Abstract
Background
Several studies have reported that birth by caesarean section is associated with increased risk of lower respiratory tract infections in the child, but it is unclear whether this applies to any caesarean section or specifically to planned caesareans. Furthermore, although infections of the upper respiratory tract are very common during childhood, there is a scarcity of studies examining whether caesarean is also a risk factor for this site of infection.
Methods
We obtained data from two UK cohorts: the Millennium Cohort Study (MCS) and linked administrative datasets of the population of Wales through the Secure Anonymised Information Linkage (SAIL) databank. The study focused on term-born singleton infants and included 15,580 infants born 2000–2002 (MCS) and 392,145 infants born 2002–2016 (SAIL). We used information about mode of birth (vaginal delivery, assisted vaginal delivery, planned caesarean and emergency caesarean) from maternal report in the MCS and from hospital birth records in SAIL. Unplanned hospital admission for lower respiratory tract infection (LRTI) was ascertained from maternal report in the MCS and from hospital record ICD codes in SAIL. Information about admissions for upper respiratory tract infection (URTI) was available from SAIL only. Cox regression was used to estimate hazard ratios for each outcome and cohort separately while accounting for a wide range of confounders. Gestational age at birth was further examined as a potential added, indirect risk of planned caesarean birth due to the early delivery.
Findings
The rate of hospital admission for LRTI was 4.6 per 100 child years in the MCS and 5.9 per 100 child years in SAIL. Emergency caesarean was not associated with LRTI admission during infancy in either cohort. In the MCS, planned caesarean was associated with a hazard ratio of 1.39 (95% CI 1.03, 1.87) which further increased to 1.65 (95% CI 1.24, 2.19) when gestational age was not adjusted for. In SAIL, the adjusted hazard ratio was 1.10 (95% CI 1.05, 1.15), which increased to 1.17 (95% CI 1.12, 1.22) when gestational age was not adjusted for. The rate of hospital admission for URTI was 5.9 per 100 child years in SAIL. Following adjustments, emergency caesarean was found to have a hazard ratio of 1.09 (95% CI 1.05, 1.14) for hospital admission for URTI. Planned caesarean was associated with a hazard ratio of 1.11 (95% CI 1.06, 1.16) which increased to 1.17 (95% CI 1.12, 1.22) when gestational age was not adjusted for.
Conclusions
The risk of severe LRTIs during infancy is moderately elevated in infants born by planned caesarean compared to those born vaginally. Infants born by any type of caesarean may also be at a small increased risk of severe URTIs. The estimated effect sizes are stronger if including the indirect effect arising from planning the caesarean birth for an earlier gestation than would have occurred spontaneously. Further studies are needed to confirm these results.
Introduction
Caesarean section rates have risen steeply in recent years, particularly in high and middle-income countries [1]. In England, for example, the caesarean rate has increased more than 3 fold in less than four decades–from 9% in 1980 [2] to 28.4% in 2018 [3]. An increase has taken place both for planned (‘elective’) caesarean sections and for unplanned, ‘emergency’ surgery. Because of this increase, the World Health Organization issued a statement in 2015 about rates of caesarean section [4] in which they called for further research to better understand the health effects of caesareans, including on longer-term child outcomes.
Acute respiratory infections are common during childhood. These infections include those manifesting in the lower respiratory tract such as bronchiolitis or pneumonia, and those of the upper respiratory tract such as the common cold, tonsillitis and laryngitis. The majority of respiratory infections are treated in primary care, however, some require hospitalisation. In Wales, for example, approximately 11% of infants were reported to require hospital admission for a respiratory infection by age 1 year [5]. These admissions were due to an infection of the upper respiratory tract in 45% of cases and due to bronchiolitis in 44% [5].
Caesarean section is a risk factor for hospital admission for lower respiratory infections (LRTIs), both during infancy [6–9] and later in childhood [10–13]. When examining elective and emergency caesareans separately, the available evidence suggests that elective caesareans are associated with an increased risk of infection [7–11], while the evidence for emergency caesarean is less conclusive [7, 8, 11]. Some studies have examined hospital admissions for any respiratory morbidity combined and found caesarean section to increase the risk of unplanned hospitalisation [5, 14]. However, only a single study that we are aware of has specifically examined the effect of mode of birth on severe upper respiratory tract infections (URTIs) [13]. This large international study identified a small increase in the risk for hospital admission for URTI in infants and preschool children born by elective or emergency caesarean section, with risks slightly higher following elective caesarean. Study findings relating to hospital admission due to LRTI or other types of infection were of a similar pattern [13].
In addition to mode of birth, other perinatal factors are also associated with respiratory morbidity. Gestational age at birth is an independent risk factor for childhood infections and respiratory illness [5, 15–17]. The severity of neonatal respiratory morbidity is inversely related to gestational week at time of caesarean [18]. Yet, despite this evidence, studies focusing on mode of birth and childhood respiratory infections have not examined the effect arising indirectly from the early delivery when a caesarean is planned [19]. Furthermore, although not being breastfed is also associated with infant infection [20, 21], most prior studies have not accounted for this factor.
The aim of the current study was to further elucidate whether birth by planned or emergency caesarean section is associated with increased risk of hospital admission due to LRTI or URTI in infancy.
Methods
The study used anonymised data from two birth cohorts analysed separately: the longitudinal UK Millennium Cohort Study and a birth cohort defined through linkage of routinely collected data for the population of Wales.
Data sources
Millennium Cohort Study
The Millennium Cohort Study (MCS) is a nationally representative longitudinal study of babies born between September 2000 and January 2002 that were alive and living in the UK at age 9 months. The sample does not include babies who died prior to this age, but these constituted ~0.5% of all births [22]. The MCS is a stratified cluster sample by electoral ward, with oversampling of ethnic minorities and disadvantaged areas, ensuring adequate representation of responses. A total of 18,818 infants were recruited from 72% of eligible families [23]. The first survey took place when most infants were 9–10 months of age. The infant’s mother was usually the main respondent and information about the family, the birth and the infant’s health was collected [24].
SAIL–Secure Anonymized Information Linkage Databank
The Secure Anonymized Information Linkage Databank (SAIL) is a data repository developed at Swansea University that stores anonymized data for the population of Wales. This databank hosts several health and administrative datasets of person-level data. Individuals can be linked between the various datasets using a unique Anonymous Linking Field (ALF) encrypted code, which is generated based on NHS number or personal identifiers [25, 26].
We defined a cohort of infants born between January 2002 and July 2016 to mothers resident in Wales as ascertained from the National Child Community Health Database (NCCHD) [27] (N = 490,826). The NCCHD is the all-Wales child health surveillance dataset and includes records of all children born, resident or treated in Wales. The records include a maternal ALF code as well as information about perinatal factors. The hospital birth record of these infants was derived from the Patient Episode Dataset for Wales (PEDW), which includes all inpatient and day case admissions in NHS Wales and those of Welsh residents in English hospitals. The relevant birth hospital admission was identified by matching on maternal ALF code with the further requirement that date of admission spanned the infant’s week of birth and that the admission record includes an Operating Procedure Codes (OPCS-4) indicating a birth. Information about maternal health was derived from diagnosis codes (ICD-10) from the birth admission. The infants identified were further linked to the Welsh Demographic Dataset (WDS) [28], a dataset including all residents registered with a General Practice (GP) in Wales, from which quintiles of the Welsh Index of Multiple Deprivation (WIMD) based on area of residency were derived, as well as information on time of emigration from Wales. Infants were also matched to the Annual District Death Extract (ADDE) [29] to derive date of death and to the Congenital Anomaly Register and Information Service for Wales (CARIS) [30] from which those with severe anomalies or genetic abnormalities were identified. The infants eligible to be included in our defined cohort were those live born and with a matching PEDW delivery record and WDS record that indicates being born to a Welsh resident (n = 442,565).
