Supplemental Digital Content is available in the text.
Keywords: apoptosis, fisetin, IκBα, NF-κB, spinal cord injury
Aim
To evaluate neuroprotective efficacy of fisetin against the experimental model of spinal cord injury (SCI).
Materials and methods
SCI was induced in male Sprague-Dawley rats by placing an aneurysm clip extradurally. Rats were treated either with vehicle or fisetin for 28 days after SCI.
Results
Treatment with fisetin significantly attenuated SCI-induced alternations in mechano-tactile and thermal allodynia, hyperalgesia and nerve conduction velocities. SCI-induced upregulated tumor necrosis factor-alpha, interleukins, inducible nitric oxide synthase, cyclooxygenase-II, Bcl-2-associated X protein and caspase-3 mRNA expressions in the spinal cord and these were markedly reduced by fisetin. Spinal nuclear factor kappa B and nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor-alpha protein levels were also significantly downregulated by fisetin. Hematoxylin and eosin staining of spinal cord suggested that fisetin significantly ameliorated histological aberrations such as neuronal degeneration, necrosis and inflammatory infiltration induced in it.
Conclusion
Fisetin exerts neuroprotection via modulation of nuclear factor kappa B/nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor-alpha pathway by inhibiting release of inflammatory mediators (inducible nitric oxide synthase and cyclooxygenase-II), proinflammatory cytokines (tumor necrosis factor-alpha and interleukins), apoptotic mediators (Bcl-2-associated X protein and caspase-3).
Introduction
Traumatic spinal cord injury (SCI) is a devastating neurophysiological disorder leading to sensory and motor dysfunction, which significantly affects the quality of life for patients [1,2]. Sometimes severe SCI may also cause loss of urinary, bladder, sexual and bowel functions [3]. Increased construction, mining and transportation industrialization, automobile accidents and sports injuries often contribute to SCI, and young adults with age >40 years are at high risk for such SCI [1,2]. The report suggested that approximately 500 000 people suffer from SCI annually, with an estimated global annual incidence of 40–80 cases per million population in most of the countries [1]. Cumulative evidence documented that almost 80% of SCI patients experienced pain, whereas almost 30% of patients reported their pain as severe [4]. Unfortunately, due to high treatment costs, SCI associate with a notable economic burden with care costs of more than $7 billion per individual per year [5].
A promising experimental strategy for the development of safe and effective treatment against SCI largely remains a major unmet need for these neurodegenerative deficits. With advancements and rapid development in the pharmaceutical sciences, emerging gene therapy, drugs and surgical methods have shown their ability to promote spinal nerve regeneration in SCI treatment. Methylprednisolone, which is the only FDA-approved glucocorticoid, which is a widely implemented agent in clinical practice for the management of acute SCI [6]. On the other hand, an inexpensive and noninvasive technique of high-frequency electrotherapy such as transcutaneous electrical nerve stimulation gains significant attention to treat pain in SCI. Though such physical therapy is most commonly used and cost-effective but provides symptomatic relief in a fraction of patients. In view of this, the surge of safe and effective treatment for SCI is presently the utmost.
Animal models play a vital role in understanding the underlying mechanism and development of effective therapeutic moieties for various maladies, including SCI [7]. Thus various animal models have been developed to determine mechanisms of motor dysfunctions, which include compression, contusion, transection and excitotoxicity models [8]. However, compression-induced SCI is well-accepted, frequently used worldwide and arguably the most prominent experimental animal model for understanding the mechanism of motor and sensory recovery by therapeutic moiety during SCI [8,9]. To mimic clinicopathological characteristics of SCI, the compression model implemented various technique which includes clip compression, calibrated forceps compression and balloon compression; however, clip compression at the thoracic level the more closely related in terms of functional and histological features of human SCI amongst all [8,9]. Clip compression is a simple, well-established, reproducible and inexpensive method for the induction of SCI [9].
In recent years, plant flavonoid has emerged as a potential therapeutic moiety for the management of various diseases [9–13]. Fisetin (3,3′,4′,7-tetrahydroxyflavone) is one such plant flavonoid that occurs naturally in various vegetables and fruits, including strawberries, apples, onions and cucumbers [14,15]. The number of evidence using renovate strategies has evinced its pharmacological potential, including antioxidant, anti-inflammatory, anticancer, antiasthmatic, antiulcer, antihyperlipidemic, anticonvulsant, cardioprotective, neuroprotective, nephroprotective and hepatoprotective effects [14–29]. Conversely, the cumulative evidence suggesting the ameliorative potential of fisetin against various maladies occurred through inhibition of nuclear factor kappa B (NF-κB) transcriptional activity [17,18,25,30,31]. Recent studies have demonstrated that fisetin exhibits its potent anti-inflammatory property via inhibition of release of various inflammatory [cyclooxygenase- II (COX-II)] and proinflammatory mediators [tumor necrosis factor-alpha (TNF-α) and interleukins (ILs)] [31,32]. Additionally, fisetin maintains neuronal function via modulation of multiple pathways, including upregulation of Nrf2 expression, attenuation of caspase-3 expression and modulation of concentrations of brain monoamines [33,34]. Nevertheless, its neuroprotective potential against traumatic injury has not been established. Thus, the present investigation was aimed to evaluate the possible mechanism underlying the neuroprotective role of fisetin against the experimental model of SCI by assessing various behavioral, biochemical and molecular parameters.
Methods
Animals
Adult male Sprague-Dawley rats were obtained from the Hebei University Animal center. They were maintained at 24 ± 1°C, with a relative humidity of 45–55% and 12:12 h dark/light cycle. The animals had free access to standard pellet chow and water throughout the experimental protocol. All experiments were carried out between 09:00 and 17:00 h. All the experimental protocol number [HBBD (2020) 12-DS] were approved by the Baoding First Central Hospital. All procedures were performed in accordance with laboratory guidelines.
Drugs and chemicals
Fisetin was purchased from Sigma-Aldrich (St. Louis, Missouri, USA) (purity: ≥98%). All other chemicals were purchased from S.D. Fine Chemicals, Mumbai, India. Methylprednisolone was obtained as a gift sample from Symed Pharmaceutical Pvt. Ltd. Total RNA Extraction kit and one-step quantitative RT-PCR kit was purchased from MP Biomedicals India Private Limited, Mumbai, India.
Preparation of drug solutions
Fisetin was suspended in a 1% aqueous solution of dimethyl sulfoxide (DMSO). An appropriate stock solution was prepared to administer a selected dosage of 10, 20 and 40 mg/kg orally for four weeks [15,19]. Methylprednisolone was administered to rats intraperitoneally at a dose of 30 mg/kg for 4 weeks [9].
Induction of spinal cord injury and drug treatment schedule
The animals were fasted for 12 h before surgery, humanely restrained and anesthetized with an intraperitoneal injection of thiopental sodium (40 mg/kg of body weight). The rectal temperature of rats was maintained at 37–38°C throughout the operative procedure. The animals were positioned in the prone position and surgery was performed under sterile conditions. A complete single level (T10) laminectomy was performed through a 2-cm incision with the aid of a dissecting microscope. SCI was induced by the extradural application of a temporary aneurysm clip with a constant closing force on the spinal cord for 60 s [9]. After removal of the clip, the skin incision was closed and the animal was allowed to recover fully from anesthesia and returned to the cage. The rats were monitored continuously with bladder palpation at least twice daily until spontaneous voiding resumed.
