Supplemental digital content is available in the text.
Key Words: NUTRITION, DIET, AEROBIC EXERCISE, RESISTANCE EXERCISE, FAT MASS, LEAN MASS
ABSTRACT
Purpose
Excess fat mass (FM) contributes to poor prostate cancer (PCa) prognosis and comorbidity. However, FM gain is a common side effect of androgen deprivation therapy (ADT). We examined the efficacy of a 12-wk weight loss intervention to reduce FM and maintain lean mass (LM) in ADT-treated obese PCa patients.
Methods
Fourteen ADT-treated obese PCa patients (72 ± 9 yr, 39.7% ± 5.4% body fat) were recruited for a self-controlled prospective study, with 11 completing the 6-wk control period, followed by a 12-wk intervention comprising 300 min·wk−1 of exercise including supervised resistance training and home-based aerobic exercise, and dietitian consultations advising a daily energy deficit (2100–4200 kJ) and protein supplementation. Body composition was assessed by dual x-ray absorptiometry. Secondary outcomes included muscle strength (one-repetition maximum), cardiorespiratory fitness (maximal oxygen consumption), and blood biomarkers.
Results
There were no significant changes during the control period. Patients attended 89% of supervised exercise sessions and 100% of dietitian consultations. No changes in physical activity or energy intake were observed. During the intervention, patients experienced significant reductions in weight (−2.8 ± 3.2 kg, P = 0.016), FM (−2.8 ± 2.6 kg, P < 0.001), and trunk FM (−1.8 ± 1.4 kg, P < 0.001), with LM preserved (−0.05 ± 1.6 kg, P = 0.805). Muscle strength (4.6%–24.7%, P < 0.010) and maximal oxygen consumption (3.5 ± 4.7 mL·min−1·kg−1, P = 0.041) significantly improved. Leptin significantly decreased (−2.2 (−2.7 to 0.5) ng·mL−1, P = 0.016) with no other changes in blood biomarkers such as testosterone and lipids (P = 0.051–0.765); however, C-reactive protein (rs = −0.670, P = 0.024) and triglycerides (r = −0.667, P = 0.025) were associated with individual changes in LM.
Conclusions
This study shows preliminary efficacy for an exercise and nutrition weight loss intervention to reduce FM, maintain LM, and improve muscle strength and cardiorespiratory fitness in ADT-treated obese PCa patients. The change in body composition may affect blood biomarkers associated with obesity and PCa progression; however, further research is required.
Overweight and obese men with prostate cancer are at increased risk of recurrence, progression to castrate resistance, advanced-stage disease, and prostate cancer-specific mortality (1–3). Obesity is also associated with the development of comorbidities such as cardiovascular disease (CVD) and diabetes (4). Although much of the evidence on obesity and poor prostate cancer outcomes relies on body mass index (BMI) as a measure of obesity (2), it is the altered metabolic environment created by excess fat mass (FM) that is critical (1,5). Accumulation of fat has been associated with increased risk of advanced and fatal prostate cancer (1,5). Although the exact mechanisms are unclear, altered insulin/insulin-like-growth-factor axis and sex hormone concentrations, and abnormal adipokine and cytokine signaling are commonly suggested (6).
Obese prostate cancer patients initiating androgen deprivation therapy (ADT) may be at a higher risk of faster cancer progression than those of normal weight on the same treatment (7). ADT reduces testosterone to castrate levels resulting in significant changes in body composition (8). Prostate cancer patients treated with ADT have been reported to gain 13.8% in FM and lose 2.4% in both lean and bone mass within the first year of treatment (9). Greater FM has also been associated with exacerbating other ADT-related side effects, including increased serum triglycerides and reduced quality of life, specifically higher fatigue and lower vitality levels (10,11). ADT may be prescribed from 3 months to several years, or indefinitely in some cases, with most patients receiving ADT at some stage after diagnosis (8). Therefore, it is fundamental to establish management strategies that can ameliorate or prevent further ADT-induced changes to improve prostate cancer patients’ quality of life and physical well-being.
Lifestyle changes involving exercise and nutrition are often strategies implemented for weight loss. Exercise can be safely performed by ADT-treated prostate cancer patients to improve physical function, quality of life, fatigue levels, and lean mass (LM) (12). Appropriate nutrition may also mitigate ADT-related side effects by inducing weight loss, supporting bone health by optimizing calcium and vitamin D intake, and potentially reducing prostate cancer progression with the consumption of specific foods or eating patterns (13,14). However, evidence for appropriate nutrition prescription is variable (14,15). To date, three combined exercise and nutrition studies have reported weight loss. Freedland et al. (16) and O’Neill et al. (17) both targeted weight loss, and Focht et al. (18) aimed to improve mobility; however, all demonstrated significant fat loss to be feasible in prostate cancer patients on ADT showing declines of 1.8–8.2 kg. Although all studies included obese patients, only Freedland et al. (16) specifically targeted patients who were overweight or obese. Cancer progression has been suggested to increase linearly with obesity status (3), and development of multiple comorbidities is also more likely for obese individuals (4). Therefore, obese patients with prostate cancer, compared with those who are considered of normal weight or overweight, are a high-risk population, and it is important to clarify how they respond to exercise and nutrition interventions, especially those expected to receive long-term ADT (7). In addition, Freedland et al. (16) reported a significant 2.1-kg loss in LM with their weight loss program, although both O’Neill et al. (17) and Focht et al. (18) found no change in LM. It is important for an intervention to stimulate both lipolysis and muscle protein synthesis, as the preservation of LM plays a key role in the maintenance of weight loss and improvement in insulin resistance and physical function (19).
Currently, men with prostate cancer are recommended to maintain a healthy weight and stay physically active during their treatment to prevent or reduce treatment-related side effects (20). If weight loss is required, patients are recommended to undertake high-volume exercise and consume a healthy balanced diet with an energy deficit, which is the same advice for the general population (20). Although these recommendations are likely beneficial for prostate cancer patients, their efficacy within this population has not been confirmed, especially for obese patients who are at increased risk. Therefore, this study aimed to examine the preliminary efficacy of a 12-wk exercise and nutrition weight loss intervention in obese prostate cancer patients on ADT to reduce FM, maintain LM, and improve physical function and blood biomarkers associated with cancer progression and obesity. We hypothesized that the weight loss program would reduce FM while preserving LM.
METHODS
Participants
Fifty-four men with prostate cancer were screened from February 2018 to June 2019 in Perth, Western Australia. Potential participants were identified through clinician referral, advertisements in local newspapers, and presentations at cancer support groups and cancer-related events. Patients were screened for eligibility over the phone (n = 54) with a recruitment package mailed to interested and eligible patients (n = 27). Inclusion criteria were as follows: receiving ADT for a minimum of 6 months, anticipated to remain on ADT for the entire study period, and being obese, defined as a body fat percentage ≥25% (21) assessed by dual x-ray absorptiometry (DXA; Horizon A, Hologic, Waltham, MA) at their first visit. Exclusion criteria were as follows: presence of bone metastases, a secondary cancer diagnosis, a musculoskeletal or uncontrolled comorbidity preventing participation in moderate-to-vigorous intensity exercise, or did not speak English. Fourteen patients were recruited to the study after written informed consent, with further medical clearance gained from their general practitioner before baseline testing. The study was approved by the Edith Cowan University Human Research Ethics Committee (ID: 18832).
