Abstract
CYP4B1 belongs to the mammalian CYP4 enzyme family and is predominantly expressed in the lungs of humans. It is responsible for the oxidative metabolism of a wide range of endogenous compounds and xenobiotics. In this study, using data from The Cancer Genome Atlas (TCGA) project and the Gene Expression Omnibus (GEO) database, a secondary analysis was performed to explore the expression profile of CYP4B1, as well as its prognostic value in patients with lung adenocarcinoma (LUAD). Based on the obtained results, a significantly decreased CYP4B1 expression was discovered in patients with LUAD when compared with their normal counterparts (p<0.05), and was linked to age younger than 65 years (p = 0.0041), history of pharmaceutical (p = 0.0127) and radiation (p = 0.0340) therapy, mutations in KRAS/EGFR/ALK (p = 0.0239), and living status of dead (p = 0.0026). Survival analysis indicated that the low CYP4B1 expression was an independent prognostic indicator of shorter survival in terms of overall survival (OS) and recurrence-free survival (RFS) in patients with LUAD. The copy number alterations (CNAs) and sites of cg23440155 and cg23414387 hypermethylation might contribute to the decreased CYP4B1 expression. Gene set enrichment analysis (GSEA) suggested that CYP4B1 might act as an oncogene in LUAD by preventing biological metabolism pathways of exogenous and endogenous compounds and enhancing DNA replication and cell cycle activities. In conclusion, CYP4B1 expression may serve as a valuable independent prognostic biomarker and a potential therapeutic target in patients with LUAD.
1. Introduction
Non-small cell lung cancer (NSCLC) is one of the most frequently diagnosed tumors and the leading cause of cancer-related deaths worldwide, accounting for approximately 85% of lung cancer cases [1]. Lung adenocarcinoma (LUAD) and squamous cell carcinoma (LUSC) are the main histological subtypes of NSCLC, with the former representing more than 40% of lung cancers, gradually replacing the latter as the most frequent histological subtype in the past 25 years for unknown causes [2]. It is well known that cigarette smoking is by far the most important risk factor for lung cancer; however, LUAD is the most common type of lung cancer observed in non-smokers, indicating that multiple nonsmoking factors, including air pollution, radiation, and environmental related carcinogens, might also be responsible for LUAD carcinogenesis [3, 4].
Owing to the insignificant early symptoms and the tendency toward hematogenous metastasis, most patients with LUAD are diagnosed in the middle and late stages when diagnosed, thus missing the ideal period for diagnosis and treatment [5]. Combined with the poor sensitivity to radiochemotherapy, the mortality of patients with LUAD at 5 years is markedly high, ranging from 43% to 95%, depending on the stage [6]. In the last three decades, extensive efforts have been made in the early detection and treatment of LUAD to improve the patient situation, especially the application of genome-guided molecularly targeted therapy [e.g., anti-vascular endothelial growth factor (VEGF) bevacizumab and anti-epidermal growth factor receptor (EGFR) necitumumab therapy] and immunotherapy [e.g., pembrolizumab, an anti-PD-1 (programmed death-1) antibody]. However, to date, no comprehensive improvement in the 5-year survival of LUAD has been achieved. Therefore, the search for new and effective biomarkers to screen out high-risk patients and predict the prognosis of LUAD has become an essential part of lung cancer prevention and treatment.
Altered cellular metabolism is a prominent feature of tumorigenesis. Cytochrome P450 (CYP), a superfamily of cysteinato-heme monooxygenases, is an essential element of in this system. It serves as the most important phase I drug metabolism enzyme system, undertaking oxidative reactions in the body [7]. Furthermore, it is widely involved in the metabolic processes of exogenous drugs, environmental poisons, carcinogens, and endogenous hormones, thus playing a pivotal role in regulating the interaction between the organism and the external environment, as well as in maintaining the homeostasis in vivo. Individual P450s often present characteristic cell type- and tissue-specific expression patterns. As an extrahepatic form of cytochrome P450, CYP4B1 is predominantly expressed in the lung, as well as in other organs in smaller amounts [8]. The contribution of CYP4B1 in cancer is of particular interest, as its expression was found to be altered in a couple of specific cancers, including lung cancer [9–14]. As the primary site of exposure to inhaled toxicants and carcinogens, the metabolic balance of the protective detoxification system of the lung organ is essential to maintain its normal physiological function; thus, it is reasonable to speculate that the dysregulation of CYP4B1 is presumably associated with carcinogenesis in the lung owing to its catalytic activity in the first step of xenobiotic processing. Accordingly, in the present study, using data from The Cancer Genome Atlas (TCGA) project and the Gene Expression Omnibus (GEO) database, we performed a secondary analysis to thoroughly analyze the CYP4B1 expression level, determine its prognostic role, explore the underlying mechanisms of its dysregulation, as well as to probe its potential functions in LUAD.
2. Materials and methods
2.1. Data mining
In this retrospective study, gene expression data with clinical information from the TCGA-LUAD project were obtained for this retrospective study from its official website (https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga). The exclusion criteria were patients who did not present primary tumors and/or had received neoadjuvant therapy. Tissues from 511 patients with primary tumors and 59 adjacent normal counterparts were extracted for RNA sequencing using Illumina HiSeq. To measure the amount of gene expression, level 3 normalized sequencing data of fragments per kilobase of transcript per million mapped reads (FPKM) were used. Of the 511 patients, 501 had intact overall survival (OS) data recorded, while 438 had intact recurrence-free survival (RFS) data recorded. Clinicopathological parameters including age, sex, pathological stage, radiation therapy, pharmaceutical therapy, tobacco smoking history, and canonical mutations in KRAS/EGFR/ALK were collected. The age of the patients at diagnosis ranged from 33 to 88 years. The pathological stage, reflecting the extent of the cancer, was graded as I, II, III, and IV based on the American Joint Committee on Cancer staging criteria [15]. The tobacco smoking history was categorized as level 1–5, describing the smoking status and history as self-reported by a patient, with level 1 indicating lifelong non-smokers, while levels 2–5 representing current smokers with different lengths of smoking. Additionally, living status, OS in days, recurrence status, and RFS in days were also downloaded for survival-related analysis. Three gene microarray datasets of lung cancer (GSE30219, GSE31210 and GSE32863) were retrieved from the GEO database (http://www.ncbi.nlm.nih.gov/geo/) for validation. To explore the underlying mechanisms of CYP4B1 dysregulation in LUAD, somatic mutations, linear copy number alterations (CNAs), and DNA methylation status in CYP4B1 were simultaneously collected from TCGA.
