Abstract
The purpose of this study was to identify and compare the diagnostic performance of gadolinium-ethoxybenzyl-diethyltriethylenetriacetic acid (Gd-EOB-DTPA) enhanced magnetic resonance imaging (MRI) and contrast-enhanced ultrasound (CEUS) in hepatocellular carcinoma (HCC).
Two researchers searched PubMed, EMBASE, and Cochrane Library databases from the inception of each database to 10 February 2020, to find comparative studies of Gd-EOB-DTPA-MRI and CEUS in detection of HCC.
The study included eight studies (374 patients). MRI is superior to CEUS in diagnostic sensitivity of HCC, P = .03. The diagnostic sensitivity of MRI in lesions with a diameter of less than 30 mm was significantly higher than that of CEUS, P = .04. MRI and CEUS had no significant difference in diagnostic specificity of HCC, P = .95. Summary Receiver Operating Characteristics (SROC) of MRI showed a larger than that of CEUS, but with P > .05.
Gd-EOB-DTPA-MRI showed higher sensitivity than CEUS for hepatocellular carcinoma lesions, especially for lesions of less than 30 mm across.
Keywords: gadoxetic acid disodium, hepatocellular carcinoma, magnetic resonance imaging, meta-analysis, ultrasonography
1. Introduction
Liver cancer is the third leading cause of death in the world and the fifth leading cause of death in China.[1] The liver is the first metastatic site of most malignant tumors.[2] Hepatocellular carcinoma (HCC) is a malignant tumor that can be diagnosed through imaging examination without biopsy.[3] Gd-EOB-DTPA-magnetic resonance imaging (MRI), which is recommended by the American Society for the Study of Liver Diseases as a first-line detection method for diagnosis of hepatocellular carcinoma,[3] offers good performance in detection and qualitative analysis of focal liver lesions.[4–6]
Contrast-enhanced ultrasound (CEUS) is a useful method for assessing focal liver lesions based on hemodynamic changes. CEUS provides good performance in detection and qualitative analysis of focal liver lesions.[7] Studies have shown that CEUS has a high diagnostic performance to arterial hypervascularity of HCC,[8] and the specificity of CEUS for nodules with hypervascular lesions for HCC is higher than that reported in computed tomography (CT) or MRI studies, especially for small (< 20 mm) HCC.[9] CEUS is inherently more sensitive to microbubbles than CT or MRI is to iodization or gadolinium contrast agent.[10] In 2018, the European Association for the Study of the Liver (EASL) and the Korean Association for Liver Cancer and the Korean National Cancer Center (KLCA-NCC) updated their guidelines to this effect. Hepatobiliary contrast-agent-enhanced MRI is now included as a first-line diagnostic method in these new guidelines, while CEUS is also included as a second-line diagnostic method in KLCA-NCC and EASL guidelines. Therefore, hepatobiliary contrast agents enhanced MRI and CEUS will be increasingly used in the non-invasive diagnosis and staging of liver cancer.[11]
Preoperative identification of the presence and absence of metastatic lesions in the liver of patients with liver cancer and the determination of the number of metastatic lesions are significantly related to the determination of the Barcelona Clinic Liver Cancer (BCLC) staging of patients with liver cancer in the formulation of surgical procedures and the prognosis of patients.[2,12] Many studies have suggested that Gd-EOB-DTPA-MRI can detect small liver lesions which may not be able to be found by CEUS, however, some have reported that CEUS is no worse than Gd-EOB-MRI in the diagnosis of HCC.[8–10] We hope to explore the performance of Gd-EOB-DTPA-MRI and CEUS in HCC diagnosis through this study.
2. Materials and methods
2.1. Literature search
JW and XY undertook a literature search on three databases to find relevant articles which were published before 10 February 2020. The databases included EMBASE, PubMed, and the Cochrane library. The search strategy and query criteria on PUBMED, one of the databases, are shown in Table 1. Institutional Review Board approval was not needed because it is a meta-analysis.
Table 1.
