Supplemental digital content is available in the text.
Key Words: PROSTATE CANCER, RESISTANCE TRAINING, DOSE–RESPONSE EFFECTS, MINIMAL DOSAGE, HEALTH-RELATED OUTCOMES
ABSTRACT
Purpose
Resistance exercise improves an array of treatment-related adverse effects in men with prostate cancer; however, the minimal dosage required is unknown. We systematically reviewed the resistance training effects in prostate cancer patients to determine the minimal dosage regarding the exercise components (type, duration, volume, and intensity) on body composition, physical function, muscle strength, cardiorespiratory fitness, body mass index, and prostate-specific antigen.
Methods
Using PRISMA guidelines, MEDLINE, CINAHL, EMBASE, SPORTDiscus, and Web of Science databases were searched. Eligible randomized controlled trials examined prostate cancer patients undertaking resistance-based exercise programs during or after treatment. Meta-analysis was undertaken when more than three studies were included. Associations between mean differences and exercise components were tested by univariate and multivariate meta-regression analysis.
Results
Twenty-three articles describing 21 trials and involving 1748 prostate cancer patients were included. Exercise improved fat mass (−1% in body fat and −0.6 kg in fat mass), lean mass (~0.5 kg in lean and appendicular lean mass), functional capacity (i.e., chair rise, 400-m test, 6-m fast walk, and stair climb tests), and fitness outcomes (i.e., V̇O2peak and muscle strength) (P = 0.040–<0.001) with no change in body mass index or prostate-specific antigen (P = 0.440–0.735). Meta-regression indicated no association between exercise type, resistance training duration, weekly volume and intensity, and primary outcomes (P = 0.075–0.965). There was a significant association between exercise intensity and chest press muscle strength (favoring moderate intensity, P = 0.012), but not in other secondary outcomes.
Conclusion
In untrained older men with prostate cancer initiating an exercise program, lower volume at moderate to high intensity is as effective as higher volume resistance training for enhancing body composition, functional capacity, and muscle strength in the short term. A low exercise dosage may help reduce barriers to exercise and enhance adherence.
The benefits of exercise medicine have been widely attested in different cancer populations (1,2). In prostate cancer patients, for example, resistance exercise alone or combined with aerobic training has been shown to reduce postsurgical impairments from prostatectomy (3), reverse the array of adverse effects from androgen deprivation therapy (ADT) (4–11), and preserve physical function in those with bone metastases (12), in addition to improvements in quality of life (5,8,12). However, although the role of exercise medicine is being expanded to include low-grade cancer patients undergoing active surveillance (13–15), or high-grade patients to enhance tumor growth suppression (16) and survival (17), information regarding the actual exercise dose–response still needs to be determined (18).
Considering the overall exercise benefits in prostate cancer patients, the assumption that a given exercise dosage will promote benefits in all outcomes is premature. In the most recent exercise guideline for cancer patients (19), a specific resistance exercise dosage (e.g., 2 sets of 8–15 reps at 60%–85% of one-repetition maximum [1-RM]) was recommended to address or counter anxiety, fatigue, and depressive symptoms based on high-quality publications. However, the disproportionately large number of breast cancer trials compared with other cancer trials from which the recommendations were derived precludes more accurate recommendations for prostate cancer patients (19). Further, the paucity of comparative trials regarding resistance training components (i.e., frequency, intensity, and volume) makes it difficult to establish the dose–response effect on commonly reported outcomes. In this report, we examined the resistance exercise dosage in body composition and functional capacity given their strong association with risk of progression and mortality in prostate cancer patients (20–23).
Thus, the aim of the present study is 1) to systematically review and analyze the resistance training effects on body composition measures, functional capacity tests, cardiorespiratory fitness, muscle strength, body mass index (BMI), and prostate-specific antigen (PSA) levels and 2) to verify the minimal dose regarding the prescribed exercise components (i.e., type, duration, volume, and intensity) and effects on these outcomes.
METHODS
Study selection procedure
The study was undertaken in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (24,25), and the method used was based on the minimum criteria established by the Cochrane Back Review Group (26). This systematic review was not registered in any prospectively systematic review database (e.g., PROSPERO).
This review included published data from randomized controlled trials that evaluated the effects of resistance-based exercise programs in prostate cancer patients at any treatment stage (e.g., presurgical, during treatment, and with bone metastases). The primary outcomes of this review were body composition (i.e., body fat percentage, fat mass, trunk fat mass, lean mass, and appendicular lean mass) and functional capacity tests (i.e., 30-s sit-to-stand test, 6-min walk, 400-m walk, 6-m usual and fast walk, timed up-and-go, stair climb, and repeated sit-to-stand where patients repeated the task 5 times). The secondary outcomes were cardiorespiratory fitness (i.e., V̇O2peak or V̇O2max), muscle strength (i.e., chest press, leg press, leg extension, and seated row), PSA, and BMI. Trials were excluded if 1) home-based exercise was used in the whole intervention period; 2) they involved mixed cancer patients without specific information on prostate cancer patient results; 3) they did not include or report the specific outcomes included in this review, or did not include sufficient information for analysis; and 4) they were written in a language other than English. Eligibility was assessed independently evaluated in duplicate, with differences resolved by consensus.
The search was conducted up to November 2019 using the following electronic databases: MEDLINE, CINAHL, EMBASE, SPORTDiscus, and Web of Science. The terms used were “prostate cancer” and “resistance training” in association with a list of sensitive terms to search for experimental studies. In addition, we performed a manual search of the reference lists provided in the selected articles as well as previous systematic reviews and meta-analytic studies (27–32) to detect studies potentially eligible for inclusion. The search strategy used is shown in Table S1 (see Supplemental Digital Content 1, Literature search strategy used for the PubMed database, http://links.lww.com/MSS/C125).
Data extraction
Titles and abstracts of all articles identified by the search strategy were independently evaluated in duplicate. Abstracts that did not provide sufficient information regarding the inclusion and exclusion criteria were selected for full-text evaluation. In the second phase, the same reviewers independently evaluated these full-text articles and selected them in accordance with the eligibility criteria. Disagreements between reviewers were resolved by consensus. The data extraction was performed via a standardized form. Information on the interventions, outcomes, and patients were collected. Study characteristics, intervention duration, components of the resistance training prescription (i.e., frequency, intensity, volume, and type), adherence (i.e., number of patients that completed the program), attendance (i.e., number of sessions attended), compliance (i.e., number of patients that successfully completed the exercise prescription), and adverse events were extracted, along with the main outcomes. The prescribed resistance training was summarized as follows: frequency (number of sessions per week), intensity (prescribed intensity of resistance training), type (resistance training, combined resistance and aerobic training, or multimodal exercise program), and volume (sets and repetitions). When studies incorporated supervised and unsupervised periods of training, information was extracted on the longest period of the supervised exercise intervention. Outcomes were extracted in their absolute units (e.g., kilograms for lean and fat mass assessments). When graphs were used instead of numerical data, the graphs were measured through their plots using a specific tool for data extraction (WebPlotDigitizer, San Francisco, CA) (33).
Assessment of risk of bias
Risk of bias of individual studies was evaluated according to the second version of the Cochrane risk of bias tool for randomized trials (RoB 2) (34), focusing on different aspects of the trial design, conduct, and reporting. Each assessment using the RoB 2 tool is focused at the outcome level. The six-item instrument evaluates 1) randomization process, 2) deviation from intended interventions, 3) missing outcome data, 4) measurement of the outcome, 5) selection of the reported result, and 6) overall bias, and it was used to evaluate each included randomized controlled trial for each outcome of interest. Risk of bias for each of the six domains was expressed as “low risk,” “some concern,” and “high risk” (34).