Study Design
This was a single-group, self-controlled 18-wk prospective study comprising a 6-wk control period during which patients undertook their usual activities and were not provided with any exercise or nutrition information, followed by a 12-wk exercise and nutrition weight loss intervention. The use of a self-controlled study design was deemed ethically appropriate for this population given the plethora of evidence highlighting exercise to be beneficial for prostate cancer patients on ADT. As such, the 6-wk control period was included in lieu of a randomized control trial design to inform whether the changes observed during the 12-wk intervention period were due to the intervention compared with activities of normal day-to-day living and usual care. Testing was conducted in the Exercise Medicine Research Institute (Edith Cowan University), over 2 to 3 nonconsecutive days at baseline (week 0), preintervention (week 6), and postintervention (week 18). Supervised exercise was conducted at exercise clinics in Joondalup or Mt Lawley (Edith Cowan University) nearest to each patient.
Exercise and Nutrition Intervention
Patients undertook combined aerobic and resistance training to accumulate 300 min of exercise per week for 12 wk. Patients attended three supervised resistance training sessions each week targeting the major muscle groups of the upper and lower body, with exercise variations provided every 3 wk. A periodized and progressive resistance training program was provided, with intensity ranging from 6 to 12 repetition maximum over 1–4 sets per exercise, with the load increased by 5%–10% based on a subjective assessment of the patient’s ability to complete the prescribed volume with the correct technique. Each session was designed to span 60 min in duration, including a 5- to 10-min aerobic-based warm-up and cool down. Patients also completed self-directed moderate-to-vigorous intensity aerobic exercise daily, defined as an RPE of 3–8 on the Borg 1–10 scale according to the Exercise and Sport Science Australia’s exercise intensity guidelines (22), using modalities of their own choice. Patients were provided with an education booklet containing information on goal setting and exercise and nutrition advice to assist with construction of a self-directed home-based routine.
Patients attended three consultations with an Accredited Practising Dietitian including an initial session to complete a diet history at preintervention (week 6) and 2 nutrition counseling sessions during the first and third weeks of the 12-wk intervention. Individual nutrition goals were developed with each patient. The advice was designed to 1) establish an estimated energy deficit of 2100–4200 kJ (500–1000 kcal), 2) reduce consumption of discretionary items including alcoholic drinks and foods containing refined sugars, and 3) maintain protein intake. Patients were also provided with a 40-g whey protein supplement (Whey Protein Concentrate; Bulk Nutrients, Tasmania, Australia) three times per week immediately after each supervised resistance exercise session to support muscle protein synthesis.
Measurements
Body composition and anthropometry
The primary outcome FM (in kilograms), in addition to secondary outcomes total body mass (in kilograms), bone-mineral free LM (in kilograms), body fat percent, trunk FM (in kilograms), visceral FM (in grams), appendicular skeletal mass (ASM; in kilograms), and bone mineral content (BMC; in grams), was assessed by DXA. ASM was calculated as the sum of upper limb and lower limb LM (23). Waist and hip circumference (in centimeters) were measured with a constant-tension tape measure, and BMI was calculated as body mass in kilograms divided by height in meters squared (kg·m−2).
Muscle strength and cardiorespiratory fitness
Upper and lower body muscle strength was assessed using the one-repetition maximum (24) for the chest press, leg press, and seated row at baseline, preintervention, and postintervention, with a familiarization session provided before baseline. Cardiorespiratory fitness was assessed using a cardiopulmonary exercise test (CPET) at preintervention and postintervention only. Patients completed a standardized progressive maximal walking test (Modified Bruce Protocol) on a motorized treadmill (25) with expired gas collected via a face mask (Hans Rudolph Inc., Shawnee, KS) to obtain maximal oxygen consumption (V˙O2max; analyzed by TrueOne 2400; Parvo Medics, Salt Lake City, UT). The protocol included monitoring of heart rhythm and rate using a 12-lead ECG (CardioDirect 12S; SpaceLabs HealthCare, Snoqualmie, WA) supervised by a medical doctor. Patients completed a 3- to 5-min warm-up at a self-selected walking pace at 0% gradient that continued into stage 1 of the test (2.7 km·h−1, 0% gradient). Speed and/or gradient was increased every 3 min until the patient reached volitional fatigue, defined as an RPE of 9 or 10 on the 10-item Borg scale. A secondary criterion for attainment of V˙O2max was a respiratory exchange ratio greater than 1.1. The test was concluded if the patient voluntarily stopped, or if signs of chest pain, dizziness, faintness, ischemic ECG changes, abnormal blood pressure, or significant symptoms of concern were evident. Total time of test (in seconds), absolute V˙O2max (in liters per minute), and relative V˙O2max (in milliliters per minute per kilogram) were recorded.
Blood biomarkers
Blood serum biomarkers were assessed at preintervention and postintervention. Patients attended a National Association of Testing Authorities–accredited phlebotomy clinic (Australian Clinical Laboratories, Perth, WA, Australia) where two serum separation tubes and one ethylenediaminetetraacetic acid tube were obtained in the morning after a minimum of 10-h overnight fast. Lipid profile including total cholesterol, HDL, LDL, triglycerides, C-reactive protein (CRP), insulin, hemoglobin A1c (HbA1c), testosterone, and prostate-specific antigen (PSA) were commercially analyzed (Australian Clinical Laboratories). Serum from one serum separation tube was stored in a −80°C alarm-controlled freezer at the Exercise Medicine Research Institute until analyzed for adiponectin, leptin, insulin-like growth factor-1 (IGF-1), IGF-binding protein-3 (IGFBP-3), and interleukin 6 (IL-6) in duplicate or triplicate, depending on agent volume available, using human serum enzyme-linked immunosorbent assays (Abcam, Cambridge, United Kingdom).
Monitoring intervention adherence
Adherence was assessed using a customized adherence questionnaire (Table, Supplemental Digital Content 1, adherence questionnaire, http://links.lww.com/MSS/C134) completed weekly during the 12-wk intervention. This questionnaire was adapted from Erdrich et al. (26) and Martínez-González et al. (27), and designed to provide an estimated frequency of consumption and number of serves of foods of interest over the previous week, based on the nutrition advice given, and whether patients completed at least 30 min·d−1 of purposeful exercise. Food items of interest included fruit, vegetables and nuts, high-protein foods, dairy, grains and cereals, beverages and alcoholic drinks, and discretionary and take-away items. It also addressed barriers and facilitators to meeting exercise and nutrition goals, which were discussed during supervised exercise sessions. Patients were asked 25 yes/no questions, where a score of 1 was given if the patient met a predetermined desired outcome or 0 if not. A higher total score indicated greater compliance, with a maximum score of 25. Adherence to the supervised resistance sessions was also recorded based on attendance and exercise volume completed each session compared with what was prescribed.
