Abstract
Palladium-catalyzed alkene difunctionalization reactions between alkenes bearing tethered aryl or alkenyl triflates and enolate nucleophiles are described. The transformations form two C–C bonds, a ring, and up to two stereocenters, while producing substituted cyclopentane derivatives that contain appended carbonyl functionality. Products are formed with up to >20:1 diastereoselectivity, and formation of sterically congested bonds between quaternary carbon atoms is feasible.
Keywords: Palladium, Cross-Coupling, Alkene Difunctionalization, Stereoselective
Graphical Abstract

INTRODUCTION
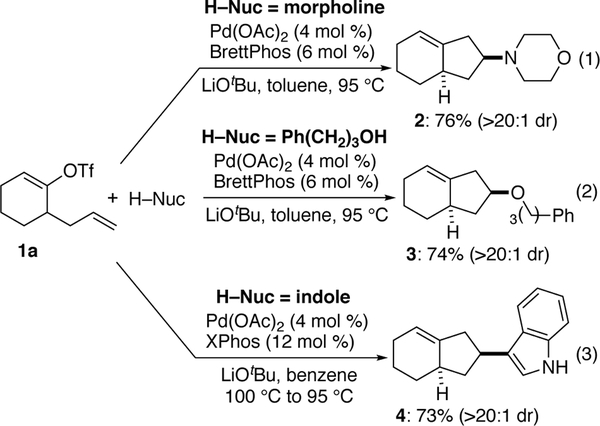
Late-transition metal catalyzed alkene difunctionalization reactions are powerful methods that have demonstrated utility for the construction of a range of heterocycles and carbocycles.1,2 We have recently reported a new class of alkene difunctionalization reactions between alkenes tethered to aryl or alkenyl triflates, and amines,3 alcohols,4 or indole5 nucleophiles. As shown in Scheme 1, these reactions afford substituted cyclopentane derivatives in good yield with high diastereoselectivity. For example, the Pd/BrettPhos-catalyzed coupling of 1a with morpholine afforded 2 in 76% yield and >20:1 dr (Scheme 1, eq 1). Similarly, treatment of 1a with 3-phenylpropanol (Scheme 1, eq 2), or with indole (Scheme 1, eq 3), provided 3 and 4 in good yield and >20:1 diastereoselectivity under appropriate conditions.
Scheme 1. Prior Studies.
In order to further explore the scope of this general class of transformations, we elected to examine the use of stabilized carbanions as nucleophiles for alkene difunctionalization. We were cautiously optimistic about the chances for success at the outset of these studies, as enolates and related compounds have previously been employed as nucleophiles in a range of other Pd-catalyzed reactions, including C-arylation6 and allylic alkylation.7 In addition, cyclopentane derivatives bearing malonate, ester, or ketone groups have previously shown utility as synthetic intermediates.8,9 In this article we describe a set of new alkene difunctionalization reactions of malonates,10 esters, and ketones, that provide substituted cyclopentane derivatives in moderate to good yield, with high levels of diastereoselectivity.
RESULTS AND DISCUSSION
Transformations of malonate nucleophiles
In order to probe the feasibility of alkene difunctionalization reactions involving enolate nucleophiles, we initially examined the coupling of diethyl malonate with 2-allylphenyl triflate 5a. We began our studies using conditions we had previously developed for similar reactions of alcohol or amine nucleophiles, and were pleased to find these conditions provided satisfactory results in this transformation. The desired product 6a was formed in 93% isolated yield on a 0.2 mmol scale, 84% isolated yield on a 1.0 mmol scale, and 88% yield on a 1.5 gram scale (eq 4).
 |
Given the successful outcome of this initial reaction, we elected not to pursue further optimization of conditions, but instead decided to explore the scope of this transformation (Scheme 2). Reactions between diethyl malonate and terminal alkene substrates (5a–d) proceeded in good yield under our standard reaction conditions, and substrate 5d was converted to 6d in >20:1 dr. In contrast, internal alkene substrates 5e–h were less reactive, and suffered from competing base-mediated isomerization of the alkene and/or cleavage of the triflate to the corresponding phenoxide. However, these problems could be alleviated to some extent through use of increased amounts of diethyl malonate (3.6 equiv) and lithium tert-butoxide (2.2 equiv), combined with a lower reaction temperature of 65 °C. Under these modified conditions, products 6e–h were obtained in >20:1 dr, albeit modest isolated yields (33–46%).11 The diminished reactivity of the internal alkene substrates is presumably due to their increased steric bulk, which decreases the facility by which the alkene is bound to palladium. Substrate 5i, which contains a methyl group at the internal alkene carbon was transformed to 6i in only 14% yield due to competing, and somewhat surprising, 5-endo Heck-type cyclization of the substrate.
Scheme 2. Reactions of Aryl Triflates with Malonatesa–c.
aConditions: 1.0 equiv 1, 1.2 equiv diethyl malonate, 1.4 equiv LiOtBu, 4 mol % Pd(OAc)2, 6 mol % BrettPhos, toluene (0.1 M), 95 °C, 16 h. Reactions were conducted on a 0.1–0.25 mmol scale. bDiastereomeric ratios were determined by 1H NMR analysis. Diastereomeric ratios were identical for crude reaction mixtures and isolated compounds unless otherwise noted. cYields are average isolated yields of two or more experiments. dThe reaction was conducted using RuPhos as ligand.
We subsequently turned our attention to cyclic alkenyl triflate substrates, which can be prepared in two steps (Pd-catalyzed enolate allylation followed by formation of the enol triflate) from the corresponding cyclic ketones (Scheme 3). Transformations of cyclic alkenyl triflates bearing pendant terminal alkenes proceeded in good to excellent yield in most cases examined. The reactions were effective with substrates derived from both 6- and 5-membered ring ketones. Products bearing fused heterocyclic rings (7b–c) were formed in good yield with high dr, and the presence of a substituent on the carbon bearing the allyl group (7e, 7g) was tolerated. The reaction between substrate 1f and diethyl 2-allyl malonate provided 7f, with formation of a bond between two quaternary carbon atoms, in 63% yield and 19:1 dr, although use of the smaller RuPhos ligand was needed to obtain acceptable results.
Scheme 3. Reactions of Alkenyl Triflates Bearing Terminal Alkenes with Malonatesa–c.
aConditions: 1.0 equiv 5, 1.2 equiv diethyl malonate, 1.4 equiv LiOtBu, 4 mol % Pd(OAc)2, 6 mol % BrettPhos, toluene (0.1 M), 95 °C, 16 h. Reactions were conducted on a 0.1–0.25 mmol scale. bDiastereomeric ratios were determined by 1H NMR analysis. Diastereomeric ratios were identical for crude reaction mixtures and isolated compounds unless otherwise noted. cYields are average isolated yields of two or more experiments. dThe reaction was conducted using (BrettPhos)Pd(allyl)(Cl) in place of Pd(OAc)2. eThe reaction was conducted using 2.2 equiv of LiOtBu and 3.6 equiv of diethyl malonate, a substrate concentration of 0.8 M, and a reaction temperature of 65 °C.
Efforts to couple alkenyl triflates bearing internal alkenes failed to provide satisfactory results either under these conditions (Scheme 4, entry 1), or under the re-optimized conditions that were modestly effective with aryl triflates. As such, we elected to examine the influence of precatalyst and ligand structure on the reaction between diethyl malonate and 1l. As shown in Scheme 4, use of other biaryl phosphines,12 including CPhos, RuPhos, and SPhos that are less sterically hindered than BrettPhos provided improved results (45–48% yield). However, use of the wide bite angle ligand Dpe-Phos afforded the desired product in 65% yield when LiHMDS was employed as base. We then examined Pd(acac)2 as a precatalyst, as we have previously observed increased efficiency in other transformations when this precatalyst was used.13 The combination of Pd(acac)2 with SPhos provided the best results, affording 7p in 79% yield and >20:1 dr.
Scheme 4. Optimization of the Reaction Between 1l and Diethyl Malonatea.
aConditions: 1.0 equiv 1l, 2.0 equiv diethyl malonate, 4 mol% Pd, 6 mol % ligand, 2.2 equiv LiOtBu, toluene (0.8 M), 95 °C, 14 h. bIsolated yield (average of two or more experiments). cDiastereomeric ratios were determined by 1H NMR analysis. dLiHMDS was used as a base instead of LiOtBu. eThe yield was determined by 1H NMR analysis of the crude reaction mixture using phenanthrene as an internal standard.
We then examined the use of these newly optimized conditions with a number of different internal alkene substrates (Scheme 5). The transformations proceed in moderate to good yield, with generally high (>20:1) diastereoselectivity. A substituent (R ≠ H) on the carbon bearing the tethered alkene was tolerated, and in some instances provided a significant increase in yield. For example, the coupling of 1l (R = CO2Et) with diethyl malonate proceeded to give 7p in 79% yield, whereas the analogous substrate 1m (R = H) was converted to 7q in only 21% yield. Not surprisingly, use of di-tert-butyl malonate as the nucleophile provided results similar to those obtained with diethyl malonate. Transformations involving diethyl 2-benzyl malonate also provided desired products 7j, 7n, and 7s in >20:1 dr, although higher reaction temperatures were required (110 °C), and chemical yields were lower than those obtained with the unsubstituted diethyl malonate nucleophile.
Scheme 5. Reactions of Alkenyl Triflates Bearing Internal Alkenes with Malonatesa–c.
aConditions: 1.0 equiv 1, 3.6 equiv diethyl malonate, 2.2 equiv LiOtBu, 4 mol % Pd(acac)2, 6 mol % SPhos, toluene (0.8 M), 95 °C, 14 h. Reactions were conducted on a 0.2 mmol scale. bDiastereomeric ratios were determined by 1H NMR analysis. Diastereomeric ratios were identical for crude reaction mixtures and isolated compounds unless otherwise noted. cYields are average isolated yields of two or more experiments. dThe reaction was conducted in xylenes solvent at 110 °C.
In prior studies involving amine or alcohol nucleophiles, we found that acyclic alkenyl triflates are also viable substrates in related alkene difunctionalization reactions.3b,4 As such, we briefly examined the reactivity of two acyclic substrates with diethyl malonate. As shown in eq 5, the coupling of 8 with diethyl malonate proceeded smoothly under standard conditions to afford spirocycle 9 in 82% and >20:1 dr. However, when gem-diester substrate 10 was treated with di-tert-butyl malonate under analogous conditions, we obtained a 4:1 mixture of 11:12 in 53% yield (eq 6). A preliminary screen of several other phosphine ligands, including X-Phos, Dpe-Phos, and P(2-furyl)3, provided similar mixtures of 11:12 (ranging from 2:1 to 1:2.5) in good yields (up to 90% for the mixture). Future studies will be directed towards improving the regioselectivity in these transformations.
 |
In addition to symmetrical malonate esters, these transformations are also effective with other stabilized carbanion nucleophiles. For example, the reaction between 1a and ethyl acetoacetate afforded 13a in 83% yield as a 1:1 mixture of stereoisomers epimeric at the stereocenter adjacent to the carbonyl group (Scheme 6, eq 7). Use of triethyl phosphonoacetate as the nucleophile, combined with LiHMDS as base, provided 13b in 55% yield on a 1.85 mmol scale (Scheme 6, eq 8). However, reactions involving other activated nucleophiles were more challenging. Ethyl cyanoacetate was much less reactive than diethyl malonate, and only small amounts of desired product were formed under standard conditions. Nonetheless, after some experimentation we were able to produce 13c in 50% yield by using (BrettPhos)Pd(allyl)Cl as the precatalyst14 along with Cs2CO3 as base (Scheme 6, eq 9). These conditions were also effective for the coupling of 2-allylphenyl triflate 5a with ethyl cyanoacetate to afford 14a in 47% yield (Scheme 6, eq 10). Efforts to employ 2,4-pentanedione (acac) as the nucleophile provided only trace amounts of product, possibly due to inhibition of catalysis by acac acting as a ligand for Pd. In contrast, 5a was successfully coupled with 2-acetyltetralone under modified conditions in which an excess (2.6 equiv) of LiHMDS was employed as base. In this case the alkylation occurred on the methyl group rather than the carbon between the carbonyls, presumably via the dianion derived from 2-acetyltetralone, to afford 14b (Scheme 6, eq 11) in 40% yield.15 The modest yield results from competing arylation6 of the methyl ketone with 2-allylphenyl triflate, rather than alkene difunctionalization. Although these other nucleophiles proved to be viable with terminal alkene substrates, efforts to couple them with internal alkene derivatives have thus far been unsuccessful.
Scheme 6. Reactions of Other Doubly Activated Nucleophiles a–c.
Transformations of ketone or ester nucleophiles
Given the successful transformations of malonates and related nucleophiles, we sought to further expand the scope of this method by employing other carbonyl-containing nucleophiles. As such, we surveyed the reactivity of a few aryl and alkenyl triflate substrates towards several different esters. Efforts to employ acetate esters gave poor results, as represented by the formation of 15a in only 24% yield. The main side reaction in this case is identical to that observed with 2-acetyltetralone – competing α-arylation of the ester with the aryl triflate substrate. In contrast, much better results were obtained with more highly substituted esters. For example, the sterically hindered methyl isobutyrate was coupled with terminal alkene substrates 5a and 1a to afford 15b and 15g in high yield. This hindered ester was also successfully coupled with substrates bearing a methyl group on the internal alkene carbon (1d, 1f, 5i, and 5j) when RuPhos was used as the ligand for Pd. These reactions afforded 15h, 15i, 15d, and 15c in 52%, 50%, 24%, and 23% yield, respectively. Although the yields in these particular transformations are modest, the reactions do affect the formation of a C–C bond between two quaternary carbon atoms, and products 15h and 15i16 were formed with high diastereoselectivity (11:1 dr and >20:1 dr, respectively). Acyclic alkenyl triflate 16 was also successfully coupled with methyl isobutyrate using the Pd(OAc)2/RuPhos catalyst to afford 17 in 52% yield and >20:1 dr (eq 10). Reactions between 1a or 5a and methyl 2-phenyl acetate afforded 15e and 15f in moderate to good yield, but 15f was obtained as a 2:1 mixture of diastereomers epimeric at the stereocenter adjacent to the ester.
 |
The coupling of 1a or 5a with several different ketones also proceeded smoothly, and this series of reactions exhibited the same general trends that were observed with ester nucleophiles. Low to moderate yields were obtained with methyl ketones (18a; 24% and 18h; 52%), and in the case of 2,2-diphenyl acetone, reaction selectively occurred at the less hindered carbon, rather than through the more highly substituted, thermodynamic enolate. However, reactions of several other ketones, including α-phenylacetophenone, α-tetralone, 6-methoxy-α-tetralone, and cyclohexanone, proceeded smoothly. In all cases examined, products were generated with essentially complete control of relative stereochemistry around the bicyclic ring system. Products 18i, 18j, and 18k, which contain a base-epimerizable stereocenter, were formed as essentially 1:1 mixtures of diastereomers epimeric at the carbonyl-bearing stereocenter. However, the reaction of 2-methyl-α-tetralone to produce 18l, which lacks a base-epimerizable proton, also proceeded with 1:1 dr. Interestingly, cyclohexanone underwent selective monoalkylation (using 2.6 equiv of cyclohexanone) to provide 18c and 18i; bis-alkylation products were not observed. This is likely due to the use of excess cyclohexanone, along with the fact that the thermodynamic enolates derived from products 18c and 18i are sterically encumbered. These hindered enolates should react much more slowly than the enolate of cyclohexanone itself, and the less-substituted “kinetic” enolates of the monoalkylated cyclohexanone products should be present in relatively low concentration under these conditions.
Mechanism and Stereochemical Model
Based on the stereochemical outcome of transformations involving internal alkenes, in which products result from anti-addition of the nucleophile and the aryl or alkenyl group to the pendant alkene, the mechanism of these transformations is probably similar to those of related reactions of amine, alcohol, or indole nucleophiles.3,4,5 As illustrated in Scheme 9, the reactions are likely initiated by oxidative addition of the aryl or alkenyl triflate to a Pd(0) complex generated by ligation and reduction of the Pd(II) precatalyst. After oxidative addition, the alkene in intermediate 19 is poised to bind to the Pd(II) center, which activates it for attack by the exogenous nucleophile in intermediate 20. This intermediate then undergoes anti-carbopalladation to provide 21, which is transformed to the desired product with regeneration of the Pd(0) catalyst through reductive elimination. The observed major product stereochemistry is consistent with carbopalladation through a chair-like conformation in which non-bonding interactions are minimized.
Scheme 9. Mechanism and Relative Stereochemistry.
Although control of relative stereochemistry of the substituents on the bicyclic ring system is generally quite high, we currently are unable to control the relative stereochemistry of stereocenters adjacent to the carbonyl of the nucleophilic component. For products such as 15f and 18i–k, which contain an acidic proton at the carbonyl α-stereocenter, the poor stereocontrol is easily ascribed to epimerization under the strongly basic reaction conditions. However, the poor selectivity in the formation of 18l, which does not contain an epimerizable α-stereocenter, reveals a second problem in stereocontrol. In this case the 1:1 dr must result from inherently low relative face selectivity when the enolate approaches intermediate 20 (Scheme 9).
SUMMARY AND CONCLUSION
In conclusion, we have developed a series of new alkene difunctionalization reactions between alkenes bearing pendant aryl or alkenyl triflates, and enolate nucleophiles derived from esters, ketones, and malonates. Reactions of terminal alkene substrates are reasonably general, and transformations involving internal alkenes provide moderate yields when malonate nucleophiles are used. The reactions generate a ring and two C–C bonds, and provide products that contain up to three stereocenters with high diastereoselectivity in most cases examined. The product stereochemistry implicates a mechanism involving anti-carbopalladation of the alkene, which occurs with high diastereoselectivity. The transformations afford several different fused-bicyclic products, including heterocycles, although reactions that generate simple monocyclic cyclopentane derivatives are currently limited in scope due to the formation of regioisomeric cyclobutene products. Future studies will be directed towards stereocontrol in reactions of prochiral enolates, regiocontrol in reactions of acyclic alkenyl triflates, and investigation of other carbon-centered nucleophiles.
EXPERIMENTAL SECTION
General Considerations: All reactions were carried out under a nitrogen atmosphere in flame-dried glassware. All reagents, palladium precatalysts, and ligands were purchased from commercial sources and were used without purification unless otherwise noted. The substrates 1a–e,3b 1f,4 1g,4 5a,3a 5c,3a 5d,3b 8,3b 10,3b 16,3b (Brettphos)Pd(allyl)(Cl),14 N-(2-pyridyl)triflimide,17 4-tert-butyl-2-allylphenol,18 and ally 1-methyl-2-oxocyclohexane-1-carboxylate19 were prepared by previously published methods. Alkenyl triflate starting materials were stored in a freezer under nitrogen. Bulk quantities of cesium carbonate, lithium tert-butoxide, and lithium hexamethyldisilazide were stored in nitrogen-filled glove box, and small amounts were removed and used within a few days, during which time they were stored in a desiccator. Toluene, THF, dichloromethane, and diethyl ether were purified using a GlassContour solvent purification system. Anhydrous dioxane was purchased from Sigma-Aldrich and was used without purification. Structural and stereochemical assignments were made on the basis of 2-D COSY and NOESY experiments. Ratios of diastereomers were determined by 1H NMR analysis. Yields refer to isolated yields of compounds estimated to be ≥95% pure as determined by 1H NMR analysis unless otherwise noted. The yields reported in the experimental section describe the result of a single experiment, whereas yields reported in Schemes 2–8 and equations 4–10 are average yields of two or more experiments. Thus, the yields reported in the experimental section may differ from those shown in Schemes 2–8 and equations 4–10.
Scheme 8. Reactions of Ketone Nucleophilesa.
aConditions: 1.0 equiv triflate substrate, 1.2 equiv ketone, 1.4 equiv LiHMDS, 4 mol % Pd(OAc)2, 6 mol % BrettPhos, toluene (0.1 M), 95 °C, 16 h. Reactions were conducted on a 0.1–0.25 mmol scale. bDiastereomeric ratios were determined by 1H NMR analysis. Diastereomeric ratios were identical for crude reaction mixtures and isolated compounds unless otherwise noted. cYields are average isolated yields of two or more experiments. dThe reaction was conducted with 2.6 equiv of the ketone.
Preparation and Characterization of Substrates
2-Allyl-4-(tert-butyl)phenyl trifluoromethanesulfonate (5b).
A flame-dried flask equipped with a stir bar was cooled under a stream of nitrogen and charged with 2-allyl-4-tert-butylphenol (0.231 g, 1.21 mmol, 1.0 equiv) and dichloromethane (1.3 mL, 1 M). Pyridine (0.117 mL, 1.48 mmol, 1.2 equiv) was added, and the reaction was cooled to 0 °C. Trifluoromethanesulfonic anhydride (0.225 mL, 1.34 mmol, 1.1 equiv) was added, the ice bath was removed, and the reaction was stirred for 15 h at rt. The mixture was quenched with ammonium chloride and extracted with DCM (x3). The organic layer was dried with MgSO4 and concentrated in vacuo to yield a brown oil. The crude material was purified via column chromatography on silica gel using 99:1 hexanes:ethyl acetate as the eluent. This procedure afforded 0.334 g (85%) of the title compound as a colorless oil. 1H NMR (500 MHz, CDCl3) δ 7.32 – 7.25 (m, 2H), 7.19 – 7.14 (m, 1H), 5.98 – 5.87 (m, 1H), 5.18 – 5.09 (m, 2H), 3.47 (d, J = 6.6 Hz, 2H), 1.31 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 151.5, 145.7, 134.9, 131.9, 128.3, 125.1, 120.7, 118.5 (q, JC–F = 321 Hz) 117.2, 77.2, 34.7, 34.3, 31.2. IR (film) 2967, 1491 cm−1. HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C14H17F3O3S 323.0850; found 323.0565
2-Cinnamylphenol (S1).
A flame-dried 2-neck flask equipped with a stir bar and a condenser was cooled under a stream of nitrogen and charged with phenol (2.0 g, 21.2 mmol, 1.0 equiv) and diethyl ether (22 mL, 0.1 M). Sodium hydride (60% in mineral oil, 1.7 g, 42.4 mmol, 2.0 equiv) was added, and the reaction stirred at rt for 30 min. Cinnamyl chloride was added and the reaction heated to 37 °C with stirring for 6 h. The reaction was cooled to rt, and the mixture was transferred to an Erlenmeyer flask containing aqueous HCl (0.1 M, 75 mL). The mixture was stirred briefly then transferred to a separatory funnel. The layers were separated, and the aqueous layer was extracted with diethyl ether (3 × 20 mL). The combined organic layers were washed with brine, dried over MgSO4, filtered and concentrated in vacuo to yield a yellow oil. The crude material was purified via column chromatography on silica gel using 95:5 -> 90:10 hexanes:ethyl acetate as the eluent. This procedure afforded 2.52 g (57%) of the title compound as a yellow semisolid, mp 57–58 °C. 1H NMR (500 MHz, CDCl3) δ 7.28 (d, J = 18.1 Hz, 4H), 7.25 – 7.11 (m, 3H), 6.91 (s, 1H), 6.82 (d, J = 8.0 Hz, 1H), 6.51 (d, J = 15.9 Hz, 1H), 6.44 – 6.34 (m, 1H), 4.89 (s, 1H), 3.58 (d, J = 6.5 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 154.0, 137.1, 131.5, 130.5, 128.5 (2 peaks), 127.9 (2 peaks), 127.3, 126.2, 121.0, 115.8, 34.1. IR (film) 3531.5, 1591.7, cm−1. HRMS (ESI− TOF) m/z: [M - H+]− calcd for C15H14O 209.0966; found 209.0972.
