Abstract
MicroRNAs (miRNAs) in the let-7 family have established regulatory roles in pregnancy; in this study, we evaluated whether miRNAs detectable in the circulation are associated with the ovarian response to stimulation.
In total, 25 patients with infertility were divided into 3 groups: poor response (n = 9), moderate response (n = 8), and hyper-response (n = 8). Serum and endometrial tissue samples on the second day of the menstrual phase, serum samples during the mid-luteal phase, and follicular fluid samples were collected from women undergoing in vitro fertilization. The levels of let-7g-5p, let-7f-5p, and let-7i-5p in were evaluated.
The levels of circulating let-7g-5p,7f-5p, and 7i-5p in the basal sera were significantly higher in the moderate ovarian response group than in the poor response group (P < .05). The expression levels of these genes tended to be down-regulated in the mid-luteal phase in the high response group (P < .05). There were no significant differences in expression levels in the endometria and follicular fluid among groups (P > .05).
Circulating let-7g-5p, 7f-5p, and 7i-5p were differentially expressed between the moderate response group and the high and poor response groups. The combination of these serum miRNAs during the menstrual phase might serve as a non-invasive predictive marker for the ovarian response to in vitro fertilization.
Keywords: controlled ovarian stimulation, in vitro fertilization, let-7, microRNAs, ovarian response
1. Introduction
The prevalence of infertility in women of all reproductive ages is about 10% to 15%[1] and can reach 20% to 50% in some populations.[2] Infertility has become a global social and medical issue. Since the success of in vitro fertilization (IVF) was first reported, rapid advances in assisted reproductive technology have been achieved. However, the live birth rate for IVF is still low. The success of embryo implantation depends on 2 important factors: the receptivity of the endometrium and the selection of embryos with high implantation potency. Choosing a viable embryo for transplantation is a critical step in assisted reproductive technology and depends on the quantity and quality of oocytes. However, patient responses to controlled ovarian stimulation (COS) vary widely; both poor and hyper-responses may adversely affect outcomes. Therefore, it is necessary to evaluate the responses of ovaries to COS to prevent poor and hyper-responses.
MicroRNAs (MiRNAs) are a class of non-coding functional RNAs of approximately 22 nucleotides. These sequences can target the 3’-untranslated region and control gene expression by regulating translation or transcription of messenger RNAs (mRNAs).[3] Recently, increasing miRNAs encoded in genes have been reported. Owing to their high variation in expression among tissue types, miRNAs are potential biomarkers.[4] miRNAs detected in IVF culture media are differentially expressed according to the fertilization method, chromosomal status, and pregnancy outcome, making them candidate predictive biomarkers for IVF success.[5] Many predictors of ovarian function, such as age, serum follicle stimulating hormone (FSH), anti-hormone (AMH), and antral follicle counts (AFC), are often insufficient for the assessment of the ovarian response owing to the complexity and diversity of symptoms.[2,6] AMH and AFC are considered promising predictors of ovarian reserve function.[7] Serum levels of phospholipids and free fatty acids (FFA) are correlated with the number of good-quality oocytes and therefore are potential biomarkers for the ovarian response to COS.[8] The importance of the interplay between miRNAs and FFAs has already been established. For example, FFA increases the hepatic expression and secretion of miR-122, which is important for energy follicular energy metabolism.[9] However, specific diagnostic biomarkers for the ovarian response are still lacking, despite the important implications for the success of individualized COS.
A number of previous studies have shown that various human diseases (such as cancer, inflammatory diseases, and pathological pregnancy) are closely related to the abnormal expression of miRNAs.[10–13] Recently, circulating miRNAs have been found in the blood and follicular fluid. These circulating miRNAs are potential noninvasive biomarkers for other conditions, including spontaneous abortion, preeclampsia, and male fertility.[14–16] The precise factors affecting circulating miRNA profiles are complex and not yet fully understood. However, cancer tissues exhibit alterations in serum/plasma miRNA profiles, indicating that miRNAs may be released from the tissue and shed into the circulation, further supporting their potential utility as biomarkers.[17,18] We have previously identified miRNAs and their target mRNAs that are differentially expressed in the endometrium and early pregnancy decidua.[19] Differentially expressed miRNAs have been identified in female miscarriage and pregnancy decidual tissues.[20] Some members of the let-7 family highly expressed in endometrial tissues and during pregnancy.[21]
Considering the high expression levels of let-7 family members in the endometrium and their important role in pregnancy, we hypothesized that let-7 miRNAs may be differentially expressed in the circulating blood, endometrium, and follicular fluid among women with different ovarian responses. We evaluated the expression of circulating let-7g-5p, 7f, and 7i in patients with high, medium, and low responses and confirmed that these miRNAs are potentially effective non-invasive biomarkers for evaluating and predicting the ovarian response to COS.