Physical activity monitoring
Physical activity and sedentary behavior were assessed using the ActiGraph wGT3X-BT (ActiGraph LLC, Pensacola, FL) at baseline, preintervention, midintervention, and postintervention. Patients wore the accelerometer on their hip for 3 consecutive days (1 weekend day and 2 weekdays) excluding water-based activities. ActiLife software (ActiLife 6; ActiGraph LLC) was used to analyze the ActiGraph data. Only wake wear time was used with a minimal data collection period set for inclusion in analysis of 1 d of at least 600 min. Nonwear time was excluded from the analysis, defined as ≥90 min of consecutive zeros with a 2-min spike tolerance (28). Commonly used cutoff points among cancer patients were used to classify sedentary time (<100 counts per minute), light physical activity (100–1951 counts per minute), and moderate-to-vigorous physical activity (≥1952 counts per minute) (29–31).
Nutrition monitoring
Patients completed a 3-d weighed food record (3d-WR) over 3 consecutive days (1 weekend day and 2 weekdays) at baseline, preintervention, midintervention, and postintervention. This information provided an estimate of total energy intake (in kilojoules per day) and macronutrients and micronutrients consumed. The 3d-WR data were analyzed using FoodWorks (FoodWorks 10 Professional; Xyris Software Pty Ltd, Brisbane, QLD, Australia).
Statistical Analysis
Sample size was determined using data from three trials completed within the Exercise Medicine Research Institute (24,32,33). Based on a total sample of 78 prostate cancer patients undertaking ADT who had completed an exercise program of 12 or 24 wk in duration, the calculated SD of change for our primary outcome FM was 2.1 kg. The goal was to achieve a ≥2-kg reduction in FM over the 12-wk intervention period, which would be considered clinically significant (≥5% reduction) (34). For a single-group study design, 12 patients were required to achieve power of 90% at an α of 0.05 (two-tailed). To account for a potential dropout of ~15%, our goal was to recruit 14 patients. Data were analyzed using IBM SPSS version 25 (SPSS Inc., IBM Corp, Armonk, NY). Normality of the distribution was assessed using the Shapiro–Wilk test. Analysis included one-way repeated-measures ANOVA followed by a Bonferroni post hoc test to account for multiple comparisons, or Friedman’s ANOVA for nonnormally distributed data followed by a Bonferroni-adjusted Wilcoxon signed rank test to locate significant differences, as appropriate. Associations between variables were assessed using Pearson correlation or Spearman rank correlation, as appropriate. Data are presented as mean ± SD, median and interquartile range (IQR), or number (percentage). All tests were two-tailed with statistical significance set at P < 0.05.
RESULTS
Fourteen men with prostate cancer age 48 to 84 yr were included in the study (Table 1). Most patients had a Gleason score of 9 (57.1%), with 42.9% diagnosed with metastatic prostate cancer in lymph nodes or organs at study entry. All patients were on ADT for a minimum of 6 months (range, 6–55 months), and 13 men had additional therapy, mainly radiation (92.9%). Two patients withdrew after baseline testing (loss to follow-up, time commitment), and a third withdrew at preintervention testing (family commitments). Eleven patients (age 63–82 yr) completed the 12-wk intervention. There was a significant increase in the weekly adherence questionnaire score from 14.6 ± 2.2 at week 1 of the intervention to 17.6 ± 2.3 at week 12 (P = 0.001). Changes for physical activity and nutrition over the control period and intervention are provided in the supplementary materials (Tables; Supplemental Digital Content 2, physical activity and sedentary data, http://links.lww.com/MSS/C135; Supplemental Digital Content 3, nutritional intake, http://links.lww.com/MSS/C136). No significant differences were observed during the 6-wk control period. Patients attended 89% of the 36 supervised resistance training sessions (range, 25–36 sessions), with a 100% compliance in consuming the whey protein supplement after every attended session. Number of sessions missed, modified, or completed as prescribed is provided in the supplemental figure (Figure, Supplemental Digital Content 4, adherence to supervised resistance exercise sessions, http://links.lww.com/MSS/C137). No significant changes in physical activity occurred during the intervention, with a nonsignificant decrease from preintervention to postintervention in sedentary behavior (68.4% ± 9.5% vs 64.9% ± 5.3%, P = 0.110) and an increase in light-intensity physical activity (31.1% ± 9.3% vs 34.5% ± 5.3%, P = 0.083). Patients attended 100% of the nutrition consultations. During the 12-wk intervention, there was a modest nonsignificant reduction in mean energy intake from 7728 ± 1131 kJ at preintervention to 7268 ± 2209 kJ at postintervention. There was a significant difference across the four time points for percent protein intake (baseline: 19.3% ± 1.6%, preintervention: 17.9% ± 2.4%, midintervention: 21.8% ± 2.9%, postintervention: 20.8% ± 4.0%, P = 0.016); however, the post hoc test was unable to locate the source of the difference. No other significant differences were found.
TABLE 1.
Baseline patient characteristics of men with prostate cancer.
| Variable | Patients (n = 14) |
|---|---|
| Age, mean ± SD, yr | 72 ± 9 |
| BMI, mean ± SD, kg·m−2 | 34.4 ± 6.4 |
| Postsecondary education, n (%) | 8 (57.1) |
| Married, n (%) | 14 (100) |
| Employed, n (%) | 4 (28.6) |
| No. medications/supplements, mean ± SD | 4.2 ± 2.7 |
| No. comorbidities, mean ± SDa | 3.0 ± 1.7 |
| Years since prostate cancer diagnosis, median (IQR), yr | 1.8 (1.2–5.6) |
| Gleason score, n (%) | |
| Gleason 7 | 4 (28.6) |
| Gleason 8 | 1 (7.1) |
| Gleason 9 | 8 (57.1) |
| Gleason 10 | 1 (7.1) |
| Contained within prostate, n (%) | 8 (57.1) |
| Lymph node metastases, n (%) | 4 (28.6) |
| Organ metastases, n (%)b | 2 (14.3) |
| ADT, n (%) | |
| Gonadotropin-releasing hormone agonist + antiandrogen | 8 (57.1) |
| Gonadotropin-releasing hormone agonist only | 5 (35.7) |
| Antiandrogen only | 1 (7.1) |
| Months on ADT, median (IQR) | 13.5 (6.7–23.3) |
| Other prostate cancer–related treatment, n (%) | |
| Surgery | 4 (28.6) |
| Radiation therapy | 13 (92.9) |
| Chemotherapy | 2 (14.3) |
aArthritis, atrial fibrillation, CVD, carpel tunnel syndrome, colitis, dyslipidemia, hypertension, sleep apnea, thyroid disease, emphysema, type 2 diabetes, peripheral neuropathy, anxiety disorder.
bLung.