2-Cinnamylphenyl trifluoromethanesulfonate (5e).
A flame-dried flask equipped with a stir bar was cooled under a stream of nitrogen and charged with 2-cinnamylphenol (S1) (0.934 g, 4.45 mmol, 1.0 equiv) and dichloromethane (20 mL, 0.2 M). Pyridine (0.72 mL, 8.9 mmol, 2.0 equiv) was added, and the reaction was cooled to 0 °C. Trifluoromethanesulfonic anhydride (1.5 mL, 8.9 mmol, 2.0 equiv) was added, the ice bath was removed, and the reaction was stirred for 15 h at rt. The purple mixture was filtered through a pad of celite, eluting with ethyl acetate. The purple filtrate was concentrated in vacuo to yield a purple oil. The crude material was purified via column chromatography on silica gel using 98:2 hexanes:ethyl acetate as the eluent. This procedure afforded 1.18 g (78%) of the title compound as a colorless oil. 1H NMR (500 MHz, CDCl3) δ 7.46 – 7.13 (m, 9H), 6.51 (d, J = 15.8 Hz, 1H), 6.35 – 6.22 (m, 1H), 3.65 (d, J = 6.8 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 147.9, 137.0, 133.1, 132.7, 131.4, 128.5, 128.2, 127.4, 126.2, 126.1, 121.4, 118.5 (q, JC–F = 319 Hz), 33.2, 29.7. IR (film) 1595.3, cm−1. HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C16H13F3O3S 342.0538; found 342.0538.
2-Cinnamyl-4-fluorophenol (S2).
The title compound was synthesized via a similar procedure described above for the preparation of 2-cinnamylphenol (S1), except using 4-fluorophenol (2.9 g, 17.86 mmol, 1.0 equiv), cinnamyl chloride (2.5 mL, 17.86 mmol, 1.0 equiv), and sodium hydride (60% in mineral oil, 1.428 g, 35.7 mmol, 2.0 equiv). The crude material was purified via column chromatography on silica gel using 95:5 -> 90:10 hexanes:ethyl acetate as the eluent. This procedure afforded 2.617 g (64%) of the title compound as a yellow solid, mp 51–52 °C. 1H NMR (500 MHz, CDCl3) δ 7.41 – 7.16 (m, 5H), 6.90 (d, J = 9.0 Hz, 1H), 6.83 (d, J = 13.6 Hz, 1H), 6.75 (d, J = 13.4 Hz, 1H), 6.51 (d, J = 15.9 Hz, 1H), 6.35 (d, J = 15.9 Hz, 1H), 4.78 (s, 1H), 3.54 (d, J = 6.6 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 158.1, 156.2, 149.8 (d, J = 2.5 Hz), 136.9, 132.0, 128.6, 127.4 (d, J = 7.5 Hz), 127.0, 126.2, 116.6 (d, J = 31 Hz), 116.5 (d, J = 18 Hz), 113.9 (d, J = 23 Hz), 34.0. IR (film) 3411.7, 1619.3 cm−1. HRMS (ESI− TOF) m/z: [M - H+]− calcd for C15H13FO 227.0872; found 227.0878.
2-Cinnamyl-4-fluorophenyl trifluoromethanesulfonate (5f).
The title compound was synthesized via a similar procedure described above for the preparation of 2-cinnamylphenyl trifluoromethanesulfonate (5e), except using 2-cinnamyl-4-fluorophenol (S2) (0.79 g, 3.47 mmol, 1.0 equiv), trifluoromethanesulfonic anhydride (1.16 mL, 6.94 mmol, 2.0 equiv), and pyridine (0.56 mL, 6.94 mmol, 2.0 equiv). The crude material was purified via column chromatography on silica gel using 100:1 -> 98:2 hexanes:ethyl acetate as the eluent. This procedure afforded 0.8474 g (68%) of the title compound as a colorless oil. 1H NMR (500 MHz, CDCl3) δ 7.52 – 7.22 (m, 6H), 7.15 (d, J = 8.7 Hz, 1H), 7.04 (s, 1H), 6.60 (d, J = 15.8 Hz, 1H), 6.31 (dd, J = 15.8, 7.0 Hz, 1H), 3.69 (d, J = 7.0 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 162.6, 160.6, 143.5 (d, J = 4 Hz), 136.8, 135.9 (d, J = 8 Hz), 133.5, 127.7, 126.3, 125.1, 123.1 (d, J = 9 Hz), 118.7 (q, J = 319 Hz), 117.9 (d, J = 24 Hz), 115.0 (d, J = 24 Hz), 33.3. IR (film) 1592.3, cm−1. HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C16H12F4O3S 361.0522; found 361.0516.
2-Cinnamyl-4-methoxyphenol (S3).
The title compound was synthesized via a similar procedure described above for the preparation of 2-cinnamylphenol (S1), except using 4-methoxyphenol (1.9 g, 14.4 mmol, 1.0 equiv), cinnamyl chloride (2.0 mL, 14.4 mmol, 1.0 equiv), and sodium hydride (60% in mineral oil, 1.15 g, 28.8 mmol, 2.0 equiv)). The crude material was purified via column chromatography on silica gel using 95:5 -> 90:10 hexanes:ethyl acetate as the eluent. This procedure afforded 1.516 g (44%) of the title compound as an orange solid, mp 74–76 °C. 1H NMR (500 MHz, CDCl3) δ 7.46 – 7.10 (m, 5H), 6.83 – 6.61 (m, 3H), 6.50 (d, J = 15.9 Hz, 1H), 6.37 (d, J = 15.8 Hz, 1H), 4.59 (s, 1H), 3.76 (s, 3H), 3.54 (d, J = 6.3 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 153.8, 137.0, 131.6, 128.6 (2 peaks), 128.5, 127.7, 126.8, 126.2, 116.5, 116.0, 112.6, 55.7, 34.3. IR (film) 3384.8, 1598.5, cm−1. HRMS (ESI− TOF) m/z: [M - H+]− calcd for C16H16O2 239.1072; found 239.1078.
2-Cinnamyl-4-methoxyphenyl trifluoromethanesulfonate (5g).
The title compound was synthesized via a similar procedure described above for the preparation of 2-cinnamylphenyl trifluoromethanesulfonate (5e), except using 2-cinnamyl-4-methoxyphenol (S3) (1.0 g, 4.17 mmol, 1.0 equiv), trifluoromethanesulfonic anhydride (1.4 mL, 8.33 mmol, 2.0 equiv), and pyridine (0.67 mL, 8.33 mmol, 2.0 equiv). The crude material was purified via column chromatography on silica gel using 98:2 -> 95:5 hexanes:ethyl acetate as the eluent. This procedure afforded 1.18 g (76%) of the title compound as a colorless oil. 1H NMR (500 MHz, CDCl3) δ 7.44 – 7.13 (m, 6H), 6.87 (s, 1H), 6.79 (d, J = 9.0 Hz, 1H), 6.51 (d, J = 15.8 Hz, 1H), 6.34 – 6.18 (m, 1H), 3.80 (s, 3H), 3.60 (d, J = 6.9 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 159.0, 141.3, 137.0, 134.4, 132.8, 128.5, 127.4, 126.2, 126.0, 122.4, 118.7 (q, JC–F = 305 Hz), 116.3, 112.8, 55.7, 33.5. IR (film) 1587.1 cm−1. HRMS (ESI+ TOF) m/z: [M + NH4+]+ calcd for C17H15F3O4S 390.0987; found 390.0981.
2-Cinnamyl-4-methylphenol (S4).
The title compound was synthesized via a similar procedure described above for the preparation of 2-cinnamylphenol (S1), except using 4-methylphenol (2.3 mL, 22.0 mmol, 1.0 equiv), cinnamyl chloride (3.0 mL, 22.0 mmol, 1.0 equiv), and sodium hydride (60% in mineral oil, 1.80 g, 44.0 mmol, 2.0 equiv). The crude material was purified via column chromatography on silica gel using 95:5 -> 90:10 hexanes:ethyl acetate as the eluent. This procedure afforded 2.68 g (54%) of the title compound as a yellow wax. 1H NMR (500 MHz, CDCl3) δ 7.44 – 7.14 (m, 5H), 7.04 – 6.88 (m, 2H), 6.72 (d, J = 8.0 Hz, 1H), 6.56 – 6.46 (m, 1H), 6.44 – 6.33 (m, 1H), 4.75 (s, 1H), 3.54 (dd, J = 6.5, 1.4 Hz, 2H), 2.28 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 151.7, 137.1, 131.4, 131.0, 130.2, 128.5, 128.2, 128.1, 127.3, 126.2, 125.4, 115.6, 34.1, 20.5. IR (film) 3214.4, 2750.3, 1495.7, 1405.2, 1275.7 cm−1. HRMS (Electron Impact Ionization TOF) m/z: [M + H+]+ calcd for C16H16O 224.1201; found 224.1201.
2-Cinnamyl-4-methylphenyl trifluoromethanesulfonate (5h).
The title compound was synthesized via a similar procedure described above for the preparation of 2-cinnamylphenyl trifluoromethanesulfonate (5e), except using 2-cinnamyl-4-methylphenol (S4) (1.02 g, 4.46 mmol, 1.0 equiv), trifluoromethanesulfonic anhydride (1.5 mL, 8.93 mmol, 2.0 equiv), and pyridine (0.72 mL, 8.93 mmol, 2.0 equiv). The crude material was purified via column chromatography on silica gel using 100:0 -> 98:2 hexanes:ethyl acetate as the eluent. This procedure afforded 1.20 g (76%) of the title compound as a colorless oil. 1H NMR (500 MHz, CDCl3) δ 7.38 (d, J = 7.0 Hz, 2H), 7.32 (t, J = 7.6 Hz, 2H), 7.28 – 7.20 (m, 1H), 7.20 – 7.13 (m, 2H), 7.10 (dd, J = 8.4, 2.2 Hz, 1H), 6.51 (d, J = 15.7 Hz, 1H), 6.35 – 6.21 (m, 1H), 3.61 (dd, J = 7.0, 1.5 Hz, 2H), 2.35 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 145.8, 138.5, 137.1, 132.6, 132.5, 131.9, 128.7, 128.5, 128.4, 127.4, 126.3, 118.6 (q, JC–F = 321 Hz), 121.1, 33.2, 20.9. IR (film) 3028.5, 2924.4, 1599.3, 1490.7, 1417.7 cm−1. HRMS (ESI+ TOF) m/z: [M + NH4+]+ calcd for C17H15F3O3S 374.1038; found 374.1032.
4-(tert-Butyl)-2-(2-methylallyl)phenyl trifluoromethanesulfonate (5i).
A flame-dried thick-walled sealable glass pressure flask equipped with a stir bar was cooled under a stream of nitrogen and charged with 1-(tert-butyl)-4-[(2-methylallyl)oxy]benzene (5.33g, 26 mmol) and dimethylformamide (5 mL, 5.2 M). The solution was heated to 200°C for 12 h, then was cooled to rt and ethyl acetate (20 mL) was added. The mixture was transferred to a separatory funnel, washed with water (3 × 20 mL), then was dried with anhydrous MgSO4 and concentrated in vacuo to yield a brown oil. The crude material was purified via column chromatography on silica gel using 80:20 hexanes:dichloromethane as the eluent. This procedure afforded 4.05 g (76%) of 4-(tert-butyl)-2-(2-methylallyl)phenol as a colorless oil with ca 3% of a side product resulting from isomerization of the alkene. 1H NMR (401 MHz, CDCl3) δ 7.14 (dd, J = 8.4, 2.5 Hz, 1H), 7.07 (d, J = 2.5 Hz, 1H), 6.75 (d, J = 8.4 Hz, 1H), 4.97 (s, 1H), 4.90 (s, 1H), 4.83 (s, 1H), 3.36 (s, 2H), 1.74 (s, 3H), 1.28 (s, 9H).
A flame-dried flask equipped with a stir bar was cooled under a stream of nitrogen and charged with 4-(tert-butyl)-2-(2-methylallyl)phenol (1.4 g, 6.85 mmol, 1.0 equiv) and dichloromethane (7 mL, 1 M). Pyridine (1.4 mL, 8.22 mmol, 1.2 equiv) was added, and the mixture was cooled to 0 °C. Trifluoromethanesulfonic anhydride (0.7 mL, 8.22 mmol, 1.2 equiv) was added, the ice bath was removed, and the reaction was stirred at rt for 15 h. The mixture was quenched with saturated aqueous ammonium chloride (10 mL) and then transferred to a separatory funnel. The mixture was extracted with dichloromethane (3 × 10 mL), then the organic layers were combined, dried with anhydrous MgSO4, and concentrated in vacuo to yield a brown oil. The crude material was purified via column chromatography on silica gel using 99:1 hexanes:ethyl acetate as the eluent. This procedure afforded 2.06 g (89%) of the title compound as a colorless oil that contained ca 6% of a side product resulting from alkene isomerization. Data are for the major isomer. 1H NMR (500 MHz, CDCl3) δ 7.32 – 7.27 (m, 2H), 7.20 – 7.14 (m, 1H), 4.89 (s, 1H), 4.66 (s, 1H), 3.40 (s, 2H), 1.72 (s, 3H), 1.31 (s, 9H); 13C NMR (126 MHz, CDCl3) δ 151.34, 146.09, 142.74, 131.42, 128.76, 125.07, 120.58, 118.5 (q, JC–F = 321 Hz), 113.05, 38.29, 34.63, 31.23, 22.22. IR (film) 2951, 2700, 1480, 1415, 1208, 729 cm−1; HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C15H19F3O3S 337.1080; found 337.1077.
Ethyl 1-cinnamyl-2-oxocyclopentane-1-carboxylate (S5).
A flame-dried flask equipped with a stir bar was cooled under a stream of nitrogen and charged with sodium hydride (60% in mineral oil, 0.534 g, 13.35 mmol, 0.89 equiv) and THF (6 mL). The mixture was cooled to 0 °C and a solution of ethyl-2-oxocyclopentanecarboxylate (1.9 mL, 15 mmol, 1 equiv) in THF (10 mL) was added dropwise over the course of 90 min. The reaction mixture was warmed to rt and stirred for 90 min, then a solution of cinnamyl bromide (2.6 mL, 17.25 mmol, 1.15 equiv) in THF (5 mL) was added and the mixture was stirred at rt for 16 h. The reaction was quenched with saturated aqueous ammonium chloride (20 mL) and transferred to a separatory funnel. The layers were separated and the aqueous layer was extracted with ethyl acetate (3 × 20 mL). The combined organic layers were washed with brine (1 × 20 mL) and then dried over Na2SO4, filtered and concentrated in vacuo to afford a yellow oil. The crude material was purified via column chromatography on silica gel using 90:10 hexanes:ethyl acetate as the eluent. This procedure afforded 1.84 g (45%) of the title compound as a colorless oil. 1H NMR (500 MHz, CDCl3) δ 7.40 – 7.25 (m, 4H), 7.21 (d, J = 14.2 Hz, 1H), 6.45 (d, J = 15.8 Hz, 1H), 6.16 – 6.01 (m, 1H), 4.25 – 4.10 (m, 2H), 2.89 – 2.73 (m, 1H), 2.59 – 2.37 (m, 3H), 2.31 – 2.16 (m, 1H), 2.04 (d, J = 14.0 Hz, 2H), 1.90 (d, J = 17.0 Hz, 1H), 1.25 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 214.6, 170.9, 137.0, 134.1, 128.5, 127.4, 126.2, 124.5, 61.5, 60.2, 38.1, 37.0, 32.3, 19.6, 14.1. IR (film) 1750.4, 1719.9, 1448.4 cm−1. HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C17H20O3 272.1413; found 273.1485.
Ethyl 1-cinnamyl-2-{[(trifluoromethyl)sulfonyl]oxy}cyclopent-2-ene-1-carboxylate (1l).
A flame-dried flask equipped with a stir bar was cooled under a stream of nitrogen and charged with THF (10 mL) and diisopropylamine (1.0 mL, 7.29 mmol, 1.7 equiv). The mixture was cooled to 0 °C, n-BuLi (2.5 M in hexanes, 2.9 mL, 7.29 mmol, 1.7 equiv) was added dropwise, and the resulting mixture was stirred at 0 °C for 30 min. The mixture was cooled to −78 °C and a solution of ethyl 1-cinnamyl-2-oxocyclopentane-1-carboxylate (S5, 1.17 g, 4.29 mmol, 1 equiv) in THF (10 mL) was added dropwise over the course of 90 min. The reaction mixture was stirred at −78 °C for 2.5 h, then N-(2-pyridyl)triflamide (2.61 g, 7.29 mmol, 1.7 equiv) in THF (5 mL) was added and the mixture was warmed to rt and stirred for 16 h. The reaction was quenched with water (20 mL) and the mixture was transferred to a separatory funnel. The layers were separated and the aqueous layer was extracted with ethyl acetate (3 × 20 mL). The combined organic layers were washed with brine (1 × 20 mL) and then dried over Na2SO4, filtered and concentrated in vacuo to afford a yellow oil. The crude material was purified via column chromatography on silica gel using 98:2 -> 95:5 hexanes:ethyl acetate as the eluent. This procedure afforded 1.18 g (68%) of the title compound as a yellow oil. 1H NMR (500 MHz, CDCl3) δ 7.42 – 7.14 (m, 5H), 6.50 (d, J = 15.7 Hz, 1H), 6.14 – 6.00 (m, 1H), 5.80 (s, 1H), 4.32 – 4.11 (m, 2H), 2.78 (d, J = 8.0 Hz, 1H), 2.63 (d, J = 14.1 Hz, 1H), 2.48 (d, J = 9.3 Hz, 2H), 2.35 (d, J = 5.5 Hz, 1H), 2.15 – 1.93 (m, 1H), 1.29 (t, J = 7.2 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 172.5, 147.9, 137.0, 134.5, 128.5, 127.5, 126.2, 123.6, 121.6 (q, JC–F = 375 Hz), 118.3, 61.6, 57.6, 38.0, 31.2, 26.2, 14.0. IR (film) 1728.6, 1602.5, 1422.8 cm−1. HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C18H19F3O5S 405.0984; found 405.0978.
2-Cinnamylcyclopentan-1-one (S6).
A flame-dried flask equipped with a stir bar was cooled under a stream of nitrogen and charged with palladium allyl chloride dimer (0.146 g, 0.4 mmol, 0.02 equiv), dppf (0.665 g, 1.2 mmol, 0.06 mmol), and methanol (80 mL). The mixture was stirred at rt for 60 min, then cinnamyl alcohol (2.8 mL, 22 mmol, 1.1 equiv) was added to the orange mixture, and the resulting mixture was stirred at rt for 30 min. Cyclopentanone (2.1 mL, 20 mmol, 1 equiv) and pyrrolidine (0.33 mL, 4 mmol, 0.2 equiv) were added, and the mixture was then heated to 45 °C with stirring for 16 h. The reaction was quenched with cold saturated aqueous ammonium chloride (20 mL). The mixture was transferred to a separatory funnel, the layers were separated, and the aqueous layer was extracted with diethyl ether (3 × 20 mL). The combined organic layers were washed with brine (1 × 20 mL) and then dried over Na2SO4, filtered, and concentrated in vacuo to afford yellow oil. The crude material was purified via column chromatography on silica gel using 98:2 -> 95:5 hexanes:ethyl acetate as the eluent. This procedure afforded 3.38 g (83%) of the title compound as a colorless oil. 1H NMR (500 MHz, CDCl3) δ 7.39 – 7.13 (m, 5H), 6.43 (d, J = 15.6 Hz, 1H), 6.24 – 6.08 (m, 1H), 2.65 (d, J = 10.0 Hz, 1H), 2.41 – 2.16 (m, 4H), 2.11 (d, J = 8.8 Hz, 1H), 2.01 (s, 1H), 1.81 (dddt, J = 14.7, 8.5, 4.3, 2.3 Hz, 1H), 1.63 (s, 1H). 13C NMR (126 MHz, CDCl3) δ 220.4, 137.4, 131.8, 128.5, 127.6, 127.1, 126.0, 49.0, 38.2, 33.1, 29.0, 20.7. IR (film) 1732.7, 1597.2 cm−1. HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C14H16O 201.1280; found 201.1274.
5-Cinnamylcyclopent-1-en-1-yl trifluoromethanesulfonate (1m).
A flame-dried flask equipped with a stir bar was cooled under a stream of nitrogen and charged with THF (20 mL) and diisopropylamine (1.05 mL, 7.5 mmol, 1.5 equiv). The mixture was cooled to 0 °C, n-BuLi (2.5 M in hexanes, 3.0 mL, 7.5 mmol, 1.5 equiv) was added dropwise, and the resulting solution was stirred at 0 °C for 30 min. The mixture was then cooled to −78 °C and a solution of 2-cinnamylcyclopentan-1-one (S6, 1.0 g, 5.0 mmol, 1 equiv) in THF (20 mL) was added dropwise over the course of 70 min. The reaction mixture was then stirred for 2 h at −78 °C, then a solution of N-(2-pyridyl)triflamide (2.61 g, 7.29 mmol, 1.2 equiv) in THF (10 mL) was added. The resulting mixture was warmed to −41 °C in CH3CN/dry ice bath and stirred for 2 h. The reaction was quenched with water (20 mL) and the mixture was transferred to a separatory funnel. The layers were separated and the aqueous layer was extracted with ethyl acetate (3 × 20 mL). The combined organic layers were washed with brine (1 × 20 mL) and then dried over Na2SO4, filtered, and concentrated in vacuo to afford a yellow oil. The crude material was purified via column chromatography on silica gel using 100:0 -> 98:2 hexanes:ethyl acetate as the eluent. This procedure afforded 0.8675 g (52%) of the title compound as a colorless oil. 1H NMR (500 MHz, CDCl3) δ 7.35 (d, J = 18.6 Hz, 4H), 7.26 (s, 1H), 6.49 (d, J = 15.8 Hz, 1H), 6.18 (d, J = 15.7 Hz, 1H), 5.71 (s, 1H), 3.04 (s, 1H), 2.57 (ddt, J = 11.1, 5.8, 2.9 Hz, 1H), 2.45 – 2.26 (m, 3H), 2.20 (s, 1H), 1.80 (d, J = 6.2 Hz, 1H). 13C NMR (126 MHz, CDCl3) δ 151.3, 137.3, 132.6, 128.5, 127.2, 126.3, 126.1, 121.1 (q, JC–F = 315 Hz), 117.4, 43.0, 35.7, 26.7, 26.6. IR (film) 1656.6, 1495.7 cm−1. HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C15H15F3O3S 333.0772; found 333.0767.
