Abstract
Objectives
The coronavirus disease 2019 (COVID-19) has become a worst pandemic. The clinical characteristics vary from asymptomatic to fatal. This study aims to examine the association between body mass index (BMI) levels and the severity of COVID-19.
Methods and study design
A cohort study included 147 adult patients with confirmed COVID-19 were categorized into 4 groups by BMI levels on admission: <18.5 (underweight), 18.5–22.9 (normal weight), 23.0–24.9 (overweight), and ≥25.0 kg/m2 (obese). Rates of pneumonia, severe pneumonia, acute kidney injury (AKI), and ICU stay during hospitalization across BMI group was determined. Logistic regression analysis was used to determine the association between BMI and severe pneumonia.
Results
Of the totals, patients having a BMI <18.5, 18.5–22.9, 23.0–24.9, and ≥25.0 kg/m2 were 12.9%, 38.1%, 17.7%, and 31.3%, respectively. The rates of pneumonia and severe pneumonia tended to be higher in patients with higher BMI, whereas the rates of AKI and ICU stay were higher in patients with BMI <18.5 kg/m2 and ≥ 25 kg/m2, when compared to patients with normal BMI. After controlling for age, sex, diabetes, hypertension and dyslipidemia in the logistic regression analysis, having a BMI ≥25.0 kg/m2 was associated with higher risk of severe pneumonia (OR 4.73; 95% CI, 1.50–14.94; p = 0.003) compared to having a BMI 18.5–22.9 kg/m2. During admission, elevated hemoglobin and alanine aminotransferase levels on day 7 and 14 of illness were associated with higher BMI levels. In contrast, rising of serum creatinine levels was observed in underweight patients on days 12 and 14 of illness.
Conclusions
Obesity in patients with COVID-19 was associated with severe pneumonia and adverse outcomes such as AKI, transaminitis and ICU stay. Underweight patients should be closely monitored for AKI. Further studies in body composition are warranted to explore the links between adiposity and COVID-19 pathogenesis.
Introduction
The Coronavirus Disease 2019 (COVID-19), an infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has become one of the worst pandemics in this century. The World Health Organization (WHO) announced the confirmation of COVID-19 as a pandemic on March 11th, 2020 [1]. As of May 26th, 2020, COVID-19 has affected over 5.5 million people worldwide, causing more than 347,000 fatalities [2]. The clinical outcomes of COVID-19 vary in severity from asymptomatic to lethal [3]. In addition to several degrees of pneumonia, COVID-19 may cause injury of many organs including liver, kidneys and heart [4].
Obesity, defined as excessive accumulation of body fat, is generally determined by body mass index (BMI), calculated by body weight (kg) divided by height squared (m2) [5]. The number of obese people is globally increasing. Adiposity affects adverse health outcomes such as coronary artery disease, cerebrovascular disease, insulin resistance, hypertension and fatty liver disease [6]. Fat accumulation does not only affect mechanical-related health complications, but the abundant adipose tissue also releases many adipokines which play a role in the inflammatory process [7]. Nonetheless, the immune system is suppressed in obese people, especially in vulnerable people with multiple comorbidities [8]. Obese people may be more susceptible to SARS-CoV-2 infection [9]. A pathophysiology of COVID-19 is an immune response dysfunction resulting in damage to multiple organs, particularly the lower airways [10]. Owing to similar pathogenesis, obesity could be correlated to adverse outcomes and severity of COVID-19.
Up to now, the data of the association between obesity and severity of COVID-19 is limited. The aims of this study were 1) to examine the characteristics of patients with COVID-19 across body mass index levels, 2) to assess the association between body mass index (BMI) and the severity of COVID-19, and 3) to evaluate the change of obese-related parameters across BMI levels during admission in patients with COVID-19.
