Abstract
Macular edema (ME) is an inflammatory disease characterized by increased microvascular permeability. Here, we proposed that plasma angiopoietin-like protein 2 (ANGPTL2) level may be related to the severity of ME patients with type 2 diabetes mellitus (T2DM). In this cross-sectional study, 172 T2DM patients were recruited and divided into clinically significant macular edema (CSME), non-CSME (nCSME), and control groups. Serum ANGPTL2 level was quantified by ELISA and best corrected vision acuity (BCVA) was detected. After adjust age, sex, body mass index (BMI), and duration of diabetes variables, ANGPTL2 performed statistics difference among CSME-, nCSME-groups, and control group (4.46 [3.97, 4.96, 95%CI] ng/mL in CSME group, 3.80 [3.42, 4.18, 95%CI] ng/mL in nCSME-group, 3.33 [3.03, 3.63, 95%CI] ng/mL in control, P < .01). After adjustment of confounding factors, high levels of circulating ANGPTL2 were related with the diagnosis of ME, BCVA, and C reactive protein (CRP) through univariate regression analysis (P < .05). Meanwhile, in the multiple regression model, ANGPTL2 took the mainly effect proportion for the diagnosis of diabetic macular edema (DME), with a LogWorth value 3.559 (P < .001). Our study suggested that elevated circulating ANGPTL2 may be associated with the development of DME and the severity of visual impairment in patients with type 2 diabetes.
Keywords: angiopoietin-like protein 2, diabetic macular edema, type 2 diabetes mellitus
1. Introduction
Diabetic macular edema (DME) is a major eye complication giving rise to visual loss and blindness.[1,2] With increasing duration of diabetes, retinal complications have probably higher incidence. It has been reported that over 50% in type 1 diabetes and 30% in type 2 diabetes potentially suffered from vision-threatening retinal pathological change.[3]
DME can be classified based on optical coherence tomography (OCT) measurements by detecting the morphology of the retina and thickness of macular. It is characterized as cystic retinal thickening and lipid deposition. In clinical, DME was divided into 2 stages: clinically significant macular edema (CSME) and non-clinically significant macular edema, according to the visual impairment and location of macular edema.[4,5] Most of the patients have already suffered visual impairment at the time of diagnosis; however, patients in the early stages of diabetes often ignore the examination due to lack of visual impairment. Therefore, there is a great clinical significance for early screening and identification of DME.
It has been confirmed that inflammatory cytokines play a key role in the pathogenesis of DME. DME is caused by multiple factors, including vascular endothelial growth factor (VEGF). As a key mediator in the pathogenesis of angiogenesis, the up-regulated VEGF expression subsequently breakdown the blood-retinal barriers (BRB) by damaging tight junctions within retinal endothelial cells.[6] Ishida et al reported that the subtype of VEGF (VEGF164) plays an important role in early diabetic retinopathy.[7] The most significant feature is hard exudates and edema on the macular region caused by accelerated retinal vascular permeability.[8] Angiopoietin-like protein 2 (ANGPTL2) is an adipokine which is abundantly produced in fat tissue, and belongs to the family of angiopoietin-like proteins (ANGPTLs).[9,10] ANGPTL2 is a secreted cytokine, which contributes to tissue homeostasis by enhancing tissue remodeling in an autocrine or paracrine manner.[9,11] It is required not only in the regulation of angiogenesis, but also in the glucose and lipid metabolism.[9] In addition, excess ANGPTL2 secretion promotes chronic inflammation and endothelial dysfunction, and results irreversible tissue remodeling subsequently. Recently, the role of ANGPTL2 draws attention to the public on clinical practice. ANGPTL2 leads to various metabolic diseases, such as obesity, metabolic syndrome, type 2 diabetes mellitus (T2DM), and atherosclerotic vascular disease.[9,12,13] Toyono et al demonstrated a significant reduction in the expression of the F4/80 and IL-1b in the corneas of ANGPTL2 knockout mice, provided evidence that ANGPTL2 is up-regulated in corneal inflammation.[14] ANGPTL2 levels have been reported to be associated with a higher risk of developing type 2 diabetes mellitus.[15,16]
To date, few studies have focus on the link between ANGPTL2 levels and macular function in type 2 diabetic patients. Our hypothesis is that circulating ANGPTL2 levels may be associated with the onset and severity of macular edema. The aim of our study was to investigate the expression of circulating ANGPTL2 and the relationship between circulating ANGPTL2 levels and diabetic macular edema in patients with T2DM.
