Abstract
Background:
Hepatitis C virus (HCV)-infected patients, including those co-infected with human immunodeficiency virus (HIV), are at increased risk of developing hepatocellular carcinoma (HCC). We evaluated the ability of agonistic human monoclonal antibodies to tumor necrosis factor-related apoptosis inducing ligand (TRAIL) receptors, mapatumumab and lexatumumab, respectively, to induce TRAIL-receptor mediated apoptosis (TRMA) in HCC (HCV-infected and -uninfected) cells and in peripheral blood cells (HIV-infected and -uninfected).
Methods:
Susceptibility to antibody-mediated TRMA was measured by caspase 3/7 activity and by confocal microscopy. Surface expression of receptors on HCV-uninfected and -infected Huh7.5 cells was measured by flow cytometry and confocal microscopy. Inhibitor of Apoptosis Protein (IAP) RNA levels were quantified by RT-PCR. DNA Microarray was performed using RNA isolated from Huh7.5 cells (HCV-infected and uninfected) using Affymetrix U133A chips.
Results:
Mapatumumab preferentially induces TRMA of HCV-infected Huh7.5 cells by binding to TRAIL-R1. Higher basal expression of TRAIL-R2 compared to that of TRAIL-R1 on HCV-uninfected Huh7.5 cells were observed. Lexatumumab induces TRMA of both HCV-infected and -uninfected cells by binding to TRAIL-R2. IFN-α has minimal effect on mapatumumab- and lexatumumab-induced TRMA. HCV infection of Huh7.5 cells up-regulates TRAIL-R1 expression and X-linked Inhibitor of apoptosis protein and survivin gene expression. Neither antibody had a pro-apoptotic effect on PBMCs from patients with HIV infection ex vivo.
Conclusion:
Both mapatumumab and lexatumumab are excellent candidates for therapy of HCC. HCV infection of Huh7.5 cells selectively up-regulates TRAIL-R1 receptor, associated with increased susceptibility to mapatumumab-mediated TRMA. HCV infection up-regulated IAP genes, offering promise for future combination therapy using TRAIL agonists and IAP inhibitors.
Keywords: hepatitis C virus cell culture system, hepatocellular carcinoma, tumor necrosis factor-related apoptosis inducing ligand agonistic antibodies
INTRODUCTION
CHRONIC HEPATITIS C virus (CHCV) infection affects approximately 180 million people worldwide,1 with cirrhosis and hepatocellular cancer developing in a significant proportion of CHCV patients. Approximately 30% of individuals with human immunodeficiency virus type 1 (HIV-1) infection in the US and Europe are also co-infected with HCV, which is a major reason for the morbidity seen in this population.2,3 Moreover, HCV/HIV co-infected subjects have a more rapid progression of liver fibrosis, and lower response rates to IFN-α based regimens when compared to that of HCV mono-infected individuals.4 While early studies with maintenance interferon (IFN)-α therapy showed a beneficial effect in reducing fibrosis progression or the development of HCC, the recently concluded HALT-C study showed no beneficial effects.5 These data suggest that a substantial number of HCV-infected subjects, especially those who are co-infected with HIV, are at a risk of progressive liver fibrosis and its complications such as cirrhosis and HCC in the future.6,7
Chronic hepatitis B and C virus infections have been the most prominent predisposing factors for hepatocarcinogenesis.8 HCC, the fifth most common malignancy worldwide, is associated with a poor prognosis due to late diagnoses and a lack of effective therapy options.9 Carcinogenesis is associated with suppression of apoptosis pathways resulting in uncontrolled proliferation of abnormal cells.10,11 Induction of apoptosis of cancer cells has been suggested as a viable therapeutic option for the treatment of malignancies.10,12 Mapatumumab and lexatumumab are monoclonal TNF-related apoptosis inducing ligand (TRAIL)-agonistic human monoclonal antibodies (mAbs), which have been shown to induce apoptosis in several cancer cell lines.13,14 Binding of mapatumumab and lexatumumab to TRAIL receptors R1 and R2 respectively results in the initiation of the extrinsic apoptosis pathway signaling cascade, leading to caspase 8 and caspase 3 activation and subsequent cell death.15–19 However, mapatumumab and lexatumumab have not yet been extensively evaluated for their abilities to induce apoptosis of human liver cancer cells infected with HCV or HBV in vitro or in vivo. A recent study demonstrated that a specific HCV isolate, JFH-1, is capable of inducing apoptosis of LH86 hepatoma cells probably by triggering an intracellular anti-viral defense mechanism mediated by enhanced susceptibility to TRAIL-mediated apoptosis.20
The effect of TRAIL and/or TRAIL receptor agonists on the susceptibility of HCV-infected Huh7.5 cells to TRAIL-mediated apoptosis (TRMA) has not been previously addressed. Since TRAIL agonist-mediated apoptosis induced by antibodies could provide a way of specifically targeting HCV-infected, poorly differentiated human HCC cells, we examined the apoptosis inducing abilities of mapatumumab and lexatumumab on Huh7.5 cells. Furthermore, while the role of IFN-α in preventing liver carcinogenesis is still debated, the molecular mechanisms underlying IFN-α activity include the up regulation of cell death receptors21 and modulation of the central apoptosis regulator NF-κB.22,23 Recent therapeutic advances that target apoptosis directly or indirectly have provided a rationale for combination therapy with IFN-α.22 We therefore explored the interactions of IFN-α2b and albinterferon alfa-2b (alb-IFN), a long acting fusion polypeptide (24), with the TRAIL pathway in this system. Finally, the impact of these antibodies in HIV-infected peripheral blood mononuclear cells (PBMC) was investigated given previous reports of enhanced sensitivity to TRAIL-mediated apoptosis in HIV-infected T cells.24,25
MATERIALS AND METHODS
Study subjects
PERIPHERAL BLOOD MONONUCLEAR Cells (PBMCs) were obtained from HIV seronegative healthy volunteers and HIV-infected patients after signing informed consents approved by the Institutional Review Boards (IRB) of the Department of Transfusion Medicine (DTM) and the National Institute of Allergy and Infectious Diseases (NIAID) at the National Institutes of Health (NIH) respectively.
Isolation of PBMCs and culture conditions
PBMCs were isolated by Ficoll Hypaque density gradient centrifugation (GE Healthcare, South Burlington, VT) and resuspended in RPMI 1640 medium (Invitrogen Corporation, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS), 1% penicillin/streptomycin, 2% glutamine and incubated at 37°C in a 5% CO2 containing incubator for 24 h in the presence or absence of control mouse anti-human IgG1 antibody (R&D Systems, Minneapolis, MN), or mapatumumab, lexatumumab and/or TRAIL –R1-Fc and TRAIL-R2-Fc proteins (R&D Systems).
Cell line and culture conditions
The Huh7.5 HCC cell line was provided by Apath LLC (Saint Louis, MO). Cells were cultured in Dulbecco’s modified eagle medium (DMEM; Invitrogen Corporation) supplemented with 10% FBS and 100 U/mL penicillin/streptomycin at 37°C in a 5% CO2 containing incubator.
HCV infectious clone
The infectious plasmid clone for the HCV J6/JFH1 infectious cell culture system was provided by Apath LLC. Transfection and subsequent infection of Huh 7.5 cells using this plasmid was performed as described elsewhere.26,27
Antibodies
Mapatumumab and Lexatumumab are agonistic, fully human IgG1 antibodies specific to TRAIL receptors TRAIL-R1 and TRAIL-R2 respectively and were provided by Human Genome Sciences (Rockville, MD). Mouse anti-human IgG1 antibody (R&D Systems) and TRAIL-R1-Fc and TRAIL-R2-Fc fusion proteins (R&D Systems) were used as a control and to block receptor binding of mapatumumab and lexatumumab to TRAIL-R1 and TRAIL-R2 respectively.
Caspase – Glo 3/7 assay (Promega)
Uninfected and J6/JFH1-infected Huh 7.5 cells were plated in 96-well solid white plates (1.8 × 104 cells/well) in 100 μl of DMEM. After overnight incubation at 37°C, 100 μl of fresh DMEM containing serial dilutions of TRAIL-R antibody were added to the cells. After 24 h of incubation, Caspase-Glo 3/7 reagent (Promega Corporation, Madison. WI) was added to each well. The contents of the plate were gently mixed using a plate shaker at 300–500 rpm for 30 s. The cells were then lysed at room temperature for 1 h. The luminescence activities (relative Caspase 3 and 7 activities) were measured using an OPTIMA plate reader (BMG Labtech, Durham, NC).