Materials and methods
Patients and design
Human Research Ethics Committee, Faculty of Medicine Ramathibodi Hospital, Mahidol University approved the study. The approval number was MURA2020/947. Consent was not obtained because the data were analyzed anonymously. A cohort study was conducted in patients with confirmed COVID-19, aged 15 years and older, and admitted to Chakri Naruebodindra Medical Institute, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Samut Prakan, Thailand, between March 12th and April 30th, 2020. Diagnosis of COVID-19 was established by detecting SARS-CoV-2 RNA in nasopharyngeal swab specimens by real-time RT-PCR amplification of SARS-CoV-2 ORF1AB and N Gene fragments using a SARS-CoV-2 nucleic acid diagnostic kit (Sansure Biotech), which was approved by the National Medical Products Administration (NMPA) and certified by the China Food and Drug Administration (CFDA). All patients diagnosed with confirmed COVID-19 were admitted to the institute and analyzed.
Baseline demographic data including underlying diseases, risk exposures, and personal history were collected. Physical examinations including body weight and height were performed by trained physicians on the admission date. Blood tests and chest radiograph were also performed on admission. BMI was categorized into 4 groups: <18.5 kg/m2 (underweight), 18.5–22.9 kg/m2 (normal weight), 23.0–24.9 kg/m2 (overweight), and ≥25.0 kg/m2 (obese), following Asia-Pacific cut-off for underweight, overweight and obesity [11]. During hospitalization, blood tests were also corrected on day 7, 10, 12, and 14 of illness.
Outcomes
Pneumonia was defined as clinical symptoms of respiratory tract infection together with abnormal lung imaging compatible with pneumonia. The patients with pneumonia were classified as severe pneumonia patients when having one of the following criteria: respiratory rate >30 breaths/min, severe respiratory distress, or an oxygen saturation ≤93% on room air [12]. Patients who had no symptom, mild symptoms, or non-severe pneumonia were described as having mild to moderate disease. Acute kidney injury (AKI) was defined as any of the following: increase in serum creatinine ≥0.3 mg/dL within 48 hours, increase in serum creatinine to ≥1.5 times from baseline, or urine volume <0.5 mL/kg/h for 6 hours [13]. Patients having an oxygen saturation <92% despite the use of an oxygen cannula 5 L/minute or respiratory rate ≥25 breaths/minute were admitted or transferred to intensive care unit (ICU). The rate of pneumonia, severe pneumonia, AKI, and ICU stay during hospitalization were determined in all patients.
Statistical analysis
To describe the characteristics of patients with COVID-19 across BMI levels, baseline characteristics of the patients including demographic data, underlying diseases, and results of laboratory investigations are presented as mean ± standard deviation (SD) for continuous variables and as frequency (%) for categorical variables, stratified by BMI groups. The mean difference of continuous variables among BMI groups was tested using ANOVA. Categorical variables were analyzed using Chi-square test to determine the differences between groups. The rates of study outcomes (pneumonia, severe pneumonia, AKI and ICU stay) are illustrated in bar chart stratified by BMI levels.
To assess the association between BMI and the severity of COVID-19, predicting factors for severe pneumonia were analyzed using logistic regression models and presented using the odds ratios (OR) and the associated 95% confidence interval (CI). The potential predicting factors including age (years), sex, diabetes, hypertension, and dyslipidemia were used in the regression models as potential covariates. The two-way and three-way interactions of age and sex with BMI levels in relation to study outcomes were also tested in the regression models.
To evaluate the change of obese-related parameters across BMI levels in patients with COVID-19 during admission, the time-related changes of hemoglobin, creatinine, albumin, and alanine aminotransferase on day 7, day 10, day 12, and day 14 across the BMI groups are illustrated in line charts.
Statistical significance was considered as p< 0.05, and all reported probability tests were two-sided. The statistical analysis was conducted using IBM SPSS Statistics for Windows, Version 24.0 (Armonk, NY: IBM Corp).