Ethyl 1-cinnamyl-2-oxocyclohexane-1-carboxylate (S7).
The title compound was synthesized via a similar procedure described above for the preparation of ethyl 1-cinnamyl-2-oxocyclopentane-1-carboxylate (S5), except using ethyl-2-oxocyclohexanecarboxylate (3.2 mL, 20 mmol, 1 equiv), cinnamyl bromide (3.4 mL, 23.0 mmol, 1.15 equiv), and sodium hydride (0.712 g, 17.8 mmol, 0.89 equiv). The crude material was purified via column chromatography on silica gel using 95:5 -> 90:10 hexanes:ethyl acetate as the eluent. This procedure afforded 3.58 g (63%) of the title compound as a yellow oil. 1H NMR (500 MHz, CDCl3) δ 7.39 – 7.12 (m, 5H), 6.37 (d, J = 15.8 Hz, 1H), 6.24 – 6.09 (m, 1H), 4.16 (q, J = 7.1 Hz, 2H), 2. 73 (tdd, J = 11.5, 7.1, 1.4 Hz, 1H), 2.56 – 2.41 (m, 3H), 2.01 (ddt, J = 9.2, 6.2, 3.1 Hz, 1H), 1.81 – 1.57 (m, 4H), 1.57 – 1.49 (m, 1H), 1.21 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 207.6, 171.5, 137.2, 133.2, 128.4, 127.2, 126.1, 125.1, 61.3, 41.2, 38.6, 36.1, 27.9, 27.5, 22.5, 14.2. IR (film) 1709.8 (2 overlapping peaks), 1597.9, 1495.7, cm−1. HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C18H22O3 287.1647; found 287.1642.
Ethyl 1-cinnamyl-2-{[(trifluoromethyl)sulfonyl]oxy}cyclohex-2-ene-1-carboxylate (1h).
The title compound was synthesized a similar procedure described above for the preparation of ethyl 1-cinnamyl-2-{[(trifluoromethyl)sulfonyl]oxy}cyclopent-2-ene-1-carboxylate (1l), except using ethyl 1-cinnamyl-2-oxocyclohexane-1-carboxylate (S7) (1.13 g, 4.0 mmol, 1 equiv), LDA (6.73 mmol, 1.7 equiv), and N-(2-pyridyl)triflamide (2.409 g, 6.73 mmol, 1.7 equiv). The crude material was purified via column chromatography on silica gel using 100:0 -> 98:2 hexanes:ethyl acetate as the eluent. This procedure afforded 1.06 g (64%) of the title compound as a colorless oil. 1H NMR (500 MHz, CDCl3) δ 7.46 – 7.12 (m, 5H), 6.49 (d, J = 15.7 Hz, 1H), 6.12 (t, J = 15.4 Hz, 1H), 5.92 (s, 1H), 4.36 – 4.11 (m, 2H), 2.82 – 2.64 (m, 2H), 2.38 – 2.10 (m, 3H), 1.82 – 1.53 (m, 3H), 1.31 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 172.6, 148.1, 137.0, 134.5, 128.5, 127.5, 126.2, 123.8, 120.3, 118.3 (q, JC–F = 318 Hz), 61.8, 50.5, 38.7, 32.3, 24.4, 18.6, 14.0. IR (film) 1730.3, 1677.1 cm−1. HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C19H21F3O5S 419.1140; found 419.1135.
2-Cinnamylcyclohexan-1-one (S8).
The title compound was synthesized via a similar procedure described above for the preparation of 2-cinnamylcyclopentan-1-one (S5), except using cyclohexanone (2.1 mL, 20 mmol, 1 equiv), cinnamyl alcohol (2.8 mL, 2.2 mmol, 1.1 equiv), palladium allyl chloride dimer (0.146 g, 0.4 mmol, 0.02 equiv), dppf (0.665 g, 1.2 mmol, 0.06 mmol), and pyrrolidine (0.33 mL, 4.0 mmol, 0.2 equiv). The crude material was purified via column chromatography on silica gel using 95:5 hexanes:ethyl acetate as the eluent. This procedure afforded 2.74 g (63%) of the title compound as a yellow oil. 1H NMR (500 MHz, CDCl3) δ 7.44 – 7.23 (m, 4H), 7.19 (s, 1H), 6.39 (d, J = 15.8 Hz, 1H), 6.20 (t, J = 15.2 Hz, 1H), 2.73 – 2.60 (m, 1H), 2.43 (d, J = 15.8 Hz, 2H), 2.32 (d, J = 19.2 Hz, 1H), 2.25 – 2.00 (m, 3H), 1.88 (s, 1H), 1.67 (s, 2H), 1.41 (d, J = 12.4 Hz, 1H). 13C NMR (126 MHz, CDCl3) δ 212.4, 137.5, 131.6, 128.5, 128.4, 128.3, 127.0, 126.0, 50.7, 42.1, 33.6, 27.9, 25.0. IR (film) 1705.24, 1597.9 cm−1. HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C15H18O 215.1436; found 215.1430.
6-Cinnamylcyclohex-1-en-1-yl trifluoromethanesulfonate (1i).
The title compound was synthesized via a similar procedure to 5-cinnamylcyclopent-1-en-1-yl trifluoromethanesulfonate (1k), except using 2-cinnamylcyclohexan-1-one (S8) (0.544 g, 2.54 mmol, 1 equiv), LDA (3.82 mmol, 1.5 equiv), and N-(2-pyridyl)triflamide (1.09 g, 3.05 mmol, 1.2 equiv). The crude material was purified via column chromatography on silica gel using 100:0 -> 98:2 -> 95:5 hexanes:ethyl acetate as the eluent. This procedure afforded 0.660 g (75%) of the title compound as a colorless oil. 1H NMR (500 MHz, CDCl3) δ 7.43 – 7.13 (m, 5H), 6.45 (d, J = 15.6 Hz, 1H), 6.23 – 6.04 (m, 1H), 5.83 (s, 1H), 2.62 (d, J = 10.1 Hz, 2H), 2.40 – 2.26 (m, 1H), 2.18 (s, 2H), 1.88 (s, 1H), 1.62 (dd, J = 49.8, 6.2 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 151.7, 137.3, 132.7, 128.5, 127.2, 126.7, 126.1, 119.5, 118.5 (q, JC–F = 319 Hz), 37.6, 35.1, 27.8, 24.3, 19.0. IR (film) 1680.2, 1494.3 cm−1. HRMS (ESI+ TOF) m/z: [M + NH4+]+ calcd for C16H17F3O3S 364.1194; found 364.1189.
Ethyl 1-cinnamyl-2-oxocyclohexane-1-carboxylate (S9).
The title compound was synthesized via a similar procedure described above for the preparation of ethyl 1-cinnamyl-2-oxocyclopentane-1-carboxylate (S4), except using ethyl-2-oxocyclohexanecarboxylate (3.4 mL, 21.1 mmol, 1 equiv), crotyl bromide (2.5 mL, 24.3 mmol, 1.15 equiv), and sodium hydride (0.75 g, 18.8 mmol, 0.89 equiv). The crude material was purified via column chromatography on silica gel using 98:2 hexanes:ethyl acetate as the eluent. This procedure afforded 2.26 g (48%) of the title compound as a brown oil. 1H NMR (500 MHz, CDCl3) δ 5.54 – 5.22 (m, 2H), 4.26 – 4.04 (m, 2H), 2.60 – 2.28 (m, 4H), 2.28 – 2.13 (m, 1H), 1.96 (ddd, J = 14.1, 5.2, 2.9 Hz, 1H), 1.81 – 1.51 (m, 6H), 1.42 (d, J = 16.2 Hz, 1H), 1.21 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 207.7, 171.6, 128.8, 125.6, 61.1, 41.1, 38.0, 35.7, 27.5, 22.5, 22.4, 17.9, 14.1. IR (film) 1710.4 (2 overlapping peaks), 1438.0 cm−1. HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C13H20O3 225.1491; found 225.1485.
(E)-Ethyl-1-(but-2-en-1-yl)-2-{[(trifluoromethyl)sulfonyl]oxy}cyclohex-2-ene-1-carboxylate (1j).
The title compound was synthesized via a similar procedure described above for the preparation of ethyl 1-cinnamyl-2-{[(trifluoromethyl)sulfonyl]oxy}cyclohex-2-ene-1-carboxylate (1l), except using ethyl 1-cinnamyl-2-oxocyclohexane-1-carboxylate (S9) (0.98 g, 4.46 mmol, 1 equiv), LDA (7.6 mmol, 1.7 equiv), and N-(2-pyridyl)triflamide (2.66 g, 7.6 mmol, 1.7 equiv). The crude material was purified via column chromatography on silica gel using 100:0 -> 98:2 hexanes:ethyl acetate as the eluent. This procedure afforded 0.623 g (40%) of the title compound as a colorless oil. 1H NMR (500 MHz, CDCl3) δ 5.86 (s, 1H), 5.57 – 5.45 (m, 1H), 5.28 (d, J = 15.0 Hz, 1H), 4.15 (d, J = 16.3 Hz, 2H), 2.47 (s, 2H), 2.29 – 2.08 (m, 3H), 1.63 (d, J = 15.5 Hz, 6H), 1.27 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 172.8, 148.2, 130.2, 124.5, 120.0, 118.3 (q, JC–F = 318 Hz), 61.5, 50.3, 38.3, 32.0, 31.9, 24.4, 18.7, 13.9. IR (film) 1731.2, 1677.4, 1414.4 cm−1. HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C14H19F3O5S 357.0984; found 357.0978.
(±)-Cinnamyl 1-methyl-2-oxocyclohexane-1-carboxylate (S10).
A flame-dried flask equipped with a stir bar was cooled under a stream of nitrogen and charged with allyl 1-methyl-2-oxocyclohexane-1-carboxylate (1.169 g, 5.97 mmol, 1 equiv), Grubbs II catalyst (0.203 g, 0.239 mmol, 0.04 equiv), and copper iodide (68.1 mg, 0.358 mmol, 0.06 equiv). The flask was evacuated and backfilled with nitrogen, then and diethyl ether (30 mL, 0.2 M) and styrene (2.05 mL, 17.9 mmol, 3.0 equiv) were added to the mixture. The septum was replaced with a condenser and the mixture was heated to 40 °C with stirring for 15 h. The reaction mixture was cooled to rt, and concentrated in vacuo to yield a purple colored crude product. The crude product was purified via column chromatography on silica gel using 95:5 hexanes:ethyl acetate as the eluent. This procedure afforded 0.904 g (63%) of the title compound as a colorless oil. 1H NMR (500 MHz, CDCl3) δ 7.42 – 7.21 (m, 5H), 6.71 – 6.58 (m, 1H), 6.31 – 6.18 (m, 1H), 4.87 – 4.72 (m, 2H), 2.57 – 2.41 (m, 2H), 2.09 – 1.95 (m, 1H), 1.83 (m, 1H), 1.78 – 1.58 (m, 2H), 1.54 – 1.42 (m, 1H), 1.32 (s, 3H), 1.04 (m, 1H). 13C NMR (126 MHz, CDCl3) δ 208.2, 172.9, 136.0, 134.8, 134.0, 128.6, 126.7, 122.4, 65.8, 57.2, 40.7, 38.2, 27.5, 22.6, 21.3. IR (film) 2935.8, 2867.1, 1709.7 (the two carbonyls are incidentally equivalent), 1495.5, 1449.5 cm−1. HRMS (ESI+ TOF) m/z: [M + Na+]+ calcd for C17H20O3Na 295.1305; found 295.1305.
2-Cinnamyl-2-methylcyclohexan-1-one (S11).
A flame-dried Schlenk flask equipped with a stir bar was cooled under a stream of nitrogen and charged with palladium acetate (27.8 mg, 0.124 mmol, 0.10 equiv), and triphenylphosphine (0.812 g, 3.10 mmol, 2.5 equiv). The flask was evacuated and backfilled with nitrogen, then tetrahydrofuran (25 mL, 0.1 M) and cinnamyl 1-methyl-2-oxocyclohexane-1-carboxylate (S10) (0.337 g, 1.24 mmol, 1.0 equiv) were added. The septum was replaced with a condenser and the reaction mixture was heated to 30 °C with stirring for 15 h. The mixture was then cooled to rt, diluted with diethyl ether, filtered through a pad of silica gel, and concentrated in vacuo to yield a yellow oil. The crude material was purified via column chromatography on silica gel using 98:2 -> 95:5 hexanes:ethyl acetate as the eluent. This procedure afforded 33.9 mg (12%) of the title compound as a colorless oil. 1H NMR (500 MHz, CDCl3) δ 7.36 – 7.25 (m, 5H), 7.19 (d, J = 7.2 Hz, 1H), 6.40 (d, J = 15.7 Hz, 1H), 6.18 – 6.06 (m, 1H), 2.47 (d, J = 15.0 Hz, 4H), 1.91 – 1.68 (m, 4H), 1.68 – 1.56 (m, 1H), 1.13 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 215.4, 137.4, 133.0, 128.4, 127.1, 126.0, 125.7, 48.9, 38.8, 38.6, 29.7, 27.4, 22.9, 21.1. IR (film) 3026.3, 2931.9, 2863.5, 1704.1, 1598.2, 1495.1 cm−1. HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C16H21O 229.1587; found 229.1586.
6-Cinnamyl-6-methylcyclohex-1-en-1-yl trifluoromethanesulfonate (1k).
The title compound was synthesized via a similar procedure to ethyl 1-cinnamyl-2-{[(trifluoromethyl)sulfonyl]oxy}cyclohex-2-ene-1-carboxylate (1l), except using 2-cinnamyl-2-methylcyclohexan-1-one (S11) (1.13 g, 4.0 mmol, 1 equiv), LDA (6.73 mmol, 1.7 equiv), and N-(2-pyridyl)triflamide (2.409 g, 6.73 mmol, 1.7 equiv). The crude material was purified via column chromatography on silica gel using 100:0 -> 98:2 hexanes:ethyl acetate as the eluent. This procedure afforded 1.06 g (64%) of a colorless oil. 1H NMR (500 MHz, CDCl3) δ 7.45 – 7.13 (m, 5H), 6.43 (d, J = 15.7 Hz, 1H), 6.14 (ddd, J = 15.4, 8.1, 7.0 Hz, 1H), 5.76 (t, J = 4.1 Hz, 1H), 2.46 (ddd, J = 13.9, 7.0, 1.4 Hz, 1H), 2.31 (ddd, J = 13.9, 8.1, 1.2 Hz, 1H), 2.17 (ddd, J = 9.6, 5.7, 3.9 Hz, 2H), 1.82 (td, J = 9.6, 4.6 Hz, 1H), 1.73 – 1.58 (m, 2H), 1.59 – 1.48 (m, 1H), 1.20 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 154.8, 137.3, 133.6, 128.5, 127.2, 126.1, 125.1, 118.4 (q, JC–F = 313 Hz), 117.3, 42.0, 38.7, 35.4, 24.7, 24.5, 18.2. IR (film) 3027.8, 2938.4, 1495.6, 1457.0, 1410.0 cm−1. HRMS (Electron Ionization Impact TOF) m/z: [M]+ calcd for C17H19F3O3S 360.1007; found 360.1024.
Preparation and Characterization of Products
General Procedure for Pd-catalyzed Alkene Dialkylation Reactions on terminal alkenes, General Procedure A.
A flame-dried 4 mL vial equipped with a stir bar was cooled under a stream of nitrogen and charged with Pd(OAc)2 (0.008 mmol, 0.04 equiv), Brettphos (0.012 mmol, 0.06 equiv), and lithium tert-butoxide (0.28 mmol, 1.4 equiv). The aryl or alkenyl triflate (0.2 mmol, 1.0 equiv) was weighed in a separate 1 dram vial and diluted with toluene (1 mL, 0.2 M). This mixture was added to the reaction vessel and the appropriate nucleophile (0.24 mmol, 1.2 equiv) was added. Toluene (1 mL, 0.2M) was used to rinse the 1 dram vial and the solution was transferred to the reaction vessel. The vial was flushed with nitrogen, capped, and heated to 95 °C with stirring overnight until the starting material had been consumed. The mixture was then cooled to rt and quenched with saturated aqueous ammonium chloride (1 mL). The aqueous layer was extracted with ethyl acetate (3 × 1 mL), dried over MgSO4, filtered and concentrated in vacuo. The crude product was purified via flash chromatography on silica gel to afford the desired product.
General procedure for Pd-catalyzed alkene dialkylation reactions on internal alkene substrates, General procedure B
A flame-dried 4 mL vial equipped with a stir bar and was cooled under a stream of nitrogen and charged with the appropriate triflate (0.2 mmol, 1.0 equiv), the appropriate palladium pre-catalyst (0.008 mmol, 0.04 equiv), the appropriate ligand (0.012 mmol, 0.06 equiv), lithium tert-butoxide (0.44 mmol, 2.2 equiv). The vial was purged with nitrogen and charged with toluene (0.8 M) and the appropriate malonate derivative (0.6 mmol, 3.6 equiv). The vial was capped and heated to the appropriate temperature with stirring until the starting material had been consumed. The mixture was cooled to rt, charged with phenanthrene (1 equiv; NMR internal standard), diluted with dichloromethane (1 mL), and quenched with saturated ammonium chloride (1 mL). The aqueous layer was extracted with dichloromethane (3 × 1 mL), dried over Na2SO4, filtered, and concentrated in vacuo. The crude product was purified via flash chromatography on silica gel to afford the desired product.
Diethyl 2-(2,3-dihydro-1H-inden-2-yl)malonate (6a).
The title compound was prepared from 2-allylphenyl triflate (53.5 mg, 0.2 mmol) and diethyl malonate (37 μL, 0.24 mmol) using General Procedure A. The crude material was purified via column chromatography on silica gel using 95:5 hexanes:ethyl acetate as the eluent. This procedure afforded 51.7 mg (93%) of the title compound as a colorless solid (mp 33 – 35°C). 1H NMR (500 MHz, CDCl3) δ 7.22 – 7.15 (m, 2H), 7.16 – 7.13 (m, 2H), 4.22 (qd, J = 7.1, 2.7 Hz, 4H), 3.45 (d, J = 9.3 Hz, 1H), 3.25 – 3.03 (m, 3H), 2.83 – 2.69 (m, 2H), 1.28 (t, J = 7.1 Hz, 6H). 13C NMR (126 MHz, CDCl3) δ 168.8, 142.1, 126.4, 124.4, 61.4, 56.8, 39.0, 37.3, 14.1; IR (film) 2980, 2840, 1744, 1727, 1476 cm−1; HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C16H20O4 277.1362; found 277.1434.
Diethyl 2-(2,3-dihydro-1H-inden-2-yl)malonate (6a).
The title compound was prepared from 2-allylphenyl triflate (267 mg, 1.0 mmol) and diethyl malonate (183 μL, 1.2 mmol) using General Procedure A. The crude material was purified via column chromatography on silica gel using 95:5 hexanes:ethyl acetate as the eluent. This procedure afforded 232 mg (84%) of the title compound as a white solid (mp 33 – 35°C). Characterization data were identical to those listed above.
Diethyl 2-(2,3-dihydro-1H-inden-2-yl)malonate (6a).
The title compound was prepared from 2-allylphenyl triflate (1.50 g, 5.63 mmol) and diethyl malonate (1.03 mL, 6.76 mmol) using General Procedure A. The crude material was purified via column chromatography on silica gel using 95:5 hexanes:ethyl acetate as the eluent. This procedure afforded 1.36 g (88%) of the title compound as a white solid (mp 33 – 35°C). Characterization data were identical to those listed above.
(±)-Diethyl 2-[5-(tert-butyl)-2,3-dihydro-1H-inden-2-yl]malonate (6b).
The title compound was prepared from 2-allyl-4-tert-butylphenyl triflate (64.5 mg, 0.2 mmol) and diethyl malonate (37 μL, 0.24 mmol) using General Procedure A. The crude material was purified via column chromatography on silica gel using 95:5 hexanes:ethyl acetate as the eluent. This procedure afforded 48.3 mg (77%) of the title compound as a colorless oil. 1H NMR (400 MHz, CDCl3) δ 7.24 (s, 1H), 7.20 (dd, J = 7.9, 1.9 Hz, 1H), 7.13 (d, J = 7.9 Hz, 1H), 4.28 – 4.16 (m, 4H), 3.46 (d, J = 8.9 Hz, 1H), 3.23 – 3.08 (m, 3H), 2.86 – 2.66 (m, 2H), 1.32 (s, 9H), 1.31 – 1.27 (m, 6H). 13C NMR (126 MHz, CDCl3) δ 168.9, 149.7, 142.1, 139.3, 124.0, 123.7, 121.4, 61.4, 57.1, 39.4, 37.6, 37.0, 34.7, 31.68, 14.3; IR (film) 1748, 1729, 1467 cm−1; HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C20H28O4 333.199; found 333.206.
(±)-Diethyl 2-(5-fluoro-2,3-dihydro-1H-inden-2-yl)malonate (6c).
The title compound was prepared from 2-allyl-4-fluorophenyl triflate (56.8 mg, 0.2 mmol) and diethyl malonate (37 μL, 0.24 mmol) using General Procedure A. The crude material was purified via column chromatography on silica gel using 95:5 hexanes:ethyl acetate as the eluent. This procedure afforded 46.5 mg (79%) of the title compound as a colorless oil. 1H NMR (500 MHz, CDCl3) δ 7.09 (dd, J = 8.3, 5.2 Hz, 1H), 6.89 – 6.83 (m, 1H), 6.82 (td, J = 8.9, 2.4 Hz, 1H), 4.25 – 4.16 (m, 4H), 3.44 (d, J = 9.1 Hz, 1H), 3.24 – 3.04 (m, 3H), 2.86 – 2.65 (m, 2H), 1.27 (t, J = 7.2 Hz, 6H). 13C NMR (126 MHz, CDCl3) δ 168.8, 163.2, 161.2, 144.2 (d, J = 8.2 Hz), 137.4 (d, J = 2.5 Hz), 125.1 (d, J = 8.8 Hz), 113.2 (d, J = 22.6 Hz), 111.4 (d, J = 21.9 Hz), 61.6, 56.8, 39.7, 37.5, 37.5, 36.6, 14.2; IR (film) 2982, 1745, 1727, 1614, 1599, 1483 cm−1; HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C16H19FO4 295.1267; found 295.134.
