Abstract
Background
The liver is a hub of metabolism and detoxification of substances. Since many redox reactions take place in the liver, it is prone to oxidative damage. Unlike conventional agents, botanicals act through several mechanisms in preventing oxidative damage. Among these Phytolacca dodecandra is the most commonly used agent in Ethiopian folk medicine.
Objective
To evaluate antioxidant and hepatoprotective activities of the 80% methanol extract of P. dodecandra root.
Methods
Male mice were divided into six groups and treated accordingly. Negative control was given 2% Tween 80, toxicant control administered with carbon tetrachloride (CCl4), positive control treated with silymarin 100 mg/kg, and test groups were treated with 100, 200, and 400 mg/kg of the extract. Then, serum levels of alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT), lactate dehydrogenase (LDH), total cholesterol (TC), triglyceride (TG), low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, albumin, total protein, and bilirubin were determined. Determination of the change in body weight and liver weight, histopathologic examination of the liver, and in vitro and in vivo antioxidant assays were also carried out.
Results
The levels of ALP, ALT, AST, GGT, LDH, and bilirubin were significantly reduced, while albumin and total protein were significantly increased after treatment with P. dodecandra root extract at the doses of 200 and 400 mg/kg in CCl4 intoxicated mice. Cholesterol metabolism and lipoprotein synthesis capabilities of the liver of mice were also returned to normal in the two doses. Besides, the 200 and 400 mg/kg doses were able to return the normal architecture and morphology of hepatocytes. Furthermore, the plant extract was found to scavenge free radicals in vitro and inhibit lipid peroxidation in vivo.
Conclusion
The results suggest that the 80% methanol extract of P. dodecandra root can be used for the management of liver disease.
Keywords: CCl4, DPPH, hepatoprotective, mice, Phytolacca dodecandra, TBARS
Background
P. dodecandra L’Her also called “gopo berry or soapberry or African soapberry” in English,1,2 “Endod” in Amharic, “Handoodee” in Afaan Oromoo,3 and “Shibti” in Tigrigna,4 belongs to the family Phytolaccaceae. It is a climbing or scrambling dioecious, semi-succulent shrub, sometimes a liana with stems up to 10–20 m long and usually glabrous, trunks up to 35 cm in diameter, and with a taproot.2 P. dodecandra is widely distributed across sub-Saharan Africa, Madagascar, Asia, and tropical America.2,5 All parts of P. dodecandra are widely used in Ethiopian traditional medicine. The leaves, roots, and fruits are used orally to treat anthrax, abdominal pain, itching,4,5 rabies, leech infestation, warts, herpes virus infection,6 liver disease, and helminthiasis.4 Several ethnobotanical studies showed that the roots,4,7–11 root barks,12 leaves,13 and berries4 of P. dodecandra are used for the management of liver problems. P. dodecandra has several pharmacologically proven activities such as antirabies,14 abortifacient,15 analgesic and anti-inflammatory,16 antibacterial,17 antioxidant,18 anti-mycelial against Histoplasma capsulatum,19 molluscicidal,20–25 spermicidal,26 antimalarial,27 larvicidal against Anopheles arabiensis28 and Anopheles gambiae,29 and insecticidal against cabbage flea beetle.30 Phytochemical analysis of the parts of P. dodecandra showed that it is found to contain terpenoids, phenolics,31 and saponins.32–34
Materials and Methods
Chemicals and Reagents
Chemicals and reagents used were 1, 1-diphenyl-2-picrylhydrazyl (DPPH) (Chemos GmbH & Co. KG, Germany), distilled water and normal saline were obtained from EPHARM (Ethiopia), ammonium hydroxide, ascorbic acid, chloroform, ethanol, ferric chloride, glacial acetic acid, hydrochloric acid, isopropyl alcohol, methanol, n-butanol, and sulfuric acid were purchased from Sigma-Aldrich (Germany), acetic anhydride, bismuth nitrate, iodine, lead acetate, mercuric chloride, potassium hydroxide, potassium iodide, sodium hydroxide, thiobarbituric acid (TBA), and trichloroacetic acid (TCA) were purchased from Fisher Scientific (UK), eosin and hematoxylin were purchased from Santa Cruz Biotechnology, Inc. (USA), ether was obtained from Puyer BioPharma Ltd., (P.R.China), 10% formalin was obtained from Novochem Engineering (India), silymarin was purchased from Silybon-140, Micro Lab Limited (India), CCl4, liquid paraffin, paraffin wax, and Tween 80 were purchased from Oxford Lab Fine Chem LLP (India), and xylene was obtained from scienTEST - bioKEMIX GmbH (Germany). All chemicals and reagents used were of analytical grade.
Plant Material
The roots of P. dodecandra were collected in May 2020 from Dessie, about 401 km north of Addis Ababa. Authentication and identification were done by a taxonomist and a voucher specimen (BGM01/2012) was deposited. Then, roots were gently washed with tap water to remove dirt and cut into pieces manually. They were allowed to dry under shade for 2 weeks and finally pulverized into coarse powder using an electrical mill.
Experimental Animals
Both male and female Swiss albino mice aged 6–8 weeks and with a weight range of 25–35 g inbred in the animal house of the Department of Pharmacy, Wollo University, were used for the experiment. The animals were housed in polypropylene cages (6 mice per cage) under standard environmental conditions (25 ± 2°C, 55 ± 5% humidity, and 12 h/12 h light/dark cycle). The animals were allowed free access to tap water and laboratory pellet and acclimatized to laboratory conditions for 1 week before the commencement of the experiment.
Preparation of Plant Extract
Three hundred grams of the coarse powder of the roots of P. dodecandra were macerated for 3 days using 80% methanol as a solvent. Then, the fluid extract was filtered using muslin cloth and Whatman® grade 1 filter paper and the marc was re-extracted for the second and third times by adding another fresh 80% methanol. The extract was concentrated in a rotary evaporator (Buchi, Rotavapor R-210/215, Switzerland), freeze-dried in a lyophilizer (OPERON, OPR-FDU-5012, Korea), and stored in a chest freezer.
