Abstract
The Aryl hydrocarbon receptor (AHR) is a major regulator of immune function within the gastrointestinal tract. Resident microbiota are capable of influencing AHR-dependent signaling pathways via production of an array of bioactive molecules that act as AHR agonists, such as indole or indole-3-aldehyde. Bacteria produce a number of quinoline derivatives, of which some function as quorum-sensing molecules. Thus, we screened relevant hydroxyquinoline derivatives for AHR activity using AHR responsive reporter cell lines. 2,8-dihydroxyquinoline (2,8-DHQ) was identified as a species-specific AHR agonist that exhibits full AHR agonist activity in human cell lines, but only induces modest AHR activity in mouse cells. Additional dihydroxylated quinolines tested failed to activate the human AHR. Nanomolar concentrations of 2,8-DHQ significantly induced CYP1A1 expression and, upon co-treatment with cytokines, synergistically induced IL6 expression. Ligand binding competition studies subsequently confirmed 2,8-DHQ to be a human AHR ligand. Several dihydroxyquinolines were detected in human fecal samples, with concentrations of 2,8-DHQ ranging between 0–3.4 pmol/mg feces. Additionally, in mice the microbiota was necessary for the presence of DHQ in cecal contents. These results suggest that microbiota-derived 2,8-DHQ would contribute to AHR activation in the human gut, and thus participate in the protective and homeostatic effects observed with gastrointestinal AHR activation.
Keywords: Ah receptor, Aryl hydrocarbon receptor, quinolinediol, dihydroxyquinoline, caco-2, tryptophan, microbiota
Graphical Abstract

INTRODUCTION
The human gastrointestinal (GI) tract is colonized by a multitude of microbial species. Host-microbiome mutualism provides numerous benefits for the host through education of the immune system, preventing allergies, improving digestion, and attenuating infection by pathogenic organisms. Dysregulation of the microbiota is thought to lead to adverse effects on the host, such as increased risk of diabetes, autoimmune diseases, and obesity.1 Microbiome community structures are variable in humans and can be influenced by host genetics, exposure history, and diet. There are numerous case examples of bacterial species that present a beneficial effect upon the host, however, the mechanisms by which bacteria interrelate within the gut environment is highly dynamic, complex, and requires further elucidation. One example is cross-talk between the intestinal mucin-associated bacteria Akkermansia muciniphila and the host resulting in an improved host metabolic profile.2 However, many of the studies that have examined this bacteria yield largely correlative results, especially in humans.3 In many cases the impact of individual species or a defined mixture of specific commensal bacterial species has been utilized, however, this does not take into account additional complex interactions between the summation of commensal species present in the intestinal tract.4, 5 Therefore, another approach is to consider the microbiota as a single entity and examine the overall gastrointestinal chemical signature.6 The microbiota collectively synthesizes or metabolizes numerous chemicals in the diet to form unique compounds, which can be referred to as the microbial metabolome. Comparative analyses of germ-free and conventional mouse models identified a number of serum metabolites associated with host microbial status. Indoxyl sulfate, p-cresol sulfate, equol sulfate, and cinnamoylglycine were only present in microbially-colonized mice.7 However, there are likely numerous additional bacterial-dependent metabolites that have not yet been identified.
The AHR is a unique ligand activated member of the bHLH-PAS transcription factor family that was originally identified as the receptor for the toxicant 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD).8 The AHR is bound in a 90 kDa heat shock protein complex in the cytoplasm and upon binding ligand the AHR translocates into the nucleus and heterodimerizes with the AHR nuclear translocator (ARNT).9 Studies have revealed that most of the toxic effects of TCDD are mediated through supra-physiological activation of the AHR, resulting in altered transcriptional activity.10–12 Initially through the use of Ahr−/− mice, the AHR has been implicated in a wide range of physiological pathways, including immune function, reproductive success, and liver vascular development. The defect that has recently received the most attention is the role of the AHR in peripheral immune cell function with specific indications pertaining to the GI tract.13 Indeed, the AHR plays a critical role in group 3 innate lymphoid cell maintenance and function. In turn, these cells produce IL22, a key signaling factor in gastrointestinal tissue regeneration in response to injury.14 The AHR is also capable of directly regulating the expression of proteins critical to innate immune responses such as Il6, Cxcl5, Ccl20 and Ptgs2.15–18 These observations suggest that under inflammatory conditions the presence of AHR ligands would mediate an enhanced innate immune response.
Due to the potential for the AHR to modulate intestinal immune function, it is critical to further characterize endogenous molecules that influence receptor activity. A number of microbiota-generated metabolites of tryptophan appear to be a major source of AHR ligands within the GI tract, particularly in the colon. The majority of these AHR ligands are indole derivatives.9 In this report we identify the microbiota-derived hydroxylated quinoline derivative, 2,8-dihydroxyquinoline (2,8-DHQ) as a human AHR ligand. Further structure-activity evaluations determined that the position of the hydroxyl groups are critical determinants of AHR activation potential.
MATERIAL AND METHODS
Materials
TCDD was provided by Dr. Stephen Safe (Texas A&M University). Quinoline derivatives were purchased from Sigma-Aldrich (St. Louis, MO). The purity of 2,8-dihydroquinoline was assessed by NMR and HPLC and was determined to be essentially pure as shown in the supplemental data section. N-[2-(3H-Inol-3-yl)ethyl]-9-isopropyl-2-(5-methyl-3-pyridyl)purin-6-amine (GNF351) and 2-methyl-8-hydroxyquinoline were synthesized as detailed in the supplemental section. Human feces samples were obtained from BioIVT (Hicksville, NY). Fresh cecal contents were collected from C57BL6/J mice obtained from Jackson Laboratories (Bar Harbor, ME) and from germ-free C57BL6/J mice housed in the Penn State Gnotobiotic Animal Research Facility.