(±)-(1S,2R)-Diethyl 2-(1-methyl-2,3-dihydro-1H-inden-2-yl)malonate (6d).
The title compound was prepared from 2-(but-3-en-2-yl)phenyl triflate (56.0 mg, 0.2 mmol), and diethyl malonate (37 μL, 0.24 mmol) using General Procedure A, except with (Brettphos)Pd(allyl)(Cl) (8.6 mg, 0.04 equiv) in place of Pd(OAc)2. The crude material was purified via column chromatography on silica gel using 95:5 hexanes:ethyl acetate as the eluent. This procedure afforded 43.1 mg (75%) of the title compound as a colorless oil. The compound was obtained as a >20:1 mixture of diastereomers as judged by 1H NMR analysis. Data are for the major isomer. 1H NMR (401 MHz, CDCl3) δ 7.23 – 7.10 (m, 4H), 4.24 – 4.15 (m, 4H) 3.51 (d, J = 8.2 Hz, 1H), 3.24 (dd, J = 16.1, 8.1 Hz, 1H), 3.08 (p, J = 6.8 Hz, 1H), 2.81 (dd, J = 16.1, 7.3 Hz, 1H), 2.69 (p, J = 8.0 Hz, 1H), 1.32 – 1.22 (m, 9H); 13C NMR (176 MHz, CDCl3) δ 169.1, 168.8, 147.0, 141.4, 126.7, 126.66, 124.5, 123.6, 61.51, 61.45, 55.6, 46.9, 43.4, 35.8, 19.8, 14.3, 14.2; IR (film) 1741, 1728, 1464 cm−1; HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C17H22O4 291.1518; found 291.1591.
(1S,2S)-Diethyl 2-(1-phenyl-2,3-dihydro-1H-inden-2-yl)malonate (6e).
The title compound was prepared from 2-cinnamylphenyl trifluoromethanesulfonate (75.2 mg, 0.22 mmol) and diethyl malonate (0.1 mL, 0.6 mmol), Pd(OAc)2 (1.8 mg, 0.008 mmol, 0.04 equiv), Brettphos (6.4 mg, 0.012 mmol, 0.06 equiv), with a reaction temperature of 65 °C for five hours using General Procedure B. The crude material was purified via column chromatography on silica gel using 98:2 -> 95:5 hexanes:ethyl acetate as the eluent. This procedure afforded 39.8 mg (51%) of the title compound as a colorless oil. The compound was obtained as a >20:1 mixture of diastereomers as judged by 1H NMR analysis. Data are for the major isomer. 1H NMR (500 MHz, Benzene-d6) δ 7.22 – 6.97 (m, 7H), 6.93 (t, J = 7.4 Hz, 1H), 6.75 (d, J = 7.5 Hz, 1H), 4.27 (d, J = 9.0 Hz, 1H), 3.92 – 3.79 (m, 2H), 3.73 – 3.52 (m, 3H), 3.45 (dd, J = 15.7, 7.9 Hz, 1H), 3.32 – 3.22 (m, 1H), 2.96 (dd, J = 15.8, 8.9 Hz, 1H), 0.83 (t, J = 7.1 Hz, 3H), 0.73 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, Benzene-d6) δ 168.1, 167.9, 145.9, 143.4, 142.0, 127.8, 127.7, 127.6, 127.5, 126.6, 124.9, 124.2, 60.7, 60.6, 55.0 (2 peaks), 48.9, 36.0, 13.7, 13.4. IR (film) 1730.5, 1601.2 cm−1. HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C22H24O4 353.1753; found 353.1747.
(±)-(1S,2S)-Diethyl 2-(5-fluoro-1-phenyl-2,3-dihydro-1H-inden-2-yl)malonate (6f).
The title compound was prepared from 2-cinnamyl 4-fluorophenyl trifluoromethanesulfonate (64.7 mg, 0.18 mmol) and diethyl malonate (0.1 mL, 0.6 mmol), palladium acetate (1.8 mg, 0.008 mmol, 0.04 equiv), Brettphos (6.4 mg, 0.012 mmol, 0.06 equiv), with a reaction temperature of 65 °C for five hours using General Procedure B. The crude material was purified via column chromatography on silica gel using 98:2 -> 95:5 hexanes:ethyl acetate as the eluent. This procedure afforded 31.0 mg (47%) of the title compound as a colorless oil. The compound was obtained as a >20:1 mixture of diastereomers as judged by 1H NMR analysis. Data are for the major isomer. 1H NMR (500 MHz, C6D6) δ 7.16 – 6.94 (m, 5H), 6.69 (dd, J = 9.0, 2.4 Hz, 1H), 6.60 (t, J = 8.7 Hz, 1H), 6.45 (d, J = 8.2 Hz, 1H), 4.13 (d, J = 8.4 Hz, 1H), 3.95 – 3.76 (m, 2H), 3.65 (dd, J = 10.8, 7.1 Hz, 1H), 3.61 – 3.46 (m, 2H), 3.29 – 3.17 (m, 2H), 2.80 (dd, J = 15.1, 8.0 Hz, 1H), 0.84 (t, J = 7.1 Hz, 3H), 0.73 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, C6D6) δ 168.0 (d, J = 34 Hz), 163.5 161.5, 144.1 (d, J = 9 Hz), 143.1, 141.3 (d, J = 1 Hz), 128.8, 127.9, 126.7, 126.0, 113.5 (d, J = 25 Hz), 111.1 (d, J = 23 Hz), 60.8 (d, J = 14 Hz), 54.5, 54.1, 49.2, 35.8 (2 peaks), 13.7, 13.4. IR (film) 1728.0, 1600.2, 1483.7 cm−1. HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C22H23FO4 371.1659; found 371.1653.
(±)-(1S,2S)-Diethyl 2-(5-methoxy-1-phenyl-2,3-dihydro-1H-inden-2-yl)malonate (6g).
The title compound was prepared from 2-cinnamyl 4-methoxyphenyl trifluoromethanesulfonate (81.6 mg, 0.23 mmol), diethyl malonate (0.1 mL, 0.6 mmol), palladium acetate (1.8 mg, 0.008 mmol, 0.04 equiv), Brettphos (6.4 mg, 0.012 mmol, 0.06 equiv), with a reaction temperature of 65 °C for five hours using General Procedure B. The crude material was purified via column chromatography on silica gel using 98:2 -> 95:5 hexanes:ethyl acetate as the eluent. This procedure afforded 29.2 mg (35%) of the title compound as a colorless oil. The compound was obtained as a >20:1 mixture of diastereomers as judged by 1H NMR analysis. Data are for the major isomer. 1H NMR (500 MHz, C6D6) δ 7.19 (d, J = 7.2 Hz, 2H), 7.10 (s, 3H), 7.01 (s, 1H), 6.69 (d, J = 19.9 Hz, 2H), 6.61 (d, J = 10.3 Hz, 1H), 4.26 (d, J = 8.5 Hz, 1H), 3.97 – 3.78 (m, 2H), 3.76 – 3.53 (m, 3H), 3.49 – 3.36 (m, 1H), 3.30 (s, 3H), 3.04 – 2.87 (m, 1H), 0.84 (t, J = 7.1 Hz, 3H), 0.74 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, C6D6) δ 168.2, 167.2, 159.5, 143.9, 143.4, 137.8, 128.8, 128.3, 127.9, 127.8, 126.5, 125.6, 113.0, 109.5, 60.7, 54.8, 54.6, 54.3, 49.4, 36.1, 13.7. IR (film) 1727.7, 1608.5, 1588.8, cm−1. HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C23H26O5 383.1859; found 383.1853.
(±)-(1S,2S)-Diethyl 2-(5-methyl-1-phenyl-2,3-dihydro-1H-inden-2-yl)malonate (6h).
The title compound was prepared from 2-cinnamyl 4-methylphenyl trifluoromethanesulfonate (5h) (81.6 mg, 0.23 mmol) and diethyl malonate (0.1 mL, 0.6 mmol) using General Procedure B. The crude material was purified via column chromatography on silica gel using 95:5 hexanes:ethyl acetate as the eluent. This procedure afforded 29.2 mg (35%) of the title compound as a colorless oil. The compound was obtained as a >20:1 mixture of diastereomers as judged by 1H NMR analysis. Data are for the major isomer. 1H NMR (500 MHz, C6D6) δ 7.23 – 7.14 (m, 1H), 7.09 (dd, J = 15.1, 7.6 Hz, 3H), 7.04 – 6.97 (m, 1H), 6.87 (s, 1H), 6.78 (d, J = 7.7 Hz, 1H), 6.70 (d, J = 7.7 Hz, 1H), 4.28 (d, J = 8.7 Hz, 1H), 3.96 – 3.78 (m, 2H), 3.73 – 3.53 (m, 3H), 3.44 (dd, J = 15.7, 7.9 Hz, 1H), 3.37 – 3.25 (m, 1H), 2.97 (dd, J = 15.7, 8.7 Hz, 1H), 2.11 (s, 3H), 0.84 (t, J = 7.2 Hz, 3H), 0.74 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, C6D6) δ 168.2, 167.9, 143.7, 142.9, 142.1, 136.2, 128.9, 128.3, 127.5, 126.5, 124.9, 124.7, 60.7, 60.6, 54.8, 54.7, 49.2, 35.9, 20.9, 13.7, 13.6. IR (film) 2980.6, 1728.9, 1602.0, 1493.5, 1453.4 cm−1. HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C23H26O4 367.1909; found 367.1904.
Diethyl 2-[5-(tert-butyl)-2-methyl-2,3-dihydro-1H-inden-2-yl]malonate (6i).
The title compound was prepared from 4-tert-butyl-2-(2-methylallyl)phenyl triflate (67.3 mg, 0.2 mmol) and diethyl malonate (37 μL, 0.24 mmol) using General Procedure A. The crude material was purified via column chromatography on silica gel using 99:1 hexanes:ethyl acetate as the eluent. This procedure afforded 9.7 mg (14%) of the title compound as a colorless oil.1H NMR (401 MHz, CDCl3) δ 7.21 – 7.15 (m, 2H), 7.12 – 7.06 (m, 2H), 4.25 – 4.12 (m, 4H), 3.56 (s, 1H), 3.24 – 3.12 (m, 2H), 2.86 – 2.74 (m, 2H), 1.30 (s, 9H), 1.29 – 1.24 (m, 9H); 13C NMR (100 MHz, CDCl3) δ 168.53, 149.43, 141.54, 138.71, 124.11, 123.37, 121.54, 61.01, 60.38, 45.55, 44.97, 44.44, 34.49, 31.55, 24.41, 14.08; IR (film) 2955, 1721, 1727, 1033 cm−1. HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C21H30O4 347.2217; found 347.2220.
(±)-(2S,3aR)-Diethyl 2-(2,3,3a,4,5,6-hexahydro-1H-inden-2-yl)malonate (7a).
The title compound was prepared from 6-allylcyclohex-1-en-1-yl triflate (54.1 mg, 0.2 mmol) and diethyl malonate (37 μL, 0.24 mmol) using General Procedure A. The crude material was purified via column chromatography on silica gel using 98:2 hexanes:ethyl acetate as the eluent. This procedure afforded 45.4 mg (84%) of the title compound as a colorless oil. The compound was obtained as a >20:1 mixture of diastereomers as judged by 1H NMR analysis. Data are for the major isomer. 1H NMR (500 MHz, CDCl3) δ 5.37 (s, 1H), 4.19 (qd, J = 7.1, 3.1 Hz, 4H), 3.17 (d, J = 9.9 Hz, 1H), 2.70 – 2.52 (m, 2H), 2.22 (br s, 1H), 2.11 – 2.01 (m, 1H), 2.03 – 1.91 (m, 4H), 1.77 (ddd, J = 12.3, 6.0, 3.2 Hz, 1H), 1.43 (m, 1H), 1.26 (td, J = 7.1, 1.9 Hz, 6H), 0.99 (m, 1H), 0.88 (q, J = 11.5 Hz, 1H); 13C NMR (125 MHz, CDCl3) δ 169.1, 169.0, 142.8, 118.1, 61.4, 57.8, 41.0, 38.6, 37.1, 35.3, 28.9, 25.3, 22.5, 14.3; IR (film) 1730, 1029 cm−1; HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C16H24O4 281.1675; found 281.1747.
(±)-(6S,7aR)-Diethyl 2-(1,3,5,6,7,7a-hexahydrocyclopenta[c]pyran-6-yl)malonate (7b).
The title compound was prepared from 3-allyl-3,6-dihydro-2H-pyran-4-yl triflate (54.4 mg, 0.2 mmol) and diethyl malonate (37 μL, 0.24 mmol) using General Procedure A. The crude material was purified via column chromatography on silica gel using 95:5 -> 90:10 hexanes:ethyl acetate as the eluent. This procedure afforded 53.6 mg (95%) of the title compound as a colorless oil. The compound was obtained as a >20:1 mixture of diastereomers as judged by 1H NMR analysis. Data are for the major isomer. 1H NMR (500 MHz, CDCl3) δ 5.40 (s, 1H), 4.25 – 4.09 (m, 6H), 4.09 – 3.99 (m, 1H), 3.19 (d, J = 9.2 Hz, 1H), 3.03 (t, J = 10.2 Hz, 1H), 2.75 – 2.63 (m, 2H), 2.56 (br s, 1H), 2.11 – 1.96 (m, 2H), 1.26 (t, J = 7.1 Hz, 6H), 0.87 (q, J = 11.5 Hz, 1H); 13C NMR (101 MHz, CDCl3) δ 168.8, 140.8, 116.8, 69.3, 65.1, 61.5, 57.4, 39.8, 37.1, 34.7, 34.0, 14.3; IR (film) 1750, 1727, 1461 cm−1; HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C15H22O5 305.1359; found 305.1362.
(±)-(6S,7aR)-Diethyl 2-[2-(tert-butoxycarbonyl)-2,3,5,6,7,7a-hexahydro-1H-cyclopenta[c]pyridin-6-yl]malonate (7c).
The title compound was prepared from tert-butyl 3-allyl-4-{[(trifluoromethyl)sulfonyl]oxy}−3,6-dihydropyridine-1(2H)-carboxylate (67.1 mg, 0.2 mmol) and diethyl malonate (37 μL, 0.24 mmol) using General Procedure A. The crude material was purified via column chromatography on silica gel using 25:75 hexanes:dichloromethane as the eluent. This procedure afforded 52.3 mg (76%) of the title compound as a colorless oil. Characterization data are for a mixture of rotamers; broadening is due to slow interconversion on the NMR time scale. 1H NMR (500 MHz, C6D6) δ 5.09 (s, 1H), 4.57 – 4.15 (m, 2H), 4.11 – 3.82 (br m, 4H), 3.43 – 3.37 (m, 1H), 3.18 – 3.15 (m, 1H), 2.81 – 2.53 (m, 2H), 2.25 (br s, 1H), 2.21 – 2.12 (m, 1H), 2.07 – 1.95 (br m, 1H), 1.94 – 1.81 (m, 1H), 1.55 – 1.38 (m, 9H), 1.04 – 0.90 (m, 6H), 0.86 – 0.74 (m, 1H). 13C NMR (100 MHz, CDCl3) δ 168.7, 79.7, 61.5, 57.3, 40.2, 37.5, 35.1, 34.8, 28.6, 28.4, 14.2; IR (film) 1751, 1729, 1693 cm−1; HRMS (ESI+ TOF) m/z: 404.2044 (404.2044 calcd for C20H31NO6, M+Na+). HRMS (ESI+ TOF) m/z: [M + Na+]+ calcd for C20H31NO6 404.2044; found 404.2044.
(±)-(6S,7aR)-diethyl 2-(2,3,5,6,7,7a-hexahydro-1H-cyclopenta[c]pyridin-6-yl)malonate (S12).
In order to confirm the connectivity and stereochemistry of 7c, the boc group was cleaved by addition of trifluoroacetic acid (3 drops) to a solution of 7c in dichloromethane; the resulting solution was stirred for 1 hour at rt. The solvent and excess trifluoroacetic acid were removed under reduced pressure and the product was washed with NaHCO3, brine, and dried with MgSO4. This procedure afforded 34.3 mg (89%) of the title compound as a colorless oil. This material was judged to be a >20:1 mixture of diastereomers by 1H NMR analysis. Data are for the major isomer. 1H NMR (400 MHz, CDCl3) δ 5.38 (s, 1H), 4.18 (q, J = 7.1 Hz, 4H), 3.37 – 3.29 (m, 2H), 3.18 (d, J = 9.5 Hz, 1H), 2.73 – 2.59 (m, 2H), 2.40 (br s, 1H), 2.33 – 2.23 (m, 1H), 2.11 – 1.97 (m, 2H), 1.49 – 1.37 (m, 1 H), 1.26 (t, J = 7.1 Hz, 6H), 0.88 (q, J = 11.3 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 168.9, 168.8, 141.8, 116.8, 61.4, 57.5, 48.3, 44.5, 40.4, 36.9, 35.7, 35.0, 14.2; IR (film) 2983, 1782, 1730, 1148 cm−1; HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C15H23NO4 282.1700; found 282.1706.
(±)-(2S,3aR)-Diethyl 2-(2-methyl-2,3,3a,4,5,6-hexahydro-1H-inden-2-yl)malonate (7d).
The title compound was prepared from 6-(2-methylallyl)cyclohex-1-en-1-yl triflate (54.1 mg, 0.2 mmol) and diethyl malonate (37 μL, 0.24 mmol) using General Procedure A. The crude material was purified via column chromatography on silica gel using 95:5 hexanes:ethyl acetate as the eluent. This procedure afforded 55.6 mg (94%) of the title compound as a colorless oil. The compound was obtained as a 9:1 mixture of diastereomers as judged by 1H NMR analysis. Data are for the major isomer. 1H NMR (500 MHz, CDCl3) δ 5.35 (s, 1H), 4.17 (q, J = 7.1 Hz, 4H), 3.32 (s, 1H), 2.48 (d, J = 17.0 Hz, 1H), 2.39 (br s, 1H), 2.28 (d, J = 17.0 Hz, 1H), 2.02 – 1.90 (m, 3H), 1.87 – 1.73 (m, 2H), 1.45 (tddd, J = 13.4, 10.3, 7.5, 2.9 Hz, 1H), 1.28 – 1.21 (m, 10H), 1.03 – 0.92 (m, 1H); 13C NMR (126 MHz, CDCl3) δ 168.8, 143.1, 118.1, 61.7, 61.3, 61.04, 61.03, 45.9, 44.1, 41.6, 38.7, 38.6, 29.0, 28.9, 25.3, 24.9, 22.6, 22.5, 14.3; IR (film) 1753, 1729 cm−1; HRMS (ESI+ TOF) m/z: [M + Na+]+ calcd for C17H26O4 317.1723; found 317.1729.
(±)-(2S,3aS)-Diethyl 2-(3a-allyl-1,2,3,3a,4,5-hexahydropentalen-2-yl)malonate (7e).
The title compound was prepared from 5,5-diallylcyclopent-1-en-1-yl triflate (60 mg, 0.2 mmol) and diethyl malonate (37 μL, 0.24 mmol) using General Procedure A. The crude material was purified via column chromatography on silica gel using 95:5 hexanes:ethyl acetate as the eluent. This procedure afforded 56.6 mg (92%) of the title compound as a colorless oil. The compound was obtained as a >20:1 mixture of diastereomers as judged by 1H NMR analysis. Data are for the major isomer. 1H NMR (700 MHz, CDCl3) δ 5.79 (dddd, J = 16.9, 10.1, 7.9, 6.6 Hz, 1H), 5.22 (s, 1H), 5.05 (d, J = 17.0 Hz, 1H), 5.02 (d, J = 10.1 Hz, 1H), 4.22 – 4.12 (m, 4H), 3.25 (d, J = 10.3 Hz, 1H), 3.09 (qt, J = 10.3, 6.4 Hz, 1H), 2.63 – 2.50 (m, 2H), 2.42 – 2.36 (m, 2H), 2.12 (dd, J = 13.8, 6.6 Hz, 1H), 2.07 (dd, J = 13.8, 8.0 Hz, 1H), 1.98 – 1.92 (m, 2H), 1.56 (dt, J = 12.4, 9.5 Hz, 1H), 1.25 (td, J = 7.1, 4.4 Hz, 6H), 1.03 (dd, J = 12.0, 10.5 Hz, 1H); 13C NMR (176 MHz, CDCl3) δ 168.9, 168.8, 153.9, 136.1, 119.1, 116.9, 61.41, 61.37, 59.3, 58.2, 41.5, 40.3, 39.9, 36.9, 36.1, 28.2, 14.3; IR (film) 1732, 1150 cm−1; HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C18H26O4 307.1904; found 307.1909.
(±)-(2S,3aR)-Diethyl 2-allyl-2-(2-methyl-1,2,3,3a,4,5-hexahydropentalen-2-yl)malonate (7f).
The title compound was prepared from (methylallyl)cyclopent-1-en-1-yl triflate (54.0 mg, 0.2 mmol), and diethyl allylmalonate (48 μL, 0.24 mmol) using General Procedure A, except with RuPhos (5.6 mg, 0.012 mmol) as ligand. The crude material was purified via column chromatography on silica gel using 95:5 hexanes:ethyl acetate as the eluent. This procedure afforded 40.8 mg (63%) of the title compound as a colorless oil. The compound was obtained as a 19:1 mixture of diastereomers as judged by 1H NMR analysis. NOTE: The alkene was observed to isomerize in CDCl3 to a 4:1 mixture of regioisomers. Data are for the major isomer. 1H NMR (401 MHz, CDCl3) δ 5.90 (ddt, J = 17.1, 10.1, 7.1 Hz, 1H), 5.22 (s, 1H), 5.14 – 4.92 (m, 2H), 4.15 (m, 4H), 3.12 – 2.95 (br m, 1H), 2.82 – 2.38 (m, 5H), 2.10 (dtd, J = 12.0, 6.8, 1.2 Hz, 1H), 1.98 (ddd, J = 17.4, 4.5, 2.3 Hz, 1H), 1.83 – 1.60 (m, 2H), 1.50 – 1.33 (m, 1H), 1.24 (q, J = 7.3 Hz, 6H), 1.15 (s, 3H); 13C NMR (126 MHz, CDCl3) δ 170.83, 170.79, 152.5, 135.2, 118.0, 117.5, 110.0, 65.5, 60.7, 51.9, 49.4, 43.2, 37.7, 37.3, 37.0, 32.4, 26.5, 14.1; IR (film) 1741, 1723, 1206 cm−1; HRMS (ESI+ TOF) m/z: [M + Na+]+ calcd for C19H28O4 343.199; found 343.188.
(±)-(1R,2S,3aR)-Diethyl 3a-(ethoxycarbonyl)-1,2,3,3a,4,5-hexahydropentalen-2-yl)malonate (7g).
