Supplemental digital content is available in the text.
Key Words: EXOGENOUS KETOSIS, KETONE MONOESTER, EXERCISE, EXERCISE INTENSITY, CYCLING EFFICIENCY
ABSTRACT
Introduction
Exogenous ketones potentially provide an alternative, energetically advantageous fuel to power exercising skeletal muscle. However, there is limited evidence regarding their relative contribution to energy expenditure during exercise. Furthermore, the effect of blood ketone concentration and exercise intensity on exogenous ketone oxidation rates is unknown.
Methods
Six athletes completed cycling ergometer exercise on three occasions within a single-blind, random-order controlled, crossover design study. Exercise duration was 60 min, consisting of 20-min intervals at 25%, 50%, and 75% maximal power output (WMax). Participants consumed (i) bitter flavored water (control), (ii) a low-dose β-hydroxybutyrate (βHB) ketone monoester (KME; 252 mg·kg BW−1, “low ketosis”), or (iii) a high-dose βHB KME (752 mg·kg BW−1, “high ketosis”). The KME contained a 13C isotope label, allowing for the determination of whole-body exogenous βHB oxidation rates through sampled respiratory gases.
Results
Despite an approximate doubling of blood βHB concentrations between low- and high-ketosis conditions (~2 mM vs ~4.4 mM), exogenous βHB oxidation rates were similar at rest and throughout exercise. The contribution of exogenous βHB oxidation to energy expenditure peaked during the 25% WMax exercise intensity but was relatively low (4.46% ± 2.71%). Delta efficiency during cycling exercise was significantly greater in the low-ketosis (25.9% ± 2.1%) versus control condition (24.1% ± 1.9%; P = 0.027).
Conclusions
Regardless of exercise intensity, exogenous βHB oxidation contributes minimally to energy expenditure and is not increased by elevating circulating concentrations greater than ~2 mM. Despite low exogenous βHB oxidation rates, exercise efficiency was significantly improved when blood βHB concentration was raised to ~2 mM.
To meet energetic requirements, most body tissues draw upon the oxidation of carbohydrate- and/or lipid-derived substrates. In states of decreased carbohydrate availability such as starvation or ketogenic diet, circulating ketone bodies—mainly β-hydroxybutyrate (βHB) and acetoacetate (AcAc)—are elevated and represent an additional major substrate (1). During starvation, the primary purpose of ketogenesis is to provide a supplementary substrate (to glucose) to support cerebral metabolism (2); however, most extrahepatic tissues, including skeletal muscle, have the capacity to oxidize ketones (1). Compared with other sites of peripheral ketone oxidation, notably the heart and kidney, skeletal muscle has comparatively low ketolytic enzyme activity (3). Furthermore, this varies widely within the spectrum of skeletal muscle phenotypes, with 3-hydroxybutyrate dehydrogenase (BDH) and 3-oxoacid-CoA transferase (SCOT) activity being, respectively, ~2.5 and ~1.5 times greater in slow-oxidative versus fast-glycolytic fibers (3). Nonetheless, given that skeletal muscle comprises ~40% of total body mass, it represents a potentially important site for the metabolic disposal of ketones.
The advent of ingestible ketones now facilitates the transient induction of ketosis minus the metabolic conditions required for hepatic ketogenesis (reduced carbohydrate stores, elevated circulating free fatty acids, and a low insulin/glucagon ratio) (1,4). Doing so creates a novel physiological state—“exogenous ketosis”—that potentially holds benefits for human metabolism and exercise performance (4,5). Ketone metabolism has energetic advantages versus lipid- and carbohydrate-derived substrates (4,6). Per mole, more energy can be liberated through the oxidation of βHB versus pyruvate. In addition, βHB oxidation exerts opposite redox effects on the respiratory chain electron carriers, NAD, and coenzyme Q, thus increasing free energy liberated by ATP hydrolysis (6). These effects are thought to explain the 25% improvement in hydraulic efficiency (J·mol O2−1) in the working rat heart perfused with a physiological ratio of ketones and glucose versus glucose alone (6). Although fat is more reduced than both pyruvate and βHB (thus, more energy can be liberated through its oxidation) (4), the ratio of O2 consumed to ATP produced is less favorable. In part, this may explain why fat oxidation is relatively low at high exercise intensities (7). As such, meaningful contributions from exogenous ketone oxidation to energy expenditure in exercising skeletal muscle might result in improved efficiency, observed as greater power output for a given volume of oxygen consumed (4).
Improvements in energy efficiency attributable to ketone metabolism may have contributed to the increased endurance exercise performance observed in athletes supplemented with a ketone monoester (KME) (5). However, the ergogenic potential of exogenous ketosis remains to be determined, with studies also finding indifferent (8–11) exercise performance after βHB KME supplementation, and worse (12) after AcAc diester supplementation. Furthermore, the observed increase in endurance exercise performance may have resulted from ketosis-mediated alterations in the availability and catabolism of other substrates, most notably the “rationing” of intramuscular glycogen stores and concomitant increase in lipid oxidation in exercising skeletal muscle (5). In other words, ketone signaling may be a greater contributor to the observed improvements in exercise performance, rather than ketone oxidation.
There is limited evidence regarding the relative contribution of exogenous ketone oxidation to overall energy expenditure during exercise. Using adjusted indirect calorimetry equations (13), βHB oxidation during 40% and 75% maximal power output (WMax) exercise has been predicted to account for 18% and 16% of total oxygen consumption, respectively (5). However, direct measures of ketone oxidation in exercising humans are warranted. In addition, factors regulating ketone oxidation during exercise are largely unknown. In particular, it is unclear how blood ketone concentration and exercise intensity affect exogenous ketone oxidation rates during exercise.
Therefore, this work sought to determine (i) the direct contribution of exogenous βHB oxidation to overall energy expenditure during exercise using a βHB stable isotope tracer, (ii) the effect of altering blood βHB concentration on exogenous βHB oxidation rates during exercise, and (iii) the effect of exercise intensity on exogenous βHB oxidation rates.
METHODS
Ethical approval
The study was approved by the Oxfordshire Clinical Research Ethics Committee (15/SC/0542). The study conformed to the standards detailed in the Declaration of Helsinki. All participants provided written informed consent.
Participants
Eligibility criteria were healthy men or women age 18–45 yr who were currently undertaking >6-h training per week in rowing, cycling, running, or triathlon. No participants had been following a ketogenic diet before enrollment in the study. Exclusion criteria included the following: cardiovascular disease, diabetes, or any other disorder that may influence their capacity to exercise; pregnancy; or current breastfeeding; or women not taking the oral contraceptive pill.
Baseline test
Between 1 and 2 wk before the first glycogen depletion visit, participants attended the laboratory at the Department of Physiology, Anatomy and Genetics, the University of Oxford, to undertake an incremental exercise test to volitional fatigue on an electronically braked bicycle ergometer (ergoselect 100; Ergoline GmbH, Bitz, Germany; Fig. 1). Participants arrived at ~0800 h having fasted overnight. Before commencing exercise, participants adjusted the saddle height and handlebar position according to personal preference. Bike geometry was recorded and kept constant for all subsequent study visits. The test began at 100 W (light intensity) and increased 25 W every 3 min. Two of the following criteria must have been attained to satisfy that exercise was undertaken to exhaustion: (i) volitional fatigue, (ii) an inability to maintain a cadence >60 rpm, (iii) the maximum heart rate (HR) attained was within 10 bpm of an age-predicted maximum (220 − age), or (iv) the maximum RER attained was >1.10. Participants were instructed to hold a cadence of 80–100 rpm during the test. The ergometer was set to the rpm-independent mode, meaning the power output was held constant during each 3-min interval regardless of the cadence held. Respiratory gases (volume of oxygen consumed (V˙O2), volume of carbon dioxide produced (V˙CO2), and minute ventilation (V˙E)) were measured continuously through indirect calorimetry. WMax achieved during the test was calculated as WStep N − 1 + (time elapsed/180 s × 25 W) and used to determine exercise intensities (% WMax) for exercise during subsequent study visits.
FIGURE 1.
