Abstract
Nuclear resonance vibrational spectroscopy (NRVS) and density functional theory (DFT) are complementary tools for studying the vibrational and geometric structure of specific isotopically-labeled molecular systems. Here we apply NRVS and DFT to characterize the trans-[57Fe(η2-H2)(H)(dppe)2][BPh4] complex. Heretofore, most NRVS observations have centered on the spectral region below 1000 cm−1 where the 57Fe signal is strongest. In this work, we show that state-of-the-art synchrotron facilities can extend the observable region to 2000 cm−1 and likely beyond, in measurements that require less than one day. The 57Fe–H stretch was revealed at 1915 cm−1, along with the asymmetric 57Fe–H2 stretch at 1774 cm−1. For a small fraction of the H2-dissociated product, the 57Fe–H stretch was detected at 1956 cm−1. The unique sensitivity to 57Fe motion and the isolated nature of the Fe–H/H2 stretching modes enabled NRVS to quantitatively analyze the sample composition.
SUBJECTS: iron hydride, molecular hydrogen, vibrational spectroscopy, NRVS, DFT
Graphical Abstract

High-energy vibrations of iron-bound hydride and molecular hydrogen were observed using synchrotron-based 57Fe NRVS technique, assisted with 2H isotope labeling. The spectral bands from a mixed sample were rationalized by DFT calculations.
Since the discovery of the first transition metal dihydrogen complexes,1 a rich chemistry has been developed for the production and interconversion of dihydrogen and hydride species.2 This information is relevant for the development of better catalysts for hydrogen conversion,3 improved hydrogen storage media,4 and also for an understanding of hydrogenase enzymes.5–7 However, characterization of metal-bound dihydrogen and hydride complexes remains a substantial challenge.8–10 Due to their low electron-scattering cross-section, hydrogen atoms remain challenging to observe by X-ray crystallography, unless very high resolution data is available.11 M(etal)–H and M–H2 stretching modes are often quite weak in infrared (IR) spectra. Although they can sometimes be observed in concentrated materials, they are too weak to be seen in dilute enzymes via IR. Raman spectroscopy can sometimes be useful, but both metal hydride and dihydrogen are susceptible to photolysis.12 Neutron diffraction (ND) and inelastic neutron scattering (INS) are powerful tools for hydrogen studies, but they require relatively large quantities of material (~1 mmol of hydrogen), and the latter is also subject to interferences from protons in the solvent or elsewhere in the sample.
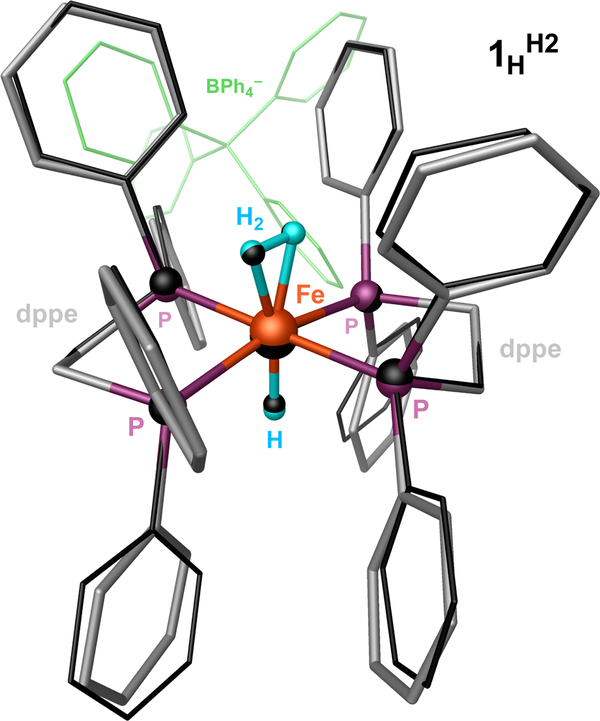
Nuclear resonance vibrational spectroscopy (NRVS)13–16 has become a popular technique for elucidating the element-selective normal modes of appropriate Mössbauer isotopes.13–18 In previous work, we have shown that 57FeNRVS can be utilized to observe Fe–H bending and Fe–H–Fe(Ni) wagging modes in various model compounds19 as well as intermediate species of [NiFe]20 and [FeFe] hydrogenases.21–23 However, to date the only reported Fe–H stretching modes were the bands around 1450 cm−1 for a doubly-bridging LFe(μ-H)2FeL complex24 and at 1468/1532 cm−1 in a singly-bridging LNi(μ-H)FeL complex.20 To better gauge the feasibility for observing even higher frequency stretching modes expected for a Fe-terminal hydride and dihydrogen, we have applied NRVS to examine a well-studied trans-[57Fe(η2-H2)(H)(dppe)2][BPh4] complex,25, 26 hereafter called 1HH2. As shown in Figure 1, our DFT modeling of 1HH2 produced an optimized structure that overlaps well with the X-ray diffraction result.26 Further we prepared the 1HD2 isotopologue with diatomic deuterium, which undergoes facile intramolecular exchange with the hydride yielding 1DHD, and then re-exchanges with molecular deuterium during a two week incubation time under the D2 atmosphere, producing 1DD2.26, 27 Although a series of alternative deuterium isotopologues can result from this transformation (the sample collectively called 1-D), it nonetheless enables us to qualitatively identify any modes pertaining to the H and H2 of the H-Fe-H2 moiety relative to the non-deuterated NRVS sample (the sample called 1-H). These samples were synthesized according to procedures described in the Supporting Information.
