Abstract
The objective of this study was to investigate the processability of hot-melt extrusion (HME) to formulate ocular inserts of valacyclovir hydrochloride and evaluate the in vivo bioavailability of the formulation. To optimize the formulation of this drug, different physical mixtures of the polymers and plasticizer were prepared. The physical mixture was extruded through a co-rotating twin-screw extruder and the obtained ocular inserts were cut with dimensions of 4 mm × 2 mm × 1 mm to enhance the formulation instillation in the eye. Ocular inserts were evaluated for drug content, weight variation, uniformity of thickness, in vitro drug release, and in vivo drug bioavailability. The ocular inserts were thermally characterized using differential scanning calorimetry (DSC). The attributes observed for the ocular inserts were within the target specifications. The ocular inserts of valacyclovir hydrochloride were successfully prepared using the HME. They provided sustained drug release along with enhanced drug permeation when compared to the eyedrop solution and dissolve completely in 8 h. Additionally, the obtained results demonstrated that the formulation of ocular inserts of valacyclovir hydrochloride using HME was reproducible, robust and effective method.
Keywords: Ocular Drug Delivery, Acyclovir, Ocular Inserts, Hot-melt Extrusion, Antiviral
Graphical Abstract
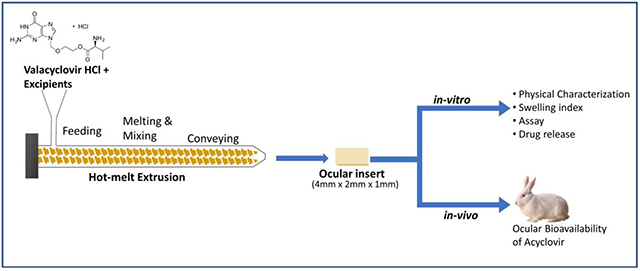
Introduction
The application of the formulation directly to the desired site, ensures that the therapeutic agent is available in higher concentration than achieved using the oral route of administration (1). Eye drops usually provide low bioavailability because the eye possesses effective protective mechanisms mediated by reflex blinking (2). This reduces the pre-corneal residence time and, thus, reduces absorption to as low as 1% of the administered drug (3). To ensure bioavailability, eye drops need to be instilled repeatedly, which often leads to low patient compliance. Semi-solid formulations such as eye ointments and gels have proven to have a higher drug bioavailability compared to the eyedrops but lack patient compliance due to sticky sensation and blurring of vision (4).
Ocular inserts are small, solid, or semi-solid polymeric devices designed to be placed in the lower fornix to deliver drugs to the corneal surface (5). The advantage of ocular inserts is increased ocular residence time of the drug, accurate dosing and reduced frequency of administration of the medication leading to better patient compliance (6). Currently available marketed ocular implants are Lacrisert® and Dextenza®.
Hot-melt extrusion (HME) offers many advantages to other pharmaceutical processing techniques as it is solvent-free and economical (7). In the extrusion process, polymers act as drug depots or release retardants following solidification (8). Solvents and water are not necessary, which reduces the number of processing steps, eliminates time-consuming drying steps and can be used for hydrolysis sensitive drugs (9). It is a promising technology for producing new drug delivery systems and improving products already on the market. HME is a continuous pharmaceutical process that involves pumping polymeric materials using a rotating screw at temperatures above their glass transition temperature (Tg) and sometimes above the melting temperature (Tm) to achieve molecular-level mixing of the active compounds and thermoplastic binders, polymers, or both (10). The intense mixing and agitation of the rotating screw causes the de-aggregation of suspended particles in the molten polymer which results in a uniform dispersion (11).
Herpes simplex virus (HSV) is one of the most common type of viral infection in humans (12). There are two subtypes of HSV. The HSV-1 is associated with orofacial infections and HSV-2 causes genital infections (13). Ocular herpes infection is a one of the predominant causes of corneal blindness in the United States of America (14). The initial ocular infection usually develops as a blepharitis, conjunctivitis or corneal epithelial keratitis. The recurrent infection is manifested as an ulcerative or stromal keratitis (15). Current therapy includes oral and topical antiviral agents (16). The prodrug approach is commonly used to transport drugs through biological membranes (17,18). Valacyclovir hydrochloride, a prodrug of acyclovir, is used in the treatment of ocular herpes (19). Valtrex® a systemic antiviral has 5 times greater bioavailability than acyclovir, allowing less frequent oral dosing (14). Valacyclovir hydrochloride is hydrolyzed to acyclovir and valine on topical application by enzymatic hydrolysis (20). The viral thymidine kinase converts acyclovir to acyclovir monophosphate, which is then converted by host cell kinases to acyclovir triphosphate (21). Acyclovir triphosphate subsequently acts to inactivate and inhibit HSV specific DNA polymerases, which prevents viral DNA synthesis further without affecting the regular cellular processes (22). Ocular penetration of the prodrug valacyclovir hydrochloride has been observed to be better than that of acyclovir in the rabbit eye via a carrier-mediated transport system (23). Valacyclovir is also known to have an affinity for peptide transporters, which explains why it has better penetration than acyclovir (24).
The main purpose of this study was to prepare ocular inserts of valacyclovir hydrochloride to treat corneal keratitis as a site-specific drug delivery system using the HME technique, which would improve patient compliance. This study is first of its kind to employ HME in development of ocular inserts using a prodrug approach.
