Abstract
Background and objectives
With the increase in the number of COVID-19 infections, the global health apparatus is facing insufficient resources. The main objective of the current study is to provide additional data regarding the clinical characteristics of the patients diagnosed with COVID-19, and in particular to analyze the factors associated with disease severity, lack of improvement, and mortality.
Methods
102 studies were included in the present meta-analysis, all of which were published before September 24, 2020. The studies were found by searching a number of databases, including Scopus, MEDLINE, Web of Science, and Embase. We performed a thorough search from early February until September 24. The selected papers were evaluated and analyzed using Stata software application version 14.
Results
Ultimately, 102 papers were selected for this meta- analysis, covering 121,437 infected patients. The mean age of the patients was 58.42 years. The results indicate a prevalence of 79.26% for fever (95% CI: 74.98–83.26; I2 = 97.35%), 60.70% for cough (95% CI: 56.91–64.43; I2 = 94.98%), 33.21% for fatigue or myalgia (95% CI: 28.86–37.70; I2 = 96.12%), 31.30% for dyspnea (95% CI: 26.14–36.69; I2 = 97.67%), and 10.65% for diarrhea (95% CI: 8.26–13.27; I2 = 94.20%). The prevalence for the most common comorbidities was 28.30% for hypertension (95% CI: 23.66–33.18; I2 = 99.58%), 14.29% for diabetes (95% CI: 11.88–16.87; I2 = 99.10%), 12.30% for cardiovascular diseases (95% CI: 9.59–15.27; I2 = 99.33%), and 5.19% for chronic kidney disease (95% CI: 3.95–6.58; I2 = 96.42%).
Conclusions
We evaluated the prevalence of some of the most important comorbidities in COVID-19 patients, indicating that some underlying disorders, including hypertension, diabetes, cardiovascular diseases, and chronic kidney disease, can be considered as risk factors for patients with COVID-19 infection. Furthermore, the results show that an elderly male with underlying diseases is more likely to have severe COVID-19.
Introduction
On December 31, 2019, a cluster of cases of pneumonia with an unknown source was reported in Wuhan, China, related to the Huanan seafood wholesale market [1, 2]. Subsequently, on January 9, 2020, the Chinese Center for Disease Control and Prevention announced its causative factor as the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This global disorder, caused by SARS-CoV-2, became known as COVID-19. Just two months later, on March 11, 2020, the Director General of the World Health Organization (WHO) designated the spread of COVID-19 as a pandemic [3, 4].
As of October 15, 2020, a total of 38,394,169 confirmed COVID-19 patients and 1,089,047 dead cases have been reported by WHO globally, and as of the time of this writing, the number of patients is increasing. In addition, 235 countries, regions, or territories with cases of COVID-19 infection have been identified worldwide [5]. The number of COVID-19 infected patients is increasing worldwide, and due to its correlation with a high morbidity and mortality rate, this trend has caused a new global phobia called Coro-phobia [6]. According to the results of a number of studies, COVID-19 infection can be diagnosed based on several symptoms, including cough, fever, fatigue, diarrhea, myalgia, and dyspnea [7–9].
Huang et al. evaluated the clinical features of 41 cases with COVID-19 infection, and their results showed that 13 (32%) cases had comorbidities, such as hypertension, chronic obstructive pulmonary disease, diabetes, and cardiovascular disease [10]. In addition, Guan et al. reported the results of 1,099 cases of COVID-19 infection, indicating that 261 (23.7%) cases had underlying diseases [11]. These studies and similar investigations show that some complications can be considered as risk factors for negative outcomes in patients with COVID-19 infection [12].
Furthermore, Lai et al. [13] realized that the mortality rate was associated with the country’s healthcare resources. However, in many countries, the intensive care units (ICUs) and the invasive ventilators are not enough to treat critically-infected patients. Consequently, clinical operators should consider the risk factors for critical cases of COVID-19, correctly assign medical resources, identify severe patients in the early stages of the disease, and devise an appropriate treatment plan to reduce the mortality rate and improve the effectiveness of the treatment [11]. Therefore, knowing the risk factors and the underlying diseases in COVID-19 patients is important for healthcare professionals, especially for immunocompromised people and the elderly. Subsequently, the evaluation of the prevalence of chronic diseases, such as hypertension, kidney disease, cardiovascular disease, and diabetes, is considered as one of the most important measures to reduce negative outcomes in COVID-19 patients [14].
Currently, we have no approved vaccines or curative drugs against the new COVID-19 infection; hence, to prevent COVID-19 infection, a systematic review and meta-analysis has been conducted to study the prevalence of common comorbidities by analyzing studies focusing on clinical risk factors for COVID-19.
Materials and methods
Study selection
We searched PubMed, Scopus, Web of Science, and Embase databases to identify relevant studies that were published on COVID-19 until September 24, 2020. The keywords for our search included: Kidney, COVID, Hypertension, Diabetes, ((Heart) OR Cardio-), and Comorbidities. Moreover, the citations in the identified studies were also scanned in order to identify references related to the subject matter.
Inclusion and exclusion criteria
All studies evaluating the clinical risk factors, underlying diseases, and comorbidities of COVID-19 have been reviewed. For our final analysis, the studies selected should report data on the prevalence of each of the risk factors for the severity of COVID-19 and its comorbidities in patients with COVID-19 infection, including hypertension, chronic kidney disease, diabetes, cardiovascular and cerebrovascular diseases, and coronary heart disease. Only human investigations were selected. The full texts of the relevant studies were assessed based on the inclusion and exclusion criteria. Studies that did not have adequate data, non-human studies, duplicate publications, and non-English publications (except for those with useful abstract data) were excluded.
Data extraction
All the selected papers were independently evaluated and screened by two authors (M.F. and K.V.), and any disagreements were resolved through discussion. The following data were collected for the final analysis: prevalence of hypertension, chronic kidney disease, diabetes, cardiovascular and cerebrovascular diseases, and coronary heart disease, along with the prevalence of symptoms such as fever, dyspnea, diarrhea, cough, fatigue, and myalgia.
Statistical analysis
Because the effect size in this study was based on proportion (i.e., prevalence of hypertension, chronic kidney disease, diabetes, cardiovascular and cerebrovascular diseases, and coronary heart disease), binomial distribution was employed to calculate the variance for each study. The prevalence in various studies were combined using the average weight. An inverse relationship between the variance of the study and its weight was observed. The I2 index was applied to evaluate the heterogeneity. In the case of heterogeneous studies, the random effects model were used. To analyze the data, Stata software application (version 14) was used. The Metaprop (meta-analysis for proportion) was used in Stata when p was close to 0 or 1. Moreover, to stabilize the variances, we used Freeman-Tukey Double Arcsine Transformation [15]. This study was performed with the approval of the Ethics Committee of Shahid Beheshti University of Medical Sciences (IR.SBMU.RETECH.REC.1399.084).
Publication bias
Publication bias was graphically defined by the Funnel plot. This funnel plot is a scatter plot with 2 variates (x, y) that evaluates the research estimate of the effect size against its sample size. A linear regression analysis was conducted for publication bias, which includes both slope and intercept parameters. It was calculated based on the following equation:
| (Eq 1) |
i = 1… r (r = the number of studies), yi = standardized estimate, xi = precision of studies, εi = error terms
Quality assessment
The Newcastle-Ottawa Scale (NOS) was employed to assess the quality of each included paper [16]. This scale includes 8 assessment items for evaluating the quality of studies, including ‘selection’, ‘comparability’, and ‘outcome’, based on the Ottawa checklist for the cross-sectional studies. Based on the score standard in NOS, cross-sectional studies can be classified as Very Good Studies (9–10 points), Good Studies (7–8 points), Satisfactory Studies (5–6 points), and Unsatisfactory Studies (0 to 4 points) (Table 1 in S1 File).
Results and discussion
Study selection
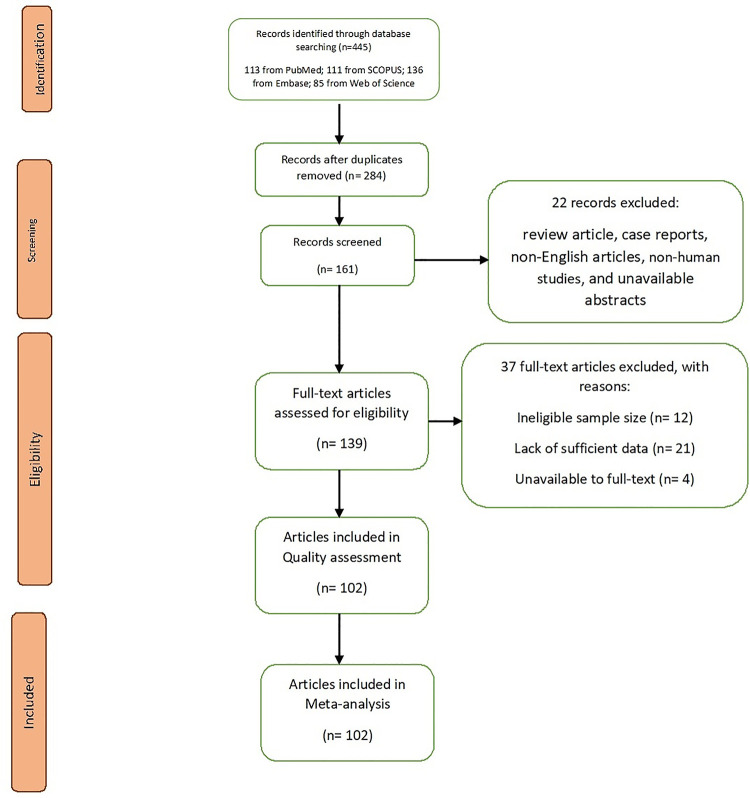
The current study was carried out based on the PRISMA checklist [17]. It should be noted that initially, 113 studies were identified through primary search on PubMed, 111 were identified by searching Scopus, 136 were identified by searching Embase, and 85 were identified by searching Web of Science. However, 284 of those 445 articles were excluded because of duplication. After screening the titles and abstracts of all identified papers, 22 more studies were excluded due to being review articles, case reports, and non-English articles, or due to unavailable abstracts. The full texts of the 139 remaining studies were reviewed, and 37 studies were excluded due to ineligible sample size (n = 12), lack of sufficient data (n = 21), or unavailable full text (n = 4). Finally, 102 articles, published from February, 2020 to July, 2020, were selected for the meta-analysis (Fig 1, Table 1).
Fig 1. Study flow diagram.
