Supplemental digital content is available in the text.
Key Words: EXERCISE, BREAST CANCER, FATIGUE, CHEMOTHERAPY, INFLAMMATION, MECHANISMS
ABSTRACT
Purpose
The randomized controlled OptiTrain trial showed beneficial effects on fatigue after a 16-wk exercise intervention in patients with breast cancer undergoing adjuvant chemotherapy. We hypothesize that exercise alters systemic inflammation and that this partially mediates the beneficial effects of exercise on fatigue.
Methods
Two hundred and forty women scheduled for chemotherapy were randomized to 16 wk of resistance and high-intensity interval training (RT-HIIT), moderate-intensity aerobic and high-intensity interval training (AT-HIIT), or usual care (UC). In the current mechanistic analyses, we included all participants with >60% attendance and a random selection of controls (RT-HIIT = 30, AT-HIIT = 27, UC = 29). Fatigue (Piper Fatigue Scale) and 92 markers (e.g., interleukin-6 [IL-6] and tumor necrosis factor α [TNF-α]) were assessed at baseline and postintervention. Mediation analyses were conducted to explore whether changes in inflammation markers mediated the effect of exercise on fatigue.
Results
Overall, chemotherapy led to an increase in inflammation. The increases in IL-6 (pleiotropic cytokine) and CD8a (T-cell surface glycoprotein) were however significantly less pronounced after RT-HIIT compared with UC (−0.47, 95% confidence interval = −0.87 to −0.07, and −0.28, 95% confidence interval = −0.57 to 0.004, respectively). Changes in IL-6 and CD8a significantly mediated the exercise effects on both general and physical fatigue by 32.0% and 27.7%, and 31.2% and 26.4%, respectively. No significant between-group differences in inflammatory markers at 16 wk were found between AT-HIIT and UC.
Conclusions
This study is the first showing that supervised RT-HIIT partially counteracted the increase in inflammation during chemotherapy, i.e., IL-6 and soluble CD8a, which resulted in lower fatigue levels postintervention. Exercise, including both resistance and high-intensity aerobic training, might be put forward as an effective treatment to reduce chemotherapy-induced inflammation and subsequent fatigue.
Fatigue is one of the most common and debilitating side effects of cancer and its treatment (1,2), with some studies reporting a prevalence as high as 70%–99% (3). It typically increases during cancer treatment and can be experienced for up to 10 yr after a cancer diagnosis (4–6). Fatigue substantially interferes with daily life activities, and as a consequence, it impairs the overall quality of life (QoL) during and after cancer treatment (7,8).
The etiology of fatigue has not been fully elucidated, and it may involve a variety of demographic, clinical, psychosocial, behavioral, and biological factors (2). Proposed underlying mechanisms include mitochondrial dysfunction, hypothalamic–pituitary–adrenal axis dysfunction, anemia, circadian rhythm disruption, disturbance of monoamine pathways, and chronic inflammation (9). To date, the underlying mechanism for fatigue that has gained most empirical attention and support is chronic inflammation.
Reviews show that elevated neutrophil and monocyte counts and higher levels of several inflammatory markers, including interleukin-6 (IL-6), IL-1β, IL-1ra, neopterin, and C-reactive protein, are associated with fatigue in cancer survivors (2,9,10). In patients with breast cancer undergoing chemotherapy specifically, changes in IL-6 were positively correlated with changes in fatigue (11,12).
Growing evidence suggests that exercise is an effective intervention to reduce levels of fatigue in patients receiving adjuvant chemotherapy (13,14). It is hypothesized that this might be partially due to its anti-inflammatory effects. Three reviews showed that regular exercise after completion of primary breast cancer treatment reduced the serum concentration of proinflammatory cytokines, including IL-2, IL-6, IL-8, and tumor necrosis factor α (TNF-α) (15), and elevated levels of anti-inflammatory cytokines, including IL-10 (9) and IL-1ra (16). Another study, including patients with breast, lung, and colon cancer, found positive correlations between changes in cytokine concentrations (i.e., IL-10:IL-6, IL-10:IL-1β, and IL-10:sTNFR1) after a 6-wk exercise intervention during adjuvant chemotherapy (17). These positive correlations were significantly greater than the correlations observed in the control group, supporting the role of exercise in moderating therapy-induced inflammation.
The randomized controlled OptiTrain trial showed beneficial effects on fatigue after a 16-wk exercise intervention during adjuvant chemotherapy in patients with breast cancer (18). Here, we investigated the effects of exercise on inflammatory markers and whether the positive effects on fatigue were mediated by changes in inflammation. In addition, we examined whether changes in inflammatory markers were correlated with changes in physiological outcomes. Finally, we aimed to identify groups of cytokines whose expression levels are correlated. We hypothesize that exercise alters systemic inflammation and that this partially mediates the beneficial effects of exercise on fatigue.
METHODS
Setting and participants
The OptiTrain study is a randomized controlled exercise trial in women with breast cancer undergoing adjuvant chemotherapy (ClinicalTrials.gov, NCT02522260). A detailed description of the OptiTrain study design (19) and the effects of the exercise intervention on fatigue, QoL, pain, and physical fitness (18,20) have been published previously. Patients were not involved in the design, conduct, and interpretation of this study. Patients were involved in dissemination of the results.
The study was conducted at Karolinska University Hospital (Stockholm, Sweden) between March 2013 and July 2016. In short, 240 women with breast cancer were randomized to 16 wk of resistance and high-intensity interval training (RT-HIIT, n = 79), moderate-intensity aerobic and high-intensity interval training (AT-HIIT, n = 80), or to usual care (UC, n = 81). The OptiTrain study included women (18–70 yr old) diagnosed with stage I–stage IIIa breast cancer, scheduled for adjuvant chemotherapy. Exclusion criteria were advanced disease, heart or lung disease, cognitive dysfunction, other health problems that would prevent safe participation in the exercise testing or training as determined by their medical doctor, or not being able to understand the Swedish language. Ethical approval was obtained from the Regional Ethical Review Board in Stockholm Sweden (Dnr 2012/1347-31/1, 2012/1347-31/2, 2013/7632-32, and 2014/408-32), and all participants gave written informed consent before enrollment.
Intervention
Participants in the exercise intervention groups commenced the exercise training 3 d after the second chemotherapy session. Patients were asked to attend 60-min exercise sessions, twice weekly, on nonconsecutive weekdays for 16 wk. Exercise sessions were supervised by an exercise physiologist or oncology nurse at the exercise clinic of Karolinska University Hospital. The exercise sessions of the AT-HIIT group consisted of 20 min of moderate-intensity aerobic exercise at an RPE of 13–15, followed by 3 × 3-min bouts of high-intensity intermittent aerobic exercise at an RPE of 16–18 interspersed with 1 min low-intensity active recovery. The exercise sessions of the RT-HIIT group consisted of eight resistance exercises followed by the 3 × 3-min bouts of high-intensity intermittent aerobic exercise. Participants performed 2–3 sets of 8–12 repetitions at an initial intensity of 70% of their estimated one-repetition maximum, progressing to 80% of one-repetition maximum when more than 12 repetitions could be performed. The UC group received information about physical activity but no supervised exercise training.