2. Subjects and methods
2.1. Patient recruitment
In our research, all procedures complied with the Helsinki Declaration for investigation of human subjects. They received ethical approval from the competent Institutional Review Boards of Beijing Luhe Hospital.
We conducted a cross-sectional study in type 2 diabetes patients. Patients were recruited from the inpatient department of endocrinology in Beijing Luhe Hospital. The major inclusion criteria were: women or men with T2DM; 7% ≤ hemoglobin A1c (HbA1c) ≤ 11%; patients without DME or untreated DME in previous history; subjects with any history or active treatment of cancer, pregnancy, cognitive inability adjusted by the interviewer, any serious medical condition, language barrier were excluded. In addition, patients with other specific types of diabetes and patients with gestational diabetes mellitus (GDM) or diagnosed with severe proliferative diabetic retinopathy (PDR) combined hemorrhage were also excluded. Demographic data were collected from 221 patients were collected their demographic data. Finally, after a match of sex, age, and body mass index (BMI), a total of 172 subjects were enrolled in our study. Then the ophthalmological examination, OCT examination as well as the whole clinical data and biological test collection were successfully conducted and collected.
2.2. Population grouping
According to the data of retinal examination, OCT and other covariates from 172 patients, we divided the subjects into 3 groups:
-
1)
CSME group: T2DM patients with diagnosis of clinically significant macular edema;
-
2)
nCSME group: T2DM patients with macular edema (ME) but not reach the diagnosis of CSME;
-
3)
DM group (control): T2DM patients without DME.
This study design was approved by the Beijing Luhe Hospital ethics committee and all participants were written informed consent.
2.3. Diagnosis and eye examination, retinal photography and OCT
DME classification (CSME and nCSME) was conducted on a senior ophthalmological report based on the OCT. Retinal photography was performed using a non-mydriatic fundus camera (Topcon, Tokyo, Japan). DME was defined as the presence of any retinal thickening or hard exudate at the posterior pole irrespective of center involvement or ischemic characteristics via OCT. The worst-injury eye should be defined according to the diagnostic criteria of DME. Central retinal thickness (CRT) was measured on an En-Face macular map 20° × 20° (5.90 mm × 5.90 mm) (Cirrus HD-OCT model 400); best corrected visual acuity (BCVA) was judged via Early Treatment Diabetic Retinopathy Study (ETDRS) protocol (logMAR 1.0, Snellen 20/200).
2.4. Clinical information and laboratory analysis
We collected epidemiological data, which including age, gender, systolic and diastolic blood pressure, BMI, the duration of diabetes and hypertension. Drug histories in relation to anti-diabetes and lipid-lowering drug (statin) and history of smoking were also evaluated. Systolic and diastolic blood pressures were measured twice at rest. Blood samples were obtained from each subject following at least 10 hours fast. Plasma were centrifuged at 3000 for 15 minutes at 4°C, aliquot, and stored at −80°C until assayed. BMI was the weight (kg) divided by the square of the height (m). The other data, such as fasting plasma total cholesterol (CHO), triglyceride (TG), high-density lipoprotein (HDL), low-density lipoprotein (LDL), serum creatinine (SCr), HbA1c, and C reactive protein (CRP) were collected according to the standard protocol.
