Abstract
Type I interferons have been shown to play a major role in anti-cancer immunity. In this issue, describe tumor-induced degradation of type I interferon receptor IFNAR1 chain as a new immune-evasion mechanism in colorectal cancers. Stabilizing IFNAR1 inhibits tumor growth and improves immunotherapy efficacy.
The role of type I interferons (IFN) against viral infections has been extensively studied since their discovery almost 60 years ago, but their immunomodulatory functions are complex and go way beyond the antiviral effects. The antitumor therapeutic potential of exogenous administered type I IFN has been exploited for several decades. However, the role of endogenous type I IFN in spontaneous or therapy-induced antitumor immune responses has been recognized more recently, and it is still not fully understood (recently reviewed in Zitvogel et al., 2015). Now, Katlinski et al. describe a mechanism by which tumor-induced degradation of type I IFN receptor IFNAR1 chain on infiltrating leukocytes contributes to the suppressive tumor microenvironment (TME) (Katlinski et al., 2017).
Resistance of solid tumors, including colorectal cancer (CRC), to immunotherapies is thought to be at least in part due to the establishment of an immunosuppressive TME. On the other hand, the presence of an intratumoral IFN signature correlates with a favorable prognosis in patients with different cancer types (Zitvogel et al., 2015). In their study, Katlinski et al. found that human CRC also had a reduced IFN-related gene signature. Interestingly, although similar IFNAR1 mRNA levels in CRC and normal tissues had been reported, Katlinski and colleagues observed decreased IFNAR1 protein levels in human colorectal adenocarcinomas compared to non-malignant tissues. The differential IFNAR1 expression had prognostic implications: low IFNAR1 levels on either the stromal or malignant cell compartment correlated with poor prognosis.
Surface levels of IFNAR1 are controlled by ubiquitination and lysosomal degradation mediated by the SCF-βTrCP2 E3 ubiquitin ligase that, in mouse, binds to phosphorylated Ser526 in the IFNAR1 cytoplasmic tail. To test the impact of IFNAR1 loss in tumor development and progression, Katlinski et al. used previously developed mice in which IFNAR1 degradation is prevented by a mutation in Ser526 (referred to as Ifnar1S526A or SA mice) (Bhattacharya et al., 2014) and well-established CRC murine models. Similar to human CRC, azoxymethane and dextran sodium (AOM/DSS)-induced tumors in wild-type (WT) animals displayed reduced levels of IFNAR1 protein with normal mRNA level. SA mice, on the other hand, developed fewer tumors with sustained high levels of IFNAR1 protein and increased expression of IFN-induced genes (Katlinski et al., 2017), thus establishing a link between TME conditions and suppressed IFN signaling via the degradation of IFNAR1 protein. Delayed tumor growth and sustained IFNAR1 expression was also observed in SA mice injected with MC38 colon adenocarcinoma cells. However, expression of IFNAR1S526A in MC38 cells had no impact on their ability to develop tumors, pointing to the need of maintaining IFNAR1 levels in the stromal compartment (Katlinski et al., 2017). This is in contrast to previous observations that both IFNAR1 expression on the stromal and malignant cell compartment is needed to control melanoma tumor growth (Katlinskaya et al., 2016) and that type I IFN in malignant cells was required for fibrosarcomas and mammary adenocarcinomas immune escape (Bidwell et al., 2012; Sistigu et al., 2014). Whether this indicates that cancer cell intrinsic or extrinsic pathways are tumor type dependent remains to be determined.
MC38 tumor growth was significantly reduced in WT mice transplanted with a mix of lymphoid and myeloid cells from SA donors, indicating the critical role of IFNAR1 stabilization in the hematopoietic compartment. Notably, previous studies using mice with a conditional deletion of Ifnar1 in intestinal epithelial cells showed that lack of IFNAR1 expression in these cells resulted in increased tumor burden induced by AOM/DSS (Tschurtschenthaler et al., 2014). It would be interesting to evaluate whether IFNAR1 stabilization in the same cell compartment would also reduce tumor development. A role for type I IFN signaling in the host hematopoietic compartment during tumor immunosurveillance has previously been shown to be mediated by the CD8α+ subset of dendritic cells (DCs) (Diamond et al., 2011; Fuertes et al., 2011). In the study by Katlinski et al., however, DCs do not seem to be the essential type I IFN-targeted cell population, but instead CD8+ T cells need to sustain IFNAR1 expression to survive in the TME. The latter observation is in line with the known direct effect of type I IFN signaling on CD8+ T cells needed for their clonal expansion and survival during viral infections (Kolumam et al., 2005).