2.2. Functional and pathway enrichment analysis
The LinkedOmics database is a unique platform containing multi-omics data and clinical data for 32 cancer types from the TCGA project, allowing biologists and clinicians to access, analyze and compare cancer multi-omics data within and across tumor types [16]. To investigate the underlying function of CYP4B1 in LUAD, we identified genes differentially co-expressed with CYP4B1 expression in TCGA-LUAD via LinkedOmics. The cut-off criteria of studies were set as a Benjamini and Hochberg false discovery rate (FDR) < 0.05 and a |Pearson’s r| > 0.2. GEPIA2 (http://gepia2.cancer-pku.cn) web server is a valuable and highly cited resource for gene expression analysis based on tumor and normal samples from the TCGA and GTEx databases [17]. In our study, it was used for further analysis of the top three genes positively correlated with CYP4B1 identified by LinkedOmics, involving their expression profiles and prognostic value in LUAD. Gene ontology (GO) analysis and gene set enrichment analysis (GSEA), categorized by the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway was performed using the LinkInterpreter module of LinkedOmics. FDR < 0.05 indicates statistically significant.
2.3. Statistical analysis
SPSS Statistics 20 (SPSS Inc., IL, USA) and GraphPad Prism 8 (GraphPad Inc., CA, USA) were used for statistical analyses. Comparison between the two groups and the association of CYP4B1 expression with clinicopathological parameters was evaluated using Welch’s t-test. Gene expression levels were categorized as low or high according to median values. Receiver operating characteristic (ROC) analysis was performed to assess the diagnostic value of CYP4B1 expression in LUAD. Kaplan-Meier curves of OS and RFS were generated using GraphPad Prism by setting the median CYP4B1 expression as the cut-off. A log-rank test was performed to examine the significance of differences between curves. The prognostic value was analyzed using univariate and multivariate Cox regression models. Factors correlated with OS outcomes in the univariate analysis were included in the multivariate analysis. Pearson correlation coefficients were calculated to evaluate the correlations between the two groups of continuous variables. p<0.05 indicates statistical significance for all statistical analyses performed.
3. Results
3.1. CPY4B1 was significantly downregulated in patients with LUAD
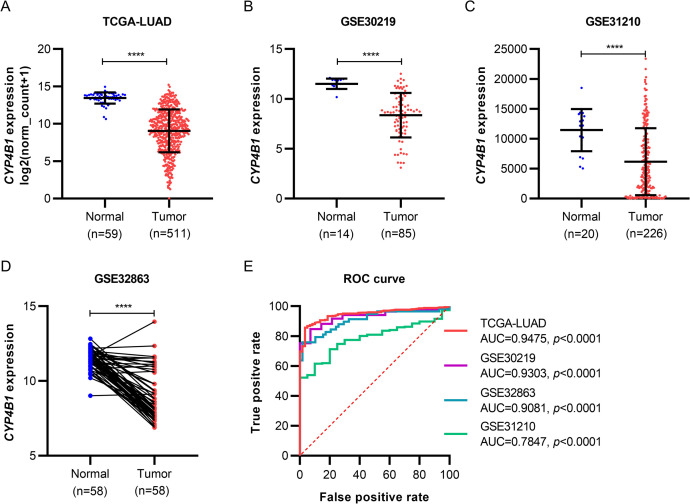
To examine the mRNA expression level of CYP4B1 in patients with LUAD, we extracted RNA-seq data from the TCGA project and microarray data of GSE30219, GSE31210, and GSE32863 from the GEO database for validation. In the TCGA-LUAD cohort, the results indicated that CYP4B1 mRNA expression was approximately 0.33-fold lower expressed in tumor tissues (n = 511) than in adjacent normal tissues (n = 59) (Fig 1A). Next, this finding was verified in specimens from the three GEO datasets (Fig 1B–1D). Moreover, the area under the curve (AUC) value of CYP4B1 expression for LUAD diagnosis was no less than 0.7847 in the four LUAD cohorts, all with a p-value<0.0001 (Fig 1E).
Fig 1. CYP4B1 mRNA expression profiles in patients with LUAD from TCGA and GEO dataset cohorts.
(A–D) CYP4B1 mRNA expression in LUAD tissues and normal controls from a TCGA cohort (A), GSE30219 cohort (B), GSE31210 cohort (C) and GSE32863 cohort (D). (E) ROC curves showing the diagnostic value of CYP4B1 in LUAD (red curve, data from TCGA cohort; purple curve, data from GSE30219 cohort; blue curve, data from GSE32863 cohort; green curve, data from GSE31210 cohort). ****p<0.0001. LUAD, lung adenocarcinoma; TCGA, The Cancer Genome Atlas; GEO, Gene Expression Omnibus; ROC, receiver operating characteristic.
Next, we explored the expression pattern of CYP4B1 in different subgroups of clinicopathological parameters. The results revealed that the significantly decreased CYP4B1 expression was more frequently observed in patients with age younger than 65 years (p = 0.0041), in those with a history of pharmaceutical therapy (p = 0.0127) and radiation therapy (p = 0.0340), mutations in KRAS/EGFR/ALK (p = 0.0239), and deaths (i.e., living status; p = 0.0026) (Table 1).