Body composition
No significant changes in body composition were observed during the 6-wk control period (Table 2). From preintervention to postintervention, there were significant reductions in total body mass (−2.8 ± 3.2 kg), FM (−2.8 ± 2.6 kg), trunk FM (−1.8 ± 1.4 kg), and visceral FM (−88 ± 87 g; all, P < 0.05), whereas LM (−0.05 ± 1.6 kg), ASM (0.06 ± 0.82 kg), and BMC (8 ± 58 g) were preserved. The mean reduction in total FM was 6.8%, and for trunk FM it was 8.8%. FM was primarily lost from the trunk (84.8%), accompanied by a significant decrease in waist (−4.8 ± 3.5 cm) and hip circumferences (−3.8 ± 4.1 cm). Individual changes in body composition are presented in Figure 1. All but one patient lost FM, whereas the results were mixed for LM, with about half of the patients gaining and half experiencing a reduction.
TABLE 2.
Body composition and anthropometry at baseline, preintervention, and postintervention.
| Variable | Baseline | Preintervention | Postintervention | P | Comparison |
|---|---|---|---|---|---|
| Total body mass, kg | 98.6 ± 15.1 | 98.3 ± 14.7 | 95.5 ± 14.1 | 0.016 | 3 < 2 |
| Total FM, kg | 40.4 ± 10.2 | 39.8 ± 10.3 | 37.0 ± 9.5 | <0.001 | 3 < 2, 1 |
| % body fat | 40.5 ± 4.7 | 40.0 ± 4.9 | 38.3 ± 4.6 | <0.001 | 3 < 2, 1 |
| Trunk fat, kg | 20.6 ± 6.3 | 20.1 ± 5.9 | 18.3 ± 5.4 | <0.001 | 3 < 2, 1 |
| Visceral fat, g | 922 ± 293 | 954 ± 372 | 866 ± 333 | 0.023 | 3 < 2 |
| Total LM, kg | 55.6 ± 6.6 | 55.9 ± 6.5 | 55.9 ± 6.2 | 0.805 | — |
| ASM, kg | 23.2 ± 3.3 | 23.3 ± 3.3 | 23.3 ± 3.1 | 0.695 | — |
| BMC, g | 2610 ± 283 | 2567 ± 278 | 2576 ± 291 | 0.082 | — |
| Waist circumference, cm | 109.2 ± 11.5 | 108.7 ± 10.8 | 103.9 ± 8.9 | 0.002 | 3 < 1, 2 |
| Hip circumference, cm | 113.8 ± 8.4 | 113.5 ± 7.6 | 109.7 ± 8.1 | 0.008 | 3 < 1, 2 |
Values are the mean ± SD or median (IQR).
1, baseline; 2, preintervention; 3, postintervention.
FIGURE 1.
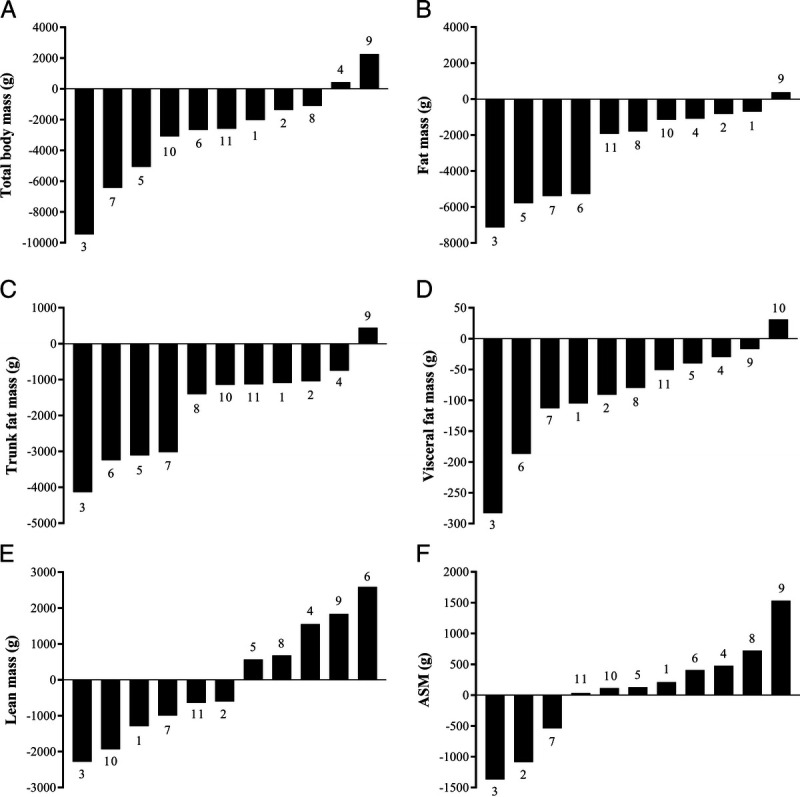
Waterfall plots of individual patients in ascending order showing change in total body mass (A), total FM (B), trunk FM (C), visceral FM (D), total LM (E), and ASM (F) over a 12-wk weight loss intervention. Individual patient numbers are identified in association with the bars.
Muscle strength and cardiorespiratory fitness
Muscle strength did not significantly change during the 6-wk control period (Table 3). After training, there was a significant increase in leg press (24.7% ± 24.5%) and chest press (19.8% ± 16.5%) strength compared with baseline and preintervention (Table 3). There was also a significant change for seated row strength (P = 0.006); however, post hoc analysis was not able to locate the source of the difference. Patients improved their cardiorespiratory fitness with a significant increase in CPET time of 83 ± 78 s from preintervention to postintervention, and a significant increase in V˙O2max of a 3.5 ± 4.7 mL·min−1·kg−1 (P = 0.041).
TABLE 3.
Muscle strength and cardiorespiratory fitness at baseline, preintervention, and postintervention.
| Variable | Baseline | Preintervention | Postintervention | P | Comparison |
|---|---|---|---|---|---|
| Muscle strength | |||||
| Leg press, kg | 90.0 (69.8–108.0) | 87.8 (72.0–120.0) | 101.3 (87.8–145.0) | <0.001 | 3 > 1, 2 |
| Chest press, kga | 41.3 ± 10.1 | 44.5 ± 12.7 | 52.4 ± 12.7 | <0.001 | 3 > 1, 2 |
| Seated row, kg | 62.6 ± 7.0 | 64.8 ± 7.3 | 67.7 ± 7.7 | 0.006 | — |
| Cardiorespiratory fitness | |||||
| CPET timea, s | — | 660 ± 173 | 743 ± 163 | 0.008 | — |
| Relative V˙O2maxa, mL·min−1·kg−1 | — | 16.5 ± 4.8 | 20.0 ± 5.0 | 0.041 | — |
| Absolute V˙O2maxa, L·min−1 | — | 1.6 ± 0.5 | 1.9 ± 0.5 | 0.071 | — |
Values are the mean ± SD or median (IQR).
aOnly n = 10 patients completed both chest press and CPET at all time points.
