Abstract
INTRODUCTION:
Patients with elderly-onset inflammatory bowel disease were previously associated with a less aggressive course of the disease. However, there are conflicting data that need further validation. We aimed to determine the association between age at diagnosis and the development of progressive disease in patients with Crohn's disease (CD) and ulcerative colitis (UC).
METHODS:
This cohort study included patients with CD and UC followed in 6 secondary and tertiary care centers in mainland Portugal. Patients were divided into a derivation (80%) cohort and a validation (20%) cohort. The primary outcome was progressive disease. Logistic regression analysis, receiver operating characteristic curves, and the areas under the curve (AUC) were performed. Odds ratios with 95% confidence intervals (CIs) were estimated.
RESULTS:
The derivation cohorts included 1245 patients with CD (68% with progressive disease) and 1210 patients with UC (37% with progressive disease), whereas the validation cohorts included 302 patients with CD and 271 patients with UC, respectively, with similar outcome proportions. In our final model, age at diagnosis older than 60 years was significantly associated with a lower risk of developing progressive disease (odds ratio 0.390, 95% CI 0.164–0.923, P = 0.032), with a high discriminative power (AUC 0.724, 95% CI 0.693–754) in patients with CD. However, according to this model, no significant associations were found between age at diagnosis and the risk of developing progressive disease in patients with UC. No differences were observed in the AUC values between the validation and the derivation cohorts.
DISCUSSION:
Patients with elderly-onset CD, but not patients with UC, were associated with a less progressive course of the disease.
INTRODUCTION
Inflammatory bowel disease (IBD) includes Crohn's disease (CD) and ulcerative colitis (UC) and is characterized by chronic inflammation of the gut, with periods of disease remission and relapse (1,2). Several factors are believed to contribute to the etiology and phenotype of IBD, including genetic, immunological and environmental factors, and the gut microbiota composition (3,4).
The prognosis of CD and UC was already associated with several factors, such as the requirement for steroids at diagnosis (5–7), complicated disease behavior (6–8), and perianal disease (5–7,9) for patients with CD and male sex (6,10–12), extensive disease (6,11–13), and steroid dependency or refractoriness for patients with UC (6,11). A younger age at diagnosis was also reported as a prognostic factor of a more aggressive course of the disease in both patients with CD and UC (5,8,14–17). However, although the Montreal classification underlies the importance of age at diagnosis in CD prognosis, defining 3 age groups (<17; 17–40; and >40 years), only the extension of the disease is considered a relevant prognostic factor for patients with UC (18). Recently, other authors highlighted the importance of age at diagnosis in UC prognosis (19,20) and the European Crohn's and Colitis Organization guidelines reflect the relevance of age at diagnosis as a prognostic factor (1).
The process of aging is associated with several physiological changes, including alterations in the gut microbiota composition and functionality (21–23)—dysbiosis—which are associated with IBD (3,24). Some studies support that patients with a late onset of IBD (generally ≥60 years, i.e., elderly-onset IBD) (4,25,26) present different pathophysiology and clinical features compared with younger onset. For instance, patients with elderly-onset IBD were associated with fewer signs and symptoms and with a less aggressive course of the disease (14–17). However, other studies reported conflicting results because a milder course of the disease was not observed in patients with elderly-onset IBD compared with patients with younger-onset IBD (16,27–29).
The concept of progressive course of the disease, originally termed disabling disease (5,7), designates a compound outcome that includes several clinical manifestations and has been previously used as an indicator of an aggressive IBD course (9,11,30,31). Specifically, regarding this outcome, some studies reported that patients with CD with an age at diagnosis younger than 40 years have an increased risk of developing progressive disease (5,32,33). For patients with UC, besides extensive disease (E3) and male sex, age at diagnosis younger than or equal to 40 years was also associated with a higher probability of developing this outcome, compared with patients diagnosed at ages older than 40 years (11). However, the effect of age at diagnosis in the course of IBD is still controversial (16,27–29,34).
In this study, we aimed to determine the association between age at diagnosis and the development of progressive disease in patients with CD and UC.
METHODS
Study design and derivation and validation cohorts
This multicentric cohort study included patients with CD and UC, followed in 6 IBD-dedicated consultations in secondary and tertiary care centers in mainland Portugal. Both outpatients and hospitalized patients were included.
Data were collected from all patients with CD included in GEDII—Portuguese Inflammatory Bowel Disease Study Group database (35)—a nationwide database where data from Portuguese patients with IBD are prospectively collected—until May 2016. The patients who met the following criteria were included: (i) had a definitive diagnosis of CD, (ii) had at least 3 years of follow-up, (iii) had at least 1 appointment with one of the study's investigators after 2010, and (iv) had performed abdominal x-ray, computed tomography, or a magnetic resonance imaging during the follow-up and/or a colonoscopy. Capsule endoscopy or double balloon endoscopy were performed when considered relevant by the clinician. We did not consider them inclusion criteria for this study because they are only relevant for the diagnosis of some patients with CD.
Regarding patients with UC, data were collected from all patients included in GEDII database (35) until April 2017, who met the following criteria: (i) had a definitive diagnosis of UC, (ii) had at least 3 years of follow-up, (iii) had at least 1 appointment with one of the study's investigators after 2010, and (iv) had at least 1 endoscopic (colonoscopy or sigmoidoscopy) with or without histological examination during the follow-up. The diagnosis of IBD was based on the European Crohn's and Colitis Organization criteria (1,36,37).
The cohorts of patients with CD and UC were randomly divided into 2 groups using IBM SPSS software version 24.0: the derivation cohort, comprising around 80% of patients, and the validation cohort, comprising the remaining 20% of patients.
The study was monitored by the national coordinator of the GEDII (Sandra Dias) and approved by the Portuguese National Data Protection Authority. This study was conducted according to the principles expressed in the Declaration of Helsinki.
Demographic and clinical variables
Demographic and clinical data were collected for each patient with CD and UC from the GEDII database (35). The main data analysis was performed based on the data collected during the worst situation faced by each patient during the follow-up time. All patients' medical records and clinical data, as well as missing or inconsistent data, were reviewed and collected by the study investigators and the GEDII national coordinator.
For both patients with CD and UC, the collected data included age at diagnosis, sex, the Montreal classification (18), and follow-up time after diagnosis. Regarding the Montreal classification (18), patients with CD were classified according to disease location and behavior, including perianal disease, and patients with UC were classified according to disease extension.