The title compound was prepared from ethyl 1-allyl-2-{[(trifluoromethyl)sulfonyl]oxy}cyclopent-2-ene-1-carboxylate (69.9 mg, 0.213 mmol) and diethyl malonate (36 μL, 0.24 mmol), Pd(OAc)2 (1.8 mg, 0.008 mmol, 0.04 equiv), BrettPhos (4.9 mg, 0.012 mmol, 0.06 equiv), at 95 °C for 14 h using General Procedure B. The crude material was purified via column chromatography on silica gel using 98:2 -> 95:5 hexanes:ethyl acetate as the eluent. This procedure afforded 48.2 mg (67%) of the title compound as a colorless oil. The compound was obtained as a >20:1 mixture of diastereomers as judged by 1H NMR analysis. Data are for the major isomer. 1H NMR (500 MHz, CDCl3) δ 5.44 (s, 1H), 4.22–4.09 (m, 6H), 3.27 (d, J = 9.7 Hz, 1H), 3.17 (qd, J = 10.3, 5.0 Hz, 1H), 2.80 (dt, J = 14.9, 8.0 Hz, 1H), 2.66 – 2.53 (m, 1H), 2.46 (m, 4H), 2.04 (dd, J = 16.4, 6.0 Hz, 1H), 1.75 (dt, J = 12.8, 9.6 Hz, 1H), 1.27–1.23 (m, 9H). 13C NMR (126 MHz, CDCl3) δ 175.6, 168.5, 168.5, 149.6, 122.9, 65.5, 61.3, 60.7, 60.6, 57.6, 40.8, 40.6, 37.6, 36.9, 36.8, 28.8, 14.2, 14.1. IR (film) 2980.3, 2933.2, 2855.3, 1724.2, 1446.3 cm−1. HRMS (ESI+ TOF) m/z: [M + Na+]+ calcd for C18H26O6Na 361.1622; found 361.1617.
(±)-(1S,2S,3aS)-Diethyl 2-[3a-(ethoxycarbonyl)-1-phenyl-2,3,3a,4,5,6-hexahydro-1H-inden-2-yl] malonate (7h).
The title compound was prepared from ethyl 1-cinnamyl-2-{[(trifluoromethyl)sulfonyl]oxy}cyclohex-2-ene-1-carboxylate (1i) (83.0 mg, 0.20 mmol) and diethyl malonate (0.1 mL, 0.6 mmol), Pd(acac)2 (2.4 mg, 0.008 mmol, 0.04 equiv), SPhos (4.9 mg, 0.012 mmol, 0.06 equiv), at 95 °C for fourteen hours using General Procedure B. The crude material was purified via column chromatography on silica gel using 95:5 -> 90:10 hexanes:ethyl acetate as the eluent. This procedure afforded 62.5 mg (73%) of the title compound as a colorless oil. The compound was obtained as a >20:1 mixture of diastereomers as judged by 1H NMR analysis. Data are for the major isomer. 1H NMR (500 MHz, C6D6) δ 7.64 (d, J = 7.6 Hz, 2H), 7.21 – 7.09 (m, 2H), 7.02 (t, J = 7.3 Hz, 1H), 5.33 (q, J = 3.2 Hz, 1H), 4.06 – 3.78 (m, 4H), 3.59 (dq, J = 10.5, 7.0 Hz, 1H), 3.49 (d, J = 6.9 Hz, 1H), 3.44 (dq, J = 10.8, 7.2 Hz, 1H), 3.14 – 2.95 (m, 2H), 2.37 (dt, J = 12.6, 3.5 Hz, 1H), 1.94 – 1.82 (m, 1H), 1.73 (dtd, J = 18.3, 7.4, 3.8 Hz, 1H), 1.61 – 1.38 (m, 3H), 1.37 – 1.22 (m, 1H), 1.12 (td, J = 13.1, 3.8 Hz, 1H), 1.01 – 0.91 (m, 3H), 0.84 (dt, J = 17.9, 7.1 Hz, 3H), 0.70 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, C6D6) δ 175.1, 167.9, 167.6, 144.5, 143.3, 129.4, 129.3, 127.5, 126.3, 123.8, 60.8, 60.6, 54.2, 53.5, 53.4, 44.6, 41.9, 33.0, 24.5, 19.2, 13.9, 13.7, 13.3. IR (film) 1724.1, 1605.6, 1446.6 cm−1. HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C25H32O6 429.2277; found 429.2272.
(±)-(1R,2S,3aR)-Diethyl 2-(1-phenyl-2,3,3a,4,5,6-hexahydro-1H-inden-2-yl)malonate (7i).
The title compound was prepared from 6-cinnamylcyclohex-1-en-1-yl trifluoromethanesulfonate (1i) (69.0 mg, 0.20 mmol) and diethyl malonate (0.1 mL, 0.6 mmol), Pd(acac)2 (2.4 mg, 0.008 mmol, 0.04 equiv), SPhos (4.9 mg, 0.012 mmol, 0.06 equiv), at 95 °C for fourteen hours using General Procedure B. The crude material was purified via column chromatography on silica gel using 98:2 hexanes:ethyl acetate as the eluent. This procedure afforded 54.1 mg (76%) of the title compound as a colorless oil. The compound was obtained as a >20:1 mixture of diastereomers as judged by 1H NMR analysis. Data are for the major isomer. 1H NMR (500 MHz, CDCl3) δ 7.32 – 7.21 (m, 2H), 7.21 – 7.11 (m, 3H), 5.23 (p, J = 2.9 Hz, 1H), 4.15 (p, J = 7.0 Hz, 2H), 3.82 (dq, J = 10.7, 7.1 Hz, 1H), 3.63 (dq, J = 10.8, 7.2 Hz, 1H), 3.40 (dt, J = 9.6, 2.6 Hz, 1H), 3.34 (d, J = 8.1 Hz, 1H), 2.78 – 2.66 (m, 1H), 2.58 – 2.45 (m, 1H), 2.31 (dt, J = 12.0, 6.1 Hz, 1H), 2.09 – 1.88 (m, 3H), 1.79 (dt, J = 12.7, 3.9 Hz, 1H), 1.51 – 1.38 (m, 1H), 1.24 (t, J = 7.1 Hz, 3H), 1.16 – 0.95 (m, 5H). 13C NMR (126 MHz, CDCl3) δ 168.6, 168.3, 147.6, 145.2, 128.2, 128.1, 126.1, 120.3, 61.2, 61.0, 55.5, 53.1, 46.4, 41.3, 37.5, 28.8, 25.1, 22.2, 14.1, 13.7. IR (film) 1730.9, 1601.5 cm−1. HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C22H28O4 357.2066; found 357.2060.
(±)-(1S,2S,3aS)-Diethyl 2-benzyl-2-[3a-(ethoxycarbonyl-1-phenyl-2,3,3a,4,5,6-hexahydro-1H-inden-2-yl]malonate (7j).
The title compound was prepared from ethyl 1-cinnamyl-2-{[(trifluoromethyl)sulfonyl]oxy}cyclohex-2-ene-1-carboxylate (1h) (85.4 mg, 0.20 mmol) and benzyl diethyl malonate (0.1 mL, 0.43 mmol), Pd(acac)2 (2.4 mg, 0.008 mmol, 0.04 equiv), SPhos (4.9 mg, 0.012 mmol, 0.06 equiv), for 14 hours using General Procedure B, except with xylenes as solvent at 110 °C. The crude material was purified via column chromatography on silica gel using 98:2 -> 95:5 hexanes:ethyl acetate as the eluent. This procedure afforded 53.3 mg (51%) of the title compound as a colorless oil. The compound was obtained as a >20:1 mixture of diastereomers as judged by 1H NMR analysis. Data are for the major isomer. 1H NMR (500 MHz, CDCl3) δ 7.38 – 7.21 (m, 4H), 7.16 – 7.08 (m, 3H), 7.04 (dd, J = 7.1, 2.4 Hz, 2H), 5.43 (d, J = 2.5 Hz, 1H), 4.15 (dd, J = 29.8, 7.1 Hz, 2H), 4.03 – 3.84 (m, 2H), 3.70 (s, 2H), 3.10 (d, J = 13.9 Hz, 1H), 3.01 (d, J = 14.1 Hz, 1H),), 2.83 (dd, J = 12.6, 6.2 Hz, 1H), 2.27 (dt, J = 12.7, 3.5 Hz, 1H), 2.18 – 1.90 (m, 2H), 1.73 – 1.46 (m, 2H), 1.25 (dt, J = 14.2, 8.3 Hz, 5H), 1.11 (t, J = 7.1 Hz, 3H), 0.97 (t, J = 7.2 Hz, 3H), 0.88 (t, J = 6.7 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 175.9, 170.0, 169.9, 144.9, 144.5, 137.0, 130.2, 128.6, 128.0, 127.8, 126.6, 123.4, 62.2, 60.9, 60.8, 52.4, 51.8, 48.2, 40.9, 40.4, 33.6, 31.6, 24.5, 22.6, 18.9, 14.1, 13.9, 13.7. IR (film) 1720.8, 1601.5, 1495.7 cm−1. HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C32H38O6 519.2747; found 519.2741.
(±)-(1R,2S,3aS)-Diethyl 2-[3a-(ethoxycarbonyl)-1-methyl-2,3,3a,4,5,6-hexahydro-1H-inden-2-yl]malonate (7k).
The title compound was prepared from ethyl (E)-1-(but-2-en-1-yl)-2-{[(trifluoromethyl)sulfonyl]oxy}cyclohex-2-ene-1-carboxylate (1j) (73.5 mg, 0.21 mmol) and diethyl malonate (0.1 mL, 0.6 mmol), Pd(acac)2 (2.4 mg, 0.008 mmol, 0.04 equiv), SPhos (4.9 mg, 0.012 mmol, 0.06 equiv), at 95 °C for fourteen hours using General Procedure B. The crude material was purified via column chromatography on silica gel using 98:2 -> 95:5 hexanes:ethyl acetate as the eluent. This procedure afforded 40.1 mg (52%) of the title compound as a colorless oil. The compound was obtained as a >20:1 mixture of diastereomers as judged by 1H NMR analysis. Data are for the major isomer. 1H NMR (500 MHz, CDCl3) δ 5.54 (q, J = 3.1 Hz, 1H), 4.17 (ddt, J = 11.1, 7.6, 4.4 Hz, 6H), 3.39 – 3.30 (m, 1H), 3.26 (d, J = 6.0 Hz, 1H), 2.44 – 2.31 (m, 2H), 2.23 (dt, J = 12.5, 3.4 Hz, 1H), 2.18 – 2.07 (m, 1H), 1.98 (ddq, J = 14.6, 7.1, 3.5 Hz, 2H), 1.76 – 1.59 (m, 2H), 1.53 (ddd, J = 13.9, 6.9, 3.5 Hz, 1H), 1.26 (t, J = 7.1 Hz, 9H), 1.12 (d, J = 6.7 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 175.5, 169.0, 168.4, 141.8, 114.8, 114.4, 61.3, 61.1 (2 peaks), 60.6, 54.8, 51.0, 49.7, 37.9, 31.7, 24.2, 19.6, 16.3, 14.1, 14.0. IR (film) 1725.2 (2 peaks), 1446.7 cm−1. HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C20H30O6 367.2121; found 367.2115.
(±)-(1S,2S,3aS)-Di-tert-butyl 2-[3a-(ethoxycarbonyl)-1-phenyl-2,3,3a,4,5,6-hexahydro-1H-inden-2-yl]malonate (7l).
The title compound was prepared from ethyl 1-cinnamyl-2-{[(trifluoromethyl)sulfonyl]oxy}cyclohex-2-ene-1-carboxylate (1h) (83.6 mg, 0.20 mmol) and di-tert-butyl malonate (0.1 mL, 0.4 mmol) using General Procedure B. The crude material was purified via column chromatography on silica gel using 95:5 hexanes:ethyl acetate as the eluent. This procedure afforded 64.1 mg (66%) of the title compound as a colorless oil. The compound was obtained as a >20:1 mixture of diastereomers as judged by 1H NMR analysis. Data are for the major isomer. 1H NMR (500 MHz, CDCl3) δ 7.44 – 7.35 (m, 2H), 7.27 (t, J = 7.4 Hz, 2H), 7.22 – 7.13 (m, 1H), 5.44 (q, J = 3.2 Hz, 1H), 4.20 (q, J = 7.2 Hz, 2H), 3.53 (dt, J = 11.0, 2.9 Hz, 1H), 3.20 (d, J = 5.8 Hz, 1H), 2.67 (dd, J = 12.2, 5.5 Hz, 1H), 2.63 – 2.49 (m, 1H), 2.34 (dt, J = 12.4, 3.3 Hz, 1H), 2.11 (ddd, J = 14.1, 7.2, 3.4 Hz, 1H), 2.03 (dq, J = 11.2, 3.8 Hz, 1H), 1.77 – 1.56 (m, 3H), 1.47 (m, 11H), 1.39 – 1.22 (m, 11H). 13C NMR (126 MHz, CDCl3) δ 176.0, 167.8, 167.7, 144.4, 143.2, 128.9, 128.2, 126.3, 123.8, 81.5, 81.3, 60.8, 55.1, 53.4, 52.9, 44.2, 40.8, 33.3, 28.0, 27.7, 24.5, 19.1, 14.3. IR (film) 2977.9, 2934.0, 1722.4 (2 peaks), 1496.0, 1453.7 cm−1. HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C29H40O6 485.2903; found 485.2898.
(±)-(1R,2S,3aR)-Di-tert-butyl 2-(1-phenyl-2,3,3a,4,5,6-hexahydro-1H-inden-2-yl)malonate (7m).
The title compound was prepared from 6-cinnamylcyclohex-1-en-1-yl trifluoromethanesulfonate (1i) (73.1 mg, 0.21 mmol) and di-tert-butyl malonate (0.1 mL, 0.4 mmol) using General Procedure B. The crude material was purified via column chromatography on silica gel using 98:2 hexanes:ethyl acetate as the eluent. This procedure afforded 53.9 mg (62%) of the title compound as a white solid, mp: 79–81 °C. The compound was obtained as a >20:1 mixture of diastereomers as judged by 1H NMR analysis. Data are for the major isomer. 1H NMR (500 MHz, CDCl3) δ 7.32 – 7.10 (m, 5H), 5.26 (m, 1H), 3.43 (dt, J = 9.2, 2.4 Hz, 1H), 3.19 (d, J = 7.0 Hz, 1H), 2.69 – 2.56 (m, 1H), 2.56 – 2.43 (m, 1H), 2.31 (dt, J = 12.2, 6.3 Hz, 1H), 2.09 – 1.92 (m, 3H), 1.78 (dt, J = 13.0, 3.7 Hz, 1H), 1.47 (d, J = 1.2 Hz, 9H), 1.26 (s, 9H), 1.22 – 1.10 (m, 2H), 1.07 (dt, J = 12.6, 2.6 Hz, 1H). 13C NMR (126 MHz, CDCl3) δ 168.0, 167.96, 147.7, 145.5, 128.3, 128.0, 126.0, 120.0, 81.4, 81.2, 56.8, 53.1, 46.1, 41.1, 36.8, 29.0, 28.0, 27.7, 25.1, 22.3. IR (film) 2977.9, 2929.2, 1720.2 (2), 1601.8, 1477.5, 1452.7 cm−1. HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C26H36O4 413.2692; found 413.2686.
(±)-(1R,2S,3aR)-Diethyl 2-benzyl-2-(1-phenyl-2,3,3a,4,5,6-hexahydro-1H-inden-2-yl)malonate (7n).
The title compound was prepared from 6-cinnamylcyclohex-1-en-1-yl trifluoromethanesulfonate (1i) (57.6 mg, 0.17 mmol) and benzyl diethyl malonate (0.1 mL, 0.43 mmol) using General Procedure B, except that the reaction was run in xylenes at 110 °C. The crude material was purified via column chromatography on silica gel using 98:2 hexanes:ethyl acetate as the eluent. This procedure afforded 39.0 mg (51%) of the title compound as a colorless oil. The compound was obtained as a >20:1 mixture of diastereomers as judged by 1H NMR analysis. Data are for the major isomer. 1H NMR (500 MHz, CDCl3) δ 7.41 – 7.06 (m, 9H), 6.95 (dd, J = 6.6, 3.0 Hz, 1H), 5.27 (q, J = 2.7 Hz, 1H), 4.05 – 3.87 (m, 3H), 3.88 – 3.76 (m, 1H), 3.70 – 3.61 (m, 1H), 3.20 – 3.14 (m, 2H), 3.09 (d, J = 14.0 Hz, 1H), 2.94 (dt, J = 10.6, 7.2 Hz, 1H), 2.40 (dt, J = 12.2, 7.2 Hz, 1H), 2.04 (ddt, J = 12.4, 5.3, 3.4 Hz, 1H), 1.98 – 1.85 (m, 1H), 1.74 (ddd, J = 12.2, 6.1, 2.9 Hz, 1H), 1.52 – 1.34 (m, 2H), 1.32 – 1.18 (m, 1H), 1.19 – 1.04 (m, 4H), 1.02 (q, J = 7.0 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 170.4, 148.1, 147.5, 136.9, 130.2, 128.3, 127.8, 126.5, 119.2, 62.8, 60.8, 54.7, 51.9, 50.3, 40.5, 39.9, 36.0, 34.6, 29.2, 25.08, 22.1, 13.9. IR (film) 3027.9, 2979.3, 2929.1, 2854.1, 1725.4, 1602.4, 1495.3 cm−1. HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C29H35O4 447.2530; found 447.2530.
(±)-(1R,2S,3aR)-Diethyl 2-(3a-methyl-1-phenyl-2,3,3a,4,5,6-hexahydro-1H-inden-2-yl)malonate (7o)
The title compound was prepared from 6-cinnamyl-6-methylcyclohex-1-en-1-yl trifluoromethanesulfonate (1k) (44.6 mg, 0.12 mmol) and benzyl diethyl malonate (0.1 mL, 0.43 mmol) using General Procedure B. The crude material was purified via column chromatography on silica gel using 98:2 -> 95:5 hexanes:ethyl acetate as the eluent. This procedure afforded 28.4 mg (62%) of the title compound as a colorless oil. The compound was obtained as a >20:1 mixture of diastereomers as judged by 1H NMR analysis. Data are for the major isomer. 1H NMR (500 MHz, CDCl3) δ 7.30 – 7.11 (m, 5H), 5.19 (td, J = 3.6, 2.1 Hz, 1H), 4.24 – 4.07 (m, 2H), 3.77 (dq, J = 10.8, 7.1 Hz, 1H), 3.58 (dq, J = 10.8, 7.2 Hz, 1H), 3.47 (dq, J = 9.0, 2.8 Hz, 1H), 3.35 (d, J = 8.1 Hz, 1H), 2.98 (dddd, J = 11.9, 10.1, 8.1, 5.8 Hz, 1H), 2.11 – 1.90 (m, 3H), 1.82 – 1.60 (m, 3H), 1.38 – 1.15 (m, 8H), 1.02 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 168.6, 168.3, 150.4, 144.3, 128.3, 128.2, 126.1, 120.6, 61.1, 61.0, 55.7, 53.5, 45.7, 43.6, 40.2, 36.8, 25.5, 24.5, 18.1, 14.1, 13.7. IR (film) 2977.3, 2933.5, 1731.4, 1492.9, 1452.0 cm−1. HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C23H31O4 371.2217; found 371.2217.
(±)-(1S,2S,3aS)-Diethyl 2-[3a-(Ethoxycarbonyl)-1-phenyl-1,2,3,3a,4,5-hexahydropentalen-2-yl] malonate (7p).
The title compound was prepared from ethyl 1-cinnamyl-2-{[(trifluoromethyl)sulfonyl]oxy}cyclopent-2-ene-1-carboxylate (1l) (70.9 mg, 0.18 mmol) and diethyl malonate (0.1 mL, 0.6 mmol), Pd(acac)2 (2.4 mg, 0.008 mmol, 0.04 equiv), SPhos (4.9 mg, 0.012 mmol, 0.06 equiv), at 95 °C for fourteen hours using General Procedure B. The crude material was purified via column chromatography on silica gel using 95:5 -> 90:10 hexanes:ethyl acetate as the eluent. This procedure afforded 53.8 mg (72%) of the title compound as a yellow oil. The compound was obtained as a >20:1 mixture of diastereomers as judged by 1H NMR analysis. Data are for the major isomer. 1H NMR (500 MHz, CDCl3) δ 7.32 – 7.08 (m, 4H), 5.58 (dt, J = 3.5, 1.7 Hz, 1H), 4.27 – 4.01 (m, 4H), 4.01 – 3.90 (m, 1H), 3.90 – 3.77 (m, 1H), 3.66 – 3.57 (m, 1H), 3.48 (d, J = 7.3 Hz, 1H), 3.24 (ddt, J = 8.8, 7.1, 1.8 Hz, 1H), 3.01 – 2.87 (m, 1H), 2.77 (dd, J = 12.1, 6.3 Hz, 1H), 2.52 (ddd, J = 15.8, 8.7, 3.4 Hz, 1H), 2.33 (dd, J = 12.6, 6.5 Hz, 1H), 1.93 – 1.78 (m, 1H), 1.45 (t, J = 11.7 Hz, 1H), 1.23 (ddt, J = 25.1, 10.9, 7.2 Hz, 7H), 1.15 – 1.01 (m, 3H). 13C NMR (126 MHz, CDCl3) δ 175.8, 168.2, 166.6, 153.3, 142.8, 128.3, 127.7, 126.3, 125.9, 64.9, 61.5, 61.4, 60.7, 55.1, 54.3, 50.2, 47.1, 41.7, 39.5, 37.1, 29.7, 14.1. IR (film) 1725.0 (2 peaks), 1597.5 cm−1. HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C24H30O6 415.2121; found 415.2115.
(±)-(1R,2S,3aR)-Diethyl 2-(1-phenyl-1,2,3,3a,4,5-hexahydropentalen-2-yl)malonate (7q).