The animals (n = 15 in each group) were divided into the following eight groups, each consisting of eight rats. Fisetin was prepared in three different dosages (10, 20 and 40 mg/kg) with 1% aqueous solution of DMSO and administered to animals orally for four weeks.
Group 1: Normal group: The spinal cord was not injured. Animals received only vehicle (10 g/kg of 1% aqueous solution of DMSO, postoperatively).
Group 2: Sham group: The spinal cord of animals was exposed but not injured. They received only vehicle (10 g/kg of 1% aqueous solution of DMSO, postoperatively).
Group 3: SCI control group: The spinal cord of animals was exposed and injured by a clip compression method. They received a vehicle (10 g/kg of 1% aqueous solution of DMSO, postoperatively).
Group 4: Methylprednisolone treated group: The spinal cord of animals was exposed and injured by a clip compression method. They were treated with methylprednisolone (30 mg/kg, intraperitoneally for 4 weeks).
Group 5: Fisetin: (10 mg/kg) treated group: The spinal cord of animals was exposed and injured by a clip compression method. They were treated with fisetin (10 mg/kg, postoperatively for 4 weeks).
Group 6: Fisetin: (20 mg/kg) treated group: The spinal cord of animals was exposed and injured by a clip compression method. They were treated with fisetin (20 mg/kg, postoperatively for 4 weeks).
Group 7: Fisetin: (40 mg/kg) treated group: The spinal cord of animals was exposed and injured by a clip compression method. They were treated with fisetin (40 mg/kg, postoperatively for 4 weeks).
The parameters of behavioral tests were recorded by an observer blind to the treatment on day −2, 0, 3, 7, 14, 21 and 24. Food intake and water intake were recorded after placing animals in the metabolic cages (Techniplast, Pontremoli, Italy).
Behavioral test
Evaluation of Basso–Beattie–Bresnahan score
The Basso–Beattie–Bresnahan (BBB) locomotor rating scale ranges from 0 to 21, where zero reflects no locomotor function, and 21 reflects a normal performance; the rating was used to evaluate the effect of fisetin on functional recovery after SCI [9].
Evaluation of allodynia and hyperalgesia
Mechano-tactile allodynia (nonnoxious mechanical stimuli) was assessed by the electronic Von Frey hair apparatus (IITC, Woodland Hills, California, USA) [35,36]. Mechanical nociceptive threshold, an index of mechano-hyperalgesia, was assessed by using Randall–Selitto paw pressure apparatus (UGO Basile SRL Biological Research Apparatus, Italy) [37]. Motor incoordination was evaluated by a Rota-Rod apparatus (25 rpm) [37]. Radiant heat hyperalgesia of the left hind paw was assessed using the radiant heat lamp source. Spinal thermal sensitivity was determined by the tail immersion test, as described previously [38].
Determination of motor and sensory nerve conduction velocity
The recording of motor nerve conduction velocity (MNCV) and sensory nerve conduction velocity (SNCV) was measured according to a previously reported method [38]. MNCV and SNCV were recorded by stimulating the sciatic and tibial nerves by 200 μs square wave pulse delivered through a pair of monopolar needle electrodes (1.0–1.5 mA, 2.0 mV/D) using a stimulator (Weltronics, Pune, India). Responses were recorded from the plantar muscles utilizing a data acquisition system (AD Instrument Pvt. Ltd., LabChart 7.3, Bella Vista, NSW, Australia).
Biochemical estimations
All animals were sacrificed at the end of the study, that is, 4th week, and the lesioned part of the spinal cord dorsal horn of each rat was immediately isolated and stored at −80°C for biochemical and RT-PCR analysis. Another lesioned portion of the spinal cord dorsal horn of three rats from each group was isolated and fixed for histopathological evaluation.
Determination of spinal cord TNF-α, IL-1β, IL-6, iNOs, COX-II, BDNF, Bax, Bcl-2 and caspase-3 mRNA expression by qRT-PCR
The mRNA expressions of TNF-α, interleukin-1β (IL-1 beta), interleukin-6 (IL-6), inducible nitric oxide synthase (iNOS), COX-II, brain-derived neurotrophic factor (BDNF), B-cell lymphoma 2 (Bcl-2), Bcl-2-associated X protein (Bax) and caspase-3 were analyzed in spinal cord tissue using qRT–PCR according to the method described elsewhere [9]. The spinal cord samples were treated with a DNase to completely remove gDNA. Nucleic acid purity was assessed by measuring the A260/A280 ratio by spectrophotometer with RNA ratio between 1.8 and 2.1. The reverse transcription was achieved using RT Kit (MP Biomedicals India Private Limited), according to the manufacturer’s instructions. All reactions took place for 1h at 37°C in a final volume of 40 μl containing 1 μg total RNA in the presence of 20 units RNase inhibitor, 1 μg random hexamers, 4 μl 10× buffer RT, 0.5 mM dNTPs and eight units reverse transcriptase. The qPCR was carried out using SYBR Green I in 96-well plates each with a total volume of 20 μl. Each well contained 5 μl of a 100-fold dilution of cDNA, 10 μl of iQ Sybr Green Supermix (2× qPCR mix contains dNTPs, 50 U/ml iTaq DNA polymerase, 6 mM MgCl2, SYBR Green I, enhancers, stabilizers, 20 nM fluorescein), 2 μl of each primer 1–3 μM and 1 μl water. All Cq values for each gene were rescaled to the lowest Cq value as an internal control, converted these rescaled Cq logarithmically into linear, relative quantities taking into account the gene-specific amplification efficiency [relative quantity = (1 + efficiency) ^ (Cqinternal control − Cqsample)].
Western blot procedure
The antibodies for phospho nuclear factor kappa B (p65-NF-κB ) (E379, ab207297), nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor-alpha (p-IκBα ) (6A920, ab12134) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH ) (EPR16891, ab181602) were purchased from Abcam, Cambridge, Massachusetts, USA. The protein expressions of p65-NF-κB and p-IκBα were estimated in spinal cord tissue according to the method described elsewhere [39].
Histopathological investigation
For the histopathological investigation of the spinal cord, tissue (n = 3 from each group) were fixed with buffered 10% formaldehyde solution, then embedded in paraffin and horizontally sectioned at 5 µm. After deparaffinization, samples were stained with hematoxylin and eosin to verify the morphological assessment of tissue. Photomicrographs of area containing the spinal cord lesions were captured under a light microscope (Nikon E200, Japan) at a magnification of 40×. The severity of spinal cord damage, including neuronal degeneration, necrosis and inflammatory infiltration, were scored (0–4) according to the method reported elsewhere (Toumpoulis, 2003 #69).