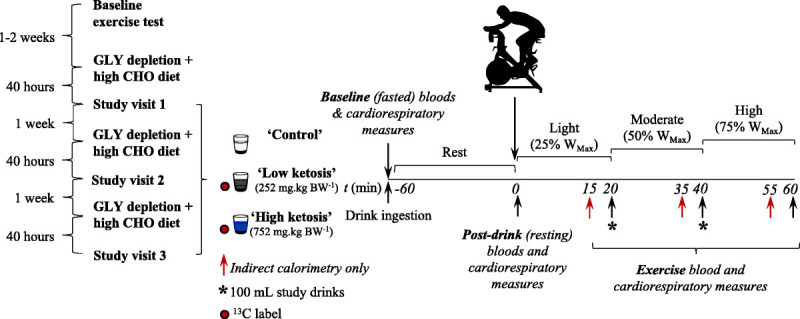
Study visit protocol. Participants (n = 6) attended the laboratory on three occasions to undertake incremental intensity cycling exercise in a random order, controlled, crossover fashion. Participants consumed either a calorie-free control drink (“control”), a low-dose KME drink (252 mg·kg BW−1, “low ketosis”), or a high-dose KME drink (752 mg·kg BW−1, “high ketosis”). KME drinks contained a 13C tracer, allowing for the subsequent determination of exogenous βHB oxidation rates in collected respiratory gas samples. Approximately 40 h before all study visits, participants performed supervised glycogen (GLY) depleting cycling exercise followed by a high carbohydrate (CHO) content diet.
Glycogen depletion visits
Approximately 40 h before each study visit, participants undertook a supervised glycogen depleting exercise protocol on the same bicycle ergometer followed by a high-carbohydrate-content diet (~70% of total energy intake) and no additional exercise (Fig. 1). These visits sought to provide a consistent level of fatigue and similar intramuscular glycogen contents for each study visit. The exercise protocol consisted of alternating 2-min “work” and “recovery” intervals commencing at 90% and 50% WMax, respectively. When the participant could no longer hold 90% WMax for 2 min (volitional fatigue or inability to hold a cadence >60 rpm), the work intensity was reduced by 10%. This continued until participants could no longer maintain 60% WMax during work intervals. The number of completed repetitions was recorded. Participants were instructed to hold a cadence of 80–100 rpm, and the bicycle ergometer was set to the rpm-independent mode. This protocol reliably depletes intramuscular glycogen stores (14).
After each glycogen depletion visit, participants were instructed to maintain a high carbohydrate diet (~70% of total energy intake) and refrain from further exercise. Glycogen depleting exercise immediately followed by a carbohydrate-rich diet is well observed to result in intramuscular glycogen “super-compensation” (15). Participants were asked to avoid foods naturally abundant in 13C (e.g., corn sugars) and to refrain from alcohol. Participants kept a food diary between the glycogen depletion and study visits (~40 h) and were asked to closely match their food intake before each study visit.
Study visits
Each subject performed three exercise trials, separated by ~1 wk (Fig. 1). Participants reported to the laboratory at ~0800 h having fasted overnight. Upon arrival, a 22-gauge catheter (BD Venflon™ Pro Safety; BD, Wokingham, Berkshire, United Kingdom) was inserted retrogradely into a dorsal vein of the hand. A three-way tap (BD Connecta™ Plus Stopcock; BD) was attached, allowing for repeated blood sampling. The catheter was flushed with ~1 mL sodium chloride (Injection BP 0.9% w/v; Kent Pharmaceuticals Ltd, Ashford, United Kingdom) after each sample was taken. To achieve arterialized venous values, participants hands were heated for 2 min before blood was drawn (16). Baseline (t = −60 min) blood samples were drawn for the analysis of blood gases (1 mL, PICO50; Radiometer UK Ltd, West Sussex, United Kingdom) and blood metabolites (3 mL, BD Plastipak 3-Part Luer Slip Syringe; BD). Respiratory gases were measured via indirect calorimetry and respiratory gas samples were collected into 12 mL Exetainer™ tubes (Labco Ltd, Lampeter, United Kingdom). Finally, resting HR and gastrointestinal discomfort, using scales previously described (17), were measured.
After completion of baseline measurements, participants consumed a 400-mL bolus study drink: (i) a calorie-free control drink (“control,” bitter flavored water), (ii) a “low”-dose KME drink (“low ketosis”; target blood ketone concentration of 2–3 mM), or (iii) a “high”-dose KME drink (“high ketosis”; target blood ketone concentration of >4 mM; Fig. 1). The order participants completed the three study conditions was randomly controlled, and participants were blinded to the condition they were undertaking. Participants then rested for 60 min before commencing exercise. After consumption of study drinks, subjects were asked to collect all urine in a provided container.
At t = 0 min, all baseline measurements (as described previously) were repeated. Participants then undertook 60 min of continuous ergometer cycling exercise, comprising 20-min intervals at 25% WMax (t = 0–20 min), 50% WMax (t = 20–40 min), and 75% WMax (t = 40–60 min) exercise intensities. Participants were instructed to maintain a cadence of 80–100 rpm, and the bicycle ergometer was set to the rpm-independent mode. Room temperature and airflow were consistent across study visits. Participants consumed water ad libitum. During exercise, indirect calorimetry (V˙O2, V˙CO2, V˙E), RPE, HR, and gastrointestinal discomfort measurements were made at t = 15, 20, 35, 40, 55, and 60 min. Breath and blood samples were collected at t = 20, 40, and 60 min. To ensure blood βHB concentrations remained stable throughout exercise, 100-mL study drinks were administered at the end of the 25% WMax and 50% WMax exercise intervals.
Drink preparations
A 600-mL study drink was prepared containing 252 mg·kg BW−1 for low ketosis and 752 mg·kg BW−1 for high ketosis of KME ((R)-3-hydroxybutyl (R)-3-hydroxybutyrate; TΔS Ltd, Oxford, United Kingdom). Thus, based on a mean participant weight of 79 ± 4 kg, the mean amounts of βHB delivered were 19.9 ± 1.0 g (95.5 ± 4.8 kcal) in the low-ketosis condition and 59.4 ± 3.0 g (285.1 ± 14.4 kcal) in the high-ketosis condition. The KME delivered the D-βHB isoform, as opposed to the less well-characterized L-βHB isoform (18) (all references made to βHB within this article are therefore related to the D-isoform only). Control study drinks were calorie-free, isovolumetric amounts of bitter flavored (product number 648352; Symrise, Holzminden, Germany) water. All drinks also included a sweetener (NutraSweet, Augusta, GA) to aid palatability. The dose response of KME drinks was predicted from previous work investigating its pharmacokinetics (19). The 600-mL study drinks were divided into a 400-mL bolus consumed before exercise (t = 0 min) and two 100-mL drinks consumed at t = 20 and 40 min.
To determine ketone oxidation rates, a 13C-labeled KME was created. Ninety-nine percent U-13C–labeled ethyl acetoacetate was purchased from Cambridge Isotope Laboratories (Tewksbury, MA). Ethyl acetoacetate was used as the precursor for the synthesis of both U-13C R-1,3-β-hydroxybutyrate and U-13C 1,3 butanediol in separate fractions. Both fractions underwent a lipase reaction to produce 50% U-13C–labeled KME with labels present in 1,3-β-hydroxybutyrate and U-13C 1,3 butanediol “halves” of the final monoester (Document, Supplemental Digital Content 1, description of stable isotope preparation and synthesis, http://links.lww.com/MSS/C114). All syntheses was performed under good manufacturing practice conditions (Sterling Pharmaceutical Solutions Ltd, England, United Kingdom). Incorporation of small aliquots of the 13C containing ester into unlabeled KME drinks, resulting in a relative enrichment of ~25%.
Blood sampling and analysis
Blood gases in 1 mL arterialized venous blood samples were immediately analyzed using a benchtop blood gas analyzer (ABL800; Radiometer UK Ltd). pH and bicarbonate (HCO3−) values were subsequently corrected using a custom MATLAB script (v2017a; MathWorks, Natick, MA), which accounted for the increased hemoglobin saturation in arterialized blood, as previously been described (8). The second blood sample (3 mL) was collected in tubes containing ethylenediaminetetraacetic acid (BD Vacutainer®; BD). Blood βHB was analyzed immediately from these samples using a portable analyzer (Freestyle Optium Neo; Abbott Laboratories Inc., Abbott Park, IL). Samples were then immersed in an ice bath until the end of the test. After completion of the visit, blood samples were centrifuged (3600 rpm for 10 min), and the plasma component was transferred to an Eppendorf tube and subsequently stored at −80°C until later analysis. Glucose, nonesterified fatty acids (NEFA), and lactate were assayed in thawed serum samples using a semiautomated benchtop analyzer (Pentra C400; Horiba Medical, Grabels, France).
Urine sampling and analysis
The total volume of urine was recorded, and a 1-mL aliquot was taken and stored at −80°C until later analysis. Urine βHB was analyzed using a semiautomated benchtop analyzer (Pentra C400; Horiba Medical).