Cell culture
The Caco-2 cells and stable DRE-driven reporter lines HepG2 40/6 and H1L1.1c2 (Hepa 1.1) were generated and cultured as previously described.19–21 Primary human hepatocytes were obtained through the Liver Tissue Cell Distribution System (University of Pittsburgh, PA). The isolated hepatocytes were seeded at ~90% confluence in 24-well collagen-coated dishes and cultured as previously described.22
Cell luciferase reporter assays
HepG2 40/6 and Hepa1.1 were each seeded into 12 well plates in complete medium and incubated in a cell culture incubator. The next day cells were treated with AHR ligands and luciferase activity measured after 4 h, as previously described.21
CYP1A1/1B1 activity assay
The P450-Glo™ CYP1A1 Assay (Promega, Madison, WI) was performed in Caco-2 cells as previously described.23
Yeast reporter assays
The human Ah receptor yeast reporter system was performed as previously described.24
Gene expression analysis
RNA was isolated, converted to cDNA, and qRT-PCR performed as previously described.25 Sequences of primers have been previously described.26–28
Cell-based AHR competition binding assay
The AHR photoaffinity ligand (PAL), 2-azido-3-[125I]iodo-7,8-dibromodibenzo-p-dioxin, was synthesized and competition binding assay performed within cells essentially as described previously.29, 30 Briefly, PAL was added directly to either Hepa 1.1 or HepG2 cultured cells, and incubated for 1 h with 10 μM of 2,8-DHQ, or 1.5 μM β-naphthoflavone. The cells were then UV photolyzed (402 nm), lysed, and lysates were subjected to gel electrophoresis, followed by transfer to polyvinylidene difluoride membrane and visualization by autoradiography.
In vitro AHR ligand competition assay
Competitive ligand competition between the PAL and benzo(a)pyrene or 2,8-DHQ were performed using cytosol isolated from AHRTtrCreAlbAhrfx/fx mouse liver as previously described.31
Assessment of dihydroxyquinolines in feces and cecal contents by UPLC-MS/MS
Dihydroxyquinolines were detected by ultra-performance liquid chromatography coupled to a tandem mass spectrometry (UPLC-MS/MS) with a C18 BEH (2.1 × 100 mm, 1.7 μm) column (Waters, Milford, MA) using the methods described previously32, with minor modifications. Briefly, human stool (~ 200 mg) and mouse cecal contents (~ 15 mg) were homogenized with 1 mm zirconium beads using a Bertin Precellys homogenizer, in 700 μL of ice cold 80% methanol containing 1 μM chloropropamide (internal standard). The samples were then centrifuged at 14,000 × g for 15 min at 4℃, and supernatants were collected for drying prior to reconstitution with 80% methanol to a final volume of 80 μL for UPLC-MS/MS.
Data Analysis
Statistical analysis was performed using one-way analysis of variance with Tukey’s multiple comparison post-test using Prism (version 6) software (GraphPad Software, Inc., San Diego, CA). Data points and error bars represent the mean +/− S.E. of three independent biological determinations. Significance is expressed either * (P < 0.05), ** (P < 0.01), or *** (P < 0.001). Alphabetical characters signify statistical comparisons between two groups. All experiments were performed at least two times.
RESULTS
2,8-DHQ activates the human AHR
The capacity for the gut microbiota to generate AHR ligands either through metabolism of dietary chemicals or de novo biosynthesis is an area of keen interest. A number of chemicals have been identified through metabolomics approaches that require the presence of bacteria in the GI tract.7 2,8-DHQ has been previously identified in stool, and at 10 μM induces AHR transcriptional activity in human HepG2 40/6 cells, a cell line with a stably integrated DRE-driven luciferase reporter vector, to a similar extent as the prototypical AHR agonist TCDD (10 nM) (Figure 1). In contrast, 10 μM 2,8-DHQ failed to induce significant AHR transcriptional activity in a DRE-driven reporter mouse Hepa 1.1 cell line. However, at higher doses a significant level of AHR activation was observed in Hepa 1.1 cells (Figure S1). TCDD and indirubin (IR) were used as full agonist controls. Differentially substituted dihydroquinolines, specifically 2,4-DHQ, 2,6-DHQ and 4,8-DHQ failed to induce significant activity in the HepG2 40/6 cell line at 10 μM. Interestingly, 4,8-DHQ was the only dihydroquinoline to induce significant AHR mediated transcriptional activity at 10 μM in Hepa 1.1 cells (Figure 1). Monosubstituted hydroxyquinolines, 2-HQ or 8-HQ displayed differential capacity to induce AHR activity compared to 2,8-DHQ in each reporter cell line. 2-HQ induced a significant level of AHR activity in both reporter cell lines, while 8-HQ failed to increase AHR transcriptional activity at 10 μM (Figure 1). However, the presence of two hydroxyl groups leads to enhanced AHR transcriptional activity relative to the mono-hydroxylated quinolones in the human reporter cell line. To evaluate if the 2-hydroxyl functional group was a critical determinant of 2,8-DHQ activity, 2-methyl-8-hydroxyquinoline was tested in human reporter cells. 2-methyl-8-hydroxyquinoline failed to activate the AHR in HepG2 40/6 cells, thus underscoring the importance of the 2-hydroxy group in AHR activation potential of 2,8-DHQ (Fig. S2). It is possible that slight variances in structure among dihydroquinolines could potentiate antagonistic rather than agonistic activity upon the AHR. Therefore, HepG2 40/6 cells were treated with various DHQ analogues in the presence or absence of TCDD. Notably, all tested DHQs failed to exhibit antagonist activity (Fig. S3).
Figure 1.

Examination of DHQ and HQ as AHR agonist in mouse and human hepatoma cell lines. The DRE-driven reporter cell lines Hepa 1.1 and HepG2 40/6 were treated with various compounds for 4 h and luciferase activity was assessed in cellular extracts. IR is the abbreviation for indirubin.
2,8-DHQ induces AHR target gene expression
Caco2 cells were treated with increasing concentrations of 2,8-DHQ or 10 nM TCDD and the relative levels of CYP1A1 and CYP1B1 mRNA were determined (Figure 2A). Exposure to 100 nM 2,8-DHQ led to a significant induction of CYP1A1 mRNA levels. To further underscore that the increase in CYP1A1 was AHR-dependent, Caco2 cells were co-treated with the AHR antagonist GNF-351, which effectively blocked TCDD- or 2,8-DHQ mediated CYP1A1 expression (Figure 2B). Treatment of primary human hepatocytes with 2,8-DHQ also resulted in significant induction of CYP1A1 gene expression (Figure S4). These results demonstrate that 2,8-DHQ exhibits agonistic AHR activity in a variety of human cell types. To illustrate that 2,8-DHQ activation of the AHR facilitates functional alteration of cellular metabolism, effects upon CYP1A1/1B1 enzymatic activity were measured. 2,8-DHQ significantly increased CYP1A1/CYP1B1 enzyme function in HepG2 40/6 cells at concentrations of 1μM or above but did not increase corresponding activity in Hepa 1.1 cells (Figure 2C).
Figure 2.