Data analysis
The pooled-effect estimates were obtained from the mean difference of baseline to the final assessment of the intervention for each group. These values were expressed as the mean difference between groups. In studies with multiple exercise interventions, the groups were divided with each respective sample size, within-group mean difference, and SD or 95% confidence interval (CI) for further analysis. Meta-analyses were conducted for overall studies, and a subgroup analysis was provided based on RoB 2.0 low-risk classification when more than three studies were included. Calculations were performed using a random-effects model (35). The level of significance was set at P ≤ 0.05. Statistical heterogeneity was assessed using the Cochran Q test. A threshold P value of 0.1 as well as values greater than 50% in the statistical test of heterogeneity (I2) were considered indicative of high heterogeneity (36). Heterogeneity between studies was explored by omitting one study at a time and comparing the pooled with the original estimates, whereas the presence of publication bias was explored by contour-enhanced funnel plots along with Egger’s test, considering a P value <0.1 as indicative of publication bias (37,38). When necessary, the trim-and-fill computation was used to estimate the effect of publication bias on the interpretation of results (39,40). Analyses were conducted using the package metan, confunnel, metabias, and metatrim from Stata 14.0 software (Stata, College Station, TX). Forest plots presented for the outcome measures are after sensitivity analysis and/or trim-and-fill procedure adjustments.
In addition, we tested the association between the mean difference effect and the exercise components to identify a dose–response relationship using univariate and multivariate meta-regression. Using one variable at a time or multivariable models, we assessed whether components such as type, intervention duration, prescribed weekly volume, and peak intensity influence the association of resistance-based exercise with the main effects. Analyses were undertaken in outcomes significantly affected by exercise provided the models had more than five studies. For intervention duration, prescribed weekly volume, and peak intensity, analyses were considered when the values presented a range higher than 5%, whereas exercise type was coded as 0 = resistance training alone and 1 = resistance training combined with other components (e.g., aerobic, flexibility, impact loading, or balance). Analyses were conducted using the package metareg from Stata 14.0 software.
RESULTS
Studies included
All studies selected reported the aim to investigate the effect of resistance training (i.e., resistance training alone, combined with aerobic exercise, or included in a multimodal exercise program) in prostate cancer patients at any treatment stage. We retrieved 1021 studies, 794 of which were retained for screening after duplicate removals. Of these, 694 were excluded and 100 full-text articles were assessed for eligibility (Fig. 1). The eligibility assessment resulted in 23 articles (describing 21 trials) (5–12,41–55), which were included in the present review and meta-analyses (see Table S2, Supplemental Digital Content 2, Study characteristics: treatment stage, sample size, exercise prescription, adherence, attendance, compliance and outcomes assessed, http://links.lww.com/MSS/C126), with 6 to 13 studies being included in the dose–response relationship analysis involving exercise type, intervention duration, prescribed weekly volume, and peak intensity.
FIGURE 1.
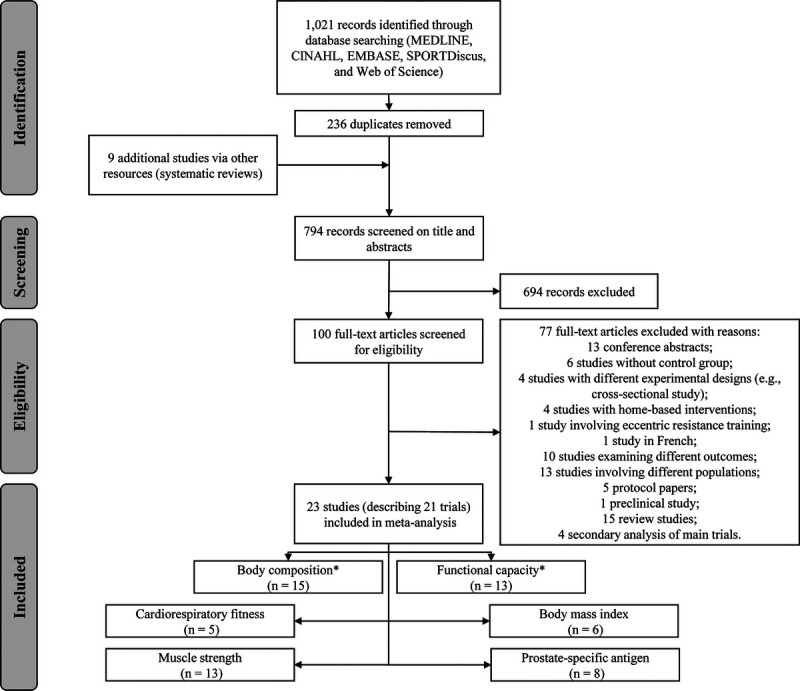
Flow chart of study selection process. *Primary outcome.
Prostate cancer patients and exercise intervention characteristics
A total of 1748 prostate cancer patients with an average age of 69.5 ± 2.1 yr participated in the included studies. Exercise interventions were predominantly undertaken in patients on ADT (17 of 23 studies) (5,7–9,11,41,43–48,51–55). Exercise modality included predominantly combined resistance and aerobic training (12 of 23 studies) (5–7,9,10,41,43,45,50–52,55) followed by multimodal exercise program (4 of 23 studies) (11,12,48,54), resistance training plus impact loading (5 of 23 studies) (7,9,44,46,49), and resistance training only (4 of 23 studies) (8,42,47,53) in a cohort of 901 patients allocated to the intervention group compared with 847 patients in the control group. In addition, three studies (41,43,48) also provided nutrition advice during the intervention. Studies were designed to compare the exercise intervention versus usual care control (15 of 23 studies) (5,8,11,12,41–43,45,47–52,55), a home-based program involving aerobic or flexibility training and physical activity (6 of 23 studies) (6,10,44,46,53,54), or to a delayed exercise group (2 of 23 studies) (7,9). Two studies compared multiple exercise interventions (7,9).
The mean exercise intervention duration was 19.5 ± 10.7 wk with an average of 2.4 ± 0.7 sessions per week. The average total prescribed resistance training volume was 9136 ± 4534 repetitions with a weekly training volume of 468 ± 177 repetitions. In addition, the mean peak intensity reached throughout the resistance training program was 79% ± 8% of 1-RM ranging from 60% to 85%. Information about resistance training frequency was not reported by one study (53), whereas four studies did not report volume (41,43,48,54) or intensity (41,50,54,55), respectively. Exercise program adherence ranged from 74% to 100% (reported in 22 of 23 studies) (5–9,11,12,41–55), whereas attendance and compliance ranged from 65% to 100% (reported in 21 of 23 studies) (5–12,41,42,44–46,48–55) and from 85% to 94% (reported in 5 of 23 studies) (41,42,47,49,53), respectively. Adverse events related to the exercise interventions were identified in 8 studies (6,8,9,45,47,50,51,54), whereas 14 studies (5,7,11,12,41–44,46,48,49,52,53,55) reported no adverse events throughout the intervention period. The adverse events were mostly related to musculoskeletal pain (e.g., back, shoulder, and knee), and only one study (53) presented a moderate adverse event with no detail provided.