Materials and Methods
Materials
Acyclovir (>98% purity) and valacyclovir hydrochloride (≥98% purity) were purchased from Ria International (East Hanover, NJ, USA). The Klucel® and Methocel® were gifts from Ashland (Wilmington, DE, USA) and Colorcon Inc. (Westpoint, PA, USA), respectively. Polyethylene Glycol 400 was purchased from Fisher Scientific®, while all other reagents used were of analytical grade.
Animal and Animal Tissue
Complete eyes of male albino New Zealand rabbits were acquired from Pel-Freez® Biologicals (Rogers, AR, USA). Male albino New Zealand rabbits weighing 2–3 kg were procured from Envigo (Indianapolis, IN, USA). All in vivo studies were conducted in accordance with protocols approved by the University of Mississippi Institutional Animal Care and Use Committee (Protocol- #17-018).
Preparation of Ocular Inserts
Valacyclovir hydrochloride and the polymer (Table 1) were sieved through a mesh (#60) and mixed with the plasticizer. The physical mixture was mixed using a mortar and pestle for 5 min, blended using a V-shell blender (Maxiblend, GlobePharma) at 25 rpm for 10 min, and then the blend was fed through the hopper in the co-rotating twin-screw extruder (Haake Minilab ThermoFisher Scientific®) at a constant feeding rate. The screw speed for extrusion process was set at 50 rpm with a film die insert. The obtained extrudate films were cut to the desired size (4 mm × 2 mm × 1 mm), sterilized using UV radiation, and packed into aluminum foils.
Table 1:
Composition and physicochemical characteristics of Valacyclovir Hydrochloride ocular inserts
| Formulations | HPCMF (%w/w) | HPMC K4M (%w/w) | Valacyclovir HCl (%w/w) | PEG 400 (%w/w) | Extrusion Temperature (°C) | Weight (mg) | Thickness (mm) | Drug Content (%) |
|---|---|---|---|---|---|---|---|---|
| FI | 75 | - | 20 | 5 | 120 | 5.09 ± 0.72 | 1.00 ± 0.38 | 98.59 ± 0.06 |
| F2 | 65 | 10 | 20 | 5 | 130 | 5.09 ± 1.02 | 1.08 ± 0.12 | 97.69 ± 0.89 |
| F3 | 55 | 20 | 20 | 5 | 130 | 4.98 ± 0.91 | 0.99 ± 0.29 | 101.59 ± 0.10 |
Evaluation of Ocular Inserts
The inserts were evaluated for appearance, weight variation, uniformity of thickness, swelling index, drug content, thermal analysis, sterility, in vitro drug release, trans-corneal permeation, in vivo bioavailability, and stability.
Physical Characterization
The surface of the ocular inserts was observed for color and surface smoothness. Nine individual ocular inserts were weighed, and the deviations were calculated. The thickness uniformity of ocular inserts was measured using a digital caliper. The uniformity was examined by placing the caliper at two different points on the ocular insert.
Drug Content
Each ocular insert was weighed, cut into pieces and placed in 50 mL of 1:1 hydro alcoholic solution. The ocular insert was dissolved by sonication for 30 min. The obtained sample was centrifuged, supernatant was diluted suitably and quantified for drug content by measuring the absorbance using an ultraviolet (UV) spectrophotometer (GENESYS 180, Thermo Scientific, Waltham, MA, USA) at 254 nm. The results were extrapolated from the standard curve to determine drug content. The ocular inserts were analyzed for drug content over the period of 30 and 60 days.
Swelling Index
To determine the swelling index of the inserts, each insert was weighed initially and then placed into freshly prepared isotonic phosphate-buffer saline (IPBS, pH 7.4) at 37 ± 0.2 °C. The insert was removed after every 5 min for up to 30 min, excess media was wiped using filter paper, and the insert was reweighed. The swelling index was calculated as (25):
The swelling index was measured on nine randomly selected ocular inserts from batch.
Differential Scanning Calorimetry
DSC was conducted to check the thermal behavior of the active pharmaceutical ingredient (API), pure polymers, and ocular inserts. The instrument used for analysis was Discovery 25 differential scanning calorimeter (TA Instruments, New Castle, DE, USA). The sample was heated from 25 °C to 160 °C at a heating rate of 10 °C/min under a nitrogen atmosphere (50 mL/min). The samples (approximately 4–5 mg) were weighed in aluminum pans and hermetically sealed while an empty pan was used as a reference.
Sterilization of Ocular Inserts
The sterilization of the ocular inserts was performed under the UV radiation (253.7 nm) for 30 min. The efficiency of sterilization was determined by incubating the sterilized and non-sterilized ocular inserts in the sodium thioglycolate medium for 48 h under aseptic conditions. After incubation, the samples were compared visually for microbial growth by comparison against the positive control and negative control (26). The study was performed in triplicate.
For preparation of sodium thioglycolate medium, 7.54 g of media was dispersed in 200 mL of sterile distilled water and boiled until the media was completely dissolved. The media was distributed into test tubes and sterilized by autoclaving at 121 °C for 15 min (27).
In Vitro Release Study
The in vitro release of valacyclovir hydrochloride from the ocular inserts was tested using 20 mL scintillation vials and a standard US #100 mesh sieve. IPBS pH 7.4 was used as the medium for the release study and sink conditions were maintained. The ocular insert was placed in a vial with the mesh placed over it; a stir bar was placed over the sieve and 10 mL of the release medium was added to the vials. The whole assembly was placed over a multi-station hot plate maintained at 34 ± 0.2 °C with continuous stirring for 8 h. 0.5 mL of the sample was collected after first 30 minutes and then after every 1 h up to 8 h to determine the amount of valacyclovir hydrochloride released. The media was replaced with the equal amount of fresh media after each withdrawal.