1, baseline; 2, preintervention; 3, postintervention.
Blood biomarkers
Mean preintervention and postintervention concentrations of blood biomarkers (lipid profile, CRP, insulin, and HbA1c) were within the recommended reference ranges, except for LDL, which was higher (reference range, <2.5 nmol·L−1; preintervention, 2.8 ± 1.4 nmol·L−1; postintervention, 2.6 ± 1.2 nmol·L−1; Table 4). There was no change in any of the biomarkers over the 12-wk intervention, except for a decrease in leptin concentration (P = 0.016; Table 4). Significant inverse associations were evident between the change in LM and change in CRP (rs = −0.670, P = 0.024) and triglycerides (r = −0.667, P = 0.025).
TABLE 4.
Serum blood biomarkers associated with obesity and prostate cancer progression.
| Variable | Preintervention | Postintervention | P | Reference Rangea |
|---|---|---|---|---|
| Insulin, mmol·L−1 | 9.0 (8.0–23.0) | 9.0 (6.0–23.0) | 0.262 | 2–12 |
| CRP, mg·L−1 | 1.3 (0.7–4.0) | 2.8 (0.7–5.8) | 0.612 | <3.0 |
| Total cholesterol, mmol·L−1 | 4.7 ± 1.5 | 4.5 ± 1.4 | 0.438 | <5.6 |
| LDL cholesterol, mmol·L−1 | 2.8 ± 1.4 | 2.6 ± 1.2 | 0.387 | <2.5 |
| HDL cholesterol, mmol·L−1 | 1.3 ± 0.5 | 1.3 ± 0.4 | 0.255 | >1.0 |
| Triglycerides, mmol·L−1 | 1.2 ± 0.5 | 1.3 ± 0.6 | 0.295 | <2.0 |
| HbA1c, mmol·mol−1 | 41.0 (38.0–48.0) | 42.0 (39.0–48.0) | 0.765 | <48 |
| Testosterone, nmol·L−1 | 0.1 (0.1–0.4) | 0.1 (0.1–0.3) | 0.257 | NA |
| PSA, μg·L−1 | 0.30 (0.02–2.27) | 0.39 (0.01–2.21) | 0.213 | NA |
| Leptin, ng·mL−1 | 6.3 (4.7–14.3) | 6.1 (3.3–11.7) | 0.016 | — |
| Adiponectin, μg·mL−1 | 65.5 ± 34.6 | 58.5 ± 24.4 | 0.215 | — |
| IGFBP-3, ng·mL−1 | 139.2 ± 55.8 | 135.3 ± 46.7 | 0.529 | — |
| IGF-1 (n = 9), ng·mL−1 | 9.3 (1.6–294.8) | 10.8 (2.0–348.4) | 0.051 | — |
| IL-6 (n = 8), pg·mL−1 | 4.7 (3.0–37.9) | 5.5 (1.7–51.1) | 0.327 | — |
Values are the mean ± SD or median (IQR).
aReference ranges were obtained from Australian Clinical Laboratory pathology reports for the standard of care blood biomarkers.
NA, not applicable.
Adverse events
No adverse events occurred during the supervised exercise sessions. One patient was referred to their general practitioner as a precaution because of an abnormal ECG result after completing the CPET but was cleared of any cardiac concerns. During home-based exercise, two patients described back pain while walking. This was addressed by reducing the volume of supervised exercise and removal of exercises involving the back until pain was reduced. One patient experienced an infected leg wound caused by resistance band exercises completed at home. Resistance band exercises were ceased, and all exercises putting pressure on the wound were removed until healed.
DISCUSSION
This study evaluated an exercise and nutrition weight loss program in obese prostate cancer patients undertaking ADT to induce fat loss while preserving LM. There were three important findings: 1) total and regional FM significantly decreased, whereas LM was preserved; 2) muscle strength and cardiorespiratory fitness significantly improved; and 3) serum leptin concentrations significantly decreased, with changes in serum CRP and triglycerides inversely associated with the individual changes in LM.
Body mass is associated with prostate cancer recurrence after prostatectomy, where weight gain of more than 2.2 kg increases the risk twofold, whereas weight loss potentially reduces risk (3). We found a significant 2.8-kg mean decrease in total body mass, which was attributed to a loss of FM. Notably, the majority of fat loss occurred in the abdominal region from a loss in trunk FM. FM loss ranged from −0.7 to −7.1 kg and was observed in 10 (90.9%) patients. O’Neill et al. (17) and Focht et al. (18) also examined combined exercise and nutrition interventions in prostate cancer patients on ADT and reported significant fat losses of 1.8 and 1.9 kg, respectively. In contrast to the individualized nutrition advice prescribed in the present study aiming to induce an energy deficit, reduce discretionary items, and optimize protein intake, both O’Neill et al. (17) and Focht et al. (18) followed healthy eating guidelines. O’Neill et al. (17) also provided tailored nutrition advice; however, only participants who were overweight were prescribed an energy deficit, whereas the Focht et al. (18) study mostly utilized group nutrition sessions. Although the nutrition and physical activity changes were modest in the present study, the individualized nutrition advice as well as the greater exercise volume (300 vs 150 min·wk−1) prescribed likely contributed to the greater FM loss. In another weight loss study conducted in overweight and obese men with prostate cancer, Freedland et al. (16) reported an FM loss of 8.2 kg. The greater fat loss was due to the carbohydrate-restricted diet utilized resulting in a significant energy deficit, in contrast to the lack of significant energy deficit in the present study. However, Freedland et al. (16) only included walking in their exercise program, which was not sufficient to stimulate muscle protein synthesis as the FM loss was accompanied by a significant 2.1-kg LM loss. We also provided data on trunk and estimated-visceral fat loss, which, to our knowledge, has not been measured in any combined exercise and nutrition study in men on ADT. Visceral fat is more metabolically active compared with subcutaneous fat and has been associated with increased risk of progression to advanced prostate cancer (5), as well as the development of comorbidities such as diabetes (35). Therefore, the observed reduction in visceral fat may be of greater clinical importance than the reduction in total FM.