Literature search strategy in PUBMED.
| Step no. | Query |
| #1 | “Ultrasonography”[Mesh] |
| #2 | “contrast-enhanced ultrasonography” OR “contrast-enhanced ultrasound” OR “enhanced ultrasound” OR “ultrasound contrast” OR “ultrasonic contrast” OR CEUS |
| #3 | “Magnetic Resonance Imaging”[Mesh]∗ |
| #4 | “Magnetic Resonance” OR MR |
| #5 | “Carcinoma, Hepatocellular”[Mesh] |
| #6 | ”hepatocellular carcinoma“ OR HCC |
| #7 | “Gadolinium DTPA”[Mesh] |
| #8 | Gd-EOB-DTPA OR “gadoxetate disodium” OR “gadoxetic acid” |
| #9 | #1 OR #2 |
| #10 | #3 OR #4 |
| #11 | #5 OR #6 |
| #12 | #7 OR #8 |
| #13 | #9 AND #10 AND #11 AND #12 |
This table provides details on how the literature was searched in various databases.
Medical subject headings.
2.2. Study selection
The two researchers independently reviewed the titles and abstracts of all the articles and the full text of some of them to determine whether, or not, they met the inclusion criteria. Where there are differences, they shall be reconciled by consensus. If agreement cannot be reached, the opinion of a third reviewer would have been sought. The inclusion criteria were as follows:
-
(1)
Gd-EOB-DTPA-MRI, and CEUS were used to diagnose HCC;
-
(2)
The number of patients in the study was not less than 10;
-
(3)
The diagnostic criteria for HCC include the following points: a. Pathology obtained by hepatectomy, liver transplantation, and/or liver biopsy; b. Imaging follow-up;
-
(4)
The true positive (TP) value and false positive (FP) value can be obtained from the article.
The exclusion criteria were as follows:
-
(1)
These types of articles include conference abstracts, comments, letters, systemic evaluations, reviews of the literature, and animal models; and
-
(2)
Absence of one of the MRI and CEUS.
2.3. Data extraction
The first author of the paper, the country in which the study was undertaken, the year of publication, the number of patients, the average number of patients, the number and size of lesions, TP, and FP were extracted from each study. FN and TN were also extracted from some studies.
2.4. Imaging follow-up
For lesions with no typical manifestations, they can only be diagnosed as benign lesions if the lesions remain unchanged after at least 6 months of follow-up. During follow-up, previously undiagnosed or new lesions can be diagnosed as HCC lesions if they meet non-invasive diagnostic criteria.
2.5. Quality assessment
Each included study was evaluated using the diagnostic accuracy study-2 (QUADAS-2) tool. The quality of each study was assessed by assessing the risk of bias in four areas.[13]
2.6. Statistical analysis
The forest plots were established using the random-effects model of New Methodology in Review Manager, and heterogeneity testing was also conducted. The comparison of sensitivity and specificity between the two methods was expressed using the odds ratio (95% confidence intervals, CIs). If the odds ratio was greater than 1, MRI was deemed superior, otherwise CEUS was deemed superior. The summary receiver operating characteristic (SROC) curve was drawn using Diagnostic Test Accuracy Review in Review Manager. The statistical software used was Review Manager (RevMan) (Version 5.3. Copenhagen: The Nordic Cochrane Centre, the Cochrane Collaboration, 2014).
We conducted sub-group analysis of sensitivity as follows: after excluding one heterogeneous article from eight articles, the seven remaining articles were classified as Sub-group 1. Studies with a lesion diameter of less than 30 mm were included in Sub-group 2. Four studies involving lesions with non-HCC were included in Sub-group 3, and another four studies that focused exclusively on HCC were included in Sub-group 4. Specificity analysis was provided in four studies in Sub-group 5.
3. Results
A total of 141 articles were retrieved: 26 of the articles were repeated and then 98 articles were eliminated by reading the titles and abstracts. A total of 9 of 17 articles were removed by reading the full text. The current meta-analysis finally included the aforementioned eight studies.[14–21] There were four studies that provided data on tumors less than 30 mm in diameter. Four of them provided specificity analysis. The research selection process is illustrated in Figures 1 and 2 shows the overall risk of bias for the eight studies.
Figure 1.

The process of study selection.
Figure 2.