Figure 1.
Molecular X-ray structure26 of 1HH2 (in black, with the BPh4– counterion shown in green) superimposed with its optimized DFT model (colored elements). The H-to-D exchangeable hydrogens of the model structure are shown in light blue, and the rest of the hydrogens are omitted for clarity.
Along with the H–H stretching mode (ν⇄), it is common to assign five normal modes for a metal complex with a side-on (η2) dihydrogen ligand, involving symmetric (ν⇈) and asymmetric (ν⇅) stretching, in-plane (δ) and out-of-plane (δ′) bending, and rotation about the M-H2 axis (τ↻).28 For 1HH2 these Fe–H2 modes will be split by coupling with Fe–H motions, as shown in Chart 1 for a minimal C2v symmetry DFT model 1HH2-C2v. Of course, a complete analysis needs to consider interactions with the dppe ligands. The modes illustrated in Chart 1 nevertheless provide a good starting point for interpretation of our NRVS spectra.
Chart 1.
Normal modes expected for a trans coordination of dihydrogen and hydride ligands in an idealized Fe(II) octahedral complex. The 9 modes are based on C2v-symmetry trans-[Fe(η2-H2)(H)(PH3)4]+ = 1HH2-C2v model calculations as described in the Supporting Information and shown in Figure S1. Rows top to bottom: arrow-style mode representations; calculated frequencies (cm−1); C2v character names; arrow-based character names presently employed for 1HH2 and its derivatives.
Solid state NRVS spectra for samples 1-H vs. 1-D in the high-frequency range are compared with each other and with corresponding DFT 57Fe-PVDOS simulations in Figure 2a,b. All of the modes expected for 1HH2 in this region are readily assigned (Table S1), as well as bands attributed to dissociation (loss of H2 forming 1H*) or substitution (exchange of H2 by N2 called 1HN2) by-products, see Figures S2 and S6–S7 for their DFT structures and individual spectra.26, 27 Starting at the low-frequency end, an isotopically sensitive band at 1053 cm−1 can be assigned to symmetric Fe–H2 stretching (ν⇈), see arrow-style representations in Figure 2; animations showing DFT-calculated normal modes with significant (>10%) either 57Fe or Fe-bound proton PVDOS characters are available as part of the Supporting Information.
Figure 2.
(a) Experimental NRVS data for high-energy (1000–2000 cm−1) bands of samples 1-H and 1-D; (b) collective DFT 57Fe-PVDOS simulations for 1-H including 52% 1HH2, 26% 1H*, and 22% 1HN2, and 1-D using equivalent contributions from 1DD2, 1D*, and 1DN2; (c) DFT stick H(+H2)-PVDOS intensities for the Fe ligand hydride (and dihydrogen in 1HH2) nuclei in the three color-coded species of 1-H, weighted with their sample composition factors. The composition (%) of the mixed sample was obtained based on the relative intensities of the experimental spectra bands in the 1000–2000 cm−1 region, with individual contributions from the three pure calculated species shown in Figures S3 and S6–S7. Top: predicted atomic motions from DFT simulations for normal modes with H(+H2)-PVDOS>10%. The mode-to-band associations are highlighted by broken lines.