Contrary to previous weight loss studies in ADT-treated prostate cancer patients reporting significant losses of LM or utilizing less accurate measuring techniques such as skinfolds to estimate LM (16,17), our study showed that LM can be preserved concurrent to significant FM loss. Despite the overall preservation of LM, five (45.5%) patients experienced a gain in total LM. Responders (LM gain) and nonresponders (LM loss) to exercise have been previously demonstrated in the prostate cancer population on ADT (36). The reasons for the difference in response in our cohort, that is, some gained LM and others lost LM, are unclear. Considerations include absolute weight at preintervention, amount of FM lost, intensity and volume of resistance and aerobic exercise, and protein intake. However, no relationships were found between these variables and change in LM. During the intervention, patients consumed a mean daily intake of 1.0 g·kg−1 body weight (BW) of protein, inclusive of the protein supplement consumed 3 d·wk−1, which is slightly lower than the 1.07 g·kg−1 BW daily recommendation for men older than 70 yr (37). It is possible this was too low to adequately support muscle protein synthesis, although daily average protein intake increased to 1.2 g·kg−1 BW on the day of resistance training when the protein supplement was provided, where similar protein intakes, in conjunction with resistance training, have been previously shown in prostate cancer patients on ADT to acutely increase muscle protein synthesis (38). However, anabolic suppression (i.e., a blunted training response) to resistance training while in an energy deficit state has been previously demonstrated even in the presence of protein supplementation and adequate daily protein intake of 1.2 g·kg−1 BW, and may have been a contributing factor to the observed LM changes in our study (39). Dawson et al. (40) conducted a resistance training intervention in prostate cancer patients on ADT using four groups, exercise, exercise and protein supplement, protein supplement, and control, providing a daily protein supplement of 50 g, which is higher than the protein supplement of 40 g provided 3 d·wk−1 in the current study. They found a significant increase in LM with exercise but no additional effect from the protein supplement. However, this study was not powered to assess the effect of protein intake across the four study arms. The optimal protein intake to effect body composition changes in prostate cancer patients requires further examination.
Although the desired loss of FM and preservation of LM are likely attributable to the intervention, the accelerometry and 3d-WR did not indicate a change in incidental physical activity or nutritional intake during the intervention, with the exception of percent of daily intake contributed to by protein. Several patients (n = 5) reported undertaking home-based cycling, and all patients attended resistance training sessions, both modes of which are not accurately captured with the ActiGraph technology used in this study and may explain the lack of change in accelerometry-measured physical activity (31). In addition, the use of individual nutrition goals may further explain the lack of a significant energy deficit. Individual goals were selected with the intention that patients would be more compliant to nutrition changes if goals were tailored to their lifestyle. Although all patients were advised to reduce meal portion sizes, specific goals such as reduction in alcohol or cake/biscuit intake, or increase amount of fruit per day, were better adhered to as indicated in discussions during supervised exercise sessions when completing the weekly adherence questionnaire. Irrespective of the lack of observable change in accelerometry and 3d-WR data, there was a high adherence to the resistance training sessions, protein supplement, and dietitian consultations demonstrating a change in exercise and nutritional habits.
LM can be substantially lost with energy-restricted diets (41). Therefore, it is important to ensure physical function is maintained, as a loss in either muscle strength or cardiorespiratory fitness is associated with clinical morbidity (42,43). Accompanying the significant loss in FM and preservation of LM, our study showed a significant increase in upper and lower body muscle strength. We also found a significant increase in the length of time patients could sustain the CPET (83 ± 78 s) and an increase in relative V˙O2max of 3.5 ± 4.7 mL·min−1·kg−1 indicating increased cardiorespiratory fitness. These findings of increased muscle strength and cardiorespiratory fitness while undergoing weight loss are consistent with other combined exercise and nutrition interventions in ADT-treated prostate cancer patients (16–18). In addition, the current study provides more comprehensive and valid results by reporting directly measured V˙O2max using a maximal CPET, rather than the submaximal tests used by other combined intervention studies.
Weight loss is recommended as a viable strategy to decrease an obese patient’s increased risk of prostate cancer progression by improving insulin/IGF axis and sex hormone concentrations, and the signaling of adipokines and cytokines (3,6). In our study, a significant decline in leptin was found, where eight (72.7%) patients showed a decrease in serum concentration. Although the change in leptin was not associated with change in body mass or FM in the current study, Santa Mina et al. (44) in a larger sample size of 44 prostate cancer patients on ADT showed a significant positive association between a reduction in leptin and reductions in BMI, waist circumference, and body mass after the completion of a home-based exercise program. Serum leptin has been associated with prostate cancer progression, mostly in androgen-independent prostate cancers; however, the evidence is inconsistent (45,46). Nevertheless, there is preliminary evidence indicating that weight loss may slow down prostate cancer progression by increasing PSA doubling time (47). Further research is required to examine whether a reduction in FM can affect prostate cancer progression, as our study found no change in IGF-1, IGFBP-3, IL-6, or adiponectin, which are potentially more strongly associated with prostate cancer progression than leptin (6). Our study also found that those who had a higher increase in CRP lost greater LM. CRP is an inflammatory biomarker with high levels associated with increased risk of CVD (48). An increase in triglycerides, which is a known risk factor for CVD (49), was also associated with a loss in LM. These associations highlight the importance of targeting both FM and LM when undertaking a weight loss program.
Strengths and limitations
This study has several strengths. The use of DXA permitted the evaluation of not only whole body but also regional changes in FM, that is, trunk FM and visceral FM. The weight loss intervention had a high adherence rate, with patients attending 100% of nutrition consultations and 89% of supervised resistance training sessions. The intervention also has external validity. Patients adopted exercise and nutrition-based lifestyle changes, which are growing in awareness as important adjuvant therapies to improve treatment-related outcomes such as body composition and physical function. Study limitations include a small sample size, although recruitment was powered for our primary outcome, and the lack of a separate control group, which prevented comparison of our results to usual care. Although this study had a small sample, the minimal recruitment goals were met. Furthermore, the study was designed to include an initial 6-wk period with no intervention, where each patient acted as their own control. As no changes were observed in any variable during the 6-wk control period, it is assumed the changes observed during the 12-wk intervention resulted from the exercise and nutrition weight loss program. Lastly, as stated previously, the ActiGraph technology used in this trial may not have been the most appropriate to assess resistance training and cycling activities.
CONCLUSIONS
This study shows preliminary efficacy for an exercise and nutrition weight loss program to induce FM loss and preserve LM in obese prostate cancer patients undergoing ADT. Muscle strength and cardiorespiratory fitness were also significantly improved. Because FM is associated with obesity-related comorbidities and prostate cancer progression, it is important to monitor body composition, particularly in patients where the treatment is likely to substantially alter FM and LM. To extend the findings of this study, larger-scale studies are required to examine the metabolic significance of purposeful FM loss in obese prostate cancer patients, as it is unclear if reductions in FM will reduce cancer progression and improve survivorship. Furthermore, the translation of this intervention to other populations such as non–ADT-treated prostate cancer, breast cancer, and colorectal cancer patients, where obesity is a contributor to poor patient outcomes, should also be of interest.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the valuable contribution of the participants, who donated their time and effort to complete the research study, and five Master of Nutrition and Dietetic students at Edith Cowan University—Thomas Hosking, Sabina Ferri, Rebecca Newton, Sarah Forrest, and Marija Karanfilovska—for their expertise and analysis of nutrition-related questionnaires.
