Abstract
Itraconazole is a triazole agent that is routinely used for treatment of nail infections and other fungal infections. Recent studies indicate that itraconazole can also inhibit the growth of basal cell carcinoma (BCC) through suppression of the Sonic Hedgehog (SHH) signaling pathway. In this study, polyglycolic acid microneedle arrays and stainless steel microneedle arrays were used for transdermal delivery of itraconazole to a human BCC model which was regenerated on mice. One-by-four arrays of 642-μm-long polyglycolic acid microneedles with sharp tips were prepared using injection molding and drawing lithography. Arrays of 85 stainless steel 800-μm-tall microneedles attached to syringes were obtained for comparison purposes. Skin grafts containing devitalized split-thickness human dermis that had been seeded with human keratinocytes transduced to express human SHH protein were sutured to the skin of immunodeficient mice. Mice with this human BCC model were treated daily for 2 weeks with itraconazole dissolved in 60% dimethylsulfoxane and 40% polyethylene glycol-400 solution; transdermal administration of the itraconazole solution was facilitated by either four 1 × 4 polyglycolic acid microneedle arrays or stainless steel microneedle arrays. The epidermal tissues treated with polyglycolic acid microneedles or stainless steel microneedles were markedly thinner than that of the control (untreated) graft tissue. These preliminary results indicate that microneedles may be used to facilitate transdermal delivery of itraconazole for localized treatment of BCC.
INTRODUCTION
Over 2 million individuals were diagnosed with basal cell carcinoma (BCC) in the United States in 2010, and 3000 deaths per year are attributed to this condition.1,2 BCC is the most common cancer among Caucasians and Hispanics.1,3 The risk of developing BCC is associated with several factors, including: (1) exposure to midrange ultraviolet B radiation, (2) family history of melanoma, (3) indoor tanning, (4) red/blond hair, (5) the presence of a higher number of extremity moles, (6) a higher susceptibility to sunburn as a child/adolescent, and (7) a higher lifetime number of severe sunburns.4,5
As noted in the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology: Basal Cell and Squamous Cell Skin Cancers, treatment of BCC is based on tumor size, depth, and location; the goals of treatment include elimination of cancerous tissue, maximal preservation of physical appearance, and maximal preservation of function.6 Surgery is commonly used to treat BCC; however, surgery may result in significant morbidity and disfiguring scarring.7 In addition, BCC on the face (e.g., near the brain or eyes) may be difficult to surgically treat without significant morbidity.4 Due to the relatively benign course of BCC, several non-surgical treatment modalities, including pharmacologic treatment options, have been developed.8 5-fluorouracil, an antineoplastic agent, was the first pharmacologic agent to be approved by the US Food and Drug Administration for topical treatment of superficial BCC.9 Unfortunately, use of 5-fluorouracil is associated with side effects such as allergic reactions, inflammation, dyspigmentation, pain, and erosion.9 In addition, in vitro studies involving yeast and bacteria cells as well as in vivo studies involving immunosuppressed syngeneic mice have demonstrated that fluorouracil causes mutations.10 A synthetic immune modulator, 5% imiquimod, has also been approved by the US Food and Drug Administration for topical treatment of superficial BCC.11 It should be noted that topical use of imiquimod may be associated with intense local inflammatory reactions such as skin weeping, erosion, and scabbing.11
The drug itraconazole is considered to be a promising alternative to conventional topical BCC agents. Itraconazole is a triazole agent that has two mechanisms of action.12 One cluster of activities, including (1) inhibition of cell membrane function, (2) interference with cytochrome P450 activity, and (3) reduction in ergosterol synthesis, imparts itraconazole with antifungal activity.12 Itraconazole has been approved for use as an antifungal agent since 1992, and has been used by tens of thousands of individuals for treatment of a wide variety of fungal infections, including onychomycosis (nail infection), blastomycosis, aspergillosis, candidiasis, crypotococcosis, histoplasmosis, and sporotrichosis.13,14 In 2010, Kim et al. elucidated a second mechanism of action for itraconazole, which involves suppression of the critical Hedgehog signaling pathway activator known as “Smoothened” (SMO).15 They studied the antineoplastic activity of itraconazole using an in vivo murine model and showed that itraconazole suppressed growth