2. Methods
2.1. Study population
Twenty-five women were enrolled after providing written informed consent. The volunteers were women of childbearing age who were infertile due to fallopian tube or male factors between October 2015 and December 2016 and underwent IVF and embryo transfer. Enrollees met the following criteria: age 25 to 45 years, no hormone therapy for at least 3 months, no history of smoking, and no history or signs of other inflammatory diseases. The exclusion criteria included previous use of hormones, organic uterine diseases, infectious diseases, inflammatory diseases, malignant tumors, and autoimmune diseases. Clinical data for each volunteer were also recorded. The ovarian response was classified as poor, moderate, or high according to patient age, AFC, AMH, gonadotrophin usage, and the number of oocytes. All patients had regular menstrual cycles. Serum and endometrial samples were collected on the second day of the menstrual phase (M2) and mid-secretory phase (progesterone level ≥3 pg/mL), and follicle fluid samples were collected. Patients who met at least 2 of the following three criteria were classified into the poor response group:
-
(1)
relatively old age (≥40 years) or have any risk factors for a poor ovarian response;
-
(2)
the ovarian response was poor during the previous ovulation induction process (less than 5 oocytes using the conventional stimulation protocol); or
-
(3)
abnormal ovarian reserve test (AFC <5–7 follicles or AMH <0.5.1.1 ng/mL).[22]
If the age or ovarian reserve function test was normal, the patient could still be diagnosed with a low ovarian response after 2 consecutive cycles of maximizing ovarian stimulation. Other risk factors were excluded: genetic or acquired diseases affecting the ovarian reserve and response to ovarian stimulation, such as abnormal numbers and structure of chromosomes, genetic mutations (eg, Turner syndrome), previous pelvic inflammatory disease, endometriosis, history of ovarian cyst surgery, radiotherapy, and chemotherapy (especially alkylated chemotherapy drugs). Patients meeting the following criteria were assigned to the moderate response group: 5 to 14 oocytes were obtained by the conventional stimulation protocol. Patients meeting at least 1 of the following criteria were classified as high responders:
-
(1)
a high ovarian response (15 or more oocytes by a conventional stimulation protocol); or
-
(2)
a high estradiol (E2) level (≥ 12000 pmol/L) or any risk factor for ovarian hyperstimulation syndrome.[23]
2.2. Ethics
The study was approved by the Institute Research Ethics Committee of Inner Mongolia Medical University (March 11, 2015, YKD2015098).
2.3. Sample collection and RNA extraction
Endometrial tissue was collected from each patient, quickly frozen in liquid nitrogen, rinsed with saline and dried with gauze, and transferred to a liquid nitrogen freezer for storage and use. The blood and follicular fluid were collected and placed in a 5-mL sterile test tube containing EDTA-K2. Samples were immediately centrifuged at 1000 × g for 10 minutes to obtain samples of serum and follicular fluid without precipitation. Samples were placed in a freezing tube and quickly put in liquid nitrogen for storage. One month later, the endometrial tissue, serum, and follicular fluid stored in liquid nitrogen were removed to prepare total RNA and then stored in a refrigerator at −80 °C for later use.
2.4. Quantitative real-time polymerase Chain reaction for miRNAs
One microgram of total RNA from each sample was used for the RT reaction to generate cDNA using a microRNA RT Kit (Takara, Kusatsu, Japan). Quantitative real-time PCR was performed in triplicate using the SYBR Green PCR Kit and specific primers (Takara) according to the manufacturer's instructions and an ABI Prism 7300HT. The relative miRNA levels were determined using the formula 2-ΔΔCT. Primers for let-7g-5p, let-7f-5p, and let-7i-5p are shown in Table 1.
Table 1.