Acute Oral Toxicity Test
Female mice were used for the acute oral toxicity test of the 80% methanol extract of P. dodecandra roots following OECD-425 guidelines.35 They fasted 3–4 h before and 1–2 h after administration of the 80% methanol extract of P. dodecandra root. First, a sighting study was performed to determine the starting dose. For this a female mouse was given a maximum dose (2000 mg/kg) of the 80% methanol extract as a single dose orally using oral gavage. Since no death was observed within 24 h, an additional four mice were used and dosed as mentioned above. Then, mice were observed continuously for 4 h at 30 min. intervals and then for 14 consecutive days with an interval of 24 h for the general signs and symptoms of toxicity (diarrhea, weight loss, tremor, lethargy, and paralysis), food and water intake, and mortality. Then, three dose levels were chosen for the extract: a middle dose; which is one-tenth of the maximum dose, a low dose; which is half of the middle dose, and a high dose that is twice the middle dose during the acute toxicity study.35
Experimental Design
Mice were randomly assigned into 6 groups (three control and three test groups) comprising of six animals per group. Negative controls were given the vehicle used for reconstitution (2% Tween 80 in water (2%TW80)) for 6 consecutive days. Positive controls were treated with the standard drug, silymarin 100 mg/kg, for 6 consecutive days and CCl4 ((CCl4 dissolved in an equal volume of liquid paraffin), 2 mL/kg, i.p.) on the 4th day. Toxicant controls were administered with 2%TW80 for 6 consecutive days and CCl4 (2 mL/kg, i.p.) on the 4th day. Three test groups were treated with the 80% methanol extract of P. dodecandra root at the doses of 100, 200, and 400 mg/kg as PDRE100, PDRE200, and PDRE400, respectively, for 6 consecutive days. All the test groups were given CCl4 (2 mL/kg, i.p.) on the 4th day. The route of administration for the test sample, the standard drug, and the vehicle was orally using oral gavage and the volume administered was 1 mL/100 g.36,37
Hepatoprotective Activity
The hepatoprotective activity of 80% methanol extract of P. dodecandra root was determined following Sintayehu et al36 and Meharie et al.37 The weight of each mouse was measured and recorded at the beginning (3–4 h of fasting) and the end of the experiment (on the 7th day) as initial and final body weight, respectively. After administration of respective treatment for 6 days as mentioned above in the “experimental design” section, mice were sacrificed under light ether anesthesia on the 7th day. Then, blood sample was collected from each mouse by cardiac puncture and transferred into heparinized sterile centrifuge tubes. The collected blood was centrifuged at 3000 rpm for 10 min and clear serum was separated. The separated serum was analyzed for the levels of biomarkers of liver function (levels of albumin, total protein, and total bilirubin) and chemistry (levels of ALP, ALT, AST, GGT, and LDH), and levels of TC, TG, HDL, and LDL using an automatic analyzer and commercial assay kits (Cobas Integra® 400 plus analyzer, Roche Diagnostics, Japan). Besides, the hepatoprotective activity of the 80% methanol extract of P. dodecandra root can be calculated as percentage protection which is; % protection = 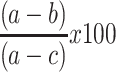 where a; is the mean value of the marker produced by hepatotoxin, b; is the mean value of the marker produced by toxin plus test material, and c; is the mean value produced by the vehicle control.37,38
where a; is the mean value of the marker produced by hepatotoxin, b; is the mean value of the marker produced by toxin plus test material, and c; is the mean value produced by the vehicle control.37,38
Liver Histopathological Examination
At the end of the experiment, all mice were sacrificed and their livers were harvested, weighed, and examined for microscopic pathology. The procedure was done as follows; individual liver samples were excised, washed with normal saline, and fixed in 10% formalin. Formalin-fixed tissue was washed in tap water. Then, it was dehydrated in serial ethanol and cleared in xylene. Finally, xylene cleared tissue was embedded in paraffin blocks. The paraffin blocks were sliced into sections of 5 μm in thickness and stained with hematoxylin and eosin. Histopathological evaluations were performed with a microscope (Olympus, Japan) and microscopic photographs were captured at 10x magnification.
Phytochemical Screening
Qualitative phytochemical investigation of the 80% methanol extract of P. dodecandra root was carried out to determine the presence of medicinally active secondary metabolites like alkaloids, cardiac glycosides, flavonoids, polyphenols, saponins, steroids, tannins, and terpenoids using standard methods.39–43
Antioxidant Activity of P. dodecandra Root Extract
In vitro DPPH Radical Scavenging Activity
The in vitro DPPH free radical scavenging activity of the 80% methanol extract of P. dodecandra root was done following Sahu et al.44 Two different stock solutions were prepared by dissolving 20 mg of DPPH in a liter of methanol and 250 mg of the 80% methanol extract of P. dodecandra root in the same liter of methanol. Then, the working solution was prepared by mixing 3.9 mL of DPPH solution with 0.1 mL of the sample solution. Blank solvent (methanol) was used to set the UV spectrophotometer (SHIMADZU UV-1800, Kyoto, Japan) at zero to cancel the solvent effect. DPPH solution was transferred into test tubes and the extract was added and followed by serial dilution. Then, the solution was incubated for 30 min at room temperature in a dark place and the absorbance was recorded at 517 nm using a UV spectrophotometer. Ascorbic acid was used as a reference standard and dissolved in methanol to make the stock solution with the same concentration.
The percent scavenging activity can be calculated as:
Analysis of Liver Lipid Peroxidation
Thiobarbituric Acid Reactive Substance (TBARS) Assay
Thiobarbituric acid reactive substance (TBARS) assay was conducted according to the method of Oakes et al.46 A molecule of malondialdehyde (MDA) forms an adduct with two TBA molecules to produce a red chromogen that absorbs ultraviolet-light at 532 nm. This assay was done by measuring the level of MDA spectrophotometrically. Primarily, 1 mL of the liver homogenate was mixed with 1 mL TCA (10% w/v) and centrifuged at 3000 rpm for 10 min. Then, 1 mL of TBA (0.67% w/v) was added to an equal volume of supernatant and incubated at 95°C for 60 min. The mixture was allowed to cool to room temperature in an ice bath for 10 min. One mL of n-butanol was added to precipitate the MDA-TBA complex. Then, n-butanol was removed and the precipitate was dissolved in water. Absorbance was recorded at 532 nm against an appropriate blank without the sample. The content of TBARS in the samples was calculated and the results were expressed as MDA equivalents in nmol/g of liver tissue.
Statistical Analysis
The results of the study are expressed as the mean ± standard error of the mean (S.E.M). Statistical analysis of the data was performed with one-way analysis of variance (ANOVA) followed by Tukey post hoc multiple comparison test to determine the difference between the mean values of different groups. P values < 0.05 were considered statistically significant.
Ethical Consideration
Mice were handled according to the international animal care and welfare,47 and the national institute of health guidelines for the care and use of laboratory animals.48
Results
Acute Oral Toxicity Test
The acute oral toxicity test of the 80% methanol extract of P. dodecandra root indicated that it did not cause gross behavioral changes and mortality within 24 h and up to 14 days later. Therefore, the LD50 was estimated to be greater than 2000 mg/kg in mice.
Hepatoprotective Activity
Effect of P. dodecandra Root Extract on Body Weight, Change in Body Weight, and Absolute and Relative Liver Weight of Mice
The effect of 80% methanol extract of P. dodecandra root on body weight, changes in body weight, and absolute and relative liver weight of mice is shown in Table 1.
Table 1.
Effect of 80% Methanol Extract of P. dodecandra Root on Body Weight, Change in Body Weight, and Absolute and Relative Liver Weight of Mice
| Group | Body Weight (g) | Change in Body Weight | Liver Weight (g) | Relative Liver Weight (%) | |
|---|---|---|---|---|---|
| Initial | Final | ||||
| 2%TW80 | 27.54 ± 0.66 | 28.86 ± 0.74 | 1.32 ± 0.38 | 2.18 ± 0.09 | 7.61 ± 0.48 |
| CCl4 | 28.42 ± 0.94 | 25.07 ± 0.90a### | −3.35 ± 0.69a### | 4.26 ± 0.16a### | 17.00 ± 0.35a### |
| Silymarin + CCl4 | 27.60 ± 0.71 | 29.92 ± 0.32b### | 2.32 ± 0.44b### | 2.20 ± 0.18b### | 7.35 ± 0.58b### |
| PDRE100 + CCl4 | 27.50 ± 0.89 | 25.34 ± 1.16a###,c###,d##,e### | −2.16 ± 0.56a###,c###,d##,e### | 4.02 ± 0.21a###,c###,d##,e### | 15.93 ± 0.68a###,c###,d##,e### |
| PDRE200 + CCl4 | 28.20 ± 0.80 | 30.04 ± 0.83b### | 1.84 ± 0.17b### | 2.46 ± 0.19b### | 8.19 ± 0.53b### |
| PDRE400 + CCl4 | 27.94 ± 0.96 | 30.26 ± 1.13b### | 2.32 ± 0.34b### | 2.24 ± 0.12b### | 7.46 ± 0.52b### |
Notes: Each value represents mean ± S.E.M; n=6; aAgainst 2%TW80; bAgainst CCl4; cAgainst standard (silymarin); dAgainst PDRE200 + CCl4; eAgainst PDRE400 + CCl4; ##p < 0.01, ###p < 0.001.
Abbreviations: PDRE100, Phytolacca dodecandra root extract 100 mg/kg; PDRE200, Phytolacca dodecandra root extract 200 mg/kg; PDRE400, Phytolacca dodecandra root extract 400 mg/kg; Silymarin, silymarin 100 mg/kg; CCl4, carbon tetrachloride 2 mL/kg; 2%TW80, 2% Tween 80 in water.