of medulloblastoma (brain tumor) at serum levels comparable to those used in antifungal treatments. In addition, the side effects associated with other drugs that act via Hedgehog pathway inhibition are apparently absent with itraconazole.16 In a subsequent paper, Kim et al. noted that itraconazole exhibits Hedgehog signaling pathway suppression activity in the presence of resistance-conferring Smoothened mutations.17 In 2012, Montoya et al. demonstrated a 30% decrease in BCC tumor diameter as well as improved wound healing among patients who received oral itraconazole.18 More recently, Kim et al. performed a clinical study in which patients with BCC tumors were treated with oral itraconazole.19,20 They showed that itraconazole decreased tumor area by 24% and reduced cell proliferation by 45%. It is important to note that use of oral itraconazole is associated with side effects, including grade 4 congestive heart failure. In addition to congestive heart failure, liver failure is a side effect of oral itraconazole therapy; in some cases, liver failure occurred in patients with no pre-existing liver condition and no underlying medical condition.21
Topical delivery may provide an advantage over systemic delivery of itraconazole. By minimizing systemic absorption, side effects and toxicity associated with itraconazole may be reduced.22 In addition, therapies based on knowledge of a given patient’s BCC lesion geometry may be more effective than therapies based on arbitrary guidelines.23 It should be noted that itraconazole is difficult to reproducibly supply to the basal layer via a conventional transdermal patch, since it exhibits poor solubility in ethanol and water24 and does not readily cross an intact epidermis.
Microneedles are <300-μm-diameter, 50-μm to 900-μm lancet-shaped or hypodermic needle-shaped structures that can be used to deliver itraconazole to the BCC tumor site.25,26 These structures are used to create channels in the 15-μm-thick keratinized stratum corneum layer of the epidermis, which typically hinders transport of pharmacologic agents through the skin.27 Bleeding and other tissue damage at the treatment site are minimized because of the small dimensions of these needles. Use of microneedles is associated with low levels of pain since they do not penetrate deeper skin layers, where Pacinian corpuscles, Meissner’s corpuscles, and large nerve endings are located.28 Delivery of pharmacologic agents using microneedles can be achieved via several protocols: (1) a “coat and poke” protocol that involves coating of a microneedle with a layer of a pharmacologic agent-containing solution, (2) a “poke and patch” protocol that involves application of a pharmacologic agent-containing patch to skin after microneedle treatment, (3) a “poke and release” protocol that involves degradation of an entire pharmacologic agent-bearing microneedle, and (4) a “poke and flow” protocol that involves movement of a pharmacologic agent through a hollow microneedle (by means of diffusion or by means of infusion).29–31
In a previous study, we examined the use of piezoelectric inkjet printing to apply an antifungal agent known as voriconazole to the surfaces of biodegradable polyglycolic acid microneedles that were fabricated using a combination of injection molding and drawing lithography.32 The hardness (588.2 MPa ± 33.8 MPa) and elastic modulus (9.9 GPa ± 0.3 GPa) values for polyglycolic acid were obtained using nanoindentation and were found to be appropriate for use in transdermal drug delivery devices.33 Voriconazole was coated on the polyglycolic acid microneedles via a piezoelectric inkjet printing approach. Unlike the vehicle-modified and unmodified microneedles, the voriconazole-coated microneedles exhibited antifungal activity against Candida albicans.
In this study, polyglycolic acid microneedles and stainless steel microneedles were used for transdermal delivery of an itraconazole-containing solution to a human BCC model which was regenerated on mice. One-by-four arrays of long polyglycolic acid microneedles with sharp tips were created using injection molding and drawing lithography. Arrays of stainless steel microneedles attached to syringes were obtained for comparison purposes. Skin grafts containing devitalized split-thickness human dermis that had been seeded with human keratinocytes transduced with retrovirus encoding human sonic hedgehog (SHH) were sutured to the skin of immunodeficient mice. Mice with this human BCC model were treated daily for 2 weeks with itraconazole dissolved in a solution containing dimethylsulfoxane and polyethylene glycol-400; transdermal administration of this solution was facilitated by either polyglycolic acid microneedle arrays or stainless steel microneedle arrays.