Statistical analysis
Data were expressed as mean ± SEM. Data analysis was performed using Graph Pad Prism (Graph Pad, San Diego, California, USA). Data of behavioral tests were statistically analyzed using two-way repeated analysis of variance (ANOVA) followed by Bonferroni’s test, while data of biochemical parameters were analyzed using one-way ANOVA and Tukey’s multiple range test was applied for post hoc analysis. A value of P < 0.05 was considered to be statistically significant.
Results
Effect of fisetin on spinal cord injury-induced alteration in body weight, food intake, water intake, urine volume and urinary protein
There was no significant difference in body weight, food intake, water intake, urine volume and urinary protein of normal and sham control rats. However, injury to the spinal cord leads to marked (P < 0.05) decreased in body weight, food intake and water intake, whereas urine volume and urinary protein increased significantly (P < 0.05) in SCI control rats as compared to normal and sham control rats. Administration of methylprednisolone significantly (P < 0.05) attenuated SCI-induced variations in body weight, food intake, water intake, urine volume and urinary protein as compared to SCI control rats. When compared with SCI control rats, fisetin (20 and 40 mg/kg) treatment also effectively (P < 0.05) inhibited SCI-induced decreased body weight, food intake and water intake and increased urine volume and urinary protein. However, methylprednisolone more effectively (P < 0.05) ameliorated SCI-induced variations in body weight, food intake, water intake, urine volume and urinary protein as compared to fisetin treated rats (Table 1).
Table 1.
Effect of fisetin on SCI-induced alteration in body weight, food intake, water intake, urine output and urine protein level
| Treatment | Normal | Sham | SCI Control | Methylprednisolone (30) | Fisetin (10) | Fisetin (20) | Fisetin (40) |
|---|---|---|---|---|---|---|---|
| Body weight (g) | 227.20 ± 2.37 | 230.20 ± 0.95 | 169.00 ± 1.93#,& | 219.20 ± 1.85*,$ | 176.80 ± 2.01 | 189.70 ± 0.92*,$ | 211.50 ± 1.75*,$ |
| Food intake (g) | 24.33 ± 0.49 | 21.33 ± 0.76 | 10.50 ± 0.62#,& | 20.00 ± 0.58*,$ | 13.67 ± 0.92 | 16.50 ± 0.67*,$ | 17.83 ± 0.79*,$ |
| Water intake (mL) | 40.67 ± 1.75 | 37.67 ± 0.71 | 23.00 ± 1.21#,& | 35.50 ± 1.50*,$ | 23.83 ± 1.35 | 29.67 ± 1.56*,$ | 34.33 ± 1.54*,$ |
| Volume of urine expressed in 24 h (ml) | 1.17 ± 0.17 | 1.67 ± 0.21 | 5.17 ± 0.17#,& | 2.33 ± 0.21*,$ | 5.00 ± 0.26 | 3.33 ± 0.21*,$ | 2.67 ± 0.21*,$ |
| Urine protein (mg/ml) | 1.66 ± 0.18 | 2.38 ± 0.16 | 6.97 ± 0.26#,& | 3.31 ± 0.16*,$ | 6.63 ± 0.20 | 4.87 ± 0.12*,$ | 3.62 ± 0.14*,$ |
Data are expressed as mean ± SEM (n = 6) and one-way ANOVA followed by Tukey’s multiple range test.
ANOVA, analysis of variance; SCI, spinal cord injury.
#P < 0.05 as compared with sham group.
&P < 0.05 as compared with normal.
*p < 0.05 as compared with the SCI control group.
$P < 0.05 as compared with one another.
Effect of fisetin on spinal cord injury-induced alteration in organs weight and sperm count
The testis, seminal vesicle, prostate gland, epididymis, kidney weight and sperm count were marked (P < 0.05) decreased whereas urinary bladder weight increased prominently (P < 0.05) in SCI control rats as compared to normal and sham control rats. However, the administration of methylprednisolone noticeably (P < 0.05) attenuated SCI-induced modification in these organs' weight and sperm count as compared to SCI control rats. Treatment with fisetin (20 and 40 mg/kg) also effectively (P < 0.05) augmented testis, seminal vesicle, prostate gland, epididymis, kidney weight and sperm count and decreased urinary bladder weight compared with SCI control rats. Fisetin treatment showed less improvement in SCI-induced variations in organ weight and sperm count as compared to methylprednisolone treated rats. When compared with normal rats, sham control rats did not show any noticeable changes in organs weight and sperm count (Supplementary File 1, Supplement digital content 1, http://links.lww.com/WNR/A614).
Effect of fisetin on spinal cord injury-induced alteration in mechano-tactile and thermal allodynia as well as hyperalgesia
SCI resulted in noticeable induction of mechano-tactile and thermal allodynia as well as hyperalgesia reflected by marked (P < 0.05) decreased in paw and tail withdrawal latency and threshold in SCI control rats as compared to normal and sham control rats. These paw and tail withdrawal latency and threshold were augmented efficiently (P < 0.05) after the administration of methylprednisolone when compared with SCI control rats. Treatment with fisetin (20 and 40 mg/kg) prominently (P < 0.05) increased paw and tail withdrawal latency and threshold when compared with SCI control rats. Methylprednisolone treatment showed more effective (P < 0.05) attenuation of SCI-induced mechano-tactile and thermal allodynia as well as hyperalgesia when compared to fisetin treated rats (Fig. 1).
Fig. 1.
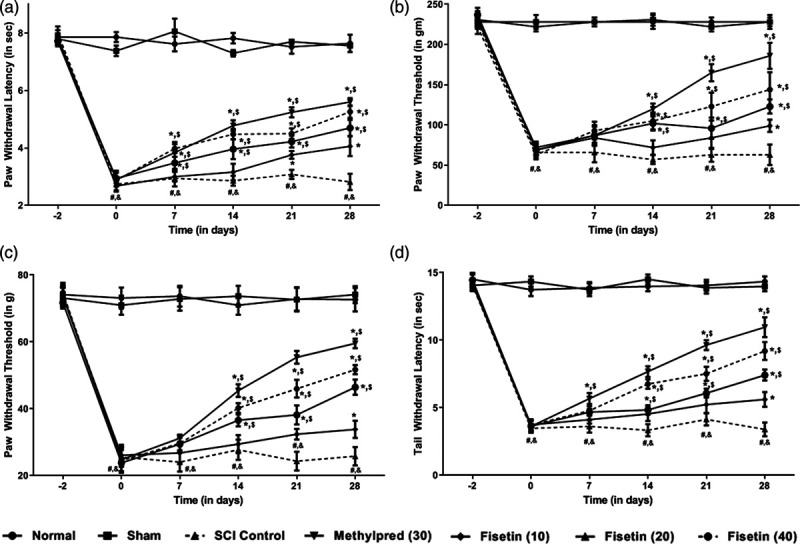
Effect of fisetin on SCI-induced alteration in thermal hyperalgesia in a plantar test (a), mechanical hyperalgesia in paw pressure test (b), mechanical allodynia in Von Frey hair test (c) and thermal hyperalgesia in tail immersion test (d). Data are expressed as mean ± SEM (n = 6) and analyzed by two-way ANOVA followed by Bonferroni’s test. *P < 0.05 as compared to SCI control group, #P < 0.05 as compared with sham group, &P < 0.05 as compared with normal and $P < 0.05 as compared with one another. ANOVA, analysis of variance; SCI, spinal cord injury.