PDRE100 treated mice did not show increment in the final body weight, whereas, PDRE200 and PDRE400 administered mice produced a significant increase in the final body weight (p<0.001), as compared to the toxicant control (Table 1). PDRE200 and PDRE400 produced a significant decrement in the absolute and relative liver weight of mice (p<0.001), as compared to the toxicant control. In contrast, the CCl4-administered group resulted in significantly (p<0.001) decreased final body weight of mice, whilst their absolute and relative liver weight significantly increased (p<0.001) as compared to the negative control (Table 1).
Effect of P. dodecandra Root Extract on Liver Function of Mice
Table 2 presents the effect of P. dodecandra root extract on the synthesis and detoxification functions of the liver of mice. PDRE100 did not show significant alteration in all of the parameters shown in Table 2 below as compared to the toxicant control. On the other hand, PDRE200 and PDRE400 treated mice showed a significantly increased level of albumin (p<0.05) and (p<0.001), respectively, and total protein (p<0.01), and a significantly decreased level of bilirubin (p<0.001) as compared to the toxicant control (Table 2).
Table 2.
Effect of P. dodecandra Root Extract on the Synthetic and Detoxifying Functions of Liver of Mice
| Group | Albumin (mg/dL) | Total Protein (g/dL) | Bilirubin (mg/dL) |
|---|---|---|---|
| 2%TW80 | 3.21 ± 0.35 | 5.62 ± 0.38 | 1.02 ± 0.42 |
| CCl4 | 1.49 ± 0.20a## | 3.78 ± 0.43a## | 3.11 ± 0.51a### |
| Silymarin + CCl4 | 3.14 ± 0.31b### | 5.40 ± 0.52b## | 1.07 ± 0.44b### |
| PDRE100 + CCl4 | 1.88 ± 0.19 a###,c###,d#,e## | 4.01 ± 0.61 a##,c##,d##,e## | 2.57 ± 0.25a###,c###,d###,e### |
| PDRE200 + CCl4 | 2.73 ± 0.32b# | 5.18 ± 0.17b## | 1.14 ± 0.39b### |
| PDRE400 + CCl4 | 3.08 ± 0.15b### | 5.35 ± 0.35b## | 1.10 ± 0.13b### |
Notes: Each value represents mean ± S.E.M; n=6; aAgainst 2%TW80; bAgainst CCl4; cAgainst standard (silymarin); dAgainst PDRE200 + CCl4; eAgainst PDRE400 + CCl4; #p < 0.05, ##p < 0.01, ###p < 0.001.
Abbreviations: PDRE100, Phytolacca dodecandra root extract 100 mg/kg; PDRE200, Phytolacca dodecandra root extract 200 mg/kg; PDRE400, Phytolacca dodecandra root extract 400 mg/kg; Silymarin, silymarin 100 mg/kg; CCl4, carbon tetrachloride 2 mL/kg; 2%TW80, 2% Tween 80 in water.
Effect of P. dodecandra Root Extract on Liver Chemistry of Mice
The 80% methanol extract of P. dodecandra root resulted in a significant decline in liver chemistry biomarkers of hepatocyte injury following hepatotoxicant administration (Table 3). Liver chemistry biomarkers of hepatocyte injury such as ALP, ALT, AST, GGT, and LDH significantly increased in CCl4 treated mice by 187.7% (p<0.001), 495.2% (p<0.001), 555.6% (p<0.001), 140.9% (p<0.001), and 165.2% (p<0.001), respectively (Table 3). In contrast, mice pre- and post-treated with 200 and 400 mg/kg of P. dodecandra root extract significantly decreased CCl4 induced elevation in ALP by 83.4% and 96.0%, ALT by 88.7% and 93.4%, AST by 85.6% and 95.4%, GGT by 66.6% and 76.9%, and LDH by 89.4% and 91.8%, respectively (Table 3). As it is presented in Table 3, PDRE400 demonstrated nearly equal hepatoprotective activity to the standard drug (silymarin).
Table 3.
Effect of P. dodecandra Root Extract on Liver Chemistry of Mice
| Group | ALP (IU/L) | ALT (IU/L) | AST (IU/L) | GGT (IU/L) | LDH (IU/L) |
|---|---|---|---|---|---|
| 2%TW80 | 94.61 ± 4.02 | 41.54 ± 3.12 | 32.86 ± 2.76 | 56.14 ± 3.69 | 214.55 ± 6.32 |
| CCl4 | 272.18 ± 7.86a### | 247.26 ± 9.32a### | 215.42 ± 8.68a### | 135.23 ± 4.45 a### | 568.92 ± 12.30 a### |
| Silymarin + CCl4 | 99.41 ± 4.09(97.3)b### | 48.74 ± 4.26(96.5)b### | 36.33 ± 3.27(98.1)b### | 66.40 ± 2.97(87.0) b### | 237.21 ± 8.42(93.6) b### |
| PDRE100 + CCl4 | 251.23 ± 4.28(11.8)a###,c###,d###,e### | 226.28 ± 5.04(10.2) a###,c###,d###,e### | 192.78 ± 6.84(12.4) a###,c###,d###,e### | 127.81 ± 5.22(9.4) a###,c###,d###,e### | 491.67 ± 10.06(21.8) a###,c###,d###,e### |
| PDRE200 + CCl4 | 124.09 ± 5.21(83.4)b###,c#,e# | 64.79 ± 4.89(88.7)b###,c# | 59.15 ± 5.74(85.6)b### | 82.58 ± 3.87(66.6) b### | 252.11 ± 9.78(89.4) b### |
| PDRE400 + CCl4 | 101.71 ± 3.96(96.0)b### | 55.12 ± 4.51(93.4)b### | 41.26 ± 3.44(95.4)b### | 74.38 ± 4.16(76.9) b### | 243.61 ± 8.74(91.8) b### |
Notes: Each value represents mean ± S.E.M; n=6; aAgainst 2%TW80; bAgainst CCl4; cAgainst standard (silymarin); dAgainst PDRE200 + CCl4; eAgainst PDRE400 + CCl4; #p < 0.05, ###p < 0.001.
Abbreviations: PDRE100, Phytolacca dodecandra root extract 100 mg/kg; PDRE200, Phytolacca dodecandra root extract 200 mg/kg; PDRE400, Phytolacca dodecandra root extract 400 mg/kg; Silymarin, silymarin 100 mg/kg; CCl4, carbon tetrachloride 2 mL/kg; 2%TW80, 2% Tween 80 in water.
Effect of P. dodecandra Root Extract on Lipid Profile of Mice
The effect of 80% methanol extract of P. dodecandra root on TC, TG, HDL, and LDL is shown in Table 4. As it is presented in Table 4 mice treated with 100 mg/kg of P. dodecandra root extract did not show a significant alteration in the levels of TC, TG, and LDL as compared to the toxicant control. Whereas, mice treated with 200 and 400 mg/kg produced significantly increased levels of TC, TG, and LDL (p<0.001) as compared to the CCl4 treated group. Similarly, the standard drug significantly increased the levels of TC, TG, and LDL (p<0.001) as compared to the toxicant control. On the contrary, neither the plant extract nor the standard drug significantly affected the HDL level as compared to the toxicant control (Table 4).
Table 4.