MATERIALS AND METHODS
Fabrication of the Polyglycolic Acid Microneedle Arrays
Microneedle arrays were fabricated from polyglycolic acid (Kureha, Tokyo, Japan) using a previously described approach.33 Polyglycolic acid pellets were injection molded with a Sesame molding machine (Trinks, De Pere, WI, USA) using steel molds into 1 × 4 arrays; in these arrays, the individual microneedles were positioned 1800 μm apart (as measured from microneedle center to microneedle center). These molded structures were in the form of half-cones on top of a rectangular base, with the faces of the half-cones oriented in a co-planar position with one of the long faces of the rectangular base.
A drawing lithography process was used to sharpen the tips of the polyglycolic acid microneedles.33 The microneedles underwent a melt-drawing process, in which the microneedle tips were lowered onto a heated surface (temperature = 220°C) that was regulated with a servomotor lift platform; the microneedles were then withdrawn while cooling to obtained sharp and high aspect ratio tips. The microneedles were placed above a Cimarec™ hotplate (Barnstead International, Dubuque, IA, USA) that was attached to an AVS125 servomotor lift platform (Aerotech, Pittsburgh, PA, USA). The microneedles were oriented such that the axis of the half-cone was positioned towards the hotplate. When the lift platform was raised, it contacted the tips of the microneedles. The platform was paused (20 s) to melt the microneedle structure; polyglycolic acid has a Tm of 220°C. The platform was subsequently raised 550 μm at a rate of 0.5 mm s−1. The hotplate was then turned off during a 3-s pause in platform motion. The platform subsequently reversed direction, moving at a rate of 1.0 mm min−1 for a distance of 1.65 mm. During this reversal in direction, the polyglycolic acid at the microneedle tips was drawn to form tapered tip structures; the microneedle tips solidified as the heat source was removed. For comparison purposes, 316L stainless steel microneedle arrays were purchased from a commercial source (AdminMed, Sunnyvale, CA, USA).34–36 Each array contains 85 microneedles 800 μm tall that were organized in 1-cm2 circular array.
Sterilization of the Microneedle Arrays
The polyglycolic acid microneedle arrays and stainless steel microneedle arrays were exposed to ethylene oxide (volume 10.5 g ± 5%) for a 4-h injection time and a 12-h hold time; the purge time exceeded 3 h. The biological indicator, which was incubated at an average temperature between 35 and 39°C for over 48 h, was negative for growth.
Preparation of the Itraconazole-Containing Solution
Itraconazole 99% (Acros Organics, NJ, USA), dimethylsulfoxide 99% (FWI, Tulsa, OK, USA), polyethylene Glycol 400 (Letco, Decatur, AL, USA), and a dimethylsulfoxide safe 0.22-μm sterilizing filter (Pall, Port Washington, NY, USA) were used to prepare the itraconazole-containing solution. A 5 mg/ml sterile solution of itraconazole was prepared in dimethylsulfoxide 60% and polyethylene glycol-400 40% solution as follows: 505 mg of itraconazole 99% was dissolved in 60.6 mL of dimethylsulfoxide 99% and brought to a final volume of 100 mL with polyethylene glycol-400. The resultant solution was passed through the dimethylsulfoxide safe 0.22-μm sterilizing filter into a vented, empty sterile glass vial (Greer Labs, Lenoir, NC, USA). The sterilizing filter was bubble point tested to ensure integrity and passed with no bubbles visible up to 275 kPa.
Animal Study
Animal studies were conducted in accordance with protocols that were approved by the Duke Animal Care and Use Committee. Immunodeficient NOD scid gamma (NSG™)-severe combined immunodeficiency mice were purchased from Duke animal facility. Surgically discarded normal human skin samples were obtained from Duke Hospital surgical units in accordance with an approved IRB protocol. For skin grafting, keratinocytes isolated from foreskin samples were transduced with retrovirus-encoding SHH as previously described was seeded onto devitalized split-thickness human dermis that had been prepared from abdominoplasty skin tissues.37 Animal surgeries were performed on anesthetized animals in a ventilated hood that was provided by the Duke Division of Laboratory Animal Resource (DLAR). After anesthesia, animals were shaved on the back skin, placed on a pile of warm and sterilized towel, and then cleaned with iodine and alcohol swabs. An appropriately 1-cm2 section of mouse skin was removed down to fascia from the dorsum of each mouse with sterile tools. The skin composite was then sutured to the mouse skin and dressed with polymoxin B antibiotic ointment, adaptic, telfa pad and Coban wrap (obtained from Duke DLAR). The bandages were removed around 2 weeks after the surgery.