Results
This study included 147 patients diagnosed with confirmed COVID-19. The mean age of patients was 39.1±13.0 years and females were 59.5% of the patients. Percentage of patients having a BMI <18.5, 18.5–22.9, 23.0–24.9, and ≥25.0 kg/m2 were 12.9%, 38.1%, 17.7%, and 31.3%, respectively. More than half of the patients were active alcohol users. Hypertension and diabetes were common comorbidities. None of the patients with a BMI <18.5 kg/m2 had hypertension, whereas most patients with a BMI ≥23.0 kg/m2 had dyslipidemia. Patients in higher BMI levels tended to be older and had a higher proportion of males. Hemoglobin, creatinine and alanine aminotransferase levels significantly elevated in patients with higher BMI levels. Baseline characteristics on admission among patients with COVID-19 across BMI levels are presented in Table 1.
Table 1. Baseline characteristics of patients with COVID-19 on admission, categorized by body mass index levels.
| Characteristics | Total (N = 147) | Body mass index (kg/m2) | P value | |||
|---|---|---|---|---|---|---|
| < 18.5 | 18.5–22.9 | 23.0–24.9 | ≥25.0 | |||
| (N = 19) | (N = 56) | (N = 26) | (N = 46) | |||
| Age (years), mean ±SD | 39.1±13.0 | 31.1±9.1 | 36.8±13.2 | 43.4±13.1 | 42.8±12.3 | <0.001 |
| Sex, male, number (%) | 61 (41.5) | 5 (26.3) | 17 (30.4) | 14 (53.8) | 25 (54.3) | 0.024 |
| Underlying conditions, number (%) | ||||||
| Diabetes | 14 (9.5) | 1 (5.3) | 4 (7.1) | 2 (7.7) | 7 (15.2) | 0.457 |
| Hypertension | 14 (9.5) | 0 (0.0) | 1 (1.8) | 3 (11.5) | 10 (21.7) | 0.003 |
| Dyslipidemia | 8 (5.4) | 0 (0.0) | 0 (0.0) | 1 (3.8) | 7 (15.2) | 0.005 |
| Active smoking | 30 (20.4) | 4 (21.1) | 13 (23.2) | 5 (19.2) | 8 (17.4) | 0.335 |
| Active alcohol drinking | 81 (55.1) | 10 (52.6) | 33 (58.9) | 14 (53.8) | 24 (52.2) | 0.957 |
| Clusters of exposure, number (%) | 0.067 | |||||
| Night club A | 44 (29.9) | 3 (15.8) | 17 (30.4) | 8 (30.8) | 16 (34.8) | |
| Boxing stadium A | 29 (19.7) | 1 (5.3) | 8 (14.3) | 7 (26.9) | 13 (28.3) | |
| Pub A | 20 (13.6) | 5 (26.3) | 12 (21.4) | 2 (7.7) | 1 (2.2) | |
| Taxi driver A | 7 (4.8) | 1 (5.3) | 4 (7.1) | 1 (3.8) | 1 (2.2) | |
| Others | 47 (32.0) | 9 (47.4) | 15 (26.8) | 8 (30.8) | 15 (32.6) | |