Indirect calorimetry and HR measurements
Participants wore a snug, comfortable fitting face mask (Hans Rudolph Inc., Shawnee, KS) attached to an indirect calorimeter (Metalyzer 3BR2; Cortex Biophysik GmbH, Leipzig, Germany) for the continuous sampling of respiratory gases, displayed in real-time using Metasoft® (v7.9.1; Cortex Biophysik GmbH). For the measurement of HR, subjects wore a chest strap (T31, Polar® Electro, Espoo, Finland) that communicated directly to the Metalyzer unit via a receiver cable attached to the bicycle ergometer. Cardiorespiratory data were averaged over 5-s intervals in Metasoft®.
Respiratory gas sampling
Participants breathed into a mouthpiece containing a one-way valve connected to a Douglas bag. Duplicate respiratory gas samples were collected into 12-mL Exetainer™ tubes (Labco Ltd). Samples were kept at room temperature until subsequent analysis (see discussion hereinafter), which occurred within ~6 wk of collection.
Calculations of exogenous βHB oxidation rates
The 13C enrichment in exogenous βHB was determined by gas chromatography–mass spectrometry after derivatization with a trimethylsilyl group (20). The tracer-to-tracee ratio in respired gases (TTr; 13CO2/12CO2) was determined using previously published methods (21). Exogenous ketone oxidation rate (in grams per minute) was calculated by the following equation:
The molar mass of βHB was assumed to be 104.1 g·mol−1 and k = the volume of CO2 produced by the oxidation of 1 g of βHB (0.86 L).
Calculations of whole-body efficiency
Energy expenditure was calculated according to the exercise intensity–dependent formulas proposed by Jeukendrup and Wallis (22):
The delta efficiency, which represents the change in energy expenditure relative to the change in work accomplished (23), was determined by calculating the individual linear regression (y = mx + b) of the relationship between energy expended per minute versus work accomplished per minute (24). Delta efficiency was then calculated from the reciprocal of the slope of this relationship (1/m) and expressed as a percentage. Delta efficiency is thought to provide a more valid reflection of changes in muscular efficiency during exercise versus other commonly used measures (23).
RPE
Subjective scores of RPE (“What is your perceived exertion?”), breathlessness (“How breathless are you?”), leg discomfort (“What is your leg discomfort?”), anxiety of breathing (“How anxious are you about your breathing?”), and anxiety of leg discomfort (“How anxious are you about your leg discomfort?”) were recorded in a random order on a linear scale of 0–10 (0 (none) to 10 (maximum)); scales are presented by Faull et al. (25).
Skeletal muscle biopsy
As the activity of BDH and SCOT (which catalyze the first and second reactions of ketolysis, respectively) is significantly greater in type I (slow-oxidative) versus types IIa and IIb skeletal muscle fibers (3), an individuals’ muscle phenotype (relative proportion of each fiber) may theoretically influence exogenous ketone oxidation rate. As such, to provide context for exogenous ketone oxidation data, we performed a skeletal muscle biopsy and subsequently determined each participants’ muscle phenotype. A skeletal muscle biopsy of vastus lateralis was performed on participants before baseline measurements at the control visit using aseptic techniques previously described (5).
Muscle fiber type
The percentage of slow-oxidative fibers in muscle biopsy samples was determined using methods adapted from Koopman and colleagues (26). Frozen vastus lateralis samples were embedded in optimal cutting temperature compound (Tissue-Tek® OCT; Labtech, Heathfield, United Kingdom), sectioned (4 μm) using a Cryostat Microtome (OTF5000; Bright Instruments Ltd, Ungland, United Kingdom), and mounted onto slides (SuperFrost® Plus; VWR International Ltd, England, United Kingdom). Samples were fixed for 1 h using a 4% formaldehyde solution. Triton X-100 (0.5%) was used to make samples permeable, before being blocked for 1 h at room temperature (1% bovine serum albumin, 0.1% Triton X-100). Samples were incubated overnight with a primary antibody (antislow skeletal myosin heavy chain, ab11083; Abcam, Cambridge, United Kingdom) diluted in the block solution (1:50). The primary antibody was internally validated on dissected rat soleus (high percentage of slow-oxidative muscle fibers) and anterior tibialis (high percentage of fast-glycolytic muscle fibers) muscle (Figure, Supplemental Digital Content 2, images of rat (A) soleus (extremely slow-oxidative phenotype) and (B) extensor digitorum longus (extremely fast phenotype) muscle stained with an antislow skeletal myosin heavy-chain primary antibody, http://links.lww.com/MSS/C115) and has previously been validated in human skeletal muscle (27). The secondary antibody (antimouse IgG (whole molecule)–fluorescein isothiocyanate produced in goat, F0257; Merck, Darmstadt, United Kingdom) diluted in the block solution (1:200) was applied for 1 h at room temperature. Finally, 4′,6-diamidino-2-phnylindole dihydrochloride (DAPI) diluted in ddH2O (1:1000) was added for 10 min to detect nuclei before a coverslip was applied.
Slides were imaged using a DeltaVision widefield fluorescence imaging system (GE Healthcare, Amersham, Buckinghamshire, United Kingdom) consisting of a 10×/0.4 NA objective lens (Olympus Life Science, Southend-on-sea, Essex, United Kingdom) fitted to an Olympus IX71 inverted microscope stand. A Lumencor SpectraX LED light engine (Lumencor Inc., Beaverton, OR) with excitation lines at 395 and 470 nm and appropriate emission filters were used to sequentially image DAPI and fluorescein isothiocyanate fluorochromes on a CoolSNAP HQ2 CCD camera (Photometrics Inc., Huntington Beach, CA). The instrument was driven using the softWoRx software package (GE Healthcare) and subsequent image analysis performed in ImageJ (NIH, Bethesda, MD). Quantification of slow-oxidative fibers was performed through manual counting (minimum of 50 fibers) and expressed as a percentage of total muscle fibers. This was performed by a single, blinded investigator.
Statistics
Results are expressed as mean ± SD. Significance was taken at P < 0.05. The effects of blood ketone concentration and exercise intensity on exogenous βHB oxidation rates were tested with two-way repeated-measures ANOVA (factors: condition (three levels) and time (five levels)). Simple linear regressions and repeated-measures one- and two-way ANOVA were used as appropriate for all other analyses. Sphericity and normality assumptions were tested in SPSS, v26 (IBM Corp., Armonk, NY), with all remaining analysis being performed in Prism v8.3 (GraphPad Software Inc., San Diego, CA). Where the assumption of sphericity was not met, a Greenhouse–Geisser correction was applied. Nonnormally distributed data were log10 transformed. In such cases, all analyses were performed on transformed data sets. Post hoc comparisons were made using Tukey or Sidak multiple comparison corrections as appropriate.
RESULTS
Participants
Six athletes (5 men, 1 woman; age, 21 ± 1 yr; height, 186 ± 4 cm; weight, 79 ± 4 kg; V˙O2 Peak, 56.73 ±4.25 mL·kg−1·min−1; WMax, 375 ± 65 W; training per week, 16 ± 6 h) completed the study. The participants’ primary sports were cycling (n = 2), running (n = 2), triathlon (n = 1), and rowing (n = 1).
Prestudy preparation
No order effects were observed for exercise performance (number of steps completed) in the glycogen depleting exercise, performed 40 h before study visits. Analysis of self-reported diet diaries showed that neither the macronutrient composition nor total energy intake for the 48-h preceding study visits differed between study conditions (Figure, Supplemental Digital Content 3, total energy intake and macronutrient composition of participants’ diets leading into each study visit, http://links.lww.com/MSS/C116). As requested, participants consumed a high-carbohydrate-content diet, representing 61.09% ± 10.44%, 64.16% ± 8.42%, and 57.09% ± 8.33% of total energy intake leading into the control, low-ketosis, and high-ketosis conditions, respectively.
Blood βHB concentration
Main condition and time effects, and a condition–time interaction effect were present for blood βHB concentration (all, P < 0.001), which diverged in a dose-dependent manner after ingestion of the study drinks (Table 1). βHB concentration was approximately twofold higher in the high-ketosis (mean ± SD of all postdrink values, 4.40 ± 0.35 mM) versus low-ketosis (1.96 ± 0.31 mM) conditions at rest and throughout exercise. Blood βHB concentrations were unaffected by ingestion of the control drink. The consumption of 100-mL study drinks at the end of the 25% WMax (20 min) and 50% WMax (40 min) exercise intervals (see study protocol in Fig. 1) meant that blood βHB concentrations remained stable during exercise in the low- and high-ketosis conditions.
TABLE 1.