Outcome assessment
At baseline (i.e., before randomization) and postintervention, participants visited Karolinska University Hospital for outcome assessment. The assessors of blood samples, but not the study investigators, were blinded to group allocation. Blood samples were drawn at both visits, in addition to performing physical measurements and completing questionnaires. At baseline, blood samples were drawn from the patients’ PICC line in conjunction with receiving their second chemotherapy session (before chemotherapy infusion). The postintervention blood samples were drawn 3 wk after the sixth (last) round of chemotherapy and 48–72 h after an exercise session. Patients were asked to not eat or drink 3 h before the blood draw. Samples were stored locally at −80°C until analysis took place.
Inflammatory markers
Plasma samples were analyzed using an immuno-oncology multiplex proximity extension assay (Olink Bioscience, Uppsala, Sweden) at the Clinical Biomarkers Facility, Science for Life Laboratory in Uppsala, Sweden. This panel measures inflammatory markers that are relevant for key processes, such as apoptosis and chemotaxis. The quantification cycles were produced by the BioMark’s real-time PCR software. The quantification cycle values from an internal control (extension control) were subtracted from the measured quantification cycle value, an interplate control was corrected for, and a correction factor was subtracted to yield a normalized, log 2-transformed protein expression value. The value is a relative quantification, meaning that no comparison of absolute levels between different proteins can be made. Ninety-two inflammatory markers were assessed. Data were censored if values were below the detection limit. If the percentage of censored values was below 25%, all values below the detection limit were substituted by the detection limit divided by the square root of 2 (21). Because some of the more relevant markers, specifically interferon γ (IFN-γ), TNF-α, IL-4, and IL-1b, showed poor detection on the Olink platform, we tested these markers on the Merck Cytomag custom made platform. Fifteen inflammatory markers had more than 25% censored values and were therefore excluded from the analyses (i.e., C-C motif chemokine 17 [CCL17], IL-1a, ADGRG1, FGF-2, IFN-β, NOS 3, IL-2, IL-5, IL-13, IL-21, IL-33, IL-35, CD28, SDF-1, and IL12RB1).
Cancer-related fatigue
Cancer-related fatigue was self-assessed using the validated Swedish version of the Piper Fatigue Scale (22). The Piper Fatigue Scale is a 22-item questionnaire and covers four dimensions of fatigue: behavioral/daily life, sensory/physical, cognitive, and affective/emotional meaning. Each item is composed of a scale from 0 to 10, with higher scores indicating higher levels of fatigue.
Physical (activity) measurements
Muscle strength was assessed using a hydraulic hand dynamometer and lower-limb muscle strength by using an isometric midthigh pull. Cardiovascular fitness, measured as predicted peak oxygen uptake (V˙O2peak), was assessed by a submaximal exercise test on a cycle ergometer. Objectively measured physical activity was assessed at baseline by an accelerometer (GT3X; ActiGraph® Corp, Pensacola, FL).
Statistical analysis
For these secondary and mechanistic analyses, we only included all participants who attended ≥60% of all exercise sessions because adherence is defined as successful if participants completed at least two-thirds of an exercise program (23). A random sample of controls was drawn. We selected all patients allocated to UC with available samples for both time points (i.e., baseline and postintervention) and used the random sampling function in Excel. Descriptive statistics were used to summarize the baseline characteristics of the study population. To assess whether the effects of exercise on fatigue were mediated by changes in inflammation, we estimated a series of linear regression equations according to Valente and MacKinnon (see Figure, Supplemental Digital Content 1, Schematic representation of the mediation model, http://links.lww.com/MSS/C101) (24). First, an ANCOVA was conducted to determine between-group differences in inflammatory markers, with postintervention values of inflammatory markers as dependent variables (M2), the randomization group as independent variable (X), and the baseline values of the inflammatory marker (M1) and outcome fatigue (Y1) as covariate. If the P value of the effect of either RT-HIIT or AT-HIIT on the inflammatory marker was below 0.20, the second regression equation was used to assess mediation. Second, an ANCOVA was conducted to determine between-group differences in the outcome fatigue, with postintervention values of fatigue as a dependent variable (Y2), the randomization group as an independent variable (X), and the baseline values of the inflammatory marker (M1) and outcome fatigue (Y1), and postintervention levels of the inflammatory marker (M2) as covariates. All models were adjusted for menopausal status and chemotherapy regimen (taxanes or nontaxane based). We did not adjust for multiple comparisons because of the exploratory nature of the current study.
The mediated effect of the intervention (X) on total and physical fatigue (Y2) through inflammation marker M2 was calculated. Because of the nonnormality of the mediated effect, resampling methods were used to construct confidence intervals around the mediated effect. The mediation effect was calculated by dividing the mediated effect by the total effect of exercise on fatigue.
The Pearson correlation coefficient was calculated to evaluate the linear relationship between physiological outcomes and inflammatory markers, and between changes in inflammatory markers. For the latter analyses, one-way hierarchical clustering was conducted using Pearson’s correlation coefficient as a proximity distance matrix to identify groups of cytokines whose expression levels are correlated. A heat map was created for all study arms using the “heat map” function in R or using GraphPad Prism. All analyses were performed using R version 3.5.1 or GraphPad Prism version 8.
RESULTS
Participants
Overall, 30 of the patients allocated to RT-HIIT attended ≥60% of all exercise sessions, whereas 27 of the patients allocated to AT-HIIT attended ≥60% of all exercise sessions (Fig. 1). On average, the RT-HIIT group attended 79.5% (SD = 20.3) of all exercise sessions, whereas the AT group attended 82.1% (SD = 17.4) of all exercise sessions.
FIGURE 1.
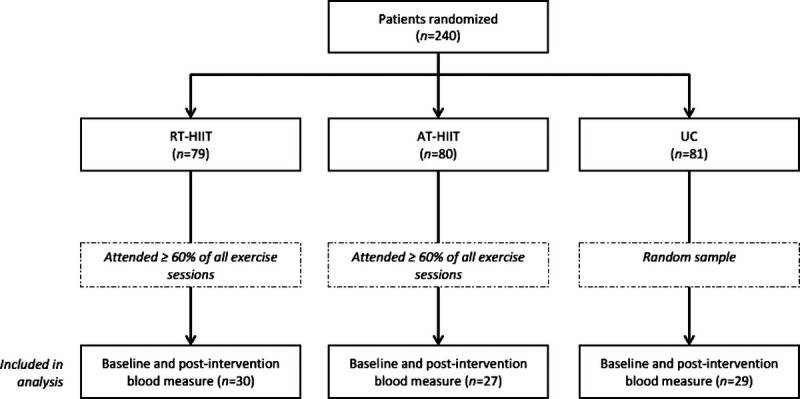
Flowchart of patients participating in the OptiTrain study and included in the current analyses.
Baseline characteristics for the patients included in the current analyses were comparable with the baseline characteristics of the patients included in the original OptiTrain study (18), except for physical activity levels. Women in the RT-HIIT group were significantly more active per day at a moderate to vigorous intensity compared with women in the UC group. Women were primarily middle-age and had on average a healthy body mass index (BMI) (Table 1). The majority of women were postmenopausal (58%) and treated with anthracyclines alone (38%) or in combination with taxanes (57%).
TABLE 1.