For both cohorts, follow-up data included the total number of surgeries (abdominal and perianal for patients with CD and colectomy for patients with UC) and IBD-related hospitalizations (i.e., hospital stay for more than 24 h), pharmacological therapy with steroids, immunomodulators or biologics (anti-tumor necrosis factor [TNF] drugs), steroid dependency or refractoriness, and the occurrence of IBD-related adverse events. For patients with CD, the occurrence of disease adverse events after the index episode (i.e., first disease episode) included stenosis, penetrating disease, or anal disease. For patients with UC, the occurrence of disease adverse events after the index episode included fibrous or mucosal bridges, stenosis, pseudopolyps (risk of cancer) (38), lead pipe or shortening, or haustral markings.
Steroid dependency and refractoriness were defined according to Van Assche and colleagues (39).
Outcomes
The primary outcome was the occurrence of progressive disease. The definitions of progressive disease are in accordance with our previous publications (9,11).
For patients with CD, progressive disease was defined as the occurrence of at least one of the following events: 1 or more surgeries in the first 5 years after diagnosis (excluding the index surgery, if applicable), more than 1 surgery during follow-up (excluding the index surgery, if applicable), more than 2 hospitalizations (excluding the index hospitalization and hospitalization for infliximab infusion), at least 2 steroid course requirements per year, steroid dependency or refractoriness, need to switch immunomodulators (azathioprine or methotrexate) or biologics (anti-TNF drugs, infliximab, or adalimumab), and occurrence of new events after the index episode including stenosis, penetrating disease, or anal disease.
For patients with UC, progressive disease was defined as the occurrence of at least one of the following events: 2 or more hospitalizations, at least 2 steroid course requirements per year, steroid dependency or refractoriness, need to switch the immunomodulators (azathioprine or methotrexate) or the biologics (anti-TNF drugs, infliximab, or adalimumab), need to add immunomodulators to anti-TNF therapy, occurrence of fibrous or mucosal bridges, stenosis, pseudopolyps (risk of cancer) (38), lead pipe or shortening or haustral markings, and colectomy.
For both patients with CD and UC, the need to switch was defined as loss of response or primary nonresponse. Other reasons such as intolerance were not considered.
The introduction of anti-TNF or immunomodulators per se was not considered progressive disease in both CD and UC.
Statistical analysis
Categorical variables were described by absolute (n) and relative (%) frequencies and continuous variables were described by the median and interquartile range.
To assess the association between age at diagnosis and the development of progressive disease, logistic regression analyses were developed for the derivation cohorts of patients with CD and UC, followed by receiver operating characteristic (ROC) curves and the areas under the curve (AUC) with 95% confidence intervals (CIs) to determine the discriminative power of each model. Age at diagnosis was used as a continuous variable and was also stratified into the age groups younger than 18, 18–30, 31–50, 51–60, and older than 60 years. Four models were built with different independent variables: model 1, including age at diagnosis (continuous variable); model 2, including age at diagnosis (continuous variable), location of disease, behavior of disease, presence of perianal disease, and follow-up time for patients with CD or age at diagnosis (continuous variable), disease extent, and follow-up time for UC patients to increase the robustness of the first model; model 3,including age at diagnosis (categorical variable) and follow-up time; and model 4, including age at diagnosis (categorical variable), location of disease, behaviour of disease, presence of perianal disease and follow-up time for patients with CD or age at diagnosis (categorical variable), disease extent, and follow-up time for UC patients to increase the robustness of the third model. To further validate our results, the model 4 was additionally performed for the age groups <17, 17–40, 41–60, and >60 years. The results were expressed as odds ratios (ORs) with 95% CI.
The final logistic regression model for CD and UC was validated by performing ROC analyses and AUCs for the validation cohorts using the same variables. The AUCs obtained for the validation cohorts were compared with those obtained with the derivation cohorts.
To address the association between the requirement of steroids at diagnosis and progressive disease, χ2 tests were performed.
To model time until progressive disease in patients with CD, survival curves showing progressive disease-free probability were generated with the Kaplan-Meier method. The survival curves by age groups were compared using the logrank test. Cox regression was also performed and age at diagnosis, location of disease, behavior of disease, presence of perianal disease, and follow-up time used as independent factors.
Statistical significance was considered at P < 0.05. All statistical analyses were performed with IBM SPSS version 24.0.
RESULTS
Demographic and clinical characteristics of the cohorts of patients with IBD
The analyzed cohorts of patients with CD and UC are described in Tables 1 and 2, respectively. The derivation cohort of patients with CD included 1,245 subjects, of which 15% were aged younger than 18 years, 45% were aged between 18 and 30 years, 33% were aged between 31 and 50 years, 5% were aged between 51 and 60 years, and 2% were aged older than 60 years at diagnosis. Furthermore, 54% of patients with CD were women, 44% presented ileal location of disease, 46% presented nonstricturing/nonpenetrating disease, and 26% presented perianal disease. Progressive disease was found in 68% of patients and the median follow-up time was 12 (7–18) years for patients with CD from the derivation cohort (Table 1).
Table 1.
Baseline characteristics and comparison between the derivation and the validation cohorts of patients with Crohn's disease
| Derivation (n = 1,245) | Validation (n = 302) | Pa | |
| Age at diagnosis, yr, n (%) | 0.740 | ||
| <18 | 183 (15) | 45 (15) | |
| 18–30 | 562 (45) | 141 (47) | |
| 31–50 | 410 (33) | 89 (29) | |
| 51–60 | 60 (5) | 18 (6) | |
| >60 | 30 (2) | 9 (3) | |
| Gender, n (%) | 0.409 | ||
| Female | 668 (54) | 179 (56) | |
| Male | 577 (46) | 132 (44) | |
| Location, n (%) | 0.246 | ||
| L1 | 542 (44) | 120 (40) | |
| L2 | 200 (16) | 44 (15) | |
| L3 | 503 (40) | 138 (46) | |
| Upper tract involvement (L4), n (%) | 152 (12) | 27 (9) | 0.111 |
| Behaviour, n (%) | 0.431 | ||
| B1 | 572 (46) | 146 (48) | |
| B2 | 308 (25) | 64 (21) | |
| B3 | 365 (29) | 92 (30) | |
| Perianal disease, n (%) | 327 (26) | 80 (26) | 0.937 |
| Progressive disease, n (%) | 849 (68) | 206 (68) | 0.995 |
| Follow-up, median (IQR), yr | 12 (7–18) | 11 (7–18) | 0.499b |
B1, nonstricturing/nonpenetrating; B2, stricturing; B3, penetrating; IQR, interquartile range; L1, ileal; L2, colonic; L3, ileocolonic; n, number of patients.