Effect of fisetin on spinal cord injury-induced alteration in Basso–Beattie–Bresnahan score and motor coordination
There was no significant difference in BBB score and motor coordination between normal and sham control rats. However, SCI caused a marked (P < 0.05) decrease in BBB score and fall off time in SCI control rats as compared to normal and sham control rats. Administration of methylprednisolone prominently (P < 0.05) improved BBB score and motor coordination as compared to SCI control rats. Fisetin (20 and 40 mg/kg) treatment augmented BBB score and fall off time effectively (P < 0.05) as compared to SCI control rats. However, SCI-induced decreased in BBB score and fall off time was more effectively (P < 0.05) attenuated by methylprednisolone treatment as compared to fisetin treated rats (Fig. 2a, b).
Fig. 2.
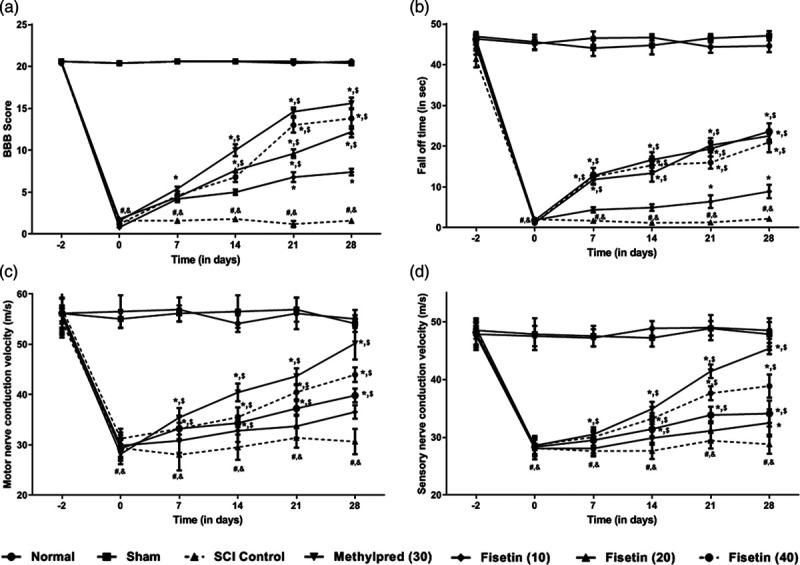
Effect of fisetin on SCI-induced alteration in locomotor behavior in BBB score (a), fall off time in motor coordination test (b), motor nerve conduction velocity (c) and sensory nerve conduction velocity (d). Data are expressed as mean ± SEM (n = 6) and analyzed by two-way ANOVA followed by Bonferroni’s test. *P < 0.05 as compared to SCI control group, #P < 0.05 as compared with sham group, &P < 0.05 as compared with normal and $P < 0.05 as compared with one another. ANOVA, analysis of variance; BBB, Basso–Beattie–Bresnahan; SCI, spinal cord injury.
Effect of fisetin on spinal cord injury-induced alteration in nerve conduction velocities
The nerve conduction velocities (MNCV and SNCV) was noticeably (P < 0.05) reduced in SCI control rats when as compared with normal and sham control rats. However, methylprednisolone treatment effectively increased (P < 0.05) MNCV and SNCV when compared with SCI control rats. As compared with SCI control rats, fisetin (20 and 40 mg/kg) treatment also effectively (P < 0.05) improved MNCV and SNCV. Methylprednisolone more prominently (P < 0.05) increased MNCV and SNCV as compared to fisetin treated rats. MNCV and SNCV did not differ significantly in normal and sham control rats (Fig. 2c, d).
Effect of fisetin on spinal cord injury-induced alteration in spinal TNF-α, IL-1β, IL-6, iNOs, COX-II, BDNF, Bax, Bcl-2 and caspase-3 mRNA expressions
The spinal mRNA expressions of TNF-α, IL-1β, IL-6, iNOs, COX-II, Bax and caspase-3 were markedly upregulated (P < 0.05) in SCI control rats as compared to normal and sham control rats. Whereas spinal BDNF and Bcl-2 mRNA expressions were marked (P < 0.05) downregulated (P < 0.05) in SCI control rats when compared to normal and sham control rats. Methylprednisolone significantly (P < 0.05) restored SCI-induced variations in spinal TNF-α, IL-1β, IL-6, iNOs, COX-II, BDNF, Bax, Bcl-2 and caspase-3 mRNA expressions as compared to SCI control rats. When compared with SCI control rats, fisetin (20 and 40 mg/kg) administration efficiently (P < 0.05) downregulated spinal TNF-α, IL-1β, IL-6, iNOs, COX-II, Bax and caspase-3 mRNA expressions whereas markedly (P < 0.05) upregulated spinal BDNF and Bcl-2 mRNA expressions. Attenuation of SCI-induced modification of spinal TNF-α, IL-1β, IL-6, iNOs, COX-II, BDNF, Bax, Bcl-2 and caspase-3 mRNA expressions were more noticeably (P < 0.05) in methylprednisolone as compared to fisetin treated rats (Table 2).
Table 2.
Effect of fisetin on SCI- induced alteration in mRNA expressions of TNF-α, IL-1β, IL-6, iNOs, COX-II, BDNF, Bax, Bcl-2 and caspase-3 in the spinal cord of a rat
| Parameter | Normal | Sham | SCI control | Methylprednisolone (30) | Fisetin (10) | Fisetin (20) | Fisetin (40) |
|---|---|---|---|---|---|---|---|
| TNF-α/β-actin ratio | 0.15 ± 0.03 | 0.33 ± 0.03 | 1.46 ± 0.02#,& | 0.38 ± 0.06*,$ | 1.37 ± 0.03 | 0.93 ± 0.02*,$ | 0.69 ± 0.05*,$ |
| IL-1β/β-actin ratio | 0.51 ± 0.07 | 0.76 ± 0.08 | 2.16 ± 0.04#,& | 0.78 ± 0.06*,$ | 2.09 ± 0.08 | 1.56 ± 0.08*,$ | 1.02 ± 0.07*,$ |
| IL-6/β-actin ratio | 0.67 ± 0.04 | 0.80 ± 0.03 | 1.49 ± 0.05#,& | 0.86 ± 0.06*,$ | 1.37 ± 0.02 | 1.02 ± 0.05*,$ | 0.92 ± 0.04*,$ |
| iNOs/β-actin ratio | 0.44 ± 0.05 | 0.73 ± 0.09 | 2.07 ± 0.07#,& | 0.78 ± 0.06*,$ | 1.92 ± 0.08 | 1.39 ± 0.07*,$ | 0.94 ± 0.02*,$ |
| COX-II/β-actin ratio | 0.15 ± 0.03 | 0.28 ± 0.02 | 1.06 ± 0.03#,& | 0.52 ± 0.05*,$ | 0.96 ± 0.04 | 0.75 ± 0.05*,$ | 0.54 ± 0.02*,$ |
| BDNF/β-actin ratio | 1.03 ± 0.09 | 1.03 ± 0.02 | 0.28 ± 0.04#,& | 0.98 ± 0.06*,$ | 0.44 ± 0.06 | 0.71 ± 0.05*,$ | 0.91 ± 0.06*,$ |
| Bax/β-actin ratio | 0.39 ± 0.06 | 0.45 ± 0.05 | 1.47 ± 0.07#,& | 0.61 ± 0.06*,$ | 1.35 ± 0.05 | 1.11 ± 0.07*,$ | 0.80 ± 0.05*,$ |
| Bcl-2/β-actin ratio | 3.41 ± 0.07 | 3.08 ± 0.04 | 0.87 ± 0.09#,& | 2.94 ± 0.10*,$ | 1.36 ± 0.13 | 1.87 ± 0.16*,$ | 2.79 ± 0.08*,$ |
| Caspase-3/β-actin ratio | 0.36 ± 0.03 | 0.41 ± 0.03 | 1.21 ± 0.02#,& | 0.60 ± 0.06*,$ | 1.06 ± 0.04 | 0.92 ± 0.05*,$ | 0.60 ± 0.03*,$ |
Data are expressed as mean ± SEM (n = 4) and one-way ANOVA followed by Tukey’s multiple range test.