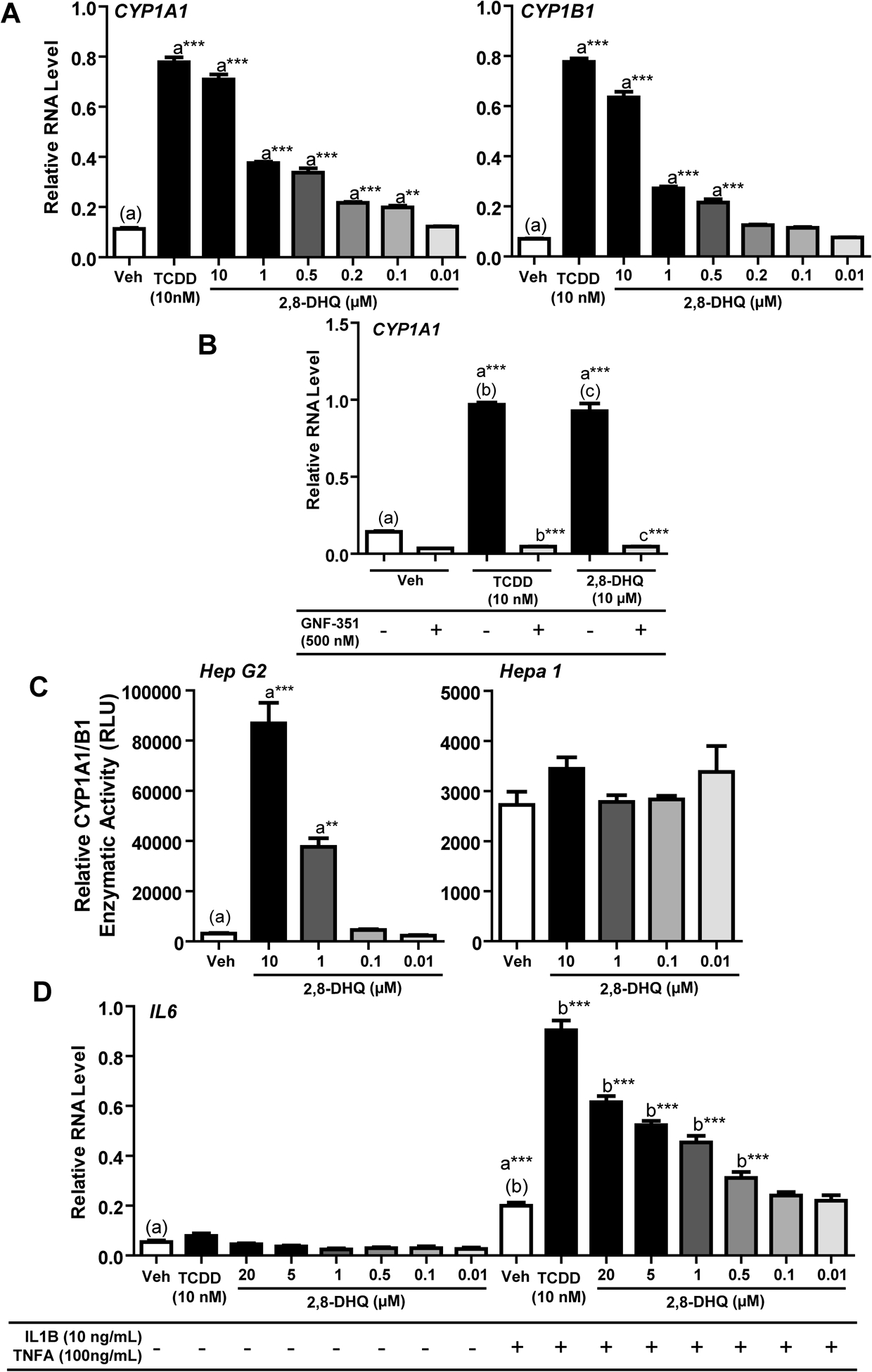
2,8-DHQ induces CYP1A1 and CYP1B1 expression and synergistically induces IL6 upon co-treatment with a cytokine in Caco2 cells. (A) Caco2 cells were treated with AHR ligands for 4 h, RNA isolated and the level of specific mRNA quantitated. (B) Caco2 cells were treated for 4 h with TCDD or 2,8-DHQ in the absence or presence of GNF-351. RNA was isolated and CYP1A1 mRNA determined. (C) HepG2 or Hepa 1.1 cells were treated with 2,8-DHQ for 24 h and the level of CYP1A1 activity determined. (D) Caco2 cells were treated as indicated for 4 h and the level of IL6 mRNA was determined.
2,8-DHQ induces combinatorial activation of IL6 in Caco-2 cells
In previous studies, we demonstrated that AHR activation enhances IL6 expression in combination with cytokine treatment.15, 33 Mechanistic studies revealed that AHR/ARNT occupation at dioxin responsive elements led to enhanced acetylation of NFkB, suggesting a epigenetic mode of gene regulation different from classical transcriptional regulation of CYP1A1. Therefore, the ability of 2,8-DHQ to induce IL6 expression in combination with cytokine treatment in Caco2 cells was examined. The combination of TCDD and cytokine treatment led to a greater than 3-fold increase in IL6 mRNA levels after 4 h (Figure 2D). A statistically significant increased level of IL6 expression was observed after co-treatment with 500 nM 2,8-DHQ. Thus, 2,8-DHQ is capable of contributing to combinatorial regulation of IL6 in a manner similar to TCDD.
2,8-DHQ exhibits additive AHR activation potential with other bacterially generated AHR ligands
We and others have identified a number of AHR ligands that are produced by the gut microbiota.9 One of the assumptions often made is that a mixture of full AHR agonists at relatively low concentrations would result in activity that is the sum of the activity exhibited by each individual compound. To test this assumption we examined the AHR transcriptional activity of 2-oxindole, 3-methyl indole, and 2,8-DHQ individually and in combination. HepG2 40/6 reporter cells were treated with 1 μM 2,8-DHQ and either 2 μM oxindole or 3-methyl indole yielding additive levels of activity, compared to treatment with each compound individually (Figure S5).
2,8-DHQ is a direct human AHR ligand
The ability of 2,8-DHQ to bind directly to the ligand-binding pocket was tested using in vitro PAL competition assays. Because the human AHR has lower affinity for [3H]-dioxin, it is difficult to perform reversible ligand competition experiments that are traditionally performed with rodent AHR (e.g., dextran/charcoal binding assays). Therefore, we utilized the PAL, which upon exposure to UV light, covalently binds to the AHR capturing the ligand binding status at equilibrium. The ability of the known AHR ligand β-naphthoflavone and 2,8-DHQ to compete with the PAL in cultured Hepa 1 and HepG2 was tested (Figure 3A). In Hepa 1 cells 10 μM 2,8-DHQ failed to compete with the PAL, while this compound effectively competed with the PAL in HepG2 cells. The ability of 2,8-DHQ to compete with the PAL was further tested using hepatic cytosol from mice expressing the human AHR.31 A relatively weak level of competition was detected with 2,8,-DHQ compared to benzo(a)pyrene (Figure 3B). To further examine the ability of 2,8-DHQ to directly activate the human AHR a yeast DRE-driven reporter system was assessed (Figure 3C). Interestingly, 2,8 DHQ displayed a potency orders of magnitude lower than β-naphthoflavone in this test system.