P values are for the χ2 test unless otherwise indicated.
P values are for Mann-Whitney test.
Table 2.
Baseline characteristics and comparison between the derivation and the validation cohorts of ulcerative colitis patients
| Derivation (n = 1,210) | Validation (n = 271) | Pa | |
| Age at diagnosis, yr, n (%) | 0.064 | ||
| <18 | 104 (9) | 10 (4) | |
| 18–30 | 380 (31) | 86 (32) | |
| 31–50 | 495 (41) | 119 (44) | |
| 51–60 | 139 (11) | 38 (14) | |
| >60 | 92 (8) | 18 (6) | |
| Gender, n (%) | 0.436 | ||
| Female | 670 (55) | 143 (53) | |
| Male | 540 (45) | 128 (47) | |
| Disease extension, n (%) | 0.351 | ||
| E1 | 498 (41) | 120 (44) | |
| E2 | 270 (22) | 50 (19) | |
| E3 | 436 (36) | 100 (37) | |
| Progressive disease, n (%) | 445 (37) | 93 (34) | 0.447 |
| Follow-up, median (IQR), yr | 12 (7–19) | 12 (7–19) | 0.505b |
E1, ulcerative proctitis; E2, left side colitis; E3, extensive ulcerative colitis; IQR, interquartile range; n, number of patients.
P values are for the χ2 test unless otherwise indicated.
P values are for Mann-Whitney test.
The validation cohort of patients with CD included 302 subjects with similar demographic and clinical characteristics and outcome proportions when compared with the derivation cohort (Table 1).
The derivation cohort of patients with UC included 1,210 subjects, of which 9% were aged younger than 18 years, 31% were aged between 18 and 30 years, 41% were aged between 31 and 50 years, 11% were aged between 51 and 60 years, and 8% were aged older than 60 years at diagnosis. Furthermore, 55% of patients with UC were women and 41% presented ulcerative proctitis. Progressive disease was found in 37% of patients, and the median follow-up time was 12 years (7–19) for patients with UC from the derivation cohort (Table 2).
The validation cohort of patients with UC consisted of 271 subjects with similar demographic and clinical characteristics and outcome proportions when compared with the derivation cohort (Table 2).
Additional descriptions of the number of patients regarding location and behavior of disease for patients with CD and disease extension for patients with UC by age groups (for both stratifications <18, 18–30, 31–50, 51–60, and >60 years and <17, 17–40, 41–60, and >60 years) are presented as Supplemental Digital Contents (see Supplementary Tables, Supplementary Digital Contents 1–4, http://links.lww.com/CTG/A500, http://links.lww.com/CTG/A501, http://links.lww.com/CTG/A502, http://links.lww.com/CTG/A503).
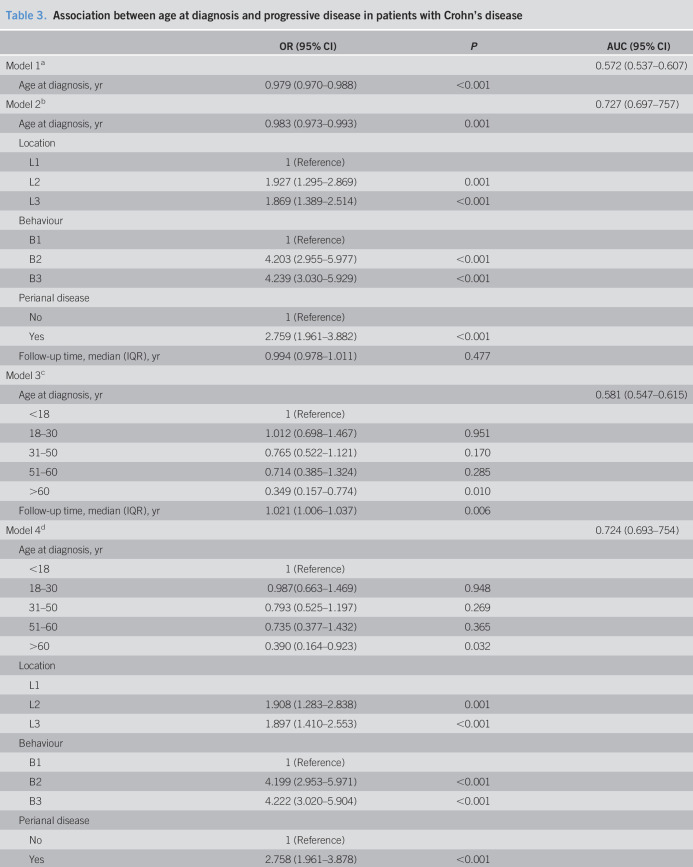
Association between age at diagnosis and progressive disease in patients with CD
Table 3 shows the association between age at diagnosis and progressive disease in patients with CD.
Table 3.
Association between age at diagnosis and progressive disease in patients with Crohn's disease
| OR (95% CI) | P | AUC (95% CI) | |
| Model 1a | 0.572 (0.537–0.607) | ||
| Age at diagnosis, yr | 0.979 (0.970–0.988) | <0.001 | |
| Model 2b | 0.727 (0.697–757) | ||
| Age at diagnosis, yr | 0.983 (0.973–0.993) | 0.001 | |
| Location | |||
| L1 | 1 (Reference) | ||
| L2 | 1.927 (1.295–2.869) | 0.001 | |
| L3 | 1.869 (1.389–2.514) | <0.001 | |
| Behaviour | |||
| B1 | 1 (Reference) | ||
| B2 | 4.203 (2.955–5.977) | <0.001 | |
| B3 | 4.239 (3.030–5.929) | <0.001 | |
| Perianal disease | |||
| No | 1 (Reference) | ||
| Yes | 2.759 (1.961–3.882) | <0.001 | |
| Follow-up time, median (IQR), yr | 0.994 (0.978–1.011) | 0.477 | |
| Model 3c | |||
| Age at diagnosis, yr | 0.581 (0.547–0.615) | ||
| <18 | 1 (Reference) | ||
| 18–30 | 1.012 (0.698–1.467) | 0.951 | |
| 31–50 | 0.765 (0.522–1.121) | 0.170 | |
| 51–60 | 0.714 (0.385–1.324) | 0.285 | |
| >60 | 0.349 (0.157–0.774) | 0.010 | |
| Follow-up time, median (IQR), yr | 1.021 (1.006–1.037) | 0.006 | |
| Model 4d | 0.724 (0.693–754) | ||
| Age at diagnosis, yr | |||
| <18 | 1 (Reference) | ||
| 18–30 | 0.987(0.663–1.469) | 0.948 | |
| 31–50 | 0.793 (0.525–1.197) | 0.269 | |
| 51–60 | 0.735 (0.377–1.432) | 0.365 | |
| >60 | 0.390 (0.164–0.923) | 0.032 | |
| Location | |||
| L1 | |||
| L2 | 1.908 (1.283–2.838) | 0.001 | |
| L3 | 1.897 (1.410–2.553) | <0.001 | |
| Behaviour | |||
| B1 | 1 (Reference) | ||
| B2 | 4.199 (2.953–5.971) | <0.001 | |
| B3 | 4.222 (3.020–5.904) | <0.001 | |
| Perianal disease | |||
| No | 1 (Reference) | ||
| Yes | 2.758 (1.961–3.878) | <0.001 | |
| Follow-up time, median (IQR), yr | 0.995 (0.979–1.012) | 0.585 |
AUC, area under the curve; B1, nonstricturing/nonpenetrating; B2, stricturing; B3, penetrating; CI, confidence interval; IQR, interquartile range; L1, ileal; L2, colonic; L3, ileocolonic; OR, odds ratio.