Risk of bias assessment
For the primary outcomes of this review, 13.3% of the studies presented some concern for risk of bias in body composition assessment (2 of 15 studies) (48,55) and 76.9% in the functional capacity tests (10 of 13 studies) (5,6,9,12,42,45,47,50,51,53). The concerns in body composition were mainly due to the measurement of the outcome as two studies (48,55) evaluated body composition outcomes through the use of bioelectrical impedance. For functional capacity, the concerns were mainly due to the measurement of the outcome as studies performed nonblinded assessments on measurement of the outcome (76.9%, 10 of 13 studies) (5,6,9,12,42,45,47,50,51,53), and one study (7.7%, 1 of 13 studies) (50) did not report the concealment of allocation in the randomization process. For the secondary outcomes, concerns were observed in cardiorespiratory fitness (some concerns: 60.0%, 3 of 5 studies) (43,53,54), muscle strength (84.6%, 11 of 13 studies) (5–8,10,12,42,45,47,50,53), and BMI (16.7%, 1 of 6 studies) (47). Concerns were not observed in the PSA assessment. The overall risk of bias assessment is shown in Table S3 (see Supplemental Digital Content 3, Risk of bias of included studies, http://links.lww.com/MSS/C127), and the individual assessment is presented in Figure S1 (see Supplemental Digital Content 4, Individual risk of bias assessment, http://links.lww.com/MSS/C128).
Exercise effects on body composition
Exercise resulted in significant positive overall effects in percent body fat (−1.0%, 95% CI = −1.3 to −0.6%), fat mass (−0.6 kg, 95% CI = −0.8 to −0.3 kg), trunk fat mass (−0.3 kg, 95% CI = −0.6 to −0.2 kg), lean mass (0.5 kg, 95% CI = 0.3 to 0.7 kg), and appendicular lean mass (0.4 kg, 95% CI = 0.2 to 0.6 kg) with heterogeneity ranging from I2 = 0% to 47% after sensitivity analysis and/or trim-and-fill procedure adjustments (Figs. 2 and 3). The samples ranged from 490 to 917 participants (see Table S4, Supplemental Digital Content 5, Overall and subgroup analysis effects on body composition, functional capacity, and the secondary outcomes in prostate cancer patients, http://links.lww.com/MSS/C129). In subgroup analysis, the main effects were significantly maintained in the outcomes (I2 = 0% to 47%; P = <0.001 to 0.025). Outliers were identified in the overall analysis for body fat percentage (6) and trunk fat mass (52) and subgroup analysis of appendicular lean mass (7), whereas publication bias and trim-and-fill procedure suggested that data from three studies were missing for appendicular lean mass (P = 0.050). These studies were omitted from the abovementioned overall and subgroup effects (Figs. 2 and 3). The meta-analysis power to detect changes in body composition was 1 − β = 1.0.
FIGURE 2.
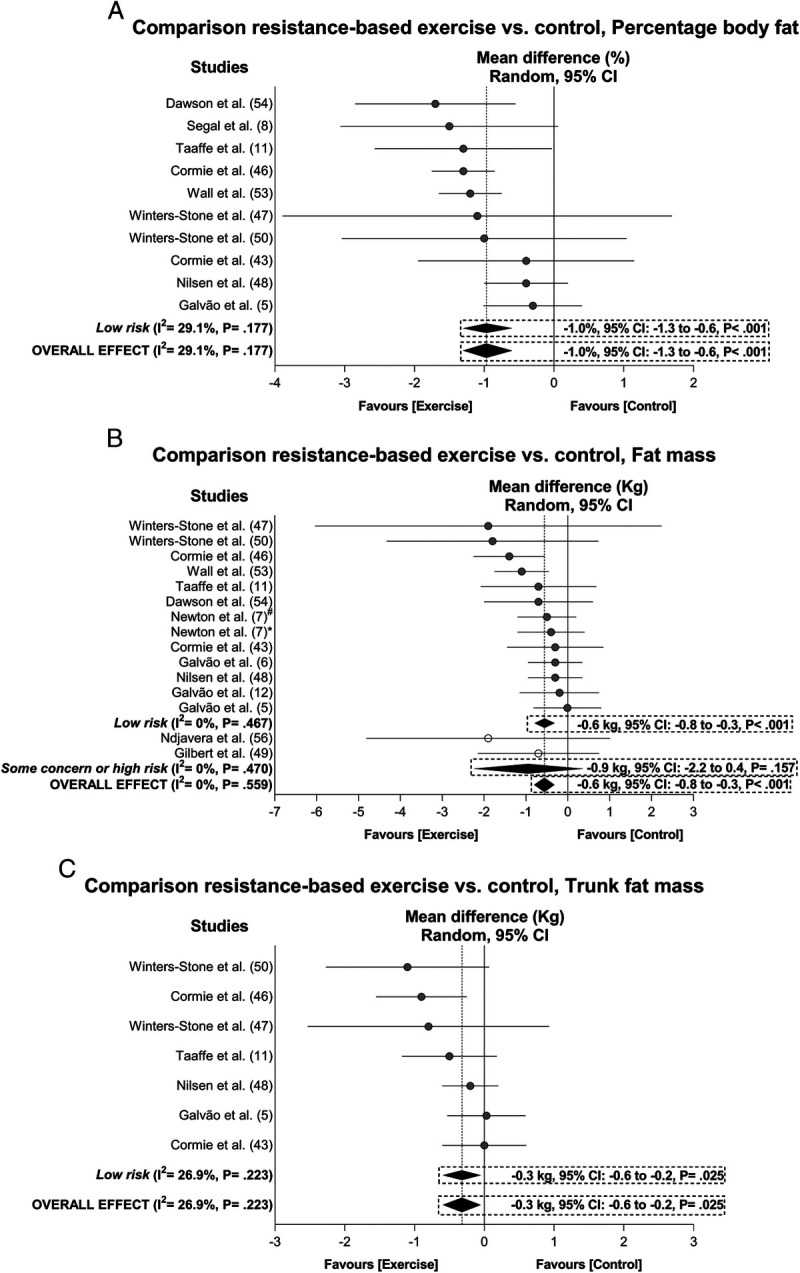
Mean difference effects of resistance-based exercise compared with control on percentage body fat (A), fat mass (B), and trunk fat mass (C). Overall and subgroup analyses conducted with a random-effects model. Gray and white circles represent study-specific estimates based on risk of bias assessment (low risk and some concern or high risk of bias, respectively); I2 represents the heterogeneity test; diamonds represent pooled estimates of random-effect meta-analysis. *Combined resistance and aerobic group. #Resistance training plus impact-loading group.
FIGURE 3.
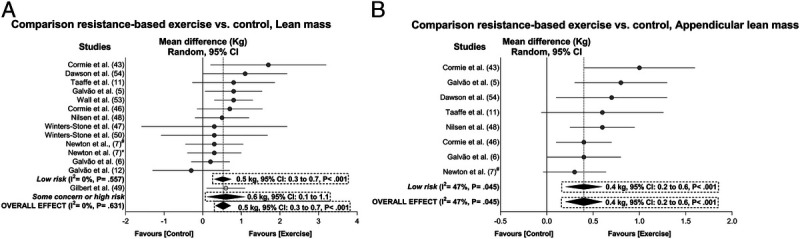
Mean difference effects of resistance-based exercise compared with control on lean mass (A) and appendicular lean mass (B). Overall and subgroup analyses conducted with a random-effects model. Gray and white circles represent study-specific estimates based on risk of bias assessment (low risk and some concern or high risk of bias, respectively); I2 represents the heterogeneity test; diamonds represent pooled estimates of random-effect meta-analysis. *Combined resistance and aerobic group. #Resistance training plus impact-loading group.