| Education, number (%) | 0.126 | |||||
| Less than secondary school | 32 (22.4) | 1 (5.3) | 11 (20.4) | 6 (23.1) | 14 (31.8) | |
| Secondary school | 65 (45.5) | 10 (52.6) | 27 (50.0) | 8 (30.8) | 20 (45.5) | |
| Bachelor degree | 39 (27.3) | 7 (36.8) | 12 (22.2) | 12 (46.2) | 8 (18.2) | |
| More than bachelor degree | 7 (4.9) | 1 (5.3) | 4 (7.4) | 0 (0.0) | 2 (4.5) | |
| Days of illness at admission (days), mean ±SD | 6.9±4.1 | 8.7±5.6 | 7.0±4.0 | 6.2±4.1 | 6.4±3.3 | 0.161 |
| Hemoglobin (g/dL), mean ±SD | 13.5±1.7 | 12.8±1.2 | 13.0±1.5 | 13.8±1.5 | 14.1±1.8 | 0.002 |
| Absolute lymphocyte count (/mm3), mean ±SD | 1914.2±770.7 | 2044.1±832.2 | 1903.6±733.0 | 1880.50±567.3 | 1892.63±898.0 | 0.890 |
| Creatinine (mg/dL), mean ±SD | 0.81±0.23 | 0.71±0.14 | 0.76±0.22 | 0.83±0.14 | 0.90±0.28 | 0.003 |
| Alkaline phosphatase (U/L), mean ±SD | 67.3±40.8 | 73.4±86.8 | 57.4±14.6 | 72.7±39.9 | 73.7±31.7 | 0.158 |
| Alanine aminotransferase (U/L), mean ±SD | 31.0±24.1 | 22.4±14.4 | 24.45±13.7 | 26.9±21.2 | 45.0±32.2 | <0.001 |
| Total protein (g/dL), mean ±SD | 78.2±5.8 | 75.1±6.8 | 78.3±6.1 | 78.7±4.8 | 79.1±5.1 | 0.076 |
| Albumin (g/dL), mean ±SD | 42.2±3.91 | 40.8±4.9 | 42.6±3.7 | 42.5±3.3 | 42.0±4.0 | 0.349 |
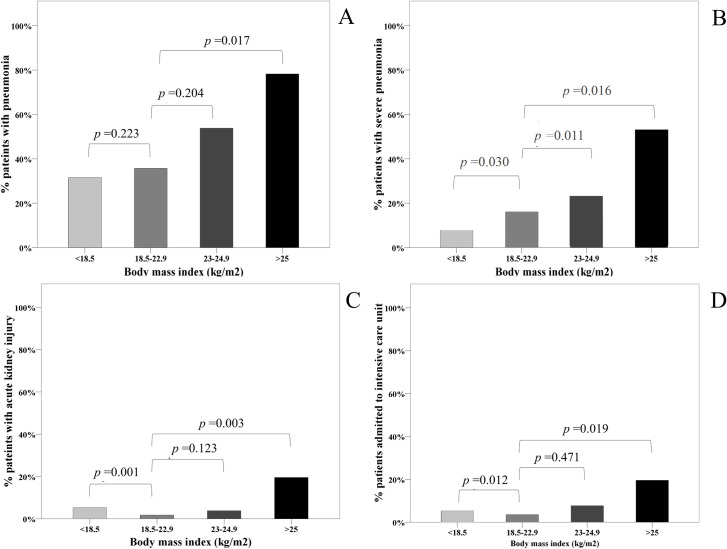
Of the total patients, 76 patients (51.7%) had pneumonia and 20 patients (13.6%) had progressed to severe pneumonia. The rates of AKI and ICU stays were 8.2% and 9.5%, respectively. The rate of pneumonia tended to increase in patients with higher BMI levels; this rate in patients with BMI ≥25.0 kg/m2 was significantly higher (78.3%) when compared to patients with BMI 18.5–22.9 kg/m2 (35.7%) (Fig 1). The rate of severe pneumonia was significantly increased in each group of patients with higher BMI, i.e. 8.1%, 16.2%, 21.6% and 54.1% in patient with BMI < 18.5, 18.5–22.9, 23.0–24.9 and ≥25.0 kg/m2, respectively. The rates of AKI and ICU stay were higher in patients with BMI <18.5 kg/m2 and ≥ 25 kg/m2, when compared to patients with BMI 18.5–22.9 kg/m2 (Fig 1).
Fig 1.
Rate of (A) pneumonia, (B) severe pneumonia, (C) acute kidney injury, and (D) ICU stay in each group of BMI levels.