2.5. Plasma ANGPTL2 level measurement
Plasma ANGPTL2 was determined by enzyme-linked immunosorbent assay (ELISA, IBL). The sensitivity for ANGPTL2 was 0.1 ng/mL, and the intra-assay coefficients of variation was 3.9% to 5.9%, the inter-assay was 6.3% to 10.5%.
2.6. Statistical analysis
For database management and statistical analysis, we used the JMP, version 13.0. The data for continuous variables are presented as means ± standard deviation, and the data for categorical variables are presented as percentage. For continuous variables consistent with normal distribution, differences in demographic characteristics and relevant factors between independent groups were calculated by the Student's t test and one-way analysis of variance (ANOVA). For skewness distribution, Tukey-Kramer HSD and Wilcoxon test were used for continuous variables. For categorical variables, χ2 test was performed. The correlation between plasma ANGPTL2 and various parameters, including BMI, fasting glucose, TG, CHO, and serum uric acid (UA) were evaluated by multiple regression analysis. A generalized linear model was used in multiple regression. Logarithmic (log) transformation of values showing a skewed distribution was carried out before the regression analysis. Both univariate and multivariate logistic regression analyses were performed to determine risk factors for the presence of DME. For multivariate logistic analysis, variables which may influence the disease we studied were further adjusted from the univariate logistic regression analysis. All statistical significance was considered as P < .05.
3. Results
3.1. Demographic data in the study
After exclusion, the criteria and matching the sex and age between groups, the study included 172 subjects finally. The population consisted in 48 (27.9%) patients in CSME group, 55 (32.0%) in nCSME group, and 69 (40.1%) in DM group. In addition, male accounts for 44.8% (77/172) of the whole population. The characteristics of the 3 groups were summarized in Table 1. Our results showed that ANGPTL2 exhibited significant statistical differences among CSME-, nCSME-, DM-groups (4.46 [3.97, 4.96, 95%CI] ng/mL in CSME group, 3.80 [3.42, 4.18, 95%CI] ng/mL in nCSME-group, 3.33 [3.03, 3.63, 95%CI] ng/mL in control, P < .01). Values of fasting glucose, duration of diabetes, and CRP showed significant differences among the 3 groups (P < .05). Also, there were significant differences in BCVA between the 3 groups (P < .05).
Table 1.
The characteristics of the 3 study groups.
| DME (N = 103) | ||||
| DM (N = 69) | nCSME (N = 55) | CSME (N = 48) | P | |
| Number in category (%) | ||||
| Female | 37 (53.62%) | 28 (50.91%) | 30 (62.50%) | >.05 |
| Smoker | 24 (34.78%) | 17 (30.91%) | 34 (70.83%) | >.05 |
| History of hyperlipidemia | 33 (47.83%) | 32 (58.18%) | 33 (68.75%) | >.05 |
| Statins use | 17 (24.63%) | 15 (27.27%) | 13 (27.08%) | >.05 |