Analytical Method for In Vitro
Valacyclovir hydrochloride was quantified using a Waters HPLC system with 600 E pump controller, 717 plus autosampler, and 2486 UV detector. The in vitro release study, drug content and the trans-corneal samples were analyzed using the Phenomenex Luna® 5 μm C18 100 A° 250 × 4.60 mm column at a flow rate of 0.75 mL/min. The mobile phase used for the analysis of valacyclovir hydrochloride was a methanol and 0.1% aqueous solution of glacial acetic acid (1:9, v/v) at 254 nm with injection volume of 20 μL. All experiments were carried out in triplicate.
Ex Vivo Trans-Corneal Permeation Study
The eyes of freshly euthanized young rabbits were obtained from Pel-Freez®. The permeation study was performed using modified vertical Franz diffusion cells (Perm Gear®). Freshly excised rabbit cornea was placed between the donor and receptor compartments so that the epithelial surface faced the donor compartment. The receptor compartment was filled with 5 mL of freshly prepared IPBS. The opening of the donor and receptor compartments was sealed using paraffin wax paper and the apparatus was maintained at 34 ± 0.2 °C with continuous stirring using magnetic stirrers. Aqueous solution of valacyclovir hydrochloride was used as a control.
The difference in formulation concentration on both sides of the cell created a pressure difference across the corneal membrane, which caused the cornea to bulge out towards the cell with less formulation, thus, mimicking the natural curvature of the cornea. The total corneal area available for diffusion was 0.64 cm2 and the test was conducted for 4 h. Samples were withdrawn hourly from the receptor compartment and replaced with the same quantity of the medium. The concentration of valacyclovir hydrochloride in the receiver chamber solution was determined using the HPLC analysis method for in vitro samples as described previously.
The steady-state flux was calculated from the rate of transport of the drug per unit surface area.
where dM is the cumulative amount of drug transported across the cornea and A is the total corneal surface area (0.64 cm2).
In Vivo Bioavailability Study
The ocular insert formulation was sterilized using UV radiation as described above and was investigated for in vivo drug bioavailability studies. An aqueous solution of valacyclovir hydrochloride was used as a control. The protocol for the use of animals was approved by the Institutional Animal Care and Use Committee (IACUC). The in vivo bioavailability of acyclovir from the prodrug valacyclovir hydrochloride was determined in male New Zealand white albino rabbits weighing approximately 2–3 kg. The ocular insert and 50 μL of the control formulation was placed in the cul-de-sac of the rabbits. At the end of 2 and 4 h, the rabbits were anesthetized using a combination of ketamine (35 mg/kg) and xylazine (3.5 mg/kg) injected intramuscularly. The anesthetized rabbits were euthanized with an overdose of pentobarbital injected through the marginal ear vein and then the eyes were enucleated and washed with Dulbecco’s PBS. The tissues were separated for further analysis and all formulations were tested in triplicate (28).
Bioanalytical Method for In Vivo Sample Analysis
Sample Preparation
To 190 μL of aqueous humor (AH) or the weighed cornea sample, 600 μL ice-cold methanol was added to precipitate the proteins from each individual tissues. The corneal tissues were cut into small pieces for effective extraction. Samples were centrifuged at 13,000 rpm for 30 min and then the supernatant was collected before analysis.
Analytical Method for Acyclovir
Calibration curves were constructed by spiking acyclovir in the blank AH and cornea. Acyclovir from the tissue was analyzed using a Waters HPLC system with a 600 E pump controller, 717 plus autosampler, and 2486 UV detector. A Phenomenex Luna® 5 μm C18 100 A° 250 × 4.60 mm column was used with a mobile phase that consisted of acetonitrile:water (1:9, v/v) at a flow rate of 0.75 mL/min. The detection wavelength was set at 254 nm and 20 μL of each sample was injected into the column. Final concentrations of the standard solutions were in the range of 10–400 ng/mL and the constructed standard curve had a coefficient of determination (R2) > 0.941.
Statistical Analysis
The results are presented as mean values ± standard deviation (SD). Statistical analysis was performed using an unpaired t-test (Graph Pad 5 Prism® Software, USA). The confidence interval set for the statistical analysis was 95%; hence, the p-value for the significant difference should be less than 0.05.
Stability Studies
A stability study of 30 and 60 days was carried out at 25 ± 2 °C. The stability samples were evaluated for drug content, and thermal analysis. The thermal analysis parameters were observed in DSC.
Results and Discussion
Physical Characterization and Drug Content
The prepared three formulations were evaluated for weight variation, uniformity of thickness and drug content (Table 1). The weight of the ocular inserts of all formulations was between 4.98 and 5.09 mg, indicating that the obtained extrudates were uniform. The expected thickness of the ocular inserts was 1 mm, which was observed with the F1 formulation with a minimum SD. The drug content varied between 97.69 % and 101.59 %. In addition, the SD of the drug content of each formulation was < 2% and, thus, demonstrated the uniformity of the drug content into the polymer matrix during the formulation process.
Differential Scanning Calorimetry
The HME process requires the polymer and drug to be heated to an elevated temperature. Hence, it is important to ensure that all the materials are stable at the processing temperature. Valacyclovir hydrochloride showed a melting peak at 129 °C, indicated by an endothermic peak, proving that the drug was in the crystalline form (Figure 1). After processing, the characteristic valacyclovir hydrochloride peak was absent in the polymeric ocular inserts, indicating the absence of crystallinity in the ocular inserts.