Inclusion criteria
Our focus was on healthy infants from birth, therefore the study included singleton infants born alive at term (37–42 gestational weeks) excluding those with a major health problem immediately following the birth (Fig 1). A major health problem was defined as a serious congenital or genetic abnormality (S1 Box in S1 File) and in the MCS also as report of neonatal special medical care and in SAIL also as low Apgar score (0–2 at 1 minute or 0–6 at 5 minutes).
Fig 1. Infants included and excluded from MCS and SAIL cohorts.
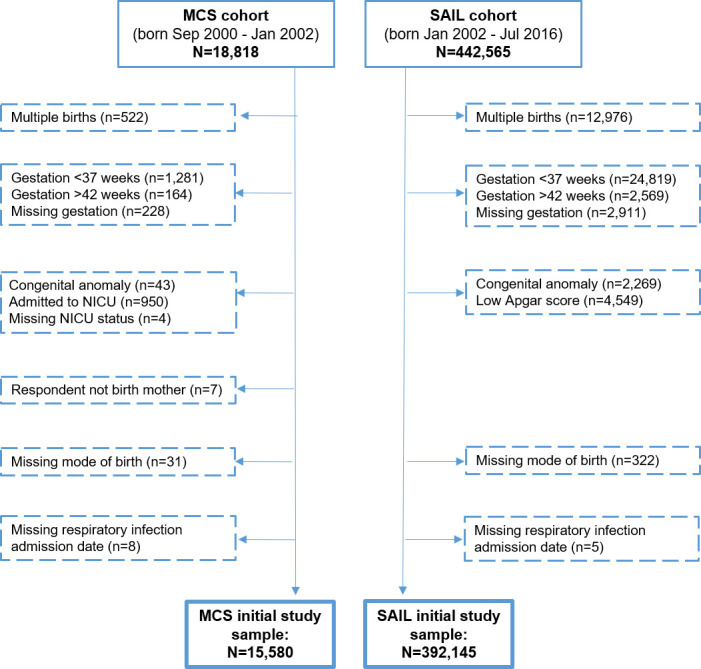
Exposure–mode of birth
Mode of birth was categorised as vaginal delivery, assisted vaginal delivery, planned caesarean section or emergency caesarean section (Table 1). In the MCS, mode of birth was ascertained from the mother’s report, which is known to be in good agreement with hospital records [31]. In the SAIL cohort, mode of birth was gathered from the birth admission operating procedure codes. When more than a single mode of birth was recorded (n = 115 in MCS and n = 293 in SAIL), the more highly medically interventional method was assigned [32].
Table 1. Definitions of exposure—mode of birth.
| Mode of birth classification | MCS | SAIL |
|---|---|---|
| Maternal report | Operating procedure codes | |
| Vaginal delivery (VD) | Normal delivery | R24 Normal delivery |
| Waterbirth | R23 Cephalic vaginal delivery with abnormal presentation of head at delivery without instrument | |
| Assisted vaginal delivery (Assisted VD) | Forceps | R19 Breech extraction delivery |
| Vacuum | R20 Other breech delivery | |
| Assisted breech | R21 Forceps cephalic delivery | |
| Other assisted delivery | R22 Vacuum delivery | |
| Planned caesarean section (Planned CS) | Planned caesarean | R17 Elective caesarean delivery |
| Emergency caesarean section (Emergency CS) | Emergency caesarean | R18 Other caesarean delivery |
| Missing | Refuse to answer, not known, irrelevant or non-codable | R25 Other method of delivery |
Outcomes–hospital admission for lower or upper respiratory tract infection
The study outcomes were hospital admission in infancy for lower respiratory infection or for upper respiratory infection. In the MCS, this was collected through mother’s report of hospital admissions due to ‘chest infection or pneumonia’ selected from a pre-defined list, together with the child’s age in months at admission. Almost all MCS sample infants (97%) were followed-up for at least 9 months.
In the SAIL cohort, information about outcomes was collected from hospital admissions recorded in PEDW. The outcomes were defined as an emergency (unplanned) admission during infancy with a diagnosis code indicating a lower respiratory tract infection (A37, J10-22) or an upper respiratory tract infection (J00-J06) (Table 2). Admissions were available from January 2002 to February 2017. There was complete follow-up until the 1st birthday for 98% of the SAIL cohort.
Table 2. Definitions of outcomes–lower and upper respiratory tract infections.
| MCS | SAIL |
|---|---|
| Maternal report | Diagnosis codes |
| Lower respiratory tract infection (LRTI) | |
| Chest infection or pneumonia | A37—Whooping cough |
| J10—Influenza due to identified influenza virus | |
| J11—Influenza, virus not identified | |
| J12—Viral pneumonia, not elsewhere classified | |
| J13—Pneumonia due to Streptococcus pneumoniae | |
| J14—Pneumonia due to Haemophilus influenzae | |
| J15—Bacterial pneumonia, not elsewhere classified | |
| J16—Pneumonia due to other infectious organisms, not elsewhere classified | |
| J17—Pneumonia in diseases classified elsewhere | |
| J18—Pneumonia, organism unspecified | |
| J20—Acute bronchitis | |
| J21—Acute bronchiolitis | |
| J22—Unspecified acute lower respiratory infection | |
| Upper respiratory tract infection (URTI) | |
| Not available | J00—Acute nasopharyngitis [common cold] |
| J01—Acute sinusitis | |
| J02—Acute pharyngitis | |
| J03—Acute tonsillitis | |
| J04—Acute laryngitis and tracheitis | |
| J05—Acute obstructive laryngitis [croup] and epiglottitis | |
| J06—Acute upper respiratory infections of multiple/unspecified sites | |
Explanatory variables
Potential explanatory variables were ascertained through parental report in the MCS and through information derived from the NCCHD, WDS or PEDW delivery record in SAIL (Table 3).