ANOVA, analysis of variance; COX-II, cyclooxygenase-II; Bax, Bcl-2-associated X; Bcl-2, B-cell lymphoma 2; BDNF, brain-derived neurotrophic factor; iNOS, inducible nitric oxide synthase; IL-1β, interleukin-1 beta; IL-6, interleukin-6; SCI, spinal cord injury; TNF-α, tumor necrosis factor-alpha.
#P < 0.05 as compared with sham group.
&P < 0.05 as compared with normal.
*P < 0.05 as compared with the SCI control group.
$P < 0.05 as compared with one another.
Effect of fisetin on spinal cord injury-induced alteration in spinal NF-kB and IkBα protein expressions
There was no significant difference in spinal NF-kB and IkBα protein expressions within normal and sham control rats. However, SCI induction resulted in a noticeable (P < 0.05) increased in spinal NF-kB and IkBα protein expressions in SCI control rats as compared to normal and sham control rats. This augmentation in spinal NF-kB and IkBα protein expressions was prominently (P < 0.05) decreased by methylprednisolone treatment as compared to SCI control rats. Fisetin (20 and 40 mg/kg) treatment also efficiently (P < 0.05) attenuated SCI-induced increased spinal NF-kB and IkBα protein expressions when compared with SCI control rats (Fig. 3).
Fig. 3.
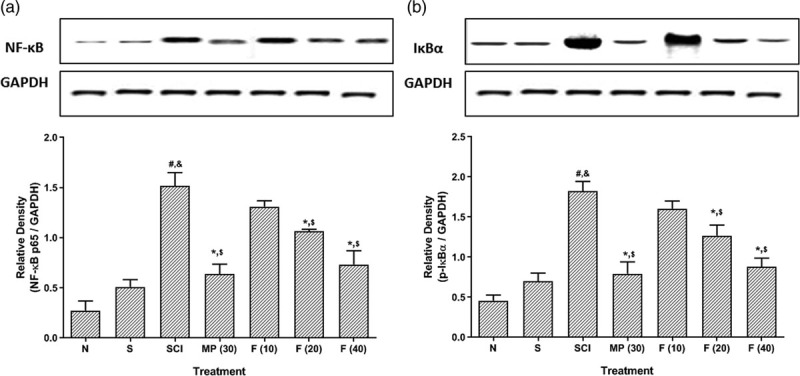
Effect of fisetin on SCI-induced alterations in NF-κB (a) and IκBα (b) protein expressions. Data are expressed as mean ± SEM (n = 6) and analyzed by two-way ANOVA followed by Bonferroni’s test. *P < 0.05 as compared to SCI control group, #P < 0.05 as compared with sham group, &P < 0.05 as compared with normal and $p < 0.05 as compared with one another. ANOVA, analysis of variance; IκBα, nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor-alpha; NF-κB, nuclear factor kappa B; SCI, spinal cord injury.
Effect of fisetin on spinal cord injury-induced alteration in spinal cord histology:
Fig. 4a, b showed normal architecture of the spinal cord from normal and sham control rats without any necrosis and inflammatory release. Injury to the spinal cord induced marked (P < 0.05) neuronal degeneration, necrosis and inflammatory infiltration in SCI control rats (Fig. 4c) as compared to normal and sham control rats. However, methylprednisolone treatment significantly (P < 0.05) attenuated SCI-induced modification in the spinal cord (Fig. 4d) as compared to SCI control rats. Fisetin (20 and 40 mg/kg) treatment also effectively (P < 0.05) lessened SCI-induced neuronal degeneration, necrosis and inflammatory infiltration (Fig. 4e, f) when compared with SCI control rats (Fig. 4g).
Fig. 4.
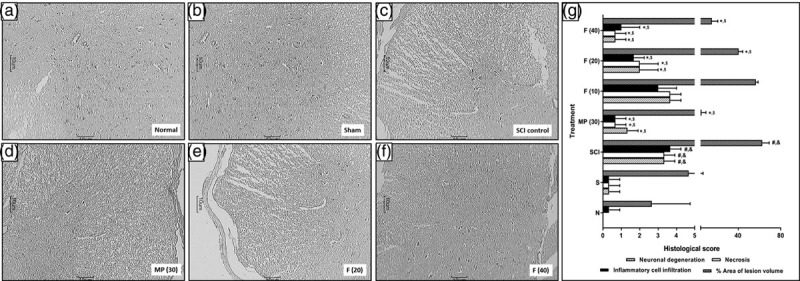
Effect of fisetin on SCI-induced alteration in spinal cord histology Spinal cord microscopic image of normal rat (a), sham control rat (b), SCI control rat (c), methylprednisolone (30 mg/kg) treated rat (d), fisetin (20 mg/kg) treated rat (e) and fisetin (40 mg/kg) treated rat (f). Spinal cord sections stained with H&E. Images at 40× are typical and representative of each study group. Data are expressed as mean ± SEM (n = 3), and one-way ANOVA followed by the Kruskal–Wallis test was applied for post hoc analysis. *P < 0.05 as compared to SCI control group, #P < 0.05 as compared with sham group, &P < 0.05 as compared with normal and $P < 0.05 as compared with one another. ANOVA, analysis of variance; H&E, hematoxylin and eosin; SCI, spinal cord injury.