Table 1. Baseline characteristics of studies included in this meta-analysis.
| study | Place | Median age | Exposure history (No.) | Patients (No.) | Symptoms (No.) | Comorbidities (No.) | Mortality rate (%.) | Total Score | Total Quality | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | Male | Female | Fever | Cough | Fatigue or Myalgia | Diarrhea | Dyspnea | All | Hypertension | Diabetes | Cardiovascular disease | Chronic kidney disease | |||||||
| [18] | China | 56 | 73 | 191 | 119 | 72 | 180 | 151 | 44 | 9 | 56 | 91 | 58 | 36 | 2 | 28.27 | 10 | Very Good | |
| [19] | China | 3 | 85 | 62 | 23 | 78 | 19 | 50 | 16 | 50 | 58 | 32 | 19 | 3 | 8 | Good | |||
| [20] | China | 57.00 | 140 | 71 | 69 | 110 | 90 | 90 | 18 | 44 | 90 | 42 | 17 | 2 | 9 | Very Good | |||
| [21] | China | 59.00 | 174 | 76 | 98 | 136 | 56 | 47 | 21 | 42 | 43 | 37 | 32 | 13 | 9 | Very Good | |||
| [22] | China | 1590 | 904 | 674 | 1351 | 1052 | 584 | 57 | 331 | 399 | 269 | 130 | 59 | 269 | 3.10 | 8 | Good | ||
| [23] | China | 51.00 | 99 | 201 | 128 | 73 | 188 | 163 | 65 | 80 | 66 | 39 | 22 | 8 | 2 | 21.90 | 9 | Very Good | |
| [24] | China | 69.00 | 339 | 166 | 173 | 311 | 179 | 135 | 43 | 138 | 138 | 54 | 53 | 13 | 19.17 | 9 | Very Good | ||
| [25] | China | 73.00 | 25 | 10 | 15 | 23 | 25 | 16 | 10 | 8 | 5 | 100.00 | 5 | satisfactory | |||||
| [26] | U.S | 7162 | 2692 | 784 | 647 | 213 | 6 | satisfactory | |||||||||||
| [27] | U.S | 180 | 153 | 155 | 62 | 48 | 144 | 159 | 79 | 47 | 45 | 20 | 9 | Very Good | |||||
| [28] | China | 50.00 | 311 | 1012 | 524 | 488 | 761 | 531 | 170 | 152 | 231 | 114 | 46 | 27 | 15 | 9 | Very Good | ||
| [1] | China | 49.00 | 27 | 41 | 30 | 11 | 40 | 31 | 18 | 1 | 22 | 13 | 6 | 8 | 6 | 14.63 | 8 | Good | |
| [29] | China | 99 | 67 | 32 | 82 | 81 | 1 | 2 | 31 | 50 | 11.00 | 7 | Good | ||||||
| [12] | China | 56.00 | 12 | 138 | 75 | 63 | 136 | 82 | 96 | 14 | 43 | 64 | 43 | 14 | 20 | 4 | 4.30 | 9 | Very Good |
| [30] | China | 17 | 9 | 8 | 12 | 13 | 4 | 2 | 3 | 1 | 7 | Good | |||||||
| [11] | China | 47.00 | 1099 | 637 | 459 | 473 | 745 | 419 | 42 | 205 | 261 | 165 | 81 | 8 | 1.40 | 8 | Good | ||
| [31] | China | 41.00 | 23 | 62 | 35 | 27 | 48 | 50 | 32 | 3 | 20 | 5 | 1 | 1 | 0.00 | 6 | satisfactory | ||
| [32] | China | 0 | 80 | 39 | 41 | 63 | 30 | 18 | 1 | 30 | 38 | 1 | 0.00 | 7 | Good | ||||
| [33] | China | 57.00 | 0 | 137 | 61 | 76 | 112 | 66 | 44 | 11 | 26 | 27 | 13 | 14 | 10 | 11.68 | 8 | Good | |
| [34] | China | 40.00 | 61 | 31 | 30 | 60 | 39 | 35 | 6 | 3 | 12 | 5 | 1 | 0.00 | 9 | Very Good | |||
| [35] | China | 52 | 35 | 17 | 51 | 40 | 6 | 33 | 21 | 9 | 5 | 61.54 | 6 | satisfactory | |||||
| [36] | China | 187 | 91 | 96 | 61 | 28 | 6 | 23.00 | 9 | Very Good | |||||||||
| [37] | Italy | 355 | 72 | 100.00 | 7 | Good | |||||||||||||
| [38] | China | 12 | 8 | 4 | 10 | 11 | 4 | 2 | 3 | 2 | 4 | 2 | 6 | satisfactory | |||||
| [39] | China | 9 | 5 | 4 | 8 | 5 | 4 | 1 | 0 | 1 | 0 | 0 | 3 | Unsatisfactory | |||||
| [40] | China | 31974 | 44672 | 22981 | 21691 | 2683 | 1102 | 873 | 2.30 | 8 | Good | ||||||||
| [10] | China | 38.00 | 0 | 78 | 39 | 39 | 34 | 8 | 5 | 7 | Good | ||||||||
| [41] | China | 63.00 | 701 | 367 | 334 | 213 | 297 | 233 | 100 | 14 | 16.10 | 9 | Very Good | ||||||
| [42] | China | 57.00 | 193 | 95 | 98 | 171 | 134 | 75 | 35 | 70 | 105 | 66.32 | 10 | Very Good | |||||
| [43] | China | 62.00 | 274 | 171 | 103 | 249 | 185 | 137 | 77 | 120 | 133 | 93 | 47 | 23 | 4 | 41.24 | 9 | Very Good | |
| [44] | China | 72.50 | 82 | 54 | 28 | 64 | 53 | 38 | 10 | 52 | 62 | 46 | 15 | 17 | 8 | Good | |||
| [45] | China | 5 | 4 | 1 | 5 | 5 | 3 | 5 | 2 | 1 | 8 | Good | |||||||
| [46] | China | 64.00 | 416 | 205 | 211 | 334 | 144 | 55 | 16 | 117 | 127 | 60 | 10 | Very Good | |||||
| [47] | China | 54.00 | 116 | 67 | 49 | 43 | 18 | 5 | 9 | Very Good | |||||||||
| [48] | US | 21 | 11 | 11 | 17 | 18 | 7 | 10 | 52.40 | 7 | Good | ||||||||
| [49] | China | 54.00 | 203 | 108 | 95 | 181 | 122 | 16 | 10 | 3 | 88 | 43 | 16 | 16 | 8 | 12.80 | 9 | Very Good | |
| [50] | China | 50.00 | 91 | 37 | 54 | 65 | 55 | 40 | 21 | 3 | 15 | 8 | 0.00 | 6 | satisfactory | ||||
| [51] | China | 58.00 | 452 | 235 | 217 | 423 | 152 | 212 | 122 | 232 | 201 | 135 | 75 | 27 | 10 | 36.70 | 9 | Very Good | |
| [52] | China | 69 | 32 | 37 | 30 | 38 | 29 | 10 | 20 | 9 | 7 | 8 | 7.50 | 8 | Good | ||||
| [53] | China | 55.00 | 8 | 221 | 108 | 113 | 200 | 136 | 156 | 25 | 64 | 78 | 54 | 22 | 22 | 5.40 | 9 | Very Good | |
| [54] | China | 50.00 | 90 | 39 | 51 | 70 | 57 | 19 | 5 | 45 | 17 | 5 | 3 | 8 | Good | ||||
| [55] | China | 109 | 74 | 35 | 99 | 77 | 58 | 29 | 75 | 65 | 34 | 9 | Very Good | ||||||
| [56] | China | 54.00 | 47 | 102 | 53 | 49 | 83 | 50 | 56 | 11 | 35 | 47 | 28 | 11 | 5 | 4 | 16.70 | 9 | Very Good |
| [57] | Italy | 63.00 | 1591 | 1304 | 287 | 1043 | 509 | 180 | 223 | 36 | 9 | Very Good | |||||||
| [58] | US | 63.00 | 5700 | 3437 | 2263 | 5700 | 3026 | 1808 | 268 | 21.00 | 9 | Very Good | |||||||
| [59] | China | 68.00 | 54 | 24 | 13 | 48.10 | 6 | satisfactory | |||||||||||
| [60] | China | 200 | 99 | 101 | 161 | 101 | 137 | 16 | 17.00 | 10 | Very Good | ||||||||
| [61] | China | 64.91 | 47 | 26 | 21 | 12 | 18 | 5 | 6 | satisfactory | |||||||||
| [62] | China | 110 | 60 | 50 | 57 | 19 | 16 | 9 | 9 | Very Good | |||||||||
| [63] | China | 61.00 | 323 | 166 | 157 | 271 | 164 | 14 | 105 | 47 | 7 | 9 | Very Good | ||||||
| [64] | China | 49 | 31 | 18 | 39 | 17 | 9 | 4 | 6 | 16 | 6 | satisfactory | |||||||
| [65] | China | 47.00 | 298 | 149 | 149 | 192 | 54 | 6 | 6 | 38 | 19 | 11 | 8 | Good | |||||
| [66] | China | 265 | 220 | 131 | 67 | 17 | 5 | 52 | 21 | 14 | 9 | Very Good | |||||||
| [67] | China | 45.00 | 242 | 119 | 123 | 162 | 132 | 72 | 15 | 21 | 36 | 15 | 9 | 0.80 | 10 | Very Good | |||
| [68] | China | 48.00 | 84 | 48 | 36 | 54 | 43 | 16 | 6 | 3 | 12 | 10 | 5 | 8 | Good | ||||
| [69] | China | 55.00 | 109 | 59 | 50 | 90 | 67 | 62 | 12 | 37 | 12 | 7 | 10 | 28.40 | 9 | Very Good | |||
| [70] | China | 60.00 | 548 | 279 | 269 | 476 | 415 | 258 | 179 | 310 | 166 | 83 | 10 | 16.50 | 9 | Very Good | |||
| [71] | China | 52.00 | 113 | 66 | 47 | 91 | 26 | 9 | 1.80 | 9 | Very Good | ||||||||
| [72] | China | 60.00 | 27 | 12 | 15 | 21 | 16 | 3 | 11 | 13 | 5 | 6 | 3 | 37.00 | 8 | Good | |||
| [73] | China | 136 | 58 | 78 | 115 | 85 | 24 | 17 | 75 | 53 | 24 | 6 | 2 | 0.00 | 9 | Very Good | |||
| [73] | China | 652 | 349 | 303 | 521 | 421 | 115 | 20 | 143 | 73 | 33 | 5 | 5 | 0.00 | 9 | Very Good | |||
| [74] | Bolivia | 39.00 | 12 | 6 | 6 | 9 | 9 | 5 | 2 | 2 | 1 | 0.00 | 6 | Satisfactory | |||||
| [75] | China | 45.00 | 104 | 161 | 80 | 81 | 122 | 101 | 64 | 17 | 23 | 33 | 22 | 7 | 0.00 | 9 | Very Good | ||
| [76] | China | 47.00 | 113 | 135 | 72 | 63 | 120 | 102 | 44 | 18 | 18 | 43 | 13 | 12 | 7 | 0.70 | 9 | Very Good | |