Table 3. Explanatory variables available for the analyses.
| Explanatory variable | MCS | SAIL |
|---|---|---|
| [Dataset] | ||
| Socio-demographic variables | ||
| Mother’s age in years | <20, 20–29, 30–39, ≥40 | <18, 18–19, 20–24, 25–29, 30–34, 35–39, ≥40 |
| [NCCHD] | ||
| Mother’s marital status | married, cohabiting, single | – |
| Mother’s education level | university degree, ‘A levels’, lower than ‘A levels’, other and overseas, no formal qualifications | – |
| Socioeconomic status (last known occupation of the mother or her partner, if higher) | managerial or professional, intermediate, routine or manual, never worked or long-term unemployed | – |
| Area deprivation quintile (Welsh Index of Multiple Deprivation—WIMD) | – | 1st (most deprived) - 5th (least deprived) |
| [WDS] | ||
| Ethnicity | white, other | white, mixed, black Indian/Pakistani/Bangladeshi, other, missing |
| [NCCHD] | ||
| Maternal and perinatal variables | ||
| Sex | male, female | male, female |
| [NCCHD] | ||
| Firstborn | yes, no | yes, no |
| [NCCHD+PEDW]* | ||
| Maternal asthma / atopic disease | eczema, hay fever or asthma | yes (ICD code J45 or J46 in any of mother’s delivery records for children included in sample), no |
| [PEDW] | ||
| Maternal diabetes | yes, no | yes (ICD codes O24, E10-E14), no |
| [PEDW] | ||
| Maternal hypertensive condition | yes, no | yes (ICD codes O10-O16, I10-I15), no |
| [PEDW] | ||
| Birthweight** | weight in Kg | weight in Kg |
| [NCCHD] | ||
| Gestational age birth** | 37–38, 39–40, 41, 42 | 37, 38, 39, 40, 41, 42 |
| [NCCHD] | ||
| Maternal smoking | non-smoker, ex-smoker, relapsed smoker, postnatal quitter, took up smoking postnatally, smoker | non-smoker, gave up in pregnancy, smokes 1–9 a day, smokes ≥10 a day, missing |
| [NCCHD] | ||
| Breastfeeding | any breastfeeding per month of follow-up (i.e. time-dependent) [52] | any breastfeeding (at birth or at 8 weeks), none, missing |
| [NCCHD] | ||
| Year of birth | – | 2002–2016 |
| [NCCHD] | ||
| Season of birth | – | January-March, April-June, |
| July-September, October-December | ||
| [NCCHD] | ||
* coded from NCCHD data on week of birth of child and sibling records, previous live and stillbirth of cohort child and sibling records and from ICD codes in PEDW delivery record indicating a prior birth
** In the MCS, gestational age was calculated by comparing mother’s report of her due date with the actual date of birth, a variable which has been previously validated with linked hospital birth records. In both cohorts, observations with implausible birthweight for gestational age were recoded to missing [53]
Statistical analysis
Hazard ratios for each mode of birth compared with vaginal delivery (the reference group) were estimated using Cox regression analysis. In the MCS, follow-up began at time of discharge from hospital of birth (median 2 days) and in SAIL follow-up began at time of birth. Follow-up ended at the first hospital admission for LRTI or URTI or was censored in the MCS at age at interview (range 6–12 months; average 9.2 months) and in SAIL at time of emigration from Wales, death, or February 2017. Statistical analyses of the MCS accounted for the complex survey design as well as weighting for non-response using Stata version 13 ‘survey commands’. Statistical analyses of the SAIL cohort accounted for within-family clustering since most infants had a sibling in the cohort (60%). The Cox regression assumption of constant hazard ratios was verified in the MCS using log-log plots and interaction with time and in SAIL using log-log plots and assessing whether hazard ratios are substantially different when follow-up is split into two time periods.
We selected variables to be included in multivariable models as follows. In the MCS, sex was included a priori and the association of other variables found to be associated (P<0.1) with the exposure and the outcome was examined in a two stage process. Initially, socio-demographic variables and maternal-perinatal variables (Table 3) were included separately in a multivariable model and then statistically significant (P<0.1) variables from each model were combined and only those that significantly improved the fit of the data were included in the final model. In the SAIL cohort all models were adjusted for year of birth and multivariable models were adjusted for those covariates listed in Table 3 that were associated (P<0.1) with both exposure and outcome.
Missing data
In the MCS, missing data about explanatory variables was minimal and therefore complete case analysis was used. In the SAIL cohort most variables had <10% missing values but the proportion was higher for smoking (60%), breastfeeding (15%) and ethnicity (11%) and therefore a category of ‘missing’ was included for these variables. Complete case analysis and multiple imputation were used in sensitivity analyses (S1 Text in S1 File).
Effect of gestational age
In the case of planned caesarean section (but less commonly in emergency caesarean), gestational age at birth is the result of the planned mode of birth. We aimed to quantify the risk of respiratory infection due to caesarean section while taking account of the potential added risk from an earlier delivery at early term (37–38 weeks). Final models were therefore repeated without adjustment for gestational age.
Sensitivity analyses
Wheezing is a common symptom of respiratory infection, therefore a sensitivity analysis was carried out in the MCS including ‘wheezing or asthma’ in the outcome definition. To address the possibility of reverse causality of a prenatal infection leading both to need for a caesarean birth and to later infant infection, we carried out an additional sensitivity analysis in the MCS excluding infants with a report of infection in the perinatal period. Further sensitivity analyses limiting to observations where mode of birth had been validated using an additional data source (S2 Text and S2 Box in S1 File) were also carried out in both cohorts.
Ethics
The MCS was granted ethics approval from Multi-Centre Research Ethics Committees [33]. Permission to use SAIL data was granted from the databank’s Information Governance Review Panel.
Results
Sample characteristics
The study included 15,580 infants for the MCS analyses and 392,145 infants for the SAIL analyses. Table 1 presents the distribution of mode of birth in the two cohorts. In the MCS, there were 71.1% vaginal deliveries, 10.1% assisted vaginal deliveries, 8.7% planned caesareans and 10.1% emergency caesareans. In the SAIL cohort there were 63.9% vaginal deliveries, 11.8% assisted vaginal deliveries, 11.0% planned caesareans and 13.3% emergency caesareans. When comparing the SAIL births taking place in 2002 (the most proximate year to when MCS children were born) to Welsh births of the MCS, the distribution of mode of birth was similar (Table 4).
Table 4. Distribution of mode of birth in MCS and SAIL cohorts.
| Proportion | VD (%) | Assisted VD (%) | Planned CS (%) | Emergency CS (%) | Total (%) |
|---|---|---|---|---|---|
| Total MCS sample* | 71.1 | 10.1 | 8.7 | 10.1 | 100 |
| Total SAIL sample | 63.9 | 11.8 | 11.0 | 13.3 | 100 |
| MCS Welsh births only** | 68.7 | 10.1 | 10.0 | 11.3 | 100 |
| SAIL 2002 births only | 67.6 | 9.6 | 10.7 | 12.1 | 100 |
* Percentages weighted using total UK weights
** Percentages weighted using country-specific weights
The characteristics of both cohorts were strongly associated with mode of birth (Table 5; P<0.001 for all variables except P<0.025 for maternal atopy). Mothers who had given birth by caesarean section (both planned and emergency) tended to be older, married, more highly educated and of higher socioeconomic status or living in a more affluent area compared to mothers who had a vaginal delivery. These mothers had more complications of pregnancy such as diabetes or hypertensive conditions but smoked less than those who had given birth vaginally. The infants born by planned caesarean were less likely to be the firstborn child compared to those born vaginally, while those born by emergency caesarean were more likely to be firstborn. The group of infants born by planned caesarean were also more likely to be born at early term (37–38 weeks), while the emergency caesarean group were more likely to be born at late term (41 weeks) and post term (42 weeks). See also S1 Table in S1 File.