Table 1. Correlation between the clinicopathological parameters and CYP4B1 expression in LUAD.
| Parameters | Variables | n | Mean ± SD | t | p-value |
|---|---|---|---|---|---|
| Age (years) | <65 | 220 | 8.588±2.896 | 2.886 | 0.0041 |
| ≥65 | 275 | 9.339±2.854 | |||
| Gender | Female | 277 | 9.255±2.778 | 1.793 | 0.0736 |
| Male | 237 | 8.801±2.961 | |||
| Smoking history | 1 | 75 | 9.607±3.153 | 1.804 | 0.0719 |
| 2/3/4/5 | 425 | 8.963±2.793 | |||
| Pathological stage | I/II | 396 | 9.162±2.863 | 1.716 | 0.0867 |
| III/IV | 110 | 8.634±2.817 | |||
| Pharmaceutical therapy | No | 280 | 9.304±2.994 | 2.504 | 0.0127 |
| Yes | 188 | 8.653±2.586 | |||
| Radiation therapy | No | 362 | 9.150±2.876 | 2.127 | 0.0340 |
| Yes | 107 | 8.308±2.762 | |||
| Mutations in KRAS/EGFR/ALK | No | 134 | 8.938±2.842 | 2.274 | 0.0239 |
| Yes | 96 | 9.756±2.572 | |||
| Recurrence status | No | 275 | 9.103±2.953 | 0.932 | 0.3523 |
| Yes | 162 | 8.838±2.826 | |||
| Living status | Living | 327 | 9.340±2.767 | 3.036 | 0.0026 |
| Dead | 187 | 8.531±2.980 |
LUAD, lung adenocarcinoma; SD, standard deviation.
3.2. Low CYP4B1 expression was associated with poor outcomes of patients with LUAD
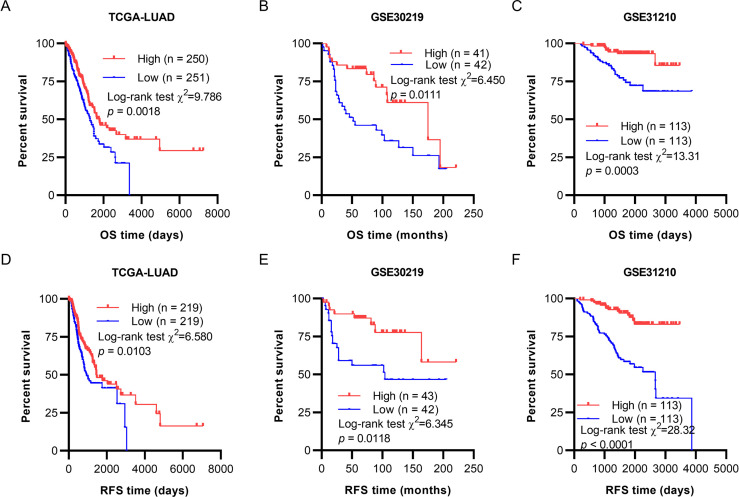
To assess the correlations between CYP4B1 mRNA expression and survival outcomes in LUAD patients, Kaplan-Meier curves were generated using clinical survival data of OS and RFS from TCGA. As shown in Fig 2A and 2D, low CYP4B1 expression was more strongly associated with shorter OS and RFS in patients with primary LUAD (p = 0.0018 and p = 0.0103, respectively). On using GEO datasets for validation, the decreased CYP4B1 expression group was found to possess remarkably inferior OS and RFS when compared with the high CYP4B1 expression group in both GSE30219 (p = 0.0111 and p = 0.0118, respectively; Fig 2B and 2E) and GSE31210 (p = 0.0003 and p<0.0001, respectively; Fig 2C & 2F) datasets.
Fig 2. Low CYP4B1 expression was associated with unfavorable survival in patients with LUAD.
(A–C) Kaplan-Meier curves of OS in patients with LUAD from a TCGA cohort (A), GSE30219 cohort (B) and GSE31210 cohort (C). (D-F) Kaplan-Meier curves of RFS in patients with LUAD from a TCGA cohort (D), GSE30219 cohort (E) and GSE31210 cohort (F). LUAD, lung adenocarcinoma; OS, overall survival; TCGA, The Cancer Genome Atlas; RFS, recurrence-free survival.
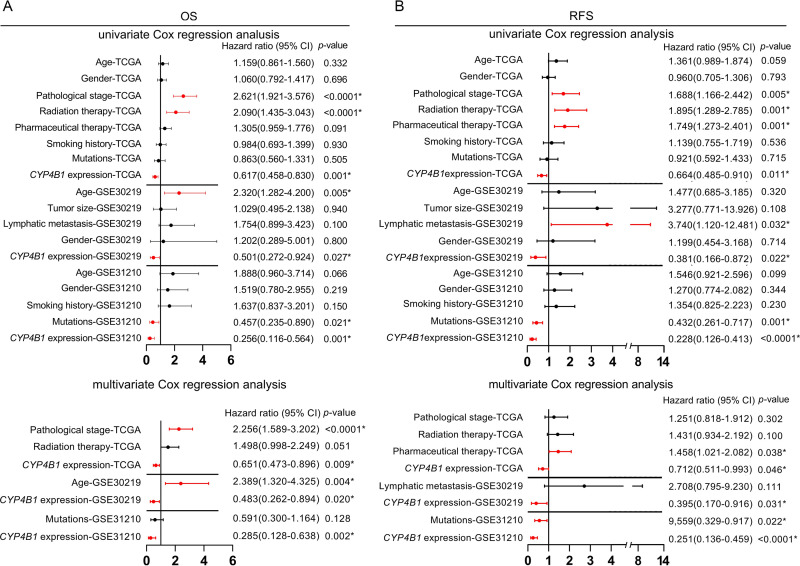
Moreover, to verify the robust prognostic value of CYP4B1 in terms of OS and RFS, univariate and multivariate analyses based on the Cox regression model were performed. Regarding OS, the decreased CYP4B1 expression was found to be linked with unfavorable OS in patients from the TCGA-LUAD cohort via univariate analysis (p = 0.001, Fig 3A). Multivariate analysis confirmed that low CYP4B1 expression could independently predict poor OS after adjustment for risk factors correlated with OS outcomes in univariate analysis (p = 0.009, Fig 3A). Subsequently, the same analysis performed on datasets from the GEO database further verified that low CYP4B1 expression was an independent prognostic indicator (pGSE30219 = 0.020 and pGSE31210 = 0.002, respectively; Fig 3A). In terms of RFS, the independent prognostic value of CYP4B1 in patients with LUAD was observed in the TCGA-LUAD cohort (p = 0.046) and was validated in the two independent GEO datasets (pGSE30219 = 0.031 and pGSE31210<0.0001, respectively; Fig 3B).
Fig 3. Forest plots of Cox regression analysis.