| [77] | China | 37 | 14 | 23 | 15 | 12 | 0 | 10 | 8 | 4 | 0.00 | 6 | Satisfactory | ||||||
| [78] | China | 214 | 87 | 127 | 132 | 107 | 41 | 83 | 51 | 30 | 15 | 6 | 0.00 | 9 | Very Good | ||||
| [79] | China | 65.00 | 112 | 57 | 55 | 98 | 79 | 63 | 36 | 19 | 0.00 | 9 | Very Good | ||||||
| [80] | China | 136 | 90 | 46 | 52 | 71 | 66 | 27 | 102 | 41 | 27 | 3 | 0.00 | 8 | Good | ||||
| [81] | China | 70.00 | 25 | 19 | 6 | 16 | 12 | 6 | 8 | 100.00 | 8 | Good | |||||||
| [81] | China | 51.00 | 149 | 60 | 89 | 36 | 25 | 11 | 8 | 0.00 | 8 | Good | |||||||
| [82] | Singapore | 47.00 | 18 | 9 | 9 | 13 | 15 | 3 | 2 | 5 | 4 | 1 | 5 | unsatisfactory | |||||
| [83] | Korea | 75.50 | 54 | 33 | 21 | 49 | 16 | 32 | 5 | 100.00 | 7 | Good | |||||||
| [84] | China | 92 | 49 | 43 | 65 | 51 | 13 | 16 | 2 | 100.00 | 7 | Good | |||||||
| [85] | China | 168 | 86 | 82 | 156 | 121 | 65 | 44 | 59 | 57 | 41 | 20 | 9 | 2 | 10.10 | 10 | Very Good | ||
| [86] | China | 13 | 10 | 3 | 13 | 10 | 8 | 2 | 12 | 2 | 1 | 38.50 | 7 | Good | |||||
| [86] | China | 12 | 2 | 10 | 11 | 7 | 8 | 3 | 8 | 0 | 0 | 0.00 | 7 | Good | |||||
| [87] | UK | 82.00 | 101 | 64 | 37 | 9 | 4 | 9 | 3 | 67 | 54 | 36 | 18 | 21 | 74.20 | 8 | Good | ||
| [88] | China | 2 | 3 | 2 | 1 | 1 | 2 | 1 | 2 | 0 | 1 | 0 | 0.00 | 6 | satisfactory | ||||
| [89] | China | 134 | 149 | 81 | 68 | 114 | 87 | 11 | 2 | 52 | 28 | 0.00 | 7 | Good | |||||
| [90] | China | 31 | 81 | 42 | 39 | 59 | 48 | 7 | 3 | 34 | 21 | 12 | 10 | 8 | 3 | 4.00 | 8 | Good | |
| [91] | Korea | 77.00 | 66 | 37 | 29 | 61 | 30 | 23 | 10 | 5 | 9 | Very Good | |||||||
| [92] | China | 52.00 | 108 | 43 | 65 | 80 | 84 | 28 | 8 | 15 | 25 | 16 | 5 | 4 | 11.10 | 9 | Very Good | ||
| [93] | China | 53.00 | 425 | 476 | 271 | 205 | 390 | 269 | 109 | 205 | 113 | 49 | 38 | 4 | 8.00 | 9 | Very Good | ||
| [94] | China | 54.00 | 37 | 155 | 86 | 79 | 126 | 97 | 60 | 7 | 50 | 37 | 15 | 15 | 6 | 0.00 | 9 | Very Good | |
| [95] | China | 186 | 487 | 259 | 228 | 99 | 29 | 11 | 7 | 0.00 | 9 | Very Good | |||||||
| [96] | USA | 72 | 167 | 55 | 112 | 74 | 38 | 68 | 43 | 21 | 8 | Good | |||||||
| [97] | Italy | 65 | 410 | 229 | 111 | 203 | 69 | 47 | 10 | Very Good | |||||||||
| [98] | USA | 45 | 100 | 56 | 44 | 71 | 87 | 21 | 10 | 38 | 19 | 10 | 6 | 8 | Good | ||||
| [99] | Italy | 99 | 80 | 19 | 39 | 63 | 30 | 15 | 9 | Very Good | |||||||||
| [100] | USA | 69 | 105 | 53 | 52 | 49 | 76 | 51 | 24 | 56 | 62 | 35 | 40 | 27 | 33 | 9 | Very Good | ||
| [101] | Greece | 21 | 17 | 4 | 7 | 2 | 0 | 0 | 9 | Very Good | |||||||||
| [101] | Greece | 24 | 18 | 6 | 11 | 4 | 3 | 1 | 9 | Very Good | |||||||||
| [101] | Greece | 45 | 37 | 8 | 27 | 11 | 16 | 3 | 9 | Very Good | |||||||||
| [102] | Italy | 68 | 236 | 177 | 59 | 230 | 144 | 83 | 37 | 127 | 15 | 10 | Very Good | ||||||
| [103] | USA | 52.7 | 13442 | 7841 | 5957 | 3043 | 2710 | 2086 | 809 | 9 | Very Good | ||||||||
| [104] | China | 83 | 44 | 39 | 72 | 65 | 15 | 7 | 9 | 15 | 5 | 7 | 1 | 8 | Good | ||||
| [105] | China | 296 | 140 | 156 | 213 | 197 | 42 | 30 | 6.4 | 9 | Very Good | ||||||||
| [106] | China | 1590 | 904 | 674 | 1351 | 1052 | 584 | 57 | 331 | 399 | 269 | 130 | 59 | 8 | Good | ||||
| [107] | Italy | 68.00 | 6272 | 3968 | 2303 | 3631 | 859 | 1891 | 181 | 9 | Very Good | ||||||||
| [108] | USA | 18472 | 7388 | 11083 | 7388 | 3509 | 2216 | 10 | Very Good | ||||||||||
| [109] | UK | 1200 | 686 | 513 | 645 | 417 | 160 | 206 | 10 | Very Good | |||||||||
| [110] | USA | 73.00 | 206 | 101 | 105 | 152 | 206 | 7131 | 10 | Very Good | |||||||||
| [5] | Spain | 69.10 | 1139 | 695 | 444 | 617 | 310 | 312 | 89 | 9 | Very Good | ||||||||
| [111] | Belgium | 86.00 | 154 | 51 | 103 | 39 | 28 | 181 | 6 | Satisfactory | |||||||||
| [112] | USA | 52.70 | 442 | 256 | 186 | 159 | 84 | 47 | 9 | Very Good | |||||||||
| [113] | UK | 68.00 | 605 | 272 | 333 | 200 | 30 | 90 | 206 | 10 | Very Good | ||||||||
| [114] | UK | 69.30 | 1474 | 787 | 686 | 728 | 228 | 138 | 7131 | 8 | Good | ||||||||
*Abbreviations: No = Number.
Demographic characteristics
Based on the data analysis, the total number of hospitalized patients covered by this analysis was 121,437, which includes 27,062 patients with hypertension, 14,300 patients with diabetes, 9,914 patients with cardiovascular diseases, 9,603 patients with chronic kidney disease, 266 patients with coronary heart disease, and 176 patients with both cardiovascular and cerebrovascular diseases. According to the results, 54.92 percent of the subjects were male (95% CI: 52.92–56.92; I2 = 96.53%), while 45.12 percent were female (95% CI: 43.12–47.12; I2 = 96.55%) (Table 2). The mean age of the subjects in all the included studies was 58.42 years. Most of the studies were conducted in China, while 11 were conducted in the United States, 6 in Italy, 4 in the UK, 2 in Korea, 1 in Spain, 1 in Belgium, 1 in Greece, 1 in Bolivia, and 1 in Singapore. We should also note that six selected studies have not reported the number of patients with both cardiovascular and cerebrovascular disease separately. Based on the papers that reported the number of individuals with a history of exposure, the prevalence of the total exposure history was calculated as 35.56% (95% CI: 21.97–50.38; I2 = 99.43%) (Table 2).
Table 2. Statistical analysis of reviewed articles.
| Number of studies | Prevalence (%) | 95% CI | I2(%) | |
|---|---|---|---|---|
| Sex | ||||
| male | 101 | 54.92 | (52.92–56.92) | 96.53 |
| female | 101 | 45.12 | (43.12–47.12) | 96.55 |
| Exposure history | 21 | 35.56 | (21.97–50.38) | 99.43 |
| Signs and symptoms | ||||
| Fever | 73 | 79.26 | (74.98–83.26) | 97.35 |
| Cough | 71 | 60.70 | (56.91–64.43) | 94.98 |
| Fatigue or Myalgia | 60 | 33.21 | (28.86–37.70) | 96.12 |
| Dyspnea | 61 | 31.30 | (26.14–36.69) | 97.67 |
| Diarrhea | 56 | 10.65 | (8.26–13.27) | 94.20 |
| Comorbidity | 54 | 45.98 | (34.47–57.71) | 99.69 |
| Hypertension | 96 | 28.30 | (23.66–33.18) | 99.58 |
| Cardiovascular and cerebrovascular diseases | 6 | 18.25 | (7.47–32.11) | 94.10 |
| Diabetes | 98 | 14.29 | (11.88–16.87) | 99.10 |
| Chronic kidney disease | 58 | 5.19 | (3.95–6.58) | 96.42 |
| Heart diseases | ||||
| Cardiovascular disease | 68 | 12.30 | (9.59–15.27) | 99.33 |
| Coronary heart disease | 17 | 7.37 | (4.86–10.28) | 86.50 |
| Mortality | 58 | 8.91 | (6.34–11.84) | 98.45 |
Clinical manifestations
The results of this analysis indicate that the prevalence of the most common symptoms of COVID-19 were 79.26% for fever (95% CI: 74.98–83.26; I2 = 97.35%), 60.70% for coughing (95% CI: 56.91–64.43; I2 = 94.98%), 33.21% for fatigue or myalgia (95% CI: 28.86–37.70; I2 = 96.12%), 31.30% for dyspnea (95% CI: 26.14–36.69; I2 = 97.67%), and 10.65% for diarrhea (95% CI: 8.26–13.27; I2 = 94.20%) (Table 2).