Effect of 80% Methanol Extract of P. dodecandra Root on Lipid Profile of Mice
| Group | TC (mg/dL) | TG (mg/dL) | HDL (mg/dL) | LDL (mg/dL) |
|---|---|---|---|---|
| 2%TW80 | 95.42 ± 3.18 | 62.39 ± 2.41 | 33.71 ± 1.48 | 42.90 ± 3.04 |
| CCl4 | 52.61 ± 6.42a### | 31.47 ± 5.36 a### | 29.62 ± 2.06 | 26.46 ± 5.13 a### |
| Silymarin + CCl4 | 93.83 ± 3.81b### | 58.26 ± 2.98 b### | 32.54 ± 1.66 | 40.28 ± 2.62 b### |
| PDRE100 + CCl4 | 58.94 ± 6.46a###,c###,d##,e### | 37.80 ± 5.29 a###,c###,d##,e### | 30.19 ± 1.80 | 28.72 ± 3.76 a##,c###,d##,e### |
| PDRE200 + CCl4 | 84.27 ± 2.87 b### | 54.54 ± 4.60 b### | 32.08 ± 2.24 | 36.39 ± 2.11 b### |
| PDRE400 + CCl4 | 91.63 ± 4.22 b### | 57.65 ± 3.21 b### | 32.36 ± 1.98 | 39.85 ± 2.19 b### |
Notes: Each value represents mean ± S.E.M; n=6; aAgainst 2%TW80; bAgainst CCl4; cAgainst standard (silymarin); dAgainst PDRE200 + CCl4; eAgainst PDRE400 + CCl4; ##p < 0.01, ###p < 0.001.
Abbreviations: PDRE100, Phytolacca dodecandra root extract 100 mg/kg; PDRE200, Phytolacca dodecandra root extract 200 mg/kg; PDRE400, Phytolacca dodecandra root extract 400 mg/kg; Silymarin, silymarin 100 mg/kg; CCl4, carbon tetrachloride 2 mL/kg; 2%TW80, 2% Tween 80 in water.
Effect of P. dodecandra Root Extract on Histology of Liver of Mice
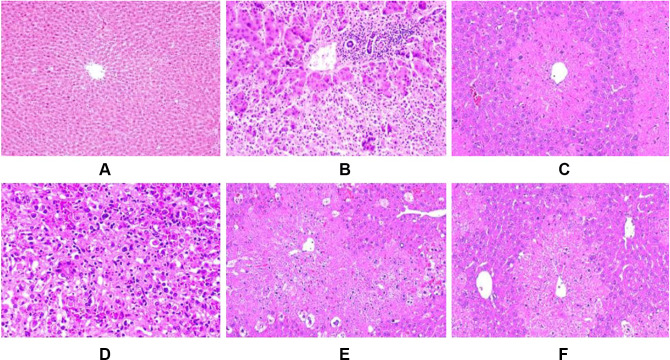
The effect of P. dodecandra root extract on the histology of the liver of mice is presented in Figure 1. In this study, histological observations provided supportive evidence for biochemical analysis. As shown in Figure 1A below, mice treated with 2%TW80 revealed normal hepatic cells with intact cytoplasm, prominent nucleus and nucleolus, and well defined central vein. In stark contrast, the toxicant group exhibited cells with prominent infiltration, necrosis, cytoplasmic vacuolization, and dilation of blood vessels Figure 1B. Administration of silymarin however returned inflamed and necrotized cells to their normal architecture (Figure 1C). In a similar manner to silymarin, the 80% methanol extract of P. dodecandra root protected the mice liver from CCl4 mediated injury as it is observed in Figure 1 below. P. dodecandra root extract normalized the defects observed in the histopathology of the liver of mice treated with CCl4 nearly to the level of the negative control group in its 200 mg/kg (Figure 1E) and 400 mg/kg (Figure 1F) doses. On the other hand, the liver section of the mouse treated with 100 mg/kg of P. dodecandra root extract revealed disarrangement of hepatocytes, well-observed hepatic lesions, infiltration of inflammatory cells, and necrosis (Figure 1D).
Figure 1.
Microphotograph of hematoxylin and eosin (H & E)-stained sections of liver. Microscopic glass slides were prepared in 50µm intervals.
Notes: (A) negative control (received 2%TW80 only), (B) toxicant control (administered with CCl4 only), (C) positive control (Silymarin + CCl4), (D) PDRE100 + CCl4, (E) PDRE200 + CCl4, (F) PDRE400 + CCl4.
Abbreviations: PDRE100, Phytolacca dodecandra root extract 100 mg/kg; PDRE200, Phytolacca dodecandra root extract 200 mg/kg; PDRE400, Phytolacca dodecandra root extract 400 mg/kg; Silymarin, silymarin 100 mg/kg; CCl4, carbon tetrachloride 2 mL/kg; 2%TW80, 2% Tween 80 in water.
Phytochemical Screening
The 80% methanol extract of P. dodecandra root was examined for the composition of medicinally active secondary metabolites and it was found to be positive for polyphenols, saponins, tannins, and terpenoids (Table 5). On the other hand, alkaloids, anthraquinone glycosides, cardiac glycosides, flavonoids, and steroids were not detected in the plant extract.
Table 5.
Phytochemical Screening of 80% Methanol Extract of P. dodecandra Root
| Phytochemical | Test Method | Observation | Result |
|---|---|---|---|
| Alkaloids | Bouchardat’s reagent test | Brown precipitate was not observed | Absence of alkaloids |
| Dragendorff’s reagent test | Red-orange precipitate was not formed | Absence of alkaloids | |
| Mayer’s reagent test | Yellowish-white precipitate was not formed | Absence of alkaloids | |
| Wagner’s reagent test | A red-brown precipitate was not formed | Absence of alkaloids | |
| Anthraquinone Glycosides | Borntrager’s test | Red color was not occurred | Absence of anthraquinone glycosides |
| Ammonium hydroxide test | Pink-red color was not formed | Absence of anthraquinone glycosides | |
| Cardiac Glycosides | Keller-Kiliani test | No formation of brown-ring between layers | Absence of cardiac glycosides |
| Flavonoids | Alkaline reagent test | Yellow fluorescence was not present | Absence of flavonoids |
| Shinoda test | Red-pink color was not formed | Absence of flavonoids | |
| Polyphenols | Lead acetate test | A bulky white precipitate was formed | Presence of polyphenols |
| Ferric chloride test | Blue-green color was formed | Presence of polyphenols | |
| Saponins | Frothing test | A stable persistent froth was formed | Presence of saponins |
| Steroids | Libermann-Buchard test | No formation of brown ring at the interface | Absence of steroids |
| Tannins | Ferric chloride test | Blue or purple or green precipitate was formed | Presence of tannins |
| Terpenoids | Salkowski test | A reddish-brown precipitate was formed | Presence of terpenoids |
Antioxidant Activity of P. dodecandra Root Extract
DPPH Radical Scavenging Activity
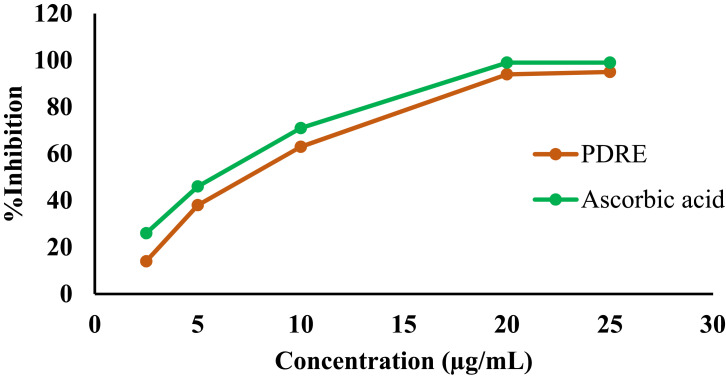
The antioxidant activity of the 80% methanol extract of P. dodecandra root was evaluated by measuring its free radical scavenging power using DPPH free radicals and the data are presented in Figure 2. It was observed that the 80% methanol extract of P. dodecandra root had a concentration-dependent free radical scavenging activity. The calculated IC50 values for the extract and standard were 7.4 μg/mL and 6.2 μg/mL, respectively. In a similar manner, the maximum percentage inhibition of free radicals by the extract and the standard were 94% and 99%, respectively, at a concentration of 20 μg/mL (Figure 2).
Figure 2.
DPPH radical scavenging activity of P. dodecandra root extract.
Abbreviations: PDRE, Phytolacca dodecandra root extract; Ascorbic acid, standard antioxidant.