Participant characteristics at baseline.
| RT-HIIT, n = 30 | AT-HIIT, n = 27 | UC, n = 29 | |
|---|---|---|---|
| Age (yr) | 52.2 ± 10.1 | 53.9 ± 7.4 | 52.9 ± 10.1 |
| BMI (kg·m−2) | 24.2 ± 3.6 | 24.2 ± 3.4 | 24.7 ± 4.4 |
| Menopausal status | |||
| Premenopausal | 16 (53.3) | 10 (37.0) | 10 (34.5) |
| Postmenopausal | 14 (46.7) | 17 (63.0) | 19 (65.5) |
| Comorbidities | 6 (20.0) | 4 (14.8) | 10 (34.5) |
| Current smoker | 1 (3.3) | 0 (0.0) | 1 (3.4) |
| Tumor profile | |||
| Triple negative | 1 (3.3) | 5 (18.5) | 6 (20.7) |
| HER2+, ER+, PR+ | 6 (20.0) | 5 (18.5) | 1 (3.4) |
| HER2+, ER−, PR− | 0 (0.0) | 2 (7.4) | 4 (13.8) |
| HER2−, ER+, PR+ | 17 (56.7) | 10 (37.0) | 14 (48.3) |
| HER2−, ER+, PR− | 3 (10.0) | 4 (14.8) | 2 (6.9) |
| HER2+, ER+, PR− | 3 (10.0) | 1 (3.7) | 1 (3.4) |
| HER2−, ER−, PR+ | 0 (0.0) | 0 (0.0) | 1 (3.4) |
| Chemotherapy regimen | |||
| Anthracycline | 12 (40.0) | 10 (37.0) | 11 (37.9) |
| Taxane | 2 (6.7) | 2 (7.4) | 0 (0.0) |
| Anthracycline + taxane | 10 (33.3) | 9 (33.3) | 11 (37.9) |
| Anthracycline + taxane + herceptin | 6 (20.0) | 6 (22.2) | 7 (24.1) |
| MVPA (min·d−1) | 92.6 ± 32.8 | 83.3 ± 28.6 | 68.0 ± 30.4 |
| SED (min·d−1) | 535.5 ± 104.4 | 555.0 ± 74.8 | 555.4 ± 93.1 |
Continuous variables are presented as mean ± SD, whereas dichotomous or categorical variables are presented as n (%).
MVPA, objectively measured moderate to vigorous physical activity; SED, objectively measured sedentary behavior.
Effects of exercise on inflammatory markers
Receiving adjuvant chemotherapy altered the plasma cytokine profile of patients with breast cancer (Fig. 2A). In general, chemotherapy led to an increase in proinflammatory cytokines in all groups (Fig. 2A and Table, Supplemental Digital Content 2, Exercise effects on 92 different cytokines, http://links.lww.com/MSS/C102), whereas other factors such as pro–epidermal growth factor (EGF) were reduced over the course of therapy. The increases in IL-6 and T-cell surface glycoprotein CD8 alpha chain (CD8a) were significantly less pronounced after RT-HIIT compared with UC (−0.47, 95% CI = −0.87 to −0.07, and −0.28, 95% CI = −0.57 to 0.004, respectively) (Fig. 2B and Table 2). No significant differences in single inflammatory markers were found between AT-HIIT and UC at 16 wk.
FIGURE 2.
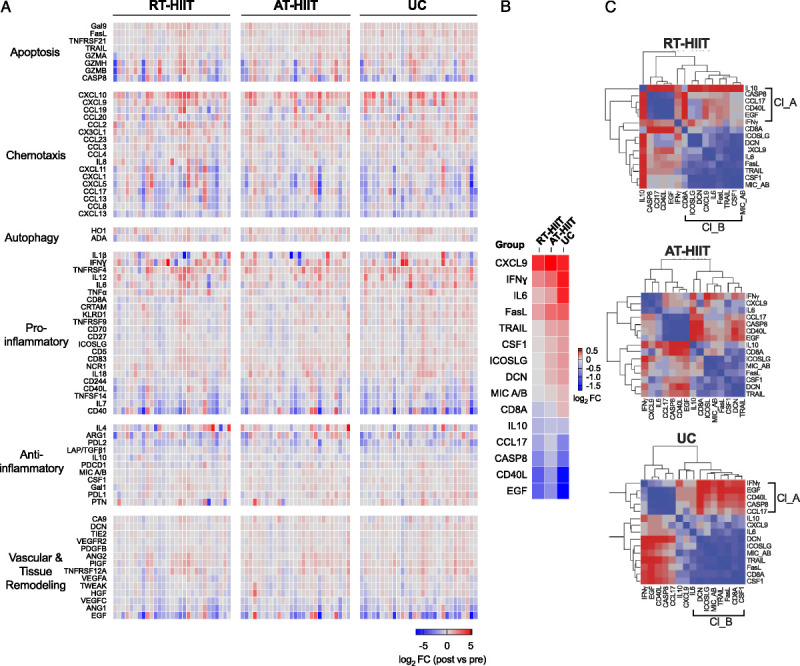
A, Heat map of changes in inflammatory marker expression after chemotherapy in exercising (RT-HIIT and AT-HIIT) and UC groups. Colors indicate log2 fold changes in post- vs prechemotherapy expression values (red—increased, blue—decreased, white—unchanged). B, Heat map of significantly altered inflammatory marker expression after chemotherapy in exercising (RT-HIIT and AT-HIIT) and UC groups. Colors indicate log2 fold changes in post- vs prechemotherapy expression values (red—increased, blue—decreased, white—unchanged). Comparisons were made using ANCOVA, and P values <0.2 were deemed significant. C, Hierarchical cluster analysis heat maps showing nearest-neighbor correlations of inflammatory markers in the RT-HIIT, AT-HIIT, and UC groups. Positive correlations are represented in graded shades of blue, whereas negative correlations are represented in graded shades of red. Clusters A and B positively correlated within the RT-HIIT and UC groups. All correlations were weaker in the AT-HIIT group.
TABLE 2.
Exercise effects on inflammatory markers.