(A) Forest plots showing univariate and multivariate analysis of OS in patients with LUAD. (B) Forest plots showing univariate and multivariate analysis of RFS in patients with LUAD. *p<0.05. OS, overall survival; LUAD, lung adenocarcinoma; RFS, recurrence-free survival.
3.3. CYP4B1 expression was regulated by CNAs and DNA methylation
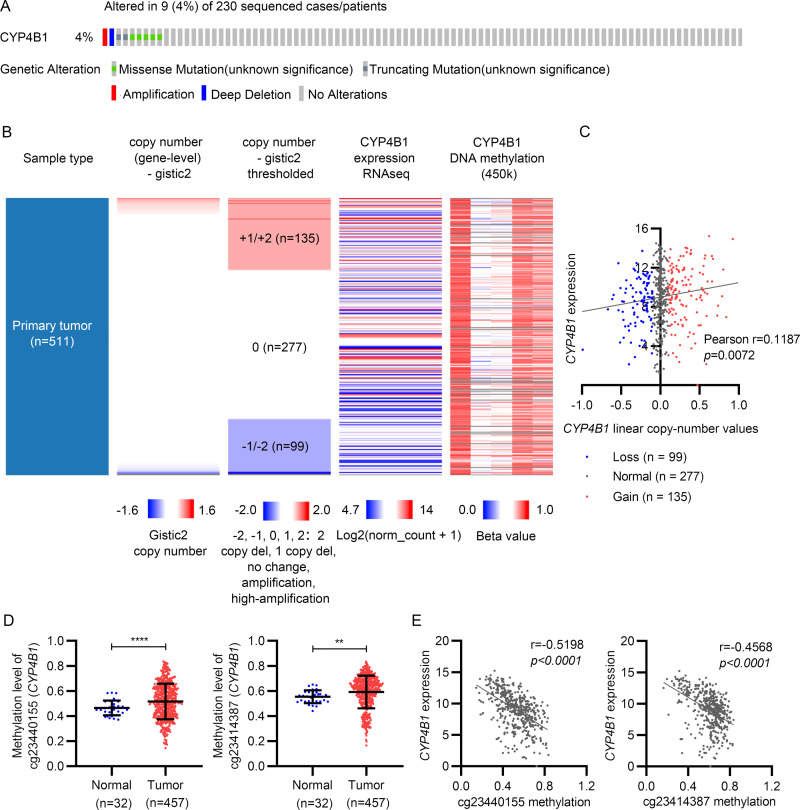
Next, we explored the potential mechanism of CYP4B1 dysregulation. First, genetic alterations in CYP4B1 were detected in TCGA-LUAD using the cBio-Portal for the Cancer Genomics platform (http://www.cbioportal.org/). CYP4B1 mutations were observed in 9 out of 230 (4%) LUAD cases, with missense mutations as the main alteration type detected (Fig 4A). On analyzing CYP4B1 DNA CNAs in 511 cases of LUAD, we observed that copy amplification occurred in 135 cases (+1/+2, 26.4%), copy deletion in 99 cases (-1/-2, 19.4%), and copy neutrality in 277 cases (0, 54.2%) (Fig 4B). Regression analysis demonstrated a significant correlation between CYP4B1 expression and linear copy number values (Pearson’s r = 0.1187, p = 0.0072, Fig 4C). Additionally, the methylation status of five CpG sites in the CYP4B1 gene was extracted to assess its role in regulating CYP4B1 expression. By comparing the methylation level between tumor (n = 457) and normal tissues (n = 32), we observed that two CpG sites, cg23440155 and cg23414387, were remarkably hypermethylated in the tumor group (Fig 4D and S1 Table). In Pearson’s regression analysis, CYP4B1 expression was found to be strongly and negatively correlated with DNA methylation in the two selected CpG sites (cg23440155: Pearson’s r = -0.5198, p<0.0001; cg23414387: Pearson’s r = -0.4568, p<0.0001) (Fig 4E).
Fig 4. CYP4B1 expression was modulated by its gene alterations and DNA methylation in LUAD.
(A) Genetic alterations of CYP4B1 in LUAD. (B) Heatmap of CYP4B1 expression, DNA copy number alterations (gistic2 linear and gistic2 thresholded), and DNA methylation (Methylation 450k). (C) CYP4B1 expression correlates with its linear copy number values by linear regression analysis. (D) DNA methylation level of cg23440155 and cg23414387 sites is significantly upregulated in LUAD tissues when compared with adjacent normal tissues. (E) CYP4B1 expression correlates with cg23440155 and cg23414387 methylation levels by a linear regression analysis. LUAD, lung adenocarcinoma.
3.4. Gene set enrichment analysis of genes co-expressed with CYP4B1 in LUAD
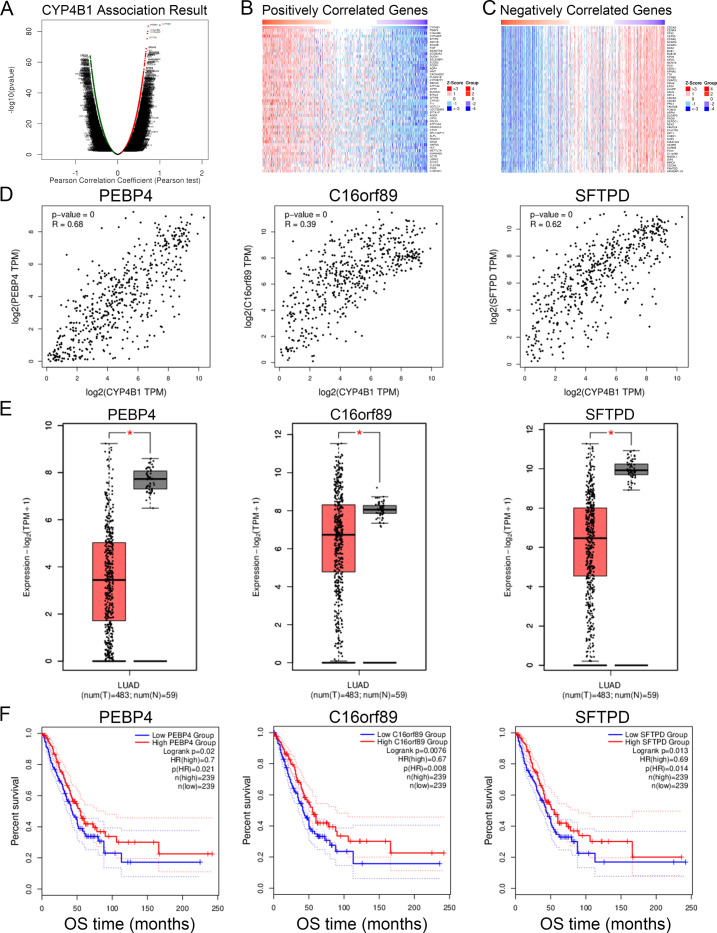
By data mining using the LinkFinder module of LinkedOmics, we identified the genes co-expressed with CYP4B1 in 515 patients with LUAD from the TCGA project (FDR<0.05, and (|Pearson’s r|)>0.2). The results indicated that a lot of 3829 and 2516 genes were positively and negatively correlated with CYP4B1 expression, respectively (Fig 5A). A heatmap of the top 50 significant genes positively or negatively associated with CYP4B1 is shown in Fig 5B and 5C. Among them, expression of PEBP4, C16orf89 and SFTPD showed the strongest positive correlation with CYP4B1 expression (PEBP4: Pearson’s r = 0.68; C16orf89: Pearson’s r = 0.39; SFTPD: Pearson’s r = 0.62) (Fig 5D). Consistent with CYP4B1 expression, the three genes mentioned above revealed lower expression in tumor tissues than in their normal counterparts. Meanwhile, the decreased expression groups were linked to an inferior OS in patients with LUAD (Fig 5E and 5F).