| Insulin use | 35 (50.72%) | 27 (49.09%) | 22 (45.83%) | >.05 |
| Mean (SD) of characteristic | ||||
| Age (yr) | 61.02 ± 10.22 | 62.27 ± 10.03 | 60.03 ± 11.29 | >.05 |
| Duration of diabetes | 10.72 ± 7.79 | 12.21 ± 7.36 | 8.17 ± 6.64 | .0078∗ |
| Body mass index (kg/m2) | 25.13 ± 5.30 | 26.30 ± 3.69 | 25.65 ± 3.15 | >.05 |
| Systolic blood pressure (mm Hg) | 130 ± 18.92 | 132 ± 17.27 | 129 ± 19.86 | >.05 |
| Diastolic blood pressure (mm Hg) | 75 ± 12.203 | 77 ± 10.21 | 75 ± 12.91 | >.05 |
| BCVA | 0.61 ± 0.13 | 0.50 ± 0.19 | 0.29 ± 0.17 | .0305∗ |
| CRT (μm) | 284.02 ± 42.80 | 296.26 ± 53.19 | 307.22 ± 63.47 | .015∗ |
| Biochemical data, mean (SD/range/95%CI) | ||||
| Total cholesterol (mmol/L) | 4.13 ± 1.13 | 4.27 ± 1.11 | 4.34 ± 1.04 | >.05 |
| LDL cholesterol (mmol/L) | 2.66 ± 0.93 | 2.72 ± 0.93 | 2.68 ± 0.85 | >.05 |
| HDL cholesterol (mmol/L) | 1.01 ± 0.26 | 1.06 ± 0.24 | 1.03 ± 0.22 | >.05 |
| Serum triglyceride (mmol/L) | 1.66 ± 1.07 | 1.53 ± 0.93 | 1.65 ± 1.22 | >.05 |
| Fasting glucose (mmol/L) | 7.47 (5.3–9.58) | 7.29 (5.37–9.06) | 7.01 (5.80–9.09) | .0237∗ |
| HbA1C (%) | 9.53 ± 2.65 | 9.08 ± 2.18 | 9.23 ± 2.28 | >.05 |
| Fasting C-peptide (ng/mL) | 1.57 (1.18,1.97) | 1.68 (1.35,1.99) | 1.71 (1.48,1.93) | >.05 |
| Serum creatinine (μmol/L) | 66.45 ± 21.95 | 66.09 ± 23.84 | 73.20 ± 19.94 | >.05 |
| Serum urea nitrogen (mmol/L) | 5.88 ± 2.29 | 5.38 ± 1.72 | 6.38 ± 2.21 | >.05 |
| Vascular and inflammatory biomarkers, mean (range/SD) | ||||
| C-reactive protein (μg/L) | 8.72 ± 1.93 | 6.47 ± 1.91 | 6.07 ± 1.78 | .0315∗ |
| ANGPTL2 (ng/mL) | 3.33 (3.03, 3.63)a | 3.80 (3.42, 4.18)b | 4.46 (3.97, 4.96)c | .0002∗ |
Data were shown as number in category in female, smoker, history of hyperlipidemia, statins, and used mean ± SD, or 95% confidence interval (95% CI) for other index. ANGPTL2, angiopoietin-like protein 2; BCVA, best corrected visual acuity; CRP, C-reactive protein; CSME, clinically significant macular edema; DME, diabetic macular edema; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
P = .0278 (DM vs nCSME).
P = .0418 (nCSME vs CSME).
P < .0001 (DM vs CSME).
Represents the statistical difference between the three groups.
3.2. Multiple regression analysis of ANGPTL2
To evaluate the potential association of circulating ANGPTL2 level with diabetic macular edema related index, we performed multiple regression analysis. By calculating of regression coefficients and P values revealed that circulating ANGPTL2 levels were correlated with DME (P < .01), and CRP (P < .05), shown in Figure 1. Also, there were linear correlation between ANGPTL2 and BCVA in the whole population (P < .05), shown in Figure 2.
Figure 1.
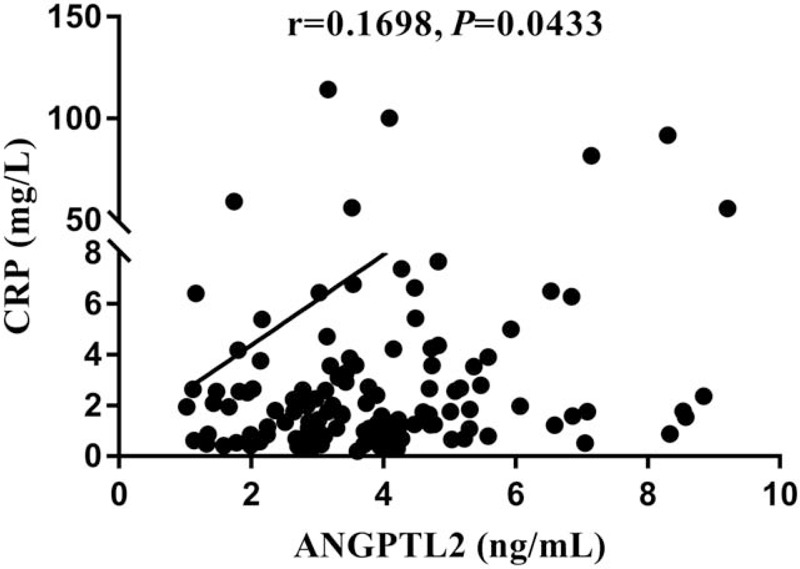
The correlation between CRP with ANGPTL2 in plasma. ANGPTL2 was positively associated with CRP in the general population.