For topical application of the itraconazole-containing solution, animals were divided into three groups and treated daily with 50 μl of 5 mg/ml itraconazole dissolved in 60% dimethylsulfoxane and 40% polyethylene glycol-400 solution for 2 weeks starting at 2 weeks after the surgery. The grafts of the first group were untreated. The grafts of the second group were poked six-eight times spaced at 1–2 mm distance with four arrays of solid 1 × 4 polyglycolic acid polymer microneedles followed by topical pipetting of the itraconazole-containing solution. The grafts of the third group were injected with 50 μl of the itraconazole-containing solution through a 1-ml syringe attached to the 316L stainless steel microneedle array. Skin grafts were collected at the end-point on euthanized animals and analyzed by hematoxylin and eosin staining (Duke Pathology Laboratory, Durham, NC, USA).
RESULTS AND DISCUSSION
Heating and melt-drawing the injection molded microneedle structures was successfully used to create arrays of microneedles with narrower tips and sharper tip radii. The microneedle arrays following the drawing lithography process were optically evaluated to determine microneedle heights and microneedle tip radii. The polyglycolic acid microneedles exhibited heights of 641 μm ± 9.5 μm (mean ± SEM, n = 60).38 As seen in Fig. 1a and b, the polyglycolic acid microneedles taper from the base, narrow toward the tip, and come to a sharp point. The 800-μm-tall microneedles in the stainless steel microneedle array also exhibit sharp tip radii; these microneedles are shown in Fig. 1c and d.
Fig. 1.
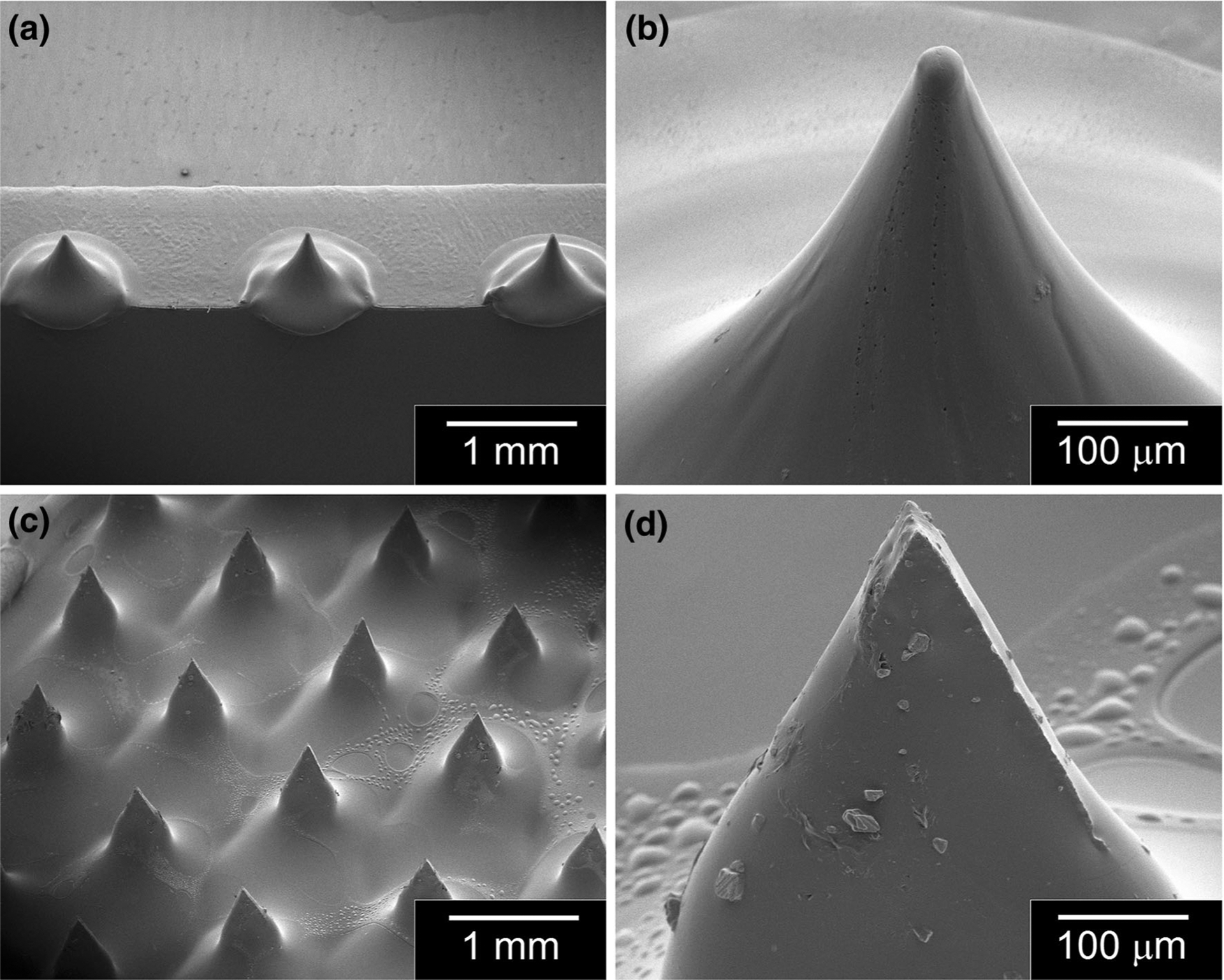
Scanning electron microscopy of images of (a) component polyglycolic acid microneedles in a 1 × 4 microneedle array, (b) an individual polyglycolic acid microneedle, (c) component stainless steel microneedles in an 85-microneedle array, and (d) an individual stainless steel microneedle.
To determine whether microneedles can be used to facilitate topical delivery of an itraconazole-containing solution, we utilized a previously established human BCC model via skin regeneration on mice.37 Primary human keratinocytes isolated from surgically discarded normal human skin were (1) transduced with retrovirus for overexpression of Sonic Hedgehog and (2) seeded on devitalized human dermis for skin grafting