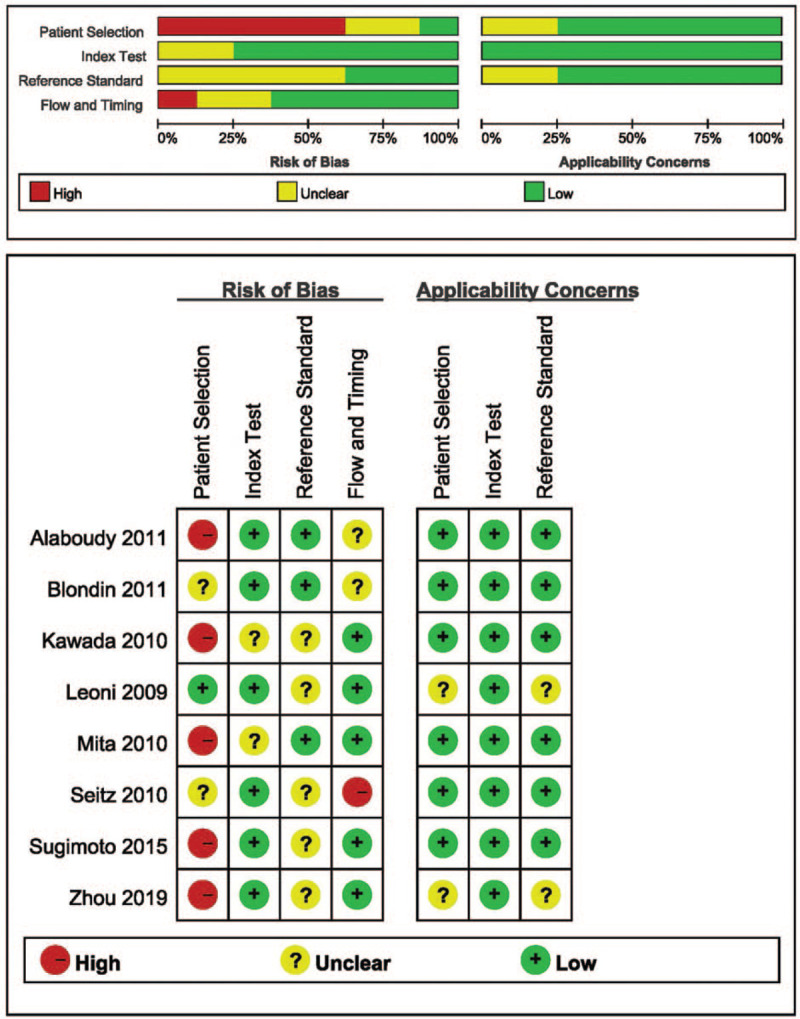
Methodological quality of the studies included (QUADAS-2 results).
3.1. Study characteristics
The demographic and baseline characteristics are shown in Tables 2 and 3. A total of 376 patients with 446 HCC lesions were included.
Table 2.
Characteristics of the included studies.
| Equipment/ | ||||||||||
| Author | Year | Patients | Lesions | HCC lesions | Lesion size (cm) | Male/ Female | Patient age | Gold standard | MRI | CEUS |
| Alaboudy | 2011 | 32 | 50 | 50 | 2.6 (0.5–11) | 23/9 | 68.3+8.1 (48–79) | P∗ | 1.5T/3.0T | Sonazoid |
| Blondin | 2011 | 33 | 47 | 41 | NA | 25/8 | 63.2 ± 11.2 | P | 1.5T | SonoVue |
| Kawada | 2010 | 13 | 15 | 15 | NA | 10/3 | 67 (51–77) | P | 3.0T | Sonazoid |
| Leoni | 2009 | 60 | 75 | 55 | 1.8 (1.0–3.0) | 52/8 | 65.2 ± 10 | P/I† | 1.5T | Sonovue |
| Mita | 2010 | 29 | 34 | 34 | 1.7 (0.8-2) | 13/16 | 70.5 ± 7.96 (55–84) | P | 1.0T | Sonazoid |
| Seitz | 2010 | 84 | 82 | 29 | NA | 53/31 | 59.6 (28 – 82) | P | 1.5T | SonoVue |
| Sugimoto | 2015 | 27 | 27 | 27 | <2 | 13/14 | 71.5 (59 – 81) | P | 1.0T | Sonazoid |
| Zhou | 2019 | 98 | 116 | 89 | <2 | 67/31 | 58.13 | P/I | 3.0T | SonoVue |
CT = computed tomography, HCC = hepatocellular carcinoma, MRI = magnetic resonance imaging, NA = not available.
P: pathological follow up.
I: imaging follow up.