Figure 2.
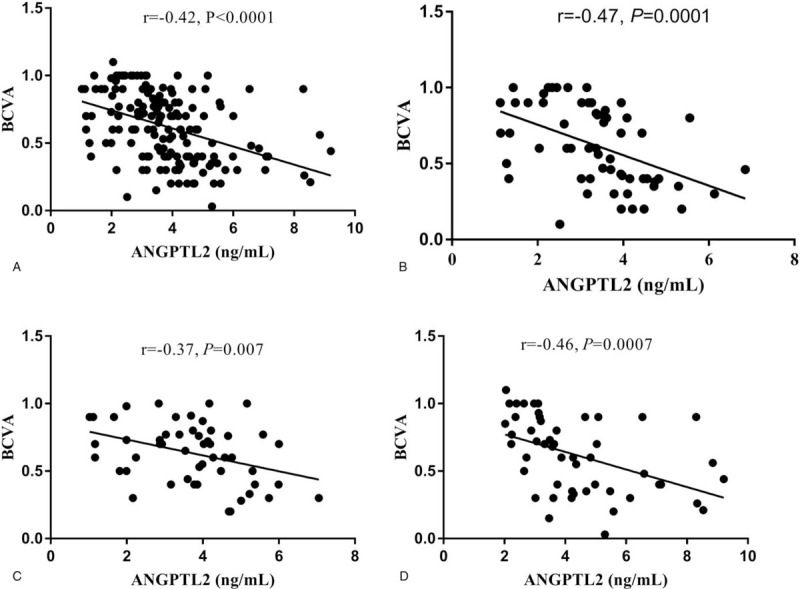
The correlation between BCVA with ANGPTL2 in plasma. (A) ANGPTL2 was negatively correlated with BCVA in the general population. (B–D) ANGPTL2 was negatively correlated with BCVA according to BCVA level in DM-, nCSME-, CSME-groups, respectively.
By contrast, circulating ANGPTL2 levels were not relative to duration of hypertension, UA, sex, age, history of smoking (P > .05). And after fully adjustment of age, sex, BMI, and duration of DM, ANGPTL2 performed statistics difference among 3 groups.
3.3. ANGPTL2 level is an independent variable in DME patients
We next examined the relationship between the development of DME and circulating ANGPTL2 levels and other clinical variables. We carried on a multiple logistic model including ANGPTL2 and index which P value below .1 from univariate logistic regression or clinical practice. Multivariate logistic regression analyses included ANGPTL2, BCVA, sex, age, history of hypertension years, duration of diabetes, history of smoking, UA. LogWorth showed the size of the effect on diagnosing of DME via JMP. In the regression model, ANGPTL2 took the mainly effect proportion for the diagnosis of DME, with the LogWorth value was 3.559 (P < .001), which shown in Table 2.
Table 2.
