD.S62750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rossi A, van der Molen T, del Olmo R, et al. INSTEAD: a randomised switch trial of indacaterol versus salmeterol/fluticasone in moderate COPD. Eur Respir J. 2014;44(6):1548–1556. doi: 10.1183/09031936.00126814 [DOI] [PubMed] [Google Scholar]
- 35.Magnussen H, Disse B, Rodriguez-Roisin R, et al. WISDOM investigators. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med. 2014;371(14):1285–1294. doi: 10.1056/NEJMoa1407154 [DOI] [PubMed] [Google Scholar]
- 36.Chapman KR, Hurst JR, Frent SM, et al. Long-term triple therapy de-escalation to indacaterol/glycopyrronium in patients with chronic obstructive pulmonary disease (SUNSET): a randomized, double-blind, triple-dummy clinical trial. Am J Respir Crit Care Med. 2018;198(3):329–339. doi: 10.1164/rccm.201803-0405OC [DOI] [PubMed] [Google Scholar]
- 37.Safka KA, McIvor RA. Non-pharmacological management of chronic obstructive pulmonary disease. Ulster Med J. 2015;84(1):13–21. [PMC free article] [PubMed] [Google Scholar]
- 38.COPD Foundation. Available from: https://www.copdfoundation.org/Learn-More/Educational-Materials-Resources/Downloads.aspx#MyCOPDActionPlan. Accessed October26, 2020.
- 39.Hanania NA, Braman S, Adams SG, et al. The role of inhalation delivery devices in COPD: perspectives of patients and health care providers. Chronic Obstr Pulm Dis. 2018;5(2):111–123. doi: 10.15326/jcopdf.5.2.2017.0168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morris JF, Temple W. Spirometric “lung age” estimation for motivating smoking cessation. Prev Med. 1985;14(5):655–662. doi: 10.1016/0091-7435(85)90085-4 [DOI] [PubMed] [Google Scholar]
- 41.Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J. 1977;1(6077):1645–1648. doi: 10.1136/bmj.1.6077.1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doherty DE. A review of the role of FEV1 in the COPD paradigm. COPD. 2008;5(5):310–318. doi: 10.1080/15412550802363386 [DOI] [PubMed] [Google Scholar]
- 43.Fletcher CM. Standardized questionnaire on respiratory symptoms: a statement prepared and approved by the MRC committee on the aetiology of chronic bronchitis (MRC breathlessness score). BMJ. 1960;2:1665.13688719 [Google Scholar]
- 44.Jones PW. Health status and the spiral of decline. COPD. 2009;6(1):59–63. doi: 10.1080/15412550802587943 [DOI] [PubMed] [Google Scholar]
- 45.COPD Foundation. Breathing Exercises and Techniques. Available from: https://www.copdfoundation.org/Learn-More/I-am-a-Person-with-COPD/Breathing-Techniques.aspx. Accessed October26, 2020.