Figure 3.

2,8-DHQ is a human AHR ligand. (A) Hepa 1.1 and HepG2 cells were co-treated with BNF or 2,8-DHQ in the presence of PAL for 1 h, exposed to UV light, total cell extracts were isolated and subjected to SDS-PAGE. The level of radioactivity in the AHR band was quantitated. (B) Liver cytosol from a transgenic mouse that expresses the human AHR and the PAL was used in an in vitro AHR ligand competition assay. (C) Dose-response activation of the human AHR by BNF and 2,8-DHQ in a yeast reporter assay system.
2,8-DHQ is present in human feces
Human fecal samples were analyzed by UPLC-MS/MS for the presence of DHQ based on parent ion, MS-MS and HPLC retention time. The presence of 2,6-, 2,4-, 2,8- and 4,8-DHQ was examined in human feces (Figure 4A). However, 2,8- and 4,8-DHQ were at the highest concentrations (Figure 4B and Figure S6). The actual levels of 2,8-DHQ varied from undetected to 3.4 pmol/mg in individual fecal samples (Figure 4B).
Figure 4.
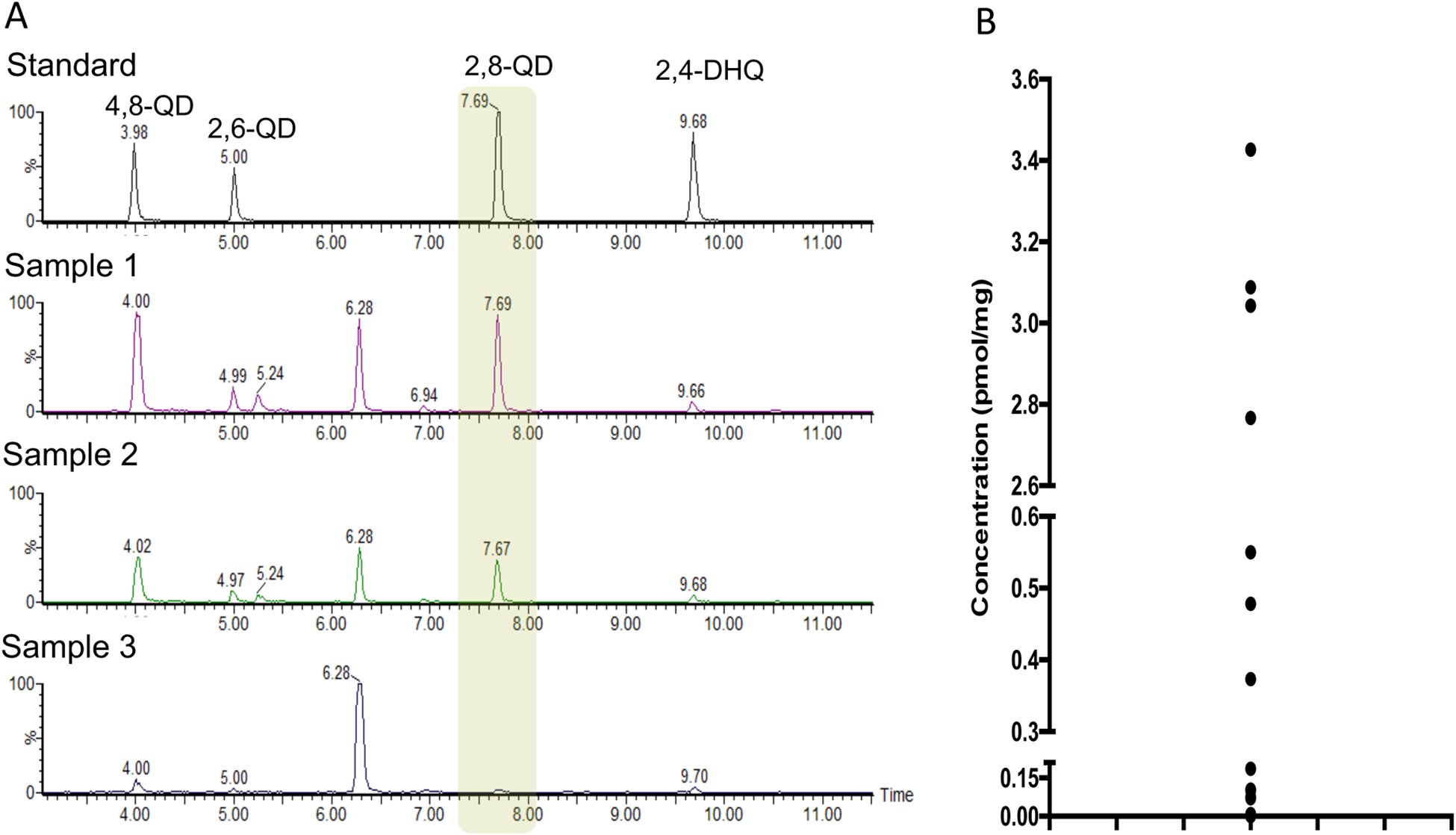
Several dihydroxyquinolines were detected in human feces. (A) Representative UPLC-MS/MS chromatographic assessment of the presence of four dihydroxyquinolines compared to authentic standards. (B) The level of 2,8-DHQ in individual human feces samples.
2,8-DHQ is a microbiota generated metabolite
Cecal contents were isolated from germ-free and pathogen-free C57BL6/J mice and the level of several dihydroquinolines were quantified using UPLC-MS/MS. Three dihydroquinolines, 2,4-, 2,8- and 2,6-DHQ were examined and all were essentially not detected in germ-free mice (Figure 5). In contrast, each dihydroquinoline examined was present in pathogen-free mouse cecal contents. The mean concentration of 2,8-DHQ detected was ~3 pmol/mg, similar to the level observed in human feces.
Figure 5.

Several dihydroquinolines are produced by the microbiota in mice. The concentration of dihydroxyquinolines in extracts from cecal contents of germ-free and pathogen free mice was examined by UPLC-MS/MS. LOQ = Level of Quantification.