In the dose–response analysis, the univariate (P = 0.075 to 0.965; see Table S5, Supplemental Digital Content 6, Univariate meta-regression on main outcomes mean difference and exercise type, resistance training duration, weekly volume and peak intensity, http://links.lww.com/MSS/C130) and multivariate meta-regression models (P = 0.203 to 0.785; see Table S6, Supplemental Digital Content 7, Multivariate meta-regression on main outcomes mean difference and exercise type, resistance training duration, weekly volume and peak intensity, http://links.lww.com/MSS/C131) did not explain the variation in body composition outcomes.
Exercise effects on functional capacity
There was a significant positive overall exercise effect for the time to perform the 30-s sit-to-stand repetitions (2.8 reps, 95% CI = 1.7 to 4.0 reps), repeated sit-to-stand test (−1.0 s, 95% CI = −1.4 to −0.6 s), 400-m walk (−8.3 s, 95% CI = −12.4 to −4.2 s), 6-m fast walk (−0.1 s, 95% CI = −0.2 to −0.0), and stair climb (−0.2 s, 95% CI = −0.3 to −0.1 s) with a heterogeneity ranging from I2 = 0% to 45.2% after sensitivity analysis and/or trim-and-fill procedure adjustments (Fig. 4). The samples ranged from 213 to 519 participants (see Table S4, Supplemental Digital Content 5, Overall and subgroup analysis effects on body composition, functional capacity, and the secondary outcomes in prostate cancer patients, http://links.lww.com/MSS/C129). Subgroup analyses were not undertaken on these outcomes as well as the overall analyses in the 6-min walk test and 6-m backwards walk test given the small number of studies included (<3). The study of Galvão et al. (12) was considered an outlier in the 6-m fast walk time analysis and omitted from the abovementioned results, whereas publication bias was only found for the 400-m walk (P = 0.063) with no trimming needed to be performed (data unchanged). The meta-analysis power to detect change in the 6-m usual walk and timed up-and-go test was 1 − β = 0.57 and 0.64, respectively, whereas a 1 − β = 1.0 was found for the remaining functional capacity outcomes.
FIGURE 4.
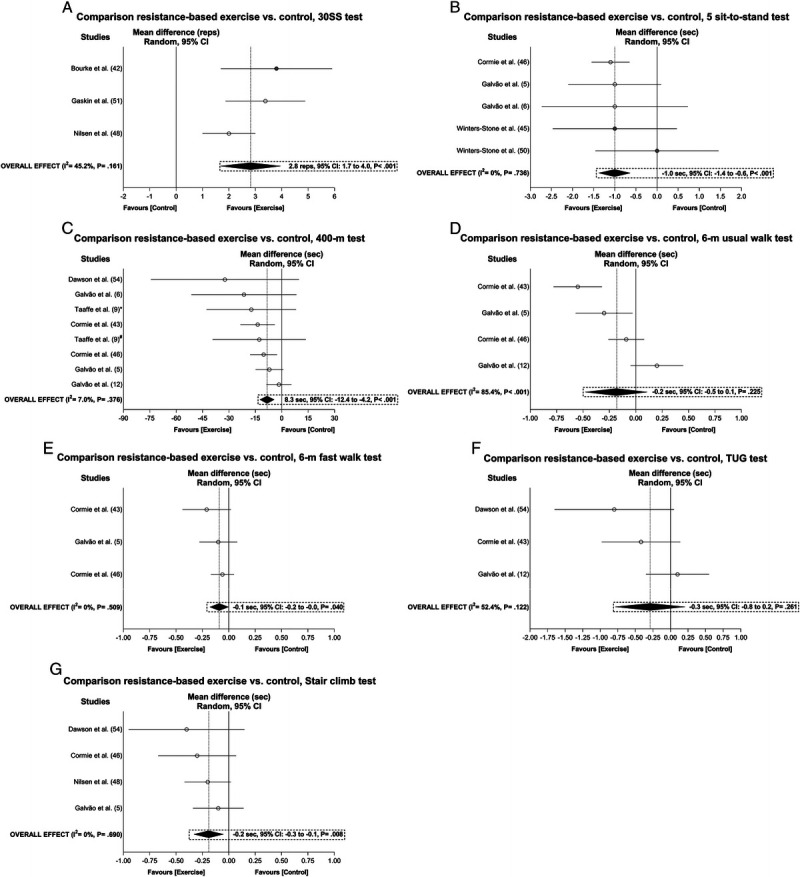
Mean difference effects of resistance-based exercise compared with control on 30-s sit-to-stand repetitions (A), 5 sit-to-stand test (B), 400-m walk test (C), 6-m usual walk test (D), 6-m fast walk test (E), timed up-and-go test (F), and stair climb test (G). Overall and subgroup analyses conducted with a random-effects model. Gray and white circles represent study-specific estimates based on risk of bias assessment (low risk and some concern or high risk of bias, respectively); I2 represents the heterogeneity test; diamonds represent pooled estimates of random-effect meta-analysis. *Combined resistance and aerobic group. #Resistance training plus impact-loading group. 30SS, 30-s sit-to-stand test; TUG, timed up-and-go test.
In the dose–response analysis, the univariate (P = 0.182 to 0.341; see Table S5, Supplemental Digital Content 6, Univariate meta-regression on main outcomes mean difference and exercise type, resistance training duration, weekly volume and peak intensity, http://links.lww.com/MSS/C130) and multivariate meta-regression models (P = 0.358; see Table S6, Supplemental Digital Content 7, Multivariate meta-regression on main outcomes mean difference and exercise type, resistance training duration, weekly volume and peak intensity, http://links.lww.com/MSS/C131) were not statistically significant in explaining the variation in 400-m test performance. Analyses of 30-s sit-to-stand, 6-min walk test, 6-m usual and fast walk, stair climb, and repeated sit-to-stand tests were not undertaken because of the small number of studies (≤5) reporting on these components. Performing univariate meta-regression resulted in nonsignificant associations between exercise type, resistance training duration, weekly volume, and peak intensity with 30-s sit-to-stand (P = 0.311 for exercise type and resistance training duration), 6-min fast walk (P = 0.165–0.793), stair climbs (P = 0.523–0.930), and repeated sit-to-stand tests (P = 0.681–0.868).
Exercise effects on secondary outcomes
There was a significant increase in chest press (3.9 kg, 95% CI = 2.9 to 4.9 kg), leg press (23.5 kg, 95% CI = 15.2 to 31.7 kg), leg extension (8.8 kg, 95% CI = 6.9 to 10.7 kg), and seated row strength (5.2 kg, 95% CI = 3.9 to 6.5 kg) with heterogeneity ranging from I2 = 0% to 77.4% after sensitivity analysis and/or trim-and-fill procedure adjustments (Fig. 5). The samples ranged from 321 to 728 participants (see Table S4, Supplemental Digital Content 5, Overall and subgroup analysis effects on body composition, functional capacity, and the secondary outcomes in prostate cancer patients, http://links.lww.com/MSS/C129). Subgroup analyses were not undertaken for these outcomes because of the small number of studies that were considered of low risk (<3). Outliers were identified in the overall analysis for chest press (8), leg extension (12), and seated row test (53). Meta-analysis power to detect change in muscle strength was 1 − β = 1.0.
FIGURE 5.