Table 5. Characteristics of SAIL and MCS cohorts by mode of birth.
| MCS* | SAIL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| VD | Assisted | Planned | Emergency | P-value | VD | Assisted VD | Planned | Emergency CS | P-value | |
| %** | VD % | CS % | CS % | % | % | CS % | % | |||
| n = 11,077 | n = 1,528 | n = 1,381 | n = 1,594 | n = 250,740 | n = 46,086 | n = 43,231 | n = 52,088 | |||
| Socio-demographics | ||||||||||
| Maternal age, mean (SD) | 28.4 (5.8) | 28.7 (5.5) | 31.1 (5.2) | 29.8 (5.7) | <0.001 | 27.5 (5.9) | 27.8 (5.9) | 30.7 (5.7) | 28.7 (6.0) | <0.001 |
| Marital status | <0.001 | |||||||||
| Married | 58.6 | 62.6 | 71.8 | 64.4 | – | – | – | – | ||
| Cohabiting | 25.9 | 26.4 | 18.7 | 24.0 | – | – | – | – | ||
| Single | 15.5 | 11.1 | 9.5 | 11.6 | – | – | – | – | ||
| Education level | <0.001 | |||||||||
| Higher | 30.4 | 41.0 | 37.5 | 40.3 | – | – | – | – | ||
| Intermediate | 14.6 | 15.1 | 11.9 | 14.0 | – | – | – | – | ||
| Lower | 38.6 | 35.4 | 37.4 | 33.8 | – | – | – | – | ||
| Other academic / vocational | 2.6 | 2.2 | 2.1 | 2.7 | – | – | – | – | ||
| None of these | 13.8 | 6.5 | 11.0 | 9.2 | – | – | – | – | ||
| Socioeconomic class | <0.001 | |||||||||
| Managerial/Professional | 42.5 | 53.9 | 48.2 | 53.7 | – | – | – | – | ||
| Intermediate | 19.8 | 18.6 | 22.8 | 18.4 | – | – | – | – | ||
| Routine/Manual | 32.5 | 24.4 | 26.3 | 24.4 | – | – | – | – | ||
| Unemployed | 5.2 | 3.1 | 2.7 | 3.6 | – | – | – | – | ||
| Area deprivation quintile | <0.001 | |||||||||
| 1st (most deprived) | – | – | – | – | 27.7 | 21.9 | 22.9 | 23.2 | ||
| 2nd | – | – | – | – | 22.2 | 21.1 | 21.2 | 21.6 | ||
| 3rd | – | – | – | – | 19.1 | 19.7 | 19.4 | 20.1 | ||
| 4th | – | – | – | – | 16.3 | 18.3 | 17.2 | 18.3 | ||
| 5th (least deprived) | – | – | – | – | 14.8 | 19.1 | 19.2 | 16.8 | ||
| White ethnicity | 86.7 | 91.2 | 89.2 | 85.9 | <0.001 | 91.7 | 91.9 | 92.5 | 91.3 | <0.001 |
| Maternal-perinatal | ||||||||||
| Male | 49.5 | 55.1 | 47.9 | 55.3 | <0.001 | 49.7 | 54.0 | 50.6 | 55.3 | <0.001 |
| Firstborn | 34.1 | 78.2 | 23.1 | 68.6 | <0.001 | 35.4 | 75.8 | 20.5 | 65.5 | <0.001 |
| Maternal asthma | – | – | – | – | 5.3 | 5.5 | 6.5 | 6.1 | <0.001 | |
| Maternal atopy | 42.2 | 46.2 | 40.7 | 40.7 | 0.025 | – | – | – | – | |
| Diabetes | 1.5 | 1.7 | 4.6 | 2.3 | <0.001 | 1.6 | 2.0 | 4.8 | 3.6 | <0.001 |
| Hypertension | 6.0 | 8.5 | 7.2 | 13.0 | <0.001 | 4.4 | 8.3 | 4.1 | 10.4 | <0.001 |
| Birthweight in kg, mean (SD) | 3.4 (0.5) | 3.5 (0.5) | 3.4 (0.5) | 3.5 (0.5) | <0.001 | 3.4 (0.5) | 3.5 (0.5) | 3.5 (0.5) | 3.5 (0.5) | <0.001 |
| Gestational age | <0.001 | <0.001 | ||||||||
| 37–38 (early term) | 17.7 | 12.1 | 54.2 | 17.8 | 16.7 | 13.4 | 34.0 | 18.5 | ||
| 39–40 (full term) | 56.3 | 53.3 | 39.3 | 46.8 | 56.5 | 51.4 | 58.8 | 44.5 | ||
| 41 (late term) | 22.6 | 29.5 | 4.9 | 28.9 | 23.1 | 28.3 | 5.7 | 28.2 | ||
| 42 (post term) | 3.5 | 5.2 | 1.7 | 6.6 | 3.7 | 7.0 | 1.4 | 8.8 | ||
| Maternal smoking | <0.001 | <0.001 | ||||||||
| Non-smoker | 65.2 | 67.5 | 69.9 | 70.7 | 74.5 | 81.5 | 82.2 | 80.9 | ||
| Gave-up in pregnancy | 12.0 | 15.7 | 11.7 | 14.5 | 3.7 | 4.1 | 2.6 | 4.0 | ||
| Smoker | 22.8 | 16.8 | 18.4 | 14.9 | 21.8 | 14.4 | 15.2 | 15.1 | ||
| Any breastfeeding | 68.5 | 75.2 | 69.5 | 74.6 | <0.001 | 56.9 | 63.9 | 56.8 | 61.8 | <0.001 |
* Percentages in MCS are weighted.
** All MCS and SAIL percentages are calculated from non-missing observations. Missing values are <10% of total observations for all variables apart from the following in SAIL: 59.8% for smoking, 15.3% for breastfeeding, 11.2% for ethnicity.
Hazard ratios of hospital admission for LRTI
Overall, 3.4% (95% CI 3.1, 3.8) of infants were admitted at least once for LRTI in the MCS sample (574 cases) and 5.6% (95% CI 5.6, 5.7) in the SAIL sample (22,154 cases). To aid comparability, the proportion of infants born in 2002 and admitted up to age 9.2 months in SAIL was examined. This proportion was found to be 4.0% (95% CI 3.7, 4.2), similar to 3.4% in the MCS. The rate of admission peaked at the 2nd-3rd month of life and later declined (S1 Fig in S1 File). In the SAIL sample, admissions were predominantly for acute bronchiolitis (J21; 83% of events) and much less frequently for unspecified LRTI (J22; 9% of events), pneumonia with unspecified organism (J18; 4% of events) or other indications. In 95% of cases, LRTI was the primary indication for admission.
The rate of hospital admission for LRTI was 4.6 per 100 child-years in the MCS and 5.9 per 100 child-years in the SAIL cohort (Table 6). In the crude model of both cohorts, infants born by assisted vaginal delivery and emergency caesarean section had a lower hazard of admission compared to those born by vaginal delivery. Following adjustment for confounders and other explanatory variables, these modes of birth were not associated with LRTI hospital admission. Infants born by planned caesarean section were more likely to be admitted to hospital (51% increased risk in the MCS and 15% in SAIL), and this increase in risk remained after accounting for other variables: adjusted HR = 1.39 (95% CI 1.03 to 1.87) in the MCS and adjusted HR = 1.10 (95% CI 1.05 to 1.15) in SAIL. The findings from SAIL using complete case analysis and from multiple imputation were similar to the main results (S2 Table in S1 File).