Comorbidities
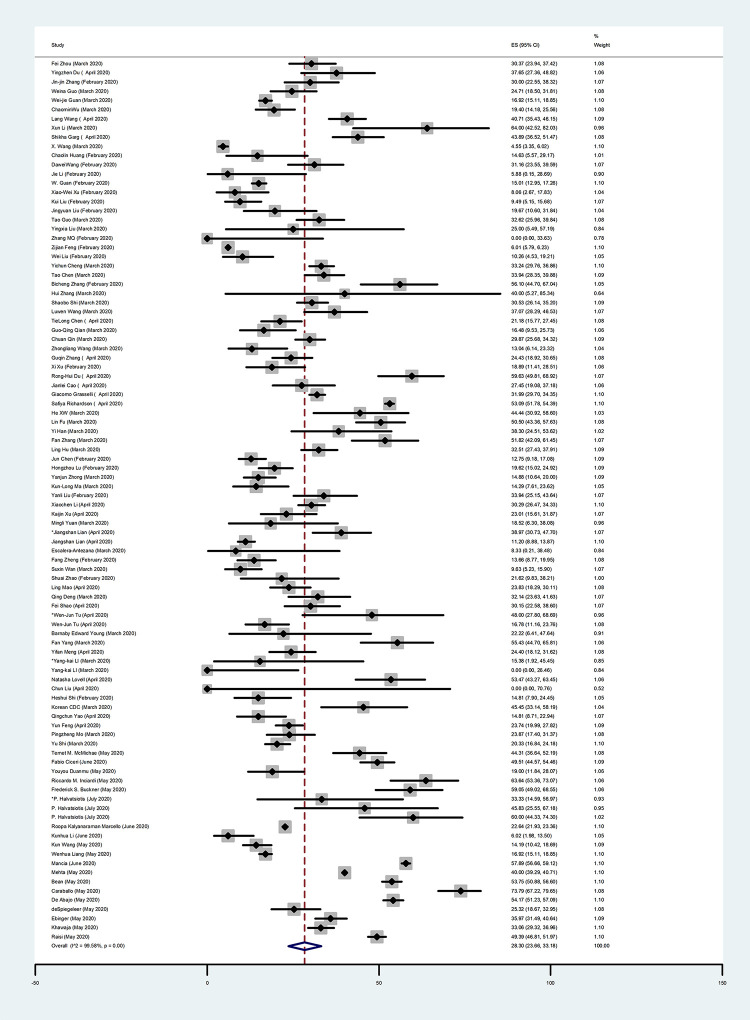
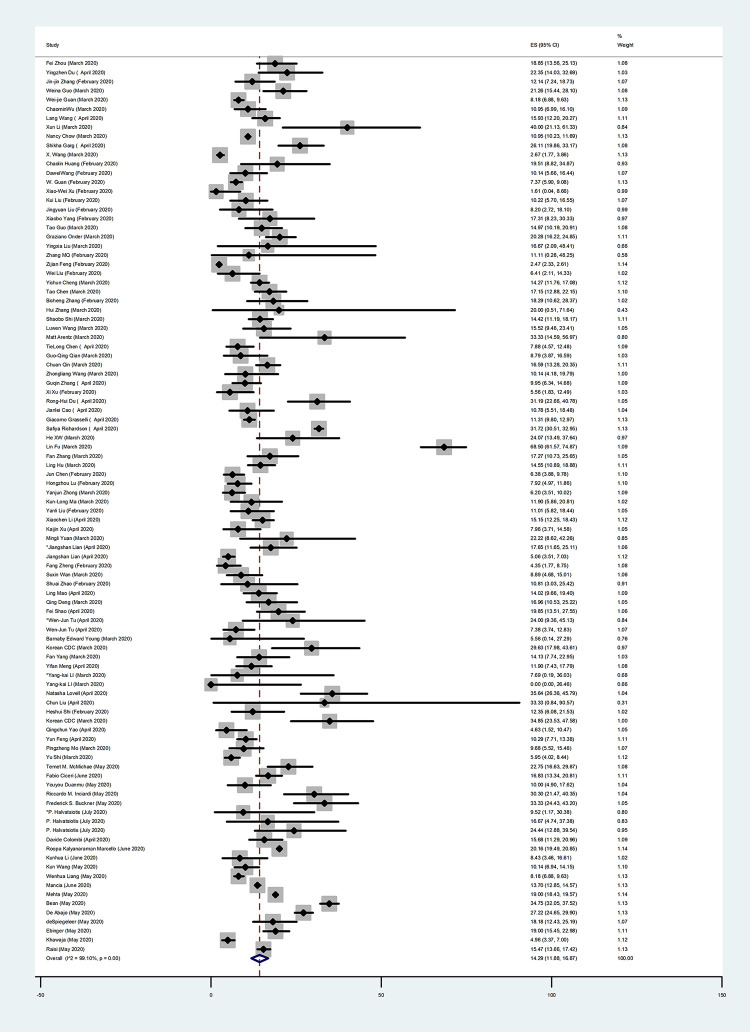
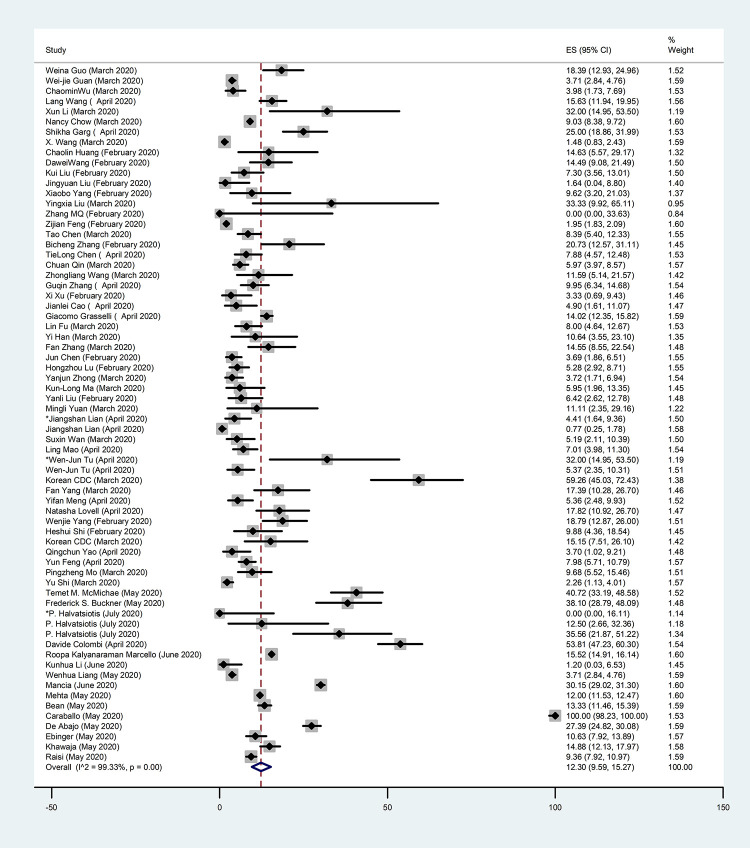
In this systematic review, we focused on the prevalence of people with comorbidities, which was 45.98% (95% CI: 34.47–57.71; I2 = 99.69%) (Table 2). The prevalence of the most common comorbidities were 28.30% for hypertension (95% CI: 23.66–33.18; I2 = 99.58%) (Fig 2, Table 2), 14.29% for diabetes (95% CI: 11.88–16.87; I2 = 99.10%) (Fig 3, Table 2), 12.30% for cardiovascular diseases (95% CI: 9.59–15.27; I2 = 99.33%) (Fig 4, Table 2), and 5.19% for chronic kidney disease (95% CI: 3.95–6.58; I2 = 96.42%) (Table 2). We also assessed the prevalence of some other comorbidities found in our included articles, such as cardiovascular and cerebrovascular diseases with 18.25% (95% CI: 7.47–32.11; I2 = 94.10%), and coronary heart disease with 7.37% (95% CI: 4.86–10.28; I2 = 86.50%) (Table 2).
Fig 2. Forest Plot of the prevalence of hypertension in COVID-19 patients.
Each square indicates the effect estimate of individual articles with their 95% CI Size of squares is proportional to the weight of each paper in the meta-analysis. In this plot, papers are indicated in the order of first author’s names and publication date (based on a random effects model).
Fig 3. Forest Plot of the prevalence of diabetes in COVID-19 patients.
Each square indicates the effect estimate of individual articles with their 95% CI Size of squares is proportional to the weight of each paper in the meta-analysis. In this plot, papers are indicated in the order of first author’s names and publication date (based on a random effects model).
Fig 4. Forest Plot of the prevalence of cardiovascular diseases in COVID-19 patients.
Each square indicates the effect estimate of individual articles with their 95% CI Size of squares is proportional to the weight of each paper in the meta-analysis. In this plot, papers are indicated in the order of first author’s names and publication date (based on a random effects model).
Outcomes
We calculated the percentage of the mortality rate based on all the selected studies reporting mortality cases, and it was 15.18% (95% CI: 10.88–20.02; I2 = 99.19%). After performing the sensitivity analysis and eliminating five studies from our selected studies because they were conducted on deceased individuals, i.e., the outlier studies, the overall mortality rate was calculated based on the remaining 58 studies as 8.91% (95% CI: 6.34–11.84; I2 = 98.45%) (Table 2).
Publication bias
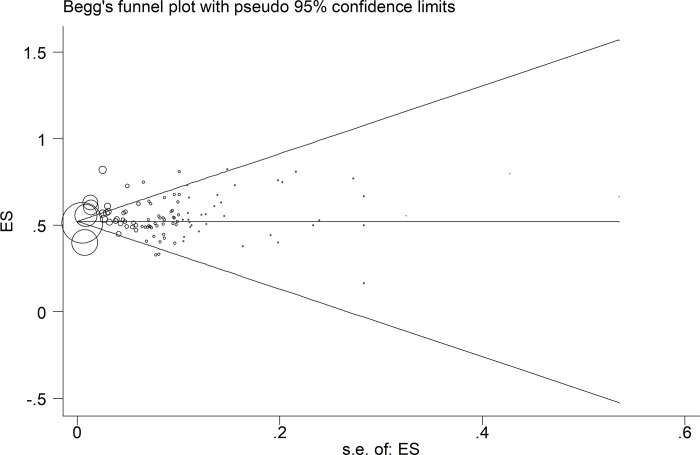
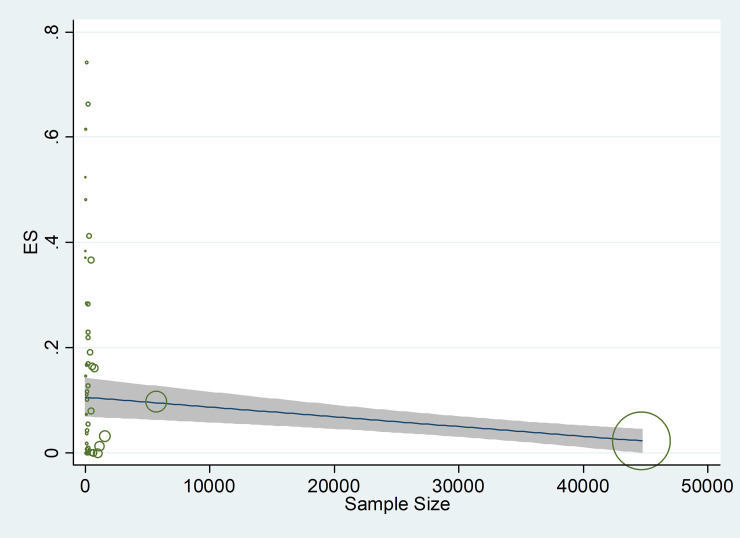
Fig 5 Shows the Begg’s funnel plot of the comorbidity studies performed on patients with COVID-19 infection. The interpretation of our Begg’s funnel plot (p = 0.780), as well as Egger’s test (p = 0.069), shows no sign of publication bias in the included studies. Therefore, it is understandable that reports have been published with both positive and negative outcomes (Fig 5). Based on the results of the meta-regression analysis, the correlation between the prevalence and the sample size was evaluated. Based on this assessment and the figure, there was no significant relation between the prevalence and the sample size (P = 0.428). It should be noted that in this figure, the circles indicate the weight of the papers (Fig 6).
Fig 5. Begg’s funnel plot for publication bias.
Fig 6. The association between prevalence of risk factors for COVID-19 and sample size, using Meta regression.