Primer sequences.
| Gene | Upstream primer sequence (5’-3’) | Downstream primer sequence (5’-3’) |
| let-7g-5p | CCGCGTGAGGTAGTAGTTTGTACAGTTAAA | GTGCAGGGTCCGAGCT |
| let-7f-5p | GCGCGCTGAGGTAGTAGATTGTATAGTTAAA | GTGCAGGGTCCGAGCT |
| let-7i-5p | CGGCTGAGGTAGTAGTTTGTGCTGTTAA | GTGCAGGGTCCGAGCT |
| miR-16-5p | UAGCAGCACGUAAAUAUUGGCG | GTGCAGGGTCCGAGCT |
2.5. Statistical analysis
Demographic variables are presented as means ± standard deviation. The obtained data were statistically evaluated by 1-way analysis of variance, where appropriate. Expression levels of miRNAs were compared by 1-way analysis of variance and non-parametric tests among 3 groups (poor, moderate, and high response groups). All statistical analyses were implemented in SPSS version 16.0. Significance was set to P <.05 (2-tailed).
3. Results
3.1. Characteristics of the patients and cycles
The clinical characteristics of the participants are shown in Table 1. There were no statistically significant differences in mean age, gravidity, body mass index, basic luteinizing hormone (LH), E2, initial FSH stimulation dose, total FSH stimulation dose, and endometrial thickness on ET day among the 3 groups. In contrast, the basic serum FSH levels were significantly higher and AMH and AFC were lower in the poor response group than in the moderate and high response groups. Days of stimulation were significantly longer and the number of collected oocytes and cleavage-stage embryos were lower in the women with a poor response than in the moderate and high response groups. The clinical pregnancy rate was significantly higher (P < .001) in women with a high response than in the other groups (Table 2).
Table 2.
Comparison of demographic and clinical findings.
| Poor responder (n = 9) | Moderate responder (n = 8) | High responder (n = 8) | P value | |
| Age | 32.33 ± 2.60 | 31.25 ± 3.69 | 29.88 ± 3.14 | .577 |
| Gravidity | 2.22 ± 1.30 | 0.75 ± 0.89 | 1.75 ± 1.49 | .262 |
| Parity | 0.22 ± 0.44 | 0 | 0.13 ± 0.35 | .009 |
| BMI (kg/m2) | 22.55 ± 4.02 | 21.95 ± 1.98 | 22.87 ± 2.54 | .081 |
| AFC | 4.78 ± 2.11 | 9.75 ± 4.86 | 13.38 ± 3.29 | .145 |
| Basic FSH | 8.93 ± 3.80 | 6.74 ± 1.95 | 6.03 ± 1.07 | .083 |
| Basic LH | 4.23 ± 2.17 | 5.19 ± 3.12 | 4.92 ± 1.41 | .138 |
| Basic E2 | 30.40 ± 13.45 | 44.50 ± 29.35 | 35.9 ± 27.23 | .676 |
| AMH | 2.16 ± 2.04 | 3.80 ± 1.97 | 5.72 ± 3.56 | .437 |
| Stimulation protocol | Long protocol | Long protocol | Long protocol | |
| Initial FSH stimulation dose (IU) | 3.44 ± 0.53 | 2.75 ± 0.85 | 2.31 ± 0.46 | .091 |
| Total FSH stimulation dose (IU) | 49.33 ± 16.48 | 40.94 ± 14.81 | 28.19 ± 8.23 | .241 |
| Days of stimulation | 13.33 ± 5.15 | 11.5 ± 1.31 | 10.88 ± 1.13 | .007 |
| Number of collected oocytes | 4.11 ± 1.45 | 11.88 ± 2.03 | 23 ± 7.89 | .002 |
| Number of cleavage embryos | 2.11 ± 1.05 | 6.62 ± 3.20 | 11.5 ± 4.54 | .009 |
| Endometrial thickness on embryo transfer day | 11.0 ± 1.58 | 11.13 ± 2.10 | 9.88 ± 1.13 | .06 |
| Clinical pregnancy rate | 44.54%(4/9) | 37.5%(3/8) | 87.5%(7/8) | <.001 |
AFC = antral follicle count, AMH = antimüllerian hormone, Basic = the second day of menstrual phase, BMI = body mass index, E2 = estradiol, FSH = Hormone, LH = luteinizing hormone.