Fig 5. Differentially expressed genes correlated with CYP4B1 in LUAD.
(A) Volcano plot showing differentially expressed genes significantly associated with CYP4B1 (FDR<0.05; |Pearson’s r|>0.2). (B, C) Heatmaps showing the top 50 significant genes positively and negatively correlated with CYP4B1 in LUAD. (D-F) The top three significant genes demonstrating positive co-expression with CYP4B1 in LUAD. (D) Pearson correlation of CYP4B1 expression with PEBP4, C16orf89, and SFTPD. (E) The mRNA expression profiles of PEBP4, C16orf89, and SFTPD in LUAD tissues when compared with normal lung tissues. (F) Survival analysis of PEBP4, C16orf89, and SFTPD in LUAD. FDR, false discovery rate; LUAD, lung adenocarcinoma.
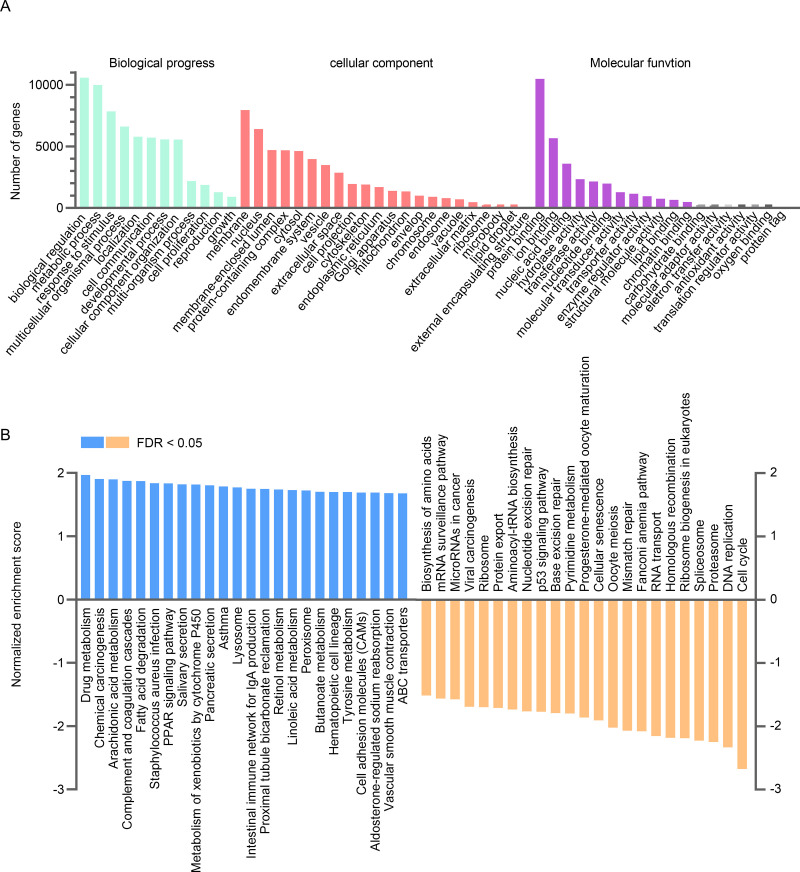
To further investigate the possible gene ontology terms and signaling pathways in which CYP4B1 might be involved, GO and GSEA analyses were performed using the LinkInterpreter module of LinkedOmics. The results of GO analysis showed that genes positively co-expressed with CYP4B1 were mainly located in the membrane, nucleus and membrane-enclosed lumen, and generally participated in biological processes of biological regulation, metabolic processes and responses to stimuli by molecular functions such as protein binding, ion binding, and nucleic acid binding (Fig 6A). Furthermore, the GSEA analysis revealed that the top five enriched KEGG pathways positively linked with CYP4B1 expression were drug metabolism, chemical carcinogenesis, arachidonic acid metabolism, complement and coagulation cascades and fatty acid degradation, while the most negatively correlated pathway was the cell cycle (Fig 6B).
Fig 6. Gene ontology and GSEA analysis categorized by the KEGG pathway of the genes co-expressed with CYP4B1 in TCGA-LUAD.
GSEA, Gene set enrichment analysis; KEGG, Kyoto Encyclopedia of Genes and Genomes; TCGA, The Cancer Genome Atlas; LUAD, lung adenocarcinoma.