Blood βHB concentration, exogenous βHB oxidation rate and contribution to total energy expenditure, and urine βHB.
| Predrink (Rest) | Post-Drink (Rest) | 25% WMax | 50% WMax | 75% WMax | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BHB, mM | BHB, mM | BHB Ox., g·min−1 | BHB, mM | BHB Ox., g·min−1 | BHB, mM | BHB Ox., g·min−1 | BHB, mM | BHB Ox., g·min−1 | Urine BHB, g | |
| Control | 0.18 ± 0.04 | 0.20 ± 0.00 | 0.18 ± 0.04 | 0.17 ± 0.05 | 0.17 ± 0.05 | 0.001 ± 0.000 | ||||
| Low ketosis | 0.20 ± 0.06 | 2.42 ± 0.53a | 0.009 ± 0.003 (2.47% ± 1.52%) |
1.83 ± 0.48a | 0.062 ± 0.031b (3.83% ± 2.12%b) |
1.73 ± 0.65a | 0.087 ± 0.053b,c (3.33% ± 2.01%) |
1.87 ± 0.73a | 0.098 ± 0.068b (2.48% ± 1.66%c,d) |
0.060 ± 0.034a |
| High ketosis | 0.17 ± 0.05 | 4.90 ± 1.03a,e | 0.012 ± 0.011 (3.37% ± 3.48%) |
4.25 ± 1.65ae | 0.079 ± 0.054b (4.46% ± 2.71%) |
4.10 ± 1.38a,e | 0.100 ± 0.058b,c (3.56% ± 1.68%c) |
4.33 ± 1.05a,e | 0.103 ± 0.044b (2.58% ± 1.05%d) |
0.318 ± 0.188a,e |
Blood βHB concentration (in millimolars), exogenous βHB oxidation rates (in grams per minute), relative contribution of βHB oxidation to energy expenditure (given in parentheses), and urine βHB (in grams) are expressed as means ± SD (n = 6).
aSignificant difference from control.
bSignificant difference from postdrink (rest).
cSignificant difference from 25% WMax.
dSignificant difference from 50% WMax.
eSignificant difference from low ketosis.
Gastrointestinal discomfort
A main effect of condition was present for nausea (P = 0.009), with post hoc analyses showing a tendency for this to be greater in the high-ketosis (mean ± SD, 2.2 ± 2.3 on a 0–10 scale) versus control condition (no reported nausea; P = 0.053). No differences were observed for heartburn, bloating, abdominal pain, dizziness, or headache.
Effect of blood βHB concentration and exercise intensity on exogenous βHB oxidation rate
Neither a main effect of condition nor a condition–time interaction effect was present for exogenous βHB oxidation rates (Table 1). Exogenous βHB oxidation rate was significantly associated with oxygen consumption (R2 = 0.402, P < 0.001). This relationship remained significant when resting values were removed (R2 = 0.111, P = 0.048; Figure, Supplemental Digital Content 4, associations between exogenous BHB oxidation rate and V˙O2 and individual BHB oxidation rates, http://links.lww.com/MSS/C117).
A main effect of time was observed for exogenous βHB oxidation rates (P < 0.001). In both the low- and high-ketosis conditions, oxidation rates rose significantly from rest to the 25% WMax exercise interval (~7-fold increase) and from 25% WMax to 50% WMax exercise intervals (~1.5-fold increase), but thereafter plateaued (Table 1). Peak exogenous βHB oxidation rates were 0.103 ± 0.044 g·min−1, observed during the 75% WMax exercise interval. In both conditions, the relative contribution to energy expenditure during exercise was highest during the 25% WMax (peak, 4.46% ± 2.71%) and lowest during the 75% WMax exercise intensities (Table 1).
Urine βHB concentration
A main effect of condition was present for urinary βHB concentration (P < 0.001), which was greater in low-ketosis versus control condition (P = 0.002) and the high- versus low-ketosis condition (P = 0.004; Table 1).
Cardiorespiratory and RPE measures
As expected, there was a main effect of time for all measured cardiorespiratory and RPE values (all, P < 0.001), which rose in accordance with exercise intensity (Table 2 and Table, Supplemental Digital Content 5, compartmentalized perceptions (leg discomfort and breathlessness) to exercise exertion and emotional affective responses to these (anxiety of leg anxiety and anxiety of breathing), http://links.lww.com/MSS/C118). However, no main effects of condition nor condition–time interaction effects were present. Importantly, no within-condition differences were observed for V˙O2, V˙E, HR, or RPE measures taken within the last 5 min of each exercise interval (e.g., t = 15 min vs t = 20 min for the light-intensity interval), indicating that athletes had achieved a physiological “steady state” at the point of respiratory gas collection for determination of exogenous βHB oxidation rates.
TABLE 2.
Effect of blood βHB concentration and exercise intensity on cardiorespiratory measures and RPE.
| Measurement | Control | Low Ketosis | High Ketosis | |
|---|---|---|---|---|
| V˙O2, L·min−1 | Predrink (rest) | 0.31 ± 0.08 | 0.28 ± 0.07 | 0.31 ± 0.03 |
| Postdrink (rest) | 0.30 ± 0.06 | 0.38 ± 0.11 | 0.37 ± 0.07 | |
| 15 min (25%) | 1.62 ± 0.25 | 1.68 ± 0.35 | 1.64 ± 0.29 | |
| 20 min (25%) | 1.55 ± 0.20 | 1.61 ± 0.25 | 1.70 ± 0.25 | |
| 35 min (50%) | 2.60 ± 0.47 | 2.60 ± 0.46 | 2.64 ± 0.55 | |
| 40 min (50%) | 2.65 ± 0.45 | 2.58 ± 0.39 | 2.66 ± 0.50 | |
| 55 min (75%) | 3.78 ± 0.86 | 3.63 ± 0.80 | 3.75 ± 0.83 | |
| 60 min (75%) | 3.89 ± 0.87 | 3.85 ± 0.77 | 3.82 ± 0.69 | |
| V˙CO2, L·min−1 | Predrink (rest) | 0.24 ± 0.08 | 0.22 ± 0.07 | 0.24 ± 0.04 |
| Postdrink (rest) | 0.25 ± 0.06 | 0.28 ± 0.29 | 0.29 ± 0.05 | |
| 15 min (25%) | 1.39 ± 0.33 | 1.37 ± 0.31 | 1.32 ± 0.32 | |
| 20 min (25%) | 1.31 ± 0.30 | 1.30 ± 0.22 | 1.38 ± 0.27 | |
| 35 min (50%) | 2.28 ± 0.46 | 2.20 ± 0.45 | 2.23 ± 0.51 | |
| 40 min (50%) | 2.34 ± 0.43 | 2.16 ± 0.41 | 2.21 ± 0.49 | |
| 55 min (75%) | 3.53 ± 0.87 | 3.34 ± 0.84 | 3.35 ± 0.86 | |
| 60 min (75%) | 3.68 ± 0.80 | 3.48 ± 0.82 | 3.44 ± 0.72 | |
| V˙E, L·min−1 | Predrink (rest) | 10.28 ± 1.58 | 8.76 ± 3.11 | 9.27 ± 2.11 |
| Postdrink (rest) | 10.78 ± 2.17 | 11.34 ± 3.46 | 12.99 ± 1.91 | |
| 15 min (25%) | 41.13 ± 5.02 | 42.05 ± 6.86 | 45.13 ± 9.40 | |
| 20 min (25%) | 38.37 ± 5.30 | 41.09 ± 4.03 | 46.46 ± 8.77 | |
| 35 min (50%) | 63.74 ± 8.39 | 64.94 ± 9.13 | 70.76 ± 11.27 | |
| 40 min (50%) | 65.52 ± 5.43 | 64.57 ± 8.37 | 68.34 ± 19.56 | |
| 55 min (75%) | 104.59 ± 20.35 | 103.22 ± 22.06 | 105.27 ± 19.56 | |
| 60 min (75%) | 107.84 ± 17.11 | 105.77 ± 19.31 | 112.34 ± 19.74 | |
| HR, bpm | Predrink (rest) | 47.33 ± 6.38 | 48.67 ± 7.26 | 47.33 ± 7.03 |
| Postdrink (rest) | 51.33 ± 5.65 | 54.50 ± 7.45 | 57.67 ± 10.05 | |
| 15 min (25%) | 97.40 ± 7.06 | 102.40 ± 8.41 | 104.40 ± 4.93 | |
| 20 min (25%) | 98.33 ± 5.01 | 97.83 ± 12.04 | 104.17 ± 7.86 | |
| 35 min (50%) | 125.83 ± 10.50 | 124.50 ± 13.49 | 128.17 ± 9.15 | |
| 40 min (50%) | 125.83 ± 10.52 | 127.50 ± 10.29 | 127.83 ± 10.30 | |
| 55 min (75%) | 156.83 ± 9.04 | 153.67 ± 11.78 | 153.00 ± 9.17 | |
| 60 min (75%) | 158.00 ± 8.79 | 156.33 ± 12.72 | 154.17 ± 8.38 | |
| RPE (0–10) | Predrink (rest) | |||
| Postdrink (rest) | ||||
| 15 min (25%) | 1.00 ± 0.82 | 1.50 ± 0.58 | 0.88 ± 0.25 | |
| 20 min (25%) | 0.92 ± 0.66 | 1.42 ± 0.66 | 0.83 ± 0.26 | |
| 35 min (50%) | 2.63 ± 0.75 | 3.25 ± 0.96 | 3.25 ± 0.87 | |
| 40 min (50%) | 2.70 ± 0.84 | 2.60 ± 1.14 | 2.60 ± 1.08 | |
| 55 min (75%) | 6.63 ± 0.48 | 6.75 ± 1.26 | 6.25 ± 0.87 | |
| 60 min (75%) | 6.33 ± 1.37 | 6.00 ± 2.00 | 5.58 ± 1.91 |
Values are expressed as means ± SD (n = 6). Exercise measurements are presented as time point with the relative workload in parentheses.