In December 2019, a new coronavirus disease, i.e., coronavirus disease 2019 (COVID-19), spread globally [115]. The city of Wuhan in China is considered to be one of the most infected areas in the world [116]. Because of this epidemic contagious disease, aggressive quarantine has been enforced by the government, which has played an important role in monitoring the prevalence of this virus. COVID-19 is not only highly contagious and insidious, but it also has the ability to give rise to cluster outbreaks [117, 118]. The SARS-CoV-2 virus can attack certain cells in the body, particularly alveolar epithelial cells, through a specific enzyme called the angiotensin-converting enzyme 2 (ACE2). ACE2 is considered to be the isoenzyme of ACE, which is mainly distributed in the testes, the kidneys, the colon, the cardiovascular system, the lungs, and other organs [119].
As of the time of writing this review, the pathogenesis and origin of COVID-19 have remained unknown, and no useful medical treatment has been offered for COVID-19 infection. Recommendations insist on intensive supportive care to reduce the risk of infection with this SARS-CoV-2 virus [10, 29]. Unfortunately, in severely-ill COVID-19 patients, the disorder develops rapidly, and the acute respiratory distress syndrome (ARDS), as one of the worst complications of COVID-19 outcomes, can occur earlier and even lead to death. In a study by Tian et al. [120], it was stated that the percentage of mild and seriously-ill patients was 73% and 18%, respectively. Furthermore, Wang et al. found that the risk of exacerbation in patients with cerebrovascular disease, hypertension, cardiovascular disease, or diabetes was higher than others [1].
This meta-analysis is based on data from 102 studies with laboratory-confirmed SARS-CoV-2 cases. The selected studies were from all over the world, and not a specific region. Based on previous studies on SARS-CoV and MERS-CoV, it is understood that these viruses affect males more than females [121, 122]. Moreover, based on our results, men with 54.92% (95% CI: 52.92–56.92; I2 = 96.53%) are more affected by SARS-CoV-2 than women with 45.12% (95% CI: 43.12–47.12; I2 = 96.55%). Furthermore, this may be due to women’s more adaptive innate immune system and robust responses [48]. Another reason for our findings may be that men have a tendency to have more harmful lifestyle habits, such as smoking, as well as underlying diseases, than women [11]. Moreover, as mentioned in Huang’s report [1], this may be associated with the risk factors that are related to men’s occupations, such as the exposure in Huanan wet market. Furthermore, based on our results, older people (mean age = 58.42) are more prone to COVID-19, which could be related to an increase in the frequency of common comorbidities [32]. As our body’s immunity weakens with age, elderly patients are more likely to have serious ailments or even die [11]. Hospitalization and further ICU admission is prevalent among sever cases of COVID-19, especially the ones with underlying health conditions and elderly patients [123]. Based on a CDC report, 8 death cases out of every 10 death cases occurring in the USA are old individuals (i.e., 65 years old and above) [124]. Additionally, it has been reported that patients with comorbid conditions, such as diabetes, hypertension, cardiovascular disease, obesity, and chronic lung diseases, are more likely to have a worse prognosis and develop severe pneumonia and further ARDS [125].
Our study provides satisfactory evidence for the risk factors in severely-ill COVID-19 patients, such as characteristics of the patients (gender, age), comorbidity (hypertension, chronic kidney disease, diabetes, cardiovascular and cerebrovascular diseases, and coronary heart disease), and signs and symptoms (fever, diarrhea, fatigue or myalgia, and dyspnea) [25]. Furthermore, we have discovered that underlying diseases such as diabetes, hypertension, cardiovascular disease, and chronic kidney disease can be considered as risk factors for the progression of this disease, which is confirmed by the analytical results of our study. Moreover, the most common clinical symptoms of COVID-19 in infected patients, including fever, diarrhea, fatigue or myalgia, and dyspnea, have been assessed in this paper. Dyspnea or shortness of breath indicates lack of oxygen and poor lung function. Therefore, if the patient is an elderly male with underlying diseases, he is more likely to have severe disorders or even face death [11].
Neurological complications can also occur as a result of COVID-19; however, they are very rare (especially in contrast to respiratory complications). According to recent reports, neurological manifestations of COVID-19 include headache, vomiting, nausea, dizziness, hyposmia, hypogeusia, and impaired consciousness [12, 78]. Although the exact neuroinvasive route of SARS-CoV-2 has not yet been established, two hypothetical routes have been suggested, i.e., (1) neural dissemination through the olfactory nerve and the cribriform plate, and (2) hematogenous dissemination through cerebral circulation [126, 127]. According to these possible routes, neural invasion of SARS-CoV-2 seems possible. Additionally, it has been proved that there is a close relationship between neurological disorders and COVID-19; however, a big question that arises in this regard is whether this relationship is causal or not (i.e., does one of them affects the mortality rate or the incident of the other one or not?). The answer to this question has not yet been given; however, there is some evidence showing that SARS-CoV-2 infection can affect the clinical spectrum of neurological disorders [128].
According to the selected papers assessed in the current study, fever with 79.26% (95% CI: 74.98–83.26; I2 = 97.35%) is the most common symptom, while hypertension with 28.30% (95% CI: 23.66–33.18; I2 = 99.58%) can be considered as the most prevalent comorbidity. When comparing our results with those of other recent meta-analysis studies, the study conducted by Rodriguez-Morales et al. [129] confirms our findings. These authors found that fever with 88.7% (95% CI 84.5–92.9) was the most prevalent symptom, and hypertension with 18.6% (95% CI 8.1–29.0) was the most common comorbidity. However, the number of selected studies in our meta-analysis was larger than the size of their dataset; hence, our findings are more reliable.
Our findings are also in line with the results of the study by Jing Yang et al. [14], in which fever with 91.3% (95% CI: 86–97) was the most prevalent symptom, and hypertension with 21.1% (95% CI: 13.0–27.2) was the most common comorbidity. In addition, in a study by Emami et al. [130], hypertension was reported as the most prevalent comorbidity with 16.37% (95%CI: 10.15–23.65). Moreover, the prevalence of diabetes mellitus and hypertension in China were 10.9% [131] and 23.2% [132] in adults, respectively.
Based on the abovementioned studies and our results, individuals with cardiovascular diseases, hypertension, chronic kidney disease, and diabetes should receive serious attention for treatments and vaccinations useful for SARS-CoV-2 in the future. Due to limited evidence, more comprehensive studies are needed to demonstrate this correlation [14].
Unfortunately, no anti-virus vaccines or medicines are yet known for the treatment of COVID-19-related diseases. At the present time, clinical management, including infection prevention, supportive care, and control measures, such as mechanical ventilation and supplementary oxygen, are available. Antibiotics are not useful in curing COVID-19 patients; however, they can be employed in the case of a secondary bacterial infection.
Favilavir
Favilavir is an antiviral drug approved in 2014 in Japan as a treatment for influenza. It is currently in use in some countries as a treatment for COVID-19 [133]. Another anti-virus drug which can have useful effects for COVID-19 is called Remdesivir. It has demonstrated efficacy by resisting two viruses that are similar to SARS-CoV2, i.e., MERS-CoV and SARS-CoV in animals [134, 135].
Remdesivir
Remdesivir was employed to cure the first case of COVID-19 infection in the US, resulting in enhancing the conditions of the patient after only one day of using Remdesivir [136]. Based on the related investigations, Remdesivir improved severe lung pathology, reduced lung viral loads, and ameliorated pulmonary function. In addition, chloroquine was suggested in the recommendations for the treatment and prevention of COVID-19 pneumonia [137]. Nevertheless, the optimal dosage of chloroquine for treating SARS-CoV-2 needs to be evaluated in future trials [138]. Hydroxychloroquine is an analog of chloroquine which is used to reduce drug-drug interactions [139]. There is no approved data about the use of corticosteroids for the treatment of SARS-CoV2 [140, 141]. Overall, there is no specific reason yet to expect that patients with COVID- 19 infection will improve by using corticosteroids; hence, these treatments may have harmful effects [142, 143].
A recent clinical trial by [144] suggests that Remdesivir is a therapeutic option for COVID-19 patients who do not receive invasive ventilation. Based on the clinical and experimental results, clinical trials for evaluating the efficacy of Remdesivir in COVID-19 patients have recently been initiated in the USA, China [145, 146], Hong Kong, the Republic of Korea, Singapore [147], Taiwan [148, 149], and France [150, 151].
Lopinavir/Ritonavir
Moreover, based on the investigations, treatment with lopinavir/ritonavir does not decrease the duration of viral RNA or viral RNA loads [11]. In addition, the number of patients treated with lopinavir/ritonavir who developed serious complications, such as secondary infections and acute kidney injury, or who required invasive or noninvasive mechanical ventilation due to respiratory failure, was lower than the number of patients who did not receive lopinavir/ritonavir treatment [11]. These observations need additional studies to find out whether treatment with lopinavir/ritonavir is able to decrease some complications in COVID-19 patients [11, 152].
On February 24, 2020, Moderna Company stated that the company’s experimental mRNA COVID-19 vaccine, which is known as mRNA-1273, was ready to be tested on human subjects. It is a considerably fast progress to produce an initial vaccine just a few weeks after determining the SARS-CoV-2 genetic sequence. However, the clinical trial of the immunogenicity and safety of mRNA-1273 in the treatment of COVID-19 needs to be evaluated more extensively (clinicaltrials.gov Identifier: NCT04283461). Additionally, a new oral SARS-CoV-2 vaccine has been successfully produced at Tianjin University, which employs food-grade safe Saccharomyces cerevisiae as a transporter and targets the S protein. There are 18 biotechnology universities and companies in China studying potential SARS-CoV-2 vaccines [143].
Current diagnostic tests for coronaviruses include real-time RT-PCR (rRT-PCR), reverse transcription loop-mediated isothermal amplification (RT-LAMP), and reverse-transcription polymerase chain reaction (RT-PCR) [153]. RT-LAMP has the same sensitivity as rRT-PCR; however, it is highly particular and is employed to detect MERS-CoV [154, 155]. Based on the current diagnosis represented by the laboratory examinations of the Chinese National Health Commission, oropharyngeal and nasopharyngeal swab tests have become a standard evaluation for the diagnosis of COVID-19 infection. A large portion of the vaccines being produced for coronaviruses target the spike glycoprotein or the S protein [156]. However, vaccine development takes too long, and no vaccines are available at the time of this pandemic [143].
In addition, COVID-19 prevents access to health care for the most vulnerable individuals due to health inequalities [157]. Although structural racism has been shown to affect the distribution of health determinants and risk factors in society, but due to the lack of understanding on the exact influence of these variables on social health, appropriate action is not given by the health system [158, 159]. Focus on addressing both unsupplied social needs (i.e. the immediate social conditions that put the greatest pressure on individuals) and social risk factors (adverse social conditions such as those associated with poor health) can help us solve this problem with a more holistic point of view [158].
Inopportunely, the unfortunate consequences of structural racism, which has been a problem since the days of the transatlantic slave trade, have continued to this day and include a more high poverty rate, an imperfect public school system, police brutality, unsafe neighborhoods and, above all, or, higher rate of chronic health conditions, obesity, and infant and maternal mortality among black American populations [160]. In compatibility with these unfortunate facts, the US national COVID-19 hospitalization rate was 4.6 times higher in Latins and the death rate was 2.1 times higher in black Americans than in whites [161]. Furthermore, it was reported that pregnancy-related complications and further death were three to four times higher in black women than in white women and were also twice as likely to die from heart disease [162–164]. Consequently, a powerful multidisciplinary and coordinated effort must be given by whoever is responsible, including politicians, policy makers, public health professionals, educators and local community leaders, to provide, systematically and actively improve health equity [161].