4. Discussion
CYP4B1, a member of the CYP450s superfamily, is responsible for the oxidative metabolism of a series of both endogenous compounds and xenobiotics, preferentially via a ω-hydroxylation pathway [18]. The gene sequence of CYP4B1 has been evolutionarily conserved, and the enzyme has been detected by immunoblotting in animals such as rabbits, guinea, mice, monkeys, and hamsters [18, 19]. The expression of CYP4B1 is predominantly extrahepatic, presenting tissue and species specificity. Studies have revealed that the human forms of CYP4B1 possess limited activity in vivo when compared with animals, rendering rabbit CYP4B1 a feasible gene therapy in humans to treat cancers in humans [20]. To date, the physiological function of innate human CYP4B1 remains unclear. Nevertheless, like other CYP4 proteins, CYP4B1 can metabolize endogenous fatty acids and hydrocarbons, as well as participates in the bioactivation of a wide variety of xenobiotics, including valproic acid (VPA), 2-aminofluorene (2-AF), 3-methylindole (3-MI), 4-ipomeanol (4-IPO) and numerous aromatic amines in rodents and ruminants. The CYP4B1 catalytic metabolites or reactive intermediates of these xenobiotics reportedly exert toxicological effects by forming adducts with DNA or proteins, eliciting organ-specific toxicities, which might contribute to the formation and progression of carcinogenesis. One example is that a high expression of CYP4B1 increases the risk of bladder tumor by activation of 2-AF [10].
Czerwinski et al. have first quantified CYP4B1 mRNA in the human lung, as well as in lung tumors, and observed 2.3-fold lower mRNA levels in tumors [9]. However, to our knowledge, the expression profile and related function of CYP4B1 have not been studied in LUAD species. In this study, we confirmed the carcinogenic role of CYP4B1 in LUAD for the first time. By analyzing RNA-seq and microarray data of LUAD, CYP4B1 was found to demonstrate a remarkably lower expression in tumor specimens than in noncancerous lung tissues. Meanwhile, the AUC value for CYP4B1 was no less than 0.7847 in the four LUAD cohorts of TCGA-LUAD, GSE30219, GSE32863, and GSE31210, revealing that the gene expression signature of CYP4B1 is a useful marker for LUAD diagnosis.
Several studies have explored the role and significance of dysregulated CYP4B1 in human cancers. In adrenocortical carcinoma (ACC), CYP4B1 expression was nearly absent when compared with that in the normal adrenal cortex. Ectopic expression of CYP4B1 reportedly promotes cytotoxicity and increases chemosensitivity in ACC cell lines, implicating the role of CYP4B1 in tumorigenesis and chemoresistance in ACC [12]. Upon injury to the cornea, the CYP4B1 enzymatic pathway was found to be activated, leading to the production of 12-hydroxyeicosatrienoic acid (12-HETrE), a potent inflammatory and angiogenic eicosanoid. By applying siRNA duplexes targeting CYP4B1 in vitro, the neovascular response and expression of corneal VEGF were notably reduced. These results strongly suggest that corneal CYP4B1 is a component of the inflammatory and neovascular cascade initiated by injury to the corneal epithelium [14]. Moreover, downregulation of CYP4B1 protein levels in urothelial carcinomas (UC) was found to be correlated with several clinicopathological factors of an advanced primary tumor, nodal metastasis, high histological grade, vascular invasion, perineural invasion, and mitotic rate. Loss of CYP4B1 expression is an independent unfavorable prognosticator of UC [13].
Currently, the relevance of the association between CYP4B1 expression and the clinical features of LUAD remains unclear. Our findings revealed that low CYP4B1 mRNA expression was linked to factors such as age less than 65 years, history of pharmaceutical therapy and radiation therapy, mutations in KRAS/EGFR/ALK, and death (living status). The correlation between CYP4B1 expression and a history of pharmaceutical therapy has implicated its crucial role in drug metabolism in LUAD. Notably, Murtha et al. have observed that the exogenous expression of CYP4B1 accentuates the apparent cytotoxicity in ACC cell lines that were treated with mitotane or cisplatin [12]. As LUAD is a drug-resistant tumor, this finding could present CYP4B1 as an attractive future target for resistant-LUAD therapeutics. Kaplan-Meier survival analyses indicated that patients with low CYP4B1 expression had remarkably shorter OS and RFS. By performing univariate and multivariate analyses, we confirmed that decreased CYP4B1 RNA expression was independently associated with inferior OS and RFS in patients with LUAD. Therefore, we infer that reduced CYP4B1 expression could serve as an independent predictor of unfavorable OS and RFS.
It is well recognized that the drug-mediated activation of CYP gene is regulated by various members of the nuclear receptor superfamily. For CYP4B1, computational analysis of its promoter region from rabbit cornea revealed sequences that could potentially bind heterodimers of the retinoic acid receptors (RARs), including retinoic acid receptor/retinoid X receptor (RAR/RXR), vitamin D receptor/RXR (VDR/RXR), and peroxisome proliferator-activated receptor/retinoid X receptor (PPAR/RXR). We then verified that the transcription of CYP4B1 in HepG2 cells was significantly activated by incubation with retinoic acid [21]. Furthermore, studies have observed that the binding activity of the promoter region of rabbit corneal CYP4B1 was induced under hypoxic conditions for hypoxia-inducible factor-1 (HIF-1), nuclear factor-kappa light chain enhancer of activated B cells (NF-κB), and activator protein-1 (AP-1) [22]. In addition to regulation at the transcriptional level, genetic and epigenetic alterations affect gene expression during carcinogenesis. In the present study, we observed that CYP4B1 DNA alterations occurred in LUAD, in which missense mutations were the major type. Genetic variants of CYP4B1 have been reported in several previous studies [23–26]. Among them, CYP4B1*2 was found to lead to a complete loss of CYP4B1 function in a cohort of French Caucasians [23]. In a Japanese population, subjects carrying the CYP4B1*1/*2 or CYP4B1*2/*2 genotypes exhibited a 1.75-fold increased risk of bladder cancer [26]. These findings suggest that genetic alteration of CYP4B1 could impact its own functionality. However, the DNA mutations observed based on TCGA-LUAD in our study are yet to be reported (data not shown). Thus, further studies are needed to evaluate inter-individual variations in the activity of CYP4B1 coding variants. Moreover, CYP4B1 dysregulation was found to be regulated by copy number variation, as well as the hypermethylation of cg23440155 and cg23414387 sites.