RER and whole-body efficiency
There was a main effect of time (P < 0.001) and tended to be a main effect of condition for RER (P = 0.051; Fig. 2A). However, a time–condition interaction effect was not present. Exploratory post hoc comparisons of main condition effects showed that RER tended to be lower in the low-ketosis versus control condition (P = 0.051). Delta efficiency also tended to be different between conditions (ANOVA, P = 0.098; Fig. 2B), with exploratory post hoc analyses showing that delta efficiency was significantly greater in the low-ketosis versus control condition (25.94% ± 2.11% vs 24.09% ± 1.91%, respectively; P = 0.027).
FIGURE 2.
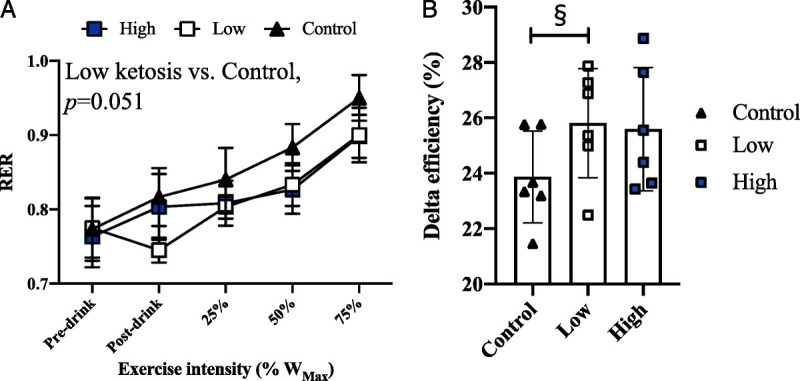
RER and exercise efficiency in the control, low-ketosis, and high-ketosis conditions. A, RER at rest and during exercise (mean ± SD). B, Delta efficiency (in percent), representing the relationship between energy expended per minute vs work accomplished per minute during exercise (mean ± SD). §Significant difference between control and low-ketosis conditions.
Plasma metabolite concentrations
There was a main effect of time (P = 0.003) and condition–time interaction (P = 0.031) effect for blood glucose concentration, which was significantly lower in the high-ketosis versus control condition at rest and after 25% WMax exercise (P = 0.036 and P = 0.035, respectively; Fig. 3A). Significant condition, time, and condition–time interaction effects (all, P < 0.001) were present for blood NEFA concentration, which fell from ~0.6 to ~0.2 mM after ingestion of the KME drinks and was significantly lower at rest (postdrink) and throughout exercise in the low-and high-ketosis versus the control conditions (Fig. 3B). There were no differences in plasma NEFA concentrations between low- and high-ketosis conditions at any time point. There was a main effect of time for plasma lactate concentration (P < 0.001), but no condition or condition–time interaction effects.
FIGURE 3.
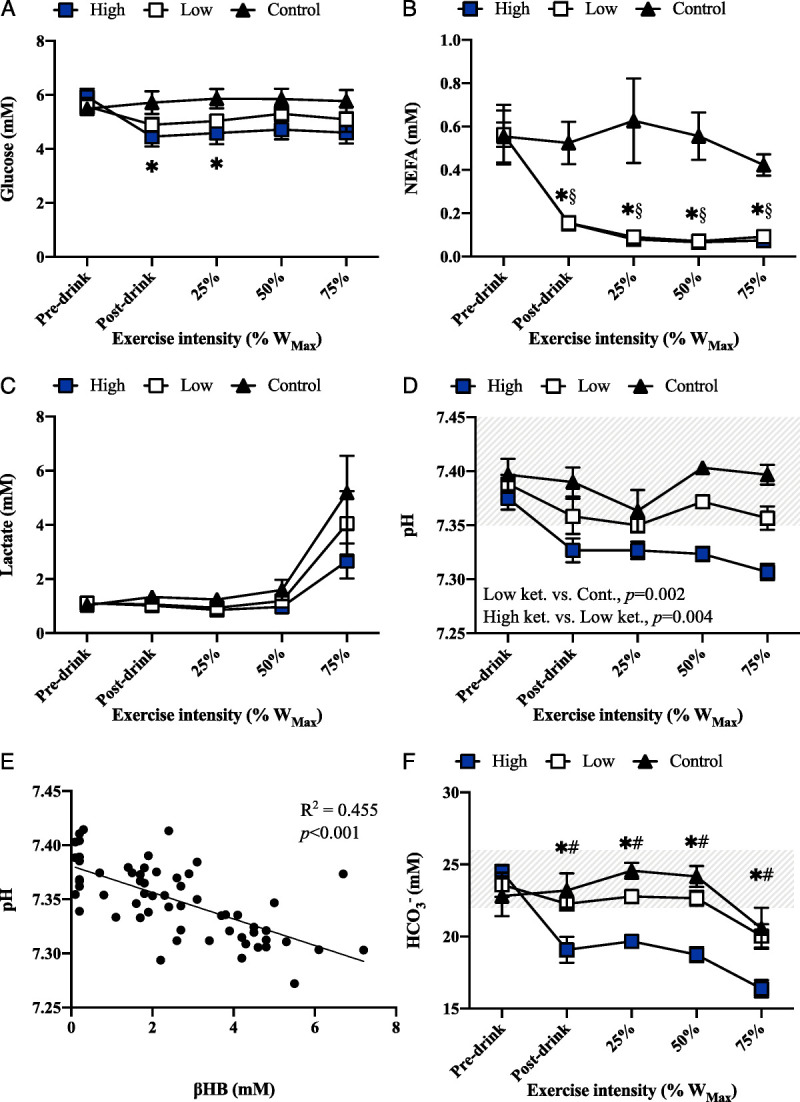
Blood metabolites and gases at rest and during cycling exercise in the control, low-ketosis, and high-ketosis conditions. A, Plasma glucose concentration (in millimolars) at rest and during exercise (mean ± SD). B, Plasma NEFA concentration (in millimolars) at rest and during exercise (mean ± SD). C, Plasma lactate concentration (in millimolars) at rest and during exercise. D, Blood pH at rest and during exercise (mean ± SD). Shaded area represents the normal blood pH range. E, Relationship between blood βHB concentration and pH in the low- and high-ketosis conditions. F, Blood HCO3− concentration (in millimolars) at rest and during exercise (mean ± SD). Shaded area represents the normal blood HCO3− range. *Significant difference between control and high-ketosis conditions. §>Significant difference between control and low-ketosis conditions. #Significant difference between low- and high-ketosis conditions.
Blood acid–base status
There were significant main effects of condition and time (both P < 0.001) for blood pH, which was reduced in the low-ketosis versus control condition (P = 0.002) and high- versus low-ketosis condition (P < 0.001; Fig. 3D). A significant negative correlation was present between blood βHB concentration and pH in the ketosis conditions (R2 = 0.455, P < 0.001; Fig. 3E). In the high-ketosis condition, participants were mildly acidotic (pH 7.33 ± 0.03) before commencing exercise. As would be expected, a similar pattern was observed for blood HCO3− where main effects of condition (P = 0.004) and time (P < 0.001), and a condition–time interaction (P < 0.001) effect were present. Blood HCO3− was lower in the high- and low-ketosis conditions versus control at rest (postdrink) and throughout exercise (Fig. 3F).