3.2. Expression of circulating has-let-7g-5p, 7f-5p, and 7i-5p in various sample types in the three groups
The levels of circulating let-7g-5p, 7f-5p, and 7i-5p in the basal sera were highest in the moderate ovarian response group and lowest in the poor response group, and the differences among groups were significant (P < .05). The expression levels of these genes tended to decrease in the mid-luteal phase in the high response group (P < .05). However, there were no significant differences in expression levels in endometrial and follicular fluid samples among the 3 groups (P > .05). (Figs. 1–3). No significant correlations were found between other clinical parameters and miRNA expression levels.
Figure 1.
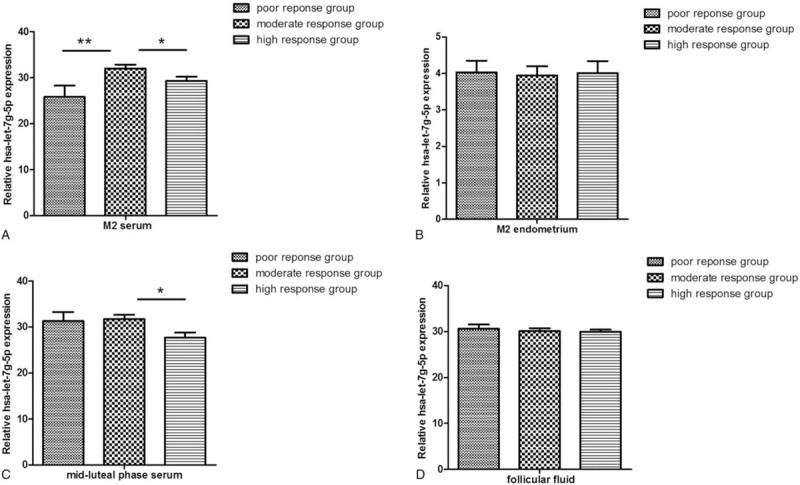
The relative expression of has-let-7g-5p in the serum during the menstrual phase and mid-luteal phase, endometria, and follicular fluid in 3 groups.
Figure 3.

The relative expression of has-let-7i-5p in the serum during the menstrual phase and mid-luteal phase, endometria, and follicular fluid in 3 groups.
Figure 2.

The relative expression of has-let-7f-5p in the serum during the menstrual phase and mid-luteal phase, endometria, and follicular fluid in 3 groups.
4. Discussion
The main goal of IVF is to provide personalized, optimal treatment based on patient characteristics to achieve the highest success rate, reduce the iatrogenic risk, and minimize the risk of cycle cancelation. Finding a reliable way to predict the ovarian response to stimuli is crucial for the success of personalized COS. Circulating miRNAs are reliable biomarkers for many human diseases; however, little is known about their associations with the ovarian response.
The let-7 miRNA family promotes cell differentiation. Some let-7 members are differentially expressed in the decidua and the endometrium, suggesting that this miRNA family plays a role in pregnancy regulation. Let-7a levels in early ectopic pregnancy are significantly decreased, and Lin28b mRNA interacts with Let-7a, suggesting that the Lin28/Let-7 axis contributes to the regulation of trophoblast invasion and placental formation.[24] Recent studies have shown that Let-7a,b,d,f,h gradually peak from stages I to V of ovarian development in Megalobrama amblycephala, followed by a significant decline at stage VI, indicating that these miRNAs play an important role in the development of this fish species.[25]
Granulosa cells (GCs) play an important role in the regulation of follicular potential, and their effects are thought to be mediated by interactions with oocytes via oocyte paracrine signaling during oocyte development.[26] Many genes involved in the response to ovarian stimulation in the IVF process are related to GCs and maternal signaling, indicating that GCs may be related to the oocyte maturation ability.[27–29] MiRNAs in GCs have the ability to regulate meiosis of oocytes during in vitro maturation in a mouse follicle model. The expression level of hsa-let-7b in the GCs of MII oocytes is lower than that in MI oocytes.[30] Accordingly, miRNAs involved in hormonal regulation during folliculogenesis and in oocyte-niche cross-talk are candidate non-invasive fertility biomarkers. However, in our study, there were no significant differences in miRNA levels in follicular fluid among the 3 groups.