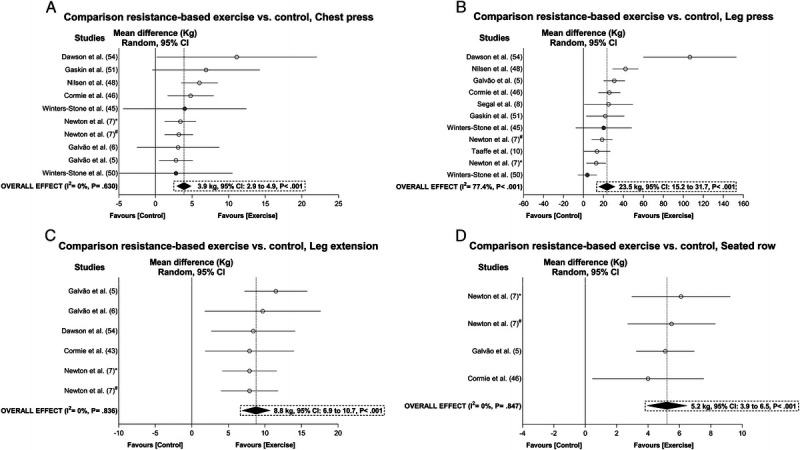
Mean difference effects of resistance-based exercise compared with control on chest press (A), leg press (B), leg extension (C), and seated row (D). Overall and subgroup analyses conducted with a random-effects model. Gray and white circles represent study-specific estimates based on risk of bias assessment (low risk and some concern or high risk of bias, respectively); I2 represents the heterogeneity test; diamonds represent pooled estimates of random-effect meta-analysis. *Combined resistance and aerobic group. #Resistance training plus impact-loading group.
Regarding V̇O2peak, there was a positive overall effect of 1.3 mL·kg−1⋅min−1 (95% CI = 0.8 to 1.7 mL·kg−1⋅min−1) after the publication bias and trim-and-fill procedure, suggesting that data were missing from two studies (P = 0.078; see Table S4, Supplemental Digital Content 5, Overall and subgroup analysis effects on body composition, functional capacity, and the secondary outcomes in prostate cancer patients, http://links.lww.com/MSS/C129, and Fig. 6). Finally, exercise did not result in a significant change in BMI or PSA levels (P = 0.440–0.735; see Table S4, Supplemental Digital Content 5, Overall and subgroup analysis effects on body composition, functional capacity, and the secondary outcomes in prostate cancer patients, http://links.lww.com/MSS/C129, and Fig. 6). Meta-analysis power to detect change in V̇O2peak was 1 − β = 1.0, whereas power for BMI and PSA was 0.25 and 0.57, respectively.
FIGURE 6.
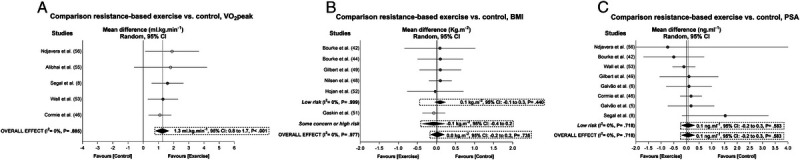
Mean difference effects of resistance-based exercise compared with control on V̇O2peak (A), BMI (B), and PSA levels (C). Overall and subgroup analyses conducted with a random-effects model. Gray and white circles represent study-specific estimates based on risk of bias assessment (low risk and some concern or high risk of bias, respectively); I2 represents the heterogeneity test; diamonds represent pooled estimates of random-effect meta-analysis.
In the univariate dose–response analysis, resistance training type and intensity (r2 = 64.0%, P = 0.010, and r2 = 100%, P < 0.001, respectively; see Table S5, Supplemental Digital Content 6, Univariate meta-regression on main outcomes mean difference and exercise type, resistance training duration, weekly volume and peak intensity, http://links.lww.com/MSS/C130) explained the variation in chest press muscle strength. In the multivariate model, gain in chest press muscle strength (r2 = 100%, P = 0.012; see Table S6, Supplemental Digital Content 7, Multivariate meta-regression on main outcomes mean difference and exercise type, resistance training duration, weekly volume and peak intensity, http://links.lww.com/MSS/C131) was greater in studies prescribing resistance training with moderate intensity (P = 0.022). Although the resistance training volume was significant in the univariate model to explain leg extension and leg press muscle strength (P = 0.043 and 0.050, respectively; see Table S5, Supplemental Digital Content 6, Univariate meta-regression on main outcomes mean difference and exercise type, resistance training duration, weekly volume and peak intensity, http://links.lww.com/MSS/C130), the results were not maintained in the multivariate meta-regression model (P = 0.147–0.204). Dose–response analyses of V̇O2peak and the seated row test were not undertaken because of the small number of studies (≤5) reporting on these components. Performing univariate meta-regression resulted in nonsignificant associations between exercise type, resistance training duration, weekly volume, and peak intensity with V̇O2peak (P = 0.598–0.651, see Table S5, Supplemental Digital Content 6, Univariate meta-regression on main outcomes mean difference and exercise type, resistance training duration, weekly volume and peak intensity, http://links.lww.com/MSS/C130), whereas seated row test variation was explained by exercise type (coefficient ± SE; −14.9 ± 2.9, P = 0.014; favoring resistance training alone), resistance training weekly volume (0.0 ± 0.1, P = 0.032; favoring higher weekly volume), but not resistance training duration (P = 0.624; see Table S5, Supplemental Digital Content 6, Univariate meta-regression on main outcomes mean difference and exercise type, resistance training duration, weekly volume and peak intensity, http://links.lww.com/MSS/C130).
DISCUSSION
The present review produced four important findings in prostate cancer patients. First, body composition is enhanced by resistance exercise (i.e., increase in whole body and regional lean mass and decrease in fat mass) regardless of type, duration, weekly volume, and peak intensity. Second, exercise promotes significant improvements in multiple components of physical function, in a nonlinear dose–response fashion. Third, muscle strength and cardiorespiratory fitness are improved with exercise, with greater effects in chest press strength resulting from resistance training performed at a moderate intensity. Finally, resistance-based exercise does not modify BMI or affect PSA levels. Therefore, the resistance training prescription combined with different exercise components is a potent therapy against an array of treatment-related adverse effects in prostate cancer patients regardless of the weekly volume prescribed when moderate to high intensity is achieved.
Obesity has been associated with an increased risk of biochemical recurrence and mortality in prostate cancer patients in a dose–response fashion (20). In the meta-analysis by Cao and Ma (20), a 5-kg·m−2 increase in BMI was associated with a 21% increased risk for biochemical recurrence and a 20% increased risk for prostate cancer–specific mortality. In our study, PSA levels did not change in response to exercise involving resistance training, indicating no impact of this exercise mode on disease progression (e.g., albeit not expected to change as most studies were short in duration with the majority of patients having local disease). In addition, few studies reported adverse events, and these were generally minor in nature. Moreover, the similar magnitude of change observed in lean mass and fat mass (i.e., increase in lean mass and decrease in fat mass) accounts for the maintenance in BMI and may result in metabolic health benefits and enhanced survival (56,57). Furthermore, the lack of relationship between resistance training weekly volume, intensity, and duration indicates the potential benefit of low-dosage resistance training to improve overall body composition. Likewise, in a previous report by Stamatakis et al. (58), a low weekly dosage of resistance training was associated with an approximately 25% reduced risk of mortality. Thus, undertaking exercise programs that include resistance training not only results in benefits for body composition in men with prostate cancer but also may provide a protective effect against cancer recurrence and cancer-specific mortality even when performed at a low weekly dosage. These results are of importance for prostate cancer patients and the prescription of exercise for this patient group as it suggests that even modest amounts of exercise may result in the accrual of significant body composition benefits, and this may also contribute to increased attendance and compliance to an exercise program.