DNA microarray analysis
J6/JFH-infected and uninfected Huh7.5 cells were used for DNA microarray analysis. Total RNA and labeled cRNA synthesis and hybridization to the Affymetrix U133A human microarray were performed according to the manufacturer’s recommended protocol. Gene expression values (log2) were determined by GC-Robust Multi-Array algorithm (Wu ZJ and Irizarray R: http://www.bioconductor.org) followed by a Loess normaliza-tion using an R package (http://www.elwood9.net/spike). Expression of TRAIL-R1, R2, R3 and R4 were compared between HCV-infected and uninfected Huh7.5 cells from four separate experiments. TRAIL-R3 mRNA signal was not detected.
Flow cytometry
The number of lymphocytes undergoing apoptosis was defined by surface staining with CD3-APC, CD4-PE-Cy7 as well as PE-conjugated Annexin-V antibody (BD Pharmingen, San Jose, CA) utilizing BD FacsArray bioanalyzer (BD Biosciences, San Jose, CA). TRAIL-R2 PE antibody (BD Pharmingen) staining of Huh 7.5 cells resuspended in EDTA-containing PBS was performed and analyzed using the FACS Canto bioanalyzer (BD Biosciences). FlowJo software (Tree Star, Ashland, OR) was used to analyze the data.
Confocal microscopy
Approximately 20 000 J6/JFH-1- infected and uninfected Huh7.5 cells were plated in Lab-Tek eight well Nunc Glass Chamber Slide (Krackeler Scientific, Albany, NY) and incubated at 37°C overnight. Cells were washed three times with PBS, and permeabilized with 0.05% Triton for 20 min. Cells were incubated with two primary antibodies (10 µg/mL goat IgG1 anti-human TRAIL-R1 (R&D Systems) and mouse IgG1 anti-HCV core antigen (Abcam)) with 1% BSA for one hour at room temperature. Cells were washed again with 1% BSA containing PBS, and incubated for 30 min at room temperature with first secondary antibody (Alexa Fluor 594 Fab fragment of rabbit anti-goat IgG (Invitrogen, Carlsbad, CA)) and subsequently with second fluorescent conjugates secondary antibody (Alexa Fluor 488 Goat anti-mouse IgG1 (Invitrogen)). Finally, cells were washed in PBS and mounted with DAPI containing mounting medium. Samples were observed under a confocal microscope (Leica SP2, Leica Microsystems, Exton, PA) using a 63X oil immersion objective NA 1.32. Images were captured with Huygens Essential software, version 3.3 (Scientific Volume Imaging BV, Hilversum).
Time-lapse DIC Microscopy
Approximately 20 000 Huh 7.5 cells were plated in Lab-Tek two well Nunc Glass Chamber Slide (Krackeler Scientific) and incubated at 37°C and 5% CO2 overnight. Differential interference contrast image sequences were collected every 5 min on an AF 6000LX, Leica Microsystems (Wetzlar) with a 20× oil immersion objective NA 0.7.
Real time PCR for HCV copy number and IAPs
The culture supernatants from HCV-infected cells were collected 48 h after treatment with the antibody and/or interferon-α and HCV copy numbers were determined using a quantitative real-time PCR as described elsewhere.28 Total RNA was extracted from HCV-uninfected, HCV-infected as well as Huh7.5 cells in the presence or absence of IFN-α2b or alb-IFN for 24 h using the mirVAna miRNA isolation kit (Applied Biosystems, Foster City, CA). 5 μg of total RNA were used for cDNA synthesis with the High-capacity cDNA Archive kit (Applied Biosystems). Primers and probes for the detection of cIAP1, cIAP2, XIAP and survivin mRNA (Applied Biosystems) by RT-PCR (ABI 7500 RT-PCR System) were used according to the manufacturer’s guidelines. The glyceraldehyde-3-phosphate dehydrogenase primer and probe set served to provide an endogenous control. Results were analyzed using the comparative Ct method for the relative quantitation of gene expression.
Statistical analysis
Analysis of variance with Tukey’s multiple comparison test was used to compare means of the independent data. The paired Student’s t-test with the Bonferroni adjustment for multiple testing was used to compare paired responses.
RESULTS
Mapatumumab preferentially induces apoptosis of HCV-infected Huh7.5 cells
WE PERFORMED A caspase 3/7 assay on HCV-infected and uninfected Huh7.5 cells treated with serial concentrations of mapatumumab or lexatumumab. As shown in Figure 1A and B, mapatumumab and lexatumumab both induced dose-dependent apoptosis of Huh7.5 cells when compared to that seen with control antibody. Mapatumumab preferentially induced higher levels of apoptosis of HCV-infected Huh7.5 cells when compared to that seen with HCV-uninfected Huh7.5 cells, which was statistically significant at higher concentrations (P < 0.05; Fig. 1A). With lexatumumab, the degree of apoptosis induced was not affected whether the cells were infected with HCV or not (P > 0.05; Fig. 1B). Relative potency of both mAb against HCV-uninfected and infected Huh 7.5 cells as expressed as EC50 values of Caspase 3/7 activity were 252 ng/mL and 137.3 ng/mL respectively for mapatumumab and 112 ng/mL and 121 ng/mL respectively for lexatumumab.
Figure 1.
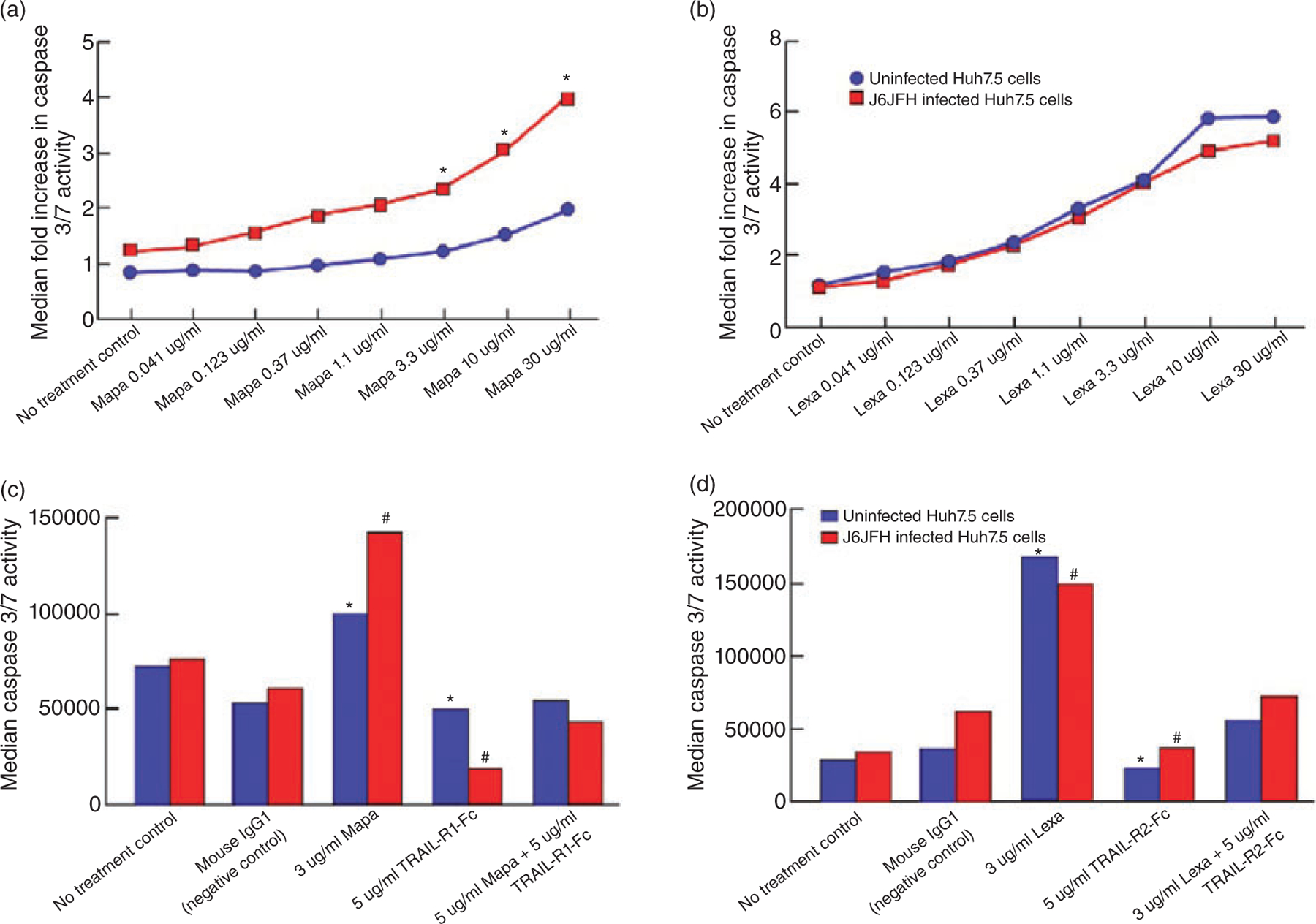
The levels of apoptosis of hepatitis C virus (HCV)-uninfected (blue line) or HCV–infected Huh7.5. cells (red line) in the absence or presence of serial concentrations of mapatumumab or lexatumumab or isotype control antibody (mouse anti-human IgG1) were determined as described in Material and Methods. The level of apoptosis is expressed as median fold increase in Caspase 3/7 activity of each experimental condition over that seen with control antibody. (a) Mapatumumab -induced dose-dependent apoptosis of both HCV-uninfected and infected Huh 7.5 cells with higher levels seen with HCV-infected Huh7.5 cells. There was statistical significance for values marked with * (P < 0.05). (b) Lexatumumab-induced dose-dependent apoptosis of both HCV-uninfected and infected Huh 7.5 cells. There was no statistical significance for any values (P > 0.05). The (median) levels of (c) mapatumumab or (d) lexatumumab -induced apoptosis of HCV-uninfected (blue bars) or infected Huh7.5 cells (red bars) in the absence or presence of tumor necrosis factor-related apoptosis inducing ligand (TRAIL)-R1-Fc were determined as described in Material and Methods. (c) The results revealed a statistically significant decline in the Caspase3/7 activity when the experiments were performed in the presence of TRAIL-R1-Fc for both HCV-uninfected (*P < 0.05) and HCV-infected (#P < 0.02) implying a direct interaction between mapatumumab and TRAIL-R1 to account for mapatumumab -induced apoptosis. (d) The results revealed a statistically significant decline in the Caspase3/7 activity when the experiments were performed in the presence of TRAIL-R2-Fc for both HCV-uninfected (*P < 0.02) and HCV-infected (#P < 0.03) implying a direct interaction between lexatumumab and TRAIL-R2 to account for lexatumumab-induced apoptosis.