Logistic regression: dependent variable—progressive disease and independent variable—age at diagnosis as a continuous variable.
Logistic regression: dependent variable—progressive disease and independent variables—age at diagnosis as a continuous variable, location of disease, behavior of disease, presence of perianal disease, and follow-up time.
Logistic regression: dependent variable—progressive disease and independent variables—age at diagnosis as a categorical variable and follow-up time.
Logistic regression: dependent variable—progressive disease and independent variables—age at diagnosis as a categorical variable, location of disease, behavior of disease, presence of perianal disease, and follow-up time.
We found a significant decrease in the odds of developing progressive disease with an increasing age at diagnosis in patients with CD (model 1, OR 0.979, 95% CI 0.970–0.988, P < 0.001). However, because the discriminative power of this model was low for progressive disease (AUC 0.572, 95% CI 0.537–0.607), we further performed a multivariate logistic regression model adjusting for location and behavior of the disease, perianal disease, and follow-up time (model 2). In this model, age at diagnosis (OR 0.983, 95% CI 0.973–0.993, P = 0.001) and the presence of perianal disease (OR 2.759, 95% CI 1.961–3.882, P < 0.001) were significantly associated with the odds of developing progressive disease. This model presented a high discriminative power (AUC 0.727, 95% CI 0.697–757) (Table 3).
Because the use of age at diagnosis as a continuous variable is difficult in the daily clinical practice, we further performed logistic regression analyses with age at diagnosis stratified into the age groups younger than 18, 18–30, 31–50, 51–60, and older than 60 years. When adjusting for follow-up time (model 3), we found that patients with CD aged greater than 60 years at diagnosis presented a decrease of 0.7-fold in the odds of developing progressive disease compared with patients younger than 18 years at diagnosis (OR 0.349, 95% CI 0.157–0.774, P = 0.010). Nevertheless, the discriminative power of this model was low for progressive disease (AUC 0.581, 95% CI 0.547–0.615). When adjusting for location and behavior of the disease, perianal disease, and follow-up time (model 4), patients with an age at diagnosis greater than 60 years had a lower risk of developing progressive disease compared with patients younger than 18 years at diagnosis (OR 0.390, 95% CI 0.164–0.923, P = 0.032). Importantly, in this model, follow-up time was not significantly associated with the odds of developing progressive disease. This final model presented a high discriminative power for progressive disease (AUC 0.724, 95% CI 0.693–754) (Table 3). Similar results were observed for model 4 when age at diagnosis was stratified into the age groups younger than 17, 17–40, 41–60, and greater than 60 years (see Supplementary Table, Supplementary Digital Content 5, http://links.lww.com/CTG/A504).
To further validate the final model with age at diagnosis stratified into the age groups younger than 18, 18–30, 31–50, 51–60, and older than 60 years, ROC analyses were performed for the validation cohort of patients with CD. No differences were found in the AUC values between the validation and the derivation cohorts because values nearly overlap (AUC 0.780, 95% CI 0.724–0.835).
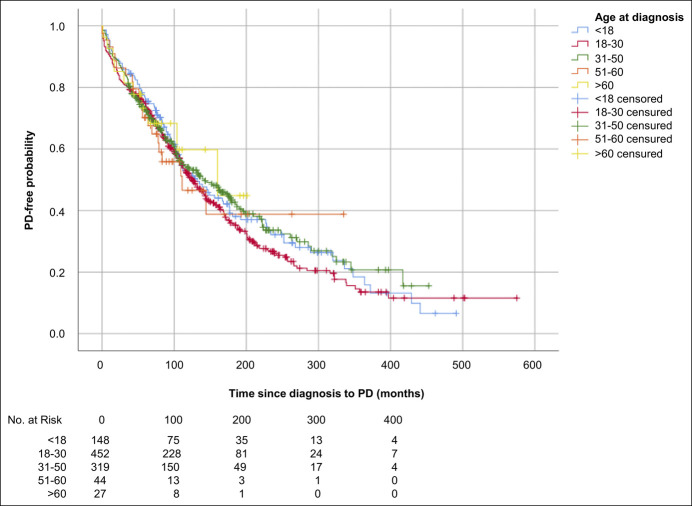
Kaplan-Meier analysis with age at diagnosis stratified both into the age groups younger than 18, 18–30, 31–50, 51–60, and older than 60 years and younger than 17, 17–40, 41–60, and older than 60 years showed no differences in the occurrence of progressive disease between age groups during the follow-up time (Figure 1 and see Supplementary Figure, Supplementary Digital Content 6, http://links.lww.com/CTG/A505, respectively). However, after performing a Cox regression analysis for the stratification of age at diagnosis into the age groups younger than 18, 18–30, 31–50, 51–60, and older than 60 years and after adjusting for location of disease, behavior of disease, presence of perianal disease, and follow-up time, we found that the older the patient at diagnosis, the slower the evolution until the outcome (hazard ratio 0.990, 95% CI 0.983–0.997, P = 0.005) (Table 4).
Figure 1.
Kaplan-Meier estimates for progressive disease (PD)-free probability in patients with Crohn's disease by age at diagnosis stratified into the age groups younger than 18 (blue line), 18–30 (red line), 31–50 (green line), 51–60 (orange line), and greater than 60 (yellow line) years (logrank test, P = 0.652).
Table 4.