There is a shoulder in the data at ~1080 cm−1, identified as ν⇈ mixed with ligand Ph–H bending. The corresponding asymmetric Fe–H2 stretching (ν⇅) bands are seen at much higher energies – 1774 and 1276 cm−1 in 1HH2 and 1DD2 respectively. The Fe–H stretching (ν↑) band for 1HH2 is clearly observed in the spectrum at 1915 cm−1. The modes from 1350–1410 cm−1 in 1-D are the ν↑-modes of the possible deuterium isotopologues and their decay products 1DD2, 1D*, and 1DN2. There is a weaker feature at ~1956 cm−1 for the 1-H sample, consistent with 1H*. An admixture of 1HN2 possibly results in a shoulder at ~1890 cm−1, yet our calculations indicate that both ν↑(1HH2) and ν↑(1HN2) modes contribute to the same 1915 cm−1 band. Here we note that the ν↑(1H*) band at 1956 cm−1 represents the highest observed NRVS frequency to date, and this augurs well for future studies aimed at characterization of iron-hydride interactions. Further, it is important to note that the NRVS specificity for Fe motion, high energy, and isolation of Fe–Hx modes affords a strong handle on the quantitative composition of our NRVS spectrum with respect to adventitious subspecies 1H* and 1HN2, that were anticipated by the IR data and supported by its DFT simulation (Figures S4 and S5). Finally, we note that compared to the IR spectrum, identification of hydrogen-based modes at these energies in NRVS (Figure 2a) is unambiguous.
In Figure 3 we present NRVS spectra for 1-H vs. 1-D in the range below 1000 cm−1, again compared with each other and with DFT simulations. As mentioned above, the Fe–H2 bending modes are split by coupling with Fe–H motions in 1HH2. Thus, different frequencies and intensities are observed for in-plane (δ) and out-of-plane (δ′) Fe–H2 bending depending on whether the associated Fe–H bending is in-phase (←⇇) or out-of-phase (→⇇), as depicted in Chart 1. Starting from high energy, an isotope-sensitive feature is observed at 823 cm−1 in 1-H. Our DFT calculation for 1HH2 indicate a coupled in-plane in-phase Fe–H2 and Fe–H bending (δ←⇇) mode at 821 cm−1, which has sufficient 57Fe motion to be observable by NRVS. The experimental feature at 733 cm−1 in 1-H is consistent with Fe–H2 and Fe–H out-of-plane in-phase bending (δ′←⇇) motion. In our calculations this feature is derived from four modes with similar character predicted in the 726–732 cm−1 range, but with varying degrees of coupling to the outer dppe ligands. Due to this coupling with the outer coordination shell, these modes are sensitive to the packing of the solid-state environment which may account for the slight shoulder at 728 cm−1 in the experimental spectrum of 1-H. The next two isotopically-sensitive features at 558 and 584 cm−1 in 1-H are identified as H–Fe–H2 out-of-phase bending modes, out-of-plane (δ′→⇇) and in-plane (δ→⇇) respectively.
Figure 3.
(a) Experimental NRVS data for low-energy (0–1000 cm−1) bands of samples 1-H and 1-D; (b) DFT 57Fe-PVDOS simulations for 1-H and 1-D; (c) DFT stick H(+H2)-PVDOS intensities in the three color-coded species of 1-H, weighted with their sample composition factors. The ×4 intensity insets in (a-b) display spectra >370 cm−1. Top: predicted atomic motions for selected normal modes of 1HH2 with H(+H2)-PVDOS>10%. Mode-to-band associations are highlighted by broken lines. Further figure details follow the legend of Figure 2.
The most prominent NRVS feature is located at 284/273 cm−1 for 1-H/D (Figure 3a,b), with its intensity approximately 100 times higher (Figure S6a) than that of the high-energy Fe–H stretching band at 1915 cm−1. This global maximum band results from vibrations reminiscent of the intense transaxial bending modes of 6-coordinate Fe complexes with a four-fold axis of symmetry.29, 30 In all the calculated species, the DFT modes that produce the most intense ~250–300 cm−1 region reveal entire [H/D-Fe-(H2/D2)/(N2)] axial fragment displacements either parallel to the equatorial plane formed by the Fe-P bonds, or perpendicular to this plane, illustrated by animations in the Supporting Information. The high intensity and lack of splitting of the 284 mode in 1HH2 (and 278 mode in 1DD2) indicate the transaxial ligands (H2, H) and slight distortion of the P-Fe-P angle in the Fe-H2 plane have no resolvable effect on this vibrational mode.