By deep sequencing of the ovaries and testes of adult Tibetan miRNA libraries, let-7i, let-7f, let-7a, and let-7c showed the highest expression levels among the top 10 unique miRNAs compared with levels in healthy follicles, let-7a, let-7b, let-7c, and let-7i levels in early amorphous and progressively amorphous porcine ovarian follicles were significantly decreased, let-7 g was highly expressed during follicular atresia, and let-7 g was highly expressed during follicle atresia.[31] These findings further suggest that the let-7-family of miRNAs may play an important role in reproductive system development. Let-7f, in particular, is significantly upregulated in cumulus cells of women with a poor ovarian response undergoing IVF.[32] The abnormal downregulation of Let-7c has also been detected in patients with premature ovarian failure,[33] indicating that let-7c plays an active role in healthy follicular development. Let-7 g is highly expressed during atresia, different from the functions of other family members.[34] Interestingly, our results show that let-7g-5p and 7f expression levels are significantly higher in basal sera of patients with proper ovarian responses than those in controls.
Reduced ovarian function stimulates the hypothalamus and pituitary and results in elevated FSH and LH levels. Due to the poor ovarian reaction and the increase in FSH levels, the growth and consumption of follicles are aggravated, since ovarian function is even further exhausted. In follicular development, FSH, LH/hCG, and estrogen can inhibit apoptosis in follicles. In contrast, androgens, gonadotropins, and gonadal hormones participate in the process of follicular atresia. FSH and AMH have been identified as accurate ovarian reserve markers in the prediction of ovarian response to stimulation; however, they do not reflect other relevant information in clinical applications.[35,36] FSH plays a crucial role in follicular development, granulosa cell proliferation, and differentiation and may be regulated by let-7a.[37] An increase in FSH reduces the number of oocytes of metaphase I and affects the ovarian response.[38] Accordingly, the ovarian reserve of oocytes and ovarian response are negatively correlated with the age of infertile women.[39] The serum FSH levels were significantly higher in women with a poor response in our study. Various clinical parameters (such as body mass index, average age, pregnancy rate, and basic LH and E2 levels) did not differ among the 3 groups, and further studies are needed to determine the ability of FSH to regulate the expression of miRNAs in follicles.
AMH is produced by GCs of developing follicles and is an established indicator of ovarian function. In women of childbearing age, the primordial follicle pool is constantly depleted during the regular recruitment process. Growing follicles are sensitive to follicle stimulating hormone and are regulated by AMH. At the same time, AMH can inhibit the initial recruitment of primordial follicles into the growth pool. Therefore, AMH may be an important regulator of female infertility and ovarian reserve; however, the regulatory mechanism involving AMH is still unclear. In our study, as the ovarian response increased, AMH levels tended to increase. Standardized AMH values may facilitate comparisons of ovarian reserves among women of different ages.[40] In a retrospective study,[41] 689 patients receiving IVF were divided into low response (0–3), medium response (4–15), and high response (more than 16) groups based on the number of eggs acquired. Based on this analysis, the level of serum FSH had limited predictive value for the ovarian response. For the prediction of the ovarian reserve, AMH and AFC are the most valuable parameters.
Kallen knocked down and overexpressed H19, combined with in vivo cross-linking and genome-wide transcriptome analysis. The experimental results showed that H19 can act as a molecular sponge for let-7, which can improve the availability of let-7 targets.[42] In the absence of H19, hyperovulation can increase the level of estradiol and increase the number of oocytes, indicating that H19 inhibits the growth and maturation of follicles and reduces the production of estradiol and the number of ovulated follicles. AMH was identified as a target of the H19/let-7 axis, and knocking down H19 in mice decreased AMH levels and fertility. Potential associations between H19/let-7 and AMH provide a new direction for exploring the molecular mechanism underlying the regulatory effects of AMH in follicular development and ovarian reserve.[43]
In our study, the expression levels of let-7g-5p,7f, and 7i in basal sera were high in the medium ovarian response group and low in the poor response group, and levels tended to decrease in the mid-luteal phase in the high response group. However, there were no significant differences in expression levels in endometria and follicular fluid among the 3 groups. In our previous study of the expression of let-7 family members in the decidua and endometrium, associations between tissue and serum expression levels were not detected. In our study, we observed significant differences in the expression levels of let-7g-5p,7f, and 7i in the serum among groups. However, the exact causes of these miRNA changes in circulation are currently unclear. Although there is evidence that miRNAs may be released from tissues before entering the circulatory system, there are clear differences in analyses of paired tissue-plasma miRNA expression profiles. Therefore, the source of circulating miRNAs is not only disease tissues or malignant tumor cells. Furthermore, the mutual regulatory relationships among miRNAs, miRNAs, tissues, etc. can also affect miRNA levels. Although little is known about these relationships, our results provide a preliminary understanding of the effects of miRNAs in circulation on communication between tissues and cells.