Discussion
Spinal cord injury is neurological deficits with debilitating pathology that induce a significant burden on a family of patients with SCI. Evidence-based on experimental studies suggests that SCI characterized by the presence of biphasic event which initiated with primary injury through a series of cascade events followed by secondary damage to the neuronal tissue [5,8]. In the lack of effective pharmacotherapy for the management of SCI, the administration of methylprednisolone provide symptomatic relief from neuropathic pain to some extent [40]; however, its high dose causes severe adverse events. Thus, the development of a safe and effective treatment remains an unmet need. Research indicated that therapeutic moieties from herbal origin provide some ray of hope and plant flavonoids, such as fisetin, nowadays gaining significant attention towards the management of neurological disorders [14,22,24,33]. In the present investigation, we have evaluated the potential of fisetin against experimental model of SCI induced by clip compression model. Findings of present study suggest that fisetin exerts its neuroprotective effect orchestrated via modulation of the NF-κB/IκBα pathway, which further inhibited the release of inflammatory mediators (iNOs and COX-II), proinflammatory cytokines (TNF-α and ILs), apoptotic mediators (Bax and caspase-3) and elevates the levels of neurotrophic factors (BDNF) to attenuate painful allodynia and hyperalgesia during SCI (Graphical Abstract, Supplement digital content 2, http://links.lww.com/WNR/A615).
Researchers have well established the relation between inflammatory release and various degenerative disorders, including SCI [33,39]. A primary mechanical injury to the spinal cord results in inflammatory influx, including macrophage adhesion and neutrophil accumulation, which triggers the secondary injury mechanism and thus causes the destruction of spinal cords. Thus, inflammation plays a central dogma role in the induction and maintenance of SCI [21,39]. The rapid upregulation in the TNF-α expression in the spinal cord immediately after traumatic SCI has been well reported previously [8,41]. This enhanced spinal TNF-α expression further induces the formation of edema, endothelial deterioration and necrosis of the spinal cord [9]. Furthermore, IL-1β has been suggested to stimulate the transcription of inflammation enzymes, including COX-2 after spinal cord injury [39]. Researchers have reported the improvement of locomotor function and reduction of apoptosis after the administration of IL-1β receptor antagonist [42]. Additionally, previous reports suggest that the IL-6 expression sharply upregulated at the early stages of SCI, thus serves as an important factor for the induction of astrocytes differentiation of neural stem cells into astrocytes [41]. Therefore, the effort has been attempted by a number of researchers to develop therapeutic moieties with inhibitory potential against these proinflammatory cytokines (TNF-α and ILs) [39,42]. In the present investigation also the expression of TNF-α, IL-1β and IL-6 was markedly elevated in the spinal cord after SCI, which was diminished by administration of fisetin by virtue of its anti-inflammatory potential. A recent report by Zhao et al. [15] suggested that fisetin exerts its beneficial effect via inhibition of release of proinflammatory cytokines via inhibition of NF-κB signaling pathways.
BDNF is a neurotrophic factor from a family of nerve growth factor, and earlier reports well documented its potent role in nerve regeneration [43]. BDNF contributes to the growth and survival of sensory and motor neurons in the spinal cord, which contributed to the improvement of the functional locomotor activity after injury [43]. Furthermore, a recent report suggested that normalization of BDNF expression is also associated with amelioration of tactile allodynia and motor dysfunction during SCI [39]. Additionally, its importance in the modulation of apoptosis and inflammation in the various biological processes has been reported during the experimental study [39,44]. In the present investigation, SCI control rats showed reduced BDNF expression in the spinal cord, followed by altered allodynia and motor dysfunction depicting its important role in neuronal deficit. However, the administration of fisetin significantly increased BDNF expression in the spinal cord, which in turn improves allodynia response. A study by Wang et al. [45] demonstrated that fisetin augmented BDNF/TrkB signaling to improve motor coordination, and the findings of the present study are in accordance with this researcher.
Primary traumatic injury by compression or contusion results in secondary injury, which ended in cellular apoptosis [42]. During the apoptosis process, genes from Bcl-2 family members, that is, Bax and Bcl-2, play a critical role in the regulation of mitochondrial apoptosis [46,47]. Bax is widely recognized as an apoptotic gene, whereas Bcl-2 as an antiapoptotic gene; thus, a balance between then considered as a determinative factor for cellular apoptosis [48]. Additionally, caspase-3 has been suggested as an important downstream protease during the apoptosis process in various neurodegenerative disorders and injuries [42]. Clinically also apoptosis has been suggested as an important target and strategy for the management of SCI [2]. In the present study, clip compression of the spinal cord resulted in upregulated Bax and caspase-3 mRNA expression depicting the induction of apoptosis after SCI. Nevertheless, fisetin treatment balances antiapoptotic and proapoptotic gene expressions, thus ameliorates neuronal insult induced in the spinal cord. Previously also fisetin exerts its neuroprotective effect via antiapoptotic potential, and the findings of the present study are in line with this investigation [14].
Evidence reported that spinal cord injury is associated with several secondary events, including urinary bladder, renal and sexual dysfunction [1,3]. Alternation in the urinary function is the most common and serious consequence of SCI, which significantly affects the quality of life of the patient [3,21]. Elevated protein levels in the urine and increased urinary excretion during SCI suggest alterations in the functional status of the kidney [3]. Additionally, reduced sperm counts and testis weight are hallmarks of abnormal semen parameters and spermatogenesis, which suggested male infertility [9]. In the present study, injury to the spinal cord is associated with a reduction in the urinary bladder, renal and sexual function, which is in line with the finding of the previous study [9]. However, the administration of fisetin increases sperm count with improvement in the urinary and bladder outcomes suggesting its possible role in the improvement of their functions.
Currently, methylprednisolone has been implemented clinically for the management of acute SCI, which acts via multiple mechanisms, including anti-inflammatory, antioxidant, and antiapoptotic pathways [6]. A study documented that high-dose methylprednisolone was implemented during the treatment of acute SCI clinically; however, it is associated with severe side effects [40]. A randomized, placebo-controlled clinical study involving the herbal preparation containing a number of herbal moieties, including ginsenoside, ursolic acid, ferulic acid, glycyrrhizic acid, provides a promising effect in the improvement of neurological function in SCI patients without any severe side effect [49]. Fisetin, a plant flavonoid, also initiated a clinical trial against the alleviation of frailty and inflammation with a dose of 20 mg/kg [50]. Thus, fisetin can be considered as a potential moiety for further clinical evaluation in the management of spinal cord injury.
Conclusion
Fisetin attenuated painful allodynia and hyperalgesia during experimental spinal cord injury, thus exerts its neuroprotective potential via modulation of the NF-κB/IκBα pathway to inhibit release of inflammatory mediators (iNOs and COX-II), proinflammatory cytokines (TNF-α and ILs) and apoptotic mediators (Bax and caspase-3).
Acknowledgements
J.C. conceptualized and designed the study and prepared, edited and reviewed the manuscript. J.F. experimented studies, acquired the data and edited and reviewed the manuscript. H.L. conceptualized and designed the study, performed the statistical analysis and reviewed the manuscript. J.Z. conceptualized the study and prepared, edited and reviewed the manuscript. J.T. conceptualized the study and edited and reviewed the manuscript.
This study was funded by Hebei Key Laboratory of Molecular Pathology and Early Diagnosis of Tumors.
Conflicts of interest
There are no conflict of interest.
Supplementary Material
Footnotes
Jing Cui and Jingshi Fan contributed equally to the writing of this manuscript.
S pplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.neuroreport.com.