Multiple logistic regression with diagnosed DME or not.
| LogWorth | P | |
| ANGPTL2 | 3.559 | .00028 |
| BCVA | 1.288 | .0326 |
| Age | 0.734 | .18446 |
| UA | 0.533 | .29279 |
| Sex | 0.377 | .41961 |
| DMY | 0.102 | .79149 |
| HTNY | 0.066 | .85945 |
| Smoke | 0.011 | .97449 |
Variables with P < .1 were put into multiple logistic regression, and LogWorth showed the size of the effect of diagnosing of DME. ANGPTL2, angiopoietin-like protein 2; BCVA, best corrected visual acuity; DME, diabetic macular edema; DMY, duration of DM; HTNY, duration of hypertension; smoke, history of smoking; UA, uric acid.
4. Discussion
In the current study, we investigated the relationship between circulating ANGPTL2 and the development of macular edema in patients with T2DM. The results showed a significant association between increased ANGPTL2 and the occurrence of DME after fully adjustment of age, sex, BMI, duration of DM. Moreover, with the aggravation of macular edema, ANGPTL2 showed an increasing trend from nCSME group to CSME group. In the research, we also found the negative correlation between high level of ANGPTL2 and injured BCVA.
In the cross-sectional study we found that, first of all, there was an upward trend of circulating ANGPTL2 in T2DM patients with DME comparing with the control. ANGPTL2 is an adipose tissue-derived secretory glycoprotein. Various studies showed that ANGPTL2 plays a role in metabolic syndrome,[10] angiogenesis,[17] and inflammation.[18] In humans, ANGPTL2 concentration in circulation is also up-regulated in obesity (particularly in visceral obesity) and correlated with the levels of systemic insulin resistance (IR) and inflammation.[10] Our clinical results also found a weak positive correlation between CRP and ANGPTL2, indicating inflammation may be the link for connecting ANGPTL2 with DME. Circulating ANGPTL2 is reported closely associated with CRP in obesity,[10] diabetes,[19] heart failure, and in general population.[20,21] Elevated serum ANGPTL2 levels were positively associated with the development of T2DM in a general population, independent of hs-CRP levels.[22] In retinal disease, ANGPTL2 had been suggested to regulate the inflammatory neovascularization in murine model of age-related macular degeneration.[23] Our research provided that ANGPTL2 has a proinflammatory effect on the development and progress of DME from clinical perspective.
For further sub-group analysis, we found that higher level of ANGPTL2 might distinguish CSME from nCSME patients. The CSME patients had worse eyesight and higher ANGPTL2 level. In CSME group, edema occurred at or near the fovea of the macular region, so the degree of visual impairment was higher than that in nCSME group. ANGTPL2 was negatively correlated with BCVA in our study. The pathogenesis of DME is retinal arteriole leakage, and ANGPTL2 is a pro-permeability cytokine which may take role in the progress of DME and damage the eyesight.
Via multivariate logistic regression analyses, we confirmed that ANGPTL2 is an independent risk factor for the development of DME by setting a fit model with sex, age, history of hypertension years, duration of diabetes, history of smoking, UA adjustment. To date, there have been few studies established the relationship between ANGPTL2 and the presence of DME. We found that ANGPTL2 accounted for the main effect size on diagnosis of DME. However, our data are not enough to indicate whether ANGPTL2 can be a specific biomarker for diagnosing of DME, but at least suggest that higher levels of ANGPTL2 in clinical practice may be a risk factor for occurrence or progression of DME. Our study maybe supplied a new sight for the inflammatory cytokines in the development of DME.
We also analyzed some factors correlated with ANGPTL2. Although inflammation plays crucial roles in progression of metabolic diseases such as impaired glycol-metabolism and obesity, a positive correlation between CRP and circulating ANGPTL2 remained statistically significant after adjustment for variables including sex, age, BMI, fasting glucose, and duration of DM, demonstrating that circulating ANGPTL2 is independently associated with inflammation in the course of macular edema. Inflammation plays a key role in the mechanism in the development of DME.[24,25]
Our current study has some potential limitations. Firstly, there was a cross-sectional study, whereas longitudinal studies are required to answer the question as to whether ANGPTL2 levels could predict the outcome of DME over years. Secondly, we could not determine the expression level of ANGPTL2 proteins in aqueous fluid, or vitreous fluid in these patients. Furthermore, other factors such as adipokines or hepatokines, hormones, or other parameters, which were not examined might also affect ANGPTL2. In addition, the sample size was not large. Based on these limitations, more large-scale population studies are needed to understand the relationship between ANGPTL2 and the presence of DME. For further study, enlarged sample size will be considered.