Cox Regression analysis for patients with Crohn's disease
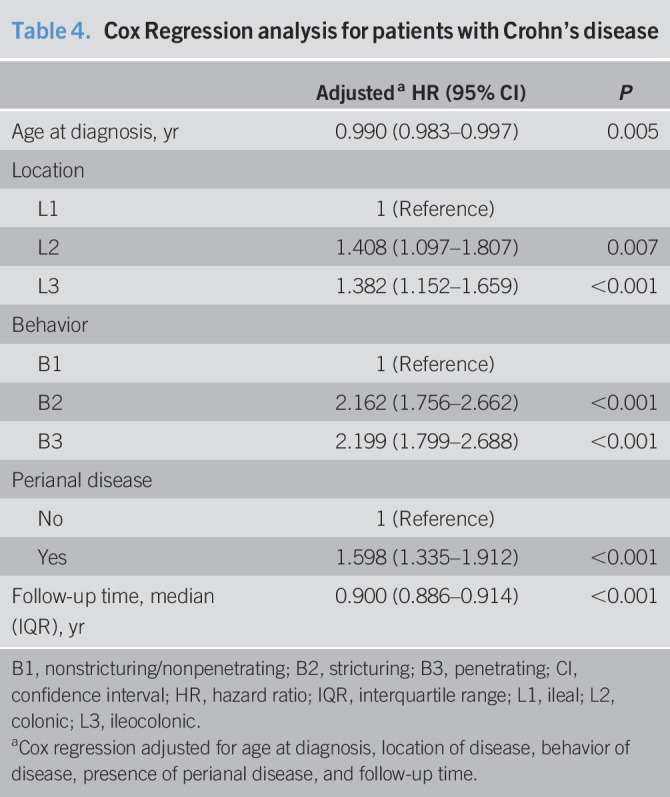
| Adjusteda HR (95% CI) | P | |
| Age at diagnosis, yr | 0.990 (0.983–0.997) | 0.005 |
| Location | ||
| L1 | 1 (Reference) | |
| L2 | 1.408 (1.097–1.807) | 0.007 |
| L3 | 1.382 (1.152–1.659) | <0.001 |
| Behavior | ||
| B1 | 1 (Reference) | |
| B2 | 2.162 (1.756–2.662) | <0.001 |
| B3 | 2.199 (1.799–2.688) | <0.001 |
| Perianal disease | ||
| No | 1 (Reference) | |
| Yes | 1.598 (1.335–1.912) | <0.001 |
| Follow-up time, median (IQR), yr | 0.900 (0.886–0.914) | <0.001 |
B1, nonstricturing/nonpenetrating; B2, stricturing; B3, penetrating; CI, confidence interval; HR, hazard ratio; IQR, interquartile range; L1, ileal; L2, colonic; L3, ileocolonic.
Cox regression adjusted for age at diagnosis, location of disease, behavior of disease, presence of perianal disease, and follow-up time.
Because the prognosis of CD and UC was already associated with the requirement for steroids at diagnosis (5–7), we also performed a χ2 test to address the association between the requirement for steroids at diagnosis and progressive disease. No association was found between these variables (P = 0.262) (see Supplementary Tables, Supplementary Digital Content 7, http://links.lww.com/CTG/A506).
Association between age at diagnosis and progressive disease in patients with UC
Table 5 shows the association between age at diagnosis and progressive disease in patients with UC.
Table 5.
Association between age at diagnosis and progressive disease in patients with ulcerative colitis
| OR (95% CI) | P | AUC (95% CI) | |
| Model 1a | 0.578 (0.544–0.611) | ||
| Age at diagnosis, yr | 0.983 (0.975–0.990) | <0.001 | |
| Model 2b | 0.714 (0.684–0.744) | ||
| Age at diagnosis, yr | 0.990 (0.982–0.999) | 0.028 | |
| Disease extension | |||
| E1 | 1 (Reference) | ||
| E2 | 3.774 (2.693–5.287) | <0.001 | |
| E3 | 5.698 (4.204–7.722) | <0.001 | |
| Follow-up time, median (IQR), yr | 1.011 (0.996–1.025) | 0.150 | |
| Model 3c | 0.566 (0.497–0.635) | ||
| Age at diagnosis, yr | |||
| <18 | 1 (Reference) | ||
| 18–30 | 0.533 (0.343–0.827) | 0.005 | |
| 31–50 | 0.451 (0.293–0.692) | <0.001 | |
| 51–60 | 0.362 (0.213–0.617) | <0.001 | |
| >60 | 0.375 (0.207–0.678) | 0.001 | |
| Follow-up time, median (IQR), yr | 1.015 (1.002–1.029) | 0.027 | |
| Model 4d | 0.716 (0.686–0.746) | ||
| Age at diagnosis, yr | |||
| <18 | 1 (Reference) | ||
| 18–30 | 0.703 (0.440–1.122) | 0.139 | |
| 31–50 | 0.637 (0.403–1.007) | 0.054 | |
| 51–60 | 0.567 (0.321–1.001) | 0.050 | |
| >60 | 0.560 (0.297–1.055) | 0.073 | |
| Disease extension | |||
| E1 | 1 (Reference) | ||
| E2 | 3.786 (2.702–5.305) | <0.001 | |
| E3 | 5.654 (4.169–7.668) | <0.001 | |
| Follow-up time, median (IQR), yr | 1.012 (0.997–1.027) | 0.106 |
AUC, area under the curve; CI, confidence interval; E1, ulcerative proctitis; E2, left side colitis; E3, extensive ulcerative colitis; IQR, interquartile range; OR, odds ratio.
Logistic regression: dependent variable—progressive disease and independent variable—age at diagnosis as a continuous variable.
Logistic regression: dependent variable—progressive disease and independent variables—age at diagnosis as a continuous variable, disease extension, and follow-up time.
Logistic regression: dependent variable—progressive disease and independent variables—age at diagnosis as a categorical variable and follow-up time.
Logistic regression: dependent variable—progressive disease and independent variables—age at diagnosis as a categorical variable, disease extension, and follow-up time.
We found a significant decrease in the odds of developing progressive disease with an increasing age at diagnosis in patients with UC (model 1, OR 0.983, 95% CI 0.975–0.990, P < 0.001). However, because the discriminative power of this model for progressive disease was low (AUC 0.578, 95% CI 0.544–0.611), we further performed a multivariate logistic regression model adjusting for disease extent and follow-up time (model 2). In this model, age at diagnosis was associated with the odds of developing progressive disease (OR 0.990, 95% CI 0.982–0.999, P = 0.028) with a high discriminative power (AUC 0.714, 95% CI 0.684–0.744) (Table 5).