Association between muscle fiber type and βHB oxidation rate
There was a significant positive association between the percentage of slow-oxidative muscle fibers in vastus lateralis muscle biopsy samples and peak exogenous βHB oxidation rate (R2 = 0.696, P = 0.039; Fig. 4 and Figure, Supplemental Digital Content 6, example images of vastus lateralis samples, http://links.lww.com/MSS/C119).
FIGURE 4.
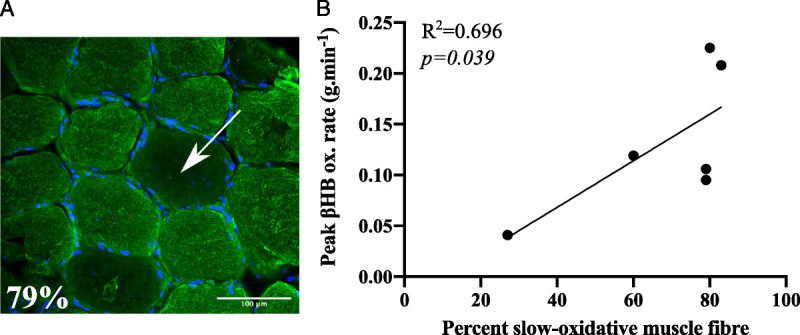
Association between skeletal muscle fiber type and peak exogenous βHB oxidation rate. A, Vastus lateralis biopsy sample stained with an antislow skeletal myosin heavy-chain primary antibody (fluorescent green). Nuclei are represented in blue (stained with DAPI). Example nonstained fibers (representing fast-glycolytic (IIa) and fast (IIb) fibers) are indicated with the white arrow. B, Relationship between peak exogenous d-βHB oxidation rate (in grams per minute) and skeletal muscle phenotype (in percent).
DISCUSSION
The main findings of this study were that (i) increasing blood βHB concentration from ~2 to ~4.4 mM did not affect exogenous βHB oxidation rates at rest or during incremental intensity exercise; (ii) the relative contribution of exogenous βHB oxidation to energy expenditure during exercise was low (2.48%–4.46%) and peaked during the 25% WMax exercise intensity; and (iii) exercise efficiency (measured as delta efficiency) was improved when blood βHB concentration was raised to ~2 mM.
Exogenous βHB oxidation contributes minimally to energy expenditure during exercise
The peak relative contribution of exogenous βHB oxidation to energy expenditure was 4.46% ± 2.71%. This is low compared with values reported for free fatty acids (31% at 40% WMax), other fat sources (27% at 40% WMax), plasma glucose (18% at 75% WMax), and muscle glycogen (58% at 75% WMax) during stepped-intensity cycling ergometer exercise (28). Thus, within the context of this study, exogenous ketones seem to contribute minimally to whole-body energy expenditure during exercise.
Previous work predicted between 16% and 18% of total oxygen consumed during cycling ergometer exercise (40% and 75% WMax, respectively) was attributable to βHB oxidation when similarly highly trained athletes were given a drink containing carbohydrate and 573 mg·kg BW−1 of the KME (5). In the present study, assuming the complete oxidation of βHB requires 4.5 mol of O2 and that 1 mol of gas occupies 22.4 L (13), the peak contribution of exogenous βHB oxidation to V˙O2 was ~4.5%. Without the capacity to directly trace whole-body exogenous βHB oxidation (as performed in this study), previous estimations utilized adjusted substrate oxidation equations from measured gaseous exchanges (13). Inherent to these equations is the assumption that βHB rate of disappearance from the blood (Rd; difference in blood βHB area under the curve (AUC; in millimoles per liter per minute) at rest and during exercise) would accurately reflect exogenous βHB oxidation. However, a substantial proportion of the blood βHB Rd may have been attributable to intermediate metabolic processes, notably, the conversion of βHB to AcAc and its subsequent spontaneous decarboxylation to acetone. Assuming AcAc was subsequently oxidized, or that any acetone produced was excreted in respiratory gases, these intermediate metabolic processes would not affect overall conclusions regarding substrate metabolism (13). However, when there is an accumulation within nonblood body compartments or nonrespiratory gas excretion from the body of these metabolites (discussed further hereinafter), respiratory gas equations would be expected to overestimate exogenous βHB oxidation rate. This may account for some of the discrepancy between findings here and those previously reported (5). However, it is important to note that other work using a 14C AcAc tracer found that ~10% of CO2 was attributable to ketone oxidation in exercising humans (29). Thus, oxidation rates here are also substantially lower than previous direct measures of whole-body exogenous ketone oxidation rates in exercising humans.
Whole-body oxidation accounted for ~27.5% (5.5 g) and ~10.7% (6.4 g) of βHB consumed in the low- and high-ketosis conditions, respectively. Assuming a βHB volume of distribution (VD) of 0.2 L·kg BW−1 (20) (although values as high as 0.5 L·kg BW−1 have been reported [30]), at the end of exercise (t = 60 min), the amount of βHB within the body fluids accounted for a further ~15.5% (~3.1 g) and ~12.0% (~7.1 g) of the βHB consumed in the low- and high-ketosis conditions, respectively. As in previous reports (18), the urinary excretion of βHB was minimal (<1% in both conditions). Given the inhibitory effects of high circulating ketone concentrations on hepatic ketogenesis (1), we assume βHB measured in urine and blood was almost entirely derived from the KME drinks. Thus, ~57% of βHB in the low-dose KME (252 mg·kg BW−1) and ~77% of βHB in the high-dose KME (752 mg·kg BW−1) remained unaccounted for.
Within the mitochondria, βHB is dehydrogenated to form AcAc in a near-equilibrium reaction (1). Previous work has demonstrated an ~4:1 ratio of blood βHB to AcAc in healthy, overnight fasted subjects after ingestion of a 395-mg·kg BW−1 dose of the KME (18), consistent with that observed during an endogenously induced physiological ketosis (1). In addition, breath acetone, formed from the spontaneous decarboxylation of AcAc, was measured at 25 ppm after 1-h rest (~7.5 mg·min−1 acetone expelled, assuming a resting V˙O2 of 0.3 L·min−1), peaking at ~70 ppm (~21 mg·min−1) after 3 h (18). The slower rate of appearance (Ra) of acetone is likely attributable to sequestration in lipid stores, represented by an acetone VD of ~0.6–0.8 L·kg−1 (13). Taken together, these unmeasured ketones may account for a substantial proportion of the unaccounted exogenous βHB.
Doubling blood ketone concentrations did not result in greater oxidation
Exogenous ketone uptake in skeletal muscle is saturated at a relatively low circulating concentration in resting subjects (~0.8 mM vs a physiological plateau of ~7 mM) (31). Inactive skeletal muscle has relatively low energy demand; however, upon commencement of exercise, a large proportion of muscle may be activated (depending on the form of exercise undertaken), leading to a dramatic increase in blood supply to and energy flux within this tissue (32). Accordingly, exercise seems to “override” resting saturation kinetics: skeletal muscle ketone oxidation is between ~5-fold (31) and ~11-fold (29) higher upon commencement of exercise, resulting in a similar uptake per kilograms of tissue as the brain (31). This suggests that skeletal muscle ketone oxidation is not restricted by ketone transport and ketolytic capacity per se. Given this, in the present study, we postulated that ketone oxidation rates during exercise would be proportional to blood concentrations (i.e., significantly greater in the high- versus low-ketosis conditions).
In agreement with previous work (5,29), we found exercise stimulated an ~6-fold increase in ketone oxidation in both low- and high-ketosis conditions. However, despite an approximate doubling of circulating βHB, oxidation rates were similar between conditions at rest and throughout all exercise intensities. Thus, we conclude that exercise but not circulating concentrations (at least >~2 mM) can stimulate an increase in ketone oxidation in skeletal muscle. This result is consistent with our observation that exogenous βHB is a minimal contributor to energy expenditure during exercise. As previously described, the primary purpose of ketogenesis is to provide a supplementary substrate to support cerebral metabolism when carbohydrate stores are challenged. Skeletal muscle is metabolically flexible and can meet most of its energy needs from the oxidation of abundant lipid stores. As such, it is logical that ketone oxidation in skeletal muscle is tightly regulated, thus preserving these respiratory fuels for the brain.