Table 3.
Various indicators of included literatures.
| Author | Year | Prospective/ Retrospective | Child-pugh class (A/B/C) | The time interval of MRI and CEUS | Nation | Enrollment Patients |
| Alaboudy | 2011 | Retrospective | NA | NA | Japanese | July 2008 and March 2010 |
| Blondin | 2011 | Retrospective | 22/9/2 | <4 weeks | Germany | January 2007 to March 2009 |
| Kawada | 2010 | Retrospective | 13 | NA | Japan | June 2008 and June 2009 |
| Leoni | 2009 | Retrospective | 40/18/2 | NA | Italy | consecutive September 2003 and November 2005 |
| Mita | 2010 | Prospective | NA | NA | Japan | April 2008 to December 2009 |
| Seitz | 2010 | Retrospective | NA | NA | Germany | May 2004 to December 2006 |
| Sugimoto | 2015 | Retrospective | NA | NA | Japan | April 2008 to December 2009 |
| Zhou | 2019 | Retrospective | NA | NA | China | February and December 2016 |
CEUS = contrast-enhanced ultrasound, MRI = magnetic resonance imaging, NA = not available.
3.2. Heterogeneity test
The homogeneity test of the sensitivity demonstrated moderate heterogeneity in the whole group and greater heterogeneity in Sub-groups 2 and 4 (I2 = 43%, I2 = 66%, I2 = 68%, respectively), and it did not demonstrate heterogeneity in Sub-groups 1 and 3. The homogeneity test of specificity demonstrated no significant heterogeneity.
Due to the whole group having moderate heterogeneity, we removed the article by Sugimoto (2015) with its high heterogeneity from the eight articles to form Sub-group 1: there was no heterogeneity in sensitivity in Sub-group 1, I2 = 0%.
3.3. Odds ratio
The total odds ratios of the whole group, and Sub-groups 1 to 5 were 1.78 (95%CI: 1.05-3.04), 1.47 (95%CI: 0.89-2.20), 2.37 (95%CI: 1.03–5.46), 1.41 (95%CI: 0.86–2.30), 2.54 (95%CI: 0.84–7.72), and 0.96 (95%CI: 0.46–2.06), respectively. The overall effects of the whole group and Sub-group 2 were 2.13 and 2.02, respectively, p < 0.05. The results are shown in Figure 3.
Figure 3.

Forest plots showing Odds Ratio with corresponding 95% Confidence Intervals (CIs) for the diagnostic performance of HCC by MRI and CEUS in each study. Overall group: including eight articles; Sub-group 1: including seven articles without heterogeneity; Sub-group 2: literature describing tumours of no more than 30 mm in diameter; Sub-group 3: literature focused exclusively on HCC. Sub-group 4: literature involving non-HCC lesions. Sub-group 5: the specific analyses of four studies.
The area under the curve (AUC) of SROC for CEUS was smaller than that of MRI in four articles, 0.88 (0.85–0.91) and 0.9 (0.87–0.92), P > .05, as shown in Figure 4.
Figure 4.

Summary receiver operating characteristic curves (SROC) for four studies. A line connects the pair of points representing the tests of MRI and CEUS from each study.
4. Discussion
Preoperative identification of the presence and absence of metastatic lesions in the liver of patients with liver cancer and the determination of the number of metastatic lesions are significantly related to the determination arising from BCLC staging, the formulation of surgical procedures, and the prognosis of patients.[2,12] CEUS and MRI can provide a standardized non-invasive diagnosis for high-risk HCC patients.[22] CEUS and MRI with liver-specific contrast media are reliable and of equal informative value in the characterization of focal liver lesions.[23,24] Some studies suggest that CEUS can be used as a first-line detection method for liver lesions.[25] There is no meta-analysis on the diagnostic performance of MRI and CEUS in HCC, so we conducted this meta-analysis on HCC diagnosis performance using Gd-EOB-DTPA-MRI with CEUS.
The total odds ratio was greater than 1 and the overall effect was significantly different in the whole group which exhibited moderate heterogeneity, necessitating the analysis thereof. After the article by Sugimoto (with its odds ratio exceeding 13) was removed, the seven remaining articles showed no heterogeneity. The total odds ratio of these seven studies was also greater 1. This suggested that the sensitivity of MRI was still better than that of CEUS and the low sensitivity of CEUS in the study by Sugimoto may be due to the fact that the tumors in this study were all less than 20 mm in diameter. The total odds ratio was less than 1 and overall effect exhibited no significant difference in Sub-group 5. This indicates that the specificity of MRI and CEUS in these four studies was similar, with no significant differences therein.