Our analysis indicates that modes with significant contribution (>10%) from the H– and H2 ligands motion do not supply 1-H spectral intensities at energies below ~570 cm−1 (Figure S6a), while the corresponding low-energy limit for the D− and D2 ligands in 57Fe-PVDOS of 1-D is ~260 cm−1 (Figure S7a). For example, the strongest mode of rotational ν↻(1HH2) character is predicted at 384 cm−1 but conveys only negligible Fe motion (Figures 3 and S6b). However, at 392 cm−1 there is a satellite mode calculated with similar, but much weaker H2 rotational character, that contains 57Fe-PVDOS and agrees well with the shoulder at the same position in the experimental spectrum of 1-H. Inspection of the 392 cm−1 normal mode displacements indicates it acquires intensity through coupling with equatorial Fe–P motion. The nearby isotopically-sensitive intensity in 1-H at 316, 332 (shoulder), and 361 cm−1 are calculated at 314, 333, and 364 cm−1 containing varying Fe–P stretching motions and, similarly to the 284 cm−1 mode, near-concerted translation of the H-Fe-H2 moiety within the Fe-P plane. The second greatest intensity in the spectrum is at 259 cm−1 in 1-H, and it is primarily correlated with P–Fe–P out-of-plane bending motion where H-Fe-H2 is collectively translating through the equatorial plane.
In summary, we have characterized trans-[57Fe(η2-H2)(H)(dppe)2][BPh4] with 57Fe NRVS combined with DFT calculations. The Fe-H stretching mode in the compound (and the H2-loss byproduct) represent the highest energy vibrational features ever observed by NRVS. Moreover, our combined approach developed a complete picture of the H-Fe-H2 vibrational structure even in the congested low energy regime. This implies that hydrogen motion in this low energy region can serve as a surrogate probe in systems that are not yet capable of being explored with high energy NRVS. There are clear accessible applications to the study of hydrogen storage materials - for example, Fe-containing metal–organic frameworks (MOFs) where the presence of framework hydrogen limits neutron-based approaches.31 Extension of this work to dilute biological systems is challenging, but with continued improvements in synchrotron radiation X-ray production it will eventually be possible to characterize Fe-H/H2 vibrational modes in enzyme intermediates.
Supplementary Material
ACKNOWLEDGMENT
M.H.C., Y.C.L. and C.C.H. acknowledge funding by the Ministry of Science and Technology of Taiwan and Academia Sinica (AS-SS-108-02-1, AS-iMate-109-22, and AS-SS-109-07). V.P. was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germanýs Excellence Strategy – EXC 2008 – 390540038 – UniSysCat. S.P.C. was funded by NIH GM65440. Some computational work was performed under the XSIM project on the CORI computing system at NERSC a U.S. Department of Energy Office of Science User Facility operated under Contract No. DE-AC02-05CH11231.
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.inorgchem.0c03006. Experimental and computational procedures, supplementary figures and tables (PDF), animations showing the calculated normal modes with significant (i) 57Fe and (ii) Fe-bound proton PVDOS characters, cartesian coordinates of the optimized structures.