To the best of our knowledge, this is the first study of the differential expression of circulating miRNAs in the endometrium, serum, and follicular fluid of patients with different ovarian responses to COS. It is interesting that periodic changes were found in the serum. The interactions between Let-7, female endocrine hormone regulation, and follicular development may affect ovarian function; however, the specific relationship and underlying mechanisms are not yet clear. Owing to the specific focus on patients who used long-term ovulation induction and the relatively small sample size, it is necessary to expand the sample to include patients who use different hyper-ovulation schemes to improve the predictive and diagnostic accuracy.
5. Conclusion
In summary, we have previously confirmed that let-7g-5p, 7f, and 7i are related to endometrial decidualization and are involved in the regulation of pregnancy. In this study, we further proved that these miRNAs can be monitored in the human serum and follicular fluid and are differentially expressed in the peripheral serum of women with different ovarian responses during menstruation and the luteal phase, with periodic differences in expression. These findings suggest that let-7g-5p, 7f, and 7i can be considered potential diagnostic biomarkers for predicting the female ovarian response to stimulation.
Author contributions
Conceptualization: Yu Wang
Formal analysis: Hongjuan Zhao
Funding acquisition: Yu Wang
Methodology: Hongjuan Zhao and Liyan Wang
Software: Hongjuan Zhao and Yu Wang.
Writing – original draft: Hongjuan Zhao
Writing – review & editing: Yu Wang.
Glossary
Abbreviations: AFC = antral follicle count, AMH = anti-hormone, BMI = body mass index, COS = controlled ovarian stimulation, FFA = free fatty acids, FSH = follicle stimulating hormone, GCs = granulosa cells, IVF = in vitro fertilization, LH = luteinizing hormone, LN2 = liquid nitrogen, M2 = the second day of menstrual phase, MiRNAs = microRNAs, MRNAs = messenger RNAs.
References
- [1].Shi Y, Sun Y, Hao C, et al. Transfer of fresh versus frozen embryos in ovulatory women. N Engl J Med 2018;378:126–36. [DOI] [PubMed] [Google Scholar]
- [2].La Marca A, Stabile G, Artenisio AC, et al. Serum anti-Mullerian hormone throughout the human menstrual cycle. Hum Reprod 2006;21:3103–7. [DOI] [PubMed] [Google Scholar]
- [3].Krutzfeldt J, Poy MN, Stoffel M. Strategies to determine the biological function of microRNAs. Nature Genetics 2006;38: (Suppl): S14–9. [DOI] [PubMed] [Google Scholar]
- [4].Kobayashi M, Sawada K, Nakamura K, et al. Exosomal miR-1290 is a potential biomarker of high-grade serous ovarian carcinoma and can discriminate patients from those with malignancies of other histological types. J Ovarian Res 2018;11:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rosenbluth EM, Shelton DN, Wells LM, et al. Human embryos secrete microRNAs into culture media--a potential biomarker for implantation. Fertil Steril 2014;101:1493–500. [DOI] [PubMed] [Google Scholar]
- [6].Nelson SM, Yates RW, Fleming R. Serum anti-Mullerian hormone and FSH: prediction of live birth and extremes of response in stimulated cycles--implications for individualization of therapy. Hum Reprod 2007;22:2414–21. [DOI] [PubMed] [Google Scholar]
- [7].Visser JA, de Jong FH, Laven JS, et al. Anti-Mullerian hormone: a new marker for ovarian function. Reproduction 2006;131:1–9. [DOI] [PubMed] [Google Scholar]
- [8].Perovic MD, Sudar-Milovanovic EM, Simonovic ED, et al. Hypothesis regarding the effects of gonadotropins on the level of free fatty acids and phospholipids in serum and follicular fluid during controlled ovarian stimulation. Med Hypotheses 2019;123:30–4. [DOI] [PubMed] [Google Scholar]