Considering the World Health Organization report (59), the concept of healthy aging should be seen as the process of developing and maintaining functional capacity. Several studies report the association between muscle strength, cardiorespiratory fitness, and functional tests with independence, hospitalization rate, and mortality (60–65). Thus, the observed gains in muscle strength, cardiorespiratory fitness, and functional capacity support the translation of exercise medicine effects into functional independence and autonomy in older prostate cancer patients. For example, the reduction in time to walk 400 m represents an increase in the safety margin before the threshold for disability and may help to reduce the risk for complications such as risk for falls and fractures (66,67) and mortality (21). Reduced risk of mortality is also associated with enhanced repeated sit-to-stand and stair climb test performance (22,23). In this way, the progression of moderate to high intensity in resistance training combined with other exercise components appears to be sufficient to achieve significant improvements in functional capacity of patients with prostate cancer regardless of the number of weekly repetitions. Thus, the present findings provide an appropriate approach for prostate cancer patients as it allows a conservative exercise prescription commencement (e.g., less repetitions per exercise at moderate to high loads) and gradual progression according to comorbidities and the patient’s treatment-related side effects (68). Furthermore, following the nonsignificant relationship between intervention duration and study outcomes, it is also possible to maintain a low-dosage resistance training program for longer periods, which may help patients to keep active during and after treatment.
One of the critical considerations in the design of exercise trials and of its potential and feasibility in cancer patients is related to the exercise dose–response (18,19,68). However, to date, the assessment and the quantification of exercise dosage as well as the lack of reporting preclude a minimal-dosage prescription for prostate cancer patients. The present review and analysis provide information that less repetitions per exercise at moderate to high intensity (i.e., 60%–85% of 1-RM) could be sufficient to achieve significant benefits for prostate cancer patients, at least in the short term. We hypothesize that because of the large window for adaptation in these undeveloped qualities, these men adapt at a similar rate within the volumes and intensities of the studies analyzed, at least over the relatively short duration of these interventions. Our results partially agree with previous studies comparing different resistance training dosages in older adults (69–71), with similar results for various dosages after 12 wk of training (69,71) but not for longer training periods such as 20 wk (70,71). This could be due to the lower threshold for muscular adaptations in untrained older participants in the initial stages of training and the need for a greater stimulus after this initial period. However, the lack of influence of intervention duration suggests the potential use of low-volume resistance training during longer periods in prostate cancer patients, different than that observed in healthy older adults (70,71). Future studies will be necessary to elucidate if higher dosage and longer duration accrue greater benefits in prostate cancer patients. Furthermore, considering the meta-analytic adjustments and heterogeneity, the positive exercise results observed in body composition and multiple components of physical function are likely to be observed across different treatment phases (e.g., during ADT or after primary treatment). Given the lower between-studies heterogeneity in the meta-analysis (I2 < 30%), the observed results in body fat, muscle mass, 6-min walk, 400-m walk, stair climb, repeated sit-to-stand, and cardiorespiratory fitness indicate that prostate cancer patients may experience similar benefits in these outcomes regardless of the treatment phase. Thus, the low-resistance training dosage could be a useful strategy to improve body composition and muscle function in patients at different treatment stages.
The strength of this review and analysis is that it included a large number of exercise trials encompassing prostate cancer patients at different disease stages (21 trials reported in 23 articles with 1748 patients included) in a conservative approach using univariate and multivariate meta-regression models, as well as sensitivity analysis to explore the common objectively assessed physical health-related outcomes. However, there are also some limitations that are worthy of comment. First, although our findings indicate a minimal dosage for health-related outcomes based on studies undertaken to date, it should not be seen as an “optimal” dosage for each of the outcomes investigated. Second, the use of prescribed dosage (not the actual dosage undertaken) may be considered a limitation in the present study. Although the compliance ranges from 65% to 94% in the included studies (41,42,47,49,53), most did not report this metric, precluding a determination of how much exercise was actually undertaken in the attended sessions. We recently reported on compliance in an exercise trial on men with prostate cancer who had bone metastases (72) and outlined the methodology and metrics that can be used in future studies. Finally, the exercise program duration was considered short in most of the included studies. Only two articles from the same trial (44,46) lasted longer than 6 months, and as a result, it is difficult to infer our results regarding exercise dosage beyond a period of 24 wk in duration. Future trials involving longer exercise durations will be necessary to confirm these results.
In conclusion, the results indicate that there is no difference in effect when prescribing low- and high-volume or moderate- and high-intensity resistance exercise in untrained older men with prostate cancer on body composition, functional capacity, and muscle strength outcomes, at least in the short term. Considering the array of benefits observed in the present study, a low-resistance training weekly volume could represent a time-efficient approach during and after active treatment, resulting in higher adherence, attendance, and compliance while accruing similar health and function benefits to that of higher volume exercise. We suggest the examination of resistance training dose–response in future trials to determine whether a minimal dose approach could culminate in substantial cancer-related benefits.
Supplementary Material
Acknowledgments
Pedro Lopez is supported by the National Health and Medical Research Council (NHMRC) Centre of Research Excellence (CRE) in Prostate Cancer Survivorship Scholarship. Daniel A. Galvão and Robert U. Newton are funded by an NHMRC CRE in Prostate Cancer Survivorship. The results of the study are presented clearly, honestly, without fabrication, falsification, or inappropriate data manipulation and do not constitute endorsement by the American College of Sports Medicine.
The authors declare no conflict of interest. The sponsors had no involvement in the study design, analysis, or interpretation of data; manuscript writing; and decision to submit the manuscript for publication.
Substantial contributions to the conception and design of the work were done by Pedro Lopez; Dennis R. Taaffe, Robert U. Newton, and Daniel A. Galvão. The work draft and revision, as well as the approval of the final version, were done by Pedro Lopez; Dennis R. Taaffe, Robert U. Newton, and Daniel A. Galvão. In addition, all aspects of this work related to the accuracy or integrity were ensured by Pedro Lopez; Dennis R. Taaffe, Robert U. Newton, and Daniel A. Galvão.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.acsm-msse.org).
Contributor Information
DENNIS R. TAAFFE, Email: d.taaffe@ecu.edu.au.
ROBERT U. NEWTON, Email: r.newton@ecu.edu.au.
DANIEL A. GALVÃO, Email: d.galvao@ecu.edu.au.