| Outcome | Group | Baseline (Mean ± SD) | Postintervention (Mean ± SD) | Baseline to Postintervention | |
|---|---|---|---|---|---|
| Within-Group Difference, Mean (95% CI) | Between-Group Difference, Mean (95% CI) | ||||
| CD40-L | RT-HIIT | 5.86 ± 1.87 | 4.90 ± 1.55 | −1.06 (−1.79 to −0.32)* | 0.19 (−0.60 to 0.99) |
| AT-HIIT | 6.07 ± 1.60 | 5.52 ± 1.18 | −0.48 (−1.30 to 0.35) | 0.74 (−0.05 to 1.53) | |
| UC | 6.52 ± 1.33 | 4.82 ± 1.58 | −1.73 (−2.47 to −0.99)* | Reference | |
| EGF | RT-HIIT | 7.60 ± 1.54 | 6.65 ± 1.35 | −1.06 (−1.76 to −0.36)* | 0.29 (−0.49 to 1.07) |
| AT-HIIT | 7.76 ± 1.50 | 7.14 ± 1.34 | −0.52 (−1.38 to 0.34) | 0.75 (−0.03 to 1.52) | |
| UC | 8.16 ± 1.46 | 6.39 ± 1.53 | −1.77 (−2.55 to −1.00)* | Reference | |
| IL-6 | RT-HIIT | 2.51 ± 0.83 | 2.54 ± 0.74 | −0.01 (−0.39 to 0.37) | −0.47 (−0.87 to −0.07)* |
| AT-HIIT | 2.85 ± 0.96 | 3.03 ± 0.75 | 0.21 (−0.09 to 0.51) | −0.15 (−0.55 to 0.25) | |
| UC | 2.59 ± 1.01 | 3.11 ± 1.01 | 0.49 (0.12 to 0.87)* | Reference | |
| TRAIL | RT-HIIT | 6.98 ± 0.29 | 7.02 ± 0.39 | −0.004 (−0.19 to 0.18) | −0.17 (−0.38 to 0.03) |
| AT-HIIT | 7.18 ± 0.55 | 7.33 ± 0.49 | 0.14 (−0.03 to 0.30) | 0.05 (−0.16 to 0.26) | |
| UC | 7.02 ± 0.51 | 7.21 ± 0.42 | 0.18 (0.03 to 0.33)* | Reference | |
| CD8a | RT-HIIT | 8.19 ± 0.86 | 8.00 ± 0.72 | −0.24 (−0.44 to −0.03)* | −0.28 (−0.57 to 0.004) |
| AT-HIIT | 8.07 ± 0.70 | 7.96 ± 0.74 | −0.12 (−0.29 to 0.06) | −0.20 (−0.48 to 0.09) | |
| UC | 7.99 ± 0.72 | 8.09 ± 0.80 | 0.10 (−0.10 to 0.30) | Reference | |
| DCN | RT-HIIT | 3.55 ± 0.33 | 3.56 ± 0.39 | −0.02 (−0.12 to 0.09) | −0.14 (−0.31 to 0.03) |
| AT-HIIT | 3.64 ± 0.50 | 3.75 ± 0.52 | 0.08 (−0.03 to 0.20) | 0.005 (−0.16 to 0.17) | |
| UC | 3.44 ± 0.54 | 3.62 ± 0.42 | 0.17 (0.03 to 0.30)* | Reference | |
| CCL17 | RT-HIIT | 7.23 ± 1.28 | 6.85 ± 1.23 | −0.45 (−0.94 to 0.03) | 0.15 (−0.44 to 0.74) |
| AT-HIIT | 7.28 ± 0.96 | 7.13 ± 1.08 | −0.12 (−0.62 to 0.38) | 0.40 (−0.19 to 0.99) | |
| UC | 7.56 ± 1.07 | 6.84 ± 1.11 | −0.73 (−1.22 to −0.24)* | Reference | |
| CASP-8 | RT-HIIT | 3.79 ± 1.25 | 3.18 ± 0.91 | −0.75 (−1.34 to −0.16)* | 0.20 (−0.25 to 0.66) |
| AT-HIIT | 3.70 ± 1.13 | 3.43 ± 0.74 | −0.27 (−0.89 to 0.35) | 0.40 (−0.05 to 0.86) | |
| UC | 3.95 ± 1.01 | 3.03 ± 0.86 | −0.96 (−1.39 to −0.53)* | Reference | |
| ICOSLG | RT-HIIT | 3.77 ± 0.42 | 3.79 ± 0.48 | −0.02 (−0.14 to 0.11) | −0.14 (−0.32 to 0.04) |
| AT-HIIT | 3.73 ± 0.55 | 3.84 ± 0.46 | 0.08 (−0.06 to 0.23) | −0.05 (−0.23 to 0.13) | |
| UC | 3.65 ± 0.49 | 3.84 ± 0.43 | 0.18 (0.05 to 0.31)* | Reference | |
| CSF-1 | RT-HIIT | 6.73 ± 0.36 | 6.77 ± 0.41 | −0.01 (−0.16 to 0.13) | −0.13 (−0.30 to 0.03) |
| AT-HIIT | 6.83 ± 0.51 | 6.92 ± 0.45 | 0.08 (−0.04 to 0.20) | −0.03 (−0.19 to 0.13) | |
| UC | 6.68 ± 0.49 | 6.86 ± 0.45 | 0.17 (0.08 to 0.27)* | Reference | |
| IFN-γa | RT-HIIT | 0.88 ± 1.59 | 1.01 ± 1.60 | 0.09 (−0.65 to 0.82) | −0.51 (−1.20 to 0.17) |
| AT-HIIT | 1.20 ± 0.98 | 1.46 ± 0.95 | 0.28 (−0.14 to 0.69) | −0.14 (−0.82 to 0.54) | |
| UC | 0.88 ± 1.65 | 1.49 ± 1.25 | 0.57 (−0.05 to 1.19) | Reference | |
| IL-10a | RT-HIIT | 0.92 ± 0.27 | 0.81 ± 0.26 | −0.08 (−0.28 to 0.13) | 0.13 (−0.02 to 0.29) |
| AT-HIIT | 0.95 ± 0.23 | 0.70 ± 0.18 | −0.29 (−0.40 to −0.19)* | −0.0004 (−0.14 to 0.14) | |
| UC | 0.92 ± 0.26 | 0.71 ± 0.22 | −0.27 (−0.41 to −0.12)* | Reference | |
| FasL | RT-HIIT | 5.18 ± 0.49 | 5.38 ± 0.58 | 0.11 (−0.05 to 0.27) | −0.15 (−0.39 to 0.08) |
| AT-HIIT | 5.06 ± 0.64 | 5.38 ± 0.53 | 0.31 (0.13 to 0.48)* | −0.02 (−0.25 to 0.21) | |
| UC | 5.06 ± 0.60 | 5.42 ± 0.63 | 0.33 (0.16 to 0.51)* | Reference | |
| CXCL9 | RT-HIIT | 6.21 ± 0.73 | 6.73 ± 0.84 | 0.48 (0.13 to 0.83)* | 0.04 (−0.37 to 0.45) |
| AT-HIIT | 6.50 ± 1.03 | 7.13 ± 0.83 | 0.66 (0.32 to 1.00)* | 0.28 (−0.13 to 0.69) | |
| UC | 6.29 ± 0.95 | 6.82 ± 0.94 | 0.47 (0.10 to 0.84)* | Reference | |
| MIC A/B | RT-HIIT | 3.11 ± 1.07 | 3.06 ± 1.04 | −0.06 (−0.19 to 0.06) | −0.14 (−0.30 to 0.03) |
| AT-HIIT | 2.84 ± 1.16 | 2.88 ± 1.16 | 0.02 (−0.09 to 0.14) | −0.06 (−0.22 to 0.11) | |
| UC | 2.97 ± 1.17 | 3.06 ± 1.29 | 0.09 (−0.03 to 0.21) | Reference | |
Baseline values, within-group differences, and between-group differences were based on participants having baseline and postintervention measurements (RT-HIIT = 30, AT-HIIT = 27, UC = 29).
*P < 0.05.
aLog-transformed.
CASP-8, caspase-8; CD40-L, CD40 ligand; CSF-1, macrophage colony stimulating factor 1; CXCL9, C-X-C motif chemokine 9; DCN, decorin; FasL, Fas antigen ligand; ICOSLG, ICOS ligand; MIC A/B, MHC class I polypeptide-related sequence A/B; TRAIL, TNF-related apoptosis-inducing ligand.
Mediation effects of inflammatory markers on fatigue
Compared with the original OptiTrain study, larger effects of the exercise intervention on fatigue were found at 16 wk in this subgroup, especially for the AT-HIIT group compared with UC (−1.59, 95% CI = −2.94 to −0.24) (Table 3). The changes in IL-6 and CD8a significantly mediated the effects of RT-HIIT on both total and physical fatigue by 32.0% and 27.7% and by 31.2% and 26.4%, respectively (Table 4).
TABLE 3.