Mapatumumab and lexatumumab induce apoptosis by direct interaction with respective cell surface receptors
As shown in Figure 1C, mapatumumab-induced apoptosis of Huh7.5 cells was blocked by addition of Fc-TRAIL-R1 (P < 0.05 for uninfected and P < 0.02 for HCV-infected Huh7.5 cells) and lexatumumab-induced apoptosis of Huh7.5 cells was blocked by Fc-TRAIL-R2 (P < 0.02 for HCV uninfected and P < 0.03 for HCV-infected Huh7.5 cells, Fig. 1D). Thus induction of apoptosis of Huh7.5 cells by mapatumumab and lexatumumab resulted from direct interactions with TRAIL-R1 and TRAIL-R2 receptors respectively.
IFN-α formulations have no effect on mapatumumab and lexatumumab-mediated apoptosis of Huh7.5 cells
We performed caspase 3/7 assays on Huh7.5 cells treated with mapatumumab or lexatumumab in the absence or presence of IFN-α2b or alb-IFN. As shown in Figure 2A and B, 24 h treatment of both IFN-α formulations had no significant effect on mapatumumab- and lexatumumab -mediated apoptosis.
Figure 2.
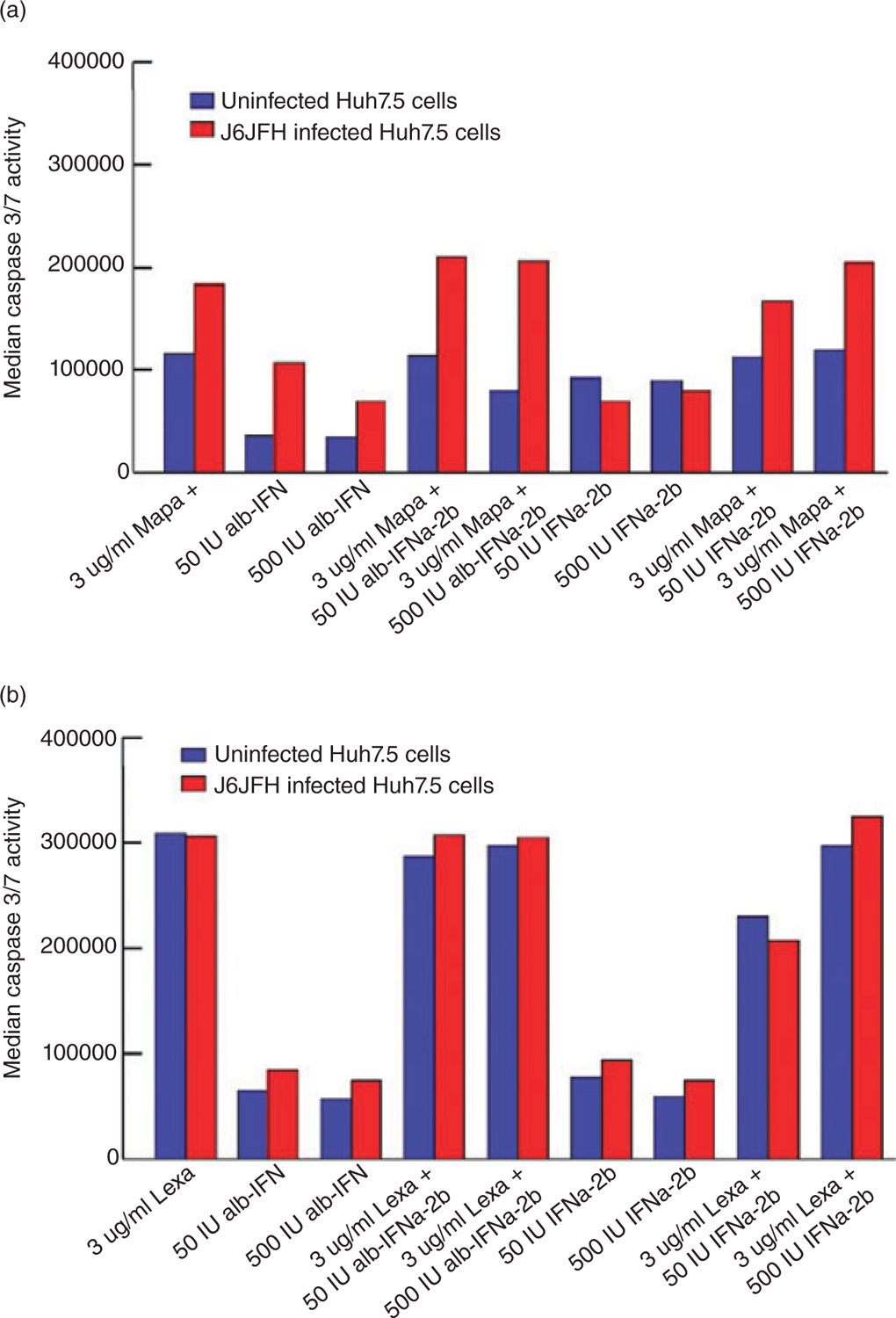
Levels of apoptosis of HCV-uninfected or infected Huh7.5 cells in the presence of (a) mapatumumab or (b) lexatumumab and in the absence or presence of different interferon (IFN)-α formulations were determined as described in Material and Methods. Different IFN-α formulations in vitro had minimal effect on mapatumumab-induced apoptosis (a) and lexatumumab-induced apoptosis (b) levels of Huh7.5 cells. There was no statistical significance for any values (P > 0.05).
Susceptibility of HCV-infected Huh7.5 cells to mapatumumab-mediated apoptosis correlated with up regulation of TRAIL-R1 receptor
To address the effects of mapatumumab treatment of HCV-infected Huh7.5 cells on HCV replication, we performed real time qPCR to quantitate the copy numbers of HCV. As shown in Figure 3A, exposure of HCV-infected Huh 7.5 cells to mapatumumab did not have any effect on levels of HCV copy numbers in the supernatants. This could be due to the release of HCV virions from apoptotic cells into the supernatant, which is then detected by PCR.
Figure 3.