The total odds ratio was greater than 1 and the overall effect was one of significant differences even for lesions with a diameter no greater than 30 mm. Four studies could be used to extract data pertaining to tumors of no greater than 30 mm in diameter, so we performed a sub-group analysis of the diagnostic sensitivity of tumors with a diameter of no more than 30 mm. Our results suggested that the sensitivity of MRI was significantly better than that of CEUS in this sub-group. Liver-specific contrast agents increased the enhancement signal of the liver parenchyma at the hepatobiliary specific stage, thereby improving the manifestation of liver space occupation, especially for small lesions with a diameter of less than 10 mm.[26] The application of Gd-EOB-DTPA has allowed considerable progress in the diagnosis of liver neoplastic lesions, especially in the diagnosis of small lesions.[27] Kudo et al[28] had confirmed that Gd-EOB-DTPA-MRI could improve the ability to detect early liver cancer lesions of less than 20 mm diameter, and they found 30 patients with liver cancer resection specimens and a diagnostic coincidence rate of 93% (28/30). Kim et al[29] found that the diagnostic coincidence rate of CEUS for lesions smaller than 20 mm was 70%. Some studies suggested that Gd-EOB-DTPA-MRI had higher sensitivity than CEUS to tumors of no greater than 20 mm in diameter.[20,21]
Some studies had suggested that MRI could detect more small lesions than CEUS, but these studies do not compare the sensitivity of the two to lesion detection. Alaboudy et al[14] found that Gd-EOB-DTPA MRI detected 12% (6/50) more lesions than CEUS. Iwamoto et al[24] found that a significantly larger number of nodules could be evaluated by Gd-EOB-DTPA-enhanced MRI than by CEUS (P < .05). Kobayashi et al[30] found that the main tumor in all patients was detected by Gd-EOB-DTPA, but the main tumor in 5.6% (5/90) of patients was not detected by CEUS.
Our results showed that the SROC of MRI was greater than that of CEUS, but there was no significant difference between them, P > .05. There was no significant difference between the two methods, which may be because only four studies provided specific analysis indicators, and the comprehensive sensitivity and specificity of the four studies did not differ between the two methods.
5. Conclusions and limitations
Gd-EOB-DTPA DCE-MRI is more sensitive than CEUS in the diagnosis of HCC, especially for lesions of no greater than 30 mm in diameter.
The lesions reported in the eight studies were lesions that could be detected by both methods. Comparative analysis was not included for some lesions that could not be detected by either method: only four studies included non-HCC lesions, thus the comparison in sensitivity is relatively biased. Future, related studies are thus warranted.
Author contributions
Conceptualization: Jiming Wang, Xiaofei Ye, Jiangfa Li, Songqing He.
Data curation: Jiming Wang, Xiaofei Ye, Jiangfa Li.
Formal analysis: Jiming Wang, Xiaofei Ye, Jiangfa Li.
Funding acquisition: Jiangfa Li, Songqing He.
Investigation: Jiming Wang, Jiangfa Li.
Methodology: Jiming Wang, Xiaofei Ye, Jiangfa Li.
Supervision: Songqing He.
Writing – original draft: Jiming Wang, Xiaofei Ye, Jiangfa Li.
Writing – review & editing: Jiangfa Li, Songqing He.
Glossary
Abbreviations: CEUS = contrast-enhanced ultrasound, CT = computed tomography, DCE = dynamic contrast enhanced, FN = false negative, FP = false positive, Gd-EOB-DTPA = gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid, HCC = hepatocellular carcinoma, MRI = magnetic resonance imaging, TN = true negative, TP = true positive.