This data is not incredible or new. Because of these statistics, the chance of death due to pregnancy-related outcomes in black women is three to four times higher than in white women. The probability of death from heart disease in black women is also twice as high as in white women [161, 163, 165, 166]. The Covid-19 epidemic shows the relationship between social risk factors, health and structural racism. The prevalence of diabetes among black Americans has been reported 1.7 times greater than in whites [166]. The prevalence of influenza-related hospitalization in black Americans has been shown to be 1.8 times that of white Americans in the past 10 flu seasons [161]. It has been hypothesized that the increased exposure to COVID-19 among the black American population is due to the higher rate of service occupations and the greater possibility of living in more crowded and dense cities [167–169]. In the current economy, people of color are more likely to lose their jobs due to the potential consequences of the prevalence of COVID-19: 16.8% of black workers and 17.6% of Hispanic workers are at risk of losing their jobs compared to approximately 12.5% of non-Hispanic whites [161]. However, more research is needed for the structural factors leading to these statistics [169].
Early diagnosis and isolation of the patients constitute a key mechanism in preventing the spread of communicable diseases; however, as SARS-Cov-2 has other transmission routes (e.g., fomite transmission), it is even harder to control its rapid global spread [170]. Recently, it has been reported that SARS-CoV-2 is also detectable in saliva, urine, stool, and gastric mucosa [11, 171, 172], which can be related to its high transmission rate. In conclusion, the best protection against infection in the population is improving personal protection and managing hygiene at the community level [170]. According to an experience in Hangzhou City, China, centralized medical observation points (medical observation of close contacts) can also be helpful in controlling the COVID-19 outbreak [173].
In summary, the results of the current review indicate that in COVID-19 patients, diabetes, hypertension, cardiovascular disease, and chronic kidney disease are the most common comorbidities, while fever, diarrhea, fatigue or myalgia, and dyspnea may be considered as the most prevalent symptoms among COVID-19 patients. Since SARS-CoV-2 has a long incubation period and the infected individuals can transmit this contagious virus even without manifesting obvious symptoms, it is highly recommended that sick patients who have underlying disorders, especially in epidemic areas, refrain from coming into contact with other people in the society. Additionally, other strategies, such as avoiding gathering in public areas and going to epidemic places, should be considered as guidelines. During the current COVID-19 outbreak, the reported statistics, related to overall mortality, incubation time, and in particular the risk factors, imply the need for serious decisions for future prevention and therapeutic actions [130].
Conclusions
This systematic review and meta-analysis summarized the current evidence on the prevalence of mortality and potential risk factors in patients with COVID-19. Based on the results of this meta-analysis, COVID-19 is closely associated with underlying diseases. According to our results, the most frequent comorbidities among hospitalized COVID-19 patients were hypertension, diabetes, cardiovascular disease, and chronic kidney disease, respectively. The results also show that old men with underlying diseases are at a higher risk of becoming infected with COVID-19. Therefore, given the high risk of death in patients with COVID-19 infection, special care and significant attention are needed in COVID-19 patients with associated conditions. Moreover, future studies are necessary to identify efficient treatments for severe COVID-19 patients who suffer from diabetes, hypertension, chronic kidney disease, or cardiovascular diseases.
Supporting information
(PDF)
(SAV)
(DOCX)
Acknowledgments
The authors of this study are thankful to Shahid Beheshti University of Medical Sciences, Tehran, Iran because of their valuable collaboration and cooperation.
Abbreviations
- WHO
World Health Organization
- ICU
intensive care unit
- ACE2
angiotensin-converting enzyme 2
- No
Number
Data Availability
The analyzed data are all available and have been selected from other studies. The SPSS file and our Analyze data (Stata software application version 11) are available as Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Huang C., et al. , Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The lancet, 2020. 395(10223): p. 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang Y., et al. , Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging, 2020. 12(7): p. 6049–6057. 10.18632/aging.103000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Organization W.H., WHO announces COVID-19 outbreak a pandemic. WHO, Geneva, Switzerland, 2020. [Google Scholar]
- 4.Kreutz R., et al. , Hypertension, the renin–angiotensin system, and the risk of lower respiratory tract infections and lung injury: implications for COVID-19European Society of Hypertension COVID-19 Task Force Review of Evidence. Cardiovascular Research, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Abajo F.J., et al. , Use of renin–angiotensin–aldosterone system inhibitors and risk of COVID-19 requiring admission to hospital: a case-population study. The Lancet, 2020. 10.1016/S0140-6736(20)31030-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelvin D.J. and Rubino S., Fear of the novel coronavirus. The Journal of Infection in Developing Countries, 2020. 14(01): p. 1–2. 10.3855/jidc.12496 [DOI] [PubMed] [Google Scholar]
- 7.Carlos W.G., et al. , Novel wuhan (2019-nCoV) coronavirus. American journal of respiratory and critical care medicine, 2020. 201(4): p. P7–P8. 10.1164/rccm.2014P7 [DOI] [PubMed] [Google Scholar]
- 8.Paules C.I., Marston H.D., and Fauci A.S., Coronavirus infections—more than just the common cold. Jama, 2020. 323(8): p. 707–708. 10.1001/jama.2020.0757 [DOI] [PubMed] [Google Scholar]
- 9.Livingston E., Bucher K., and Rekito A., Coronavirus Disease 2019 and Influenza 2019–2020. Jama, 2020. 323(12): p. 1122–1122. 10.1001/jama.2020.2633 [DOI] [PubMed] [Google Scholar]
- 10.Liu W., et al. , Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chinese medical journal, 2020. 10.1097/CM9.0000000000000775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guan W.-j., et al. , Clinical characteristics of coronavirus disease 2019 in China. New England journal of medicine, 2020. 382(18): p. 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang D., et al. , Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Jama, 2020. 323(11): p. 1061–1069. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lai C.-C., et al. , Global epidemiology of coronavirus disease 2019: disease incidence, daily cumulative index, mortality, and their association with country healthcare resources and economic status. International Journal of Antimicrobial Agents, 2020: p. 105946 10.1016/j.ijantimicag.2020.105946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang J., et al. , Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. International Journal of Infectious Diseases, 2020. [Google Scholar]
- 15.Freeman M.F. and Tukey J.W., Transformations related to the angular and the square root. The Annals of Mathematical Statistics, 1950: p. 607–611. [Google Scholar]
- 16.Wells G., The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analysis. http://www.ohri.ca/programs/clinical_epidemiology.oxford.htm, 2004. [Google Scholar]
- 17.Liberati A., et al. , The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Annals of internal medicine, 2009. 151(4): p. W-65–W-94. [DOI] [PubMed] [Google Scholar]
- 18.Zhou F., et al. , Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The lancet, 2020. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Du Y., et al. , Clinical features of 85 fatal cases of COVID-19 from Wuhan: a retrospective observational study. American journal of respiratory and critical care medicine, 2020(ja). 10.1164/rccm.202003-0543OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J.-j., et al. , Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy, 2020. 10.1111/all.14238 [DOI] [PubMed] [Google Scholar]
- 21.Guo W., et al. , Diabetes is a risk factor for the progression and prognosis of COVID‐19. Diabetes/metabolism research and reviews, 2020. 10.1002/dmrr.3319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guan W.-j., et al. , Comorbidity and its impact on 1590 patients with Covid-19 in China: A Nationwide Analysis. European Respiratory Journal, 2020. 10.1183/13993003.00547-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu C., et al. , Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA internal medicine, 2020. 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L., et al. , Coronavirus Disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. Journal of Infection, 2020. 10.1016/j.jinf.2020.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X., et al. , Clinical characteristics of 25 death cases with COVID-19: a retrospective review of medical records in a single medical center, Wuhan, China. International Journal of Infectious Diseases, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chow N., et al. , Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019—United States, February 12–March 28, 2020. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garg S., Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019—COVID-NET, 14 States, March 1–30, 2020. MMWR. Morbidity and Mortality Weekly Report, 2020. 69 10.15585/mmwr.mm6915e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X., et al. , Clinical characteristics of non-critically ill patients with novel coronavirus infection (COVID-19) in a Fangcang Hospital. Clinical Microbiology and Infection, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen N., et al. , Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet, 2020. 395(10223): p. 507–513. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J., et al. , Epidemiological and Clinical Characteristics of 17 Hospitalized Patients with 2019 Novel Coronavirus Infections Outside Wuhan, China. medRxiv, 2020. [Google Scholar]
- 31.Xu X.-W., et al. , Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. bmj, 2020. 368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu J., et al. , Clinical Characteristics of Imported Cases of Coronavirus Disease 2019 (COVID-19) in Jiangsu Province: A Multicenter Descriptive Study. Clinical Infectious Diseases, 2020. 10.1093/cid/ciaa199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu K., et al. , Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chinese medical journal, 2020. 10.1097/CM9.0000000000000744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu J., et al. , Neutrophil-to-lymphocyte ratio predicts severe illness patients with 2019 novel coronavirus in the early stage. MedRxiv, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang X., et al. , Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. The Lancet Respiratory Medicine, 2020. 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo T., et al. , Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA cardiology, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Onder G., Rezza G., and Brusaferro S., Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. Jama, 2020. 10.1001/jama.2020.4683 [DOI] [PubMed] [Google Scholar]
- 38.Liu Y., et al. , Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Science China Life Sciences, 2020. 63(3): p. 364–374. 10.1007/s11427-020-1643-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang M.Q., et al. , [Clinical features of 2019 novel coronavirus pneumonia in the early stage from a fever clinic in Beijing]. Zhonghua Jie He He Hu Xi Za Zhi, 2020. 43(0): p. E013 10.3760/cma.j.issn.1001-0939.2020.0013 [DOI] [PubMed] [Google Scholar]
- 40.Surveillances V., The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19)—China, 2020. China CDC Weekly, 2020. 2(8): p. 113–122. [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng Y., et al. , Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney International, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Z., et al. , Caution on kidney dysfunctions of COVID-19 patients. 2020. [Google Scholar]
- 43.Chen T., et al. , Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. Bmj, 2020. 368 10.1136/bmj.m1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang B., et al. , Clinical characteristics of 82 death cases with COVID-19. medRxiv, 2020. [Google Scholar]
- 45.Zhang H., et al. , Identification of kidney transplant recipients with coronavirus disease 2019. European Urology, 2020. 10.1016/j.eururo.2020.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shi S., et al. , Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA cardiology, 2020. 10.1001/jamacardio.2020.0950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang L., et al. , Coronavirus disease 19 infection does not result in acute kidney injury: an analysis of 116 hospitalized patients from Wuhan, China. American journal of nephrology, 2020. 51(5): p. 343–348. 10.1159/000507471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arentz M., et al. , Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. Jama, 2020. 323(16): p. 1612–1614. 10.1001/jama.2020.4326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen T., et al. , Clinical characteristics and outcomes of older patients with coronavirus disease 2019 (COVID-19) in Wuhan, China (2019): a single-centered, retrospective study. The Journals of Gerontology: Series A, 2020. 10.1093/gerona/glaa089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qian G.-Q., et al. , Epidemiologic and Clinical Characteristics of 91 Hospitalized Patients with COVID-19 in Zhejiang, China: A retrospective, multi-centre case series. QJM: An International Journal of Medicine, 2020. 10.1093/qjmed/hcaa089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qin C., et al. , Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clinical Infectious Diseases, 2020. 10.1093/cid/ciaa248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Z., et al. , Clinical Features of 69 Cases with Coronavirus Disease 2019 in Wuhan, China. Clin Infect Dis, 2020. 10.1093/cid/ciaa272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang G., et al. , Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. Journal of Clinical Virology, 2020: p. 104364 10.1016/j.jcv.2020.104364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu X., et al. , Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. European journal of nuclear medicine and molecular imaging, 2020: p. 1–6. 10.1007/s00259-020-04735-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Du R.-H., et al. , Hospitalization and Critical Care of 109 Decedents with COVID-19 Pneumonia in Wuhan, China. Annals of the American Thoracic Society, 2020(ja). 10.1513/AnnalsATS.202003-225OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cao J., et al. , Clinical Features and Short-term Outcomes of 102 Patients with Corona Virus Disease 2019 in Wuhan, China. Clinical Infectious Diseases, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grasselli G., et al. , Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. Jama, 2020. 10.1001/jama.2020.5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Richardson S., et al. , Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.He X.W., et al. , [Impact of complicated myocardial injury on the clinical outcome of severe or critically ill COVID-19 patients]. Zhonghua Xin Xue Guan Bing Za Zhi, 2020. 48(0): p. E011. [DOI] [PubMed] [Google Scholar]