When age at diagnosis was stratified into the age groups younger than 18, 18–30, 31–50, 51–60, and older than 60 years and after adjusting for follow-up time (model 3), we found an overall decrease of the odds of developing progressive disease with increasing age at diagnosis, compared with patients aged younger than 18 years at diagnosis. As for CD patients, the discriminative power of this model was low for progressive disease (AUC 0.566, 95% CI 0.497–0.635). When further adjusted for disease extent and follow-up time (model 4), no significant associations were found between age at diagnosis and the odds of developing progressive disease, but disease extension was significantly associated with an increase in the odds of developing progressive disease (E2: OR 3.786, 95% CI 2.702–5.305, P < 0.001; E3: OR 5.654, 95% CI 4.169–7.668, P < 0.001). Importantly, in this model, follow-up time was not significantly associated with the odds of developing progressive disease. This model presented a high discriminative power for progressive disease (AUC 0.716, 95% CI 0.686–0.746) (Table 5).
As for patients with CD, the AUC values of the validation cohort of patients with UC nearly overlap those of the derivation cohort (AUC 0.753, 95% CI 0.693–0.812).
Similar results were observed when age at diagnosis was stratified into the age groups younger than 17, 17–40, 41–60, and older than 60 years, with the exception of patients with an age at diagnosis between 41 and 60 years that presented a lower risk of developing progressive disease compared to patients younger than 17 years at diagnosis (OR 0.572, 95% CI 0.345–0.950, P = 0.031) (see Supplementary Table, Supplementary Digital Content 8, http://links.lww.com/CTG/A507).
Regarding the association between the requirement for steroids at diagnosis and progressive disease, a significant association was found between these variables for patients with UC (P = 0.009), in which progressive disease was more likely to occur in patients without the requirement of steroids at the time of diagnosis (see Table, Supplemental Digital Content 7, http://links.lww.com/CTG/A506).
DISCUSSION
Several studies have previously assessed the relevance of age at diagnosis as a prognostic factor for the course of IBD. However, most of the published studies assessed simple outcomes such as disease location and behavior, disease extension, the use of biologics or immunomodulators, hospitalizations, or surgeries (14–17,27–29,40). In this study, we aimed to further determine the relevance of age at diagnosis as a prognostic factor for IBD course, by using the compound outcome progressive disease in patients with CD and UC. This outcome included several clinical manifestations and was previously used as an indicator of an aggressive IBD course (9,11,30,31).
We found that patients with CD aged older than 60 years at diagnosis were associated with a lower risk of developing progressive disease on bivariate and multivariate analyses, whereas for patients with UC, this association was only observed on bivariate analysis. Cox regression for CD also shows that the older the patient at diagnosis, the slower the evolution until progressive disease. Nevertheless, for CD, we must highlight that the number of B1 patients at the time of diagnosis did not act as a confounder because our analysis was also adjusted for disease behavior. Therefore, our data suggest that age at diagnosis is more important for the course of CD than UC and that patients with elderly-onset CD are associated with a less progressive course of the disease.
Previous studies supported that patients with elderly-onset IBD are associated with fewer signs and symptoms and with a less aggressive course of the disease (14–17). Nevertheless, conflicting results were also reported for both CD (27,28,40) and UC (27,28,34), associating older ages at diagnosis with higher rates of hospitalization (27,28,40) and a higher absolute risk of bowel surgery (27) but also with a lower use of biologics and immunomodulators, when compared with patients diagnosed at a younger age. Moreover, Kariyawasam et al. (28) suggested that the delayed use of immunomodulators in elderly-onset patients are associated with comorbidities, rather than age at diagnosis. In our study, the definition of progressive disease did not include the requirement of biologics or immunomodulators, given the shifting from a step-up approach to a top-down approach in IBD management (41). Instead, our definition included the need to switch from biologics or immunomodulators, thus using a stricter definition of the outcome.
By using the compound outcome including several clinical manifestations, we also shown that for patients with UC disease extension is more important for the course of the disease than age at diagnosis. In fact, several studies reported disease extension as a prognostic factor of UC course (6,11–13).
It should be noted that elderly-onset IBD has been mainly defined as older than 60 years (14,15) or older than or equal to 60 years (16,17,27,28), but other cutoffs were also reported (29,40). Specifically, regarding the outcome progressive disease, a cutoff of ≥40 years at diagnosis was associated with a decreased risk of developing 5-year progressive disease for patients with CD (5,32,33). For patients with UC, besides extensive disease (E3) and male sex, age at diagnosis younger than or equal to 40 years was associated with a higher probability of developing this outcome, compared with patients diagnosed at ages older than 40 years (11). Nevertheless, these studies used different age categories, hindering direct comparisons between studies. It should be also highlighted that we have stratified the patients into the age groups younger than 18, 18–30, 31–50, 51–60, and older than 60 years to address as much as possible the association between the different age groups and the occurrence of progressive disease. Importantly, when stratifying patients by the Montreal classification (<17, 17–40, ≥40 years), further dividing the A3 group into 41–60, and older than 60 years, we found similar results.
The key strengths of our study were the analysis of large multicentric cohorts with prolonged follow-up times (median of 12 years) and the validation of our results using an independent validation cohort. In addition, this study had some limitations that must be acknowledged: the referral center-based cohorts, the retrospective analysis of the outcome, the reduced size of some subgroups of patients and the reduced occurrence of progressive disease in patients with UC, which may have limited the power for statistical analyses, and the noninclusion of neoplastic complications strictly related to IBD in the definition of the outcome of progressive disease because our database lacks sufficiently robust data on this parameter.
Additional studies should be conducted to assess the multiple comorbidities of patients with elderly-onset IBD and to further evaluate the relevance of age at diagnosis as a prognostic factor for the course of CD and UC. In addition, given the association of ageing with B and T cells immunosenescence (42–50) and the role of dysbiosis in the pathogenesis of IBD (3,24), future studies should assess their association with the course of CD and UC.
In conclusion, we observed that age at diagnosis was more important for the course of CD than UC, in which patients with elderly-onset CD were associated with a less progressive course of the disease. However, for patients with UC, the extension of the disease was more important for the prediction of the course of the disease than age at diagnosis. Such prognostic factors are crucial in IBD management because therapeutic decisions may be implemented sooner for patients at higher risk of progressive courses of the disease, hence potentially altering the course of the disease in those patients with IBD.
DATA SHARING AND DATA ACCESSIBILITY
All data relevant to the study are included in the article. Further information can be obtained from the corresponding author.