5. Conclusions
In conclusion, our data demonstrate higher level of circulating ANGPTL2 may be associated with macular edema in patients with type 2 diabetes.
Acknowledgments
We gratefully thank Prof. Xiaojiang Quan for her assistance in language modification of this article.
Author contributions
Ning Zhang and Dong Zhao generated the random allocation sequence, Dawei Zhang and Wenying Zhao enrolled participants, and Ruili Yin and Jing Ke assigned participants to interventions.
Conceptualization: Dong Zhao.
Data curation: Ruili Yin, Ning Zhang.
Formal analysis: Ruili Yin.
Funding acquisition: Dong Zhao.
Methodology: Ning Zhang, Dawei Zhang.
Supervision: Dawei Zhang, Wenying Zhao, Jing Ke.
Writing – original draft: Ruili Yin.
Writing – review & editing: Dong Zhao, Jing Ke.
Glossary
Abbreviations: ANGPTL2 = angiopoietin-like protein 2, BMI = body mass index, BRB = blood-retinal barriers, CHO = total cholesterol, CRP = C reactive protein, CRT = central retinal thickness, CSME = clinically significant macular edema, DME = diabetic macular edema, GDM = gestational diabetes mellitus, HDL = high-density lipoprotein, LDL = low-density lipoprotein, ME = macular edema, OCT = optical coherence tomography, SCr = serum creatinine, T2DM = type 2 diabetes mellitus, TG = triglyceride, UA = uric acid, VEGF = vascular endothelial growth factor.
References
- [1].Klein R, Klein BE, Moss SE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. XV. The long-term incidence of macular edema. Ophthalmology 1995;102:7–16. [DOI] [PubMed] [Google Scholar]
- [2].Browning DJ, Stewart MW, Lee C. Diabetic macular edema: evidence-based management. Indian J Ophthalmol 2018;66:1736–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Nentwich MM, Ulbig MW. Diabetic retinopathy – ocular complications of diabetes mellitus. World J Diabetes 2015;6:489–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Browning DJ, McOwen MD, Bowen RM, Jr, et al. Comparison of the clinical diagnosis of diabetic macular edema with diagnosis by optical coherence tomography. Ophthalmology 2004;111:712–5. [DOI] [PubMed] [Google Scholar]
- [5].Brown JC, Solomon SD, Bressler SB, et al. Detection of diabetic foveal edema: contact lens biomicroscopy compared with optical coherence tomography. Arch Ophthalmol 2004;122:330–5. [DOI] [PubMed] [Google Scholar]
- [6].Theodossiadis PG, Theodoropoulou S, Neamonitou G, et al. Hemodialysis-induced alterations in macular thickness measured by optical coherence tomography in diabetic patients with end-stage renal disease. Ophthalmologica 2012;227:90–4. [DOI] [PubMed] [Google Scholar]
- [7].Ishida S, Usui T, Yamashiro K, et al. VEGF164 is proinflammatory in the diabetic retina. Invest Ophthalmol Vis Sci 2003;44:2155–62. [DOI] [PubMed] [Google Scholar]
- [8].Campochiaro PA, Hafiz G, Mir TA, et al. Pro-permeability factors in diabetic macular edema; the diabetic macular edema treated with ozurdex trial. Am J Ophthalmol 2016;168:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kadomatsu T, Endo M, Miyata K, et al. Diverse roles of ANGPTL2 in physiology and pathophysiology. Trends Endocrinol Metab 2014;25:245–54. [DOI] [PubMed] [Google Scholar]
- [10].Tabata M, Kadomatsu T, Fukuhara S, et al. Angiopoietin-like protein 2 promotes chronic adipose tissue inflammation and obesity-related systemic insulin resistance. Cell Metab 2009;10:178–88. [DOI] [PubMed] [Google Scholar]