Exercise effects on fatigue and physiological outcomes.
| Baseline (Mean ± SD) | Baseline to Postintervention | |||
|---|---|---|---|---|
| Within-Group Differences, Mean (95% CI) | Between-Group Differences, Mean (95% CI) | |||
| Fatigue | ||||
| Total fatigue | RT-HIIT | 2.59 ± 3.46 | −0.02 (−1.45 to 1.42) | −1.25 (−2.58 to 0.09) |
| AT-HIIT | 1.70 ± 2.31 | 0.31 (−0.95 to 1.57) | −1.59 (−2.94 to −0.24)* | |
| UC | 2.27 ± 2.82 | 1.57 (0.72 to 2.41)* | Reference | |
| Physical fatigue | RT-HIIT | 2.53 ± 3.35 | 0.05 (−1.44 to 1.54) | −1.48 (−2.98 to −0.02)* |
| AT-HIIT | 1.76 ± 2.56 | 0.52 (−0.89 to 1.94) | −1.60 (−3.12 to −0.09)* | |
| UC | 2.62 ± 3.22 | 1.64 (0.63 to 2.65)* | Reference | |
| Physiological outcomes | ||||
| Cardiorespiratory fitness (L·min−1) | RT-HIIT | 2.34 ± 0.49 | −0.09 (−0.23 to 0.05) | 0.21 (−0.01 to 0.43) |
| AT-HIIT | 2.23 ± 0.48 | 0.03 (−0.09 to 0.15) | 0.31 (0.09 to 0.53)* | |
| UC | 2.26 ± 0.49 | −0.29 (−0.44 to −0.14)* | Reference | |
| Lower-limb muscle strength (kg) | RT-HIIT | 88.87 ± 28.94 | 16.21 (8.72 to 23.81)* | 21.65 (10.04 to 33.26)* |
| AT-HIIT | 81.27 ± 24.17 | 12.68 (7.11 to 18.25)* | 17.53 (6.03 to 29.04)* | |
| UC | 87.31 ± 26.02 | −6.00 (−13.49 to 1.49) | Reference | |
| Handgrip strength (kg) | RT-HIIT | 29.30 ± 5.82 | 2.03 (0.95 to 3.11)* | 3.07 (1.12 to 5.02)* |
| AT-HIIT | 29.65 ± 4.57 | 0.19 (−1.13 to 1.50) | 1.31 (−0.66 to 3.28) | |
| UC | 29.64 ± 5.72 | −1.14 (−2.28 to 0.01) | Reference | |
| BMI (kg·m−2) | RT-HIIT | 24.22 ± 3.55 | 0.19 (−0.15 to 0.52) | −0.68 (−1.32 to −0.05)* |
| AT-HIIT | 24.22 ± 3.32 | 0.10 (−0.24 to 0.44) | −0.67 (−1.31 to −0.02)* | |
| UC | 24.73 ± 4.35 | 0.75 (0.26 to 1.24)* | Reference | |
Baseline values, within-group differences, and between-group differences were based on participants having baseline and postintervention measurements (RT-HIIT = 30, AT-HIIT = 27, UC = 29). For exercise effects on fatigue and physiological outcomes in the whole OptiTrain study population, we refer to the original publications (18,20).
*P < 0.05.
TABLE 4.
The mediating effects of inflammatory markers on the relationship between exercise and fatigue.
| Total Fatigue, Estimate (95% CI) | Proportion Mediated (%) | Physical Fatigue, Estimate (95% CI) | Proportion Mediated (%) | |
|---|---|---|---|---|
| Effect of RT-HIIT on fatigue | ||||
| Total effect | −1.25 (−2.58 to 0.09) | −1.48 (−2.98 to −0.02)* | ||
| Indirect effect through CD40-L | −0.01 (−0.34 to 0.12) | 0.8 | −0.03 (−0.49 to 0.11) | 2.0 |
| Indirect effect through IL-6 | −0.40 (−1.11 to −0.04)* | 32.0 | −0.41 (−1.01 to −0.03)* | 27.7 |
| Indirect effect through EGF | −0.006 (−0.29 to 0.14) | 0.5 | −0.03 (−0.41 to 0.08) | 2.0 |
| Indirect effect through TRAIL | −0.13 (−0.69 to 0.04) | 10.4 | −0.13 (−0.78 to 0.06) | 8.8 |
| Indirect effect through CD8a | −0.39 (−1.11 to −0.02)* | 31.2 | −0.39 (−1.20 to −0.004)* | 26.4 |
| Indirect effect through DCN | −0.13 (−0.77 to 0.05) | 10.4 | −0.12 (−0.69 to 0.08) | 8.1 |
| Indirect effect through CCL17 | −0.009 (−0.30 to 0.10) | 0.7 | −0.02 (−0.43 to 0.09) | 1.4 |
| Indirect effect through CASP-8 | −0.05 (−0.52 to 0.06) | 4.0 | −0.07 (−0.60 to 0.06) | 4.7 |
| Indirect effect through ICOSLG | −0.17 (−0.81 to 0.05) | 13.6 | −0.15 (−0.72 to 0.05) | 10.1 |
| Indirect effect through CSF-1 | −0.30 (−0.91 to 0.03) | 24.0 | −0.29 (−0.93 to 0.07) | 19.6 |
| Indirect effect through IFN-γ | −0.22 (−0.86 to 0.07) | 17.6 | −0.24 (−1.00 to 0.08) | 0.16 |
| Indirect effect through IL-10 | −0.03 (−0.36 to 0.56) | 2.4 | −0.06 (−0.78 to 0.26) | 4.1 |
| Indirect effect through FasL | −0.11 (−0.61 to 0.08) | 8.8 | −0.15 (−0.73 to 0.12) | 10.1 |
| Indirect effect through CXCL9 | −0.03 (−0.25 to 0.38) | 2.4 | −0.05 (−0.27 to 0.44) | 3.4 |
| Indirect effect through MIC A/B | −0.24 (−0.68 to 0.02) | 19.2 | −0.23 (−0.73 to 0.01) | 15.5 |
| Effect of AT-HIIT on fatigue | ||||
| Total effect | −1.59 (−2.94 to −0.24)* | −1.60 (−3.12 to −0.09)* | ||
| Indirect effect through CD40-L | −0.05 (−0.51 to 0.21) | 3.1 | −0.11 (−0.64 to 0.17) | 6.9 |
| Indirect effect through IL-6 | −0.13 (−0.60 to 0.18) | 8.2 | −0.12 (−0.64 to 0.16) | 7.5 |
| Indirect effect through EGF | −0.02 (−0.45 to 0.24) | 0.9 | −0.08 (−0.65 to 0.17) | 4.8 |
| Indirect effect through TRAIL | −0.04 (−0.09 to 0.47) | 2.5 | −0.05 (−0.11 to 0.51) | 3.1 |
| Indirect effect through CD8a | −0.22 (−0.82 to 0.03) | 13.8 | −0.22 (−0.95 to 0.06) | 13.8 |
| Indirect effect through DCN | −0.005 (−0.21 to 0.27) | 0.3 | −0.007 (−0.17 to 0.37) | 0.4 |
| Indirect effect through CCL17 | −0.02 (−0.46 to 0.16) | 1.3 | −0.06 (−0.65 to 0.12) | 3.8 |
| Indirect effect through CASP-8 | −0.10 (−0.64 to 0.10) | 6.3 | −0.14 (−0.74 to 0.08) | 8.8 |
| Indirect effect through ICOSLG | −0.06 (−0.53 to 0.11) | 3.8 | −0.06 (−0.49 to 0.08) | 3.8 |
| Indirect effect through CSF-1 | −0.07 (−0.50 to 0.22) | 4.4 | −0.07 (−0.50 to 0.27) | 4.4 |
| Indirect effect through IFN-γ | −0.06 (−0.50 to 0.16) | 3.8 | −0.06 (−0.53 to 0.20) | 3.8 |
| Indirect effect through IL-10 | −0.01 (−0.20 to 0.22) | 0.01 | −0.001 (−0.17 to 0.24) | 0.1 |
| Indirect effect through FasL | −0.01 (−0.37 to 0.18) | 0.6 | −0.006 (−0.40 to 0.30) | 0.4 |
| Indirect effect through CXCL9 | −0.18 (−0.02 to 0.64) | 11.3 | −0.23 (−0.03 to 0.72) | 14.4 |
| Indirect effect through MIC A/B | −0.10 (−0.57 to 0.17) | 6.3 | −0.09 (−0.57 to 0.11) | 5.6 |
*P < 0.05.