The TME-induced downregulation of IFNAR1 observed in MC38 tumor-bearing WT animals correlated with reduced frequencies of tumor-infiltrating CD8+ T cells, NK cells, and CD11b+ Ly6ChiLy6G− cells while no significant systemic differences in CD8+ T cells were observed. Although a contribution of IFNAR1 signaling in the myeloid cell compartment could not be ruled out, transfer of lymphocytes carrying the Ifnar1S526A allele mixed with WT myeloid cells was sufficient to improve tumor growth control. Furthermore, the benefit of stabilizing IFNAR1 expression was lost when SA animals were depleted of CD8+ T cells, but not when depleted of NK cell. CD8+ T cells from WT, but not SA animals, incubated in the presence of supernatant from tumor explants or MC38 cells conditioned medium expressed decreased surface levels of IFNAR1. Additionally, tumor-infiltrating WT CD3+ CD8+ cells displayed lower IFNAR1 surface levels compared to splenic CD3+ CD8+ T cells isolated from the same animals. Further analysis showed that cytotoxic T lymphocytes (CTLs) from SA mice exhibited higher levels of interleukin 2 (IL2) receptor α mRNA and produced more IL2 compared to WT CTLs. When cultured in vitro in the presence of anti-IL2R blocking antibodies, the increased survival of SA CTLs was lost, suggesting that IFNAR1 stabilization improves CTL survival within the TME by enhancing the pro-survival effects of IL2.
The study by Katlinski et al. presents a mechanism employed by colorectal cancers to promote the immunosuppressive TME by diminishing CTL survival via the degradation of surface IFNAR1. If extended to other tumor types, it could help explain the variability observed in the therapeutic efficacy of systemic administration of type I IFNs in different cancers and at the same time opens avenues for therapeutic interventions targeting IFNAR1 stabilization within the TME. The latter is of particular importance considering the significant inflammatory and autoimmune side effects triggered by systemic type I IFNs and the ongoing effort to develop locally delivered type I IFN-based therapies. In this regard, using the MC38 model, the authors showed that genetic stabilization of IFNAR1 leads to greater therapeutic effects of adoptive cell transfer-based therapies and, consistent with the improved tumor infiltrating CTL survival, enhanced efficacy of checkpoint blockade with anti-PD1. These experiments served as a proof of concept that local stabilization of IFNAR1 might be a plausible approach to improve can-cer therapy. To assess this in a more clinically applicable way, Katlinski and colleagues blocked IFNAR1 degradation by simultaneously inhibiting the p38 and protein-kinase-2 (PKD) pathways involved in IFNAR1 phosphorylation. In vivo administration of LY2228820 (p38) and SD-208 (PKD) inhibitors in MC38 tumor bearing WT, but not Ifnar1-deficient mice, resulted in increased frequencies of intratumoral CTLs with higher surface expression of IFNAR1 and suppressed tumor growth (Figure 1).
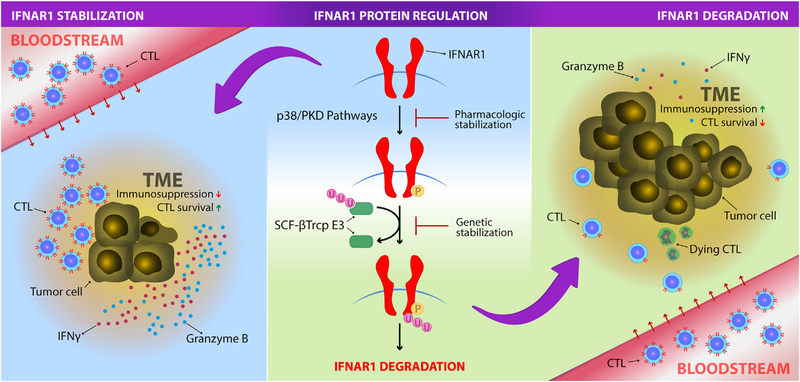
Figure 1. IFNAR1 Surface Expression Downmodulation Is Required for the Establishment of the Immune-Privileged Tumor Microenvironment.