CONFLICTS OF INTEREST
Guarantor of the article: Fernando Magro, MD, PhD.
Specific author contributions: F.M. and P.M.: contributed to study conception or design. P.M., F.P., S.F., S.B., F.P., P.L., I.R., E.T., L.C., and F.M.: involved in data collection. C.A., F.M., and C.C.D.: contributed to data analysis. F.M. and C.C.D.: contributed to data interpretation. F.M. and P.M.: contributed to the drafting of the manuscript. All authors critical revised the manuscript. All authors approved the final version of the manuscript and the authorship list and take responsibility for the accuracy and integrity of any part of the work.
Financial support: This work was fully funded by the Portuguese Inflammatory Bowel Diseases Study Group (GEDII).
Potential competing interests: P. Ministro, F. Portela, S. Fernandes, S. Bernardo, P. Lago, I. Rosa, E. Trindade, and L. Correia received a fee for presenting from ABBvie, Ferring, Yansen, Phfizer, MSD, Schering, Lab, and Takeda. F. Magro received a fee for presenting from AbbVie, Ferring, Falk, Hospira, PharmaKern, MSD, Schering, Lab, Vitoria, Vifor, and OmPharma. All other authors declare no conflicts of interest.
Study Highlights.
WHAT IS KNOWN
✓ Patients with elderly-onset inflammatory bowel disease were previously associated with a less aggressive course of the disease.
✓ There are conflicting data that need further validation.
WHAT IS NEW HERE
✓ Age at diagnosis was more important for the course of CD than UC.
✓ Patients with elderly-onset CD, but not patients with UC, were associated with a less progressive course of the disease.
TRANSLATION IMPACT
✓ Therapeutic decisions may be implemented sooner for patients at higher risk of progressive disease.
ACKNOWLEDGEMNTS
The authors thank all investigators at the hospitals who provided data for the study, the GEDII for all the support, and Sandra Dias for supervising data collection. The authors would also like to acknowledge Ana Morgado, PhD, and Sofia Nunes, PhD, from Scientific ToolBox Consulting (Lisbon, Portugal) for providing medical writing assistance and technical editing.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A500; http://links.lww.com/CTG/A501; http://links.lww.com/CTG/A502; http://links.lww.com/CTG/A503; http://links.lww.com/CTG/A504; http://links.lww.com/CTG/A505; http://links.lww.com/CTG/A506; http://links.lww.com/CTG/A507
REFERENCES
- 1.Magro F, Gionchetti P, Eliakim R, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: Definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis 2017;11:649–70. [DOI] [PubMed] [Google Scholar]
- 2.Weimers P, Munkholm P. The natural history of IBD: Lessons learned. Curr Treat Options Gastroenterol 2018;16:101–11. [DOI] [PubMed] [Google Scholar]
- 3.Ruel J, Ruane D, Mehandru S, et al. IBD across the age spectrum: Is it the same disease? Nat Rev Gastroenterol Hepatol 2014;11:88–98. [DOI] [PubMed] [Google Scholar]
- 4.Butter M, Weiler S, Biedermann L, et al. Clinical manifestations, pathophysiology, treatment and outcome of inflammatory bowel diseases in older people. Maturitas 2018;110:71–8. [DOI] [PubMed] [Google Scholar]
- 5.Beaugerie L, Seksik P, Nion-Larmurier I, et al. Predictors of Crohn's disease. Gastroenterology 2006;130:650–6. [DOI] [PubMed] [Google Scholar]
- 6.Torres J, Caprioli F, Katsanos KH, et al. Predicting outcomes to optimize disease management in inflammatory bowel diseases. J Crohns Colitis 2016;10:1385–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loly C, Belaiche J, Louis E. Predictors of severe Crohn's disease. Scand J Gastroenterol 2008;43:948–54. [DOI] [PubMed] [Google Scholar]
- 8.Solberg IC, Vatn MH, Hoie O, et al. Clinical course in Crohn's disease: Results of a Norwegian population-based ten-year follow-up study. Clin Gastroenterol Hepatol 2007;5:1430–8. [DOI] [PubMed] [Google Scholar]
- 9.Dias CC, Rodrigues PP, Coelho R, et al. Development and validation of risk matrices for Crohn's disease outcomes in patients who underwent early therapeutic interventions. J Crohns Colitis 2017;11:445–53. [DOI] [PubMed] [Google Scholar]
- 10.Targownik LE, Singh H, Nugent Z, et al. The epidemiology of colectomy in ulcerative colitis: Results from a population-based cohort. Am J Gastroenterol 2012;107:1228–35. [DOI] [PubMed] [Google Scholar]
- 11.Magro F, Dias CC, Portela F, et al. Development and validation of risk matrices concerning ulcerative colitis outcomes-bayesian network analysis. J Crohns Colitis 2019;13:401–9. [DOI] [PubMed] [Google Scholar]
- 12.Dias CC, Rodrigues PP, da Costa-Pereira A, et al. Clinical predictors of colectomy in patients with ulcerative colitis: Systematic review and meta-analysis of cohort studies. J Crohns Colitis 2015;9:156–63. [DOI] [PubMed] [Google Scholar]
- 13.Cesarini M, Collins GS, Ronnblom A, et al. Predicting the individual risk of acute severe colitis at diagnosis. J Crohns Colitis 2017;11:335–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lakatos PL, David G, Pandur T, et al. IBD in the elderly population: Results from a population-based study in Western Hungary, 1977–2008. J Crohns Colitis 2011;5:5–13. [DOI] [PubMed] [Google Scholar]
- 15.Charpentier C, Salleron J, Savoye G, et al. Natural history of elderly-onset inflammatory bowel disease: A population-based cohort study. Gut 2014;63:423–32. [DOI] [PubMed] [Google Scholar]
- 16.Gower-Rousseau C, Vasseur F, Fumery M, et al. Epidemiology of inflammatory bowel diseases: New insights from a French population-based registry (EPIMAD). Dig Liver Dis 2013;45:89–94. [DOI] [PubMed] [Google Scholar]
- 17.Song EM, Kim N, Lee SH, et al. Clinical characteristics and long-term prognosis of elderly-onset Crohn's disease. Scand J Gastroenterol 2018;53:417–25. [DOI] [PubMed] [Google Scholar]
- 18.Satsangi J, Silverberg MS, Vermeire S, et al. The Montreal classification of inflammatory bowel disease: Controversies, consensus, and implications. Gut 2006;55:749–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Portela F, Magro F, Lago P, et al. Ulcerative colitis in a Southern European country: A national perspective. Inflamm Bowel Dis 2010;16:822–9. [DOI] [PubMed] [Google Scholar]