Table 6. Association between mode of birth and hospital admission for LRTIs and URTIs in the MCS and SAIL cohorts.
| Hospital admission for | Hospital admission for | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lower Respiratory Tract Infection | Upper Respiratory Tract Infection | ||||||||||||||
| MCS | SAIL | SAIL | |||||||||||||
| No. of events | Child-years | Rate per 100 child-years | Crude hazard ratio | Adjusted hazard ratio * | No. of events | Child-years | Rate per 100 child-years | Crude hazard ratio ** | Adjusted hazard ratio *** | No. of events | Child-years | Rate per 100 child-years | Crude hazard ratio ** | Adjusted hazard ratio **** | |
| (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | ||||||||||
| P-value | P-value | P-value | P-value | P-value | P-value | ||||||||||
| n = 15,580 | n = 15,531 | n = 392,145 | n = 364,651 | n = 392,145 | n = 364,654 | ||||||||||
| Overall | 574 | 11,664 | 4.6 | 22,154 | 374,489 | 5.9 | 22,278 | 377,422 | 5.9 | ||||||
| VD | 420 | 8,308 | 4.6 | 1.00 | 1.00 | 14,697 | 239,134 | 6.2 | 1.00 | 1.00 | 14,073 | 241,384 | 5.8 | 1.00 | 1.00 |
| Assisted VD | 43 | 1,181 | 3.8 | 0.82 (0.57,1.19) | 1.18 (0.79, 1.75) | 2,024 | 44,348 | 4.6 | 0.73 (0.69, 0.76) | 0.97 (0.93, 1.02) | 2,495 | 44,425 | 5.6 | 0.95 (0.91, 0.99) | 1.03 (0.98, 1.07) |
| P = 0.301 | P = 0.420 | P<0.001 | P = 0.304 | P = 0.024 | P = 0.292 | ||||||||||
| Planned CS | 61 | 999 | 7.0 | 1.51 (1.13, 2.03) | 1.39 (1.03, 1.87) | 2,942 | 41,005 | 7.2 | 1.15 (1.11, 1.20) | 1.10 (1.05, 1.15) | 2,717 | 41,481 | 6.6 | 1.12 (1.07, 1.16) | 1.11 (1.06, 1.16) |
| P = 0.006 | P = 0.030 | P<0.001 | P<0.001 | P<0.001 | P<0.001 | ||||||||||
| Emergency CS | 50 | 1,176 | 3.7 | 0.83 (0.58, 1.20) | 1.14 (0.79, 1.65) | 2,491 | 50,002 | 5.0 | 0.80 (0.77, 0.84) | 1.03 (0.98, 1.08) | 2,993 | 50,132 | 6.0 | 1.02 (0.98, 1.06) | 1.09 (1.05, 1.14) |
| P = 0.328 | P = 0.494 | P<0.001 | P = 0.244 | P = 0.326 | P<0.001 | ||||||||||
* Adjusted for maternal age, firstborn, infant’s sex, maternal smoking, gestational age and breastfeeding per month of follow-up
** Adjusted for year of birth
***Adjusted for maternal age, area deprivation quintile, firstborn, smoking, maternal asthma, hypertensive conditions, infant’s sex, ethnicity, gestational age, birthweight, breastfeeding, season and year of birth
**** Adjusted for maternal age, area deprivation quintile, firstborn, smoking, maternal asthma, infant’s sex, ethnicity, gestational age, birthweight, breastfeeding, season and year of birth
Findings from further sensitivity analyses were consistent with the main results. These included using validated mode of birth data in both cohorts (S3 Table in S1 File), and in the MCS—excluding infants with a perinatal infection (S4 Table in S1 File) and using a broader definition of the outcome (S5 Table in S1 File).
Hazard ratios of hospital admission for URTI
Hospital admission for URTI was available solely in the SAIL cohort. Overall, the proportion of infants who were admitted at least once for URTI was 5.7% (95% CI 5.6 to 5.8) (22,278 cases). The rate of admission increased towards the infant’s 1st birthday (S2 Fig in S1 File). Admissions were predominantly for acute URTI of multiple or unspecified sites (J06; 72% of events) and much less frequently due to tonsillitis (J03; 12% of events), croup (J05; 11% of events) or other indications. The URTI was the primary indication for admission in 91% of cases. This group of infants had little overlap with the group admitted for LRTI—only 3% of infants admitted for URTI had a diagnosis code for LRTI too.
The rate of URTI hospital admission was 5.9 per 100 child-years (Table 6). Infants born by assisted vaginal delivery did not have a different risk of admission in comparison to the risk of infants born vaginally, either before or after adjustments. Infants born by emergency caesarean had a similar risk to the vaginal delivery group, however after adjustment for other variables the excess risk slightly increased (adjusted HR = 1.09; 95% CI 1.05 to 1.14). Infants born by planned caesarean had a small increased risk (12%) of hospital admission for URTI, and this elevated risk remained the same following adjustment (adjusted HR = 1.11 95% CI 1.06 to 1.16). Results from multiple imputation and complete case analysis were in agreement with the main findings (S2 Table in S1 File) as well as those restricted to validated observations (S3 Table in S1 File).
Indirect effect of mode of birth through earlier delivery
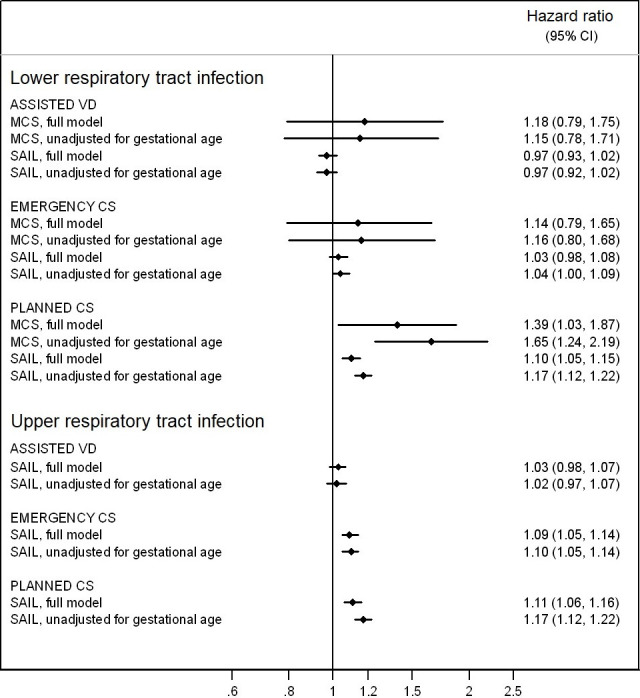
To obtain hazard ratios that also comprise the indirect effect of the planned caesarean through the earlier timing of the delivery (e.g. 37–38 weeks), final models were repeated without adjusting for gestational age. The size of effects found in assisted vaginal delivery and emergency caesarean were unchanged (Fig 2). However, in infants born by planned caesarean, the hazard ratio for LRTI increased from 1.39 to 1.65 in the MCS and from 1.10 to 1.17 in SAIL when gestational age was removed from the model. Likewise, the hazard ratio for URTI increased from 1.11 in the fully adjusted model to 1.17 in the model unadjusted for gestational age in SAIL.