The title compound was prepared from 5-cinnamylcyclopent-1-en-1-yl trifluoromethanesulfonate (1m) (62.4 mg, 0.19 mmol) and diethyl malonate (0.1 mL, 0.6 mmol), Pd(acac)2 (2.4 mg, 0.008 mmol, 0.04 equiv), SPhos (4.9 mg, 0.012 mmol, 0.06 equiv), at 95 °C for fourteen hours using General Procedure B. The crude material was purified via column chromatography on silica gel using 98:2 -> 95:5 hexanes:ethyl acetate as the eluent. This procedure afforded 18.8 mg (30%) of the title compound as a colorless oil. The compound was obtained as a >20:1 mixture of diastereomers as judged by 1H NMR analysis. Data are for the major isomer. 1H NMR (500 MHz, CDCl3) δ 7.33 – 7.22 (m, 3H), 7.22 – 7.10 (m, 3H), 5.25 (tt, J = 3.2, 1.7 Hz, 1H), 4.23 – 4.05 (m, 2H), 3.98 – 3.84 (m, 1H), 3.84 – 3.71 (m, 1H), 3.47 (dd, J = 21.1, 8.3 Hz, 2H), 3.20 (d, J = 6.6 Hz, 1H), 3.10 – 2.97 (m, 1H), 2.59 (ddd, J = 7.0, 3.4, 1.7 Hz, 1H), 2.49 (dd, J = 9.7, 6.2 Hz, 1H), 2.29 (dt, J = 12.3, 6.4 Hz, 1H), 2.15 (dt, J = 13.2, 6.9 Hz, 2H), 1.24 (t, J = 7.1 Hz, 3H), 1.05 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 168.5, 168.4, 155.7, 144.6, 128.3, 127.6, 126.2, 120.2, 61.2, 61.1, 55.7, 52.3, 51.5, 47.2, 37.2, 36.7, 32.1, 14.1, 13.7. IR (film) 2932.7, 2849.4, 1730.9, 1600.8, 1495.7, 1452.3 cm−1. HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C21H26O4 343.1909; found 343.1904.
(±)-(1S,2S,3aS)-Di-tert-butyl 2-[3a-(ethoxycarbonyl)-1-phenyl-1,2,3,3a,4,5-hexahydropentalen-2-yl]malonate (7r).
The title compound was prepared from ethyl 1-cinnamyl-2-{[(trifluoromethyl)sulfonyl]oxy}cyclopent-2-ene-1-carboxylate (1l) (80.8 mg, 0.2 mmol) and di-tert-butyl malonate (0.1 mL, 0.4 mmol) using General Procedure B. The crude material was purified via column chromatography on silica gel using 95:5 hexanes:ethyl acetate as the eluent. This procedure afforded 54.5 mg (58%) of the title compound as a pale yellow oil. The compound was obtained as a >20:1 mixture of diastereomers as judged by 1H NMR analysis. Data are for the major isomer. δ 7.29 – 7.21 (m, 4H), 7.16 (p, J = 3.5, 2.8 Hz, 1H), 5.63 – 5.55 (m, 1H), 4.09 (q, J = 7.2 Hz, 2H), 3.64 (d, J = 8.4 Hz, 1H), 3.33 (dd, J = 6.8, 1.5 Hz, 1H), 3.20 – 3.08 (m, 1H), 2.94 (t, J = 8.5 Hz, 1H), 2.79 (dd, J = 12.3, 6.5 Hz, 1H), 2.53 (ddd, J = 15.7, 9.0, 2.8 Hz, 1H), 2.35 (dd, J = 12.6, 6.5 Hz, 1H), 1.95 – 1.81 (m, 1H), 1.56 – 1.40 (m, 10H), 1.31 (d, J = 1.6 Hz, 9H), 1.17 (td, J = 7.1, 1.5 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 175.9, 167.8, 167.7, 153.3, 143.0, 128.3, 127.7, 126.2, 125.7, 81.6, 81.5, 64.8, 60.6, 56.9, 49.9, 47.0, 39.4, 39.1, 37.1, 28.0, 27.7, 14.1. IR (film) 2977.8, 2933.2, 1721.1 (the carbonyl peaks are incidentally equivalent), 1596.3, 1497.0, 1454.5 cm−1. HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C28H38O6 471.2747; found 471.2741.
(±)-(1S,2S,3aS)-Diethyl 2-benzyl-2-[3a-(ethoxycarbonyl)-1-phenyl-1,2,3,3a,4,5-hexahydro pentalen-2-yl]malonate (7s).
The title compound was prepared from ethyl 1-cinnamyl-2-{[(trifluoromethyl)sulfonyl]oxy}cyclopent-2-ene-1-carboxylate (1l) (89.2 mg, 0.22 mmol) and benzyl diethyl malonate (0.1 mL, 0.43 mmol) using General Procedure B, except that the reaction was run in xylenes at 110 °C. The crude material was purified via column chromatography on silica gel using 98:2 -> 95:5 hexanes:ethyl acetate as the eluent. This procedure afforded 45.4 mg (41%) of the title compound as a colorless oil. The compound was obtained as a >20:1 mixture of diastereomers as judged by 1H NMR analysis. Data are for the major isomer. 1H NMR (500 MHz, CDCl3) δ 7.30 – 7.02 (m, 10H), 5.54 (dt, J = 3.2, 1.5 Hz, 1H), 4.14 – 3.96 (m, 3H), 3.98 – 3.82 (m, 3H), 3.73 – 3.61 (m, 1H), 3.52 (dt, J = 10.3, 7.2 Hz, 1H), 3.19 (s, 2H), 3.02 – 2.81 (m, 2H), 2.52 (ddd, J = 15.6, 8.8, 3.3 Hz, 1H), 2.30 (dd, J = 12.7, 6.8 Hz, 1H), 1.84 (dt, J = 12.7, 9.4 Hz, 1H), 1.62 – 1.49 (m, 1H), 1.17 (t, J = 7.1 Hz, 3H), 1.09 (t, J = 7.2 Hz, 3H), 0.89 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 176.2, 170.3, 170.2, 153.8, 143.7, 136.6, 130.2, 128.2, 127.9, 127.5, 126.7, 125.9, 124.4, 64.0, 63.0, 61.0, 60.8, 60.6, 52.4, 46.0, 40.6, 39.6, 37.9, 37.2, 14.0 (2 peaks), 13.5. IR (film) 2980.3, 1719.6, 1602.1, 1495.9, 1454.1 cm−1. HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C31H36O6 505.2590; found 505.2585.
(±)-(5S,7R)-Diethyl 2-(9-methylene-1-oxaspiro[4.4]nonan-7-yl)malonate (9).
The title compound was prepared from 1-(2-allyltetrahydrofuran-2-yl)vinyl triflate (57.3 mg, 0.2 mmol) and diethyl malonate (37 μL, 0.24 mmol) using General Procedure A. The crude material was purified via column chromatography on silica gel using 95:5 -> 90:10 hexanes:ethyl acetate as the eluent. This procedure afforded 49.0 mg (82%) of the title compound as a colorless oil. The compound was obtained as a >20:1 mixture of diastereomers as judged by 1H NMR analysis. Data are for the major isomer. 1H NMR (500 MHz, C6D6) δ 4.96 (s, 1H), 4.88 (s, 1H), 4.07 – 3.84 (m, 4H), 3.75 – 3.59 (m, 2H), 3.31 – 3.18 (m, 1H), 3.23 – 3.09 (m, 1H), 2.89 (dd, J = 16.4, 7.8 Hz, 1H), 2.29 (dd, J = 12.9, 6.5 Hz, 1H), 2.16 (ddt, J = 16.3, 9.5, 2.6 Hz, 1H), 1.87 – 1.76 (m, 1H), 1.71 – 1.52 (m, 3H), 1.46 (dd, J = 12.9, 10.7 Hz, 1H), 0.91 (t, J = 7.1 Hz, 6H). 13C NMR (126 MHz, C6D6) δ 168.12, 168.09, 154.1, 106.3, 88.0, 66.3, 60.63, 60.62, 57.0, 43.8, 36.8, 35.7, 34.4, 26.0, 13.7; IR (film) 1749, 1728, 1445, 1025 cm−1; HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C16H24O5 297.1624; found 297.1697.
(±)-Diethyl 4-(1,3-di-tert-butoxy-1,3-dioxopropan-2-yl)-2-methylenecyclopentane-1,1-dicarboxylate (11) and (±)-Diethyl 3-(3-(tert-butoxy)-2-(tert-butoxycarbonyl)-3-oxopropyl)-2-methylenecyclobutane-1,1-dicarboxylate (12).
The title compounds were prepared from diethyl 2-allyl-2-(1-{[(trifluoromethyl)sulfonyl]oxy}vinyl)malonate (37.3 mg, 0.1 mmol) and di-tert-butyl malonate (26.7 μL, 0.12 mmol) using General Procedure A except XPhos (6 mol %) was used as ligand, 1,4-dioxane was used as solvent, and the reaction was conducted for 1 hr. The crude material was purified via column chromatography on silica gel using 95:5 hexanes:ethyl acetate as the eluent. This procedure afforded 33.4 mg (76%) combined yield of [11] and [12] in a 1:2.5 regioisomeric mixture which was determined by 1H NMR analysis. Characterization data are for the mixture. 1H NMR (500 MHz, CDCl3) δ 5.35 – 5.3 (ddd, J = 12.4, 2.9, 1.3 Hz, 1.4H), 5.25 (t, J = 2.0 Hz, 0.43H), 5.14 (dd, J = 2.4, 1.2 Hz, 1H), 4.30 – 4.15 (m, J = 14.2, 7.2, 5.9, 3.2 Hz, 5.7H), 3.16 (t, J = 7.6 Hz, 1H), 3.09 – 2.95 (m, 1.4H), 2.75 – 2.55 (m, 2.3H), 2.31 – 2.18 (m, 1.4H), 2.15 (ddd, J = 13.9, 7.8, 6.1 Hz, 1H), 2.09 – 2.00 (m, 0.8H), 1.94 (ddd, J = 13.9, 9.4, 7.4 Hz, 1H), 1.50 – 1.41 (m, 26H), 1.25 (td, J = 7.2, 6.1 Hz, 9.7H); 13C NMR (126 MHz, CDCl3) δ 169.6, 169.1, 168.7, 168.6, 147.8, 146.5, 112.8, 110.6, 81.75, 81.73, 81.72, 61.84, 61.78, 61.76, 61.7, 59.3, 58.6, 51.8, 40.0, 38.5, 36.9, 32.9, 32.1, 29.8, 28.1, 19.0, 14.19, 14.14, 14.12.
(±)-Ethyl (2S,3aR)-2-(2,3,3a,4,5,6-hexahydro-1H-inden-2-yl)-3-oxobutanoate (13a).
The title compound was prepared from 6-allylcyclohex-1-en-1-yl triflate (54.1 mg, 0.2 mmol) and ethyl acetoacetate (31 μL, 0.24 mmol) using the General Procedure A. The crude material was purified via column chromatography on silica gel using 99:1 -> 95:5 hexanes:ethyl acetate as the eluent. This procedure afforded 40.5 mg (81%) of the title compound. The compound was obtained as a 1:1 mixture of diastereomers, epimeric at the stereocenter between the carbonyl groups as indicated by the structure above, as judged by 1H NMR analysis. Data are for the mixture. 1H NMR (401 MHz, CDCl3) δ 5.35 (s, 0.5H), 5.32 (s, 0.5H), 4.28 – 4.10 (m, 2H), 3.25 (dd, J = 10.1, 8.2 Hz, 1H), 2.75 – 2.50 (m, 2H), 2.22 (d, J = 3.1 Hz, 4H), 2.10 – 1.71 (m, 6H), 1.51 – 1.34 (m, 1H), 1.29 – 1.25 (m, 3H), 1.05 – 0.89 (m, 1H), 0.88 – 0.71 (m, 1H); 13C NMR (126 MHz, CDCl3) δ 203.1, 202.9, 169.33, 169.28, 142.7, 142.5, 118.3, 118.2, 66.4, 66.2, 61.4, 41.1, 40.8, 38.6, 38.59, 36.9, 35.2, 35.2, 29.3, 29.0, 28.9, 25.30, 25.29, 22.5, 22.4, 14.3; IR (film) 1735, 1710 cm−1; HRMS (ESI+ TOF) m/z: [M + Na+]+ calcd for C15H22O3 273.1569; found 273.1461.
(±)-(2S,3aR)-Ethyl 2-(diethoxyphosphoryl)-2-(2,3,3a,4,5,6-hexahydro-1H-inden-2-yl)acetate (13b).
The title compound was prepared from 6-allylcyclohex-1-en-1-yl triflate (500 mg, 1.85 mmol), and triethyl phosphonoacetate (440 μL, 2.22 mmol) using General Procedure A except with lithium hexamethyldisilazide (774 mg, 4.625 mmol) as base. The crude material was purified via column chromatography on silica gel using 50:50 -> 15:85 hexanes:ethyl acetate as the eluent. This procedure afforded 332 mg (52%) of the title compound as a yellow oil. The compound was obtained as a 1:1 mixture of diastereomers, epimeric at the stereocenter adjacent to the ester group as indicated by the structure above, as judged by 1H NMR analysis. Data are for the mixture 1H NMR (401 MHz, CDCl3) δ 5.39 (s, 0.5H), 5.35 (s, 0.5H), 4.35 – 3.98 (m, 6H), 2.84 – 2.54 (m, 2.5H), 2.35 – 2.09 (m, 2H), 2.07 – 1.86 (m, 4H), 1.85 – 1.71 (m, 1H), 1.50 – 1.37 (m, 1H), 1.41 – 1.14 (m, 9H), 1.14 – 0.73 (m, 2.5H); 13C NMR (126 MHz, CDCl3) δ 169.21, 169.06, 142.83, 142.20, 117.97, 62.46, 61.21, 52.14, 51.09, 41.32, 40.64, 39.63, 39.51, 39.42, 36.28, 36.24, 36.15, 36.11, 35.92, 35.79, 35.43, 28.65, 28.58, 25.19, 25.14, 22.32, 22.30, 16.40, 16.36, 14.17; IR (film) 1731 cm−1; HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C17H29O5P 345.1753; found 345.1825.
(±)-(2S, 3aR)-Ethyl 2’-cyano-2–2,3,3a,4,5,6-hexahydro-1H-inden-2-yl acetate (13c).
The title compound was prepared from 6-allylcyclohex-1-en-1-yl triflate (54.1 mg, 0.2 mmol), ethyl cyanoacetate (26 μL, 0.24 mmol), using General Procedure A, except with Pd(allyl)BrettphosCl (8.6 mg, 0.012 mmol), as catalyst, BrettPhos was added (6.4 mg, 0.012 mmol), cesium carbonate (91.2 mg, 0.28 mmol) as base, and 1,4 dioxane (2 mL, 0.1M) as solvent. The crude material was purified via column chromatography on silica gel using 65:35 -> 50:50 hexanes:dichloromethane as the eluent. This procedure afforded 22.8 mg (50%) of the title compound as a colorless oil. The compound was obtained as a 1:1 mixture of diastereomers, epimeric at the CN-bearing stereocenter, as judged by 1H NMR analysis. Data are for the mixture. 1H NMR (401 MHz, CDCl3) δ 5.43 (s, 1H), 4.26 (q, J = 7.1 Hz, 2H), 3.48 (dd, J = 11.3, 6.5 Hz, 1H), 2.69 – 2.50 (m, 2H), 2.35 – 1.91 (m, 6H), 1.85 – 1.71 (m, 1H), 1.51 – 1.34 (m, 1H), 1.32 (t, J = 7.1 Hz, 3H), 1.16 – 0.97 (m, 2H); 13C NMR (126 MHz, CDCl3) δ 166.0, 141.3, 141.1, 119.32, 119.27, 115.9, 62.8, 42.70, 42.65, 41.1, 41.0, 38.5, 37.9, 37.7, 34.8, 34.0, 28.7, 25.2, 22.3, 14.2; IR (film) 2248, 1743, 1453 cm−1; HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C14H19NO2 234.1416; found 234.1493.
(±)-Ethyl 2-cyano-2-(2,3-dihydro-1H-inden-2-yl)acetate (14a).
The title compound was prepared from 2-allylphenyl triflate (53.3 mg, 0.2 mmol), ethyl cyanoacetate (26 μL, 0.24 mmol), using General Procedure A, except with Pd(allyl)BrettphosCl (8.6 mg, 0.012 mmol) as catalyst, additional BrettPhos (6.4 mg, 0.012 mmol), Cs2CO3 (91.2 mg, 0.28 mmol) as base, and dioxane (2 mL, 0.1M) as solvent. The crude material was purified via column chromatography on silica gel using 95:5 hexanes:ethyl acetate as the eluent. This procedure afforded 22.0 mg (47%) of the title compound as a colorless oil. The compound was obtained as a 1:1 mixture of diastereomers, epimeric at the CN-bearing stereocenter, as judged by 1H NMR analysis. Data are for the mixture. 1H NMR (500 MHz, CDCl3) δ 7.25 – 7.15 (m, 4H), 4.28 (q, J = 7.2 Hz, 2H), 3.64 (dd, J = 6.8, 1.7 Hz, 1H), 3.25 – 3.08 (m, 3H), 2.99 (dd, J = 15.5, 7.4 Hz, 1H), 2.92 (dd, J = 14.5, 6.7 Hz, 1H), 1.34 (t, J = 7.2, 3H); 13C NMR (126 MHz, CDCl3) δ 165.59, 140.95, 140.93, 126.91, 126.87, 124.54, 124.46, 115.72, 62.85, 42.08, 42.04, 39.96, 37.07, 36.56, 14.02; IR (film) 2908, 2242, 1737, 1460, 743 cm−1; HRMS (ESI+ TOF) m/z: [M + Na+]+ calcd for C14H15NO2 247.1441; found 247.1445.
(±)-2-[2-(2,3-Dihydro-1H-inden-2-yl)acetyl]-3,4-dihydronaphthalen-1(2H)-one (14b).
The title compound was prepared from 2-allylphenyl triflate (53.5 mg, 0.2 mmol) and 2-acetyl-1-tetralone (45.2 mg, 0.24 mmol) using General Procedure A, except with LiHMDS (87.0 mg, 0.52 mmol) as the base, and xylenes (2 mL) as the solvent, and a reaction temperature of 130 °C. The crude material was purified via column chromatography on silica gel using 65:35 hexanes:dichloromethane as the eluent. This procedure afforded 24.5 mg (40%) of the title compound as a yellow solid, mp 77–80 °C, that contained ca 5% of inseparable impurities. 1H NMR (401 MHz, CDCl3) δ 7.95 – 7.91 (m, 1H), 7.37 (td, J = 7.4, 1.5 Hz, 1H), 7.33 – 7.26 (m, 1H), 7.20 – 7.14 (m, 3H), 7.14 – 7.08 (m, 2H), 3.15 (dd, J = 15.6, 7.7 Hz, 2H), 3.01 – 2.91 (m, 1H), 2.85 – 2.79 (m, 2H), 2.73 – 2.61 (m, 4H), 2.58 (dd, J = 8.3, 6.3 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 194.95, 177.67, 142.75, 140.82, 131.88, 131.30, 127.51, 126.85, 126.26, 125.90, 124.50, 106.06, 41.46, 39.10, 36.22, 28.28, 22.52; IR (film) 3062, 2926, 2833, 1712, 1666, 1586, 1554, 1294 cm−1; HRMS (ESI+ TOF) m/z: [M + Na+]+ calcd for C21H20O2 327.1356; found 327.1360.
tert-Butyl 2-(2,3-dihydro-1H-inden-2-yl)acetate (15a).
The title compound was prepared from 2-allylphenyl triflate (266.5 mg, 1 mmol) and tert-butyl acetate (165 μL, 1.4 mmol) using General Procedure A, except with LiHMDS (368 mg, 2.2 mmol) as the base. The crude material was purified via column chromatography on silica gel using 95:5 hexanes:ethyl acetate as the eluent to afford a mixture of the desired product and a side product resulting from C-arylation of the ester. In order to separate these compounds, the material was further reacted with 0.5 g of AD-alpha-mix in a 1:1 solution of tert-butyl alcohol and water (1.6 mL: 1.6 mL) for 12 h at rt to oxidize the alkene in the undesired product. The oxidation reaction was then quenched with sodium sulfite and stirred at rt for 30 min. The aqueous layer was extracted with ethyl acetate (3 × 5 mL), dried with MgSO4, and concentrated in vacuo. The crude material was purified via column chromatography on silica gel using 80:20 hexanes:dichloromethane. This procedure afforded 24.5 mg (24%) of the title compound as a colorless oil.1H NMR (401 MHz, CDCl3) δ 7.22 – 7.16 (m, 2H), 7.16 – 7.10 (m, 2H), 3.12 (dd, J = 15.5, 7.8 Hz, 2H), 2.85 (p, J = 7.6 Hz, 1H), 2.64 (dd, J = 15.5, 7.4 Hz, 2H), 2.41 (d, J = 7.5 Hz, 1H), 1.47 (s, 9H); 13C NMR (126 MHz, CDCl3) δ 172.27, 142.80, 126.20, 124.44, 80.22, 41.41, 38.92, 36.34, 28.13; IR (film) 2248, 1743, 1453 cm−1; IR (film) 2969, 2921, 1724, 1480 cm−1; HRMS (ESI+ TOF) m/z: [M + Na+]+ calcd for C15H20O2 255.1356; found 255.1360.
Methyl 2-(2,3-dihydro-1H-inden-2-yl)-2-methylpropanoate (15b).
The title compound was prepared from 2-allylphenyl triflate (53.3 mg, 0.2 mmol) and methyl isobutyrate (28 μL, 0.24 mmol) using General Procedure A, except with LiHMDS (73.6 mg, 0.44 mmol) as the base. The crude material was purified via column chromatography on silica gel using 95:5 hexanes:ethyl acetate as the eluent. This procedure afforded 37.1 mg (85%) of the title compound as a colorless oil.1H NMR (500 MHz, CDCl3) δ 7.20 – 7.15 (m, 2H), 7.14 – 7.10 (m, 2H), 3.68 (s, 3H), 2.96 – 2.86 (m, 2H), 2.85 – 2.74 (m, 3H), 1.23 (s, 6H); 13C NMR (126 MHz, CDCl3) δ 178.11, 142.89, 126.33, 124.45, 77.41, 77.16, 76.91, 51.85, 47.83, 44.52, 34.56, 23.09; IR (film) 1726 cm−1; HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C14H18O2 219.3180; found 219.1380.
(±)-Methyl 2-(5-fluoro-2-methyl-2,3-dihydro-1H-inden-2-yl)-2-methylpropanoate (15c).
The title compound was prepared from 4-fluoro-2-(2-methylallyl)phenyl triflate (56.8 mg, 0.2 mmol) and methyl isobutyrate (28 μL, 0.24 mmol) using General Procedure A, except with LiHMDS (73.6 mg, 0.44 mmol) as the base and RuPhos (5.6 mg, 0.012 mmol) as the ligand. The crude material was purified via column chromatography on silica gel using 80:20 hexanes:dichloromethane as the eluent. This procedure afforded 11.5 mg (23%) of the title compound as a colorless oil. 1H NMR (401 MHz, CDCl3) δ 7.08 (dd, J = 8.2, 5.3 Hz, 1H), 6.90 – 6.75 (m, 2H), 3.65 (s, 3H), 3.23 (dd, J = 25.1, 16.1 Hz, 2H), 2.48 (dd, J = 16.0, 11.5 Hz, 2H), 1.25 (s, 6H), 1.03 (s, 3H); 13C NMR (176 MHz, CDCl3) δ 177.52, 162.65, 161.27, 144.36 (d, J = 8.0 Hz), 137.55, 125.58 (d, J = 8.7 Hz), 112.94 (d, J = 22.2 Hz), 111.71 (d, J = 21.8 Hz), 51.51, 48.95, 47.81, 47.56, 42.50, 42.49, 41.50, 29.69, 23.75, 22.09, 22.07, 19.01; IR (film) 2929, 1723, 1141 cm−1; HRMS (ESI+ TOF) m/z: [M + Na+]+ calcd for C15H19FO2 273.1369; found 273.1267.