on NOD scid gamma (NSG™)-severe combined immunodeficient mice. Starting at 2 weeks after the surgery, the skin grafts underwent daily treatment with the itraconazole-containing solution (250 μg/graft in 50 μl solution) following skin pretreatment with the polyglycolic acid microneedle arrays. Alternatively, grafts were injected with the itraconazole-containing solution through an array of stainless steel microneedles attached to a 1-ml syringe. The skin grafts were collected for histological analysis 2 weeks after the treatment. During the course of the treatment, 1–2 animals of each treatment group were lost due to unknown reasons, suggesting that the animal loss may not be related to the treatment. We observed that the polyglycolic acid microneedles induced skin injuries in all four animals as evidenced by traces of blood on the skin surface following each treatment. At the end of the 2-week treatment, only one of the two remaining polyglycolic acid microneedle-treated animals had an apparent human skin graft.
As shown by hematoxylin and eosin staining (Fig. 2a), the epidermis of SHH-expressing control grafts displayed basaloid overgrowth that resembles human BCC. In contrast, the epidermal tissues treated with the polyglycolic acid microneedle array- and stainless steel microneedle array-administered itraconazole were markedly thinner than those of the control graft (Fig. 2b and c). In addition, there was an increased presence of likely immune cells in the dermal compartment of the polyglycolic acid microneedle array-treated tissue. A wounding response was not apparent following administration of itraconazole with the stainless steel microneedle arrays.
Fig. 2.
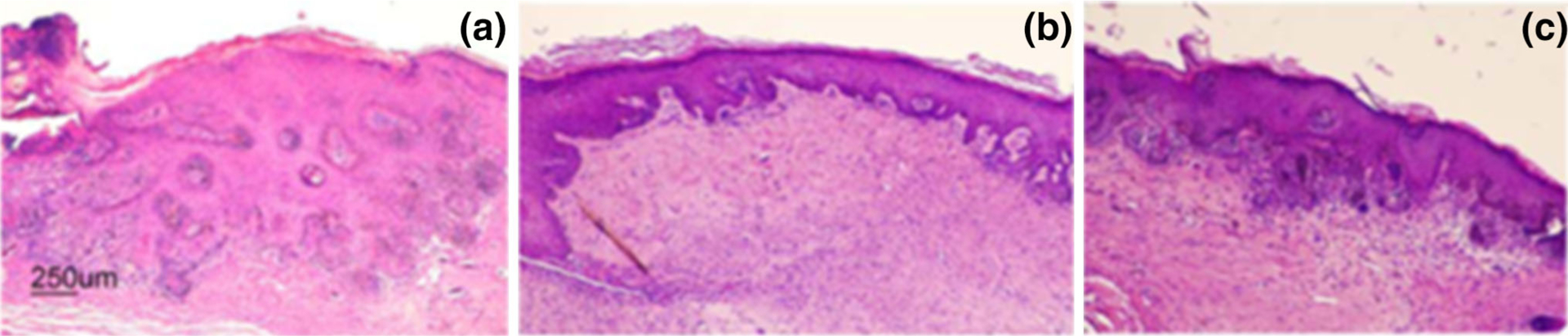
Microneedle-facilitated delivery of itraconzole to regenerated human basal cell carcinoma. Skin grafts expressing Sonic Hedgehog were generated on immunodeficient mice; topical treatment of itraconazole was facilitated by either four 1 × 4 polyglycolic acid microneedle arrays or stainless steel microneedle arrays. Hematoxylin and eosin staining of 4-week-old regenerated human skin grafts of: (a) untreated control, (b) polyglycolic acid microneedle array-facilitated delivery of itraconazole-containing solution, and (c) stainless steel microneedle array-facilitated delivery of itraconazole-containing solution.