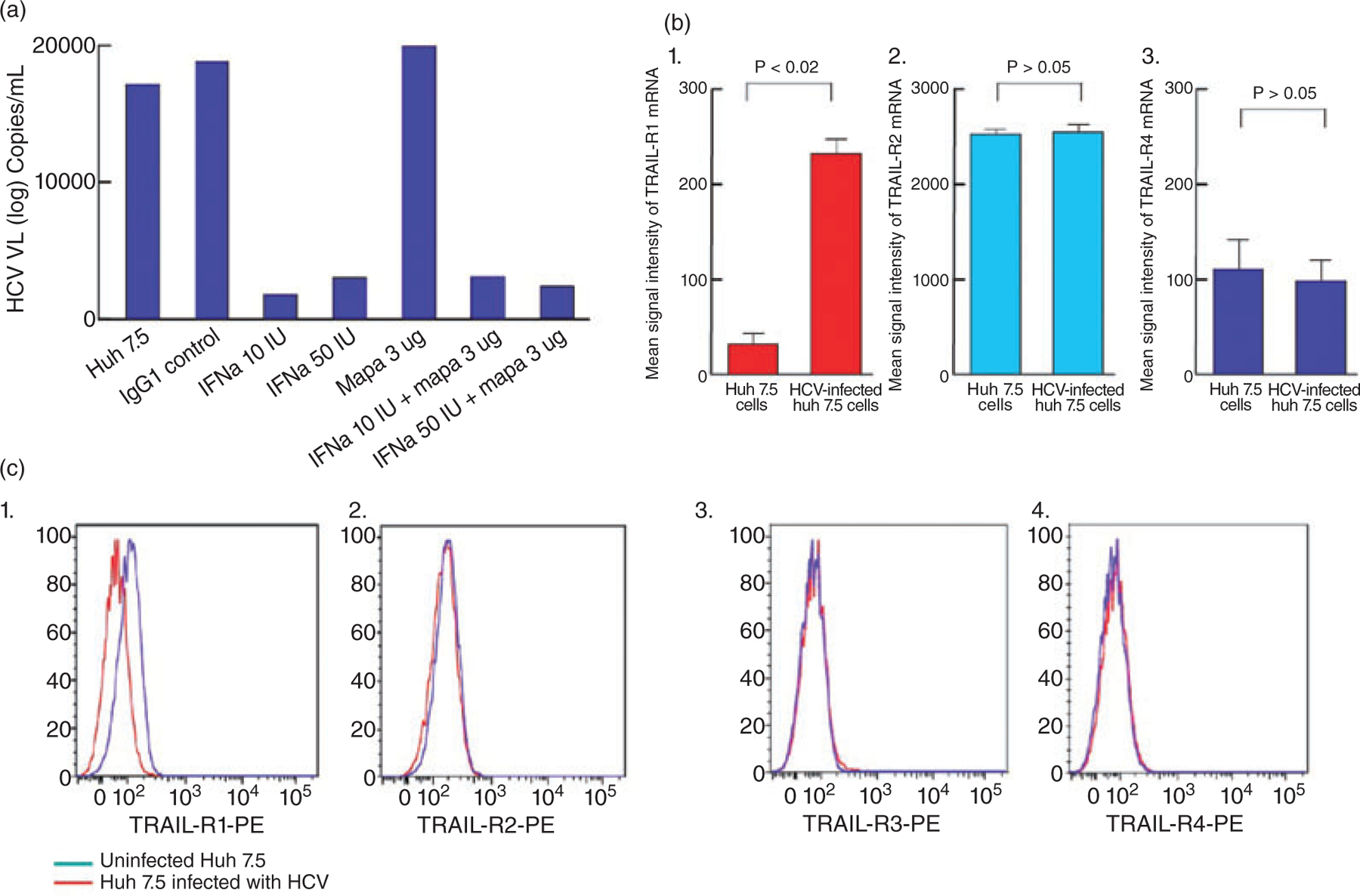
(a) Levels of hepatitis C virus (HCV) replication in J6/JFH HCV-infected Huh 7.5 cells in the presence or absence of control antibody, interferon (INF)-α and/or mapatumumab (3 µg/mL) was measured as described in the Materials and Methods. As expected, IFN-α suppressed HCV replication in JFH-infected Huh7.5 cells, while Mapatumumab did not have any effect on HCV replication. Representative data from 4 separate experiments shown. (b) The levels of mRNA transcripts for tumor necrosis factor-related apoptosis inducing ligand (TRAIL)-receptors (R1 to R4) in Huh 7.5 cells (HCV-uninfected and infected). While there was a statistically significant increase in the levels of TRAIL-R1 mRNA transcripts in infected Huh7.5 cells (P < 0.02), there were no differences in the levels of transcripts of TRAIL-R2, and R4 detected in HCV uninfected and infected Huh7.5 cells (P > 0.05 for both). TRAIL-R3 mRNA signal was not detected in this microarray experiment. (c) The level of expression of TRAIL receptors (TRAIL R1 and 2 and TRAIL DcR 3 and 4) on the surface of HCV-uninfected or infected Huh7.5 cells was measured as described in Material and Methods. TRAIL-R1 surface expression levels were substantially up regulated in HCV-infected compared to uninfected Huh7.5 cells (C (1)), while HCV-infection had no effect on the levels of expression of TRAIL-R2 (C (2)), TRAIL-R3 (C (3)) and TRAIL-R4 (C (4)).
To explore underlying mechanisms of enhanced susceptibility of HCV-infected Huh7.5 cells to mapatumumab- mediated apoptosis, we analyzed the levels of mRNA of TRAIL receptors on HCV-uninfected and HCV-infected Huh7.5 cells. As shown in Figure 3B, HCV-infected Huh7.5 cells expressed higher levels of TRAIL-R1 mRNA (P < 0.02; Fig. 3B) compared to uninfected cells, while the levels of expression of TRAIL-R2 (Fig. 3B) mRNA remained unchanged. Likewise, surface expression of TRAIL-R1 receptor on HCV-infected Huh7.5 cells were higher compared to uninfected cells (Fig. 3C). Modulation of expression of TRAIL decoy receptors (DcR), TRAIL-R3 and R4 has also been suggested as a mechanism that may result in altered susceptibility of tumor cells to TRMA.23 As shown in Figure 3B and C, HCV infection of Huh7.5 cells did not modulate the expression of TRAIL DcR (Fig. 3B,C). Given the suppression of HCV replication by IFN-α, one might anticipate a decrease in TRAIL-R1 expression and TRMA in IFN-α-treated HCV-infected Huh7.5 cells. However, the levels of TRAIL-R1 expression before and after IFN-α treatment remain unchanged (data not shown).
Confocal microscopy confirms HCV-infection of Huh7.5 cells up-regulates TRAIL-R1 and are susceptible to Mapatumumab-mediated apoptosis
To visualize and confirm the effects of HCV infection on TRAIL-R1 expression of Huh 7.5 cells, we performed confocal microscopy of HCV-uninfected and infected Huh7.5 cells after labelling cells for HCV core protein and TRAIL-R1 simultaneously. As shown in Figure 4A, TRAIL-R1 (red) expression was increased on HCV-infected (green) as compared to that seen on HCV uninfected Huh7.5 cells (left panel). Figure 4B shows the levels of apoptosis induced by mapatumumab as detected by direct visualization and quantitation was higher in HCV-infected than uninfected Huh7.5 cells. We also obtained time-lapse video of mapatumumab exposed Huh7.5 cells (HCV uninfected and infected) over time, which shows a more extensive and rapid induction of apoptosis of HCV-infected Huh7.5 cells (Supplemental video S1,S2).
Figure 4.