Analysis of Liver Lipid Peroxidation
TBARS Assay
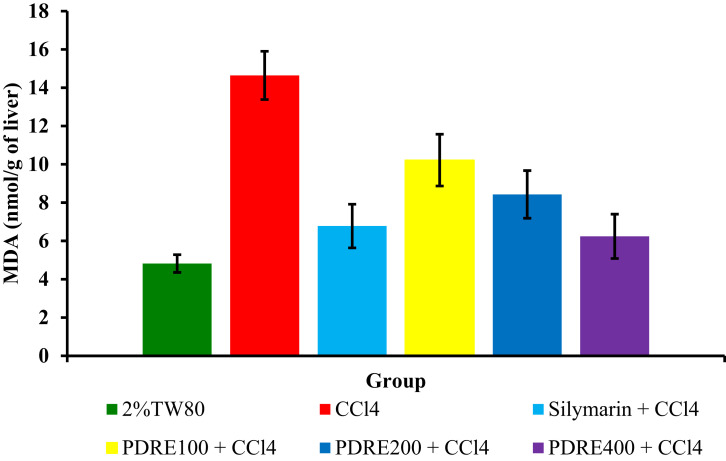
Figure 3 below shows the effect of P. dodecandra root extract on the inhibition of lipid peroxidation reaction in CCl4 induced hepatotoxicity.
Figure 3.
Effect of P. dodecandra root extract on MDA levels.
Notes: Each value represents mean ± S.E.M; n=6.
Abbreviations: PDRE100, Phytolacca dodecandra root extract 100 mg/kg; PDRE200, Phytolacca dodecandra root extract 200 mg/kg; PDRE400, Phytolacca dodecandra root extract 400 mg/kg; Silymarin, silymarin 100 mg/kg; CCl4, carbon tetrachloride 2 mL/kg; 2%TW80, 2% Tween 80 in water.
A significantly increased level of MDA (14.64 ± 1.26 nmol/g of the liver) was obtained in the CCl4 intoxicated group as compared to the negative control (4.82 ± 0.46 nmol/g of the liver) (p<0.001). But PDRE200 and PDRE400 showed a significant decrement in the levels of MDA 8.43 ± 1.24 and 6.24 ± 1.16 nmol/g of liver tissue (p<0.01) respectively, as compared to the toxicant control. The MDA level of mice treated with 100 mg/kg of the 80% methanol extract of P. dodecandra root is significantly higher than that of the standard (10.22 ± 1.35 nmol/g of liver vs 6.78 ± 1.14 nmol/g of the liver) (p<0.01) (Figure 3).
Discussion
The liver has a crucial role in life because it performs a vast array of biochemical and metabolic functions, including synthesis and elimination of numerous substances.49 Since it is the first port of call for most nutrients absorbed across the gut wall and site of oxidation of most compounds, it is highly susceptible to oxidative stress and related damage.49 So, it is highly imperative to demonstrate the hepatoprotective activity of the plant extract in the presence of hepatotoxicant that causes oxidative damage.
P. dodecandra is the most commonly used plant in Ethiopian folk medicine for the treatment of liver disease.4,7–11 Its hepatoprotective activity was investigated pharmacologically by using a hepatotoxic agent, CCl4. Since CCl4 is a widely used hepatotoxicant in an experimental model of liver injury.36,37 The highly reactive metabolites of CCl4 such as trichloromethyl (CCl3⋅) and trichloromethyl peroxyl (CCl3O2⋅) radicals are the main players in hepatocyte injury. They deactivate antioxidant enzymes and thereby alter the antioxidant state of hepatocytes.50,51
As per the traditional claim, the 80% methanol extract of P. dodecandra root was administered to mice in three different doses pre- and post-dose of CCl4. As it is presented in Table 1, CCl4 intoxicated mice had decreased final body weight and increased change in body weight and both absolute and relative liver weight as compared to the negative control. Proliferation, cytoplasmic vacuolization, infiltration of inflammatory cells, and deposition of collagen fibers in the hepatocytes are critical determinants for the survival of the liver from an injury.51 This was evidenced by the histopathology of the liver of mice given CCl4 in which there was a profound disarrangement, inflammation, and infiltration of liver cells (Figure 1B). Therefore, up-regulation of hepatocyte activity in response to CCl4 toxicity is likely to be the cause of the recorded increase in liver weight.
In stark contrast, mice treated with P. dodecandra root extract at the doses of 200 and 400 mg/kg pre- and post-dose of CCl4 were returned to their normal final body weight and absolute and relative liver weight as compared to the toxicant control (Table 1). Similarly, the hepatocyte architecture of mice treated with PDRE200 and PDRE400 was returned to normal (Figure 1E and F, respectively). On the other hand, P. dodecandra root extract administration to mice pre- and post-dose of CCl4 normalized the liver’s metabolic capability in a dose-dependent manner (Table 2). In the injured liver, there are depressed levels of total protein and albumin due to disturbances in carbohydrate, protein, and lipid metabolism or perturbed protein biosynthesis.52 The marked reduction of total protein and albumin synthesis and substantially increased bilirubin levels in the CCl4 treated mice agreed with the early observations.36,37
The liver plays an essential role in lipid metabolism, synthesis and oxidation of cholesterol, and synthesis of lipoproteins.52 However, mice intoxicated with CCl4 showed significantly reduced levels of TC, TG, and LDL which is reasonably expected since cholesterol biosynthesis in the liver has been reduced. Whereas treatment with P. dodecandra root extract at the doses of 200 and 400 mg/kg returned the metabolic function of the liver as evidenced in the levels of TC, TG, LDL, and HDL, which were normalized as compared to the toxicant control (Table 4).
CCl4 is known to damage liver cells via oxidation of membrane lipids and other cellular components.51 The damage of the hepatocyte membrane resulted in the release of cellular components and enzymes. The serum level of liver enzymes helps to diagnose and monitor liver disease or damage. Alteration in the structure and function of the liver resulted in a marked elevation in the levels of ALP, ALT, AST, GGT, and LDH.53 An agent that returns the levels of these enzymes to normal is considered hepatoprotective since these enzymes are at a high level in plasma following hepatocyte injury.53 According to this study, mice received P. dodecandra root extract at the doses of 200 and 400 mg/kg showed a significant reduction in the levels of liver enzymes (Table 3). This can be evidenced from the percent reduction of the levels of ALP, ALT, AST, GGT, and LDH for the two mentioned doses, where PDRE400 displayed a better activity than PDRE200 (96.0%, 93.4%, 95.4%, 76.9%, and 91.8% vs 83.4%, 88.7%, 85.6%, 66.6%, and 89.4%), respectively (Table 3). Probably P. dodecandra root extract might stabilize hepatocyte membranes and interrupt the release of enzymes from the liver into blood especially in the two larger doses. However, PDRE100 did not produce a visible effect in all biomarkers of hepatocyte injury. This is probably due to the lower dose might contain a low level of active principles and the other two doses might have enough concentration of active substances that can elicit the observed effect.
Results of histopathological examination served as compelling evidence in supporting the biochemical markers of liver injury. PDRE200 and PDRE400 treated mice resulted in a marked decrease in the hepatocyte ballooning, cytoplasmic vacuolization, derangement of hepatocytes, and infiltration of hepatocyte membrane with inflammatory cells and collagen fibers (Figure 1E and F, respectively). Besides treatment with P. dodecandra root extract inhibits CCl4 induced formation of lipid peroxides in the liver of mice. Since the toxicity mechanism of CCl4 is peroxidation of membrane lipids after bio-activated to its toxic metabolites.51 In this study peroxidation of lipids was determined by measuring the levels of MDA, an end product of lipid peroxidation, in hepatic tissues. CCl4 intoxication induced a marked increase in tissue MDA levels as compared with the negative control (P<0.001; Figure 3). On the contrary, treatment with P. dodecandra root extract returned the increased MDA to its normal level. This showed that CCl4 mediated oxidative damage is reversed by administration of the plant extract and probably prevents the liver from free radical-induced injury and subsequent pathological changes.