Figure 1:
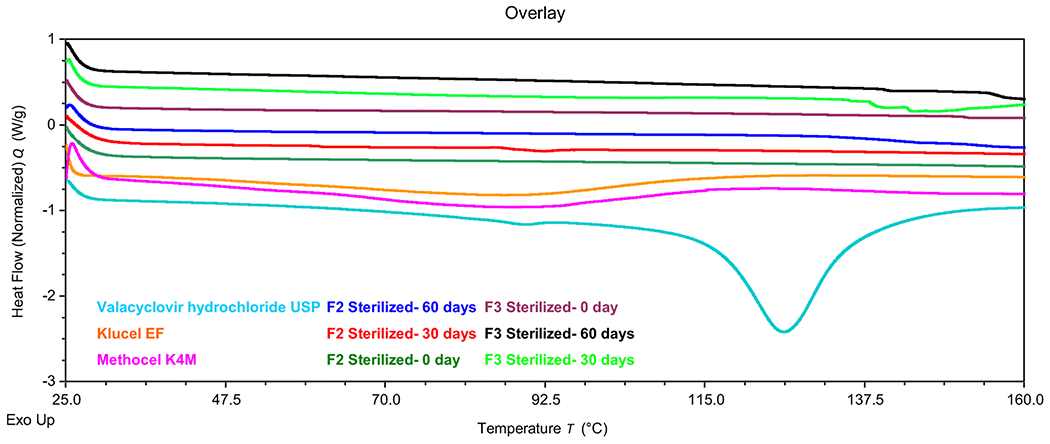
DSC thermogram of valacyclovir hydrochloride, pure polymers (HPC and HPMC), formulations F2 and F3 for day 0, day 30 and day 60 after sterilization
Swelling Index
The swelling index represents the water-retaining capacity of the polymers (29). Water absorption and swelling increase with time owing to polymer hydrophilicity. The integrity of the matrix and swelling process is greatly influenced by the viscosity of the polymer. Thus, determining the swelling index of the formulation is important as it is an indicator of the adhesion properties of the ocular inserts. The inserts did not break after swelling, hydrated quickly, and formulations F2 and F3 reached hydration of approximately 30% in 20 min (Figure 2). In contrast, formulation F1 only reached 15% of hydration in 30 min.
Figure 2:
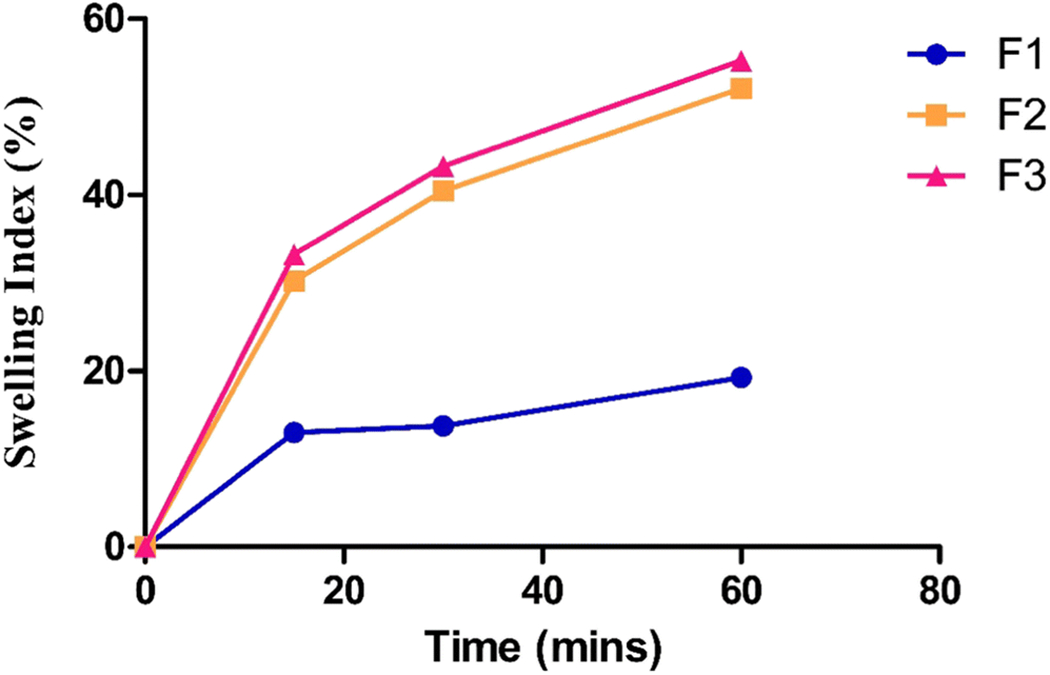
Swelling index of ocular inserts in isotonic phosphate buffer saline
A very high swelling index was observed with ocular inserts containing HPMC K4M, which is a hydrophilic swellable polymer with a high molecular weight that forms a viscous gel layer on hydration. Hence, increasing the amount of this polymer in the ocular insert increased the swelling behavior. Polymer swelling is necessary to initiate the bio-adhesive characteristic of the inserts (24). The swelling index of formulations F2 and F3 followed a similar trend. It was difficult to measure the swelling index of F1 after 30 min because it broke into pieces and reached maximum hydration of 20%. HPC is a low-molecular-weight excipient that undergoes faster erosion and hence shows a lower swelling index than that of HPMC.