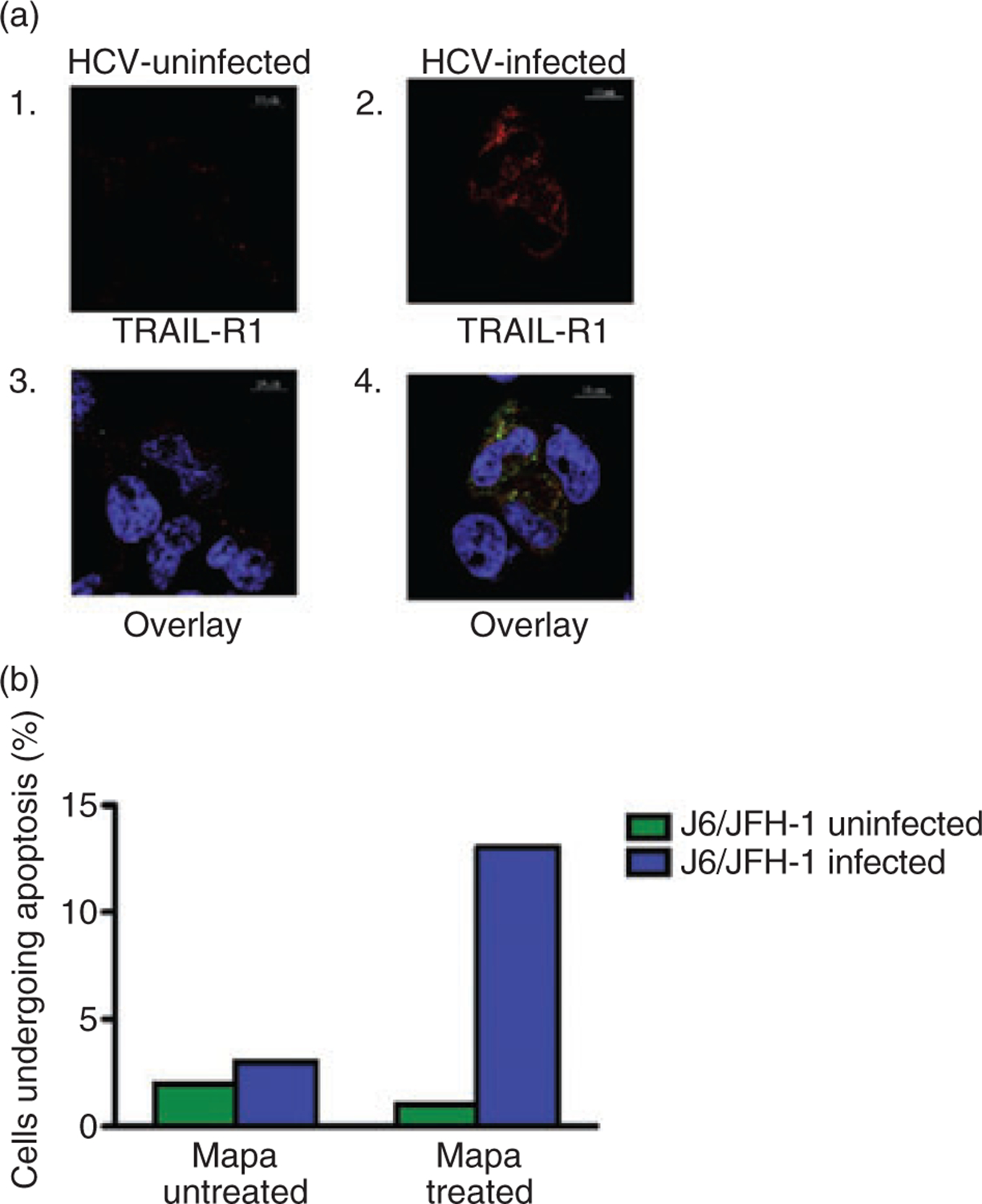
A. The levels of tumor necrosis factor-related apoptosis inducing ligand (TRAIL)-R1 and intracellular hepatitis C virus (HCV) core proteins were determined using confocal microscopy as described in the Materials and Methods. HCV-uninfected Huh7.5 cells (A (1)) had higher expression of tumor necrosis factor-related apoptosis inducing ligand (TRAIL)-R1 expression (in red) when compared to that seen with HCV-uninfected cells (a(2)). Further, when the images for intracellular HCV core protein and TRAIL-R1 surface expression were overlaid, the image clearly showed that HCV-infected (a(4)) and not HCV uninfected Huh7.5 cells (a(3)), that express high levels of TRAIL-R1 receptor. (b) When the number of HCV uninfected and infected Huh7.5 cells undergoing apoptosis was counted visually using confocal microscopy and using a software as described in the Materials and Methods there was an increased percentage of HCV-infected Huh7.5cells per field undergoing apoptosis when compared to that seen with HCV uninfected cells.
Increased basal expression of TRAIL-R2 receptors on Huh7.5 cells
Our results have demonstrated that HCV infection of Huh7.5 cells neither increases the expression of TRAIL-R2 receptor nor enhances the susceptibility to lexatumumab mediated apoptosis. As shown in Figure 5, both mRNA (P < 0.001) and cell surface expression of TRAIL-R2 was significantly higher on the surface of HCV uninfected Huh7.5 cells. These results suggest that level of expression and density of TRAIL receptors on Huh7.5 cells may explain the differential susceptibility of Huh7.5 cells to mapatumumab and lexatumumab mediated apoptosis.
Figure 5.
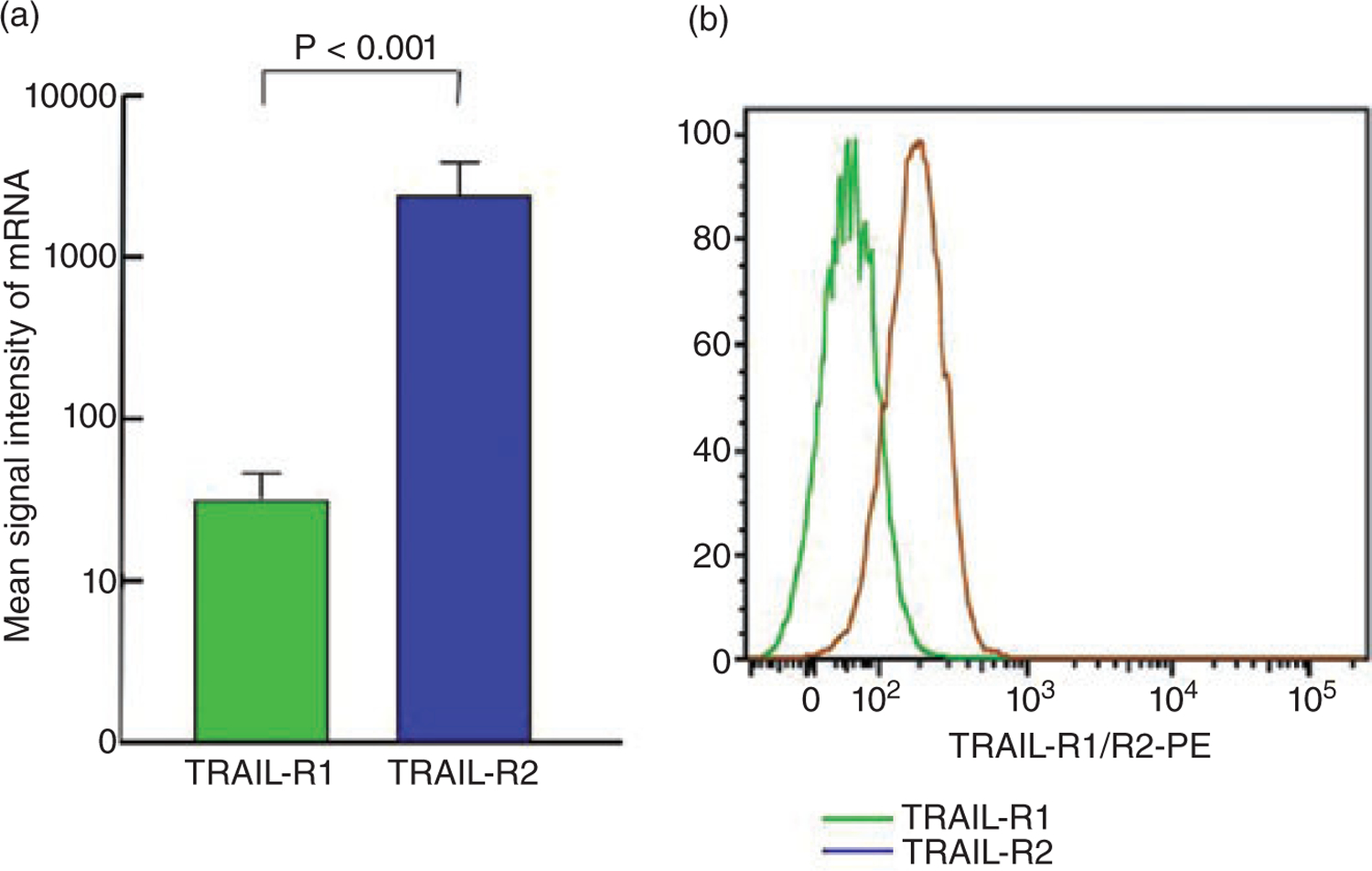
The levels of mRNA transcripts and cell surface expression of tumor necrosis factor-related apoptosis inducing ligand (TRAIL) receptors (TRAIL R1 and 2) in hepatitis C virus (HCV)-uninfected Huh7.5 cells was measured as described in Material and Methods. Huh7.5 cells expressed significantly higher levels of TRAIL mRNA ((a) P < 0.001) and (b) TRAIL-R1 receptor expression on the surface (b).