R. L. W. is supported by an Australian Government Research Training Program Scholarship. R. U. N. is supported by a Vice-Chancellor Professorial Research Fellowship. N. H. H. is supported by a Cancer Council of Western Australia Postdoctoral Research Fellowship. NHMRC Centre for Research Excellence funding supported this project in addition to allocated funds from Edith Cowan University.
The authors declare they have no conflict of interest.
The results of the present study do not constitute endorsement by the American College of Sports Medicine. The authors declare that the results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
Ethical approval was gained from Human Research Ethics Committee at Edith Cowan University (ID: 18832). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Written informed consent was obtained from participating patients after reading an information letter outlining study procedures, risks, and benefits.
The contributions of each author are as follows: R. L. W., significant manuscript writer, concept and design, data acquisition, and data analysis and interpretation; R. U. N., significant manuscript reviewer/revisor and concept and design; D. R. T., significant manuscript reviewer/revisor, concept and design, and statistical expertise; N. H. H., significant manuscript reviewer/revisor and concept and design; P. L.- W., significant manuscript reviewer/revisor, concept and design, and data acquisition; D. A. G., significant manuscript reviewer/revisor and concept and design.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.acsm-msse.org).
Contributor Information
ROBERT U. NEWTON, Email: r.newton@ecu.edu.au.
DENNIS R. TAAFFE, Email: d.taaffe@ecu.edu.au.
NICOLAS H. HART, Email: n.hart@ecu.edu.au.
PHILIPPA LYONS-WALL, Email: p.lyons-wall@ecu.edu.au.
DANIEL A. GALVÃO, Email: d.galvao@ecu.edu.au.
REFERENCES
- 1.Salji M, Hendry J, Patel A, Ahmad I, Nixon C, Leung HY. Peri-prostatic fat volume measurement as a predictive tool for castration resistance in advanced prostate cancer. Eur Urol Focus. 2018;4(6):858–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Cancer Research Fund, American Institute for Cancer Research Continuous Update Project Expert Report 2018. Diet, nutrition, physical activity and prostate cancer. 2018. Available from: https://www.wcrf.org/dietandcancer/prostate-cancer. Accessed May 20, 2020. [Google Scholar]
- 3.Joshu CE Mondul AM Menke A, et al. Weight gain is associated with an increased risk of prostate cancer recurrence after prostatectomy in the PSA era. Cancer Prev Res (Phila). 2011;4(4):544–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bray GA. Medical consequences of obesity. J Clin Endocrinol Metab. 2004;89(6):2583–9. [DOI] [PubMed] [Google Scholar]
- 5.Dickerman BA Torfadottir JE Valdimarsdottir UA, et al. Body fat distribution on computed tomography imaging and prostate cancer risk and mortality in the AGES-Reykjavik study. Cancer. 2019;125(16):2877–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allott EH, Masko EM, Freedland SJ. Obesity and prostate cancer: weighing the evidence. Eur Urol. 2013;63(5):800–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keto CJ Aronson WJ Terris MK, et al. Obesity is associated with castration-resistant disease and metastasis in men treated with androgen deprivation therapy after radical prostatectomy: results from the SEARCH database. BJU Int. 2012;110(4):492–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rhee H Gunter JH Heathcote P, et al. Adverse effects of androgen-deprivation therapy in prostate cancer and their management. BJU Int. 2015;115(5 Suppl):3–13. [DOI] [PubMed] [Google Scholar]
- 9.Galvao DA Spry NA Taaffe DR, et al. Changes in muscle, fat and bone mass after 36 weeks of maximal androgen blockade for prostate cancer. BJU Int. 2008;102(1):44–7. [DOI] [PubMed] [Google Scholar]
- 10.Newton RU Jeffery E Galvao DA, et al. Body composition, fatigue and exercise in patients with prostate cancer undergoing androgen-deprivation therapy. BJU Int. 2018;122(6):986–93. [DOI] [PubMed] [Google Scholar]
- 11.Galvao DA, Taaffe DR, Spry N, Joseph D, Newton RU. Acute versus chronic exposure to androgen suppression for prostate cancer: impact on the exercise response. J Urol. 2011;186(4):1291–7. [DOI] [PubMed] [Google Scholar]
- 12.Bourke L Smith D Steed L, et al. Exercise for men with prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016;69(4):693–703. [DOI] [PubMed] [Google Scholar]
- 13.Lin PH, Aronson W, Freedland SJ. Nutrition, dietary interventions and prostate cancer: the latest evidence. BMC Med. 2015;13(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hackshaw-McGeagh LE Perry RE Leach VA, et al. A systematic review of dietary, nutritional, and physical activity interventions for the prevention of prostate cancer progression and mortality. Cancer Causes Control. 2015;26(11):1521–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barnes KA, Ball LE, Galvao DA, Newton RU, Chambers SK. Nutrition care guidelines for men with prostate cancer undergoing androgen deprivation therapy: do we have enough evidence? Prostate Cancer Prostatic Dis. 2019;22(2):221–34. [DOI] [PubMed] [Google Scholar]
- 16.Freedland SJ Howard L Allen J, et al. A lifestyle intervention of weight loss via a low-carbohydrate diet plus walking to reduce metabolic disturbances caused by androgen deprivation therapy among prostate cancer patients: Carbohydrate and Prostate Study 1 (CAPS1) randomized controlled trial. Prostate Cancer Prostatic Dis. 2019;22(3):428–37. [DOI] [PubMed] [Google Scholar]
- 17.O’Neill RF, Haseen F, Murray LJ, O’Sullivan JM, Cantwell MM. A randomised controlled trial to evaluate the efficacy of a 6-month dietary and physical activity intervention for patients receiving androgen deprivation therapy for prostate cancer. J Cancer Surviv. 2015;9(3):431–40. [DOI] [PubMed] [Google Scholar]
- 18.Focht BC Lucas AR Grainger E, et al. Effects of a group-mediated exercise and dietary intervention in the treatment of prostate cancer patients undergoing androgen deprivation therapy: results from the IDEA-P trial. Ann Behav Med. 2018;52(5):412–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soenen S, Martens EA, Hochstenbach-Waelen A, Lemmens SG, Westerterp-Plantenga MS. Normal protein intake is required for body weight loss and weight maintenance, and elevated protein intake for additional preservation of resting energy expenditure and fat free mass. J Nutr. 2013;143(5):591–6. [DOI] [PubMed] [Google Scholar]
- 20.Skolarus TA Wolf AM Erb NL, et al. American Cancer Society prostate cancer survivorship care guidelines. CA Cancer J Clin. 2014;64(4):225–49. [DOI] [PubMed] [Google Scholar]
- 21.Okorodudu DO Jumean MF Montori VM, et al. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: a systematic review and meta-analysis. Int J Obes (Lond). 2010;34(5):791–9. [DOI] [PubMed] [Google Scholar]
- 22.Exercise and Sport Science Australia Exercise intensity guidelines. 2011 [cited 2018]. Available from: https://www.essa.org.au/wp-content/uploads/2014/06/Exercise-Intensity-Guildines.pdf.