REFERENCES
- 1.Schmitz KH Courneya KS Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409–26. [DOI] [PubMed] [Google Scholar]
- 2.Schmitz KH Campbell AM Stuiver MM, et al. Exercise is medicine in oncology: engaging clinicians to help patients move through cancer. CA Cancer J Clin. 2019;69(6):468–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh F Newton RU Baker MK, et al. Feasibility of presurgical exercise in men with prostate cancer undergoing prostatectomy. Integr Cancer Ther. 2017;16(3):290–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galvão DA, Newton RU, Taaffe DR, Spry N. Can exercise ameliorate the increased risk of cardiovascular disease and diabetes associated with ADT? Nat Clin Pract Urol. 2008;5(6):306–7. [DOI] [PubMed] [Google Scholar]
- 5.Galvão DA, Taaffe DR, Spry N, Joseph D, Newton RU. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: a randomized controlled trial. J Clin Oncol. 2010;28(2):340–7. [DOI] [PubMed] [Google Scholar]
- 6.Galvão DA Spry N Denham J, et al. A multicentre year-long randomised controlled trial of exercise training targeting physical functioning in men with prostate cancer previously treated with androgen suppression and radiation from TROG 03.04 RADAR. Eur Urol. 2014;65(5):856–64. [DOI] [PubMed] [Google Scholar]
- 7.Newton RU Galvão DA Spry N, et al. Exercise mode specificity for preserving spine and hip bone mineral density in prostate cancer patients. Med Sci Sports Exerc. 2019;51(4):607–14. [DOI] [PubMed] [Google Scholar]
- 8.Segal RJ Reid RD Courneya KS, et al. Randomized controlled trial of resistance or aerobic exercise in men receiving radiation therapy for prostate cancer. J Clin Oncol. 2009;27(3):344–51. [DOI] [PubMed] [Google Scholar]
- 9.Taaffe DR Newton RU Spry N, et al. Effects of different exercise modalities on fatigue in prostate cancer patients undergoing androgen deprivation therapy: a year-long randomised controlled trial. Eur Urol. 2017;72(2):293–9. [DOI] [PubMed] [Google Scholar]
- 10.Taaffe DR Buffart LM Newton RU, et al. Time on androgen deprivation therapy and adaptations to exercise: secondary analysis from a 12-month randomized controlled trial in men with prostate cancer. BJU Int. 2018;121(2):194–202. [DOI] [PubMed] [Google Scholar]
- 11.Taaffe DR Galvão DA Spry N, et al. Immediate versus delayed exercise in men initiating androgen deprivation: effects on bone density and soft tissue composition. BJU Int. 2019;123(2):261–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galvão DA Taaffe DR Spry N, et al. Exercise preserves physical function in prostate cancer patients with bone metastases. Med Sci Sports Exerc. 2018;50(3):393–399.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galvão DA Taaffe DR Spry N, et al. Enhancing active surveillance of prostate cancer: the potential of exercise medicine. Nat Rev Urol. 2016;13(5):258–65. [DOI] [PubMed] [Google Scholar]
- 14.Galvão DA Hayne D Frydenberg M, et al. Can exercise delay transition to active therapy in men with low-grade prostate cancer? A multicentre randomised controlled trial. BMJ Open. 2018;8(4):e022331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang DW, Fairey AS, Boulé NG, Field CJ, Courneya KS. Exercise during active surveillance for prostate cancer—the ERASE trial: a study protocol of a phase II randomised controlled trial. BMJ Open. 2019;9(7):e026438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hart NH Newton RU Spry NA, et al. Can exercise suppress tumour growth in advanced prostate cancer patients with sclerotic bone metastases? A randomised, controlled study protocol examining feasibility, safety and efficacy. BMJ Open. 2017;7(5):e014458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newton RU Kenfield SA Hart NH, et al. Intense exercise for survival among men with metastatic castrate-resistant prostate cancer (INTERVAL-GAP4): a multicentre, randomised, controlled phase III study protocol. BMJ Open. 2018;8(5):e022899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Lancet Oncology Exercise and cancer treatment: balancing patient needs. Lancet Oncol. 2018;19(6):715. [DOI] [PubMed] [Google Scholar]
- 19.Campbell KL Winters-Stone KM Wiskemann J, et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc. 2019;51(11):2375–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao Y, Ma J. Body mass index, prostate cancer-specific mortality, and biochemical recurrence: a systematic review and meta-analysis. Cancer Prev Res (Phila). 2011;4(4):486–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newman AB Simonsick EM Naydeck BL, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295(17):2018–26. [DOI] [PubMed] [Google Scholar]
- 22.De Buyser SL, Petrovic M, Taes YE, Toye KR, Kaufman JM, Goemaere S. Physical function measurements predict mortality in ambulatory older men. Eur J Clin Invest. 2013;43(4):379–86. [DOI] [PubMed] [Google Scholar]
- 23.Stessman J, Rottenberg Y, Jacobs JM. Climbing stairs, handrail use, and survival. J Nutr Health Aging. 2017;21(2):195–201. [DOI] [PubMed] [Google Scholar]
- 24.Liberati A Altman DG Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Page MJ McKenzie JE Bossuyt PM, et al. Mapping of reporting guidance for systematic reviews and meta-analyses generated a comprehensive item bank for future reporting guidelines. J Clin Epidemiol. 2020;118:60–8. [DOI] [PubMed] [Google Scholar]
- 26.Furlan AD Pennick V Bombardier C van Tulder M, Editorial Board, Cochrane Back Review Group . 2009 updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine (Phila Pa 1976). 2009;34(18):1929–41. [DOI] [PubMed] [Google Scholar]
- 27.Bourke L Smith D Steed L, et al. Exercise for men with prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016;69(4):693–703. [DOI] [PubMed] [Google Scholar]
- 28.Chen Z, Zhang Y, Lu C, Zeng H, Schumann M, Cheng S. Supervised physical training enhances muscle strength but not muscle mass in prostate cancer patients undergoing androgen deprivation therapy: a systematic review and meta-analysis. Front Physiol. 2019;10:843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keilani M Hasenoehrl T Baumann L, et al. Effects of resistance exercise in prostate cancer patients: a meta-analysis. Support Care Cancer. 2017;25(9):2953–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang B, Wang J. Effects of exercise on cancer-related fatigue and quality of life in prostate cancer patients undergoing androgen deprivation therapy: a meta-analysis of randomized clinical trials. Chin Med Sci J. 2017;32(1):13–21. [DOI] [PubMed] [Google Scholar]
- 31.Ying M, Zhao R, Jiang D, Gu S, Li M. Lifestyle interventions to alleviate side effects on prostate cancer patients receiving androgen deprivation therapy: a meta-analysis. Jpn J Clin Oncol. 2018;48(9):827–34. [DOI] [PubMed] [Google Scholar]
- 32.Yunfeng G, Weiyang H, Xueyang H, Yilong H, Xin G. Exercise overcome adverse effects among prostate cancer patients receiving androgen deprivation therapy: an update meta-analysis. Medicine (Baltimore). 2017;96(27):e7368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drevon D, Fursa SR, Malcolm AL. Intercoder reliability and validity of WebPlotDigitizer in extracting graphed data. Behav Modif. 2017;41(2):323–39. [DOI] [PubMed] [Google Scholar]
- 34.Sterne JAC Savović J Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 35.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. [DOI] [PubMed] [Google Scholar]
- 36.Higgins JPT Thomas J Chandler J, et al. Cochrane handbook for systematic reviews of interventions version 6.0 (updated July 2019). Cochrane. 2019. [Google Scholar]
- 37.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol. 2008;61(10):991–6. [DOI] [PubMed] [Google Scholar]