Gene expression of IAPs XIAP and survivin is significantly up-regulated in HCV-infected compared to HCV-uninfected Huh7.5 cells
To investigate whether HCV infection of HCC cells modulated the expression of IAP genes, we performed real time PCR to quantitate the relative expression of different IAP genes in HCC cells infected or uninfected with HCV, in the presence or absence of IFN-α. As illustrated in Figure 6, HCV infection of Huh7.5 cells was associated with an up-regulation of gene expression for XIAP (P < 0.01) and survivin (P < 0.03). Both IFN-α and albinterferon had no significant effect on IAP gene expression of Huh 7.5 cells after 24 h exposure (P > 0.05).
Figure 6.
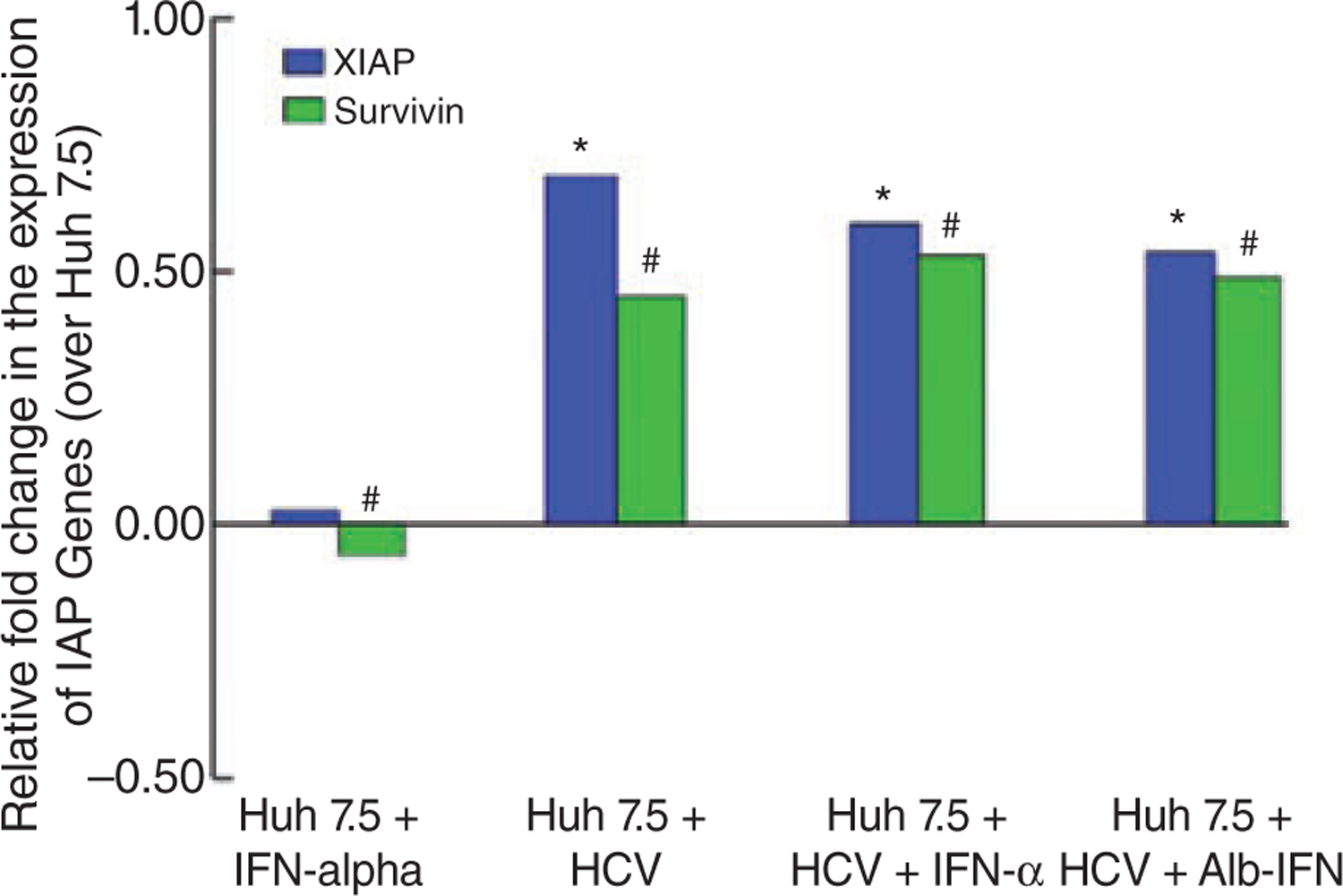
The gene expression levels of all four inhibitors of apoptosis protein (IAP) genes were performed by RT-PCR as described in the Materials and Methods. The relative fold change in IAP gene expression in hepatitis C virus (HCV)-infected compared to uninfected Huh7.5 cells revealed significant up-regulation of X-linked IAP (*P < 0.01) and survivin (#P < 0.03) gene expression. Interferon formulations had minimal effect on the IAP gene expression on HCV-uninfected (P > 0.05 for both) and HCV-infected Huh7.5 cells (P > 0.05 for both).
Mapatumumab does not induce apoptosis of PBMCs from HIV-negative and HIV viremic individuals
Since recent studies have suggested that HIV-infection may trigger TRAIL-mediated apoptosis of peripheral T cells in vitro, we evaluated the degree of apoptosis in PBMCs from HIV-infected viremic and HIV negative subjects resulting from exposure to mapatumumab in vitro. As shown in Figure 7A through 7D there was no significant degree of mapatumumab-mediated apoptosis of B, T, NK cells or monocytes from both HIV-negative and HIV viremic individuals. Similar results were observed with lexatumumab (data not shown).
Figure 7.
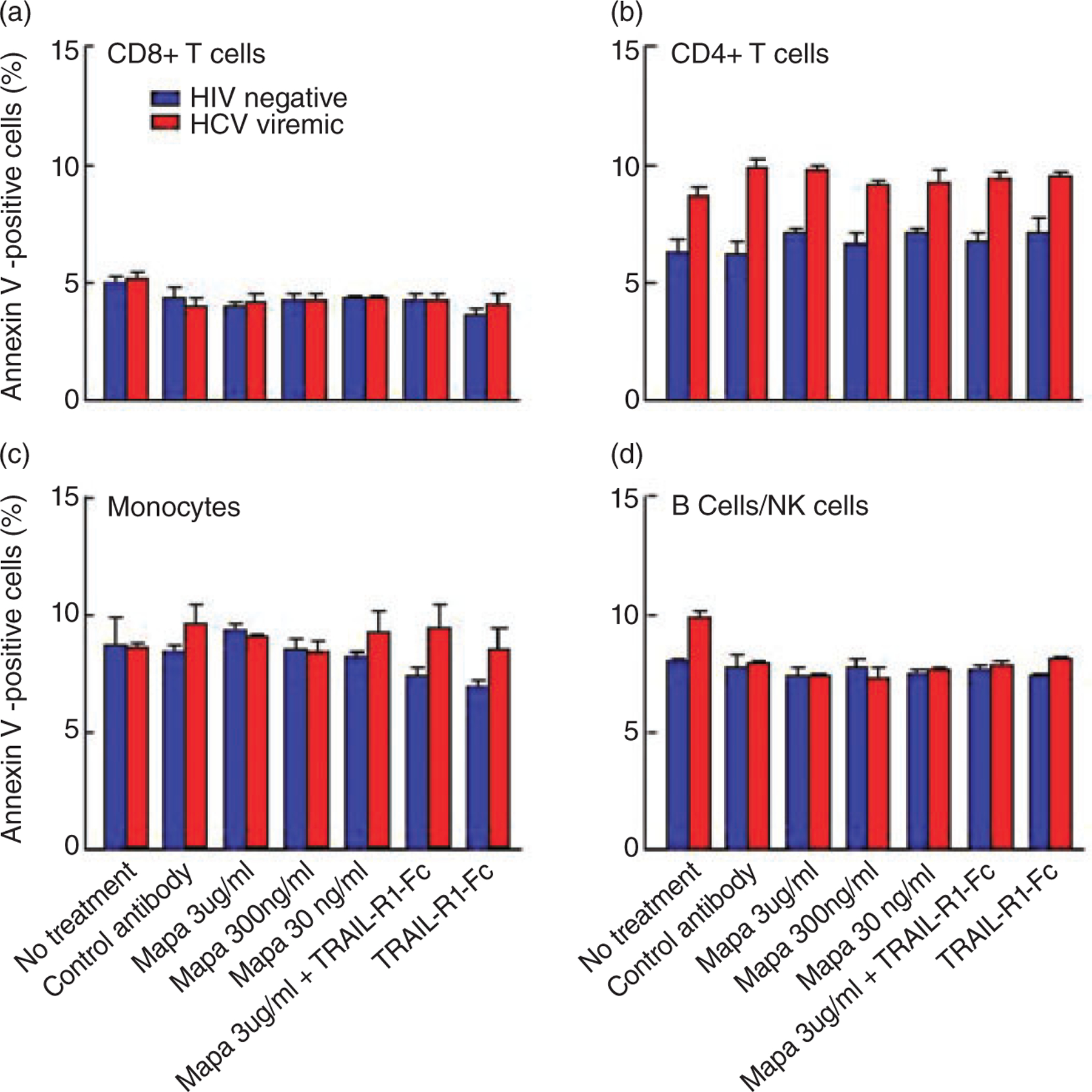
In vitro apoptosis levels of PBMCs from human immunodeficiency virus (HIV)-negative or HIV-infected viremic individuals were determined by staining for Annexin V as described in Material and Methods in the absence or presence of mapatumumab or control isotype antibody (mouse anti-human IgG1). Mapatumumab did not induce apoptosis of (a) CD8 T cells, (b) CD4 T cells, (c) Monocytes, (d) B cells/NK cells.