DISCUSSION
Bacteria are known to influence mammalian physiology through the production of signaling components that modulate mammalian receptor systems (e.g., short-chain fatty acids and lipopolysaccharides).34, 35 There is now considerable evidence that the AHR is not only a sensor of environmental contaminants, but also a sensor for the presence of microbes on or within the host. For example, naturally occurring yeast found on human skin are capable of producing seven potent AHR ligands from tryptophan.36 Furthermore, activation of the AHR can work in concert with pattern recognition receptors to mount an innate immune response.37 Thus, the AHR has evolved to maintain host-microbiome homeostasis in epithelial tissues and contribute to the mounting of the innate immune response upon pathogenic infection. The results presented here establish that 2,8-DHQ can induce expression of CYP1A1/1B1 and thus likely contribute to xenobiotic metabolism. Whether 2,8-DHQ and indeed other quinolines are metabolized by CYP1A1/1B1 should be examined. Additionally, 2,8-DHQ in combination with cytokine exposure induces IL6 expression, this is consistent with our previous studies that AHR ligands can activate IL6 expression upon combinatoral treatment15, 33. These results illustrate the proposed role of the AHR in the innate immune response to invasive bacteria and demonstrate that 2,8-DHQ through the AHR can contribute to the regulation of the AHR gene battery.
If AHR “sensing” of microbiota-derived molecules is important to an innate immune response, it would likely be evolutionarily conserved. Notably, a divergence of AHR ligand selectivity has occurred over the course of mammalian evolution. We have previously shown that the human AHR possesses a conserved V381 residue instead of an A381 observed in other primates, including Neanderthals.30 Amino acid residue 381 is located in the middle of the ligand-binding pocket and this change in the human AHR leads to a dramatic decrease in affinity for polycyclic aromatic hydrocarbons (e.g. benzo(a)pyrene, 2,3,7,8-tetrachlorodibenzofuran), compared to the Neanderthal AHR. However, primate, Neanderthal, and human AHR sensitivity to tryptophan metabolites produced by the microbiota or the host (e.g., indole, indirubin) was conserved and not influenced by this evolutionary change in the AHR ligand binding domain.30 This class of endogenous ligands exhibit greater activation potential for primate AHR compared with the rodent AHR.9, 31, 38 This would suggest that conserved binding of this latter class of ligands is important from a physiological perspective.
A gradient of AHR and CYP1A1 is observed in the small intestines of rodents with decreasing levels observed from the duodenum to the distal ileum, however the functional significance of this gradient is not yet known.39 Mice genetically altered to constitutively express elevated CYP1A1 in the intestinal tract facilitates diminished AHR transcriptional activity, which is likely associated with increased capacity to metabolize and deplete levels of endogenous or dietary AHR ligands.40 In addition, this study revealed that constitutive intestinal CYP1A1 activity leads to reduced numbers of AHR-dependent type 3 innate lymphoid cells (ILC), similar to what has been observed in AHR null mice.41 ILCs are predominantly located at mucosal tissues and implicated in numerous functions, such as controlling pathogenic infection, progression of autoimmune disease, and carcinogenesis. Selective genetic ablation of ARNT expression from enterocytes leads to constitutive activation of the AHR in distal tissues, suggesting AHR activation in enterocytes serves as a means to control systemic circulation of endogenous and dietary AHR ligands.42 The direct effects of AHR activation on enterocyte populations, beyond activation of xenobiotic metabolism are poorly understood. Although, studies have revealed that TCDD can inhibit development of cultured mouse intestinal organoids.43
The GI tract is a significant source of AHR ligands with several distinct origins; host, dietary and microbial metabolites. For example, the AHR ligands, kynurenic acid and kynurenine, are both derived from mammalian tryptophan metabolism through the indoleamine-2,3-dioxygenase pathways. Raw cruciferous vegetables, such as broccoli and cabbage, are important sources of dietary AHR ligands, such as indolo[3,2b]carbazole.44 It has been recently shown that mouse diets containing 15% broccoli lead to a significant increase in AHR activation throughout the intestinal tract and that such AHR activation is a contributing factor to the attenuation of dextran sodium sulfate mediated intestinal distress.45 These results would suggest that the presence of AHR ligands, at an appropriate concentration, can protect the gut from a toxic insult. Notably, increased levels of 2,8-DHQ were detected in the urine of rats fed a diet containing corn, suggesting endogenous levels of 2,8-DHQ may also have a dietary component.46 However, it has not been confirmed if a similar effect is observed in humans.
Fecal bacteria are capable of forming AHR ligands from tryptophan.44 More recently, it has been shown that microbial tryptophan metabolites, indole, 3-methyl indole and 2-oxindole exhibit greater activation potential for the human AHR compared with the mouse AHR.9 A combination of microbial and mammalian metabolism of indoles leads to the production of indigo and indirubin, which are potent AHR ligands. Endogenous AHR ligand production in the G.I. tract appears to be dependent on the types of bacteria present; a recent report has established that Lactobacillus bulgaricus OLL1181 bacteria and other lactic acid bacteria produce AHR ligands.47 In addition, studies have revealed that intestinal microbial metabolism of tryptophan generated indole-3-aldehyde, which contributes to IL22 production through AHR activation.48 Colonization by this particular bacteria strain was able to ameliorate DSS mediated gut dysfunction, although it was not determined if these protective effects were directly due to the presence of AHR ligands. These results support the concept that the intestinal microbiota may metabolize indole-based compounds, such as indole-3-carbinol and tryptophan, to a variety of AHR ligands.
Tryptophan is metabolized by bacteria to substituted quinolines, many of these products are quorum sensing molecules.49 Production of 2,4-dihydroxyquinoline by Pseudomonas aeruginosa has been shown to be important in maintaining pathogenicity in infection models.50 The precursor of 2,8-dihydroxyquinoline formation in the gut has not been established. However, the quinoline based AHR ligands kynurenic acid and xanthurenic acid can be formed by enzymes isolated from E. coli.51 Furthermore, increasing amounts of kynurenic acid are found in the small intestine as you move towards the distal ileum, consistent with increasing bacterial population numbers and production of these metabolites.52 With these results in mind, it is plausible that 2,8-DHQ is a product of bacterial tryptophan metabolism. A number of published studies have detected the presence of 2,8-DHQ in mouse feces and found fecal levels can be modulated by a combination of factors such as diet, PPARα expression, tempol and dehydrodisoeugenol exposure.53–56 Thus, the level of 2,8-DHQ in humans is likely to significantly vary among individuals. The presence of 2,8-DHQ would contribute to the overall pool of bacterially generated AHR ligands (e.g. kynurenic acid, 3-methyl indole, 2-oxindole) in the gastrointestinal tract. Just how important the production of 2,8-DHQ is to the total pool of AHR ligands in the cecum is currently under investigation.