- 23.Heymsfield SB Smith R Aulet M, et al. Appendicular skeletal muscle mass: measurement by dual-photon absorptiometry. Am J Clin Nutr. 1990;52(2):214–8. [DOI] [PubMed] [Google Scholar]
- 24.Galvão DA, Taaffe DR, Spry N, Joseph D, Newton RU. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: a randomized controlled trial. J Clin Oncol. 2010;28(2):340–7. [DOI] [PubMed] [Google Scholar]
- 25.Lerman J, Bruce RA, Sivarajan E, Pettet GE, Trimble S. Low-level dynamic exercises for earlier cardiac rehabilitation: aerobic and hemodynamic responses. Arch Phys Med Rehabil. 1976;57(8):355–60. [PubMed] [Google Scholar]
- 26.Erdrich S, Bishop KS, Karunasinghe N, Han DY, Ferguson LR. A pilot study to investigate if New Zealand men with prostate cancer benefit from a Mediterranean-style diet. PeerJ. 2015;3:e1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martínez-González MA García-Arellano A Toledo E, et al. PREDIMED Study Investigators . A 14-item Mediterranean diet assessment tool and obesity indexes among high-risk subjects: the PREDIMED trial. PLoS One. 2012;7(8):e43134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi L, Liu Z, Matthews CE, Buchowski MS. Validation of accelerometer wear and nonwear time classification algorithm. Med Sci Sports Exerc. 2011;43(2):357–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matthews CE Chen KY Freedson PS, et al. Amount of time spent in sedentary behaviors in the United States, 2003–2004. Am J Epidemiol. 2008;167(7):875–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc. 1998;30(5):777–81. [DOI] [PubMed] [Google Scholar]
- 31.Peddle-McIntyre CJ Cavalheri V Boyle T, et al. A review of accelerometer-based activity monitoring in cancer survivorship research. Med Sci Sports Exerc. 2018;50(9):1790–801. [DOI] [PubMed] [Google Scholar]
- 32.Taaffe DR Newton RU Spry N, et al. Effects of different exercise modalities on fatigue in prostate cancer patients undergoing androgen deprivation therapy: a year-long randomised controlled trial. Eur Urol. 2017;72(2):293–9. [DOI] [PubMed] [Google Scholar]
- 33.Cormie P Galvao DA Spry N, et al. Can supervised exercise prevent treatment toxicity in patients with prostate cancer initiating androgen-deprivation therapy: a randomised controlled trial. BJU Int. 2015;115(2):256–66. [DOI] [PubMed] [Google Scholar]
- 34.Jensen MD Ryan DH Donato KA, et al. Executive summary: guidelines (2013) for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society published by the Obesity Society and American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Based on a systematic review from The Obesity Expert Panel, 2013. Obesity. 2014;22(S2):S5–39. [DOI] [PubMed] [Google Scholar]
- 35.Bray GA Jablonski KA Fujimoto WY, et al. Relation of central adiposity and body mass index to the development of diabetes in the Diabetes Prevention Program. Am J Clin Nutr. 2008;87(5):1212–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taaffe DR, Newton RU, Spry N, Joseph DJ, Galvao DA. Responsiveness to resistance-based multimodal exercise among men with prostate cancer receiving androgen deprivation therapy. J Natl Compr Canc Netw. 2019;17(10):1211–20. [DOI] [PubMed] [Google Scholar]
- 37.Capra S, Members of the Working Party . Nutrient Reference Values for Australia and New Zealand: Including Recommended Dietary Intakes. Canberra, Australia: Commonwealth of Australia; 2006. [Google Scholar]
- 38.Hanson ED Nelson AR West DW, et al. Attenuation of resting but not load-mediated protein synthesis in prostate cancer patients on androgen deprivation. J Clin Endocrinol Metab. 2017;102(3):1076–83. [DOI] [PubMed] [Google Scholar]
- 39.Murphy C, Koehler K. Caloric restriction induces anabolic resistance to resistance exercise. Eur J Appl Physiol. 2020;120(5):1155–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dawson JK, Dorff TB, Todd Schroeder E, Lane CJ, Gross ME, Dieli-Conwright CM. Impact of resistance training on body composition and metabolic syndrome variables during androgen deprivation therapy for prostate cancer: a pilot randomized controlled trial. BMC Cancer. 2018;18(1):368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weinheimer EM, Sands LP, Campbell WW. A systematic review of the separate and combined effects of energy restriction and exercise on fat-free mass in middle-aged and older adults: implications for sarcopenic obesity. Nutr Rev. 2010;68(7):375–88. [DOI] [PubMed] [Google Scholar]
- 42.Ruiz JR Sui X Lobelo F, et al. Association between muscular strength and mortality in men: prospective cohort study. BMJ. 2008;337(7661):a439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davidson T, Vainshelboim B, Kokkinos P, Myers J, Ross R. Cardiorespiratory fitness versus physical activity as predictors of all-cause mortality in men. Am Heart J. 2018;196:156–62. [DOI] [PubMed] [Google Scholar]
- 44.Santa Mina D Connor MK Alibhai SM, et al. Exercise effects on adipokines and the IGF axis in men with prostate cancer treated with androgen deprivation: a randomized study. Can Urol Assoc J. 2013;7(11–12):E692–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li H Stampfer MJ Mucci L, et al. A 25-year prospective study of plasma adiponectin and leptin concentrations and prostate cancer risk and survival. Clin Chem. 2010;56(1):34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Di Sebastiano KM, Pinthus JH, Duivenvoorden WC, Patterson L, Dubin JA, Mourtzakis M. Elevated C-peptides, abdominal obesity, and abnormal adipokine profile are associated with higher Gleason scores in prostate cancer. Prostate. 2017;77(2):211–21. [DOI] [PubMed] [Google Scholar]
- 47.Freedland SJ Allen J Jarman A, et al. A randomized controlled trial of a 6-month low-carbohydrate intervention on disease progression in men with recurrent prostate cancer: Carbohydrate And Prostate Study 2 (CAPS2). Clin Cancer Res. 2020;26(12):3035–43. [DOI] [PubMed] [Google Scholar]
- 48.Ridker PM, Bassuk SS, Toth PP. C-reactive protein and risk of cardiovascular disease: evidence and clinical application. Curr Atheroscler Rep. 2003;5(5):341–9. [DOI] [PubMed] [Google Scholar]
- 49.Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet. 2014;384(9943):626–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