Fig 2. Hazard ratios of admission for LRTI and URTI, fully adjusted and unadjusted for gestational age at birth.
Discussion
Our study demonstrated that birth by planned caesarean section is an independent risk factor associated with 10–39% increase in risk for LRTI hospital admission during infancy. Our study further showed that birth by planned or emergency caesarean section is an independent risk factor associated with a 9–11% increase in risk for URTI admission. When the effect of gestational age at delivery is not controlled, but rather included in the overall effect arising from the plan to deliver by caesarean, the effect size found is stronger.
Comparison with literature
Prior studies have also identified planned caesarean as a risk factor for LRTI, as described here. However, the evidence regarding emergency caesarean is less conclusive, with some studies finding no increased risk, as in our study, and others identifying emergency caesarean as a risk factor as well.
Large population-based studies from Australia concluded that both types of caesarean are risk factors for LRTI admission. A study from New South Wales [9] found an increased risk in the magnitude of 12–13% while a study from Western Australia [10] reported that elective caesarean is associated with 34% increased risk of admission and emergency caesarean with 20% elevated risk. Likewise, an international study pooling data from several high-income countries found 15% elevated risk of admission associated with elective caesarean and a slightly lower risk of 9% associated with emergency caesarean [13].
Other studies highlighted elective caesarean, but not emergency caesarean, as a risk factor for LRTI, similarly to our study. Studies conducted in Denmark [8, 11] and Western Australia [7] examined infant and child admissions for bronchiolitis or respiratory syncytial virus, which is responsible for most bronchiolitis cases [34]. These studies found an 11–29% increased risk of admission associated with elective caesarean, but no increased risk for emergency caesarean. Likewise, a study examining recurrent episodes of LRTIs during early childhood in a Norwegian birth cohort reported 19% increased risk (although not statistically significant) in the elective caesarean group, and no association in the emergency caesarean group [35].
We are aware of only a single study that had examined the association of mode of birth with severe URTIs using population data. Similarly to our findings, the study found 12% elevated risk for admission in children born by emergency caesarean and a slightly larger 18% increased risk in children born by elective caesarean. A smaller study followed up 334 children in Copenhagen and did not find an association between caesarean section (any type) and URTIs, although an association with LRTI was found [36]. Our results are therefore novel in highlighting hospital admission for URTIs as a potential adverse outcome of caesarean sections. Minor infectious episodes of the upper respiratory tract are generally more frequent than those of the lower respiratory tract [36] and admission for this indication during infancy is as common as admission for LRTI (both of a prevalence of 5.6–5.7% in our sample). Therefore, although the excess risk for URTI admission arising from the caesarean birth is small (10%), this evidence of a possible adverse outcome of caesarean section is important.
Some infants experiencing a hospital admission for respiratory infection will continue to have persistent episodes of infection or wheeze later in childhood. In certain cases, the admissions during infancy may be an early sign of subsequent asthma diagnosis [37]. Analogous to our findings, studies focusing on asthma have identified a 20% elevated risk in children born by caesarean, in particular when elective [11, 38].
Birth at every successive week earlier than 39 or 40 weeks is associated with increased risk of childhood infections [5, 15]. During the years when many of the children included in our study were born (particularly the MCS sample), a relatively high proportion of planned caesareans were carried out at early term gestation (54.2% in the MCS and 34.0% in SAIL). Notably, this proportion decreased in the UK in more recent years due to guidelines recommending to refrain from routinely scheduling caesareans for earlier than 39 weeks [39, 40]. Our results therefore demonstrate the added effect of the timing of delivery to that of the caesarean birth and the significance of reporting the long-term effects of planned caesarean unadjusted for gestational age.
Strengths and limitations
The main strength of our study is the use of two complementary cohorts to examine the association of mode of birth with LRTIs. The MCS includes rich data which enabled us to include variables such as breastfeeding that have not been previously well accounted for. The SAIL cohort was large enough to allow detection of modest effect sizes and provided a platform to investigate admission for URTIs. A further strength is our focus on term-born neonates, since prematurity is likely a strong confounder, as it influences the choice of mode of birth and is a risk factor for poor respiratory health [5]. Moreover, the exclusion of ill neonates helps address bias from confounding by indication. Complications during pregnancy and birth may indicate a medically-assisted delivery and at the same time lead to complications in the neonate, which may predispose to later disease risk. Lastly, the definitions and validation of the exposure and outcome variables are also strengths of the study. The exposure was validated using an additional data source, therefore increasing confidence that planned and emergency caesareans were correctly classified. The outcome focused on infections that require hospital admission, which are not likely influenced by parental health-seeking behaviour.
Limitations of the study include the unavailability of information about URTI admissions in the MCS and examination of this outcome solely through the SAIL datasets. The SAIL cohort is limited in having only area-level information about socio-economic factors, crude data on breastfeeding and under measurement of variables such as maternal asthma, which was collected through diagnosis codes at delivery. For LRTI, the MCS is limited by measurement of this outcome through maternal report, although this might not be a serious limitation since it has been shown that mothers generally recall their child’s hospital admission well [41], particularly considering the short recall period of less than one year. Nevertheless, we cannot rule out some degree of misclassification between various respiratory diagnoses in our outcome measurements. Lastly, residual confounding is a possible source of bias in both cohorts since potentially important covariates such as fetal or maternal complications, prophylactic antibiotics given at birth and induction of labour, were unavailable [14, 42].
Mechanisms
Several biological mechanisms could potentially explain how a caesarean birth might affect subsequent risk of respiratory infection. First, the hormones of labour and the physical forces of contractions which squeeze amniotic fluid out of the lungs of the fetus, both have a role in establishing normal pulmonary function in the newborn [43]. A birth where this hormonal secretion or physical force does not occur (i.e. caesarean) may result in retained lung fluids, which can lead to transient tachypnoea of the newborn or, in severe cases, respiratory distress syndrome [44]. These conditions are more likely following planned caesarean section, especially at earlier gestations, compared to other modes of birth. If the respiratory health of the neonate affects later susceptibility to lower respiratory tract infection, this may explain the results found in the current study. Second, infants born by caesarean section show altered microbiota colonization as they do not benefit from the natural transmission of microbes from their mother’s vaginal flora [45] and have higher abundancy of opportunistic pathogens common in the hospital environment [46]. There is also emerging evidence to suggest that differences in microbial composition are especially pronounced following an elective caesarean, perhaps since amniotic membranes are still intact prior to delivery [47, 48]. Although the gut microbiota is the body area most studied in relation to mode of birth, other areas such as the upper airways also exhibit delayed development and reduced abundancy of beneficial bacterial strains in infants born by caesarean [49]. Dysbiosis of the microbiota has been linked to various adverse health conditions, including respiratory infections [50, 51].
Conclusion
Planned caesarean section is a small but important risk factor for hospital admission for lower respiratory infections during infancy. Both planned and emergency caesarean section may be small risk factors for admissions due to upper respiratory infections. The risk of both types of respiratory infection is further elevated in infants born by planned caesarean due to the indirect increased risk from birth at an earlier gestational week. Although the effect sizes are modest, these excess risks are important in view of the large number of caesareans carried out globally.