The positions of the hydroxyl groups are a critical determinant for potent activation of the AHR by dihydroxyquinolines. This suggests that optimal positioning of hydroxyl groups and corresponding electrostatic interactions within the ligand binding pocket are necessary for receptor activation. Interestingly, 2-hydroxyquinoline is capable of activating the human AHR. In contrast, if the second hydroxyl group is in the 4 or 6 position instead of the 8 position of DHQ, agonist activity is suppressed. In vitro competition with the photoaffinity ligand suggests that 2,8-DHQ is able to directly bind the AHR ligand-binding domain and act as a weak ligand, however, this was not consistent with its activation potential in cultured cells. In contrast, 2,8-DHQ effectively competes with the photoaffinity ligand in cultured cells, suggesting that it is indeed a direct ligand for the AHR. The likely reason for the differing results in these assays is that 2,8-DHQ has greater water solubility compared to the competing hydrophobic radioligand, thus the latter would exhibit very high affinity at relatively dilute protein concentrations in the in vitro assay, which has been observed previously.29, 57 Although it is still possible that cellular metabolism may alter the structure of the parent compound to enhance its activation potential. The kinetics of ligand binding in vivo may differ from what is observed in vitro due to the temperature of the assay or the different physical properties of the ligand within a cell. To further explore this difference, we examined the activation potential of 2,8-DHQ in a yeast reporter system that expresses the human AHR, and found that 2,8-DHQ was a very weak inducer of the AHR. This result may indicate that activation potential of 2,8-DHQ is context specific by factors that remain to be determined.
CONCLUSIONS
This study establishes that 2,8-DHQ is a human AHR ligand that would contribute to the total pool of compounds that can regulate AHR activation within the gastrointestinal tract. This compound is yet another example of an AHR ligand generated by the microbiota that preferentially activates the human AHR relative to the mouse AHR. This further reinforces the concept that the human AHR has evolved to sense and respond to microbiota-generated tryptophan metabolites.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. Curt Omiecinski and Denise Coslo for advice and technical assistance with the procurement and maintenance of primary human hepatocyte cultures from the Liver Tissue Cell Distribution System at the University of Pittsburgh, Pittsburg, PA, which is funded by National Institutes of Health Contract HHSN276201200017C. We thank Dr. Michael Denison for providing Hepa 1.1 cells. We also thank Marcia H. Perdew for excellent editorial assistance. This work was supported by the National Institutes of Health Grants ES004869 (GHP), ES028244 (GHP), ES028288 (ADP) and ES026684 (ADP). This work was supported by the USDA National Institute of Food and Federal Appropriations under Project PEN04607 and Accession number 1009993.
Footnotes
Supporting information
The Supporting Information is available free of charge on the ACS Publications website: https://na01.safelinks.protection.outlook.com/?url=http%3A%2F%2Fpubs.acs.org&data=02%7C01%7Cghp2%40psu.edu%7C2a377ca12b2d45905d8f08d68ceba931%7C7cf48d453ddb4389a9c1c115526eb52e%7C0%7C0%7C636851341129033323&sdata=D49tzpVEYlvSStGXB0sB5xTVdFp9k3m9ZlGBzzjIzq8%3D&reserved=0
Supporting Methods and Results; purity and identification information; Figures S1-S6; Supporting Reference (PDF)
The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.de Vos WM; de Vos EA, Role of the intestinal microbiome in health and disease: from correlation to causation. Nutr Rev 2012, 70 Suppl 1, S45–56. [DOI] [PubMed] [Google Scholar]
- 2.Everard A; Belzer C; Geurts L; Ouwerkerk JP; Druart C; Bindels LB; Guiot Y; Derrien M; Muccioli GG; Delzenne NM; de Vos WM; Cani PD, Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A 2013, 110, (22), 9066–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Vos WM, Microbe Profile: Akkermansia muciniphila: a conserved intestinal symbiont that acts as the gatekeeper of our mucosa. Microbiology 2017, 163, (5), 646–648. [DOI] [PubMed] [Google Scholar]
- 4.Sarma-Rupavtarm RB; Ge Z; Schauer DB; Fox JG; Polz MF, Spatial distribution and stability of the eight microbial species of the altered schaedler flora in the mouse gastrointestinal tract. Appl Environ Microbiol 2004, 70, (5), 2791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wymore Brand M; Wannemuehler MJ; Phillips GJ; Proctor A; Overstreet AM; Jergens AE; Orcutt RP; Fox JG, The Altered Schaedler Flora: Continued Applications of a Defined Murine Microbial Community. ILAR J 2015, 56, (2), 169–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vernocchi P; Del Chierico F; Putignani L, Gut Microbiota Profiling: Metabolomics Based Approach to Unravel Compounds Affecting Human Health. Front Microbiol 2016, 7, 1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wikoff WR; Anfora AT; Liu J; Schultz PG; Lesley SA; Peters EC; Siuzdak G, Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci U S A 2009, 106, (10), 3698–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beischlag TV; Luis Morales J; Hollingshead BD; Perdew GH, The aryl hydrocarbon receptor complex and the control of gene expression. Crit Rev Eukaryot Gene Expr 2008, 18, (3), 207–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hubbard TD; Murray IA; Perdew GH, Indole and Tryptophan Metabolism: Endogenous and Dietary Routes to Ah Receptor Activation. Drug Metab Dispos 2015, 43, (10), 1522–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peters JM; Narotsky MG; Elizondo G; Fernandez-Salguero PM; Gonzalez FJ; Abbott BD, Amelioration of TCDD-induced teratogenesis in aryl hydrocarbon receptor (AhR)-null mice. Toxicol Sci 1999, 47, (1), 86–92. [DOI] [PubMed] [Google Scholar]