CASP-8, caspase-8; CD40-L, CD40 ligand; CSF-1, macrophage colony stimulating factor 1; CXCL9, C-X-C motif chemokine 9; DCN, decorin; FasL, Fas antigen ligand; ICOSLG, ICOS ligand; MIC A/B, MHC class I polypeptide-related sequence A/B; TRAIL, TNF-related apoptosis-inducing ligand.
Correlations between physiological outcomes and inflammatory markers
Postintervention, lower-body muscle strength significantly improved in both the RT-HIIT and AT-HIIT group compared with UC, whereas cardiorespiratory fitness significantly improved in the AT-HIIT group only and handgrip strength improved in the RT-HIIT group (Table 3). Correlations between changes in physiological outcomes and changes in inflammatory markers are shown in Table 5. A significant inverse correlation was found between change in cardiorespiratory fitness and change in serum levels of IFN-γ (r = −0.33, P = 0.005). A significant positive correlation was found between change in handgrip strength and change in serum levels of CCL17 (r = 0.22, P = 0.045). No significant correlations between changes in BMI and lower-limb muscle strength and changes in inflammatory markers were found.
TABLE 5.
Pearson product–moment correlations between changes in physiological outcomes and changes in inflammatory markers
| BMI (kg·m−2) | Cardiorespiratory Fitness (L·min−1) | Lower-Limb Muscle Strength (kg) | Handgrip Strength (kg) | |
|---|---|---|---|---|
| CASP-8 | −0.04 | −0.05 | −0.19 | −0.13 |
| CCL17 | −0.05 | −0.10 | −0.13 | −0.22** |
| CD8a | −0.11 | −0.06 | −0.23* | −0.18* |
| CD40-L | −0.07 | −0.07 | −0.11 | −0.14 |
| CSF-1 | −0.08 | −0.02 | −0.16 | −0.16 |
| CXCL9 | −0.14 | −0.01 | −0.04 | −0.03 |
| DCN | −0.004 | −0.21* | −0.13 | −0.03 |
| EGF | −0.05 | −0.03 | −0.11 | −0.19* |
| ICOSLG | −0.003 | −0.22* | −0.14 | −0.12 |
| IFN-y | −0.04 | −0.33** | −0.06 | −0.04 |
| IL-6 | −0.03 | −0.10 | −0.14 | −0.01 |
| IL-10 | −0.02 | −0.13 | −0.03 | −0.01 |
| MIC A/B | −0.10 | −0.02 | −0.14 | −0.16 |
| TRAIL | −0.14 | −0.10 | −0.13 | −0.16 |
*P < 0.10.
**P < 0.05.
CASP-8, caspase-8; CD40-L, CD40 ligand; CSF-1, macrophage colony stimulating factor 1; CXCL9, C-X-C motif chemokine 9; DCN, decorin; FasL, Fas antigen ligand; ICOSLG, ICOS ligand; MIC A/B, MHC class I polypeptide-related sequence A/B; TRAIL, TNF-related apoptosis-inducing ligand.
Cluster analysis of cytokine correlations
Two major clusters, which are groups of inflammatory markers whose expression levels are correlated, were found within the RT-HIIT and UC groups (Fig. 2C). The first cluster (cluster A) included anti-inflammatory markers: caspase-8, CCL17, CD40 ligand, and EGF. The second cluster (cluster B) included proinflammatory markers: CD8a, ICOS ligand, decorin, C-X-C motif chemokine 9, IL-6, Fas antigen ligand, TNF-related apoptosis-inducing ligand, macrophage colony stimulating factor 1, and MHC class I polypeptide-related sequence A/B. A positive correlation was observed between cytokines within each cluster. A negative correlation was observed between cytokines in clusters A and B in the UC group, whereas weaker correlations between the two clusters were observed in the RT-HIIT group. The AT-HIIT group exhibited weaker correlations between inflammatory markers.
DISCUSSION
This study showed that chemotherapy led to a general increase in inflammation; however, the increases in IL-6 and CD8a were less pronounced after 16 wk of RT-HIIT compared with UC. Furthermore, we found that these two inflammatory markers partially mediated the previously proven beneficial effect of exercise on total and physical fatigue. We did not observe the same effects of AT-HIIT on inflammatory markers.
Our work extends current knowledge from previous studies on inflammation during cancer treatment, with studies reporting increased levels of IL-6 and decreased levels of IL-1RA in patients with breast cancer undergoing chemotherapy (11,25). Weekly paclitaxel has been shown to increase plasma levels of IL-10, whereas higher dose treatment, administered every 3 wk, resulted in the upregulation of plasma levels of IL-6 and IL-8 (26). By contrast, circulating levels of IL-6 have not been shown to increase after anthracycline-based treatment (27,28). Because of the wide variety of chemotherapy regimens in our study and the small study population, we were not able to examine the effects of specific cytostatic therapies. As demonstrated by within-group differences in all groups, we observed a substantial increase in proinflammatory markers (e.g., IL-6, IL-12, IFN-γ, and TNF-α) and a decrease in anti-inflammatory markers (e.g., IL-10, EGF, ANG-1, and caspase-8), suggesting that chemotherapy induces an inflammatory environment.
Exercise has been shown to prevent or diminish inflammation in both healthy individuals (29,30) and patients with cancer (15,17,31,32). This study showed that RT-HIIT counteracted an increase in IL-6 and CD8a. This finding is not in line with results from two previous studies, which showed that combined resistance and aerobic training during adjuvant chemotherapy led to serum levels of IL-6 comparable to the UC group (12) or to elevated levels of IL-6 in patients with breast cancer in general (17). In contrast to the aforementioned studies, we used a per-protocol analysis and included patients who attended ≥60% of all exercise sessions, and additionally, we implemented a more vigorous exercise program. Both aspects could have contributed to larger effects found in the current study. Other studies on the effects of exercise on inflammation had a different timing of the intervention period or included patients who received different treatment regimens. A recent meta-analysis showed that exercise, implemented after primary breast cancer treatment, reduced serum concentrations of IL-6, TNF-α, and IL-8 (15). Furthermore, Schmidt et al. (31) observed that the increase in IL-6 during radiation therapy was counteracted by resistance exercise training in women with breast cancer. The latter study also assessed the mediating role of IL-6 in the development of fatigue during radiation therapy and the moderation by resistance exercise. Schmidt and colleagues observed that IL-6 mediated the effect of exercise on physical fatigue by 22%, which is in agreement with our observation that IL-6 mediated the effect by 27.7%.