- 39.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–63. [DOI] [PubMed] [Google Scholar]
- 41.Bourke L, Doll H, Crank H, Daley A, Rosario D, Saxton JM. Lifestyle intervention in men with advanced prostate cancer receiving androgen suppression therapy: a feasibility study. Cancer Epidemiol Biomarkers Prev. 2011;20(4):647–57. [DOI] [PubMed] [Google Scholar]
- 42.Cormie P, Newton RU, Spry N, Joseph D, Taaffe DR, Galvão DA. Safety and efficacy of resistance exercise in prostate cancer patients with bone metastases. Prostate Cancer Prostatic Dis. 2013;16(4):328–35. [DOI] [PubMed] [Google Scholar]
- 43.Bourke L Gilbert S Hooper R, et al. Lifestyle changes for improving disease-specific quality of life in sedentary men on long-term androgen-deprivation therapy for advanced prostate cancer: a randomised controlled trial. Eur Urol. 2014;65(5):865–72. [DOI] [PubMed] [Google Scholar]
- 44.Winters-Stone KM, Dieckmann N, Maddalozzo GF, Bennett JA, Ryan CW, Beer TM. Resistance exercise reduces body fat and insulin during androgen-deprivation therapy for prostate cancer. Oncol Nurs Forum. 2015;42(4):348–56. [DOI] [PubMed] [Google Scholar]
- 45.Cormie P Galvão DA Spry N, et al. Can supervised exercise prevent treatment toxicity in patients with prostate cancer initiating androgen-deprivation therapy: a randomised controlled trial. BJU Int. 2015;115(2):256–66. [DOI] [PubMed] [Google Scholar]
- 46.Winters-Stone KM Dobek JC Bennett JA, et al. Resistance training reduces disability in prostate cancer survivors on androgen deprivation therapy: evidence from a randomized controlled trial. Arch Phys Med Rehabil. 2015;96(1):7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nilsen TS Raastad T Skovlund E, et al. Effects of strength training on body composition, physical functioning, and quality of life in prostate cancer patients during androgen deprivation therapy. Acta Oncol. 2015;54(10):1805–13. [DOI] [PubMed] [Google Scholar]
- 48.Gilbert SE Tew GA Fairhurst C, et al. Effects of a lifestyle intervention on endothelial function in men on long-term androgen deprivation therapy for prostate cancer. Br J Cancer. 2016;114(4):401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Winters-Stone KM Lyons KS Dobek J, et al. Benefits of partnered strength training for prostate cancer survivors and spouses: results from a randomized controlled trial of the exercising together project. J Cancer Surviv. 2016;10(4):633–44. [DOI] [PubMed] [Google Scholar]
- 50.Gaskin CJ, Fraser SF, Owen PJ, Craike M, Orellana L, Livingston PM. Fitness outcomes from a randomised controlled trial of exercise training for men with prostate cancer: the ENGAGE study. J Cancer Surviv. 2016;10(6):972–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hojan K, Kwiatkowska-Borowczyk E, Leporowska E, Milecki P. Inflammation, cardiometabolic markers, and functional changes in men with prostate cancer: a randomized controlled trial of a 12-month exercise program. Pol Arch Intern Med. 2017;127(1):25–35. [DOI] [PubMed] [Google Scholar]
- 52.Wall BA Galvão DA Fatehee N, et al. Exercise improves VO2max and body composition in androgen deprivation therapy-treated prostate cancer patients. Med Sci Sports Exerc. 2017;49(8):1503–10. [DOI] [PubMed] [Google Scholar]
- 53.Dawson JK, Dorff TB, Todd Schroeder E, Lane CJ, Gross ME, Dieli-Conwright CM. Impact of resistance training on body composition and metabolic syndrome variables during androgen deprivation therapy for prostate cancer: a pilot randomized controlled trial. BMC Cancer. 2018;18(1):368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alibhai SMH Santa Mina D Ritvo P, et al. A phase II randomized controlled trial of three exercise delivery methods in men with prostate cancer on androgen deprivation therapy. BMC Cancer. 2019;19(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ndjavera W Orange ST O’Doherty AF, et al. Exercise-induced attenuation of treatment side-effects in patients with newly diagnosed prostate cancer beginning androgen-deprivation therapy: a randomised controlled trial. BJU Int. 2020;125(1):28–37. [DOI] [PubMed] [Google Scholar]
- 56.Cespedes Feliciano EM Kroenke CH Bradshaw PT, et al. Postdiagnosis weight change and survival following a diagnosis of early-stage breast cancer. Cancer Epidemiol Biomarkers Prev. 2017;26(1):44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tanaka M Okada H Hashimoto Y, et al. Relationship between metabolic syndrome and trunk muscle quality as well as quantity evaluated by computed tomography. Clin Nutr. 2019;39(6):1818–25. [DOI] [PubMed] [Google Scholar]
- 58.Stamatakis E Lee IM Bennie J, et al. Does strength-promoting exercise confer unique health benefits? A pooled analysis of data on 11 population cohorts with all-cause, cancer, and cardiovascular mortality endpoints. Am J Epidemiol. 2018;187(5):1102–12. [DOI] [PubMed] [Google Scholar]
- 59.Beard JR, Officer AM, Cassels AK. The world report on ageing and health. Gerontologist. 2016;56(2 Suppl):S163–6. [DOI] [PubMed] [Google Scholar]
- 60.Guralnik JM Simonsick EM Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–94. [DOI] [PubMed] [Google Scholar]
- 61.Simonsick EM Lafferty ME Phillips CL, et al. Risk due to inactivity in physically capable older adults. Am J Public Health. 1993;83(10):1443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spirduso WW, Cronin DL. Exercise dose–response effects on quality of life and independent living in older adults. Med Sci Sports Exerc. 2001;33(6 Suppl):S598–608. [DOI] [PubMed] [Google Scholar]
- 63.Jensen MT, Holtermann A, Bay H, Gyntelberg F. Cardiorespiratory fitness and death from cancer: a 42-year follow-up from the Copenhagen male study. Br J Sports Med. 2017;51(18):1364–9. [DOI] [PubMed] [Google Scholar]
- 64.Kim Y White T Wijndaele K, et al. The combination of cardiorespiratory fitness and muscle strength, and mortality risk. Eur J Epidemiol. 2018;33(10):953–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Versteeg KS Blauwhoff-Buskermolen S Buffart LM, et al. Higher muscle strength is associated with prolonged survival in older patients with advanced cancer. Oncologist. 2018;23(5):580–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352(2):154–64. [DOI] [PubMed] [Google Scholar]
- 67.Ward PR, Wong MD, Moore R, Naeim A. Fall-related injuries in elderly cancer patients treated with neurotoxic chemotherapy: a retrospective cohort study. J Geriatr Oncol. 2014;5(1):57–64. [DOI] [PubMed] [Google Scholar]
- 68.Hayes SC, Newton RU, Spence RR, Galvão DA. The Exercise and Sports Science Australia Position Statement: exercise medicine in cancer management. J Sci Med Sport. 2019;22(11):1175–99. [DOI] [PubMed] [Google Scholar]
- 69.Cunha PM Nunes JP Tomeleri CM, et al. Resistance training performed with single and multiple sets induces similar improvements in muscular strength, muscle mass, muscle quality, and IGF-1 in older women: a randomized controlled trial. J Strength Cond Res. 2020;34(4):1008–16. [DOI] [PubMed] [Google Scholar]
- 70.Galvão DA, Taaffe DR. Resistance exercise dosage in older adults: single- versus multiset effects on physical performance and body composition. J Am Geriatr Soc. 2005;53(12):2090–7. [DOI] [PubMed] [Google Scholar]
- 71.Radaelli R Botton CE Wilhelm EN, et al. Time course of low- and high-volume strength training on neuromuscular adaptations and muscle quality in older women. Age (Dordr). 2014;36(2):881–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fairman CM Nilsen TS Newton RU, et al. Reporting of resistance training dose, adherence, and tolerance in exercise oncology. Med Sci Sports Exerc. 2020;52(2):315–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