The greatest relative contribution of exogenous βHB oxidation is during light-intensity exercise
We previously reported that after ingestion of 573 mg·kg BW−1 KME, βHB AUC was significantly decreased at greater exercise intensities and subsequently, negatively correlated with oxygen consumption (5). In agreement with this work, here we found a significant association between exogenous βHB oxidation rate and V˙O2. Notably, this relationship remained significantly nonzero even when resting values were excluded (although the variation in exogenous βHB oxidation explained was substantially lower, representing just ~11%). However, contrary to our previous report (5), exogenous βHB oxidation was not increased when the exercise intensity was raised from 50% WMax to 75% WMax. As described, previous estimates of exogenous ketone oxidation relied on βHB Rd from the blood. It is possible that the inhibition of ketolysis downstream of the BDH reaction could be increased at higher exercise intensities, resulting in an accumulation of AcAc and, in turn, acetone. Inhibitory mechanisms may include a depletion of the succinyl-CoA pool, which is a substrate for the SCOT catalyzed reaction, or product inhibition from increased acetyl-CoA (primarily derived from carbohydrate oxidation) on the acetyl-CoA acetyltransferase 1 catalyzed reaction. In such a case, changes in blood βHB Rd would overestimate βHB oxidation.
Arguably of more practical importance is the relative contribution of exogenous βHB oxidation to overall energy expenditure, which was low throughout exercise and declined with successive increments in exercise load. This observation is consistent with the increased importance of the ATP synthesized/oxygen consumed ratio (P/O ratio) at higher exercise intensities (7), which is greater for pyruvate versus βHB (4).
Exercise efficiency is increased during ketosis
Elevating blood ketone concentrations to ~2 mM resulted in an ~7% improvement in exercise efficiency versus the control condition. This effect was driven by small, nonsignificant reductions in both V˙O2 and V˙CO2 at the 50% and 75% WMax exercise intensities during ketosis, leading to an improvement in the ratio of the change of work accomplished per minute (which was fixed) and the change in energy expended per minute (i.e., delta efficiency [23]). βHB is more chemically reduced than pyruvate and its combustion increases the electron transport redox span, thus creating a greater ΔG′ATP for the generation of ATP (4,6). In the working rat heart perfused with a physiological ratio of ketones and glucose versus glucose alone, these effects were reported to increase hydraulic efficiency (J·mol O2−1) by 25% (6). Thus, a convenient explanation for the observed improvement in exercise efficiency is that exogenous ketone oxidation in exercising skeletal muscle resulted in lower oxygen consumption at the same (fixed) power output. However, it is unclear whether a peak ~4.5% contribution from exogenous βHB oxidation to energy expenditure would be sufficient to drive the observed improvements in exercise efficiency.
It is plausible that improvements in exercise efficiency resulted from ketosis-mediated alterations in carbohydrate and fat metabolism. However, the oxidation of fat seems to be augmented during exogenous ketosis (5), which has a lower P/O ratio versus carbohydrate (reflecting a reduction in the ATP produced vs oxygen consumed) (7) and would therefore be expected to lower exercise efficiency (33). Further work is required to both confirm the observed improvement in exercise efficiency during exogenous ketosis and determine the mechanisms contributing to this effect.
Skeletal muscle fiber phenotype may influence exogenous βHB oxidation rate
The activities of BDH and SCOT (which catalyze the first and second reactions of ketolysis, respectively) are, respectively, ~1.5 and ~2.5 times greater in slow-oxidative versus fast glycolytic rat skeletal muscle fibers (3). As such, it is postulated that the uptake and utilization of ketones are greater in athletes with a slow-oxidative skeletal muscle phenotype (34). In this study, we provide preliminary data supporting this hypothesis. Using simple linear regression, participants’ muscle phenotype explained ~70% of variation in exogenous βHB oxidation. Although a plausible finding, these data should be interpreted with caution as the association was driven by one participant with an extremely fast muscle phenotype (former national-level sprinter, undertaking training for 5- to 10-km running at the time of enrollment). Notably, this individual had an ~1.5-fold increase in blood βHB AUC and an ~2-fold increase in blood lactate AUC versus the mean for the remaining participants, and consistently lower blood pH. These results are consistent with a reduced uptake and oxidation of exogenous βHB in this athlete. Further work is warranted to test our preliminary results indicating that skeletal muscle phenotype is an important modulator of exogenous βHB oxidation in exercising humans.
Ketosis-mediated changes in glucose, nonesterified fatty acids, and lactate concentrations were not affected by elevating circulating ketone concentrations
Ketones have pleiotropic effects on metabolism, including acting as signaling molecules to alter the availability and catabolism of other substrates. Both βHB and AcAc are agonists for the hydroxycarboxylic acid receptor 2 on adipocyte cell membranes, which through a decrease in cyclic adenosine monophosphate signaling leads to a reduction in lipolytic rate and consequently, circulating NEFA and glycerol concentrations (35). Glucose concentrations also fall during exogenous ketosis (5), although the mechanisms have not yet been established. Ketone metabolism also inhibits glycolysis (5), potentially via glucose-fatty acid cycle–like mechanisms (36), which is commonly observed as a reduction in blood lactate.
Previous work has shown sequential decreases in blood glucose and glycerol concentrations with successive increases in blood ketone concentrations of ~0.5, 0.8, and 1.7 mM (31). Notably, the greatest decreases were observed from baseline (preinfusion) to ~0.5 mM. In this study, no differences were observed for glucose, NEFA, or lactate concentrations between blood ketone concentrations of ~2 and ~4.4 mM, either at rest or during exercise. Taken together, these results provide evidence for a blood ketone concentration threshold for ketosis-mediated alterations in circulating substrate concentrations, which is likely to occur <2 mM.
Acid–base regulation
Consumption of the KME drinks caused dose-dependent falls in blood pH (ketoacidemia). Notably, participants in the high-ketosis condition were acidotic before commencing exercise (pH 7.32). Furthermore, although blood buffering capacity corrected toward control values in the low-ketosis condition, HCO3− remained significantly depressed throughout exercise in the high-ketosis condition. Unlike previous work (25), ketoacidemia did not alter participants’ perceptions of exercise exertion at any exercise intensity, nor were they affected by the degree of ketoacidemia induced (low vs high ketosis). We suggest that this was due to the comparatively lower exercise intensities and longer exercise intervals used in this study, with even minor changes in the relationship between time and power output having been shown to yield differences in the magnitudes of reported exercise exertion (37). Moreover, the rationale for including RPE measures within this study was to help ascertain (together with other cardiorespiratory measures) whether participants had achieved a “steady state” at the end of each exercise interval, not to determine the effects of blood ketone concentrations on subjective perceptions of exercise exertion, which, given the design used (three conditions, six time points), the study was underpowered to achieve.
Limitations and future directions
It is important that exogenous βHB oxidation results presented here are interpreted in the context of an exogenously induced ketosis. It is very likely that whole-body oxidation rates during exercise would be different in the setting of a starvation- or dietary-induced ketosis. Participants recruited had been consuming a standard carbohydrate-rich diet before enrollment. Skeletal muscle is a highly plastic tissue, capable of altering its morphology and metabolism in response to a range of environmental stimuli, including diet (38). As such, it is possible that exogenous βHB oxidation rates and relative contributions to overall energy expenditure would be greater in individuals chronically exposed to hyperketonemia (“keto-adapted”), for example, through adoption of a ketogenic diet or even chronic consumption of exogenous ketones. Although preliminary data demonstrated that skeletal muscle phenotype influences exogenous βHB oxidation rates, further work in a wider population of athletes is warranted to test this association. Lastly, stable isotope tracers provide an invaluable tool for determining in vivo substrate oxidation. However, findings must be interpreted within the context of the inherent assumptions that apply to this methodology (e.g., that the 13C-labeled KME will behave identically to the nonlabeled KME).
CONCLUSIONS
We report that exogenous βHB oxidation contributes minimally to energy expenditure at rest and during exercise in highly trained athletes. Furthermore, we find that oxidation rate is not affected by doubling blood βHB from ~2 to ~4.4 mM. Although peak exogenous βHB oxidation occurs at a light-moderate exercise intensity (50% WMax), the greatest relative contribution is during light exercise (25% WMax). Finally, despite low βHB oxidation rates, exercise efficiency was improved when blood βHB concentration was elevated to ~2 mM versus control.
Supplementary Material
Acknowledgments
K. C. is director of TΔS Ltd, a spin-out company of the University of Oxford, which seeks to develop and commercialize products based on the ketone monoester. D. D. is a current employee of TΔS Ltd and O. H. a former employee.
The authors would like to thank Brianna Stubbs, Edward Stace, Rita Alonaizan, and Edward Rolls for their valuable contributions to this research. Funding for this work was provided by TΔS Ltd and The Royal Commission for the Exhibition of 1851.
The authors declare that the results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation and do not constitute endorsement by the American College of Sports Medicine.
D. D., O. H., and P. C. were involved in the experimental design and data collection. D. D. performed data analysis and was responsible for the manuscript preparation. L. H. and A. J. assisted with data analysis and manuscript preparation. K. C. was involved in the manuscript preparation and support for the study. All authors reviewed the manuscript.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.acsm-msse.org).