CONCLUSION
Arrays of polyglycolic acid microneedles were successfully fabricated using drawing lithography. Scanning electron microscopy of the microneedle arrays showed that the polyglycolic acid microneedles and the commercially obtained stainless steel microneedles exhibited sharp tips. The preliminary in vivo results indicate that microneedles may be used to facilitate delivery of itraconazole for localized treatment of BCC. Additional studies are underway to maximize the efficacy of this approach by optimizing (1) the concentration of itraconazole in the itraconazole-containing solution and (2) the number of times that the itraconazole-containing solution is administered over the course of treatment. In addition, microneedle-based transdermal delivery of itraconazole may potentially be useful for prophylactic treatment of precancerous regions on the skin.
ACKNOWLEDGEMENTS
The authors would like to thank Gigi Davidson, Director of Clinical Pharmacy Services at the NC State College of Veterinary Medicine, and her staff for their efforts to develop and prepare the itraconazole formulation. We thank Paul Khavari and Anthony Oro of Stanford University for providing the SHH expression construct.
REFERENCES
- 1.“Basal cell carcinoma” (The Skin Cancer Foundation, 2015), http://www.skincancer.org/skin-cancer-information/basal-cell-carcinoma. Accessed 4 January 2016.
- 2.Mohan SV and Chang ALS, Curr. Dermatol. Rep 3, 40 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gloster HM and Neal K, J. Am. Acad. Dermatol 55, 741 (2006). [DOI] [PubMed] [Google Scholar]
- 4.Wu S, Han J, Li WQ, Li T, and Qureshi AA, Am. J. Epidemiol 178, 890 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wehner MR, Chren MM, Nameth D, Choudhry A, Gaskins M, Nead KT, Boscardin WJ, and Linos E, JAMA Dermatol. 150, 390 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bader RS, Santacroce L, Kennedy AS, “Basal Cell Carcinoma Treatment & Management” (Medscape, 2016), http://emedicine.medscape.com/article/276624-treatment. Accessed 4 Jan 2016.
- 7.Bath-Hextall F, Ozolins M, Armstrong SJ, Colver GB, Perkins W, Miller PS, and Williams HC, Lancet Oncol. 15, 96 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Shumack SP, Aust. Prescr 34, 6 (2011). [Google Scholar]
- 9.Korgavkar K, Firoz EF, Xiong M, Lew R, Marcolivio K, Burnside N, Dyer R, Weinstock MA, and VAKCC Trial Group, J. Cutan. Med. Surg 18, 229 (2014).25008439 [Google Scholar]
- 10.“Fluorouracil Cream” (Drugs.com, 2016), http://www.dmgs.com/pro/fluorouracil-cream.html. Accessed 4 Jan 2016.
- 11.“Aldara” (Drugs.com, 2016), http://www.drugs.com/pro/aldara.html. Accessed 4 Jan 2016.
- 12.“Itraconazole” (Merck Sharp & Dohme Corp., 2016), http://www.merckmanuals.com/professional/lexicomp/itraconazole.html. Accessed 4 Jan 2016.
- 13.“Itraconazole”, (Drugs.com, 2016). http://www.drugs.com/pro/itraconazole.html. Accessed 4 Jan 2016.