- [9].Chai C, Rivkin M, Berkovits L, et al. Metabolic circuit involving free fatty acids, microRNA 122, and triglyceride synthesis in liver and muscle tissues. Gastroenterology 2017;153:1404–15. [DOI] [PubMed] [Google Scholar]
- [10].Scholer N, Langer C, Dohner H, et al. Serum microRNAs as a novel class of biomarkers: a comprehensive review of the literature. Exp Hematol 2010;38:1126–30. [DOI] [PubMed] [Google Scholar]
- [11].Galliano D, Pellicer A. MicroRNA and implantation. Fertil Steril 2014;101:1531–44. [DOI] [PubMed] [Google Scholar]
- [12].Hull ML, Nisenblat V. Tissue and circulating microRNA influence reproductive function in endometrial disease. Reprod Biomed Online 2013;27:515–29. [DOI] [PubMed] [Google Scholar]
- [13].Cretoiu D, Xu J, Xiao J, et al. Circulating MicroRNAs as potential molecular biomarkers in pathophysiological evolution of pregnancy. Dis Markers 2016;2016:3851054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tsatsanis C, Bobjer J, Rastkhani H, et al. Serum miR-155 as a potential biomarker of male fertility. Hum Reprod 2015;30:853–60. [DOI] [PubMed] [Google Scholar]
- [15].Qin W, Tang Y, Yang N, et al. Potential role of circulating microRNAs as a biomarker for unexplained recurrent spontaneous abortion. Fertil Steril 2016;105:1247–54. [DOI] [PubMed] [Google Scholar]
- [16].Murphy MS, Casselman RC, Tayade C, et al. Differential expression of plasma microRNA in preeclamptic patients at delivery and 1 year postpartum. Am J Obstet Gynecol 2015;213:367. [DOI] [PubMed] [Google Scholar]
- [17].Resnick KE, Alder H, Hagan JP, et al. The detection of differentially expressed microRNAs from the serum of ovarian cancer patients using a novel real-time PCR platform. Gynecol Oncol 2009;112:55–9. [DOI] [PubMed] [Google Scholar]
- [18].Wang F, Zheng Z, Guo J, et al. Correlation and quantitation of microRNA aberrant expression in tissues and sera from patients with breast tumor. Gynecol Oncol 2010;119:586–93. [DOI] [PubMed] [Google Scholar]
- [19].Wang Y, Lv Y, Gao S, et al. MicroRNA profiles in spontaneous decidualized menstrual endometrium and early pregnancy decidua with successfully implanted embryos. PLoS One 2016;11:e0143116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lv Y, Gao S, Zhang Y, et al. miRNA and target gene expression in menstrual endometria and early pregnancy decidua. Eur J Obstet Gynecol Reprod Biol 2016;197:27–30. [DOI] [PubMed] [Google Scholar]
- [21].Wang Y, Lv Y, Wang L, et al. MicroRNAome in decidua: a new approach to assess the maintenance of pregnancy. Fertil Steril 2015;103:980–9. [DOI] [PubMed] [Google Scholar]
- [22].Ferraretti AP, La Marca A, Fauser BC, et al. ESHRE consensus on the definition of ’poor response’ to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod 2011;26:1616–24. [DOI] [PubMed] [Google Scholar]
- [23].Chen QJ, Sun XX, Li L, et al. Effects of ovarian high response on implantation and pregnancy outcome during controlled ovarian hyperstimulation (with GnRH agonist and rFSH). Acta Obstet Gynecol Scand 2007;86:849–54. [DOI] [PubMed] [Google Scholar]
- [24].Lozoya T, Dominguez F, Romero-Ruiz A, et al. The Lin28/Let-7 system in early human embryonic tissue and ectopic pregnancy. PLoS One 2014;9:e87698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lan T, Chen YL, Gul Y, et al. Comparative expression analysis of let-7 microRNAs during ovary development in Megalobrama amblycephala. Fish Physiol Biochem 2019;45:1101–15. [DOI] [PubMed] [Google Scholar]
- [26].Adriaenssens T, Mazoyer C, Segers I, et al. Differences in collagen expression in cumulus cells after exposure to highly purified menotropin or recombinant follicle-stimulating hormone in a mouse follicle culture model. Biol Reprod 2009;80:1015–25. [DOI] [PubMed] [Google Scholar]