- 60.Fu L., et al. , Influence factors of death risk among COVID-19 patients in Wuhan, China: a hospital-based case-cohort study. medRxiv, 2020: p. 2020.03.13.20035329. [Google Scholar]
- 61.Han Y., et al. , Lactate dehydrogenase, a Risk Factor of Severe COVID-19 Patients. medRxiv, 2020. 10.18632/aging.103372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang F., et al. , Myocardial injury is associated with in-hospital mortality of confirmed or suspected COVID-19 in Wuhan, China: A single center retrospective cohort study. MedRxiv, 2020. [Google Scholar]
- 63.Hu L., et al. , Risk factors associated with clinical outcomes in 323 COVID-19 patients in Wuhan, China. Medrxiv, 2020. 10.1093/cid/ciaa539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ji D., et al. , Clinical Characteristics Predicting Progression of COVID-19. 2020. [Google Scholar]
- 65.Cai Q., et al. , 2019-nCoV Pneumonia in a Normal Work Infectious Diseases Hospital Besides Hubei Province, China. 2020. [Google Scholar]
- 66.Lu H., et al. , A descriptive study of the impact of diseases control and prevention on the epidemics dynamics and clinical features of SARS-CoV-2 outbreak in Shanghai, lessons learned for metropolis epidemics prevention. medRxiv, 2020. [Google Scholar]
- 67.Wang G., et al. , Epidemiological and Clinical Features of Corona Virus Disease 2019 (COVID-19) in Changsha, China. China (3/1/2020), 2020. [Google Scholar]
- 68.Ma K.-L., et al. , COVID-19 myocarditis and severity factors: an adult cohort study. medRxiv, 2020. [Google Scholar]
- 69.Liu Y., et al. , Clinical features and progression of acute respiratory distress syndrome in coronavirus disease 2019. MedRxiv, 2020. [Google Scholar]
- 70.Li X., et al. , Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. Journal of Allergy and Clinical Immunology, 2020. 10.1016/j.jaci.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu K., et al. , Factors associated with prolonged viral RNA shedding in patients with COVID-19. Clinical Infectious Diseases, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yuan M., et al. , Association of radiologic findings with mortality of patients infected with 2019 novel coronavirus in Wuhan, China. PLoS One, 2020. 15(3): p. e0230548 10.1371/journal.pone.0230548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lian J., et al. , Analysis of Epidemiological and Clinical features in older patients with Corona Virus Disease 2019 (COVID-19) out of Wuhan. Clinical Infectious Diseases, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Escalera-Antezana J.P., et al. , Clinical features of cases and a cluster of Coronavirus Disease 2019 (COVID-19) in Bolivia imported from Italy and Spain. Travel Medicine and Infectious Disease, 2020: p. 101653 10.1016/j.tmaid.2020.101653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zheng F., et al. , Clinical characteristics of 161 cases of corona virus disease 2019 (COVID-19) in Changsha. Eur Rev Med Pharmacol Sci, 2020. 24(6): p. 3404–3410. 10.26355/eurrev_202003_20711 [DOI] [PubMed] [Google Scholar]
- 76.Wan S., et al. , Clinical Features and Treatment of COVID‐19 Patients in Northeast Chongqing. Journal of medical virology, 2020. 10.1002/jmv.25783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao S., et al. , Anesthetic management of patients with suspected or confirmed 2019 novel coronavirus infection during emergency procedures. J Cardiothorac Vasc Anesth, 2020. 28: p. 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mao L., et al. , Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA neurology, 2020. 10.1001/jamaneurol.2020.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Deng Q., et al. , Suspected myocardial injury in patients with COVID-19: Evidence from front-line clinical observation in Wuhan, China. International Journal of Cardiology, 2020. 10.1016/j.ijcard.2020.03.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shao F., et al. , In-hospital cardiac arrest outcomes among patients with COVID-19 pneumonia in Wuhan, China. Resuscitation, 2020. 10.1016/j.resuscitation.2020.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tu W.-J., et al. , Clinicolaboratory study of 25 fatal cases of COVID-19 in Wuhan. Intensive care medicine, 2020: p. 1–4. 10.1007/s00134-020-06023-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Young B.E., et al. , Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. Jama, 2020. 323(15): p. 1488–1494. 10.1001/jama.2020.3204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Park S., et al. , Analysis on 54 Mortality Cases of Coronavirus Disease 2019 in the Republic of Korea from January 19 to March 10, 2020. J Korean Med Sci, 2020. 35(12): p. e132 10.3346/jkms.2020.35.e132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang F., et al. , Analysis of 92 deceased patients with COVID‐19. Journal of Medical Virology, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Meng Y., et al. , Sex-specific clinical characteristics and prognosis of coronavirus disease-19 infection in Wuhan, China: A retrospective study of 168 severe patients. PLoS pathogens, 2020. 16(4): p. e1008520 10.1371/journal.ppat.1008520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li Y.-K., et al. , Clinical and transmission characteristics of Covid-19—a retrospective study of 25 cases from a single thoracic surgery department. Current Medical Science, 2020: p. 1–6. 10.1007/s11596-020-2140-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lovell N., et al. , Characteristics, symptom management and outcomes of 101 patients with COVID-19 referred for hospital palliative care. Journal of Pain and Symptom Management, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu C., et al. , Clinical features and multidisciplinary treatment outcome of COVID-19 pneumonia: A report of three cases. Journal of the Formosan Medical Association, 2020. 10.1016/j.jfma.2020.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang W., et al. , Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): A multi-center study in Wenzhou city, Zhejiang, China. Journal of Infection, 2020. 10.1016/j.jinf.2020.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shi H., et al. , Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. The Lancet Infectious Diseases, 2020. 10.1016/S1473-3099(20)30086-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Choe Y.J., Coronavirus disease-19: The First 7,755 Cases in the Republic of Korea. medRxiv, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yao Q., et al. , Retrospective study of risk factors for severe SARS-Cov-2 infections in hospitalized adult patients. Polish archives of internal medicine, 2020. 10.20452/pamw.15312 [DOI] [PubMed] [Google Scholar]
- 93.Feng Y., et al. , COVID-19 with Different Severity: A Multi-center Study of Clinical Features. American Journal of Respiratory and Critical Care Medicine, 2020(ja). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mo P., et al. , Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clinical Infectious Diseases, 2020. 10.1093/cid/ciaa270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shi Y., et al. , Host susceptibility to severe COVID-19 and establishment of a host risk score: findings of 487 cases outside Wuhan. Critical Care, 2020. 24(1): p. 1–4. 10.1186/s13054-019-2683-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McMichael T.M., et al. , Epidemiology of Covid-19 in a long-term care facility in King County, Washington. New England Journal of Medicine, 2020. 382(21): p. 2005–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fabio C., et al. , Early predictors of clinical outcomes of COVID-19 outbreak in Milan, Italy. Clinical Immunology, 2020: p. 108509 10.1016/j.clim.2020.108509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Duanmu Y., et al. , Characteristics of Emergency Department Patients With COVID‐19 at a Single Site in Northern California: Clinical Observations and Public Health Implications. Academic Emergency Medicine, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Inciardi R.M., et al. , Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy. European heart journal, 2020. 41(19): p. 1821–1829. 10.1093/eurheartj/ehaa388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Buckner F.S., et al. , Clinical Features and Outcomes of 105 Hospitalized patients with COVID-19 in Seattle, Washington. Clinical Infectious Diseases, 2020. 10.1093/cid/ciaa632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Halvatsiotis P., et al. , Demographic and clinical features of critically ill patients with COVID-19 in Greece: The burden of diabetes and obesity. Diabetes research and clinical practice, 2020. 166: p. 108331 10.1016/j.diabres.2020.108331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Colombi D., et al. , Well-aerated lung on admitting chest CT to predict adverse outcome in COVID-19 pneumonia. Radiology, 2020: p. 201433 10.1148/radiol.2020201433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Marcello R.K., et al. , Characteristics and Outcomes of COVID-19 Patients in New York City’s Public Hospital System. medRxiv, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li K., et al. , The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Investigative radiology, 2020. 10.1097/RLI.0000000000000672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang K., et al. , Clinical and laboratory predictors of in-hospital mortality in patients with COVID-19: a cohort study in Wuhan, China. Clinical infectious diseases, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liang W., et al. , Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Internal Medicine, 2020. 10.1001/jamainternmed.2020.2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mancia G., et al. , Renin–angiotensin–aldosterone system blockers and the risk of Covid-19. New England Journal of Medicine, 2020. 10.1056/NEJMoa2006923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mehta N., et al. , Association of use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID-19). JAMA cardiology, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bean D., et al. , ACE-inhibitors and Angiotensin-2 Receptor Blockers are not associated with severe SARS-COVID19 infection in a multi-site UK acute Hospital Trust. MedRxiv, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Caraballo C., et al. , COVID-19 Infections and Outcomes in a Live Registry of Heart Failure Patients Across an Integrated Health Care System. medRxiv, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.De Spiegeleer A., et al. , The effects of ARBs, ACEIs and statins on clinical outcomes of COVID-19 infection among nursing home residents. medRxiv, 2020. 10.1016/j.jamda.2020.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ebinger J.E., et al. , Pre-Existing Characteristics Associated with Covid-19 Illness Severity. medRxiv, 2020. 10.1371/journal.pone.0236240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Khawaja A.P., et al. , Associations with covid-19 hospitalisation amongst 406,793 adults: the UK Biobank prospective cohort study. medRxiv, 2020. [Google Scholar]
- 114.Raisi-Estabragh Z., et al. , NON-WHITE ETHNICITY, MALE SEX, AND HIGHER BODY MASS INDEX, BUT NOT MEDICATIONS ACTING ON THE RENIN-ANGIOTENSIN SYSTEM ARE ASSOCIATED WITH CORONAVIRUS DISEASE 2019 (COVID-19) HOSPITALISATION: REVIEW OF THE FIRST 669 CASES FROM THE UK BIOBANK. medRxiv, 2020. [Google Scholar]
- 115.Ghebreyesus T.A., WHO Director-General’s opening remarks at the media briefing on COVID-19-11 March 2020. World Health Organization; https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-atthe-media-briefing-on-covid-19—11-march-2020, 2020. [Google Scholar]
- 116.Li J. and Xu G., Lessons from the Experience in Wuhan to Reduce Risk of COVID-19 Infection in Patients Undergoing Long-Term Hemodialysis. Clinical Journal of the American Society of Nephrology, 2020. 10.2215/CJN.03420320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chan J.F.-W., et al. , A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. The Lancet, 2020. 395(10223): p. 514–523. 10.1016/S0140-6736(20)30154-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guan W., et al. , China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease, 2019. [Google Scholar]
- 119.Tipnis S., Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem, 2000. 275: p. 33238–33243. 10.1074/jbc.M002615200 [DOI] [PubMed] [Google Scholar]
- 120.Tian S., et al. , Characteristics of COVID-19 infection in Beijing. Journal of Infection, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Channappanavar R., et al. , Sex-based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. The Journal of Immunology, 2017. 198(10): p. 4046–4053. 10.4049/jimmunol.1601896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Badawi A. and Ryoo S.G., Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. International Journal of Infectious Diseases, 2016. 49: p. 129–133. 10.1016/j.ijid.2016.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jaillon S., Berthenet K., and Garlanda C., Sexual dimorphism in innate immunity. Clinical reviews in allergy & immunology, 2017: p. 1–14. [DOI] [PubMed] [Google Scholar]
- 124.CDC. Symptoms of Coronavirus. 2020 12 Ooctober 2020]; Available from: https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html.