(±)-Methyl 2-[5-(tert-butyl)-2-methyl-2,3-dihydro-1H-inden-2-yl]-2-methylpropanoate (15d).
The title compound was prepared from 4-tert-butyl-2-(2-methylallyl)phenyl triflate (67.3 mg, 0.2 mmol) and methyl isobutyrate (28 μL, 0.24 mmol) using General Procedure A except with LiHMDS (73.6 mg, 0.44 mmol) as the base and RuPhos (5.6 mg, 0.012 mmol) as the ligand. The crude material was purified via column chromatography on silica gel using 99:1 hexanes:ethyl acetate as the eluent. This procedure afforded 13.7 mg (24%) of the title compound as a colorless oil. 1H NMR (401 MHz, CDCl3) δ 7.22 – 7.15 (m, 2H), 7.10 (d, J = 7.8 Hz, 1H), 3.65 (s, 3H), 3.31 – 3.20 (m, 2H), 2.54 – 2.43 (m, 2H), 1.31 (s, 9H), 1.26 (s, 6H), 1.04 (s, 3H); 13C NMR (126 MHz, CDCl3) δ 177.71, 149.22, 142.08, 139.24, 124.23, 123.15, 121.69, 51.44, 48.47, 47.61, 42.47, 41.89, 34.49, 31.59, 23.94, 22.14; IR (film) 2952, 1728, 1144 cm−1; HRMS (ESI+ TOF) m/z: [M + Na+]+ calcd for C19H28O2 306.2428; found 306.2436.
(±)-Methyl 2-(2,3-dihydro-1H-inden-2-yl)-2-phenylacetate (15e).
The title compound was prepared from 2-allylphenyl triflate (53.3 mg, 0.2 mmol) and methyl 2-phenylacetate (35 μL, 0.24 mmol) using General Procedure A, except with LiHMDS (73.6 mg, 0.44 mmol) as the base. The crude material was purified via column chromatography on silica gel using 80:20 hexanes:dichloromethane -> 65:35 hexanes:dichloromethane as the eluent. This procedure afforded 45.8 mg (86%) of the title compound as a white solid, mp 70–71 °C. 1H NMR (400 MHz, CDCl3) δ 7.41 – 7.28 (m, 4H), 7.24 – 7.19 (m, 1H), 7.18 – 7.06 (m, 3H), 3.70 (s, 3H), 3.56 (d, J = 10.5 Hz, 1H), 3.34 – 3.15 (m, 2H), 2.84 – 2.66 (m, 2H), 2.50 (dd, J = 15.7, 7.6 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 174.03, 142.53, 142.33, 138.30, 128.70, 128.28, 127.45, 126.32, 126.29, 124.48, 124.34, 56.98, 51.96, 42.92, 38.19, 37.06; IR (film) 2941, 1725, 1577, 1451, 1433 cm−1; HRMS (ESI+ TOF) m/z: [M + Na+]+ calcd for C18H18O2 289.1199; found 289.1203.
(±)-(2S,3aR)-Methyl 2-(2,3,3a,4,5,6-hexahydro-1H-inden-2-yl)-2-phenylacetate (15f).
The title compound was prepared from 6-allylcyclohex-1-en-1-yl triflate (54.1 mg, 0.2 mmol) and methyl 2-phenylacetate (35 μL, 0.24 mmol) using General Procedure A, except with LiHMDS (73.6 mg, 0.44 mmol) as the base. The crude material was purified via column chromatography on silica gel using 80:20 hexanes:dichloromethane -> 65:35 hexanes:dichloromethane as the eluent. This procedure afforded 29.2 mg (54%) of the title compound as a colorless oil. The compound was obtained as a 2:1 mixture of diastereomers epimeric at the stereocenter alpha to the carbonyl group, as judged by 1H NMR analysis. Data are for the mixture. 1H NMR (500 MHz, CDCl3) δ 7.38 – 7.30 (m, 4H), 7.30 – 7.24 (m, 1H), 5.40 (m, 0.67H), 5.30 (s, 0.34H), 3.66 (s, 3H), 3.29 (d, J = 10.6 Hz, 1H), 2.84 – 2.61 (m, 1.67H), 2.28 (br s, 0.33H), 2.24 – 2.09 (m, 1.35H), 2.05 – 1.94 (m, 3H), 1.92 – 1.83 (m, 0.67H), 1.83 – 1.70 (m, 1.34H), 1.66 – 1.55 (m, 1H), 1.51 – 1.34 (m, 1H), 1.09 – 0.86 (m, 1.34H), 0.67 (q, J = 11.6 Hz, 0.67H); 13C NMR (126 MHz, CDCl3) δ 174.15, 143.38, 143.06, 138.54, 128.51, 128.19, 128.14, 127.25, 127.22, 117.78, 117.75, 58.17, 57.91, 51.86, 51.83, 41.21, 40.88, 40.82, 40.74, 39.42, 38.66, 35.92, 35.01, 28.91, 28.83, 25.19, 25.17, 22.38, 22.35; IR (film) 2914, 2833, 1731, 1430 cm−1; HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C18H22O2 271.1693; found 271.1695.
(±)-(2S,3aR)-Methyl 2-(2,3,3a,4,5,6-hexahydro-1H-inden-2-yl)-2-methylpropanoate (15g).
The title compound was prepared from 6-allylcyclohex-1-en-1-yl triflate (54.1 mg, 0.2 mmol) and methyl isobutyrate (28 μL, 0.24 mmol) using General Procedure A, except with LiHMDS (73.6 mg, 0.44 mmol) as the base. The crude material was purified via column chromatography on silica gel using 80:20 hexanes:dichloromethane -> 65:35 hexanes:dichloromethane as the eluent. This procedure afforded 39.1 mg (88%) of the title compound as a colorless oil. The compound was obtained as a >20:1 mixture of diastereomers as judged by 1H NMR analysis. Data are for the major isomer. 1H NMR (500 MHz, CDCl3) δ 5.34 (s, 1H), 3.65 (s, 3H), 2.38 – 2.12 (m, 3H), 2.09 – 1.90 (m, 4H), 1.87 – 1.70 (m, 2H), 1.50 – 1.36 (m, 1H), 1.13 (s, 3H), 1.12 (s, 3H), 1.06 – 0.95 (m, 1H), 0.94 – 0.83 (m, 1H); 13C NMR (126 MHz, CDCl3) δ 178.47, 143.62, 117.60, 77.41, 77.16, 76.91, 51.72, 45.66, 44.10, 41.42, 35.44, 31.85, 28.98, 25.39, 22.74, 22.61, 22.57; IR (film) 1730, 1151 cm−1; HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C14H22O2 223.1693; found 223.1682.
(±)-(2S,3aR)-Methyl 2-methyl-2-(2-methyl-2,3,3a,4,5,6-hexahydro-1H-inden-2-yl)propanoate (15h).
The title compound was prepared from 6-(2-methylallyl)cyclohex-1-en-1-yl triflate (56.9 mg, 0.2 mmol) and methyl isobutyrate (28 μL, 0.24 mmol) using General Procedure A, except with LiHMDS (73.6 mg, 0.44 mmol) as the base and RuPhos (5.6 mg, 0.012 mmol) as the ligand. The crude material was purified via column chromatography on silica gel using 95:5 hexanes:ethyl acetate as the eluent. This procedure afforded 24.7 mg (52%) of the title compound as a colorless oil. The compound was obtained as an 11:1 mixture of diastereomers as judged by 1H NMR analysis. Data are for the major isomer. 1H NMR (401 MHz, CDCl3) δ 5.32 (s, 1H), 3.63 (s, 3H), 2.65 – 2.54 (m, 1H), 2.38 (br s, 1H), 2.05 – 1.88 (m, 4H), 1.82 – 1.72 (m, 1H), 1.52 (dd, J = 12.1, 7.0 Hz, 1H), 1.48 – 1.37 (m, 1H), 1.33 (t, J = 12.1 Hz, 1H), 1.14 (s, 6H), 1.01 (s, 3H), 1.00 – 0.94 (m, 1H); 13C NMR (126 MHz, CDCl3) δ 177.90, 143.91, 117.22, 51.28, 47.96, 45.16, 42.34, 40.66, 38.95, 29.04, 25.17, 24.87, 22.47, 21.61, 21.58; IR (film) 1922, 1726, 1432, 1135 cm−1; HRMS (ESI+ TOF) m/z: [M + Na+]+ calcd for C19H28O2 306.2428; found 306.2436.
(±)-(2S,3aR)-Methyl 2-methyl-2-(2-methyl-1,2,3,3a,4,5-hexahydropentalen-2-yl)propanoate (15i).
The title compound was prepared from 5-(2-methylallyl)cyclopent-1-en-1-yl triflate (54.0 mg, 0.2 mmol) and methyl isobutyrate (28 μL, 0.24 mmol) using General Procedure A, except with LiHMDS (73.6 mg, 0.44 mmol) as the base and RuPhos (5.6 mg, 0.012 mmol) as the ligand. The crude material was purified via column chromatography on silica gel using 95:5 hexanes:ethyl acetate as the eluent. This procedure afforded 22.2 mg (50%) of the title compound as a colorless oil. The compound was obtained as a >20:1 mixture of diastereomers as judged by 1H NMR analysis. Data are for the major isomer. NMR spectra were acquired in C6D6, as some (ca 5%) isomerization of the alkene occurred when spectra were acquired in CDCl3. 1H NMR (401 MHz, C6D6) δ 5.24 (s, 1H), 3.31 (s, 3H), 2.95 (br s, 1H), 2.80 – 2.67 (m, 1H), 2.66 – 2.53 (m, 1H), 2.51 – 2.38 (m, 1H), 2.11 – 2.05 (m, 1H), 1.84 – 1.74 (m, 1H), 1.52 – 1.29 (m, 4H), 1.14 (s, 6H), 1.03 (s, 3H); 13C NMR (126 MHz, C6D6) δ 176.54, 152.91, 117.81, 52.08, 50.53, 49.78, 48.01, 41.82, 37.24, 34.74, 32.35, 25.21, 21.47, 21.43; IR (film) 2943, 2839, 1726, 1132 cm−1; HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C14H22O2 223.1693; found 223.1690.
(±)-(1S,4R)-Methyl 2-methyl-2-(1-methyl-3-methylene-4-phenylcyclopentyl)propanoate (17).
The title compound was prepared from 5-methyl-3-phenylhexa-1,5-dien-2-yl triflate (64.1 mg, 0.2 mmol) and methyl isobutyrate (28 μL, 0.24 mmol) using General Procedure A, except with LiHMDS (73.6 mg, 0.44 mmol) as the base and RuPhos (5.6 mg, 0.012 mmol) as the ligand. The crude material was purified via column chromatography on silica gel using 80:20 hexanes:dichloromethane as the eluent. This procedure afforded 28 mg (52%) of the title compound as a colorless oil. The compound was obtained as a >20:1 mixture of diastereomers as judged by 1H NMR analysis. Data are for the major isomer. 1H NMR (500 MHz, CDCl3) δ 7.33 – 7.27 (m, 2H), 7.23 – 7.17 (m, 3H), 5.00 (s, 1H), 4.59 (s, 1H), 3.79 – 3.70 (m, 1H), 3.67 (s, 3H), 2.83 (d, J = 16.4 Hz, 1H), 2.21 (d, J = 16.3 Hz, 1H), 2.03 – 1.94 (m, 1H), 1.92 – 1.84 (m, 1H), 1.23 (s, 3H), 1.22 (s, 3H) 1.06 (s, 3H); 13C NMR (126 MHz, CDCl3) δ 177.64, 155.32, 145.32, 128.35, 128.14, 126.05, 108.75, 51.39, 48.86, 47.74, 45.59, 44.87, 43.20, 22.23, 21.63, 21.60; IR (film) 2968, 1726, 1142 cm−1; HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C18H24O2 273.1849; found 273.1847.
3-(2,3-Dihydro-1H-inden-2-yl)-1,1-diphenylpropan-2-one (18a).
The title compound was prepared from 2-allylphenyl triflate (533 mg, 2.0 mmol) and 1,1-diphenylacetone (505 mg, 2.4 mmol) using General Procedure A, except with LiHMDS (736 mg, 4.4 mmol) as the base. The crude material was purified via column chromatography on silica gel using 99:1 hexanes:ethyl acetate as the eluent to afford a mixture of the desired product and a side product resulting from C-arylation of the ester. In order to separate these compounds, the material was further reacted with 1.2 g of AD-alpha-mix in a 1:1 solution of tertbutyl alcohol and water (4 mL: 4 mL) for 12 h at rt to oxidize the alkene in the undesired product. The oxidation reaction was then quenched with sodium sulfite and stirred at rt for 30 min. The aqueous layer was extracted with ethyl acetate (3 × 8 mL), dried with MgSO4, and concentrated in vacuo. The crude material was purified via column chromatography on silica gel using 99:1 hexanes:ethyl acetate. This procedure afforded 158 mg (24%) of the title compound as a colorless oil. 1H NMR (401 MHz, CDCl3) δ 7.34 – 7.27 (m, 4H), 7.26 – 7.19 (m, 6H), 7.14 – 7.05 (m, 4H), 5.10 (s, 1H), 3.08 (dd, J = 15.6, 7.8 Hz, 2H), 2.90 (dt, J = 14.9, 7.5 Hz, 1H), 2.74 (d, J = 7.1 Hz, 2H), 2.46 (dd, J = 15.5, 7.3 Hz, 2H); 13C NMR (126 MHz, CDCl3) δ 207.95, 142.79, 138.35, 129.01, 128.78, 127.30, 126.28, 124.48, 64.35, 48.73, 39.07, 35.10; IR (film) 3020, 2902, 2833, 1713, 1492, 903 cm−1; HRMS (ESI+ TOF) m/z: [M + Na+]+ calcd for C24H22O 349.1563; found 349.1566.
(±)-2-(2,3-Dihydro-1H-inden-2-yl)-1,2-diphenylethan-1-one (18b).
The title compound was prepared from 2-allylphenyl triflate (53.3 mg, 0.2 mmol) and deoxybenzoin (47.1 mg, 0.24 mmol) using General Procedure A, except with LiHMDS (73.6 mg, 0.44 mmol) as the base. The crude material was purified via column chromatography on silica gel using 80:20 hexanes:dichloromethane as the eluent. This procedure afforded 50.1 mg (81%) of the title compound as a colorless oil. 1H NMR (401 MHz, CDCl3) δ 8.01 – 7.95 (m, 2H), 7.52 – 7.45 (m, 1H), 7.43 – 7.36 (m, 4H), 7.35 – 7.28 (m, 2H), 7.26 – 7.20 (m, 1H), 7.18 – 7.08 (m, 4H), 4.57 (d, J = 10.7 Hz, 1H), 3.53 – 3.37 (m, 1H), 3.27 (dd, J = 15.8, 7.7 Hz, 1H), 2.77 – 2.54 (m, 3H); 13C NMR (126 MHz, CDCl3) δ 199.76, 142.91, 142.54, 138.59, 137.06, 132.94, 128.97, 128.65, 128.59, 128.55, 127.27, 126.27, 126.24, 124.54, 124.37, 58.86, 43.16, 38.65, 37.04; IR (film) 3026, 2938, 2836, 1675, 1596, 698 cm−1; HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C23H20O 313.1587; found 313.1592.
(±)-2-(2,3-Dihydro-1H-inden-2-yl)cyclohexan-1-one (18c).
The title compound was prepared from 2-allylphenyl triflate (53.3 mg, 0.2 mmol) and cyclohexanone (50 μL, 0.48 mmol) using General Procedure A, except with LiHMDS (73.6 mg, 0.44 mmol) as the base. The crude material was purified via column chromatography on silica gel using 65:35 hexanes:dichloromethane as the eluent. The product was further purified via column chromatography on silica gel using 99:1 hexanes:ethyl acetate. This procedure afforded 30.5 mg (71%) of the title compound as a white solid, mp: 67–69°C. 1H NMR (500 MHz, CDCl3) δ 7.21 – 7.15 (m, 2H), 7.15 – 7.10 (m, 2H), 3.24 (dd, J = 15.9, 7.9 Hz, 1H), 3.03 (dd, J = 15.3, 7.9 Hz, 1H), 2.76 (p, J = 8.5 Hz, 1H), 2.64 (dd, J = 15.4, 9.2 Hz, 1H), 2.55 (dd, J = 15.9, 8.9 Hz, 1H), 2.49 – 2.39 (m, 2H), 2.39 – 2.30 (m, 1H), 2.19 – 2.11 (m, 1H), 2.09 – 2.00 (m, 1H), 1.95 – 1.87 (m, 1H), 1.80 – 1.64 (m, 2H), 1.60 – 1.48 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 213.08, 143.51, 142.67, 126.14, 126.00, 124.29, 124.13, 56.25, 42.23, 39.38, 38.22, 36.64, 32.17, 28.24, 24.64; IR (film) 2931, 2857, 1704, 744 cm−1; HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C15H18O 215.1430; found 215.1432.
2-(2,3-Dihydro-1H-inden-2-yl)-2-methyl-1-phenylpropan-1-one (18d).
The title compound was prepared from 2-allylphenyl triflate (53.3 mg, 0.2 mmol) and phenyl isopropyl ketone (36 μL, 0.24 mmol) using General Procedure A, except with LiHMDS (73.6 mg, 0.44 mmol) as the base. The crude material was purified via column chromatography on silica gel using 90:10 hexanes:dichloromethane -> 80:20 hexanes:dichloromethane as the eluent. This procedure afforded 35.5 mg (67%) of the title compound as a colorless oil. 1H NMR (500 MHz, CDCl3) δ 7.72 – 7.66 (m, 2H), 7.51 – 7.45 (m, 1H), 7.44 – 7.38 (m, 2H), 7.21 – 7.16 (m, 2H), 7.15 – 7.11 (m, 2H), 3.24 – 3.13 (m, 1H), 2.99 – 2.78 (m, 4H), 1.37 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 209.32, 142.62, 139.58, 130.65, 128.12, 127.43, 126.25, 124.33, 49.99, 46.61, 34.30, 22.93; IR (film) 2914, 1669, 1593, 956, 740 cm−1; HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C19H20O 265.1587; found 265.1592.
(±)-2-(2,3-Dihydro-1H-inden-2-yl)-6-methoxy-3,4-dihydronaphthalen-1(2H)-one (18e).
The title compound was prepared from 2-allylphenyl triflate (53.3 mg, 0.2 mmol) and 6-methoxy-1-tetralone (42.3 mg, 0.24 mmol) using General Procedure A, except with LiHMDS (73.6 mg, 0.44 mmol) as the base. The crude material was purified via column chromatography on silica gel using 65:35 hexanes:dichloromethane -> 50:50 hexanes:dichloromethane as the eluent. This procedure afforded 42.1 mg (72%) of the title compound as a colorless oil. 1H NMR (500 MHz, CDCl3) δ 8.04 (d, J = 8.7 Hz, 1H), 7.23 – 7.17 (m, 2H), 7.15 – 7.11 (m, 2H), 6.84 (dd, J = 8.8, 2.5 Hz, 1H), 6.71 – 6.67 (m, 1H), 3.85 (s, 3H), 3.18 (dd, J = 15.7, 7.9 Hz, 1H), 3.15 – 2.91 (m, 4H), 2.87 (dd, J = 15.7, 8.4 Hz, 1H), 2.74 (dd, J = 15.0, 8.2 Hz, 1H), 2.62 (ddd, J = 9.8, 7.4, 4.1 Hz, 1H), 2.24 – 2.17 (m, 1H), 2.02 – 1.92 (m, 1H); 13C NMR (126 MHz, CDCl3) δ 198.64, 163.38, 146.16, 143.53, 142.78, 129.87, 126.34, 126.19, 126.09, 124.28, 124.16, 113.15, 112.39, 55.41, 51.94, 38.75, 37.81, 36.74, 28.45, 26.21; IR (film) 2963, 2832, 1660, 1601, 1251, 742 cm−1; HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C20H20O2 293.1536; found 293.1541.
(±)-(2S,3aR)-2-(2,3,3a,4,5,6-Hexahydro-1H-inden-2-yl)-2-methyl-1-phenylpropan-1-one (18f).
The title compound was prepared from 6-allylcyclohex-1-en-1-yl triflate (54.1 mg, 0.2 mmol) and phenyl isopropyl ketone (36 μL, 0.24 mmol) using General Procedure A, except with LiHMDS (73.6 mg, 0.44 mmol) as the base. The crude material was purified via column chromatography on silica gel using 50:50 hexanes:dichloromethane as the eluent under high pressure. This procedure afforded 28.4 mg (53%) of the title compound as a colorless oil. The compound was obtained as a >20:1 mixture of diastereomers as judged by 1H NMR analysis. Data are for the major isomer. 1H NMR (500 MHz, CDCl3) δ 7.63 – 7.59 (m, 2H), 7.46 – 7.42 (m, 1H), 7.41 – 7.35 (m, 2H), 5.35 (s, 1H), 2.63 – 2.53 (m, 1H), 2.33 – 2.25 (m, 1H), 2.21 – 2.13 (m, 1H), 2.08 – 1.90 (m, 4H), 1.86 – 1.73 (m, 2H), 1.42 (m, 1H), 1.27 (s, 3H), 1.23 (s, 3H), 1.07 – 0.90 (m, 2H); 13C NMR (126 MHz, CDCl3) δ 209.74, 143.29, 139.83, 130.43, 128.01, 127.34, 117.60, 49.61, 44.55, 41.15, 35.16, 31.72, 28.81, 25.22, 23.03, 22.38; IR (film) 2925, 2837, 1673, 958 cm−1; HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C19H24O 269.1900; found 269.1905.
(±)-2-(2,3-Dihydro-1H-inden-2-yl)-3,4-dihydronaphthalen-1(2H)-one (18g).