After controlling for age, sex, diabetes, hypertension and dyslipidemia in the logistic regression analysis, having a BMI ≥25.0 kg/m2 was associated with higher risk of severe pneumonia (OR 4.73; 95% CI, 1.50–14.94; p = 0.003) compared to having a BMI 18.5–22.9 kg/m2 (Table 2). Clinical characteristics of patients with severe pneumonia versus those with mild to moderate disease are summarized in S1 Table. The rates of pneumonia, severe pneumonia, AKI and ICU stay across the BMI groups are also shown in S2 Table.
Table 2. Association between body mass index and severe pneumonia, using multivariable-adjusted logistic regression analyses.
| Severe pneumonia | ||||
|---|---|---|---|---|
| Model 1 | Model 2 | |||
| Beta-coefficient (95%CI) | p-value | Beta-coefficient (95%CI) | p-value | |
| Body mass index (kg/m2) | ||||
| <18.5 | 2.73 (0.54–13.70) | 0.224 | 2.56 (0.50–13.01) | 0.257 |
| 18.5–22.9 | reference | reference | ||
| 23.0–24.9 | 2.73 (0.76–9.83) | 0.125 | 2.83 (0.77–10.36) | 0.116 |
| ≥25.0 | 5.33 (1.76–16.12) | 0.003 | 4.73 (1.50–14.94) | 0.008 |
Model 1 was adjusted for age and sex.
Model 2 was adjusted for age, sex, diabetes, hypertension and dyslipidemia.
We did not find any two-way interactions of either age or sex with BMI levels in relation to pneumonia, severe pneumonia, AKI, and ICU stay, nor any three-way interactions of both age and sex with BMI levels in relation to pneumonia, severe pneumonia, AKI, and ICU stay.
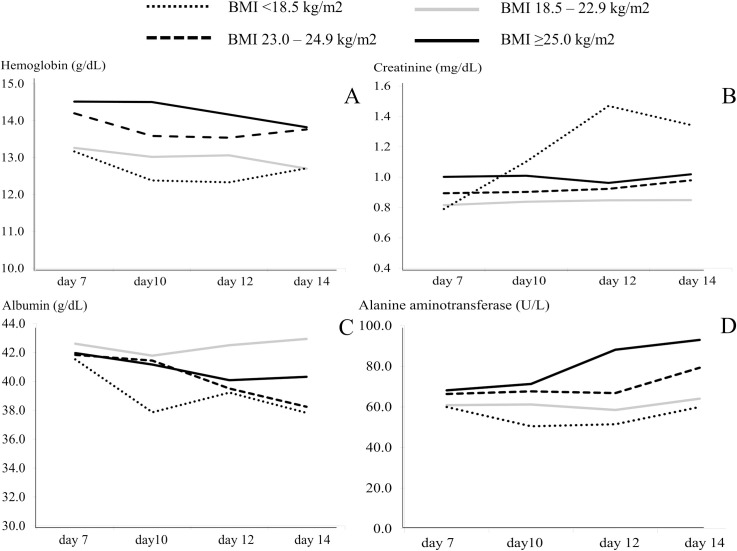
The mean of hemoglobin and albumin levels was significantly decreased from day 7 to day 14 of illness (p<0.001 and p = 0.04, respectively). Patients with higher BMI levels had higher hemoglobin and alanine aminotransferase levels during day 7 and day 14 of illness. Creatinine levels in patients having a BMI <18.5 kg/m2 tended to increase between day 7 and day 14 of illness and were significantly higher on day 12 and day 14 of illness, comparing with patients having a BMI ≥18.5 kg/m2 (p<0.05). Creatinine levels in patients having a BMI ≥25.0 kg/m2 tended to increase between day 14 and day 21 of illness and were significantly higher on day 21, comparing with patients having a BMI <25.0 kg/m2 (p = 0.019). Fig 2 illustrates the time-related changes of hemoglobin, creatinine, albumin, and alanine aminotransferase on day 7, day 10, day 12, and day 14 in each group of BMI levels.
Fig 2.