- 14.Mulcahy N, “Common Antifungal Drug Works in Basal Cell Carcinoma” (Medscape, 2016) http://www.medscape.com/viewarticle/820444. Accessed 4 Jan 2016.
- 15.Kim J, Tang JY, Gong RY, Kim J, Lee JJ, Clemons KV, Chong CR, Chang KS, Fereshteh M, Gardner D, Reya T, Liu JO, Epstein EH, Stevens DA, and Beachy PA, Cancer Cell 17, 388 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dirix L, J. Clin. Oncol 32, 720 (2014). [DOI] [PubMed] [Google Scholar]
- 17.Kim J, Aftab BT, Tang JY, Kim D, Lee AH, Rezaee M, Kim J, Chen B, King EM, Borodovsky A, Riggins GJ, Epstein EH, Beachy PA, and Rudin CM, Cancer Cell 23,23 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montoya J, Molgo M, Uribe P, and Gonzalez S, J. Am. Acad. Dermatol 66, aB155 (2012). [Google Scholar]
- 19.Kim DJ, Kim J, Spaunhurst K, Montoya J, Khodosh R, Chandra K, Fu T, Gilliam A, Molgo M, Beachy PA, and Tang JY, J. Clin. Oncol 32, 745 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Kim DJ, Kim J, Spaunhurst K, Montoya J, Khodosh R, Fu T, Gilliam A, Molgo M, Beachy PA, and Tang JY, J. Invest. Dermatol 582, S101 (2014). [DOI] [PubMed] [Google Scholar]
- 21.“Sporanox & Lamisil Public Health Advisory issued.” (Medscape 2016), http://www.medscape.com/viewarticle/446823. Accessed 4 Jan 2016.
- 22.Kesisoglou F, Zhou SY, Niemiec S, Lee JW, Zimmermann EM, and Fleisher D, Pharm. Res 22, 1320 (2005). [DOI] [PubMed] [Google Scholar]
- 23.Hong H, Sun J, and Cai W, Clin. Cosmet. Investig. Dermatol 1, 1 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.“Itraconazole” (Selleck Chemicals, 2016), http://www.selleckchem.com/products/Itraconazole(Sporanox).html. Accessed 4 Jan 2016.
- 25.Khanna P, Strom JA, Malone JI, and Bhansali S, J. Diabetes Sci. Technol 2, 1122 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baria SH, Gohel MC, Mehta TA, and Sharma OP, J. Pharmacol. Pharmacother 64, 11 (2011). [Google Scholar]
- 27.Arora A, Prausnitz M, and Mitragotri S, Int. J. Pharm 364, 227 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gill HS, Denson DD, Burris BA, and Prausnitz MR, Clin. J. Pain 24, 585 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bariya SH, Gohel MC, Mehta TA, and Sharma OP, J. Pharm. Pharmacol 64, 11 (2011). [DOI] [PubMed] [Google Scholar]
- 30.Kim YC, Park JH, and Prausnitz MR, Adv. Drug Deliver. Rev 64, 1547 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tuan-Mahmood TM, McCrudden MT, Torrisi BM, McAlister E, Garland MJ, Singh TR, and Donnelly RF, Eur. J. Pharm. Sci 50, 623 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boehm RD, Daniels J, Stafslien S, Nasir A, Lefebvre J, and Narayan RJ, Biointerphases 10, 011004 (2015). [DOI] [PubMed] [Google Scholar]
- 33.Park JH, Allen MG, and Prausnitz MR, J. Control. Release 104, 51 (2005). [DOI] [PubMed] [Google Scholar]
- 34.Yuzhakov VV, Microneedle array, patch, and applicator for transdermal drug delivery, US Patent 7658728 B2, February 9, 2010.
- 35.Yuzhakov VV, Tissue conforming microneedle array and patch for transdermal drug delivery or biological fluid collection, US Patent 7785301 B2, August 31, 2010.
- 36.Yuzhakov VV, Method of making microneedle array and device for applying microneedle array to skin, US Patent US8414548 B2, April 9, 2013.
- 37.Boehm RD, Jaipan P, Skoog SA, Stafslien S, Vanderwal L, and Narayan RJ, Biointerphases 11, 011008 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Fan H, Oro AE, Scott MP, and Khavari PA, Nat. Med 3, 788 (1997). [DOI] [PubMed] [Google Scholar]