- 125.Sanyaolu A., et al. , Comorbidity and its Impact on Patients with COVID-19. SN comprehensive clinical medicine, 2020: p. 1–8. 10.1007/s42399-020-00363-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Baig A.M., et al. , Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host–virus interaction, and proposed neurotropic mechanisms. ACS chemical neuroscience, 2020. 11(7): p. 995–998. 10.1021/acschemneuro.0c00122 [DOI] [PubMed] [Google Scholar]
- 127.Netland J., et al. , Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. Journal of virology, 2008. 82(15): p. 7264–7275. 10.1128/JVI.00737-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ferini-Strambi L. and Salsone M., COVID-19 and neurological disorders: are neurodegenerative or neuroimmunological diseases more vulnerable? Journal of neurology, 2020: p. 1–11. 10.1007/s00415-020-10070-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rodriguez-Morales A.J., et al. , Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel medicine and infectious disease, 2020: p. 101623 10.1016/j.tmaid.2020.101623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Emami A., et al. , Prevalence of underlying diseases in hospitalized patients with COVID-19: a systematic review and meta-analysis. Archives of Academic Emergency Medicine, 2020. 8(1). [PMC free article] [PubMed] [Google Scholar]
- 131.Liu M., et al. , Burden of diabetes, hyperglycaemia in China from to 2016: findings from the 1990 to 2016, global burden of disease study. Diabetes & metabolism, 2019. 45(3): p. 286–293. 10.1016/j.diabet.2018.08.008 [DOI] [PubMed] [Google Scholar]
- 132.Hu S., et al. , Summary of the 2018 report on cardiovascular diseases in China. Chin Circulation J, 2019. 34: p. 209. [Google Scholar]
- 133.Elfiky A.A., Anti-HCV, nucleotide inhibitors, repurposing against COVID-19. Life sciences, 2020: p. 117477 10.1016/j.lfs.2020.117477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang M., et al. , Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell research, 2020. 30(3): p. 269–271. 10.1038/s41422-020-0282-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Abd El-Aziz T.M. and Stockand J.D., Recent progress and challenges in drug development against COVID-19 coronavirus (SARS-CoV-2)-an update on the status. Infection, Genetics and Evolution, 2020: p. 104327 10.1016/j.meegid.2020.104327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Holshue M.L., et al. , First case of 2019 novel coronavirus in the United States. New England Journal of Medicine, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gao J., Tian Z., and Yang X., Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Bioscience trends, 2020. 10.5582/bst.2020.01047 [DOI] [PubMed] [Google Scholar]
- 138.Colson P., et al. , Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents, 2020. 105932(10.1016). 10.1016/j.ijantimicag.2020.105932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jallouli M., et al. , Determinants of hydroxychloroquine blood concentration variations in systemic lupus erythematosus. Arthritis & rheumatology, 2015. 67(8): p. 2176–2184. 10.1002/art.39194 [DOI] [PubMed] [Google Scholar]
- 140.Lee N., et al. , Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. Journal of clinical virology, 2004. 31(4): p. 304–309. 10.1016/j.jcv.2004.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lee D.T., et al. , Factors associated with psychosis among patients with severe acute respiratory syndrome: a case-control study. Clinical infectious diseases, 2004. 39(8): p. 1247–1249. 10.1086/424016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Russell C.D., Millar J.E., and Baillie J.K., Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. The Lancet, 2020. 395(10223): p. 473–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhai P., et al. , The epidemiology, diagnosis and treatment of COVID-19. International journal of antimicrobial agents, 2020: p. 105955 10.1016/j.ijantimicag.2020.105955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.GREIN J., OHMAGARI N., and SHIN D., original: Compassionate Use of Remdesivir for Patients with Severe Covid-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.NCT04252664, ClinicalTrials.gov, (2020), Feb 5.
- 146.NCT04257656, ClinicalTrials.gov, (2020), Feb 6.
- 147.NCT04280705, ClinicalTrials.gov, (2020), Feb 21.
- 148.NCT04292730, ClinicalTrials.gov, (2020), Mar 3.
- 149.NCT04292899, ClinicalTrials.gov, (2020), Mar 3.
- 150.NCT04314817, ClinicalTrials.gov, (2020), Mar 19.
- 151.NCT04315948, ClinicalTrials.gov, (2020), Mar 20.
- 152.McKee D.L., et al. , Candidate drugs against SARS-CoV-2 and COVID-19. Pharmacological Research, 2020: p. 104859 10.1016/j.phrs.2020.104859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bhadra S., et al. , Real-time sequence-validated loop-mediated isothermal amplification assays for detection of Middle East respiratory syndrome coronavirus (MERS-CoV). PLoS One, 2015. 10(4): p. e0123126 10.1371/journal.pone.0123126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Huang P., et al. , A rapid and specific assay for the detection of MERS-CoV. Frontiers in microbiology, 2018. 9: p. 1101 10.3389/fmicb.2018.01101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lee S.H., et al. , One-pot reverse transcriptional loop-mediated isothermal amplification (RT-LAMP) for detecting MERS-CoV. Frontiers in microbiology, 2017. 7: p. 2166 10.3389/fmicb.2016.02166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Du L., et al. , The spike protein of SARS-CoV—a target for vaccine and therapeutic development. Nature Reviews Microbiology, 2009. 7(3): p. 226–236. 10.1038/nrmicro2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Diabetes T.L., COVID-19 and Racism—a double edged dagger. The Lancet. Diabetes & Endocrinology, 2020. 10.1016/S2213-8587(20)30243-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gravlee C.C., Systemic racism, chronic health inequities, and COVID‐19: A syndemic in the making? American Journal of Human Biology, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bailey Z., KN (2017). Structural racism and health inequities in the USA: evidence and interventions. Lancet. 389: p. 1453–1463. 10.1016/S0140-6736(17)30569-X [DOI] [PubMed] [Google Scholar]
- 160.Laurencin C.T. and McClinton A., The COVID-19 pandemic: A call to action to identify and. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Johnson-Agbakwu C.E., et al. , Racism, COVID-19, and Health Inequity in the USA: a Call to Action. Journal of racial and ethnic health disparities, 2020: p. 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ferdinand K.C. and Nasser S., Brief Review Racial/ethnic disparities in prevalence and care of patients with type 2 diabetes mellitus. 2014. [DOI] [PubMed] [Google Scholar]
- 163.Wingo S.N., Black uteri matter. 2019, LWW. [DOI] [PubMed] [Google Scholar]
- 164.Callaghan W.M. Overview of maternal mortality in the United States. in Seminars in perinatology. 2012. Elsevier; 10.1053/j.semperi.2011.09.002 [DOI] [PubMed] [Google Scholar]
- 165.Maddali M.M., et al. , Outcomes after rigid bronchoscopy in children with suspected or confirmed foreign body aspiration: a retrospective study. Journal of Cardiothoracic and Vascular Anesthesia, 2011. 25(6): p. 1005–1008. 10.1053/j.jvca.2011.02.005 [DOI] [PubMed] [Google Scholar]
- 166.Song X., et al. , Overall survival in patients with metastatic melanoma. Current Medical Research and Opinion, 2015. 31(5): p. 987–991. 10.1185/03007995.2015.1021904 [DOI] [PubMed] [Google Scholar]
- 167.Alderwick H. and Gottlieb L.M., Meanings and misunderstandings: a social determinants of health lexicon for health care systems. The Milbank Quarterly, 2019. 97(2): p. 407 10.1111/1468-0009.12390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Price-Haywood E.G., et al. , Hospitalization and mortality among black patients and white patients with Covid-19. New England Journal of Medicine, 2020. 10.1056/NEJMsa2011686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Egede L.E. and Walker R.J., Structural racism, social risk factors, and Covid-19—a dangerous convergence for black Americans. New England Journal of Medicine, 2020. 383(12): p. e77 10.1056/NEJMp2023616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Guan W.-j., Chen R.-c., and Zhong N.-s., Strategies for the prevention and management of coronavirus disease 2019. 2020, Eur Respiratory Soc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Xie C., et al. , Comparison of different samples for 2019 novel coronavirus detection by nucleic acid amplification tests. International Journal of Infectious Diseases, 2020. 10.1016/j.ijid.2020.02.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.To K.K.-W., et al. , Consistent detection of 2019 novel coronavirus in saliva. Clinical Infectious Diseases, 2020. 10.1093/cid/ciaa149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hui J., Qing-xin K., and Hui-min W., COVID-19 prevention and control strategy: Management of close contacts in Hangzhou City, China. Journal of Infection and Public Health, 2020. 10.1016/j.jiph.2020.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(SAV)
(DOCX)
Data Availability Statement
The analyzed data are all available and have been selected from other studies. The SPSS file and our Analyze data (Stata software application version 11) are available as Supporting Information files.