Time-related changes of (A) hemoglobin, (B) creatinine, (C) albumin, and (D) alanine aminotransferase, stratified by body mass index levels.
Discussion
The present study included all patients with confirmed COVID-19 in Faculty of Medicine Ramathibodi Hospital, Mahidol University, during the study period. The majority of patients were middle aged. Approximately two thirds of the patients were either over or under normal weight. Nearly a half of the patients were diagnosed with pneumonia and a quarter of the patients with pneumonia had progressed to severe pneumonia. A higher BMI was significantly associated with severe pneumonia, after controlling for age, sex, diabetes, hypertension, and dyslipidemia.
The present study has demonstrated an increased rate of pneumonia and severe pneumonia in higher BMI levels. Moreover, obese patients (BMI ≥25 kg/m2) with COVID-19 were at higher risk of severe pneumonia, comparing to COVID-19 patients with normal weight. Similar to a previous study [14], our study demonstrated the dose response relationship between increase BMI and severity of COVID-19 focusing on severe pneumonia. The pathogeneses linked between obesity and severe COVID-19 could be immune dysregulation, comorbidities, and an impaired respiratory system [15]. Adiposity is related to immune dysregulation, including elevated inflammation and impaired host immune response. Fat cells, particularly visceral adipocytes, can induce macrophages to release interleukin (IL)-1, IL-6, IL-8, IL-10, tumor necrosis factor-α, c-reactive protein, and resistin. The overproduction of these proinflammatory cytokines, as a cytokine storm, is also a mechanism of lung injury as well as multiorgan failure in COVID-19 [16, 17]. Conceivably, chronic inflammatory state in obese people might be related to more severe inflammation and results in worsened outcomes in COVID-19. On the other hand, obese people are less adept at mounting immune response against microbes [8]. So SARS-CoV-2 and superimposed bacteria could infect and replicate more efficiently among this population. Additionally, patients having underlying medical conditions (e.g. serious heart conditions, diabetes, chronic kidney disease, etc.) might be at higher risk for severe COVID-19 [18, 19]. Obesity is associated with an increased risk of diabetes mellitus, hypertension, and cardiovascular disease [6]. The greater number of comorbidities in obese patients therefore increases the severity of COVID-19. The respiratory system is also changed in obese people. Altered respiratory mechanics, increased airway resistance, and decreased lung volume can impair gas exchange [20].
Our study revealed both underweight and obese patients with COVID-19 had a higher rate of AKI, after controlling for age, sex, diabetes, hypertension, and dyslipidemia. Both underweight and obese patients had increasing creatinine levels in the 2nd or 3rd week of illness. The causes of AKI in COVID-19 were proposed in various mechanisms including prerenal azotemia, acute tubular necrosis, direct viral injury, and thrombotic microangiopathy [21]. The increased severity of COVID-19 in obese patients related to higher rates of AKI. Severe pneumonia, acute respiratory distress syndrome (ARDS), and invasive mechanical ventilation use are predisposing factors of AKI [22]. As well, obesity-related inflammatory mediators could impair kidney function [15]. These pathogeneses might be an explanation to why the rate of AKI increased and the creatinine levels on the 3rd week of illness were higher among obese patients with COVID-19, comparing with normal weight patients. Even so, the reason of creatinine level elevation among underweight patients with COVID-19 could differ. Patients with low BMI may be considered as malnutrition. The risk of AKI can be increased in malnourished patients when hospitalized [23]. Nausea, anosmia, and dysgeusia are common presenting symptoms of COVID-19 in the first period of illness, resulting in less appetite [24]. Reduced consumption, especially water, might result in dehydration and serum creatinine level increase on 2nd week of illness in underweight patients with COVID-19.