The title compound was prepared from 2-allylphenyl triflate (53.3 mg, 0.2 mmol) and 1-tetralone (32 μL, 0.24 mmol) using General Procedure A, except with LiHMDS (73.6 mg, 0.44 mmol) as the base. The crude material was purified via column chromatography on silica gel using 80:20 hexanes:dichloromethane as the eluent. This procedure afforded 37.8 mg (72%) of the title compound as a white solid, mp 61–63 °C. 1H NMR (500 MHz, CDCl3) δ 8.08 (dd, J = 7.9, 1.4 Hz, 1H), 7.49 (td, J = 7.5, 1.5 Hz, 1H), 7.37 – 7.32 (m, 1H), 7.29 – 7.25 (m, 1H), 7.25 – 7.20 (m, 2H), 7.18 – 7.13 (m, 2H), 3.22 (dd, J = 15.7, 8.0 Hz, 1H), 3.18 – 2.97 (m, 4H), 2.89 (dd, J = 15.8, 8.5 Hz, 1H), 2.78 (dd, J = 15.2, 8.4 Hz, 1H), 2.69 (ddd, J = 10.1, 7.6, 4.2 Hz, 1H), 2.30 – 2.22 (m, 1H), 2.08 – 1.97 (m, 1H); 13C NMR (126 MHz, CDCl3) δ 199.93, 143.76, 143.47, 142.74, 133.21, 132.72, 128.71, 127.46, 126.64, 126.26, 126.16, 124.32, 124.21, 52.32, 38.72, 37.83, 36.73, 28.12, 26.25; IR (film) 3009, 2877, 1671, 1597, 734 cm−1; HRMS (ESI+ TOF) m/z: [M + Na+]+ calcd for C19H18O 285.1250; found 285.1255.
(±)-(2S,3aR)-2-(2,3,3a,4,5,6-Hexahydro-1H-inden-2-yl)-1-phenylethan-1-one (18h).
The title compound was prepared from 6-allylcyclohex-1-en-1-yl triflate (54.1 mg, 0.2 mmol) and acetophenone (28 μL, 0.24 mmol) using General Procedure A, except with LiHMDS (73.6 mg, 0.44 mmol) as the base. The crude material was purified via column chromatography on silica gel using 99.5:0.5 hexanes:ethyl acetate as the eluent. This procedure afforded 25.0 mg (52%) of the title compound as a colorless oil. The compound was obtained as a >20:1 mixture of diastereomers as judged by 1H NMR analysis. Data are for the major isomer. 1H NMR (700 MHz, CDCl3) δ 7.95 (d, J = 7.7 Hz, 2H), 7.58 – 7.53 (m, 1H), 7.49 – 7.43 (m, 2H), 5.37 (s, 1H), 3.06 – 2.94 (m, 2H), 2.73 – 2.64 (m, 1H), 2.56 – 2.47 (m, 1H), 2.23 (br s, 1H), 2.16 – 2.09 (m, 1H), 2.06 – 1.94 (m, 3H), 1.93 – 1.86 (m, 1H), 1.82 – 1.74 (m, 1H), 1.49 – 1.40 (m, 1H), 1.05 – 0.96 (m, 1H), 0.84 (q, J = 11.4 Hz, 1H); 13C NMR (176 MHz, CDCl3) δ 200.22, 143.91, 137.35, 133.03, 128.69, 128.22, 117.73, 45.15, 41.34, 40.73, 37.19, 33.57, 29.15, 25.39, 22.62; IR (film) 1682, 1448 cm−1; HRMS (ES) [M]+ calcd for C17H20O 240.1514; found 240.1517.
(±)-(2S,3aR)-2-(2,3,3a,4,5,6-Hexahydro-1H-inden-2-yl)cyclohexan-1-one (18i).
The title compound was prepared from 6-allylcyclohex-1-en-1-yl triflate (54.1 mg, 0.2 mmol) and cyclohexanone (50 μL, 0.48 mmol) using General Procedure A, except with LiHMDS (73.6 mg, 0.44 mmol) as the base. The crude material was purified via column chromatography on silica gel using 80:20 hexanes:dichloromethane as the eluent. This procedure afforded 29.5 mg (68%) of the title compound as a colorless oil. The compound was obtained as a 1:1 mixture of diastereomers epimeric at the stereocenter alpha to the carbonyl group, as judged by 1H NMR analysis. Data are for the mixture. 1H NMR (500 MHz, CDCl3) δ 5.38 – 5.30 (m, 1H), 2.75 – 2.66 (m, 0.5H), 2.56 – 2.47 (m, 0.5H), 2.41 – 2.33 (m, 1H), 2.33 – 2.12 (m, 4H), 2.13 – 2.06 (m, 1H), 2.06 – 1.91 (m, 5H), 1.90 – 1.69 (m, 4H), 1.69 – 1.59 (m, 1H), 1.57 – 1.37 (m, 2H), 1.02 – 0.92 (m, 1H), 0.76 – 0.64 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 213.62, 213.49, 144.03, 143.49, 117.41, 117.06, 57.36, 56.70, 42.09, 41.90, 41.47, 40.87, 39.04, 38.06, 36.80, 36.63, 36.24, 34.37, 32.21, 32.13, 28.93, 28.80, 28.32, 28.28, 25.25, 25.20, 24.56, 24.11, 22.42; IR (film) 2920, 2853, 1709, 1447 cm−1; HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C15H22O 219.1743; found 219.1747.
(±)-(2S,3aR)-2-(2,3,3a,4,5,6-Hexahydro-1H-inden-2-yl)-1,2-diphenylethan-1-one (18j).
The title compound was prepared from 6-allylcyclohex-1-en-1-yl triflate (54.1 mg, 0.2 mmol) and deoxybenzoin (47.1 mg, 0.24 mmol) using General Procedure A, except with LiHMDS (73.6 mg, 0.44 mmol) as the base. The crude material was purified via column chromatography on silica gel using 99:1 hexanes:ethyl acetate as the eluent. The material was further purified via column chromatography on silica gel using 90:10 hexanes:dichloromethane -> 80:20 hexanes:dichloromethane as the eluent. This procedure afforded 45.4 mg (72%) of the title compound as a viscous, colorless oil. The compound was obtained as a 1:1 mixture of diastereomers epimeric at the stereocenter alpha to the carbonyl group, as judged by 1H NMR analysis. Data are for the mixture. 1H NMR (500 MHz, CDCl3) δ 8.02 – 7.94 (m, 2H), 7.53 – 7.47 (m, 1H), 7.45 – 7.33 (m, 4H), 7.33 – 7.26 (m, 2H), 7.25 – 7.19 (m, 1H), 5.34 (s, 1H), 4.39 – 4.29 (m, 1H), 2.98 – 2.84 (m, 1H), 2.84 – 2.73 (m, 0.5H), 2.30 (br s, 0.5H), 2.24 – 2.13 (m 1H), 2.05 – 1.73 (m, 5H), 1.66 – 1.55 (m, 1H), 1.50 – 1.38 (m, 1H), 1.04 – 0.92 (m, 1H), 0.89 – 0.73 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 200.18, 199.77, 143.95, 143.37, 138.83, 138.80, 137.24, 137.05, 132.81, 132.78, 128.76, 128.73, 128.60, 128.51, 128.49, 128.46, 128.35, 127.01, 117.58, 117.48, 60.37, 59.63, 41.36, 41.08, 41.00, 40.58, 39.80, 38.93, 36.45, 34.99, 28.97, 25.22, 22.38; IR (film) 2914, 2847, 1678, 1596, 659 cm−1; HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C23H24O 317.1900; found 317.1906.
(±)-(2S,3aR)-2-(2,3,3a,4,5,6-Hexahydro-1H-inden-2-yl)-3,4-dihydronaphthalen-1(2H)-one (18k).
The title compound was prepared from 6-allylcyclohex-1-en-1-yl triflate (54.1 mg, 0.2 mmol) and 1-tetralone (32 μL, 0.24 mmol) using General Procedure A, except with LiHMDS (73.6 mg, 0.44 mmol) as the base. The crude material was purified via column chromatography on silica gel using 80:20 hexanes:dichloromethane as the eluent. This procedure afforded 42.1 mg (79%) of the title compound as a colorless oil. The compound was obtained as a 1:1 mixture of diastereomers epimeric at the stereocenter alpha to the carbonyl group, as judged by 1H NMR analysis. Data are for the mixture. 1H NMR (500 MHz, CDCl3) δ 8.0 – 7.98 (m, 1H), 7.47 – 7.42 (m, 1H), 7.32 – 7.27 (m, 1H), 7.25 – 7.20 (m, 1H), 5.37 (s, 1H), 3.11 – 3.02 (m, 1H), 3.00 – 2.88 (m, 1H), 2.68 – 2.54 (m, 1H), 2.50 – 2.35(m, 2H), 2.28 – 2.17 (m, 2H), 2.17 – 2.08 (m, 1H) 2.08 – 1.92 (m, 5H), 1.84 – 1.73 (m, 1H), 1.50 – 1.37 (m, 1H), 1.08 – 0.82 (m, 2H); 13C NMR (126 MHz, CDCl3) δ 200.29, 143.83, 143.71, 143.69, 143.47, 133.03, 132.99, 132.71, 132.67, 128.64, 128.61, 127.43, 127.39, 126.51, 117.61, 117.32, 52.62, 52.09, 41.43, 41.08, 38.76, 38.01, 36.25, 36.10, 35.60, 34.27, 28.90, 28.80, 27.95, 27.41, 26.31, 26.01, 25.27, 25.23, 22.44; IR (film) 2934, 2869, 1675, 1598 cm−1; HRMS (ESI+ TOF) m/z: [M + Na+]+ calcd for C19H22O 289.1563; found 223.1569.
(±)-(2S,3aR)-2-(2,3,3a,4,5,6-Hexahydro-1H-inden-2-yl)-2-methyl-3,4-dihydronaphthalen-1(2H)-one (18l).
The title compound was prepared from 6-allylcyclohex-1-en-1-yl triflate (54.1 mg, 0.2 mmol) and 2-methyl-1-tetralone (37 μL, 0.24 mmol) using General Procedure A, except with LiHMDS (73.6 mg, 0.44 mmol) as the base. The crude material was purified via column chromatography on silica gel using 80:20 hexanes:dichloromethane as the eluent. This procedure afforded 31.6 mg (56%) of the title compound as a colorless oil. The compound was obtained as a 1:1 mixture of diastereomers epimeric at the stereocenter alpha to the carbonyl group, as judged by 1H NMR analysis. Data are for the mixture. 1H NMR (401 MHz, CDCl3) δ 8.06 – 7.99 (m, 1H), 7.48 – 7.41 (m, 1H), 7.33 – 7.26 (m, 1H), 7.23 – 7.18 (m, 1H), 5.35 (s, 1H), 3.12 – 2.98 (m, 1H), 2.97 – 2.87 (m, 1H), 2.56 – 2.36 (m, 1.5H), 2.25 – 2.12 (m, 2H), 2.10 – 2.04 (m, 1H), 2.03 – 1.84 (m, 5H), 1.82 – 1.72 (m, 1H), 1.71 – 1.63 (m, 0.5H), 1.49 – 1.33 (m, 1H), 1.13 (s, 1.5H), 1.12 (s, 1.5H), 1.08 – 0.94 (m, 2H); 13C NMR (126 MHz, CDCl3) δ 202.51, 202.36, 188.00, 143.51, 143.46, 143.22, 143.14, 132.86, 132.83, 132.03, 128.55, 128.00, 127.97, 126.57, 117.49, 117.43, 46.25, 46.02, 41.23, 41.15, 40.74, 40.62, 35.00, 34.65, 32.15, 32.09, 31.64, 30.97, 28.89, 28.82, 25.28, 25.26, 25.22, 22.43, 22.39, 18.75; IR (film) 2926, 2856, 1680, 1600, 1222, 747 cm−1; HRMS (ESI+ TOF) m/z: [M + H+]+ calcd for C20H24O 281.1900; found 281.1902.
Supplementary Material
Scheme 7. Reactions of Ester Nucleophilesa–c.
aConditions: 1.0 equiv triflate substrate, 1.2 equiv ester, 2.2 equiv LiHMDS, 4 mol % Pd(OAc)2, 6 mol % BrettPhos, toluene (0.1 M), 95 °C, 16 h. Reactions were conducted on a 0.1–0.25 mmol scale. bDiastereomeric ratios were determined by 1H NMR analysis. Diastereomeric ratios were identical for crude reaction mixtures and isolated compounds unless otherwise noted. cYields are average isolated yields of two or more experiments. dRuPhos was used in place of BrettPhos as the ligand.
ACKNOWLEDGMENTS
The authors thank the NIH-NIGMS (GM 124030) for financial support of this work. EMH was partially supported by a University of Michigan Rackham dissertation fellowship.
Footnotes
Supporting Information
The supporting Information is available free of charge on the ACS Publications Website at DOI: 10.1021/xxx.
Description of stereochemical assignments and copies of 1H and 13C NMR spectra for all new compounds (PDF).
The authors declare no competing financial interests.
REFERENCES
- (1).For recent reviews, see:; (a) Dhungana RK; KC S; Basnet P; Giri R Transition Metal-Catalyzed Dicarbofunctionalization of Unactivated Olefins. Chem. Rec 2018, 18, 1314. [DOI] [PubMed] [Google Scholar]; (b) Garlets ZJ; White DR; Wolfe JP Recent Developments in Pd0-Catalyzed Alkene Carboheterofunctionalization Reactions. Asian. J. Org. Chem 2017, 6, 636. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kaur N; Wu F; Alom N-E; Ariyarathna JP; Saluga SJ; Li W Intermolecular Alkene Difunctionalizations for the Synthesis of Saturated Heterocycles. Org. Biomol. Chem 2019, 17, 1643. [DOI] [PubMed] [Google Scholar]; (d) Chemler SR; Karyakarte SD; Khoder ZM Stereoselective and Regioselective Synthesis of Heterocycles via Copper-Catalyzed Additions of Amine Derivatives and Alcohols to Alkenes. J. Org. Chem 2017, 82, 11311. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Yin G; Mu X; Liu G Palladium(II)-Catalyzed Oxidative Difunctionalization of Alkenes: Bond Forming at a High-Valent Palladium Center. Acc. Chem. Res 2016, 49, 2413. [DOI] [PubMed] [Google Scholar]; (f) Muniz K; Martinez C Development of Intramolecular Vicinal Diamination of Alkenes: From Palladium to Bromine Catalysis. J. Org. Chem 2013, 78, 2168. [DOI] [PubMed] [Google Scholar]; (g) Balme G; Bouyssi D; Monteiro N Palladium-Mediated Cascade or Multicomponent Reactions: A New Route to Carbo- and Heterocyclic Compounds. Pure. Appl. Chem 2006, 78, 231. [Google Scholar]
- (2).For selected recent examples that form two C–C bonds, see:; (a) KC S; Basnet P; Thapa S; Shrestha B; Giri R Ni-Catalyzed Regioselective Dicarbofunctionalization of Unactivated Olefins by Tandem Cyclization/Cross-Coupling and Application to the Concise Synthesis of Lignan Natural Products. J. Org. Chem 2018, 83, 2920. [DOI] [PubMed] [Google Scholar]; (b) Kuang Y; Wang X; Anthony D; Diao T Ni-Catalyzed two-Component Reductive Dicarbofunctionalization of Alkenes via Radical Cyclization. Chem. Commun 2018, 54, 2558. [DOI] [PubMed] [Google Scholar]; (c) Wang K; Ding Z; Zhou Z; Kong W Ni-Catalyzed Enantioselective Reductive Diarylation of Activated Alkenes by Domino Cyclization/Cross-Coupling. J. Am. Chem. Soc 2018, 140, 12364. [DOI] [PubMed] [Google Scholar]; (d) Basnet P; Dhungana RK; Thapa S; Shrestha B; KC S; Sears JM; Giri R Ni-Catalyzed Regioselective β,δ-Diarylation of Unactivated Olefins in Ketimines via Ligand-Enabled Contraction of Transient Nickellacycles: Rapid Access to Remotely Diarylated Ketones. J. Am. Chem. Soc 2018, 140, 7782. [DOI] [PubMed] [Google Scholar]; (e) Derosa J; Tran VT; Boulous MN; Chen JS; Engle KM Nickel-Catalyzed β,γ-Dicarbofunctionalization of Alkenyl Carbonyl Compounds via Conjunctive Cross-Coupling. J. Am. Chem. Soc 2017, 139, 10657. [DOI] [PubMed] [Google Scholar]; (f) Derosa J; van der Puyl VA; Tran VT; Liu M; Engle KM Direct Nickel-Catalyzed 1,2-Dialkylation of Alkenyl Carbonyl Compounds Chem. Sci 2018, 9, 5278. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Liu Z; Zeng T; Yang KS; Engle KM β,γ-Vicinal Dicarbofunctionalization of Alkenyl Compounds via Directed Nucleopalladation. J. Am. Chem. Soc 2016, 138, 15122. [DOI] [PubMed] [Google Scholar]; For related transformations between aryl halide and malonates bearing tethered alkenes, see:; (h) Balme G; Bouyssi D; Faure R; Gore J; Van Hemelryck B Formation de Cyclopentans a Partier de Malonates δ-Ethyeniques par un Processus Catalytique en Palladium(0) Stereochemie et Mecanisme. Tetrahedron 1992, 48, 3891. [Google Scholar]
- 3.(a) White DR; Hutt JT; Wolfe JP Asymmetric Pd-Catalyzed Alkene Carboamination Reactions for the Synthesis of 2-Aminoindane Derivatives. J. Am. Chem. Soc 2015, 137, 11246. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) White DR; Wolfe JP Stereocontrolled Synthesis of Amino-Substituted Carbocycles by Pd-Catalyzed Alkene Carboamination Reactions. Chem. Eur. J 2017, 23, 5419. [DOI] [PubMed] [Google Scholar]
- 4.White DR; Herman MI; Wolfe JP Palladium-Catalyzed Alkene Carboalkoxylation Reactions of Phenols and Alcohols for the Synthesis of Carbocycles. Org. Lett 2017, 19, 4311. [DOI] [PubMed] [Google Scholar]
- 5.Kirsch JK; Manske JL; Wolfe JP Pd-Catalyzed Alkene Carboheteroarylation Reactions for the Synthesis of 3-Cyclopentylindole Derivatives. J. Org. Chem 2018, 83, 13568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.For a recent review, see:; Prim D; Marque S; Gaucher A; Campagne J-M Transition-Metal-Catalyzed α-Arylation of Enolates. Org. React 2012, 76, 49. [Google Scholar]
- 7.(a) Braun M; Meier T Tsuji-Trost Allylic Alkylation with Ketone Enolates. Angew. Chem., Int. Ed 2006, 45, 6952. [DOI] [PubMed] [Google Scholar]; (b) Trost BM Asymmetric Allylic Alkylation, an Enabling Methodology. J. Org. Chem 2004, 69, 5813. [DOI] [PubMed] [Google Scholar]
- 8.(a) Iwata C; Kubota H; Yamada M; Takemoto Y; Uchida S; Tanaka T; Imanishi T Stereocontrolled Total Synthesis of (±)-Lubiminol, a Spirovetivane Phytoalexin. Tetrahedron Lett. 1984, 25, 3339. [Google Scholar]; (b) Iwata C; Takemoto Y; Kubota H Kuroda T; Imanishi T A Stereoselective Total Synthesis of (±)-Oxylubimin. Tetrahedron Lett. 1985, 26, 3231. [Google Scholar]; (c) Muraoka M; Toshio N; Sugie A; Ono K; Yamamoto M Carbacyclin Analogs. US Patent 4,680,307, 1987. [Google Scholar]; (d) Srikrishna A; Beeraiah B An Enantiospecific Synthesis of a Komarovispirane. Tetrahedron: Asymmetry 2007, 18, 2587. [Google Scholar]; (e) Shoji M; Suzuki E; Ueda M Total Synthesis of (±)-5-Deoxystrigol via Reductive Carbon–Carbon Bond Formation. J. Org. Chem 2009, 74, 3966. [DOI] [PubMed] [Google Scholar]
- 9.Related 2-cyclopentylacetic acid derivatives that would derive from saponification and decarboxylation of the products have also found utility as synthetic intermediates. For representative examples, see:; (a) Taber DF; Paquette CM Synthesis of the Pentacyclic Core of (+)-Salvileucalin B. J. Org. Chem 2014, 79, 3410. [DOI] [PubMed] [Google Scholar]; (b) Srikrishna A; Beeraiah B Synthetic Approaches to Komarovispiranes. Enantiospecific Synthesis of Bicyclo[3.3.0]octanespiro[3/1’]cyclohexanes. Tetrahedron Lett. 2007, 48, 2291. [Google Scholar]
- 10.A portion of these studies have been previously communicated. See:; White DR; Hinds EM; Bornowski EC; Wolfe JP Pd-Catalyzed Alkene Difunctionalization Reactions of Malonate Nucleophiles: Synthesis of Substituted Cyclopentanes via Alkene Aryl-Alkylation and Alkenyl-Alkylation. Org. Lett 2019, 21, 3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. In our prior studies involving alcohol, phenol, or indole nucleophiles, we demonstrated only one example of a reaction involving a 1,2-disubstituted alkene. That transformation also proceeded in low yield (36%). See reference 3b.
- 12.(a) Surry DS; Buchwald SL Biaryl Phosphane Ligands in Palladium-Catalyzed Amination. Angew. Chem., Int. Ed 2008, 47, 6338. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Martin R; Buchwald SL Palladium-Catalyzed Suzuki-Miyaura Cross-Coupling Reactions Employing Dialkylbiaryl Phosphine Ligands. Acc. Chem. Res 2008, 41, 1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peterson LJ; Kirsch JK; Wolfe JP Pd-Catalyzed Alkene Diamination Reactions of Nitrogen Electrophiles: Synthesis of Cyclic Guanidines and Ureas Bearing Dialkylaminomethyl Groups. Org. Lett 2018, 20, 3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeAngelis AJ; Glidner PG; Chow R; Colacot TJ Generating Active “L-Pd(0)” via Neutral or Cationic π-Allylpalladium Complexes Featuring Biaryl/Bipyrazolylphosphines: Synthetic, Mechanistic, and Structure-Activity Studies in Challenging Cross-Coupling Reactions. J. Org. Chem 2015, 80, 6794. [DOI] [PubMed] [Google Scholar]
- 15. This material contained ca 5% of an inseparable impurity.
- 16. Fused cyclopentane products such as 15i were quite sensitive to acid-mediated isomerization of the double bond, to the point that CDCl3 could not be used as a NMR solvent without leading to some isomerization of the product. As such, NMR data for these compounds were collected in C6D6.
- 17.Comins DL; Dehghani A; Foti CJ; Joseph SP Pyridine-Derived Triflating Reagents: N-(2-Pyridyl)-Triflimide and N-(5-Chloro-2-Pyridyl)Triflamide. Org. Synth 1997, 74, 77. [Google Scholar]
- 18.Ramachary DB; Narayana VV; Ramakumar K A New One-Pot Synthetic Approach to the Highly Functionalized (Z)-(Buta-1,3-Dienyl)Phenols and 2-Methyl-2H-Chromenes: Use of Amine, Ruthenium, and Base-Catalysis. Eur. J. Org. Chem 2008, 23, 3907. [Google Scholar]
- 19.Krout MR; Mohr JT; Stoltz BM Preparation of (S)-tert-ButylPHOX Org. Synth 2009, 86, 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.