- [27].Pacella L, Zander-Fox DL, Armstrong DT, et al. Women with reduced ovarian reserve or advanced maternal age have an altered follicular environment. Fertil Steril 2012;98:986–94. e1-2. [DOI] [PubMed] [Google Scholar]
- [28].Yerushalmi GM, Maman E, Yung Y, et al. Molecular characterization of the human ovulatory cascade-lesson from the IVF/IVM model. J Assist Reprod Genet 2011;28:509–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gebhardt KM, Feil DK, Dunning KR, et al. Human cumulus cell gene expression as a biomarker of pregnancy outcome after single embryo transfer. Fertil Steril 2011;96:47–52. e2. [DOI] [PubMed] [Google Scholar]
- [30].Kim YJ, Ku SY, Kim YY, et al. MicroRNA profile of granulosa cells after ovarian stimulation differs according to maturity of retrieved oocytes. Geburtshilfe Frauenheilkd 2016;76:704–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Li M, Liu Y, Wang T, et al. Repertoire of porcine microRNAs in adult ovary and testis by deep sequencing. Int J Biol Sci 2011;7:1045–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Karakaya C, Guzeloglu-Kayisli O, Uyar A, et al. Poor ovarian response in women undergoing in vitro fertilization is associated with altered microRNA expression in cumulus cells. Fertil Steril 2015;103:1469–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Dang Y, Zhao S, Qin Y, et al. MicroRNA-22-3p is down-regulated in the plasma of Han Chinese patients with premature ovarian failure. Fertil Steril 2015;103:802–7. [DOI] [PubMed] [Google Scholar]
- [34].Cao R, Wu WJ, Zhou XL, et al. Expression and preliminary functional profiling of the let-7 family during porcine ovary follicle atresia. Mol Cells 2015;38:304–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Broer SL, van Disseldorp J, Broeze KA, et al. Added value of ovarian reserve testing on patient characteristics in the prediction of ovarian response and ongoing pregnancy: an individual patient data approach. Hum Reprod Update 2013;19:26–36. [DOI] [PubMed] [Google Scholar]
- [36].Broer SL, Dolleman M, van Disseldorp J, et al. Prediction of an excessive response in in vitro fertilization from patient characteristics and ovarian reserve tests and comparison in subgroups: an individual patient data meta-analysis. Fertil Steril 2013;100:420–9. [DOI] [PubMed] [Google Scholar]
- [37].Yao N, Lu CL, Zhao JJ, et al. A network of miRNAs expressed in the ovary are regulated by FSH. Front Biosci (Landmark Ed) 2009;14:3239–45. [DOI] [PubMed] [Google Scholar]
- [38].Gingold JA, Lee JA, Whitehouse MC, et al. Maximum basal FSH predicts reproductive outcome better than cycle-specific basal FSH levels: waiting for a “better” month conveys limited retrieval benefits. Reprod Biol Endocrinol 2015;13:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Raeissi A, Torki A, Moradi A, et al. Age-specific serum anti-mullerian hormone and follicle stimulating hormone concentrations in infertile Iranian women. Int J Fertil Steril 2015;9:27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Helden JV, Weiskirchen R. Age-independent anti-Mullerian hormone (AMH) standard deviation scores to estimate ovarian function. Eur J Obstet Gynecol Reprod Biol 2017;213:64–70. [DOI] [PubMed] [Google Scholar]
- [41].Vural B, Cakiroglu Y, Vural F, et al. Hormonal and functional biomarkers in ovarian response. Arch Gynecol Obstet 2014;289:1355–61. [DOI] [PubMed] [Google Scholar]
- [42].Kallen AN, Zhou XB, Xu J, et al. The imprinted H19 lncRNA antagonizes let-7 microRNAs. Mol Cell 2013;52:101–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Qin C, Xia X, Fan Y, et al. A novel, noncoding-RNA-mediated, post-transcriptional mechanism of anti-Mullerian hormone regulation by the H19/let-7 axis. Biol Reprod 2019;100:101–11. [DOI] [PMC free article] [PubMed] [Google Scholar]