- 11.Bunger MK; Glover E; Moran SM; Walisser JA; Lahvis GP; Hsu EL; Bradfield CA, Abnormal liver development and resistance to 2,3,7,8-tetrachlorodibenzo-p-dioxin toxicity in mice carrying a mutation in the DNA-binding domain of the aryl hydrocarbon receptor. Toxicol Sci 2008, 106, (1), 83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walisser JA; Bunger MK; Glover E; Harstad EB; Bradfield CA, Patent ductus venosus and dioxin resistance in mice harboring a hypomorphic Arnt allele. J Biol Chem 2004, 279, (16), 16326–31. [DOI] [PubMed] [Google Scholar]
- 13.Stockinger B; Di Meglio P; Gialitakis M; Duarte JH, The aryl hydrocarbon receptor: multitasking in the immune system. Annu Rev Immunol 2014, 32, 403–32. [DOI] [PubMed] [Google Scholar]
- 14.Nikoopour E; Bellemore SM; Singh B, IL-22, cell regeneration and autoimmunity. Cytokine 2015, 74, (1), 35–42. [DOI] [PubMed] [Google Scholar]
- 15.DiNatale BC; Schroeder JC; Francey LJ; Kusnadi A; Perdew GH, Mechanistic insights into the events that lead to synergistic induction of interleukin 6 transcription upon activation of the aryl hydrocarbon receptor and inflammatory signaling. J Biol Chem 2010, 285, (32), 24388–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith KJ; Boyer JA; Muku GE; Murray IA; Gowda K; Desai D; Amin SG; Glick AB; Perdew GH, Editor’s Highlight: Ah Receptor Activation Potentiates Neutrophil Chemoattractant (C-X-C Motif) Ligand 5 Expression in Keratinocytes and Skin. Toxicol Sci 2017, 160, (1), 83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lahoti TS; Boyer JA; Kusnadi A; Muku GE; Murray IA; Perdew GH, Aryl Hydrocarbon Receptor Activation Synergistically Induces Lipopolysaccharide-Mediated Expression of Proinflammatory Chemokine (c-c motif) Ligand 20. Toxicol Sci 2015, 148, (1), 229–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Degner SC; Kemp MQ; Hockings JK; Romagnolo DF, Cyclooxygenase-2 promoter activation by the aromatic hydrocarbon receptor in breast cancer mcf-7 cells: repressive effects of conjugated linoleic acid. Nutr Cancer 2007, 59, (2), 248–57. [DOI] [PubMed] [Google Scholar]
- 19.Long WP; Pray-Grant M; Tsai JC; Perdew GH, Protein kinase C activity is required for aryl hydrocarbon receptor pathway-mediated signal transduction. Mol Pharmacol 1998, 53, (4), 691–700. [DOI] [PubMed] [Google Scholar]
- 20.Garrison PM; Tullis K; Aarts JM; Brouwer A; Giesy JP; Denison MS, Species-specific recombinant cell lines as bioassay systems for the detection of 2,3,7,8-tetrachlorodibenzo-p-dioxin-like chemicals. Fundam Appl Toxicol 1996, 30, (2), 194–203. [DOI] [PubMed] [Google Scholar]
- 21.Smith KJ; Murray IA; Tanos R; Tellew J; Boitano AE; Bisson WH; Kolluri SK; Cooke MP; Perdew GH, Identification of a high-affinity ligand that exhibits complete aryl hydrocarbon receptor antagonism. J Pharmacol Exp Ther 2011, 338, (1), 318–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Girer NG; Murray IA; Omiecinski CJ; Perdew GH, Hepatic Aryl Hydrocarbon Receptor Attenuates Fibroblast Growth Factor 21 Expression. J Biol Chem 2016, 291, (29), 15378–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hubbard TD; Murray IA; Bisson WH; Lahoti TS; Gowda K; Amin SG; Patterson AD; Perdew GH, Adaptation of the human aryl hydrocarbon receptor to sense microbiota-derived indoles. Sci Rep 2015, 5, 12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fox JE; Burow ME; McLachlan JA; Miller CA 3rd, Detecting ligands and dissecting nuclear receptor-signaling pathways using recombinant strains of the yeast Saccharomyces cerevisiae. Nat Protoc 2008, 3, (4), 637–45. [DOI] [PubMed] [Google Scholar]
- 25.Murray IA; Krishnegowda G; DiNatale BC; Flaveny C; Chiaro C; Lin JM; Sharma AK; Amin S; Perdew GH, Development of a selective modulator of aryl hydrocarbon (Ah) receptor activity that exhibits anti-inflammatory properties. Chem Res Toxicol 2010, 23, (5), 955–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murray IA; Flaveny CA; DiNatale BC; Chairo CR; Schroeder JC; Kusnadi A; Perdew GH, Antagonism of aryl hydrocarbon receptor signaling by 6,2’,4’-trimethoxyflavone. J Pharmacol Exp Ther 2010, 332, (1), 135–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith KJ; Murray IA; Boyer JA; Perdew GH, Allelic variants of the aryl hydrocarbon receptor differentially influence UVB-mediated skin inflammatory responses in SKH1 mice. Toxicology 2018, 394, 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dinatale BC; Perdew GH, Ah receptor antagonism inhbits constitutive and cytokine inducible IL6 production in head and neck tumor cell lines. Molecular Carcinogenesis 2011, 50, 173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramadoss P; Perdew GH, Use of 2-azido-3-[125I]iodo-7,8-dibromodibenzo-p-dioxin as a probe to determine the relative ligand affinity of human versus mouse aryl hydrocarbon receptor in cultured cells. Mol Pharmacol 2004, 66, (1), 129–36. [DOI] [PubMed] [Google Scholar]
- 30.Hubbard TD; Murray IA; Bisson WH; Sullivan AP; Sebastian A; Perry GH; Jablonski NG; Perdew GH, Divergent Ah Receptor Ligand Selectivity during Hominin Evolution. Mol Biol Evol 2016, 33, (10), 2648–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flaveny CA; Murray IA; Chiaro CR; Perdew GH, Ligand selectivity and gene regulation by the human aryl hydrocarbon receptor in transgenic mice. Mol Pharmacol 2009, 75, (6), 1412–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang J; Reijmers T; Chen L; Van Der Heijden R; Wang M; Peng S; Hankemeier T; Xu G; Van Der Greef J, Systems toxicology study of doxorubicin on rats using ultra performance liquid chromatography coupled with mass spectrometry based metabolomics. Metabolomics 2009, 5, (4), 407–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hollingshead BD; Beischlag TV; Dinatale BC; Ramadoss P; Perdew GH, Inflammatory signaling and aryl hydrocarbon receptor mediate synergistic induction of interleukin 6 in MCF-7 cells. Cancer Res 2008, 68, (10), 3609–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen LJ; Esterhazy D; Kim SH; Lemetre C; Aguilar RR; Gordon EA; Pickard AJ; Cross JR; Emiliano AB; Han SM; Chu J; Vila-Farres X; Kaplitt J; Rogoz A; Calle PY; Hunter C; Bitok JK; Brady SF, Commensal bacteria make GPCR ligands