- [11].Okada T, Tsukano H, Endo M, et al. Synoviocyte-derived angiopoietin-like protein 2 contributes to synovial chronic inflammation in rheumatoid arthritis. Am J Pathol 2010;176:2309–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tian Z, Miyata K, Kadomatsu T, et al. ANGPTL2 activity in cardiac pathologies accelerates heart failure by perturbing cardiac function and energy metabolism. Nat Commun 2016;7:13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Huang CL, Wu YW, Wu CC, et al. Serum angiopoietin-like protein 2 concentrations are independently associated with heart failure. PLoS One 2015;10:e0138678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Toyono T, Usui T, Yokoo S, et al. Angiopoietin-like protein 2 is a potent hemangiogenic and lymphangiogenic factor in corneal inflammation. Invest Ophthalmol Vis Sci 2013;54:4278–85. [DOI] [PubMed] [Google Scholar]
- [15].El-Lebedy D. Association of serum angiopoietin-like protein 2 with elevated risk of cardiovascular diseases in subjects with type 2 diabetes. J Diabetes Complications 2019;33:107421. [DOI] [PubMed] [Google Scholar]
- [16].Yoshinaga T, Niou T, Niihara T, et al. Angiopoietin-like protein 2 is a useful biomarker for pancreatic cancer that is associated with type 2 diabetes mellitus and inflammation. J Cancer 2018;9:4736–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Oike Y, Yasunaga K, Suda T. Angiopoietin-related/angiopoietin-like proteins regulate angiogenesis. Int J Hematol 2004;80:21–8. [DOI] [PubMed] [Google Scholar]
- [18].Lee HJ, Kim JH, Kim JH, et al. Angiopoietin-like protein 2, a chronic inflammatory mediator, is a new target induced by TGF-beta1 through a Smad3-dependent mechanism. Biochem Biophys Res Commun 2013;430:981–6. [DOI] [PubMed] [Google Scholar]
- [19].Jung CH, Lee WJ, Lee MJ, et al. Association of serum angiopoietin-like protein 2 with carotid intima-media thickness in subjects with type 2 diabetes. Cardiovasc Diabetol 2015;14:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Usui T, Ninomiya T, Nagata M, et al. Angiopoietin-like protein 2 is associated with chronic kidney disease in a general Japanese population: the Hisayama Study. Circ J 2013;77:2311–7. [DOI] [PubMed] [Google Scholar]
- [21].Hata J, Mukai N, Nagata M, et al. Serum angiopoietin-like protein 2 is a novel risk factor for cardiovascular disease in the community: the Hisayama study. Arterioscler Thromb Vasc Biol 2016;36:1686–91. [DOI] [PubMed] [Google Scholar]
- [22].Doi Y, Ninomiya T, Hirakawa Y, et al. Angiopoietin-like protein 2 and risk of type 2 diabetes in a general Japanese population: the Hisayama study. Diabetes Care 2013;36:98–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hirasawa M, Takubo K, Osada H, et al. Angiopoietin-like protein 2 is a multistep regulator of inflammatory neovascularization in a murine model of age-related macular degeneration. J Biol Chem 2016;291:7373–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cabral T, Mello LGM, Lima LH, et al. Retinal and choroidal angiogenesis: a review of new targets. Int J Retina Vitreous 2017;3:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ioanna Z, Christian S, Christian G, et al. Plasma levels of hypoxia-regulated factors in patients with age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 2018;256:325–32. [DOI] [PMC free article] [PubMed] [Google Scholar]