RT-HIIT, but not AT-HIIT, was effective in moderating chemotherapy-induced inflammation, which can be explained by the underlying biology. It is generally known that resistance training primarily recruits type II muscle fibers compared with aerobic exercise, which mostly recruits type I fibers (33). Evidence suggests that muscles express inflammatory markers in a fiber-type–specific manner (34), which might explain the different effects found for RT-HIIT and AT-HIIT on inflammatory markers compared with UC. During exercises with sufficient load, the skeletal muscle secretes myokines, such as IL-6 (35). The rise in circulating IL-6 is responsible for the release of anti-inflammatory marker IL-10, which downregulates the expression of several proinflammatory markers, including IL-6 (16). Because this cascade of inflammatory cytokines is assumed to be less active after AT-HIIT, this might explain the smaller changes in concentrations of inflammatory markers found in this study arm. Indeed, there was a significant loss of IL-10 in both the UC and the AT-HIIT groups and a trend towards a moderated IL-10 loss in the RT-HIIT group compared with UC, whereas there was no effect of AT-HIIT compared with UC. Future studies are needed to further explore potential mechanisms that can underpin the beneficial effects of RT-HIIT on inflammatory markers.
In the present study, we also explored correlations between inflammatory markers and physiological outcomes because the influence of exercise on the inflammatory pathway might be more pronounced in the patients showing a physiological response. Of note, we found correlations between changes in lower limb muscle and handgrip strength and immunogenic markers, including CD8a. These results are confirmed by a recent pilot study from Narsale et al. (36), who showed that naïve, memory, and regulatory T-cells correlate with muscle strength and performance, suggesting that engagement in muscular strengthening activities might have beneficial effects on inflammatory markers. We did not find a correlation between changes in BMI and inflammatory markers, as suggested by earlier research (37). We speculate that BMI might not be a sufficiently sensitive parameter compared with changes in adipose tissue. Furthermore, it should be noted that we investigated correlations between changes in physiological outcomes and inflammatory markers over time. We assume that muscle strength is more likely to change over a 16-wk period compared with BMI, which might explain why we did not find any correlations between BMI and inflammatory markers. This study highlights that exercise influences markers not only via BMI as shown in previous research (38) but also via muscle strength (39). It can be speculated that as a result of the exercise-induced reduction of inflammation in blood, muscle strength will not be negatively affected by the catabolic effects of inflammation. Many studies examined the association between weight loss and inflammation and by putting this evidence and our findings into perspective, we suggest that it would be interesting to examine whether inflammation influences muscle strength by its effects on body composition in future studies.
Strengths and limitations
The results of this study should be viewed in the context of several strengths and limitations. First, this study captured many key and non-key exercise-related inflammatory markers, which enabled us to explore exercise effects on inflammation during adjuvant chemotherapy in greater detail. As a result, we hope that these results will guide future studies by helping them to decide which inflammatory markers might be or might not be interesting to examine using more sensitive methods in the context of exercise during chemotherapy for breast cancer. Second, this is a randomized controlled trial, suggesting a causal relationship between the exercise interventions and inflammation. Limitations are that we only included women who attended more than 60% of all exercise sessions, thereby compromising randomization (40). Nevertheless, women included in the current analyses were comparable with the women in the original OptiTrain study with respect to all baseline characteristics, except for baseline physical activity levels. Patients in the intervention groups were more often moderately to vigorously active compared with the UC group. It is intuitive that patients who participated in physical activities before inclusion in the study would be more likely to adhere to the exercise program. Because this is a mechanistic study and our particular interest lies on the effects of performed exercise on inflammation, we did not adjust for this difference at baseline. Evidence suggests that exercise effects on inflammatory markers might be different for specific cytostatic therapies (taxanes vs nontaxane based); however, we were not able to stratify our results for these two types of therapies due to the small sample size. Future studies are needed to further explore this. Although we captured many inflammatory markers, we missed a few interesting markers due to undetectable cytokine concentrations (e.g., IL-13, IL-1ra, and IFN-β). Because of the exploratory nature of the current analyses, the probability of a type I error was increased (41). Finally, cancer-related fatigue is a complex and multifactorial syndrome, and therefore additional potential mechanistic mediators should be investigated.
Clinical relevance
This present study helps to explain the beneficial effects of exercise on fatigue, and as a consequence this might enhance exercise promotion and adherence. Knowledge about the underlying mechanisms and the link between relevant inflammatory markers and physiological response may potentially move exercise training in cancer patients beyond a “one size fits all” approach because it provides us with extensive knowledge of the molecular effects on fatigue that are induced by different dosages, intensities, and modes of exercise. Ultimately, the development of targeted interventions to ameliorate fatigue will improve the long-term QoL in the growing population of patients with cancer.
CONCLUSIONS
In conclusion, our study shows that chemotherapy induced an inflammatory environment in general. Resistance training and HIIT concomitant to chemotherapy are suggested to be effective interventions to reduce chemotherapy-induced inflammation and subsequent fatigue. The beneficial effect of exercise on fatigue seemed to be partially mediated by IL-6 and CD8a. Future studies are needed to confirm our findings and to assess the long-term effects of exercise on the inflammatory environment.
Supplementary Material
Acknowledgments
The authors thank the women who participated in this clinical intervention trial, the oncological rehabilitation unit at the University hospital in Stockholm (Sweden) for providing the exercise facilities, and Daniele Cardinale at the Swedish Sports Confederation (Lidingö, Sweden) for technical support.
This work was supported by the Swedish Cancer Society (130452 to Y. W.), the Cancer Society of Stockholm (131242 to Y. W.), the Swedish Cancer and Traffic Accident Foundation (F-C-001225 to Y. W.), and the Swedish Society for Medical Research (SLS 50514 to H. R.).
The authors declare no conflict of interest and have read and approved the manuscript. The authors acknowledge that the result of the present study do not constitute endorsement by the American College of Sports Medicine. The results are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.acsm-msse.org).
Contributor Information
ANOUK E. HIENSCH, Email: a.e.hiensch-2@umcutrecht.nl.
SARA MIJWEL, Email: sara.mijwel@ki.se.
DAVID BARGIELA, Email: david.bargiela@ki.se.
YVONNE WENGSTRÖM, Email: yvonne.wengstrom@ki.se.
ANNE M. MAY, Email: a.m.may@umcutrecht.nl.