- 20.Barreiro-de Acosta M, Magro F, Carpio D, et al. Ulcerative colitis in northern Portugal and Galicia in Spain. Inflamm Bowel Dis 2010;16:1227–38. [DOI] [PubMed] [Google Scholar]
- 21.Biagi E, Candela M, Fairweather-Tait S, et al. Aging of the human metaorganism: The microbial counterpart. Age (Dordr) 2012;34:247–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Odamaki T, Kato K, Sugahara H, et al. Age-related changes in gut microbiota composition from newborn to centenarian: A cross-sectional study. BMC Microbiol 2016;16:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maffei VJ, Kim S, Blanchard Et, et al. Biological aging and the human gut microbiota. J Gerontol A Biol Sci Med Sci 2017;72:1474–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishida A, Inoue R, Inatomi O, et al. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin J Gastroenterol 2018;11:1–10. [DOI] [PubMed] [Google Scholar]
- 25.Nimmons D, Limdi JK. Elderly patients and inflammatory bowel disease. World J Gastrointest Pharmacol Ther 2016;7:51–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taleban S, Colombel JF, Mohler MJ, et al. Inflammatory bowel disease and the elderly: A review. J Crohns Colitis 2015;9:507–15. [DOI] [PubMed] [Google Scholar]
- 27.Everhov AH, Halfvarson J, Myrelid P, et al. Incidence and treatment of patients diagnosed with inflammatory bowel diseases at 60 years or older in Sweden. Gastroenterology 2018;154:518–28.e5. [DOI] [PubMed] [Google Scholar]
- 28.Kariyawasam VC, Kim S, Mourad FH, et al. Comorbidities rather than age are associated with the use of immunomodulators in elderly-onset inflammatory bowel disease. Inflamm Bowel Dis 2019;25:1390–8. [DOI] [PubMed] [Google Scholar]
- 29.Ananthakrishnan AN, Shi HY, Tang W, et al. Systematic review and meta-analysis: Phenotype and clinical outcomes of older-onset inflammatory bowel disease. J Crohns Colitis 2016;10:1224–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magro F, Dias CC, Coelho R, et al. Impact of early surgery and immunosuppression on Crohn's disease disabling outcomes. Inflamm Bowel Dis 2017;23:289–97. [DOI] [PubMed] [Google Scholar]
- 31.Dias CC, Pereira Rodrigues P, Fernandes S, et al. The risk of disabling, surgery and reoperation in Crohn's disease - a decision tree-based approach to prognosis. PLoS One 2017;12:e0172165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang CH, Ding J, Gao Y, et al. Risk factors that predict the requirement of aggressive therapy among Chinese patients with Crohn's disease. J Dig Dis 2011;12:99–104. [DOI] [PubMed] [Google Scholar]
- 33.Dias CC, Rodrigues PP, da Costa-Pereira A, et al. Clinical prognostic factors for disabling Crohn's disease: A systematic review and meta-analysis. World J Gastroenterol 2013;19:3866–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Waterman M, Knight J, Dinani A, et al. Predictors of outcome in ulcerative colitis. Inflamm Bowel Dis 2015;21:2097–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.GEDII data base [database on the Internet]. 2019. (https://gediibasedados.med.up.pt/). Accessed 2019. [Google Scholar]
- 36.Gionchetti P, Dignass A, Danese S, et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn's disease 2016: Part 2: Surgical management and special situations. J Crohns Colitis 2017;11:135–49. [DOI] [PubMed] [Google Scholar]
- 37.Maaser C, Sturm A, Vavricka SR, et al. ECCO-ESGAR guideline for diagnostic assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis 2019;13:144–64. [DOI] [PubMed] [Google Scholar]
- 38.Fort Gasia M, Ghosh S, Iacucci M. Colorectal polyps in ulcerative colitis and Crohn's colitis. Minerva Gastroenterol Dietol 2015;61:215–22. [PubMed] [Google Scholar]
- 39.Van Assche G, Dignass A, Panes J, et al. The second European evidence-based consensus on the diagnosis and management of Crohn's disease: Definitions and diagnosis. J Crohns Colitis 2010;4:7–27. [DOI] [PubMed] [Google Scholar]
- 40.Viola A, Monterubbianesi R, Scalisi G, et al. Late-onset Crohn's disease: A comparison of disease behaviour and therapy with younger adult patients. Eur J Gastroenterol Hepatol 2019;31:1. [DOI] [PubMed] [Google Scholar]
- 41.Berg DR, Colombel J-F, Ungaro R. The role of early biologic therapy in inflammatory bowel disease. Inflamm Bowel Dis 2019;25:1896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Butcher SK, Chahal H, Nayak L, et al. Senescence in innate immune responses: Reduced neutrophil phagocytic capacity and CD16 expression in elderly humans. J Leukoc Biol 2001;70:881–6. [PubMed] [Google Scholar]
- 43.Kawanishi H, Kiely J. Immune-related alterations in aged gut-associated lymphoid tissues in mice. Dig Dis Sci 1989;34:175–84. [DOI] [PubMed] [Google Scholar]
- 44.Koga T, McGhee JR, Kato H, et al. Evidence for early aging in the mucosal immune system. J Immunol 2000;165:5352–9. [DOI] [PubMed] [Google Scholar]
- 45.McDonald KG, Leach MR, Huang C, et al. Aging impacts isolated lymphoid follicle development and function. Immun Ageing 2011;8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmucker DL, Daniels CK, Wang RK, et al. Mucosal immune response to cholera toxin in ageing rats. I. Antibody and antibody-containing cell response. Immunology 1988;64:691–5. [PMC free article] [PubMed] [Google Scholar]
- 47.Thoreux K, Owen RL, Schmucker DL. Intestinal lymphocyte number, migration and antibody secretion in young and old rats. Immunology 2000;101:161–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Kruiningen HJ, West AB, Freda BJ, et al. Distribution of Peyer's patches in the distal ileum. Inflamm Bowel Dis 2002;8:180–5. [DOI] [PubMed] [Google Scholar]
- 49.Van Kruiningen HJ, Ganley LM, Freda BJ. The role of Peyer's patches in the age-related incidence of Crohn's disease. J Clin Gastroenterol 1997;25:470–5. [DOI] [PubMed] [Google Scholar]
- 50.Dukes C, Bussey HJR. The number of lymphoid follicles of the human large intestine. J Pathol Bacteriol 1926;29:111–6. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data relevant to the study are included in the article. Further information can be obtained from the corresponding author.