Additionally, the prognostic role of CYP4B1 indicated that it might act as a tumor-driven gene in LUAD. We then identified its co-expressed genes and performed the GO and GSEA enrichment analyses to explore the underlying oncogenic properties of CYP4B1 in LUAD. In our study, three genes, PEBP4, C16orf89, and SFTPD, were found to be significantly and positively co-expressed with CYP4B1. These genes all showed remarkable downregulation in tumor tissues and were linked to the unfavorable OS in LUAD. PEBP4 and SFTPD have been reported as oncogenes in lung cancer [27–33]. However, C16orf89 is a rarely investigated gene and its role in LUAD needs to be further confirmed. As suggested by GO and GSEA enrichment analyses, genes positively co-expressed with CYP4B1 were predominantly enriched in biological metabolism-related activities and regulation of exogenous and endogenous compounds, including drug metabolism, chemical carcinogenesis, and arachidonic acid metabolism. This finding is consistent with the physiological functions of CYP4B1. Moreover, one of the top pathways that was negatively correlated with CYP4B1 expression was the cell cycle. Disruption of multiple biological pathways is a hallmark of several tumors, including LUAD. These results suggest that inhibition of CYP4B1 in LUAD promotes tumorigenesis by preventing metabolism and enhancing DNA replication and cell cycle activities. Overall, our findings provide new insights into the role of CYP4B1 in the development and treatment of LUAD. However, this study had several limitations. First, the number of subjects included in our study cohort was relatively small, which probably limits the statistical power, especially in the subgroup analyses. Second, we observed that both CNAs and DNA methylation contributed to the decreased expression of CYP4B1 in LUAD; however, we were unable to determine whether additional genetic or epigenetic mechanisms influence its transcription. Third, the results of this study were not externally validated; thus, experimental studies are required to further explore the role and underlying mechanisms if action of CYP4B1 in LUAD.
5. Conclusion
CYP4B1 expression may serve as a valuable independent prognostic biomarker and a potential therapeutic target in patients with LUAD.
Supporting information
(DOCX)
Acknowledgments
We gratefully thank Chen cen (emergency management bureau, Jining) for helpful suggestions for graphs drawing work in this study and Maria Brazelina Nandini in Editage for professional language editing service.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by the NSFC cultivation project of Jining Medical University (JYP2019KJ10), the Medical and Health Science and Technology Development Project of Shandong Province (2019WS357), the open project of Shanxi Provincial Key Laboratory of Forensic Science, China (SFM2019002) and the Student Innovation Training Program Project of Shandong Province (S202010443043). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ferly J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941–53. 10.1002/ijc.31937 [DOI] [PubMed] [Google Scholar]
- 2.Devita VT, Lawrence TS, Rosenberg SA. DeVita, Hellman, and Rosenberg’s Cancer: Principles & Practice of Oncology. 10th ed Philadelphia: Wolters Kluwer; 2015. [Google Scholar]
- 3.Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends—an update. Cancer Epidemiol Biomarkers Prev. 2016;25(1):16–27. 10.1158/1055-9965.EPI-15-0578 [DOI] [PubMed] [Google Scholar]
- 4.Chalela R, Curull V, Enríquez C, Pijuan L, Bellosillo B, Gea J. Lung adenocarcinoma: from molecular basis to genome-guided therapy and immunotherapy. J Thorac Dis. 2017;9(7):2142–58. 10.21037/jtd.2017.06.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denisenko TV, Budkevich IN, Zhivotovsky B. Cell death-based treatment of lung adenocarcinoma. Cell Death Dis. 2018;9(2):117–30. 10.1038/s41419-017-0063-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cancer Facts & Figures 2020. American Cancer Society. 2020. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2020.html
- 7.Adhikari A, De M. Association of cytochrome P450 with cancer induced by Betel quid (BQ): a review. Am J Pharmacol Pharmacother. 2015;2:81–94. [Google Scholar]
- 8.Wiek C, Schmidt EM, Roellecke K, Freund M, Nakano M, Kelly EJ, et al. Identification of amino acid determinants in CYP4B1 for optimal catalytic processing of 4-ipomeanol. Biochem J. 2015;465(1):103–14. 10.1042/BJ20140813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Czerwinski M, McLemore TL, Gelbion HV, Gonzalez FJ. Quantification of CYP2B7, CYP4B1, and CYPOR messenger RNAs in normal human lung and lung tumors. Cancer Res. 1994;54(4):1085–91. [PubMed] [Google Scholar]
- 10.Imaoka S, Yoneda Y, Sugimoto T, Hiroi T, Yamamoto K, Nakatani T, et al. CYP4B1 is a possible risk factor for bladder cancer in humans. Biochem Biophys Res Commun. 2000;277(3):776–80. 10.1006/bbrc.2000.3740 [DOI] [PubMed] [Google Scholar]
- 11.Iscan M, Klaavuniemi T, Coban T, Kapucuoglu N, Pelkonen O, Raunio H. The expression of cytochrome P450 enzymes in human breast tumours and normal breast tissue. Breast Cancer Res Treat. 2001;70(1):47–54. 10.1023/a:1012526406741 [DOI] [PubMed] [Google Scholar]
- 12.Murtha TD, Korah R, Carling T. Suppression of cytochrome P450 4B1: An early event in adrenocortical tumorigenesis. Surgery. 2017;161(1):257–63. 10.1016/j.surg.2016.04.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin JT, Chan TC, Li CF, Huan SKH, Tian YF, Liang PI, et al. Downregulation of the cytochrome P450 4B1 protein confers a poor prognostic factor in patients with urothelial carcinomas of upper urinary tracts and urinary bladder. APMIS. 2019;127(4):170–80. 10.1111/apm.12939 [DOI] [PubMed] [Google Scholar]