Contributor Information
OLIVIA K. HARRISON, Email: faull@biomed.ee.ethz.ch.
LEANNE HODSON, Email: leanne.hodson@ocdem.ox.ac.uk.
ANDREW JEFFERSON, Email: jeffersona@cardiff.ac.uk.
KIERAN CLARKE, Email: kieran.clarke@dpag.ox.ac.uk.
PETE J. COX, Email: petecox123@hotmail.com.
REFERENCES
- 1.Robinson A, Williamson D. Physiological roles of ketone bodies as substrates and signals in mammalian tissues. Physiol Rev. 1980;60(1):143–87. [DOI] [PubMed] [Google Scholar]
- 2.Owen OE, Morgan AP, Kemp HG, Sullivan JM, Herrera MG, Cahill GF. Brain metabolism during fasting. J Clin Investig. 1967;46(10):1589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winder W, Holloszy J, Baldwin K. Enzymes involved in ketone utilization in different types of muscle: adaptation to exercise. Eur J Biochem. 1974;47(3):461–7. [DOI] [PubMed] [Google Scholar]
- 4.Cox PJ, Clarke K. Acute nutritional ketosis: implications for exercise performance and metabolism. Extrem Physiol Med. 2014;3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cox PJ Kirk T Ashmore T, et al. . Nutritional ketosis alters fuel preference and thereby endurance performance in athletes. Cell Met. 2016;24(2):256–68. [DOI] [PubMed] [Google Scholar]
- 6.Sato K Kashiwaya Y Keon CA, et al. . Insulin, ketone bodies, and mitochondrial energy transduction. FASEB J. 1995;9(8):651–8. [DOI] [PubMed] [Google Scholar]
- 7.Spriet LL. New insights into the interaction of carbohydrate and fat metabolism during exercise. Sport Med. 2014;44(1 Suppl):S87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dearlove DJ, Faull OK, Rolls E, Clarke K, Cox PJ. Nutritional ketoacidosis during incremental exercise in healthy athletes. Front Physiol. 2019;10:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans M, McSwiney FT, Brady AJ, Egan B. No benefit of ingestion of a ketone monoester supplement on 10-km running performance. Med Sci Sports Exerc. 51(12):2506–15. [DOI] [PubMed] [Google Scholar]
- 10.Evans M, Egan B. Intermittent running and cognitive performance after ketone ester ingestion. Med Sci Sports Exerc. 2018;50(11):2330–8. [DOI] [PubMed] [Google Scholar]
- 11.Poffé C, Ramaekers M, Bogaerts S, Hespel P. Exogenous ketosis impacts neither performance nor muscle glycogen breakdown in prolonged endurance exercise. J Appl Physiol. 2020;128(6):1643–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leckey JJ, Ross ML, Quod M, Hawley JA, Burke LM. Ketone diester ingestion impairs time-trial performance in professional cyclists. Front Physiol. 2017;8:806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol Respir Env Exerc Physiol. 1983;55(2):628–34. [DOI] [PubMed] [Google Scholar]
- 14.Holdsworth DA, Cox PJ, Kirk T, Stradling H, Impey SG, Clarke K. A ketone ester drink increases postexercise muscle glycogen synthesis in humans. Med Sci Sport Exerc. 2017;49(9):1789–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergstrom J, Hultman E. Diet, muscle glycogen and physical performance. Acta Physiol Scand. 1967;71(2):140–50. [DOI] [PubMed] [Google Scholar]
- 16.Liu D, Moberg E, Kollind M, Lins P, Adamson U, Macdonald I. Arterial, arterialized venous, venous and capillary blood glucose measurements in normal man during hyperinsulinaemic euglycaemia and hypoglycaemia. Diabetologia. 1992;35:287–90. [DOI] [PubMed] [Google Scholar]
- 17.Stubbs BJ, Cox PJ, Kirk T, Evans RD, Clarke K. Gastrointestinal effects of exogenous ketone drinks are infrequent, mild, and vary according to ketone compound and dose. Int J Sport Nutr Exerc Metab. 2019;29(6):596–603. [DOI] [PubMed] [Google Scholar]
- 18.Stubbs BJ Cox PJ Evans RD, et al. . On the metabolism of exogenous ketones in humans. Front Physiol. 2017;8:848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shivva V, Cox PJ, Clarke K, Veech RL, Tucker IG, Duffull SB. The population pharmacokinetics of d-β-hydroxybutyrate following administration of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate. AAPS J. 2016;18(3):678–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beylot M, Beaufrère B, Normand S, Riou JP, Cohen R, Momex R. Determination of human ketone body kinetics using stable-isotope labelled tracers. Diabetologia. 1986;29(2):90–6. [DOI] [PubMed] [Google Scholar]
- 21.Chong MF, Fielding BA, Frayn KN. Mechanisms for the acute effect of fructose on postprandial lipemia. Am J Clin Nutr. 2007;85(6):1511–20. [DOI] [PubMed] [Google Scholar]
- 22.Jeukendrup AE, Wallis GA. Measurement of substrate oxidation during exercise by means of gas exchange measurements. Int J Sport Med. 2005;26(1 Suppl):S28–37. [DOI] [PubMed] [Google Scholar]
- 23.Moseley L, Jeukendrup AE. The reliability of cycling efficiency. Med Sci Sports Exerc. 2001;33(4):621–7. [DOI] [PubMed] [Google Scholar]
- 24.Coyle EF, Sidossis LS, Horowitz JF, Beltz J. Cycling efficiency is related to the percetnage of type I muscle fibers. Med Sci Sports Exerc. 1992;24(7):782–8. [PubMed] [Google Scholar]
- 25.Faull OK, Dearlove DJ, Clarke K, Cox PJ. Beyond RPE: the perception of exercise under normal and ketotic conditions. Front Physiol. 2019;10:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koopman R, Schaart G, Hesselink MK. Optimisation of oil red O staining permits combination with immunofluorescence and automated quantification of lipids. Histochem Cell Biol. 2001;116(1):63–8. [DOI] [PubMed] [Google Scholar]
- 27.Takano Y, Kobayashi H, Yuri T, Yoshida S, Naito A, Kiyoshige Y. Fat infiltration in the gluteus minimus muscle in older adults. Clin Interv Aging. 2018;13:1011–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Loon L, Greenhaff P, Constantin-Teodosiu D, Saris W, Wagenmakers A. The effects of increasing exercise intensity on muscle fuel utilisation in humans. J Physiol. 2001;536(1):295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balasse EO, Fery F, Neef M. Changes induced by exercise in rates of turnover and oxidation of ketone bodies in fasting man. J Appl Physiol Respir Env Exerc Physiol. 1978;44(1):5–11. [DOI] [PubMed] [Google Scholar]
- 30.Cobelli C Nosadini R Toffolo G, et al. . Model of the kinetics of ketone bodies in humans. Am J Physiol. 1982;243(1):R7–17. [DOI] [PubMed] [Google Scholar]
- 31.Mikkelsen KH, Seifert T, Secher NH, Grøndal T, Van Hall G. Systemic, cerebral and skeletal muscle ketone body and energy metabolism during acute hyper-d-β-hydroxybutyratemia in post-absorptive healthy males. J Clin Endocrinol Metab. 2015;100(2):636–43. [DOI] [PubMed] [Google Scholar]
- 32.Frayn KN, Evans RD. Human Metabolism: A Regulatory Perspective. 4th ed Hoboken (NJ): Wiley-Blackwell; 2019. [Google Scholar]
- 33.Burke LM Ross ML Garvican-Lewis LA, et al. . Low carbohydrate, high fat diet impairs exercise economy and negates the performance benefit from intensified training in elite race walkers. J Physiol. 2017;595(9):2785–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Evans M, Cogan KE, Egan B. Metabolism of ketone bodies during exercise and training: physiological basis for exogenous supplementation. J Physiol. 2017;595(1):2857–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taggart AK Kero J Gan X, et al. . (d)-beta-hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J Biol Chem. 2005;280(29):26649–52. [DOI] [PubMed] [Google Scholar]
- 36.Randle P, Garland P, Hales C, Newsholme E. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1(7285):785–9. [DOI] [PubMed] [Google Scholar]
- 37.Borg E, Kaijser L. A comparison between three rating scales for perceived exertion and two different work tests. Scand J Med Sci Sport. 2006;16(1):57–69. [DOI] [PubMed] [Google Scholar]
- 38.Egan B, Zierath JR. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Met. 2013;17(2):162–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