Supporting information
(DOCX)
Acknowledgments
We are grateful to the families of the Millennium Cohort Study and to the Centre for Longitudinal Studies. This study also makes use of anonymised data held in the Secure Anonymised Information Linkage (SAIL) Databank, which bears no responsibility for the further analysis or interpretation of these data. We would like to acknowledge all the data providers who make anonymised data available for research.
Data Availability
The data underlying the results presented in the study are available through the UK Data Service https://www.ukdataservice.ac.uk/ and through The Secure Anonymised Information Linkage Databank (SAIL) https://saildatabank.com/.
Funding Statement
JJK and MAQ: This research is part funded by the National Institute for Health Research (NIHR) Policy Research Programme, conducted through the Policy Research Unit in Maternal Health and Care, 108/0001. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. NA: DPhil studies funded through the support of the Clarendon Fund Scholarships and the Nuffield Department of Population Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Betrán AP, Ye J, Moller A-B, Zhang J, Metin Gülmezoglu A, Torloni MR. The Increasing Trend in Caesarean Section Rates: Global, Regional and National Estimates: 1990–2014. 2016; 10.1371/journal.pone.0148343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Government Statistical Service. NHS Maternity Statistics, England: 1998–99 to 2000–01. 2002.
- 3.NHS Digital. NHS Maternity Statistics, England 2017–18. NHS Maternity Statistics, England. 2018.
- 4.World Health Organization. WHO Statement on casarean Section Rate. Geneva; 2015.
- 5.Paranjothy S, Dunstan F, Watkins WJ, Hyatt M, Demmler JC, Lyons RA, et al. Gestational Age, Birth Weight, and Risk of Respiratory Hospital Admission in Childhood. Pediatrics. 2013. December 18;132(6):e1562–9. 10.1542/peds.2013-1737 [DOI] [PubMed] [Google Scholar]
- 6.Green CA, Yeates D, Goldacre A, Sande C, Parslow RC, McShane P, et al. Admission to hospital for bronchiolitis in England: trends over five decades, geographical variation and association with perinatal characteristics and subsequent asthma. Arch Dis Child. 2016. February 1;101(2):140–6. 10.1136/archdischild-2015-308723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore HC, de Klerk N, Holt P, Richmond PC, Lehmann D. Hospitalisation for bronchiolitis in infants is more common after elective caesarean delivery. Arch Dis Child. 2012. May 1;97(5):410–4. 10.1136/archdischild-2011-300607 [DOI] [PubMed] [Google Scholar]
- 8.Kristensen K, Fisker N, Haerskjold A, Ravn H, Simões EAF, Stensballe L. Caesarean section and hospitalization for respiratory syncytial virus infection: a population-based study. Pediatr Infect Dis J. 2015. February;34(2):145–8. 10.1097/INF.0000000000000552 [DOI] [PubMed] [Google Scholar]
- 9.Roberts CL, Algert CS, Ford JB, Nassar N. Mode of delivery may be the risk factor for infant infectious morbidity. Arch Dis Child. 2012. August 1;97(8):759. 10.1136/archdischild-2011-301458 [DOI] [PubMed] [Google Scholar]
- 10.Moore HC, de Klerk N, Richmond P, Lehmann D. A retrospective population-based cohort study identifying target areas for prevention of acute lower respiratory infections in children. BMC Public Health. 2010. January 7;10(1):757. 10.1186/1471-2458-10-757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kristensen K, Henriksen L. Cesarean section and disease associated with immune function. J Allergy Clin Immunol. 2016. September 11;137(2):587–90. 10.1016/j.jaci.2015.07.040 [DOI] [PubMed] [Google Scholar]
- 12.Haataja P, Korhonen P, Ojala R, Hirvonen M, Korppi M, Gissler M, et al. Hospital admissions for lower respiratory tract infections in children born moderately/late preterm. Pediatr Pulmonol. 2018. February;53(2):209–17. 10.1002/ppul.23908 [DOI] [PubMed] [Google Scholar]
- 13.Miller JE, Goldacre R, Moore HC, Zeltzer J, Knight M, Morris C, et al. Mode of birth and risk of infection-related hospitalisation in childhood: A population cohort study of 7.17 million births from 4 high-income countries. Smith GC, editor. PLOS Med. 2020. November 19;17(11):e1003429. 10.1371/journal.pmed.1003429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peters LL, Rm CT, Jonge A De, Khashan A, Mark, Mbbs T, et al. The effect of medical and operative birth interventions on child health outcomes in the first 28 days and up to 5 years of age: A linked data population-based cohort study. Birth. 2018;1–11. 10.1111/birt.12348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller JE, Hammond GC, Strunk T, Moore HC, Leonard H, Carter KW, et al. Association of gestational age and growth measures at birth with infection-related admissions to hospital throughout childhood: a population-based, data-linkage study from Western Australia. Lancet Infect Dis. 2016. August 1;16(8):952–61. 10.1016/S1473-3099(16)00150-X [DOI] [PubMed] [Google Scholar]
- 16.Tickell KD, Lokken EM, Schaafsma TT, Goldberg J, Lannon SMR. Lower respiratory tract disorder hospitalizations among children born via elective early-term delivery. J Matern Fetal Neonatal Med. 2015;7058(June):1–6. [DOI] [PubMed] [Google Scholar]
- 17.Edwards MO, Kotecha SJ, Lowe J, Richards L, Watkins WJ, Kotecha S. Early-term birth is a risk factor for wheezing in childhood: A cross-sectional population study. J Allergy Clin Immunol. 2015;136(3):581–587.e2. 10.1016/j.jaci.2015.05.005 [DOI] [PubMed] [Google Scholar]
- 18.Zanardo V, Simbi A, Franzoi M, Soldá G, Salvadori A, Trevisanuto D. Neonatal respiratory morbidity risk and mode of delivery at term: influence of timing of elective caesarean delivery. Acta Paediatr. 2004. May 1;93(5):643–7. 10.1111/j.1651-2227.2004.tb02990.x [DOI] [PubMed] [Google Scholar]
- 19.Kotecha SJ, Gallacher DJ, Kotecha S. The respiratory consequences of early-term birth and delivery by caesarean sections. Paediatr Respir Rev. 2016;19:49–55. 10.1016/j.prrv.2015.12.002 [DOI] [PubMed] [Google Scholar]
- 20.Duijts L, Ramadhani MK, Moll HA. Breastfeeding protects against infectious diseases during infancy in industrialized countries. A systematic review. Matern Child Nutr. 2009. July;5(3):199–210. 10.1111/j.1740-8709.2008.00176.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Payne S, Quigley MA. Breastfeeding and infant hospitalisation: analysis of the UK 2010 Infant Feeding Survey. Matern Child Nutr. 2017. January;13(1). 10.1111/mcn.12263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cullis A. A Technical Report Infant Mortality in the Millennium Cohort Study (MCS) Sample Areas. 2007. [Google Scholar]
- 23.Plewis I, Calderwood L, Hawkes D, Hughes G, Joshi H. The Millennium Cohort Study: Technical Report on Sampling. Centre for Longitudinal Studies; 2007. [Google Scholar]
- 24.Centre for Longitudinal Studies. Millennium Cohort Study [Internet]. [cited 2019 Jul 16]. Available from: https://cls.ucl.ac.uk/cls-studies/millennium-cohort-study/