- 14.Seta F, Patil K, Bellner L, Mezentsev A, Kemp R, Dunn MW, et al. Inhibition of VEGF expression and corneal neovascularization by siRNA targeting cytochrome P450 4B1. Prostaglandins Other Lipid Mediat. 2007;84(3–4):116–27. 10.1016/j.prostaglandins.2007.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, et al. AJCC Cancer Staging Manual. 8th ed Springer; 2018. [Google Scholar]
- 16.Vasaikar SV, Straub P, Wang J, Zhang B. LinkedOmics: analyzing multi-omics data within and across 32 cancer types. Nucleic Acids Res. 2018;46(D1):D956–D63. 10.1093/nar/gkx1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang ZF, Kang BX, Li CW, Chen TX, Zhang ZM. GEPIA2: an enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019;47(W1):W556–W60. 10.1093/nar/gkz430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baer BR, Rettie AE. CYP4B1: an enigmatic P450 at the interface between xenobiotic and endobiotic metabolism. Drug Metab Rev. 2006;38(3):451–76. 10.1080/03602530600688503 [DOI] [PubMed] [Google Scholar]
- 19.Nelson DR, Zeldin DC, Hoffman SMG, Maltais LJ, Wain HM, Nebert DW. Comparison of cytochrome P450 (CYP) genes from the mouse and human genomes, including nomenclature recommendations for genes, pseudogenes and alternative-splice variants. Pharmacogenetics. 2004;14(1):1–18. 10.1097/00008571-200401000-00001 [DOI] [PubMed] [Google Scholar]
- 20.Rainov NG, Dobberstein KU, Sene-Esteves M, Herrlinger U, Kramm CM, Philpot RM, et al. New prodrug activation gene therapy for cancer using cytochrome P450 4B1 and 2-aminoanthracene/4-ipomeanol. Hum Gene Ther. 1998;9(9):1261–73. 10.1089/hum.1998.9.9-1261 [DOI] [PubMed] [Google Scholar]
- 21.Ashkar S, Mesentsev A, Zhang WX, Mastyugin V, Dunn MW, Laniado-Schwartzman M. Retinoic acid induces corneal epithelial CYP4B1 gene expression and stimulates the synthesis of inflammatory 12-hydroxyeicosanoids. J Ocul Pharmacol Ther. 2004;20(1):65–74. 10.1089/108076804772745473 [DOI] [PubMed] [Google Scholar]
- 22.Mastyugin V, Mezentsev A, Zhang WX, Ashkar S, Dunn MW, Laniado-Schwartzman M. Promoter activity and regulation of the corneal CYP4B1 gene by hypoxia. J Cell Biochem. 2004;91(6):1218–38. 10.1002/jcb.20018 [DOI] [PubMed] [Google Scholar]
- 23.Lo-Guidice JM, Allorge D, Cauffiez C, Chevalier D, Laffite JJ, Lhermitte M, et al. Genetic polymorphism of the human cytochrome P450 CYP4B1: evidence for a non-functional allelic variant. Pharmacogenetics. 2002;12(5):367–74. 10.1097/00008571-200207000-00004 [DOI] [PubMed] [Google Scholar]
- 24.Hiratsuka M, Nozawa H, Konno Y, Saito T, Konno S, Mizugaki M. Human CYP4B1 gene in the japanese population analyzed by denaturing HPLC. Drug Metab Pharmacokinet. 2004;19(2):114–9. 10.2133/dmpk.19.114 [DOI] [PubMed] [Google Scholar]
- 25.Tamaki Y, Arai T, Sugimura H, Sasaki T, Honda M, Muroi Y, et al. Association between cancer risk and drug-metabolizing enzyme gene (CYP2A6, CYP2A13, CYP4B1, SULT1A1, GSTM1, and GSTT1) polymorphisms in cases of lung cancer in Japan. Drug Metab Pharmacokinet. 2011;26(5):516–22. 10.2133/dmpk.dmpk-11-rg-046 [DOI] [PubMed] [Google Scholar]
- 26.Sasaki T, Horikawa M, Orikasa K, Sato M, Arai Y, Mitachi Y, et al. Possible relationship between the risk of Japanese bladder cancer cases and the CYP4B1 genotype. Jpn J Clin Oncol. 2008;38(9):634–40. 10.1093/jjco/hyn081 [DOI] [PubMed] [Google Scholar]
- 27.Yu GP, Zhong N, Chen GQ, Huang B, Wu S. Downregulation of PEBP4, a target of miR-34a, sensitizes drug-resistant lung cancer cells. Tumour Biol. 2014;35(10):10341–9. 10.1007/s13277-014-2284-3 [DOI] [PubMed] [Google Scholar]
- 28.Jian W, Bai YL, Li X, Kang J, Lei YF, Xue Y. Phosphatidylethanolamine-binding protein 4 promotes the epithelial-to-mesenchymal transition in non-small cell lung cancer cells by activating the sonic hedgehog signaling pathway. J Cell Biochem. 2019;120(4):5386–95. 10.1002/jcb.27817 [DOI] [PubMed] [Google Scholar]
- 29.Yu GP, Huang B, Chen GQ, Mi YD. Phosphatidylethanolamine-binding protein 4 promotes lung cancer cells proliferation and invasion via PI3K/Akt/mTOR axis. J Thorac Dis. 2015;7(10):1806–16. 10.3978/j.issn.2072-1439.2015.10.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu GP, Huang B, Chen GQ, Wu S, Ji Y, Shen ZY. PEBP4 gene expression and its significance in invasion and metastasis of non-small cell lung cancer. Tumour Biol. 2012;33(1):223–8. 10.1007/s13277-011-0265-3 [DOI] [PubMed] [Google Scholar]
- 31.Umeda Y, Hasegawa Y, Otsuka M, Ariki S, Takamiya R, Saito A, et al. Surfactant protein D inhibits activation of non-small cell lung cancer-associated mutant EGFR and affects clinical outcomes of patients. Oncogene. 2017;36(46):6432–45. 10.1038/onc.2017.253 [DOI] [PubMed] [Google Scholar]
- 32.Wei XP, Wang C, Feng HM, Li B, Jiang P, Yang JB, et al. Effects of ALOX5, IL6R and SFTPD gene polymorphisms on the risk of lung cancer: A case-control study in China. Int Immunopharmacol. 2020;79:106155 10.1016/j.intimp.2019.106155 [DOI] [PubMed] [Google Scholar]
- 33.Ishii T, Hagiwara K, Ikeda S, Arai T, Mieno MN, Kumasaka T, et al. Association between genetic variations in surfactant protein d and emphysema, interstitial pneumonia, and lung cancer in a Japanese population. COPD. 2012;9(4):409–16. 10.3109/15412555.2012.676110 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.