Though the active principles present in the P. dodecandra root extract are not identified, polyphenols, saponins, tannins, and terpenoids were found to have antioxidant and hepatoprotective activities via several mechanisms.
Qualitative phytochemical analysis of the 80% methanol extract of P. dodecandra root for the presence and absence of several medicinally active secondary metabolites was done and polyphenols, saponins, tannins, and terpenoids were found in the plant extract (Table 5). Probably phytochemicals present in the plant extract act individually or in synergy to produce the observed antioxidant, lipid peroxidation inhibition, and hepatoprotective effects.
Previous studies revealed that phenolic compounds have several antioxidant, anti-inflammatory, and immunomodulatory activities. They prevent oxidative induced cellular damage by inhibiting enzymes involved in the production of reactive oxygen species (ROS), through neutralizing ROS, and up-regulation of antioxidant defenses.55–57 Oxidative stress promotes inflammation via activation of genes and thereby the expression of several proteins (enzymes) involved in the inflammatory pathways.58 Polyphenols also have an anti-inflammatory effect via inhibition of enzymes involved in the inflammatory process following membrane damage such as cyclooxygenase-2 (COX-2), lipoxygenase (LOX), and nitric oxide synthase (NOS).59 In addition, they are also known for donating hydrogen atoms in the prevention of lipid peroxidation.60
Tannins are also hepatoprotective secondary active metabolites either by their antioxidant activity (donate hydrogen atom or electrons, chelate metal ions, and interfere in the Fenton reaction)61 or via inhibition of apoptosis through increased expression of the anti-apoptotic protein Bcl-2.62 Besides, they also inhibit lipid peroxidation via inhibition of COX-2.61 Terpenoids and saponins are also natural hepatoprotective compounds.63,64 They inhibit the formation of ROS, scavenge free radicals, inhibit peroxidation of lipids, suppress prooxidants and increase antioxidants, up-regulate nuclear factor E2-related factor 2 (Nrf2)-antioxidant signaling,65 and inhibit apoptosis through activating the mitogen-activated protein kinase (MAPK) signaling pathway.66
To confirm the antioxidant activity of P. dodecandra root extract, DPPH free radical scavenging activity was carried out. In this assay, P. dodecandra root extract was observed to inhibit DPPH free radicals with the maximum value of 94% at the concentration of 20 µg/mL (Figure 2) and had a calculated IC50 value of 7.4 μg/mL, which is nearly similar to the calculated IC50 value of the standard antioxidant, ascorbic acid, i.e. 6.2 μg/mL. The presence of potent phytochemicals in the extract may be responsible for the observed antioxidant activity.
To sum up, the 80% methanol extract of P. dodecandra root possessed comparable antioxidant and hepatoprotective activities with the respective standards used in the study. Based on the results of this study P. dodecandra root extract showed a dose-dependent reduction in the biomarkers of liver damage and MDA level and increment in the metabolic functions of the liver. Even though the plant exact might have several mechanisms of hepatoprotective actions, lipid peroxidation inhibition and free radical scavenging activities are among the demonstrated mechanisms. Above all, the plant extract would be rewarded as safe based on the results of the acute oral toxicity study. Also, isolation and characterization of the specific hepatoprotective compound will be done in future studies by using HPLC/LC-MS techniques.
Conclusion
The results of serum biochemical analysis, histopathological investigations, and both in vitro and in vivo antioxidant studies provide evidence for the folkloric use of P. dodecandra for the management of liver problems. The middle and larger doses of P. dodecandra root extract produced remarkable hepatoprotective and antioxidant activities that were comparable to silymarin. The presence of medicinally active secondary metabolites in the plant extract may justify the observed hepatoprotective, lipid peroxidation inhibition, and in vitro free radical scavenging activities.
Funding Statement
No funding was received for this work.
Abbreviations
ALP, Alkaline phosphatase; ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; CCl4, Carbon tetrachloride; DPPH, 1, 1-diphenyl-2-picrylhydrazyl; GGT, Gamma-glutamyl transferase; HDL, High-density lipoprotein; LDH, Lactate dehydrogenase; LDL, Low-density lipoprotein; MDA, Malondialdehyde; ROS, Reactive oxygen species; TBARS, Thiobarbituric acid reactive substance; TC, Total cholesterol; TG, Triglyceride.
Data Sharing Statement
All the necessary data and materials can be obtained from the corresponding author with a reasonable request.
Ethics Approval
The study was approved by the Ethical Review Board of the College of Medicine and Health Sciences of Wollo University.
Consent for Publication
The authors have consented to the publication of this manuscript.
Author Contributions
All authors made a significant contribution from the conception and design of the study to the execution, acquisition, analysis, and interpretation of data. Besides, they took part in drafting, revising, or critically reviewing the manuscript, gave final approval of the version to be published, have agreed on the journal to which the manuscript has been submitted, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest for this work.
References
- 1.Esser KB, Semagn K, Wolde-Yohannes L. Medicinal use and social status of the soap berry endod (Phytolacca dodecandra) in Ethiopia. J Ethnopharmacol. 2003;85(2–3):269–277. doi: 10.1016/S0378-8741(03)00007-2 [DOI] [PubMed] [Google Scholar]
- 2.Karunamoorthi K, Bishaw D, Mulat T. Laboratory evaluation of Ethiopian local plant Phytolacca dodecandra extract for its toxicity effectiveness against aquatic macroinvertebrates. Eur Rev Med Pharmacol Sci. 2008;12(6):381–386. [PubMed] [Google Scholar]
- 3.Gijan M, Dalle G. Ethnobotanical study of medicinal plants in Nagelle Arsi District, West Arsi Zone of Oromia, Ethiopia. J Nat Sci Res. 2019;9(13):1–18. [Google Scholar]
- 4.Giday K, Lenaerts L, Gebrehiwot K, Yirga G, Verbist B, Muys B. Ethnobotanical study of medicinal plants from degraded dry afromontane forest in northern Ethiopia: species, uses and conservation challenges. J Herb Med. 2016;6(2):96–104. doi: 10.1016/j.hermed.2016.03.004 [DOI] [Google Scholar]
- 5.Worku AM. A review on significant of traditional medicinal plants for human use in case of Ethiopia. J Plant Pathol Microbiol. 2019;10(6):1–12. [Google Scholar]
- 6.Zenebe G, Zerihun M, Solomon Z. An ethnobotanical study of medicinal plants in Asgede Tsimbila district, Northwestern Tigray, northern Ethiopia. Ethnobot Res Appl. 2012;10:305–320. doi: 10.17348/era.10.0.305-320 [DOI] [Google Scholar]
- 7.Birhan Y, Kitaw S, Alemayehu Y, Mengesha N. Ethnobotanical study of medicinal plants used to treat human diseases in Enarj Enawga district, East Gojjam zone, Amhara region, Ethiopia. SM J Med Plant Stud. 2017;1(1):1–9. doi: 10.36876/smjmps.1006 [DOI] [Google Scholar]