References
- 1.Badhiwala JH, Wilson JR, Fehlings MG. Global burden of traumatic brain and spinal cord injury. Lancet Neurol. 2019; 18:24–25. [DOI] [PubMed] [Google Scholar]
- 2.Donovan J, Kirshblum S. Clinical trials in traumatic spinal cord injury. Neurotherapeutics. 2018; 15:654–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taweel WA, Seyam R. Neurogenic bladder in spinal cord injury patients. Res Rep Urol. 2015; 7:85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Masri R, Keller A. Chronic pain following spinal cord injury. Adv Exp Med Biol. 2012; 760:74–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El Tecle NE, Dahdaleh NS, Bydon M, Ray WZ, Torner JC, Hitchon PW. The natural history of complete spinal cord injury: a pooled analysis of 1162 patients and a meta-analysis of modern data. J Neurosurg Spine. 2018; 28:436–443. [DOI] [PubMed] [Google Scholar]
- 6.Ulndreaj A, Badner A, Fehlings MG. Promising neuroprotective strategies for traumatic spinal cord injury with a focus on the differential effects among anatomical levels of injury. F1000Res. 2017; 6:1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kandhare AD, Raygude KS, Ghosh P, Gosavi TP, Bodhankar SL. Patentability of animal models: India and the globe. Int J Pharm Biol Arc. 2011; 2:1024–1032. [Google Scholar]
- 8.Ahmed RU, Alam M, Zheng YP. Experimental spinal cord injury and behavioral tests in laboratory rats. Heliyon. 2019; 5:e01324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kandhare AD, Ghosh P, Bodhankar SL. Naringin, a flavanone glycoside, promotes angiogenesis and inhibits endothelial apoptosis through modulation of inflammatory and growth factor expression in diabetic foot ulcer in rats. Chem Biol Interact. 2014; 219:101–112. [DOI] [PubMed] [Google Scholar]
- 10.Cui J, Wang G, Kandhare AD, Mukherjee-Kandhare AA, Bodhankar SL. Neuroprotective effect of naringin, a flavone glycoside in quinolinic acid-induced neurotoxicity: possible role of PPAR-γ, Bax/Bcl-2, and caspase-3. Food Chem Toxicol. 2018; 121:95–108. [DOI] [PubMed] [Google Scholar]
- 11.Li W, Kandhare AD, Mukherjee AA, Bodhankar SL. Hesperidin, a plant flavonoid accelerated the cutaneous wound healing in streptozotocin-induced diabetic rats: role of TGF-ß/Smads and Ang-1/Tie-2 signaling pathways. EXCLI J. 2018; 17:399–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohod SM, Kandhare AD, Bodhankar SL. Gastroprotective potential of Pentahydroxy flavone isolated from Madhuca indica J. F. Gmel. Leaves against acetic acid-induced ulcer in rats: the role of oxido-inflammatory and prostaglandins markers. J Ethnopharmacol. 2016; 182:150–159. [DOI] [PubMed] [Google Scholar]
- 13.Mukherjee AA, Kandhare AD, Bodhankar SL. Elucidation of protective efficacy of pentahydroxy flavone isolated from Madhuca indica against arsenite-induced cardiomyopathy: role of Nrf-2, PPAR-γ, c-fos and c-jun. Environ Toxicol Pharmacol. 2017; 56:172–185. [DOI] [PubMed] [Google Scholar]
- 14.Rajendran M, Ramachandran R. Fisetin protects against rotenone-induced neurotoxicity through signaling pathway. Front Biosci (Elite Ed). 2019; 11:20–28. [DOI] [PubMed] [Google Scholar]
- 15.Zhao L, Kandhare AD, Mukherjee AA, Bodhankar SL. Anti-allergic potential of fisetin in a murine model of OVA-induced allergic rhinitis via inhibition of GATA-3 and Th2 cytokines. Biomedica. 2018; 34:88–101. [Google Scholar]
- 16.Farsad-Naeimi A, Alizadeh M, Esfahani A, Darvish Aminabad E. Effect of fisetin supplementation on inflammatory factors and matrix metalloproteinase enzymes in colorectal cancer patients. Food Funct. 2018; 9:2025–2031. [DOI] [PubMed] [Google Scholar]
- 17.Goh FY, Upton N, Guan S, Cheng C, Shanmugam MK, Sethi G, et al. Fisetin, a bioactive flavonol, attenuates allergic airway inflammation through negative regulation of NF-κB. Eur J Pharmacol. 2012; 679:109–116. [DOI] [PubMed] [Google Scholar]
- 18.Huang W, Li ML, Xia MY, Shao JY. Fisetin-treatment alleviates airway inflammation through inhbition of MyD88/NF-κB signaling pathway. Int J Mol Med. 2018; 42:208–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kandhare AD, Raygude KS, Ghosh P, Bodhankar SL. The ameliorative effect of fisetin, a bioflavonoid, on ethanol-induced and pylorus ligation-induced gastric ulcer in rats. Inter J Green Pharmacy. 2011; 5:236–243. [Google Scholar]
- 20.Kim JH, Kim MY, Kim JH, Cho JY. Fisetin suppresses macrophage-mediated inflammatory responses by blockade of Src and Syk. Biomol Ther (Seoul). 2015; 23:414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehta P, Pawar A, Mahadik K, Bothiraja C. Emerging novel drug delivery strategies for bioactive flavonol fisetin in biomedicine. Biomed Pharmacother. 2018; 106:1282–1291. [DOI] [PubMed] [Google Scholar]
- 22.Maher P, Dargusch R, Bodai L, Gerard PE, Purcell JM, Marsh JL. ERK activation by the polyphenols fisetin and resveratrol provides neuroprotection in multiple models of Huntington’s disease. Hum Mol Genet. 2011; 20:261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prasath GS, Subramanian SP. Antihyperlipidemic effect of fisetin, a bioflavonoid of strawberries, studied in streptozotocin-induced diabetic rats. J Biochem Mol Toxicol. 2014; 28:442–449. [DOI] [PubMed] [Google Scholar]
- 24.Raygude KS, Kandhare AD, Ghosh P, Bodhankar SL. Anticonvulsant effect of fisetin by modulation of endogenous biomarkers. Biomed Prev Nutr. 2012; 2:215–222. [Google Scholar]
- 25.Sahu BD, Kalvala AK, Koneru M, Mahesh Kumar J, Kuncha M, Rachamalla SS, Sistla R. Ameliorative effect of fisetin on cisplatin-induced nephrotoxicity in rats via modulation of NF-κB activation and antioxidant defence. PLoS One. 2014; 9:e105070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shanmugam K, Ravindran S, Kurian GA, Rajesh M. Fisetin confers cardioprotection against myocardial ischemia reperfusion injury by suppressing mitochondrial oxidative stress and mitochondrial dysfunction and inhibiting glycogen synthase kinase 3β activity. Oxid Med Cell Longev. 2018; 2018:9173436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suh Y, Afaq F, Khan N, Johnson JJ, Khusro FH, Mukhtar H. Fisetin induces autophagic cell death through suppression of mTOR signaling pathway in prostate cancer cells. Carcinogenesis. 2010; 31:1424–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu MY, Hung SK, Fu SL. Immunosuppressive effects of fisetin in ovalbumin-induced asthma through inhibition of NF-κB activity. J Agric Food Chem. 2011; 59:10496–10504. [DOI] [PubMed] [Google Scholar]