that mimic human signalling molecules. Nature 2017, 549, (7670), 48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gonzalez FJ; Jiang C; Xie C; Patterson AD, Intestinal Farnesoid X Receptor Signaling Modulates Metabolic Disease. Dig Dis 2017, 35, (3), 178–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mexia N; Gaitanis G; Velegraki A; Soshilov A; Denison MS; Magiatis P, Pityriazepin and other potent AhR ligands isolated from Malassezia furfur yeast. Arch Biochem Biophys 2015, 571, 16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moura-Alves P; Fae K; Houthuys E; Dorhoi A; Kreuchwig A; Furkert J; Barison N; Diehl A; Munder A; Constant P; Skrahina T; Guhlich-Bornhof U; Klemm M; Koehler AB; Bandermann S; Goosmann C; Mollenkopf HJ; Hurwitz R; Brinkmann V; Fillatreau S; Daffe M; Tummler B; Kolbe M; Oschkinat H; Krause G; Kaufmann SH, AhR sensing of bacterial pigments regulates antibacterial defence. Nature 2014, 512, (7515), 387–92. [DOI] [PubMed] [Google Scholar]
- 38.Schroeder JC; Dinatale BC; Murray IA; Flaveny CA; Liu Q; Laurenzana EM; Lin JM; Strom SC; Omiecinski CJ; Amin S; Perdew GH, The uremic toxin 3-indoxyl sulfate is a potent endogenous agonist for the human aryl hydrocarbon receptor. Biochemistry 2010, 49, (2), 393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang QY; Wikoff J; Dunbar D; Fasco M; Kaminsky L, Regulation of cytochrome P4501A1 expression in rat small intestine. Drug Metab Dispos 1997, 25, (1), 21–6. [PubMed] [Google Scholar]
- 40.Schiering C; Wincent E; Metidji A; Iseppon A; Li Y; Potocnik AJ; Omenetti S; Henderson CJ; Wolf CR; Nebert DW; Stockinger B, Feedback control of AHR signalling regulates intestinal immunity. Nature 2017, 542, (7640), 242–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kiss EA; Vonarbourg C; Kopfmann S; Hobeika E; Finke D; Esser C; Diefenbach A, Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science 2011, 334, (6062), 1561–5. [DOI] [PubMed] [Google Scholar]
- 42.Ito S; Chen C; Satoh J; Yim S; Gonzalez FJ, Dietary phytochemicals regulate whole-body CYP1A1 expression through an arylhydrocarbon receptor nuclear translocator-dependent system in gut. J Clin Invest 2007, 117, (7), 1940–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park JH; Choi AJ; Kim SJ; Cheong SW; Jeong SY, AhR activation by 6-formylindolo[3,2-b]carbazole and 2,3,7,8-tetrachlorodibenzo-p-dioxin inhibit the development of mouse intestinal epithelial cells. Environ Toxicol Pharmacol 2016, 43, 44–53. [DOI] [PubMed] [Google Scholar]
- 44.Perdew GH; Babbs CF, Production of Ah receptor ligands in rat fecal suspensions containing tryptophan or indole-3-carbinol. Nutr Cancer 1991, 16, (3–4), 209–18. [DOI] [PubMed] [Google Scholar]
- 45.Hubbard TD; Murray IA; Nichols RG; Cassel K; Podolsky M; Kuzu G; Tian Y; Smith P; Kennett MJ; Patterson AD; Perdew GH, Dietary Broccoli Impacts Microbial Community Structure and Attenuates Chemically Induced Colitis in Mice in an Ah receptor dependent manner. J Funct Foods 2017, 37, 685–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Inagami K; Kaihara M; Price JM, The identification of 2,8-quinolinediol in the urine of rats fed a diet containing corn. J Biol Chem 1965, 240, (9), 3682–4. [PubMed] [Google Scholar]
- 47.Takamura T; Harama D; Fukumoto S; Nakamura Y; Shimokawa N; Ishimaru K; Ikegami S; Makino S; Kitamura M; Nakao A, Lactobacillus bulgaricus OLL1181 activates the aryl hydrocarbon receptor pathway and inhibits colitis. Immunol Cell Biol 2011, 89, (7), 817–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zelante T; Iannitti RG; Cunha C; De Luca A; Giovannini G; Pieraccini G; Zecchi R; D’Angelo C; Massi-Benedetti C; Fallarino F; Carvalho A; Puccetti P; Romani L, Tryptophan Catabolites from Microbiota Engage Aryl Hydrocarbon Receptor and Balance Mucosal Reactivity via Interleukin-22. Immunity 2013, 39, (2), 372–85. [DOI] [PubMed] [Google Scholar]
- 49.Heeb S; Fletcher MP; Chhabra SR; Diggle SP; Williams P; Camara M, Quinolones: from antibiotics to autoinducers. FEMS Microbiol Rev 2011, 35, (2), 247–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gruber JD; Chen W; Parnham S; Beauchesne K; Moeller P; Flume PA; Zhang YM, The role of 2,4-dihydroxyquinoline (DHQ) in Pseudomonas aeruginosa pathogenicity. PeerJ 2016, 4, e1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Han Q; Fang J; Li J, Kynurenine aminotransferase and glutamine transaminase K of Escherichia coli: identity with aspartate aminotransferase. Biochem J 2001, 360, (Pt 3), 617–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuc D; Zgrajka W; Parada-Turska J; Urbanik-Sypniewska T; Turski WA, Micromolar concentration of kynurenic acid in rat small intestine. Amino Acids 2008, 35, (2), 503–5. [DOI] [PubMed] [Google Scholar]
- 53.Fardet A; Llorach R; Martin JF; Besson C; Lyan B; Pujos-Guillot E; Scalbert A, A liquid chromatography-quadrupole time-of-flight (LC-QTOF)-based metabolomic approach reveals new metabolic effects of catechin in rats fed high-fat diets. J Proteome Res 2008, 7, (6), 2388–98. [DOI] [PubMed] [Google Scholar]
- 54.Zhen Y; Krausz KW; Chen C; Idle JR; Gonzalez FJ, Metabolomic and genetic analysis of biomarkers for peroxisome proliferator-activated receptor alpha expression and activation. Mol Endocrinol 2007, 21, (9), 2136–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li F; Pang X; Krausz KW; Jiang C; Chen C; Cook JA; Krishna MC; Mitchell JB; Gonzalez FJ; Patterson AD, Stable isotope- and mass spectrometry-based metabolomics as tools in drug metabolism: a study expanding tempol pharmacology. J Proteome Res 2013, 12, (3), 1369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lv QQ; Yang XN; Yan DM; Liang WQ; Liu HN; Yang XW; Li F, Metabolic profiling of dehydrodiisoeugenol using xenobiotic metabolomics. J Pharm Biomed Anal 2017, 145, 725–733. [DOI] [PubMed] [Google Scholar]
- 57.Bradfield CA; Kende AS; Poland A, Kinetic and equilibrium studies of Ah receptor-ligand binding: use of [125I]2-iodo-7,8-dibromodibenzo-p-dioxin. Mol Pharmacol 1988, 34, (2), 229–37. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