In Vitro Release Study
After the 2-h time point, the drug release from the ocular inserts varied from 36.15% to 78.07% (Figure 3). For formulation F1, 100% drug release was observed within 5 h. However, the release of formulations F2 and F3 reached 100% by 8 h because of the presence of HPMC K4M in the ocular inserts. The release of the drug was affected by the presence of HPMC K4M in the formulation as it acts as a release retardant. Based on the film characteristics and in vitro drug release profiles, formulations F2 and F3 were selected for further ex vivo tissue permeation studies.
Figure 3:
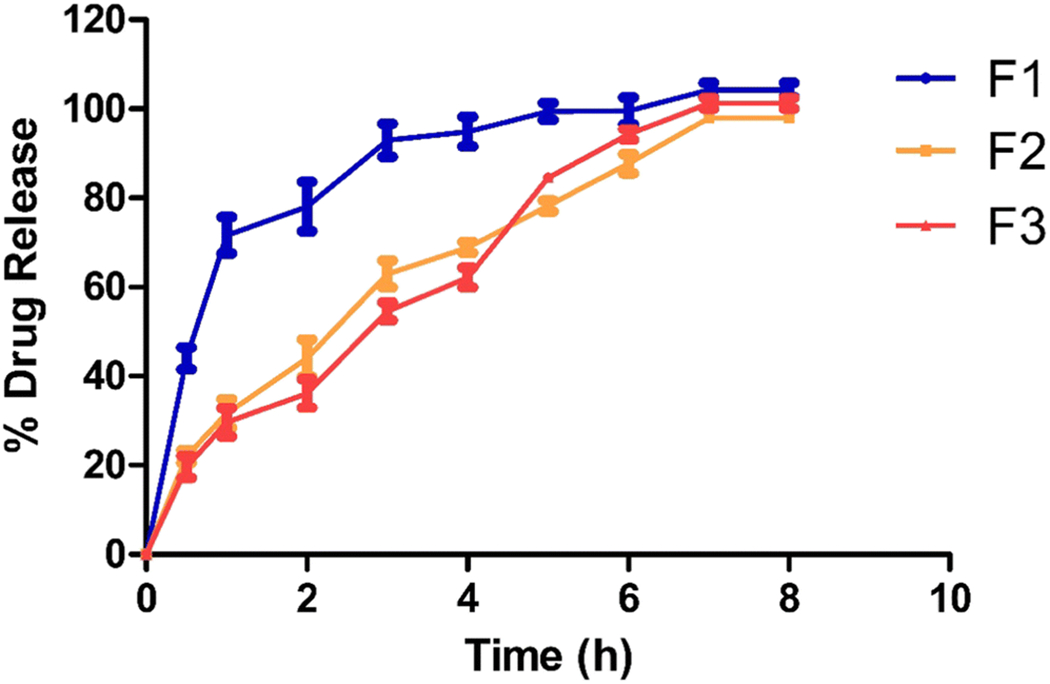
In vitro drug release from ocular insert
Ex Vivo Trans-Corneal Permeation
Of the prepared formulations, only F2 and F3 were tested for trans-corneal permeation. The plot of the cumulative amount of drug that permeated the cornea versus time was used to obtain the rate of transport through the rabbit cornea.
The trans-corneal studies were performed in triplicate and the data are expressed as means ± SD. The trans-corneal flux of the F2 and F3 ocular inserts was 0.662 ± 0.087 and 0.606 ± 0.084 μg·min−1·cm(2)−1, respectively while that of the control eyedrop formulation was 0.069 ± 0.002 μg·min−1·cm(2)−1. The flux of the F2 and F3 ocular inserts was 9.5- and 8.7-fold more than that of the control formulation (Figure 4). The ocular inserts are in close proximity to the corneal tissues and thus create a localized high drug concentration. This drug accumulation near corneal surface enhances the trans-corneal flux as compared to the control solution.
Figure 4:
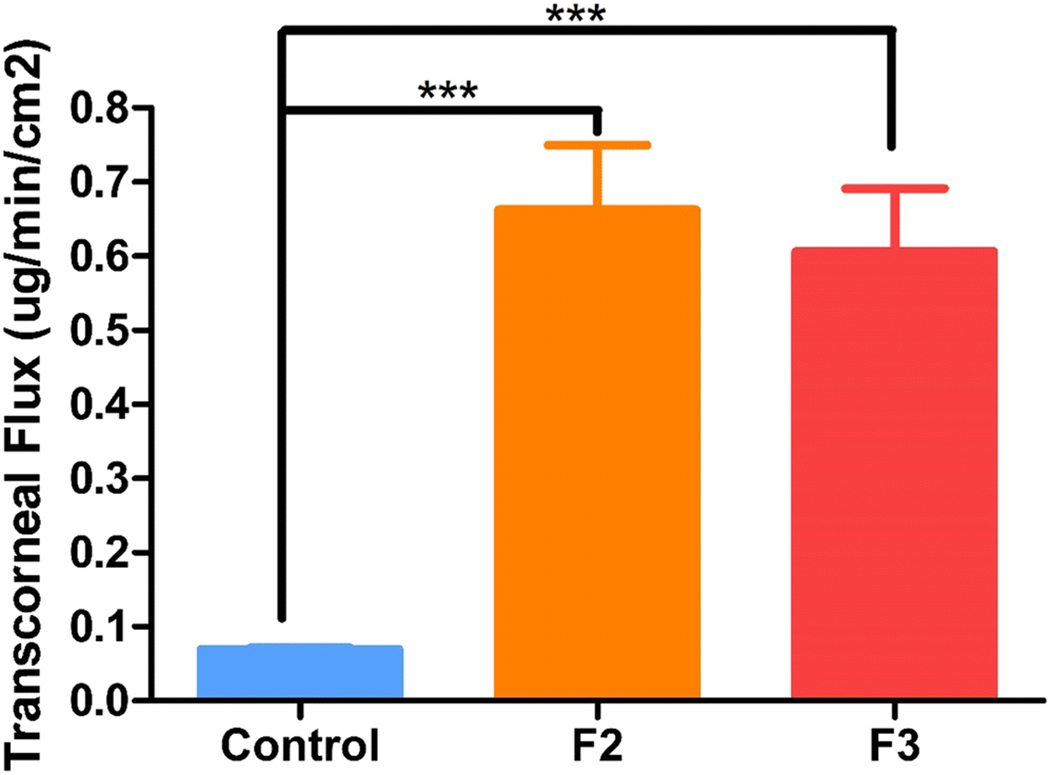
Trans-corneal flux obtained from control and ocular inserts at equivalent doses using valia-chien cells at 34°C.