DISCUSSION
IN THIS STUDY we show that HCV infection of Huh7.5 cells results in preferential up regulation of TRAIL-R1 receptor, which correlates with enhanced susceptibility to mapatumumab-induced apoptosis compared to HCV-uninfected cells. Although lexatumumab is capable of inducing apoptosis of Huh7.5 cells more effectively than mapatumumab, apoptosis was not increased by HCV infection. However, HCV-infected Huh 7.5 cells have increased basal expression of TRAIL-R2 than TRAIL-R1, which could explain the greater potency of lexatumumab-mediated apoptosis than mapatumumab in uninfected Huh7.5 cells. HCV infection of Huh7.5 cells up regulates the surface expression of TRAIL-R1 receptor as well as gene expression of IAPs, XIAP and survivin. IFN-α treatment does not appear to affect the susceptibility of Huh7.5 cells to undergo TRMA. Further, mapatumumab does not induce apoptosis of PBMCs from HIV-negative and HIV-infected viremic individuals.
Previous studies have shown that HCV infection of primary HCC cells results in apoptosis of HCC cells in a TRAIL receptor dependent and independent manner.20,28 One study showed that HCV infection of primary hepatoma cells up-regulate gene expression of TRAIL receptors and enhance their susceptibility for TRMA.20 Our results demonstrate that the susceptibility of HCV-infected hepatoma cells to undergo TRMA is associated with up-regulation of TRAIL-R1 receptor. We were able to demonstrate an increase in TRAIL-R1 mRNA and cell surface expression in HCV-infected Huh7.5 cells by flow cytometry as well as by confocal microscopy. However, it is not known whether HCV-infection can also affect down-stream events that could enhance the susceptibility of hepatoma cells to TRMA. Elevated apoptosis levels in HCV-infected Huh7.5 cells might also result from down regulation of TRAIL decoy receptor (DcR) surface expression.29 However our results show that HCV infection of Huh7.5 cells does not have any effect on TRAIL-DcR expression.
Interestingly, mapatumumab or lexatumumab had no effect on the levels of HCV copy numbers in the supernatant of HCV-infected Huh 7.5 cells in vitro. This likely reflects the HCV virions that are released from Huh 7.5 cells undergoing TRMA into the culture supernatant. However, the impact of reducing HCV viremia in vivo by eliminating HCV-infected hepatocytes remains unknown. Thus, efforts to investigate whether HCV-infected primary hepatocytes are also susceptible to TRMA will help define the potential role of TRAIL-agonists in vivo for enhancing rates of HCV eradication in combination with IFN-based regimens for the treatment of chronic HCV infection.
IAPs XIAP and survivin, have been shown to be associated with protection from apoptosis through both extrinsic and intrinsic pathways and have generated interest as potential targets for chemotherapeutic agents for cancer.30 Recent studies have suggested roles for both XIAP and survivin expression on regulating apoptosis of hepatoma cells in vitro.31,32 Since these proteins can specifically block TRMA mediated by TRAIL or TRAIL agonists, their inhibitors could have a significant role as adjuvant therapeutic agents with TRAIL agonists in vivo in the treatment of HCC.33 Our data suggest that HCV infection up-regulates the expression of IAP genes, XIAP and survivin. This up regulation of IAPs (XIAP and survivin) is an escape/survival mechanism adopted by the tumor cells. Hence the net effect is a balance of both the effect of TRMA and IAP-mediated inhibition of apoptosis. At the time frame of the experiments, we performed, TRMA dominates the effect of IPA-mediated inhibition of apoptosis, however, this could be different when the IAP expression increases over time and Huh 7.5 cells could become resistant to TRMA. Hence blocking of IAP function could synergistically aid in further augmenting TRMA mediated by mapatumumab or lexatumumab. Future studies will provide insights into whether IAP protein expression and function in HCC cells are modulated by HCV infection.
Our in vitro results do not suggest synergy of mapatumumab or lexatumumab with IFNα-2b or alb-IFN. Within the time frame of our experiments, IFNα formu lations did not affect expression of TRAIL-R1 or XIAP and survivin genes. Further investigations will be needed to determine whether inhibition of viral replication by IFNα26,27 impacts different components of the apoptotic pathway. It remains to be determined, whether novel agents such as IL-21 or mAbs agonistically mimicking the effect of CD40 or CD137 ligands, which have recently been found to exhibit synergistic effects with agonistic mAbs against TRAIL-R2 in mice13 will exhibit synergy with mapatumumab or lexatumumab in vitro.
An increase in the incidence of HCC among HCV/HIV co-infected patients is anticipated over the coming decade. Since peripheral immune cells of HIV-infected patients have been described to be more susceptible to TRAIL-mediated apoptosis,24,25 TRAIL agonists in vivo could result in worsening of HIV-associated immunodeficiency. Our results suggest that mapatumumab has minimal effect on the degree of apoptosis in PBMCs from healthy and HIV viremic individuals. However, whether HCC from HIV-infected individuals exhibit the same degree of susceptibility to TRMA is not clearly understood. Further clinical studies will be required to ascertain in vivo safety of mapatumumab and lexatumumab in the treatment of HCC in uninfected, HCV-mono-infected and HCV/HIV co-infected patients. Future studies will also address whether HCV-infection of a wide spectrum of HCC cell lines and primary hepatocytes may have similar effect of TRAIL-mediated apoptosis.
In summary, our study shows in vitro evidence for the therapeutic potential of mapatumumab and lexatumumab TRAIL-receptor agonistic antibodies in the treatment of HCC. To date, there are several studies that have demonstrated toxicological profiles of these antibodies in Phase I clinical trials.14,17,34 Both antibodies are under investigation in various experimental cancer combination therapies35–37 and this study suggests that mapatumumab has the potential to be a therapeutic agent for specifically targeting HCC in HCV-infected subjects.
Supplementary Material
ACKNOWLEDGMENT
THIS RESEARCH WAS supported in whole by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases. We would also like to thank Apath LLC (St. Louis, MO) and Toray Industries, Japan for providing us with the Huh7.5cells and J6/JFH-1 HCV strains.
Footnotes
Publisher's Disclaimer: Disclaimer: The content of this publication does not necessarily reflect the views of policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organization imply endorsement by the US Government.
Conflict of Interest Statement: G. Mani Subramanian is an employee of Human Genome Sciences. No other authors have a conflict of interest.