REFERENCES
- 1.Kubas GJ; Ryan RR; Swanson BI; Vergamini PJ; Wasserman HJ Characterization of the 1st Examples of Isolable Molecular-Hydrogen Complexes, Mo(CO)3(Pcy3)2(H2), W(CO)3(Pcy3)2(H2),Mo(CO)3 (Pi-Pr3)2(H2),W(CO)3(Pi-Pr3)2(H2) - Evidence for a Side-On Bonded H2 Ligand. J. Am. Chem. Soc. 1984, 106 (2), 451–452. [Google Scholar]
- 2.Kubas GJ Activation of dihydrogen and coordination of molecular H2 on transition metals. J. Organomet. Chem. 2014, 751, 33–49. [Google Scholar]
- 3.Bullock RM; Appel AM; Helm ML Production of hydrogen by electrocatalysis: making the H-H bond by combining protons and hydrides. Chem. Comm. 2014, 50 (24), 3125–3143. [DOI] [PubMed] [Google Scholar]
- 4.Kubas GJ Hydrogen activation on organometallic complexes and H2 production, utilization, and storage for future energy. J. Organomet. Chem. 2009, 694 (17), 2648–2653. [Google Scholar]
- 5.Haumann M; Stripp ST The Molecular Proceedings of Biological Hydrogen Turnover. Acc. Chem. Res. 2018, 51 (8), 1755–1763. [DOI] [PubMed] [Google Scholar]
- 6.Lubitz W; Ogata H; Rudiger O; Reijerse E Hydrogenases. Chem. Rev. 2014, 114 (8), 4081–4148. [DOI] [PubMed] [Google Scholar]
- 7.Peters JW; Schut GJ; Boyd ES; Mulder DW; Shepard EM; Broderick JB; King PW; Adams MWW [FeFe]- and [NiFe]-hydrogenase diversity, mechanism, and maturation. Biochim. Biophys. Acta 2015, 1853 (6), 1350–1369. [DOI] [PubMed] [Google Scholar]
- 8.Crabtree RH Dihydrogen Complexation. Chem. Rev. 2016, 116 (15), 8750–8769. [DOI] [PubMed] [Google Scholar]
- 9.Kaesz HD; Saillant RB Hydride Complexes of Transition Metals. Chem. Rev. 1972, 72 (3), 231–281. [Google Scholar]
- 10.Heinekey DM; Oldham WJ Coordination Chemistry of Dihydrogen. Chem. Rev. 1993, 93 (3), 913–926. [Google Scholar]
- 11.Woinska M; Grabowsky S; Dominiak PM; Wozniak K; Jayatilaka D Hydrogen atoms can be located accurately and precisely by x-ray crystallography. Sci. Adv. 2016, 2 (5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perutz RN; Procacci B Photochemistry of Transition Metal Hydrides. Chem. Rev. 2016, 116 (15), 8506–8544. [DOI] [PubMed] [Google Scholar]
- 13.Seto M; Yoda Y; Kikuta S; Zhang XW; Ando M Observation of Nuclear Resonant Scattering Accompanied by Phonon Excitation Using Synchrotron Radiation. Phys. Rev. Lett. 1995, 74, 3828–3831. [DOI] [PubMed] [Google Scholar]
- 14.Chumakov A; Rüffer R Nuclear inelastic scattering. Hyp. Int. 1998, 113 (1), 59–79. [Google Scholar]
- 15.Scheidt WR; Li JF; Sage JT What Can Be Learned from Nuclear Resonance Vibrational Spectroscopy: Vibrational Dynamics and Hemes. Chem. Rev. 2017, 117 (19), 12532–12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cramer SP Nuclear Resonance Vibrational Spectroscopy Chapter 10 in X-Ray Spectroscopy with Synchrotron Radiation: Fundamentals and Applications; Springer International Publishing: Cham, 2020, pp. 257–278. [Google Scholar]
- 17.Sage JT; Paxson C; Wyllie GRA; Sturhahn W; Durbin SM; Champion PM; Alp EE; Scheidt WR Nuclear resonance vibrational spectroscopy of a protein active-site mimic. J. Phys.: Condens. Matter 2001, 13, 7707–7722. [Google Scholar]
- 18.Leu BM; Zgierski MZ; Wyllie GRA; Scheidt WR; Sturhahn W; Alp EE; Durbin SM; Sage JT Quantitative Vibrational Dynamics of Iron in Nitrosyl Porphyrins. J. Am. Chem. Soc. 2004, 126 (13), 4211–4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gee LB; Pelmenschikov V; Wang H; Mishra N; Liu Y-C; Yoda Y; Tamasaku K; Kaupp M; Chiang M-H; Cramer SP Vibrational and electronic characterization of a diiron bridging hydride complex – a model for hydrogen catalysis. Chem. Sci. 2020, 11 (21), 5487–5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogata H; Krämer T; Wang H; Schilter D; Pelmenschikov V; van Gastel M; Neese F; Rauchfuss TB; Gee LB; Scott AD; Yoda Y; Tanaka Y; Lubitz W; Cramer SP Hydride bridge in [NiFe]-hydrogenase observed by nuclear resonance vibrational spectroscopy. Nat. Commun. 