- 8.Mekuanent T, Zebene A, Solomon Z. Ethnobotanical study of medicinal plants in Chilga district, Northwestern Ethiopia. J Nat Remedies. 2015;15(2):88–112. doi: 10.18311/jnr/2015/476 [DOI] [Google Scholar]
- 9.Garedew B, Bizuayehu B. AReview on Ethnobotanical study of traditional medicinal plants used for treatment of liver problems in Ethiopia. Eur J Med Plants. 2018;1–18. doi: 10.9734/EJMP/2018/38153 [DOI] [Google Scholar]
- 10.Megersa M, Asfaw Z, Kelbessa E, Beyene A, Woldeab B. An ethnobotanical study of medicinal plants in Wayu Tuka district, east Welega zone of oromia regional state, West Ethiopia. J Ethnobiol Ethnomed. 2013;9(1):68. doi: 10.1186/1746-4269-9-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teklehaymanot T. Ethnobotanical study of knowledge and medicinal plants use by the people in Dek Island in Ethiopia. J Ethnopharmacol. 2009;124(1):69–78. doi: 10.1016/j.jep.2009.04.005 [DOI] [PubMed] [Google Scholar]
- 12.Wubetu M, Abula T, Dejenu G. Ethnopharmacologic survey of medicinal plants used to treat human diseases by traditional medical practitioners in Dega Damot district, Amhara, Northwestern Ethiopia. BMC Res Notes. 2017;10(1):157. doi: 10.1186/s13104-017-2482-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teklehaymanot T, Giday M. Ethnobotanical study of medicinal plants used by people in Zegie Peninsula, Northwestern Ethiopia. J Ethnobiol Ethnomed. 2007;3(1):12. doi: 10.1186/1746-4269-3-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Admasu P, Deressa A, Mengistu A, Gebrewold G, Feyera T. In vivo antirabies activity evaluation of hydroethanolic extract of roots and leaves of Phytolacca dodecandra. Glob Vet. 2014;12(1):12–18. doi: 10.5829/idosi.gv.2014.12.01.81150. [DOI] [Google Scholar]
- 15.Namulindwa A, Nkwangu D, Oloro J. Determination of the abortifacient activity of the aqueous extract of Phytolacca dodecandra (LHer) leaf in Wistar rats. Afr J Pharm Pharmacol. 2015;9(3):43–47. doi: 10.5897/AJPP2014.4227 [DOI] [Google Scholar]
- 16.Nakalembe L, Kasolo JN, Nyatia E, Lubega A, Bbosa GS. Analgesic and anti-inflammatory activity of total crude leaf extract of Phytolacca dodecandra in Wistar Albino Rats. Neurosci Med. 2019;10(3):259–271. doi: 10.4236/nm.2019.103020 [DOI] [Google Scholar]
- 17.Bernard N, Emmerance N, Ingabire A. Comparative bacterial inhibition by bioactive extracts from Datura stramonium and Phytolacca dodecandra. Virol Res J. 2018;2(1):1–5. [Google Scholar]
- 18.Iteku JB, Mbayi O, Bongo GN, et al. Phytochemical analysis and assessment of antibacterial and antioxidant activities of Phytolacca dodecandra L. Herit Leaf extracts (Phytolaccaceae). Int J Biomed Eng Clin Sci. 2019;5(3):31. doi: 10.11648/j.ijbecs.20190503.11 [DOI] [Google Scholar]
- 19.Jaleta H, Ameni G, Arage M, Giday M, Girma M, Sori T. In vitro evaluation of the effects of selected plants on the growth of the mycelial form of histoplasma capsulatum variety farciminosum in Ethiopia. J Equine Vet Sci. 2020;91:103139. doi: 10.1016/j.jevs.2020.103139. [DOI] [PubMed] [Google Scholar]
- 20.Lemma A. Laboratory and field evaluation of the molluscicidal properties of Phytolacca dodecandra. Bull World Health Organ. 1970;42(4):597. [PMC free article] [PubMed] [Google Scholar]
- 21.Kariuki S, Kariuki J, Mailu B, Muchiri D. Phytolacca octandra (L.), Phytolacca dodecandra (LHerit) and Balanites aegypiaca (L.) extracts as potential molluscicides of schistosomiasis transmitting snails. J Med Plants Res. 2016;10(44):823–828. doi: 10.5897/JMPR2016.6237 [DOI] [Google Scholar]
- 22.Lemma A, Brody G, Newell GW, Parkhurst R, Skinner W. Studies on the molluscicidal properties of endod (Phytolacca dodecandra): I. Increased potency with butanol extraction. J Parasitol. 1972;58(1):104–107. doi: 10.2307/3278251 [DOI] [PubMed] [Google Scholar]
- 23.Thiilborg ST, Christensen SB, Cornett C, Olsen CE, Lemmich E. Molluscicidal saponins from Phytolacca dodecandra. Phytochemistry. 1993;32(5):1167–1171. doi: 10.1016/S0031-9422(00)95085-4 [DOI] [PubMed] [Google Scholar]
- 24.Madhina D, Shiff C. Prevention of snail miracidia interactions using Phytolacca dodecandra (L’Herit)(endod) as a miracidiacide: an alternative approach to the focal control of schistosomiasis. Trop Med Int Health. 1996;1(2):221–226. doi: 10.1111/j.1365-3156.1996.tb00030.x [DOI] [PubMed] [Google Scholar]
- 25.Pezzuto JM, Swanson SM, Farnsworth NR. Evaluation of the mutagenic potential of endod (Phytolacca dodecandra), a molluscicide of potential value for the control of schistosomiasis. Toxicol Lett. 1984;22(1):15–20. doi: 10.1016/0378-4274(84)90039-0 [DOI] [PubMed] [Google Scholar]
- 26.Stolzenberg S, Parkhurst R. Spermicidal actions of extracts and compounds from Phytolacca dodecandra. Contraception. 1974;10(2):135–143. doi: 10.1016/0010-7824(74)90069-9 [DOI] [PubMed] [Google Scholar]
- 27.Adinew GM. Antimalarial activity of methanolic extract of Phytolacca dodecandra leaves against Plasmodium berghei infected Swiss albino mice. Int J Pharmacol Clin Sci. 2014;3(3):39–45. [Google Scholar]
- 28.Zeleke AJ, Shimo BA, Gebre DY. Larvicidal effect of endod (Phytolacca dodecandra) seed products against Anopheles arabiensis (Diptera: culicidae) in Ethiopia. BMC Res Notes. 2017;10(1):449. doi: 10.1186/s13104-017-2792-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Owiti YJ, Barack -O-OJ, Auma AC, India JJ, Pamela W-K, John VM. Larviciding potency of water and ethanol extracts of Phytolacca dodecandra (L’Herit) on Anopheles gambiae (Diptera: culicidae). J Mosq Res. 2015;5(2):1–6. [Google Scholar]
- 30.Raja N, Masresha G, Jemberie W. Insecticidal activity of Phytolacca dodecandra L. Herit (Phytolaccaceae) plant extracts against cabbage flea beetle Phyllotreta cruciferae Goeze (Coleoptera: chrysomilidae). Am Eurasian J Sci Res. 2015;10(5):325–331. [Google Scholar]
- 31.Matebie WA, Zhang W, Xie G. Chemical composition and antimicrobial activity of essential oil from Phytolacca dodecandra collected in Ethiopia. Molecules. 2019;24(2):342. doi: 10.3390/molecules24020342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perret C, Wolfender JL, Hostettmann K. LC/ES‐MS analysis of triterpene glycosides: rapid estimation of the saponin content of dried berries of Phytolacca dodecandra. Phytochem Anal. 1999;10(5):272–278. doi: [DOI] [Google Scholar]
- 33.Dorsaz AC, Hostettmann K. Further saponins from Phytolacca dodecandra L′ Herit. Helv Chim Acta. 1986;69(8):2038–2047. doi: 10.1002/hlca.19860690827 [DOI] [Google Scholar]
- 34.Domon B, Hostettmann K. New saponins from Phytolacca dodecandra L’Herit. Helv Chim Acta. 1984;67(5):1310–1315. doi: 10.1002/hlca.19840670517 [DOI] [Google Scholar]
- 35.Guideline OO. 425: acute oral toxicity—up-and-down procedure. OECD Guidelines Test Chem. 2001;2:12–16. [Google Scholar]
- 36.Sintayehu B, Bucar F, Veeresham C, Asres K. Hepatoprotective and free radical scavenging activities of extracts and a major compound isolated from the leaves of Cineraria abyssinica Sch. Bip. exA. Rich. Pharmacogn J. 2012;4(29):40–46. doi: 10.5530/pj.2012.29.6 [DOI] [Google Scholar]