- 25.Ford D V, Jones KH, Verplancke J-P, Lyons RA, John G, Brown G, et al. The SAIL Databank: building a national architecture for e-health research and evaluation. BMC Health Serv Res. 2009. December 4;9(1):157. 10.1186/1472-6963-9-157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lyons RA, Jones KH, John G, Brooks CJ, Verplancke J-P, Ford D V, et al. The SAIL databank: linking multiple health and social care datasets. BMC Med Inform Decis Mak. 2009. December 16;9(1):3. 10.1186/1472-6947-9-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Statistics for Wales. Births in Wales 2017: Data from the National Community Child Health Database. Welsh Government; 2018. [Google Scholar]
- 28.NHS Wales Informatics Service. Handling patient details [Internet]. NHS Wales Informatics Service; 2017 [cited 2018 Nov 7]. Available from: http://www.wales.nhs.uk/nwis/page/52552
- 29.Office for National Statistics. Deaths registered in England and Wales: 2017 [Internet]. [cited 2018 Dec 5]. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/bulletins/deathsregistrationsummarytables/2017
- 30.Public health Wales. Congenital Anomaly Register and Information Service (CARIS) [Internet]. [cited 2018 Nov 7]. Available from: http://www.caris.wales.nhs.uk/home
- 31.Quigley MA, Hockley C, Davidson LL. Agreement between hospital records and maternal recall of mode of delivery: evidence from 12 391 deliveries in the UK Millennium Cohort Study. BJOG. 2007;114(2):195–200. 10.1111/j.1471-0528.2006.01203.x [DOI] [PubMed] [Google Scholar]
- 32.Essex HN, Green J, Baston H, Pickett KE. Which women are at an increased risk of a caesarean section or an instrumental vaginal birth in the UK: an exploration within the Millennium Cohort Study. BJOG. 2013. May;120(6):732–42. 10.1111/1471-0528.12177 [DOI] [PubMed] [Google Scholar]
- 33.Shepherd P, Gilbert E. Millennium Cohort Study—Ethical review and consent. 2019. [Google Scholar]
- 34.Miller EK, Gebretsadik T, Carroll KN, Dupont WD, Mohamed YA, Morin L-L, et al. Viral etiologies of infant bronchiolitis, croup and upper respiratory illness during 4 consecutive years. Pediatr Infect Dis J. 2013. September;32(9):950–5. 10.1097/INF.0b013e31829b7e43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Magnus MC, Håberg SE, Stigum H, Nafstad P, London SJ, Vangen S, et al. Delivery by Cesarean section and early childhood respiratory symptoms and disorders: the Norwegian mother and child cohort study. Am J Epidemiol. 2011. December 1;174(11):1275–85. 10.1093/aje/kwr242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vissing NH, Chawes BL, Rasmussen MA, Bisgaard H. Epidemiology and Risk Factors of Infection in Early Childhood. Pediatrics. 2018. June 1;141(6):e20170933. 10.1542/peds.2017-0933 [DOI] [PubMed] [Google Scholar]
- 37.Inoue Y, Shimojo N. Epidemiology of virus-induced wheezing/asthma in children. Front Microbiol. 2013. December 16;4:391. 10.3389/fmicb.2013.00391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keag OE, Norman JE, Stock SJ. Long-term risks and benefits associated with cesarean delivery for mother, baby, and subsequent pregnancies: Systematic review and meta-analysis. Myers JE, editor. PLOS Med. 2018. Jan 23;15(1):e1002494. 10.1371/journal.pmed.1002494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.National Collaborating Centre for Women’s and Children’s Health. Caesarean section NICE clinical guideline (CG132). 2011. [Google Scholar]
- 40.Gurol-Urganci I, Cromwell DA, Edozien LC, Onwere C, Mahmood TA, van der Meulen JH. The timing of elective caesarean delivery between 2000 and 2009 in England. BMC Pregnancy Childbirth. 2011. January;11:43. 10.1186/1471-2393-11-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.D’Souza-Vazirani D, Minkovitz CS, Strobino DM. Validity of maternal report of acute health care use for children younger than 3 years. Arch Pediatr Adolesc Med. 2005. February 1;159(2):167–72. 10.1001/archpedi.159.2.167 [DOI] [PubMed] [Google Scholar]
- 42.Bokulich NA, Chung J, Battaglia T, Henderson N, Jay M, Li H, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med. 2016. June 15;8(343):343ra82. 10.1126/scitranslmed.aad7121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jain L, Eaton DC. Physiology of Fetal Lung Fluid Clearance and the Effect of Labor. Semin Perinatol. 2006. February 1;30(1):34–43. 10.1053/j.semperi.2006.01.006 [DOI] [PubMed] [Google Scholar]
- 44.Ramachandrappa A, Jain L. Elective cesarean section: its impact on neonatal respiratory outcome. Clin Perinatol. 2008. Jun;35(2):373–93, vii. 10.1016/j.clp.2008.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci. 2010. June 29;107(26):11971–5. 10.1073/pnas.1002601107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shao Y, Forster SC, Tsaliki E, Vervier K, Strang A, Simpson N, et al. Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature. 2019. October;574(7776):117–21. 10.1038/s41586-019-1560-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Azad MB, Konya T, Maughan H, Guttman DS, Field CJ, Chari RS, et al. Gut microbiota of healthy Canadian infants: profiles by mode of delivery and infant diet at 4 months. CMAJ. 2013. March 19;185(5):385–94. 10.1503/cmaj.121189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chu DM, Ma J, Prince AL, Antony KM, Seferovic MD, Aagaard KM. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat Med. 2017. January 23;23(3):314–26. 10.1038/nm.4272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bosch A, Levin E, Van Houten MA, Hasrat R, Kalkman G, Biesbroek G, et al. Development of Upper Respiratory Tract Microbiota in Infancy is Affected by Mode of Delivery. EBioMedicine. 2016. July;9:336–45. 10.1016/j.ebiom.2016.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Unger SA, Bogaert D. The respiratory microbiome and respiratory infections. J Infect. 2017. June 1;74:S84–8. 10.1016/S0163-4453(17)30196-2 [DOI] [PubMed] [Google Scholar]
- 51.Fonseca W, Lukacs NW, Ptaschinski C. Factors Affecting the Immunity to Respiratory Syncytial Virus: From Epigenetics to Microbiome. Front Immunol. 2018;9:226. 10.3389/fimmu.2018.00226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quigley MA, Kelly YJ, Sacker A. Breastfeeding and Hospitalization for Diarrheal and Respiratory Infection in the United Kingdom Millennium Cohort Study. Pediatrics. 2007. April;119(4):e837–42. 10.1542/peds.2006-2256 [DOI] [PubMed] [Google Scholar]
- 53.Poulsen G, Kurinczuk JJ, Wolke D, Boyle EM, Field D, Alfirevic Z, et al. Accurate reporting of expected delivery date by mothers 9 months after birth. J Clin Epidemiol. 2011. December 1;64(12):1444–50. 10.1016/j.jclinepi.2011.03.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
The data underlying the results presented in the study are available through the UK Data Service https://www.ukdataservice.ac.uk/ and through The Secure Anonymised Information Linkage Databank (SAIL) https://saildatabank.com/.