References
- [1].Zhou M, Wang H, Zeng X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019;394:1145–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Battaglia V, Cervelli R. Liver investigations: Updating on US technique and contrast-enhanced ultrasound (CEUS). Eur J Radiol 2017;96:65–73. [DOI] [PubMed] [Google Scholar]
- [3].Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67:358–80. [DOI] [PubMed] [Google Scholar]
- [4].Son J, Hwang SH, Park S, et al. Imaging features of hepatocellular carcinoma: quantitative and qualitative comparison between MRI-enhanced with Gd-EOB-DTPA and Gd-DTPA. Invest Radiol 2019;54:494–9. [DOI] [PubMed] [Google Scholar]
- [5].Li J, Li X, Weng J, et al. Gd-EOB-DTPA dynamic contrast-enhanced magnetic resonance imaging is more effective than enhanced 64-slice CT for the detection of small lesions in patients with hepatocellular carcinoma. Medicine (Baltimore) 2018;97:e13964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Li J, Wang J, Lei L, et al. The diagnostic performance of gadoxetic acid disodium-enhanced magnetic resonance imaging and contrast-enhanced multi-detector computed tomography in detecting hepatocellular carcinoma: a meta-analysis of eight prospective studies. Eur Radiol 2019;29:6519–28. [DOI] [PubMed] [Google Scholar]
- [7].Strobel D, Bernatik T, Blank W, et al. Diagnostic accuracy of CEUS in the differential diagnosis of small (</= 20 mm) and subcentimetric (</= 10 mm) focal liver lesions in comparison with histology. Results of the DEGUM multicenter trial. Ultraschall Med 2011;32:593–7. [DOI] [PubMed] [Google Scholar]
- [8].Sugimoto K, Moriyasu F, Shiraishi J, et al. Assessment of arterial hypervascularity of hepatocellular carcinoma: Comparison of contrast-enhanced US and gadoxetate disodium-enhanced MR imaging. Eur Radiol 2012;22:1205–13. [DOI] [PubMed] [Google Scholar]
- [9].Wilson SR, Kim TK, Jang HJ, et al. Enhancement patterns of focal liver masses: discordance between contrast-enhanced sonography and contrast-enhanced CT and MRI. AJR Am J Roentgenol 2007;189:W7–12. [DOI] [PubMed] [Google Scholar]
- [10].Kim TK, Noh SY, Wilson SR, et al. Contrast-enhanced ultrasound (CEUS) liver imaging reporting and data system (LI-RADS) 2017 - a review of important differences compared to the CT/MRI system. Clin Mol Hepatol 2017;23:280–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kim TH, Yoon JH, Lee JM. Emerging role of hepatobiliary magnetic resonance contrast media and contrast-enhanced ultrasound for noninvasive diagnosis of hepatocellular carcinoma: emphasis on recent updates in major guidelines. Korean J Radiol 2019;20:863–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kim H-D, Lim Y-S, Han S, et al. Evaluation of early-stage hepatocellular carcinoma by magnetic resonance imaging with gadoxetic acid detects additional lesions and increases overall survival. Gastroenterology 2015;148:1371–82. [DOI] [PubMed] [Google Scholar]
- [13].Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529–36. [DOI] [PubMed] [Google Scholar]
- [14].Alaboudy A, Inoue T, Hatanaka K, et al. Usefulness of combination of imaging modalities in the diagnosis of hepatocellular carcinoma using Sonazoid(-enhanced ultrasound, gadolinium diethylene-triamine-pentaacetic acid-enhanced magnetic resonance imaging, and contrast-enhanced computed tomography. Oncology 2011;81: (SUPPL. 1): 66–72. [DOI] [PubMed] [Google Scholar]
- [15].Blondin D, Erhardt A, Crynen K, et al. Diagnosis of focal liver lesions in cirrhotic patients: Comparison of contrast-enhanced ultrasound using sulphur hexafluoride (SF6) microbubbles and MRI using Gd-EOB-DTPA. Zeitsch Gastroenterol 2011;49:23–9. [DOI] [PubMed] [Google Scholar]
- [16].Kawada N, Ohkawa K, Tanaka S, et al. Improved diagnosis of well-differentiated hepatocellular carcinoma with gadolinium ethoxybenzyl diethylene triamine pentaacetic acid-enhanced magnetic resonance imaging and Sonazoid contrast-enhanced ultrasonography. Hepatol Res 2010;40:930–6. [DOI] [PubMed] [Google Scholar]