- 29.Zhao X, Li XL, Liu X, Wang C, Zhou DS, Ma Q, et al. Antinociceptive effects of fisetin against diabetic neuropathic pain in mice: engagement of antioxidant mechanisms and spinal GABAA receptors. Pharmacol Res. 2015; 102:286–297. [DOI] [PubMed] [Google Scholar]
- 30.Chou RH, Hsieh SC, Yu YL, Huang MH, Huang YC, Hsieh YH. Fisetin inhibits migration and invasion of human cervical cancer cells by down-regulating urokinase plasminogen activator expression through suppressing the p38 MAPK-dependent NF-κB signaling pathway. PLoS One. 2013; 8:e71983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suh Y, Afaq F, Johnson JJ, Mukhtar H. A plant flavonoid fisetin induces apoptosis in colon cancer cells by inhibition of COX2 and Wnt/EGFR/NF-kappaB-signaling pathways. Carcinogenesis. 2009; 30:300–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee JD, Huh JE, Jeon G, Yang HR, Woo HS, Choi DY, Park DS. Flavonol-rich RVHxR from Rhus verniciflua Stokes and its major compound fisetin inhibits inflammation-related cytokines and angiogenic factor in rheumatoid arthritic fibroblast-like synovial cells and in vivo models. Int Immunopharmacol. 2009; 9:268–276. [DOI] [PubMed] [Google Scholar]
- 33.Maher P. Modulation of multiple pathways involved in the maintenance of neuronal function during aging by fisetin. Genes Nutr. 2009; 4:297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhen L, Zhu J, Zhao X, Huang W, An Y, Li S, et al. The antidepressant-like effect of fisetin involves the serotonergic and noradrenergic system. Behav Brain Res. 2012; 228:359–366. [DOI] [PubMed] [Google Scholar]
- 35.Raygude KS, Kandhare AD, Ghosh P, Ghule AE, Bodhankar SL. Evaluation of ameliorative effect of quercetin in experimental model of alcoholic neuropathy in rats. Inflammopharmacology. 2012; 20:331–341. [DOI] [PubMed] [Google Scholar]
- 36.Visnagri A, Kandhare AD, Chakravarty S, Ghosh P, Bodhankar SL. Hesperidin, a flavanoglycone attenuates experimental diabetic neuropathy via modulation of cellular and biochemical marker to improve nerve functions. Pharm Biol. 2014; 52:814–828. [DOI] [PubMed] [Google Scholar]
- 37.Visnagri A, Kandhare AD, Kumar VS, Rajmane AR, Mohammad A, Ghosh P, et al. Elucidation of ameliorative effect of co-enzyme Q10 in streptozotocin-induced diabetic neuropathic perturbation by modulation of electrophysiological, biochemical and behavioral markers. Biomed Aging Pathol. 2012; 2:157–172. [Google Scholar]
- 38.Kandhare AD, Ghosh P, Ghule AE, Bodhankar SL. Elucidation of molecular mechanism involved in neuroprotective effect of coenzyme Q10 in alcohol-induced neuropathic pain. Fundam Clin Pharmacol. 2013; 27:603–622. [DOI] [PubMed] [Google Scholar]
- 39.Liang K, Kandhare AD, Mukherjee-Kandhare AA, Bodhankar SL, Xu D. Morin ameliorates ovalbumin-induced allergic rhinitis via inhibition of STAT6/SOCS1 and GATA3/T-bet signaling pathway in BALB/c mice. J Funct Foods. 2019; 55:391–401. [Google Scholar]
- 40.Young W, Bracken MB. The second national acute spinal cord injury study. J Neurotrauma. 1992; 9 (Suppl 1):S397–S405. [PubMed] [Google Scholar]
- 41.Mukaino M, Nakamura M, Yamada O, Okada S, Morikawa S, Renault-Mihara F, et al. Anti-IL-6-receptor antibody promotes repair of spinal cord injury by inducing microglia-dominant inflammation. Exp Neurol. 2010; 224:403–414. [DOI] [PubMed] [Google Scholar]
- 42.Nesic-Taylor O, Svrakic N, Xu G-Y, McAdoo D, Westlund K, Hulsebosch C, et al. IL-1 receptor antagonist prevents apoptosis of spinal cord cells and caspase-3 activation after injury. J Neurochem. 2001; 78:65–65. [DOI] [PubMed] [Google Scholar]
- 43.Garraway SM, Huie JR. Spinal plasticity and behavior: BDNF-induced neuromodulation in uninjured and injured spinal cord. Neural Plast. 2016; 2016:9857201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Furuya M, Masuda H, Hara K, Uchida H, Sato K, Sato S, et al. ZEB1 expression is a potential indicator of invasive endometriosis. Acta Obstet Gynecol Scand. 2017; 96:1128–1135. [DOI] [PubMed] [Google Scholar]
- 45.Wang Y, Wang B, Lu J, Shi H, Gong S, Wang Y, et al. Fisetin provides antidepressant effects by activating the tropomyosin receptor kinase B signal pathway in mice. J Neurochem. 2017; 143:561–568. [DOI] [PubMed] [Google Scholar]
- 46.Visnagri A, Kandhare AD, Bodhankar SL. Renoprotective effect of berberine via intonation on apoptosis and mitochondrial-dependent pathway in renal ischemia reperfusion-induced mutilation. Ren Fail. 2015; 37:482–493. [DOI] [PubMed] [Google Scholar]
- 47.Ghule AE, Kandhare AD, Jadhav SS, Zanwar AA, Bodhankar SL. Omega-3-fatty acid adds to the protective effect of flax lignan concentrate in pressure overload-induced myocardial hypertrophy in rats via modulation of oxidative stress and apoptosis. Int Immunopharmacol. 2015; 28:751–763. [DOI] [PubMed] [Google Scholar]
- 48.Kandhare AD, Raygude KS, Kumar VS, Rajmane AR, Visnagri A, Ghule AE, et al. Ameliorative effects quercetin against impaired motor nerve function, inflammatory mediators and apoptosis in neonatal streptozotocin-induced diabetic neuropathy in rats. Biomed Aging Pathol. 2012; 2:173–186. [Google Scholar]
- 49.Li YL, Li LT, Yu M, Wang YZ, Ge HY, Song CQ. Beneficial effects of the herbal medicine Di Huang Yin Zi in patients with spinal cord injury: a randomized, placebo-controlled clinical study. J Int Med Res. 2012; 40:1715–1724. [DOI] [PubMed] [Google Scholar]
- 50.ClinicalTrials.gov. Alleviation by Fisetin of Frailty, Inflammation, and Related AQ4 Measures in Older Adults (AFFIRM-LITE). NCT036757242018. 2020. https://clinicaltrials.gov/ct2/show/results/NCT03675724?view=results. [Accessed 24 August 2020]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