This study presented patients with higher BMI levels had higher alanine aminotransferase levels both on admission and during hospitalization, comparing with patients with lower BMI level. The mechanisms of liver injury in patients with COVID-19 are inflammatory response, direct viral cytotoxicity, anoxia, and reactivation of pre-existing chronic liver disease [25]. Obesity is also related with chronic inflammation [26] and non-alcoholic fatty liver disease [27]. Viral hepatitis B and C profiles were tested in all patients with abnormal liver function test but none of the patients had HBV or HCV infection. The percentages of patients with active alcohol drinking were not different between different BMI levels. Abnormal alanine aminotransferase should be concerned in obese patients with COVID-19.
This is a well-designed prospective cohort. Since we included all patients with confirmed COVID-19 range from asymptomatic to fatal, the patients in this study are representative of all COVID-19 cases. This cohort followed until all patients clinically improved or died; therefore, the final outcomes were accurately determined and all data were completed when analyzed. Nevertheless, this study has some limitations that should be of concern. First, the sample size was modest; however, this study enrolled all adult patients from one of the largest cohorts in Thailand. Second, the treatment regimens were not evaluated in the analysis; nonetheless, all patients were treated as to the Thai National Guidelines recommended. Third, we did not assess the body composition of the patients due to the limited access to the patients during hospitalization.
Conclusions
Obesity in patients with COVID-19 is associated with severe disease particularly severe pneumonia. The rates of adverse outcomes including AKI and ICU stay also increase in obese patients with COVID-19. The pathogenesis of AKI between underweight and obese patients may differ. Patients with a higher BMI are at higher risk for transaminitis. Further studies in body composition are warranted to explore the links between adiposity and severity of COVID-19.
Supporting information
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We are grateful to all study patients and all attending staffs, fellows, residents, nurses and dietitian of Chakri Naruebodindra Medical Institute, Faculty of Medicine Ramathibodi Hospital, Mahidol University.
Abbreviations
- AKI
acute kidney injury
- ARDS
acute respiratory distress syndrome
- BMI
body mass index
- COVID-19
Coronavirus Disease 2019
- ICU
intensive care unit
- IL
interleukin
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- SD
standard deviation
- WHO
World Health Organization
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. Coronavirus COVID-19 global cases by Johns Hopkins CSSE. [cited 2020/5/17]; Available from: https://coronavirus.jhu.edu/map.html.
- 2.World Health Organization. Coronavirus disease (COVID-19) situation report-124. May 23, 2020. [cited 2020/5/26]; Available from https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports.
- 3.Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, et al. Clinical characteristics of Covid-19 in New York City. N Engl J Med 2020; 382(24):2372–2374. 10.1056/NEJMc2010419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang T, Du Z, Zhu F, Cao Z, An Y, Gao Y, et al. Comorbidities and multi-organ injuries in the treatment of COVID-19. Lancet 2020; 395(10228):e52 10.1016/S0140-6736(20)30558-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keys A, Fidanza F, Karvonen MJ, Kimura N, Taylor HL. Indices of relative weight and obesity. J Chron Dis 1972; 25(6): 329–43. 10.1016/0021-9681(72)90027-6 [DOI] [PubMed] [Google Scholar]
- 6.Ghoorah K, Campbell P, Kent A, Maznyczka A, Kunadian. Obesity and Cardiovascular Outcomes: A Review. Eur Heart J Acute Cardiovasc Care 2016; 5(1):77–85. 10.1177/2048872614523349 [DOI] [PubMed] [Google Scholar]
- 7.Nimptsch K, Konigorski S, Pischon T. Diagnosis of Obesity and Use of Obesity Biomarkers in Science and Clinical Medicine. Metabolism 2019; 92:61–70. 10.1016/j.metabol.2018.12.006 [DOI] [PubMed] [Google Scholar]
- 8.Huttunen R, Syrjänen J. Obesity and the risk and outcome of infection. Int J Obes (Lond) 2013; 37(3):333–340. 10.1038/ijo.2012.62 [DOI] [PubMed] [Google Scholar]
- 9.Misumi I, Starmer J, Uchimura T, Beck MA, Magnuson T, Whitmire JK. Obesity expands a distinct population of T cells in adipose tissue and increases vulnerability to infection. Cell Rep 2019; 27:514–524. 10.1016/j.celrep.2019.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong CK, Lam CW, Wu AK, Ip WK, Lee NL, Chan IH, et al. Plasma Inflammatory Cytokines and Chemokines in Severe Acute Respiratory Syndrome. Clin Exp Immunol 2004; 136(1):95–103. 10.1111/j.1365-2249.2004.02415.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO, IOTF/IASO. The Asia-Pacific perspective: Redefining Obesity and its treatment. 2000.