SUPPORTING INFORMATION
ADDITIONAL SUPPORTING INFORMATION may be found in the online version of this article:
Video Clip S1 and S2 Time lapse DIC microscopy was performed of HCV-uninfected and infected Huh 7.5 cells in the presence of mapatumumab was performed over a period of 7 h. The video is condensed data for the complete 7 h for HCV-uninfected (file1) and HCV-infected (file2). The unstained cells undergoing apoptosis was monitored and counted. As clearly demonstrated in the time-lapse video, HCV-infected Huh7.5 cells undergo apoptosis (defined as membrane blebbing and nuclear condensation) much rapidly and profoundly when compared to that observed with HCV-uninfected cells after exposure to mapatumumab.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
REFERENCES
- 1.Schiff ER. Introduction: advances in antiviral therapy for chronic hepatitis c infection—the influence of genotype and HIV co-infection. Nat Clin Prac Gastroenterol Hepatol 2007; 4: S1–2. [Google Scholar]
- 2.Blackard JT, Sherman KE. HCV/HIV co-infection: time to re-evaluate the role of HIV in the liver? J Viral Hepat 2008; 15: 323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sherman KE, Rouster SD, Chung RT, Rajicic N. Hepatitis C virus prevalence among patients infected with human immunodeficiency virus: a cross-sectional analysis of the US adult AIDS Clinical Trials Group. Clin Infect Dis 2002; 34: 831–7. [DOI] [PubMed] [Google Scholar]
- 4.Benhamou Y, Bochet M, Di Martino V et al. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology 1999; 30: 1054–8. [DOI] [PubMed] [Google Scholar]
- 5.Yu ML, Huang CF, Dai CY, Huang JF, Chuang WL. Long-term effects of interferon-based therapy for chronic hepatitis C. Oncology 2007; 72 (Suppl. 1): 16–23. [DOI] [PubMed] [Google Scholar]
- 6.Ghany MG, Kleiner DE, Alter H et al. Progression of fibrosis in chronic hepatitis C. Gastroenterology 2003; 124: 97–104. [DOI] [PubMed] [Google Scholar]
- 7.Sherman KE. HCV and HIV: a tale of two viruses. Rev Gastroenterol Disord 2004; 4 (Suppl. 1): S48–54. [PubMed] [Google Scholar]
- 8.Coleman WB. Mechanisms of human hepatocarcinogenesis. Curr Mol Med 2003; 3: 573–88. [DOI] [PubMed] [Google Scholar]
- 9.Farazi PA, Depinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer 2006; 6: 674–87. [DOI] [PubMed] [Google Scholar]
- 10.Almasan A, Ashkenazi A. Apo2L/TRAIL: apoptosis signaling, biology, and potential for cancer therapy. Cytokine Growth Factor Rev 2003; 14: 337–48. [DOI] [PubMed] [Google Scholar]
- 11.Igney FH, Krammer PH. Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer 2002; 2: 277–88. [DOI] [PubMed] [Google Scholar]
- 12.Okano H, Shiraki K, Inoue H et al. Over-expression of Smac promotes TRAIL-induced cell death in human hepatocellular carcinoma. Int J Mol Med 2003; 12: 25–8. [PubMed] [Google Scholar]
- 13.Cretney E, Shanker A, Yagita H, Smyth MJ, Sayers TJ. TNF-related apoptosis-inducing ligand as a therapeutic agent in autoimmunity and cancer. Immunol Cell Biol 2006; 84: 87–98. [DOI] [PubMed] [Google Scholar]
- 14.Humphreys RC, Halpern W. Trail receptors: targets for cancer therapy. Adv Exp Med Biol 2008; 615: 127–58. [DOI] [PubMed] [Google Scholar]
- 15.Georgakis GV, Li Y, Humphreys R et al. Activity of selective fully human agonistic antibodies to the TRAIL death receptors TRAIL-R1 and TRAIL-R2 in primary and cultured lymphoma cells: induction of apoptosis and enhancement of doxorubicin- and bortezomib-induced cell death. Br J Haematol 2005; 130: 501–10. [DOI] [PubMed] [Google Scholar]
- 16.Greco FA, Bonomi P, Crawford J et al. Phase 2 study of mapatumumab, a fully human agonistic monoclonal antibody which targets and activates the TRAIL receptor-1, in patients with advanced non-small cell lung cancer. Lung Cancer 2008; 61: 82–90. [DOI] [PubMed] [Google Scholar]
- 17.Hotte SJ, Hirte HW, Chen EX et al. A phase 1 study of mapatumumab (fully human monoclonal antibody to TRAIL-R1) in patients with advanced solid malignancies. Clin Cancer Res 2008; 14: 3450–5. [DOI] [PubMed] [Google Scholar]
- 18.Pukac L, Kanakaraj P, Humphreys R et al. HGS-ETR1, a fully human TRAIL-receptor 1 monoclonal antibody, induces cell death in multiple tumour types in vitro and in vivo. Br J Cancer 2005; 92: 1430–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Srivastava RK. TRAIL/Apo-2L: mechanisms and clinical applications in cancer. Neoplasia 2001; 3: 535–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu H, Dong H., Eksioglu E et al. Hepatitis C virus triggers apoptosis of a newly developed hepatoma cell line through antiviral Defense system. Gastroenterology 2007. [DOI] [PubMed]
- 21.Herr I, Schemmer P, Buchler MW. On the TRAIL to therapeutic intervention in liver disease. Hepatology 2007; 46: 266–74. [DOI] [PubMed] [Google Scholar]
- 22.Ryan CW, Goldman BH, Lara PN Jr et al. Sorafenib with interferon alfa-2b as first-line treatment of advanced renal carcinoma: a phase II study of the Southwest Oncology Group. J Clin Oncol 2007; 25: 3296–301. [DOI] [PubMed] [Google Scholar]
- 23.Yen TS. Nuclear factor kappaB and hepatitis C – is there a connection? Hepatology 2000; 31: 785–7. [DOI] [PubMed] [Google Scholar]
- 24.Herbeuval JP, Boasso A, Grivel JC et al. TNF-related apoptosis-inducing ligand (TRAIL) in HIV-1-infected patients and its in vitro production by antigen-presenting cells. Blood 2005; 105: 2458–64. [DOI] [PubMed] [Google Scholar]
- 25.Herbeuval JP, Hardy AW, Boasso A et al. Regulation of TNF-related apoptosis-inducing ligand on primary CD4+ T cells by HIV-1: role of type I IFN-producing plasmacytoid dendritic cells. Proc Natl Acad Sci USA 2005; 102: 13974–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindenbach BD, Evans MJ, Syder AJ et al. Complete replication of hepatitis C virus in cell culture. Science 2005; 309: 623–6. [DOI] [PubMed] [Google Scholar]
- 27.Wakita T, Pietschmann T, Kato T et al. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat Med 2005; 11: 791–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Belyanskaya LL, Marti TM, Hopkins-Donaldson S, Kurtz S, Felley-Bosco E, Stahel RA. Human agonistic TRAIL receptor antibodies Mapatumumab and Lexatumumab induce apoptosis in malignant mesothelioma and act synergistically with cisplatin. Mol Cancer 2007; 6: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kimberley FC, Screaton GR. Following a TRAIL: update on a ligand and its five receptors. Cell Res 2004; 14: 359–72. [DOI] [PubMed] [Google Scholar]
- 30.Vucic D Targeting IAP (inhibitor of apoptosis) proteins for therapeutic intervention in tumors. Curr Cancer Drug Targets 2008; 8: 110–17. [DOI] [PubMed] [Google Scholar]
- 31.Wu L, Wang Y, Tian D. Knockdown of survivin expression by siRNA induces apoptosis of hepatocellular carcinoma cells. J Huazhong Univ Sci Technolog Med Sci 2007; 27: 403–6. [DOI] [PubMed] [Google Scholar]
- 32.Sakemi R, Yano H, Ogasawara S et al. X-linked inhibitor of apoptosis (XIAP) and XIAP-associated factor-1 expressions and their relationship to apoptosis in human hepatocellular carcinoma and non-cancerous liver tissues. Oncol Rep 2007; 18: 65–70. [PubMed] [Google Scholar]
- 33.Fernandez-Luna JL. Apoptosis regulators as targets for cancer therapy. Clin Transl Oncol 2007; 9: 555–62. [DOI] [PubMed] [Google Scholar]
- 34.Plummer R, Attard G, Pacey S et al. Phase 1 and pharmacokinetic study of lexatumumab in patients with advanced cancers. Clin Cancer Res 2007; 13: 6187–94. [DOI] [PubMed] [Google Scholar]
- 35.Gajewski T On the TRAIL toward death receptor-based cancer therapeutics. J Clin Oncol 2007; 25: 1305–7. [DOI] [PubMed] [Google Scholar]
- 36.Marini P, Denzinger S, Schiller D et al. Combined treatment of colorectal tumours with agonistic TRAIL receptor antibodies HGS-ETR1 and HGS-ETR2 and radiotherapy: enhanced effects in vitro and dose-dependent growth delay in vivo. Oncogene 2006; 25: 5145–54. [DOI] [PubMed] [Google Scholar]
- 37.Vulfovich M, Saba N. Technology evaluation: mapatumumab, Human Genome Sciences/GlaxoSmithKline/Takeda. Curr Opin Mol Ther 2005; 7: 502–10. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