***Symbol represented on control and ocular inserts indicate statistically significant difference of the flux (p <0.001).
We can conclude that the flux of the investigated ocular inserts was higher than that of the control formulation. The ocular inserts provided sustained drug release and improved the corneal drug permeation by enhancing retention.
Sterility Study
In the sterilization study we revealed that the developed ocular inserts were sterile after the UV sterilization. The positive control showed microbial growth on incubation for 48 h and compared to that the negative control remained clear and did not show any growth. This shows effective sterility of the ocular inserts.
In Vivo Bioavailability Study
The ocular insert of size 4×2×1 mm were placed in the cul-de-sac of New Zealand albino rabbits. For the control formulation, 50 μL of the formulation was instilled into the cul-de-sac. The films adhered to the cul-de-sac within 5 to 10 min of contact with the tear fluid and hence did not interfere with vision. The concentration of the active form, acyclovir, was determined in the rabbit cornea and aqueous humor, and the drug content was analyzed at 2 and 4 h. The amount of acyclovir in cornea treated with F2 after 2 and 4 hours was 0.95 ± 0.14 and 1.24 ± 0.067 μg/g, respectively. For F3, the amount of acyclovir in the cornea was 1.02 ± 0.08 and 1.34 ± 0.07 μg/g at 2 and 4 h, respectively. Similarly, for the control solution, the amount of acyclovir in the cornea was 1.51 ± 0.40 and 0.83 ± 0.02 μg/g at 2 and 4 h, respectively (Figure 5).
Figure 5:
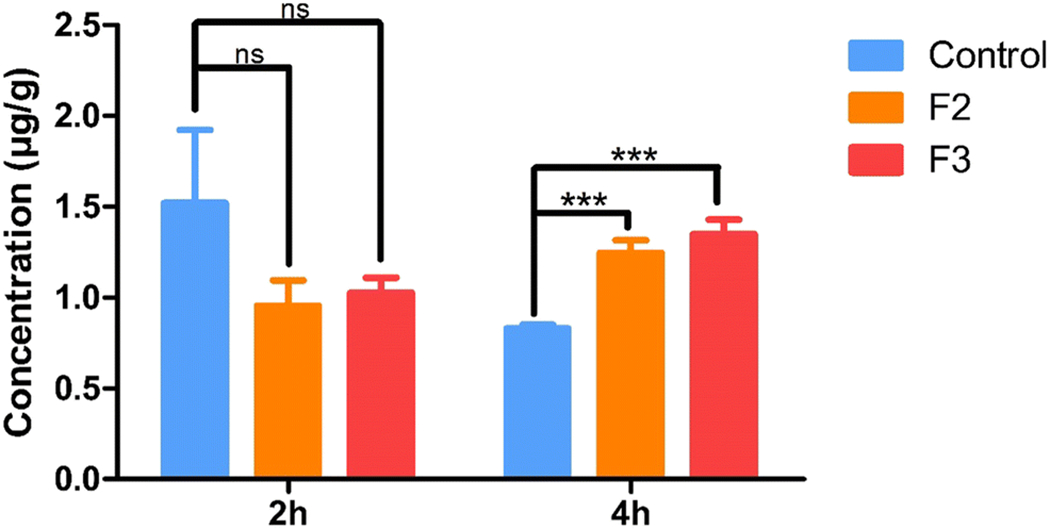
In vivo acyclovir concentrations obtained in cornea from ocular inserts and control solution at equivalent dose, 2 h and 4 h after topical application in conscious rabbit model. All experiments were performed in triplicate. *** Symbol represented indicates statistically significant difference in ocular inserts and control (p < 0.001).
Similarly, the amount of acyclovir was measured in the aqueous humor of the rabbit. The acyclovir concentrations of the F2 and F3 formulations at 2 h were 0.122 ± 0.01 and 0.13 ± 0.02 μg/mL, respectively, while the values at 4 h were 0.295 ± 0.01 and 0.288 ± 0.01 μg/mL, respectively. For the control solution the concentration of acyclovir in the aqueous humor was 0. 27 ± 0.02 and 0.22 ± 0.014 μg/mL at the 2- and 4-h timepoints respectively (Figure 6).