Cytotoxic T lymphocytes (CTLs) expressing normal surface IFNAR1 protein levels in the periphery (bloodstream) downregulate its expression when they enter the tumor microenviroenment (TME). This impairs their survival and leads to immunosuppression. The immune-privileged niche generated in the TME leads to tumor progression (right panel). IFNAR1 protein regulation is controlled by different cellular and soluble factors, including elements released under TME stress. Degradation of IFNAR1 is controlled by phosphorylation induced via p38 and PKD pathways and consequent ubiquitination mediated by SCF-βTcrp E3 ligase (middle panel). Different approaches can be used to stabilize IFNAR1 on the cell surface. Genetic stabilization by a single point mutation in the phosphorylation site of IFNAR1 protein or pharmacological inhibition of p38 and PKD pathways prevent IFNAR1 degradation improving CTL survival and increasing IFN γ and granzyme expression in the TME, which results in antitumoral immunity (left panel). IFNAR1 stabilization also improves adoptive cell transfer- or checkpoint-based therapies.
Therapies aiming at improving CTL function and reverting the immunosuppressive TME are among the most promising cancer therapy approaches. In addition, certain chemotherapies and radiotherapy have been shown to rely on host type I IFN for their optimal effectiveness. Different mechanisms involving cancer cell autonomous, distinct stromal processes or even a combination of both seem to be engaged probably depending on the specific tumor-elicited microenvironment. Nevertheless, strategies aiming at preventing IFNAR1 surface degradation hold promise, and new compounds with such an effect could have an important impact on cancer therapy efficacy.
ACKNOWLEDGMENTS
This work was supported by the intramural research program of the NCI. We are grateful to Juan Pablo Borrelli for help with the illustration.
REFERENCES
- Bhattacharya S, Katlinski KV, Reichert M, Takano S, Brice A, Zhao B, Yu Q, Zheng H, Carbone CJ, Katlinskaya YV, et al. (2014). EMBO Mol. Med. 6, 384–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidwell BN, Slaney CY, Withana NP, Forster S, Cao Y, Loi S, Andrews D, Mikeska T, Mangan NE, Samarajiwa SA, et al. (2012). Nat. Med. 18, 1224–1231. [DOI] [PubMed] [Google Scholar]
- Diamond MS, Kinder M, Matsushita H, Mashayekhi M, Dunn GP, Archambault JM, Lee H, Arthur CD, White JM, Kalinke U, et al. (2011). J. Exp. Med. 208, 1989–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuertes MB, Kacha AK, Kline J, Woo SR, Kranz DM, Murphy KM, and Gajewski TF (2011). J. Exp. Med. 208, 2005–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katlinskaya YV, Katlinski KV, Yu Q, Ortiz A, Beiting DP, Brice A, Davar D, Sanders C, Kirkwood JM, Rui H, et al. (2016). Cell Rep. 15, 171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katlinski KV, Gui J, Katlinskaya YV, Ortiz A, Chakraborty R, Bhattacharya S, Carbone CJ, Beiting DP, Girondo MA, Peck AR, et al. (2017). Cancer Cell 31, this issue, 194–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolumam GA, Thomas S, Thompson LJ, Sprent J, and Murali-Krishna K (2005). J. Exp. Med. 202, 637–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sistigu A, Yamazaki T, Vacchelli E, Chaba K, Enot DP, Adam J, Vitale I, Goubar A, Baracco EE, Remédios C, et al. (2014). Nat. Med. 20, 1301–1309. [DOI] [PubMed] [Google Scholar]
- Tschurtschenthaler M, Wang J, Fricke C, Fritz TM, Niederreiter L, Adolph TE, Sarcevic E, Künzel S, Offner FA, Kalinke U, et al. (2014). Gut 63, 1921–1931. [DOI] [PubMed] [Google Scholar]
- Zitvogel L, Galluzzi L, Kepp O, Smyth MJ, and Kroemer G (2015). Nat. Rev. Immunol. 15, 405–414. [DOI] [PubMed] [Google Scholar]