- 12.World Health Organization. Clinical management of severe acute respiratory infection when Novel coronavirus (nCoV) infection is suspected: interim guidance. January 28, 2020. [cited 2020/4/15]; Available from: https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected.
- 13.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group KDIGO clinical practice guidelines for acute kidney injury. Kidney Int. Suppl. 2012; 2:1–138. [Google Scholar]
- 14.Pranata R, Lim MA, Yonas E, Vania R, Lukito AA, Siswanto BB, et al. Body mass index and outcome in patients with COVID-19: A dose-response meta-analysis. Diabetes Metab 2020; S1262-3636(20)30097-5. 10.1016/j.diabet.2020.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stefan N, Birkenfeld AL, Schulze MB, Ludwig DS. Obesity and Impaired Metabolic Health in Patients With COVID-19. Nat Rev Endocrinol 2020; 16(7):341–342. 10.1038/s41574-020-0364-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strissel KJ, DeFuria J, Shaul ME, Bennett G, Greenberg AS, Obin MS. T ‑cell recruitment and Th1 polarization in adipose tissue during diet‑induced obesity in C57BL/6 mice. Obesity (Silver Spring). 2010;18(10):1918‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGonagle D, Sharif K, O’Regan A, Bridgewood C. The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease. Autoimmun Rev. 2020;19(6):102537 10.1016/j.autrev.2020.102537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;e200994 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323(11):1061‐9. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murugan AT, Sharma G. Obesity and respiratory diseases. Chron Respir Dis 2008; 5(4):233–42. 10.1177/1479972308096978 [DOI] [PubMed] [Google Scholar]
- 21.Batlle D, Soler MJ, Sparks MA, Hiremath S, South AM, Welling PA, et al. Acute Kidney Injury in COVID-19: Emerging Evidence of a Distinct Pathophysiology. JASN May 2020, ASN.2020040419 10.1681/ASN.2020040419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joannidis M, Forni LG, Klein SJ, Honore PH, Kashani K, Ostermann M, et al. Lung-kidney interactions in critically ill patients: consensus report of the Acute Disease Quality Initiative (ADQI) 21 Workgroup. Intensive Care Med. 2020;46(4):654‐72. 10.1007/s00134-019-05869-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li C, Xu L, Guan C, Zhao L, Luo C, Zhou B, et al. Malnutrition screening and acute kidney injury in hospitalised patients: a retrospective study over a 5-year period from China. Br J Nutr. 2020. February 14;123(3):337–46. 10.1017/S000711451900271X [DOI] [PubMed] [Google Scholar]
- 24.Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, De Siati DR, Rodriguez A, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;1‐11. 10.1007/s00405-020-05965-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun J, Aghemo A, Forner A, Valenti L. COVID-19 and liver disease. Liver Int. 2020. April 6. [DOI] [PubMed] [Google Scholar]
- 26.Cox AJ, West NP, Cripps AW. Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol. 2015;3(3):207‐15. 10.1016/S2213-8587(14)70134-2 [DOI] [PubMed] [Google Scholar]
- 27.Dietrich P, Hellerbrand C. Non-alcoholic fatty liver disease, obesity and the metabolic syndrome. Best Pract Res Clin Gastroenterol. 2014. August;28(4):637–53. 10.1016/j.bpg.2014.07.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.