Figure 6:
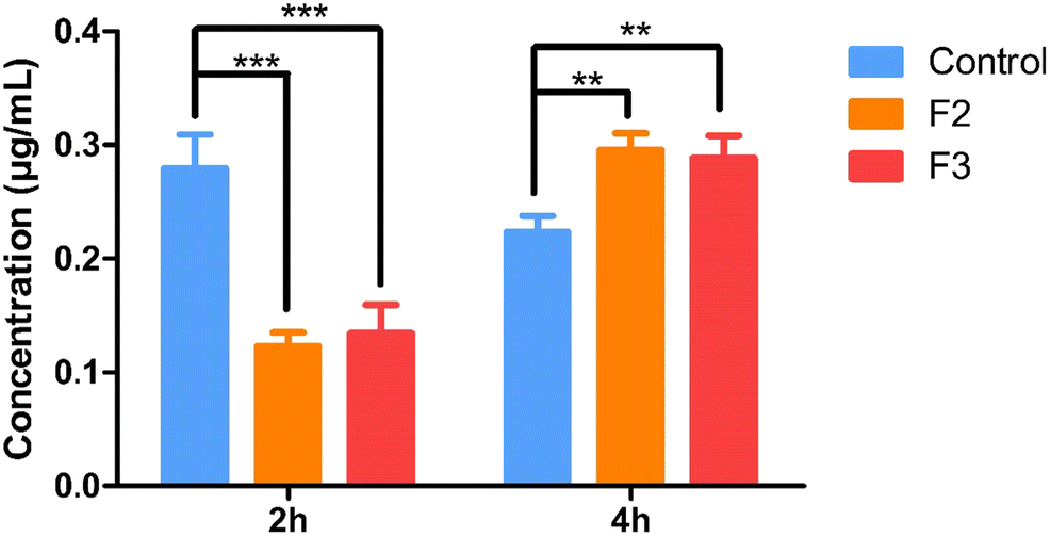
In vivo acyclovir concentrations obtained in aqueous humor from ocular inserts and control solution at equivalent dose, 2 h and 4 h after topical application in conscious rabbit model. All experiments were performed in triplicate. **Symbol represented indicated statistically significant difference in ocular inserts and control (p < 0.01).
***Symbol represented indicated statistically significant difference in ocular inserts and control (p < 0.001).
The ocular inserts provided sustained drug release with improved retention of the drug. The control initially showed a higher drug permeation owing to the drug already present in the solution form, whereas with the inserts, the drug had to first overcome the matrix barriers of the film. This resulted in a slow but steady release of the drug from the inserts. Thus, the permeation and subsequent retention of the drug were higher in the inserts than in the control. No signs of irritation and redness were observed during the experiment in the rabbit eyes.
Stability
The formulations were stored for the stability assessment for 30 and 60 days. The drug content was observed to be 98.85 ± 0.09 % for 30 days and 98.87 ± 0.06 % for 60 days which were within the expected limits. The stability was further confirmed by the absence of crystallization in the thermograms (Figure 1).
Conclusion
Valacyclovir hydrochloride ocular inserts were successfully developed using the HME technique, which did not require a solvent for preparation and used minimal excipients. The ocular inserts showed improved flux compared with those of the control formulation. The ocular inserts dissolved completely within 8 h, proving their biodegradability. The drug was released over a period of time and, thus, would improve patient compliance compared to that of using eyedrops and eye ointments. The ocular inserts were soft in texture and assumed a gel-like consistency in the eye. The polymer and plasticizer used in the formulation were nonirritant and non-greasy and, hence, would improve therapy and the acceptability of the formulation. Thus, this study demonstrated the novelty and functionality of the widely applicable HME technique in the production of ocular inserts.
Acknowledgments
This project was partially supported by Core E, Grant Number P20GM104932 from the National Institute of General Medical Sciences (NIGMS), a component of NIH.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of interest
The authors declare no conflict of interest.