REFERENCES
- 1.Berger AM Mooney K Alvarez-Perez A, et al. Cancer-related fatigue, version 2.2015. J Natl Compr Canc Netw. 2015;13:1012–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bower JE. Cancer-related fatigue: mechanisms, risk factors, and treatments. Nat Rev Clin Oncol. 2014;11:597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barsevick AM Irwin MR Hinds P, et al. Recommendations for high-priority research on cancer-related fatigue in children and adults. J Natl Cancer Inst. 2013;105:1432–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bower JE Ganz PA Desmond KA, et al. Fatigue in long-term breast carcinoma survivors: a longitudinal investigation. Cancer. 2006;106:751–8. [DOI] [PubMed] [Google Scholar]
- 5.Servaes P Gielissen MFM Verhagen S, et al. The course of severe fatigue in disease-free breast cancer patients: a longitudinal study. Psychooncology. 2007;16:787–95. [DOI] [PubMed] [Google Scholar]
- 6.Fabi A Falcicchio C Giannarelli D, et al. The course of cancer related fatigue up to ten years in early breast cancer patients: what impact in clinical practice? Breast. 2017;34:44–52. [DOI] [PubMed] [Google Scholar]
- 7.Bower J Ganz P Desmond K, et al. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J Clin Oncol. 2000;18:743–53. [DOI] [PubMed] [Google Scholar]
- 8.Hofman M Ryan JL Figueroa-Moseley CD, et al. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12(1 Suppl):4–10. [DOI] [PubMed] [Google Scholar]
- 9.LaVoy ECP, Fagundes CP, Dantzer R. Exercise, inflammation, and fatigue in cancer survivors. Exerc Immunol Rev. 2016;22:82–92. [PMC free article] [PubMed] [Google Scholar]
- 10.Saligan LN, Kim HS. A systematic review of the association between immunogenomic markers and cancer-related fatigue. Brain Behav Immun. 2012;26:830–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu L Mills PJ Rissling M, et al. Fatigue and sleep quality are associated with changes in inflammatory markers in breast cancer patients undergoing chemotherapy. Brain Behav Immun. 2012;26:706–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Vulpen JK Schmidt ME Velthuis MJ, et al. Effects of physical exercise on markers of inflammation in breast cancer patients during adjuvant chemotherapy. Breast Cancer Res Treat. 2017;1–11. [DOI] [PubMed] [Google Scholar]
- 13.Mustian KM Alfano CM Heckler C, et al. Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: a meta-analysis. JAMA Oncol. 2017;3:961–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meneses-Echávez JF, González-Jiménez E, Ramírez-Vélez R. Supervised exercise reduces cancer-related fatigue: a systematic review. J Physiother. 2015;61:3–9. [DOI] [PubMed] [Google Scholar]
- 15.Meneses-Echavez JF Correa-Bautista JE Gonzalez-Jimenez E, et al. The effect of exercise training on mediators of inflammation in breast cancer survivors: a systematic review with meta-analysis. Cancer Epidemiol Biomarkers Prev. 2016;25:1009–17. [DOI] [PubMed] [Google Scholar]
- 16.Gleeson M Bishop NC Stensel DJ, et al. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11:607–10. [DOI] [PubMed] [Google Scholar]
- 17.Kleckner IR Kamen C Cole C, et al. Effects of exercise on inflammation in patients receiving chemotherapy: a nationwide NCORP randomized clinical trial. Support Care Cancer. 2019;27(12):4615–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mijwel S Backman M Bolam KA, et al. Adding high-intensity interval training to conventional training modalities: optimizing health-related outcomes during chemotherapy for breast cancer: the OptiTrain randomized controlled trial. Breast Cancer Res Treat. 2017;1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wengström Y Bolam KA Mijwel S, et al. Optitrain: a randomised controlled exercise trial for women with breast cancer undergoing chemotherapy. BMC Cancer. 2017;17:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mijwel S Backman M Bolam KA, et al. Highly favorable physiological responses to concurrent resistance and high-intensity interval training during chemotherapy: the OptiTrain breast cancer trial. Breast Cancer Res Treat. 2018;169:93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Croghan C, Egeghy P. Methods of dealing with values below the limit of detection using SAS. Southeastern SAS User Group; St. Petersburg, FL; 2003. pp. 22–4. [Google Scholar]
- 22.Jakobsson S Taft C Östlund U, et al. Performance of the Swedish version of the revised Piper Fatigue Scale. Eur J Oncol Nurs. 2013;17:808–13. [DOI] [PubMed] [Google Scholar]
- 23.Hawley-Hague H Horne M Skelton DA, et al. Review of how we should define (and measure) adherence in studies examining older adults’ participation in exercise classes. BMJ Open. 2016;6:e011560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valente M, MacKinnon D. Comparing models of change to estimate the mediated effect in the pretest-posttest control group design. Struct Equ Modeling. 2017;24:428–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyon D Cohen R Chen H, et al. Relationship of systemic cytokine concentrations to cognitive function over two years in women with early stage breast cancer. J Neuroimmunol. 2016;301:74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pusztai L Mendoza TR Reuben JM, et al. Changes in plasma levels of inflammatory cytokines in response to paclitaxel chemotherapy. Cytokine. 2004;25:94–102. [DOI] [PubMed] [Google Scholar]
- 27.Mills PJ Parker B Dimsdale JE, et al. The relationship between fatigue and quality of life and inflammation during anthracycline-based chemotherapy in breast cancer. Biol Psychol. 2005;69:85–96. [DOI] [PubMed] [Google Scholar]
- 28.Mills P Ancoli-Israel S Parker B, et al. Predictors of inflammation in response to anthracycline-based chemotherapy for breast cancer. Brain Behav Immun. 2008;22:98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng G Qiu P Xia R, et al. Effect of aerobic exercise on inflammatory markers in healthy middle-aged and older adults: a systematic review and meta-analysis of randomized controlled trials. Front Aging Neurosci. 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petersen AMW, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol (1985). 2005;98:1154–62. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt ME Meynköhn A Habermann N, et al. Resistance exercise and inflammation in breast cancer patients undergoing adjuvant radiation therapy: mediation analysis from a randomized, controlled intervention trial. Int J Radiat Oncol Biol Phys. 2016;94:329–37. [DOI] [PubMed] [Google Scholar]
- 32.Fairey AS Courneya KS Field CJ, et al. Effect of exercise training on C-reactive protein in postmenopausal breast cancer survivors: a randomized controlled trial. Brain Behav Immun. 2005;19:381–8. [DOI] [PubMed] [Google Scholar]
- 33.Wilson JM Loenneke JP Jo E, et al. The effects of endurance, strength, and power training on muscle fiber type shifting. J Strength Cond Res. 2012;26:1724–9. [DOI] [PubMed] [Google Scholar]
- 34.Plomgaard P, Penkowa M, Pedersen BK. Fiber type specific expression of TNF-alpha, IL-6 and IL-18 in human skeletal muscles. Exerc Immunol Rev. 2005;11:53–63. [PubMed] [Google Scholar]
- 35.Pedersen BK, Fischer CP. Beneficial health effects of exercise—the role of IL-6 as a myokine. Trends Pharmacol Sci. 2007;28:152–6. [DOI] [PubMed] [Google Scholar]
- 36.Narsale A Moya R Ma J, et al. Cancer-driven changes link T cell frequency to muscle strength in people with cancer: a pilot study. J Cachexia Sarcopenia Muscle. 2019;10(4):827–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Connor M Bower J Cho H, et al. To assess, to control, to exclude: effects of biobehavioral factors on circulating inflammatory markers. Brain Behav Immun. 2009;23:887–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Gemert WA May AM Schuit AJ, et al. Effect of weight loss with or without exercise on inflammatory markers and adipokines in postmenopausal women: the SHAPE-2 trial, a randomized controlled trial. Cancer Epidemiol Biomarkers Prev. 2016;25:799–806. [DOI] [PubMed] [Google Scholar]
- 39.Norman K Stobäus N Kulka K, et al. Effect of inflammation on handgrip strength in the non-critically ill is independent from age, gender and body composition. Eur J Clin Nutr. 2014;68:155–8. [DOI] [PubMed] [Google Scholar]
- 40.Ranganathan P, Pramesh CS, Aggarwal R. Common pitfalls in statistical analysis: intention-to-treat versus per-protocol analysis. Perspect Clin Res. 2016;7:144–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bender R, Lange S. Adjusting for multiple testing—when and how? J Clin Epidemiol. 2001;54:343–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