- 37.Meharie BG, Amare GG, Belayneh YM. Evaluation of hepatoprotective activity of the crude extract and solvent fractions of Clutia abyssinica (Euphorbiaceae) leaf against CCl4-induced hepatotoxicity in Mice. J Exp Pharmacol. 2020;12:137–150. doi: 10.2147/JEP.S248677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agrawal A, Rao M, Jasdanwala S, Mathur A, Eng M. Cephalexin induced cholestatic jaundice. Case Rep Gastrointest Med. 2014;2014:1–3. doi: 10.1155/2014/260743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maria R, Shirley M, Xavier C, et al. Preliminary phytochemical screening, total phenolic content and antibacterial activity of thirteen native species from Guayas province Ecuador. J King Saud Univ Sci. 2018;30(4):500–505. doi: 10.1016/j.jksus.2017.03.009 [DOI] [Google Scholar]
- 40.Tiwari P, Kumar B, Kaur M, Kaur G, Kaur H. Phytochemical screening and extraction: a review. Int Pharm Sci. 2011;1(1):98–106. [Google Scholar]
- 41.Rohini MV, Padmini E. Preliminary phytochemical screening of selected medicinal plants of polyherbal formulation. J Pharmacogn Phytochem. 2016;5(5):277–282. [Google Scholar]
- 42.Hossain MA, AL-Raqmi KAS, AL-Mijizy ZH, Weli AM, Al-Riyami Q. Study of total phenol, flavonoids contents and phytochemical screening of various leaves crude extracts of locally grown Thymus vulgaris. Asian Pac J Trop Biomed. 2013;3(9):705–710. doi: 10.1016/S2221-1691(13)60142-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gul R, Jan SU, Faridullah S, Sherani S, Jahan N. Preliminary phytochemical screening, quantitative analysis of alkaloids, and antioxidant activity of crude plant extracts from Ephedra intermedia indigenous to Balochistan. Sci World J. 2017;2017:1–7. doi: 10.1155/2017/5873648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sahu RK, Kar M, Routray R. DPPH free radical scavenging activity of some leafy vegetables used by tribals of Odisha, India. J Med Plants. 2013;1(4):21–27. [Google Scholar]
- 45.Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181(4617):1199–1200. doi: 10.1038/1811199a0 [DOI] [Google Scholar]
- 46.Oakes KD, Van Der Kraak GJ. Utility of the TBARS assay in detecting oxidative stress in white sucker (Catostomus commersoni) populations exposed to pulp mill effluent. Aquat Toxicol. 2003;63(4):447–463. doi: 10.1016/S0166-445X(02)00204-7 [DOI] [PubMed] [Google Scholar]
- 47.Vogel HG. Guidelines for the Care and Use of Laboratory Animals. In: Vogel HG, editor. Drug Discovery and Evaluation. Heidelberg: Springer-Verlag Berlin Heidelberg; 2007:2023-2037. doi: 10.1007/978-3-54070995-4_18. [DOI] [Google Scholar]
- 48.National Research Council. Guide for the Care and Use of Laboratory Animals. National Academies Press; 2011. Available from: https://grants.nih.gov/grants/olaw/guide-for-the-care-and-use-of-laboratory-animals.pdf. Accessed November, 2020. [Google Scholar]
- 49.Brinkløv S, Warrant E. Oilbirds. Curr Biol. 2017;27(21):R1145–R7. doi: 10.1016/j.cub.2017.08.071 [DOI] [PubMed] [Google Scholar]
- 50.Boll M, Lutz W, Becker E, Stampfl A. Mechanism of carbon tetrachloride-induced hepatotoxicity. Hepatocellular damage by reactive carbon tetrachloride metabolites. Z Naturforsch C. 2001;56(7–8):649–659. doi: 10.1515/znc-2001-7-826 [DOI] [PubMed] [Google Scholar]
- 51.Weber LW, Boll M, Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Rev Toxicol. 2003;33(2):105–136. doi: 10.1080/713611034 [DOI] [PubMed] [Google Scholar]
- 52.Ghadir MR, Riahin AA, Havaspour A, Nooranipour M, Habibinejad AA. The relationship between lipid profile and severity of liver damage in cirrhotic patients. Hepat Mon. 2010;10(4):285. [PMC free article] [PubMed] [Google Scholar]
- 53.Dutta S, Chakraborty AK, Dey P, et al. Amelioration of CCl4 induced liver injury in swiss albino mice by antioxidant rich leaf extract of Croton bonplandianus Baill. PLoS One. 2018;13(4):e0196411. doi: 10.1371/journal.pone.0196411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gupta A, Pandey AK. Chapter 3 - Plant Secondary Metabolites With Hepatoprotective Efficacy. In: Galanakis CM editor. Nutraceuticals and Natural Product Pharmaceuticals Academic Press; 2019:71–104. 10.1016/B978-0-12-816450-1.00003-9. [DOI] [Google Scholar]
- 55.Santos-Sánchez NF, Salas-Coronado R, Villanueva-Cañongo C, Hernández-Carlos B. Antioxidant Compounds and Their Antioxidant Mechanism. Antioxidants: IntechOpen; 2019:1–28. [Google Scholar]
- 56.Perron NR, Brumaghim JL. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem Biophys. 2009;53(2):75–100. [DOI] [PubMed] [Google Scholar]
- 57.Yahfoufi N, Alsadi N, Jambi M, Matar C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients. 2018;10(11):1618. doi: 10.3390/nu10111618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hong S-Y, Roze LV, Linz JE. Oxidative stress-related transcription factors in the regulation of secondary metabolism. Toxins. 2013;5(4):683–702. doi: 10.3390/toxins5040683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yoon J-H, Baek SJ. Molecular targets of dietary polyphenols with anti-inflammatory properties. Yonsei Med J. 2005;46(5):585–596. doi: 10.3349/ymj.2005.46.5.585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ozgová Š, Heřmánek J, Gut I. Different antioxidant effects of polyphenols on lipid peroxidation and hydroxyl radicals in the NADPH-, Fe-ascorbate-and Fe-microsomal systems. Biochem Pharmacol. 2003;66(7):1127–1137. doi: 10.1016/S0006-2952(03)00425-8 [DOI] [PubMed] [Google Scholar]
- 61.Amarowicz R. Tannins: the new natural antioxidants? Eur J Lipid Sci Technol. 2007;109(6):549–551. doi: 10.1002/ejlt.200700145 [DOI] [Google Scholar]
- 62.Sobeh M, Mahmoud MF, Hasan RA, et al. Tannin-rich extracts from Lannea stuhlmannii and Lannea humilis (Anacardiaceae) exhibit hepatoprotective activities in vivo via enhancement of the anti-apoptotic protein Bcl-2. Sci Rep. 2018;8(1):1–16. doi: 10.1038/s41598-018-27452-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Krishnamurthy PT, Bajaj J, Sharma A, Manimaran S, Ravanappa PKB, Pottekad V. Hepatoprotective activity of terpenoids and terpenoid fractions of Scoparia dulcis L. Orient Pharm Exp Med. 2010;10(4):263–270. [Google Scholar]
- 64.Qu L, Xin H, Zheng G, Su Y, Ling C. Hepatoprotective activity of the total saponins from Actinidia valvata dunn root against carbon tetrachloride-induced liver damage in Mice. Evid Based Complement Alternat Med. 2012;2012:1–13. doi: 10.1155/2012/216061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hussain T, Tan B, Liu G, et al. Modulatory mechanism of polyphenols and Nrf2 signaling pathway in LPS challenged pregnancy disorders. Oxid Med Cell Longev. 2017;2017:1–14. doi: 10.1155/2017/8254289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cui Y, Liu B, Sun X, et al. Protective effects of alfalfa saponins on oxidative stress-induced apoptotic cells. Food Funct. 2020;11(9):8133–8140. doi: 10.1039/D0FO01797C [DOI] [PubMed] [Google Scholar]