- [17].Leoni S, Piscaglia F, Golfieri R, et al. The impact of vascular and nonvascular findings on the noninvasive diagnosis of small hepatocellular carcinoma based on the EASL and AASLD criteria. Am J Gastroenterol 2010;105:599–609. [DOI] [PubMed] [Google Scholar]
- [18].Mita K, Kim SR, Kudo M, et al. Diagnostic sensitivity of imaging modalities for hepatocellular carcinoma smaller than 2 cm. World J Gastroenterol 2010;16:4187–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Seitz K, Bernatik T, Strobel D, et al. Contrast-enhanced ultrasound (CEUS) for the characterization of focal liver lesions in clinical practice (DEGUM Multicenter Trial): CEUS vs. MRI--a prospective comparison in 269 patients. Ultraschall Med 2010;31:492–9. [DOI] [PubMed] [Google Scholar]
- [20].Sugimoto K, Kim SR, Imoto S, et al. Characteristics of hypovascular versus hypervascular well-differentiated hepatocellular carcinoma smaller than 2 cm - focus on tumor size, markers and imaging detectability. Digest Dis 2015;33:721–7. [DOI] [PubMed] [Google Scholar]
- [21].Zhou Y, Jing X, Zhang X, et al. Combining the arterial phase of contrast-enhanced ultrasonography, gadoxetic acid-enhanced magnetic resonance imaging and diffusion-weighted imaging in the diagnosis of hepatic nodules ≤20 mm in patients with cirrhosis. Ultrasound Med Biol 2019;45:693–701. [DOI] [PubMed] [Google Scholar]
- [22].Schellhaas B, Hammon M, Strobel D, et al. Interobserver and intermodality agreement of standardized algorithms for non-invasive diagnosis of hepatocellular carcinoma in high-risk patients: CEUS-LI-RADS versus MRI-LI-RADS. Eur Radiol 2018;28:4254–64. [DOI] [PubMed] [Google Scholar]
- [23].Beyer LP, Wassermann F, Pregler B, et al. Characterization of focal liver lesions using CEUS and MRI with liver-specific contrast media: experience of a single radiologic center. Ultraschall Med 2017;38:619–25. [DOI] [PubMed] [Google Scholar]
- [24].Iwamoto T, Imai Y, Kogita S, et al. Comparison of contrast-enhanced ultrasound and gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced mri for the diagnosis of macroscopic type of hepatocellular carcinoma. Dig Dis 2016;34:679–86. [DOI] [PubMed] [Google Scholar]
- [25].Sporea I, Sirli R, Martie A, et al. How useful is contrast enhanced ultrasonography for the characterization of focal liver lesions? JGLD 2010;19:393–8. [PubMed] [Google Scholar]
- [26].Huppertz A, Balzer T, Blakeborough A, et al. Improved detection of focal liver lesions at MR imaging: multicenter comparison of gadoxetic acid-enhanced MR images with intraoperative findings. Radiology 2004;230:266–75. [DOI] [PubMed] [Google Scholar]
- [27].Golfieri R, Renzulli M, Lucidi V, et al. Contribution of the hepatobiliary phase of Gd-EOB-DTPA-enhanced MRI to Dynamic MRI in the detection of hypovascular small (</= 2 cm) HCC in cirrhosis. Eur Radiol 2011;21:1233–42. [DOI] [PubMed] [Google Scholar]
- [28].Kudo M. The 2008 Okuda lecture: Management of hepatocellular carcinoma: from surveillance to molecular targeted therapy. J Gastroenterol Hepatol 2010;25:439–52. [DOI] [PubMed] [Google Scholar]
- [29].Kim SR, Mita K, Maekawa Y, et al. Comparison of diagnostic capability of contrast-enhanced CT, sonazoid contrast-enhanced us, Gd-EOB-DTPA MRI and CT arterioportal angiography in detecting histologically proven HCC nodules smaller than 2 cm. J Hepatol 2010;52:S221–2. ((Kim S.R., asahi-hp@arion.ocn.ne.jp; Mita K.; Imoto S.; Nakajima T.; Ando K.; Fukuda K.) Department of Gastroenterology, Kobe Asahi Hospital, Kobe, Japan). [Google Scholar]
- [30].Kobayashi T, Aikata H, Hatooka M, et al. Usefulness of combining gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging and contrast-enhanced ultrasound for diagnosing the macroscopic classification of small hepatocellular carcinoma. European Radiol 2015;25:3272–81. [DOI] [PubMed] [Google Scholar]