2015, 6, 7890 (1–8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reijerse EJ; Pham CC; Pelmenschikov V; Gilbert-Wilson R; Adamska-Venkatesh A; Siebel JF; Gee LB; Yoda Y; Tamasaku K; Lubitz W; Rauchfuss TB; Cramer SP Direct observation of an iron bound terminal hydride intermediate in [FeFe] hydrogenase. J. Am. Chem. Soc. 2017, 139 (12), 4306–4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pelmenschikov V; Birrell JA; Pham CC; Mishra N; Wang HX; Sommer C; Reijerse E; Richers CP; Tamasaku K; Yoda Y; Rauchfuss TB; Lubitz W; Cramer SP Reaction Coordinate Leading to H2 Production in [FeFe] Hydrogenase Identified by Nuclear Resonance Vibrational Spectroscopy and Density Functional Theory. J. Am. Chem. Soc. 2017, 139 (46), 16894–16902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pham CC; Mulder DW; Pelmenschikov V; King PW; Ratzloff MW; Wang H; Mishra N; Alp EE; Zhao J; Hu MY; Tamasaku K; Yoda Y; Cramer SP Terminal Hydride Species in [FeFe]-Hydrogenases are Vibrationally Coupled to the Active Site Environment. Angew. Chem. Int. Ed. 2018, 57, 10605–10609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pelmenschikov V; Gee LB; Wang H; MacLeod KC; McWilliams SF; Skubi KL; Cramer SP; Holland PL High-Frequency Fe–H Vibrations in a Bridging Hydride Complex Characterized by NRVS and DFT. Angew. Chem., Int. Ed. 2018, 57 (30), 9367–9371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abrecht DG; Munoz JA; Smith HL; Fultz B Spin-State Effects on the Thermal Dihydrogen Release from Solid-State [MH(η2-H2)dppe2]+ (M = Fe, Ru, Os) Organometallic Complexes for Hydrogen Storage Applications. J. Phys. Chem. C 2014, 118 (4), 1783–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ricci JS; Koetzle TF; Bautista MT; Hofstede TM; Morris RH; Sawyer JF Single-Crystal X-Ray and Neutron Diffraction Studies of an η2-Dihydrogen Transition-Metal Complex: trans-[Fe(η2-H2)(H)(PPH2CH2CH2PPh2)2]BPh4. J. Am. Chem. Soc. 1989, 111 (24), 8823–8827. [Google Scholar]
- 27.Bautista MT; Cappellani EP; Drouin SD; Morris RH; Schweitzer CT; Sella A; Zubkowski J Preparation and Spectroscopic Properties of the Eta-2-Dihydrogen Complexes [Mh(Eta-2-H2)(Pr2ch2ch2pr2)2]+ (M = Fe, Ru, R = Ph, Et) and Trends in Properties down the Iron Group Triad. J. Am. Chem. Soc. 1991, 113 (13), 4876–4887. [Google Scholar]
- 28.Kubas GJ Fundamentals of H2 binding and reactivity on transition metals underlying hydrogenase function and H2 production and storage. Chem. Rev. 2007, 107 (10), 4152–4205. [DOI] [PubMed] [Google Scholar]
- 29.Bell CB; Wong SD; Xiao Y; Klinker EJ; Tenderholt AL; Smith MC; Rohde J-U; Que L; Cramer SP; Solomon EI A combined NRVS and DFT study of Fe(IV)=O model complexes: a diagnostic method for the elucidation of non-heme iron enzyme intermediates. Angew. Chem., Int. Ed. 2008, 47 (47), 9071–9074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeng W; Barabanschikov A; Zhang Y; Zhao J; Sturhahn W; Alp EE; Sage JT Synchrotron-Derived Vibrational Data Confirm Unprotonated Oxo Ligand in Myoglobin Compound II. J. Am. Chem. Soc. 2008, 130 (6), 1816–1817. [DOI] [PubMed] [Google Scholar]
- 31.Gygi D; Bloch ED; Mason JA; Hudson MR; Gonzalez MI; Siegelman RL; Darwish TA; Queen WL; Brown CM; Long JR Hydrogen Storage in the Expanded Pore Metal–Organic Frameworks M2(dobpdc) (M = Mg, Mn, Fe, Co, Ni, Zn). Chem. Mater. 2016, 28 (4), 1128–1138. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.