References
- 1.Wen H, Jung H, Li X. Drug Delivery Approaches in Addressing Clinical Pharmacology-Related Issues: Opportunities and Challenges. AAPS J. 2015. November;17(6):1327–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta H, Aqil M, Khar RK, Ali A, Bhatnagar A, Mittal G, et al. Development and Characterization of 99mTc-timolol Maleate for Evaluating Efficacy of In Situ Ocular Drug Delivery System. AAPS PharmSciTech. 2009. June;10(2):540–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agrahari V, Mandal A, Agrahari V, Trinh HM, Joseph M, Ray A, et al. A comprehensive insight on ocular pharmacokinetics. Drug Delivery and Translational Research. 2016. December;6(6):735–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baranowski P, Karolewicz B, Gajda M, Pluta J. Ophthalmic drug dosage forms: Characterisation and research methods. The Scientific World Journal. 2014. January;2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumari A, Sharma PK, Garg VK, Garg G. Ocular inserts — Advancement in therapy of eye diseases. J Adv Pharm Technol Res. 2010. July;1(3):291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saettone MF, Salminen L. Ocular inserts for topical delivery. Advanced Drug Delivery Reviews. 1995. August;16(1):95–106. [Google Scholar]
- 7.Repka MA, Bandari S, Kallakunta VR, Vo AQ, McFall H, Pimparade MB, et al. Melt extrusion with poorly soluble drugs – An integrated review. Int J Pharm. 2018. January;535(1-2):68:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patil H, Tiwari RV., Repka MA. Hot-Melt Extrusion: from Theory to Application in Pharmaceutical Formulation. AAPS PharmSciTech. 2016. February;17(1):20–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Repka MA, Battu SK, Upadhye SB, Thumma S, Crowley MM, Zhang F, et al. Pharmaceutical Applications of Hot-Melt Extrusion: Part II. Drug Dev Ind Pharm. 2007. January;33(10):1043–57. [DOI] [PubMed] [Google Scholar]
- 10.Feng X, Vo A, Patil H, Tiwari RV., Alshetaili AS, Pimparade MB, et al. The effects of polymer carrier, hot melt extrusion process and downstream processing parameters on the moisture sorption properties of amorphous solid dispersions. J Pharm Pharmacol. 2016. May;68(5):692–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crowley MM, Zhang F, Repka MA, Thumma S, Upadhye SB, Battu SK, et al. Pharmaceutical applications of hot-melt extrusion: Part I. Drug Development and Industrial Pharmacy. 2007. January;33(9):909–26. [DOI] [PubMed] [Google Scholar]
- 12.D. Vadlapudi A, K. Vadlapatla R, K. Mitra A. Update On Emerging Antivirals For The Management Of Herpes Simplex Virus Infections: A Patenting Perspective. Recent Pat Antiinfect Drug Discov. 2013. April;8(1):55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whitley RJ, Roizman B. Herpes simplex virus infections. Lancet. 2001. May;357(9267):1513–8. [DOI] [PubMed] [Google Scholar]
- 14.Fatahzadeh M, Schwartz RA. Human herpes simplex virus infections: Epidemiology, pathogenesis, symptomatology, diagnosis, and management. Journal of the American Academy of Dermatology. 2007. November;57(5):737–63. [DOI] [PubMed] [Google Scholar]
- 15.Kaye S, Choudhary A. Herpes simplex keratitis. Progress in Retinal and Eye Research. 2006. July;25(4):355–80. [DOI] [PubMed] [Google Scholar]
- 16.Koganti R, Yadavalli T, Shukla D. Current and emerging therapies for ocular herpes simplex virus type-1 infections. Microorganisms. 2019. October;7(10):429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Müller CE. Prodrug approaches for enhancing the bioavailability of drugs with low solubility. In: Chemistry and Biodiversity. 2009. November;6(11):2071–83. [DOI] [PubMed] [Google Scholar]
- 18.Taskar P, Tatke A, Majumdar S. Advances in the use of prodrugs for drug delivery to the eye. Expert Opinion on Drug Delivery. 2017. January;14(1):49–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsatsos M, MacGregor C, Athanasiadis I, Moschos MM, Hossain P, Anderson D. Herpes simplex virus keratitis: an update of the pathogenesis and current treatment with oral and topical antiviral agents. Clinical and Experimental Ophthalmology. 2016. December;44(9):824–37. [DOI] [PubMed] [Google Scholar]
- 20.Kapanigowda UG, Nagaraja SH, Ramaiah B, Boggarapu PR, Subramanian R. Enhanced Trans-Corneal Permeability of Valacyclovir by Polymethacrylic Acid Copolymers Based Ocular Microspheres: In Vivo Evaluation of Estimated Pharmacokinetic/Pharmacodynamic Indices and Simulation of Aqueous Humor Drug Concentration-Time Profile. J Pharm Innov. 2016. March;11(1):82–91. [Google Scholar]
- 21.Elion GB. Mechanism of action and selectivity of acyclovir. Am J Med. 1982. July;73(1):7–13. [DOI] [PubMed] [Google Scholar]
- 22.Paintsil E, Cheng YC. Antiviral agents. In: Encyclopedia of Microbiology. 2019:176. [Google Scholar]
- 23.Anand BS, Mitra AK. Mechanism of corneal permeation of L-Valyl ester of acyclovir: Targeting the oligopeptide transporter on the rabbit cornea. Pharm Res. 2002. August;19(8):1194–202. [DOI] [PubMed] [Google Scholar]
- 24.Anand BS, Nashed YE, Mitra AK. Novel dipeptide prodrugs of acyclovir for ocular herpes infections: Bioreversion, antiviral activity and transport across rabbit cornea. In: Current Eye Research. 2003. January;26(3-4):151–63. [DOI] [PubMed] [Google Scholar]
- 25.Juliano C, Cossu M, Pigozzi P, Rassu G, Giunchedi P. Preparation, in vitro characterization and preliminary in vivo evaluation of buccal polymeric films containing chlorhexidine. AAPS PharmSciTech. 2008;9(4):1153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jethava J, Jethava G. Design, formulation, and evaluation of novel sustain release bioadhesive in-situ gelling ocular inserts of ketorolac tromethamine. Int J Pharm Investig. 2014. October;4(4):226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thioglycollate broth: Principle, Composition, Preparation and Uses - Learn Microbiology Online. Available from: https://microbeonline.com/thioglycollate-broth-principle-composition-preparation-uses/
- 28.Balguri SP, Adelli GR, Tatke A, Janga KY, Bhagav P, Majumdar S. Melt-Cast Noninvasive Ocular Inserts for Posterior Segment Drug Delivery. J Pharm Sci. 2017. December;106(12):3515–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma R, Kamboj S, Singh G, Rana V. Development of aprepitant loaded orally disintegrating films for enhanced pharmacokinetic performance. Eur J Pharm Sci. 2016. March;84:55–69. [DOI] [PubMed] [Google Scholar]


