Supplemental Digital Content is available in the text
Keywords: clopidogrel, comedication, ethnicity, proton pump inhibitors, stent implantation
Abstract
Background:
Pharmacokinetic and pharmacodynamic study showed a lower clopidogrel response when coprescribed with proton pump inhibitors (PPIs). Despite this, PPIs is necessary for patients treated with long term dual antiplatelet therapy (DAPT). Ethnic variance also played a different effect on clopidogrel response. Our study evaluated the effect of concomitant use of DAPT and PPIs and assessed whether ethnic variance exert different effect on clinical outcomes.
Methods:
We carefully searched EMBASE, PubMed/Medline databases, and the Cochrane library in April 2019. The primary endpoint was major adverse cardiovascular and cerebrovascular events (MACCE) and individual endpoints reported. We also focused on bleeding events. Studies were excluded if the follow-up were <12 months and patients were not treated with clopidogrel after stent implantation.
Results:
A total of 18 studies were included in the systematic review (involving 79,670 patients). No randomized controlled trials (RCTs) were included. PPIs comedication were associated with increased MACCE (odds ratio [OR] = 1.38; 95% confidence interval [CI] = 1.28–1.49) while not associated with decreased bleeding risks, such as gastrointestinal bleeding (OR = 1.05; 95% CI = 0.53–2.11). PPIs comedication were associated with increased risk for all endpoints among Caucasian population while not with increased risk for MACE (OR = 1.20; 95% CI = 0.99–1.39), all-cause death (OR = 1.24; 95% CI = 0.74–2.06), cardiac-death (OR = 1.29; 95% CI = 0.64–2.57) among Asian population.
Conclusion:
PPIs comedication were associated with adverse clinical outcomes, and ethnic variance may exert different effect on clinical outcomes. Subgroup analysis indicated that concomitant use of PPI might be suitable for Asian patients after stent implantation.
1. Introduction
The crucial role played by clopidogrel in preventing ischemic events have been fully demonstrated in the past 2 decades and clinical guidelines recommended that clopidogrel should be given for at least 12 months after stent implantation.[1] Extended antiplatelet agents decrease the risk for major adverse cardiovascular and cerebrovascular events (MACCE), very late stent thrombosis, and myocardial infarction,[2] while risk for upper and lower gastrointestinal bleeding (GIB) increased.[3] Therefore proton pump inhibitors (PPIs) are often comedicated to protect gastrointestinal (GI) tract. However, studies showed a potential drug-interaction which would attenuate clopidogrel antiplatelet function and result in adverse clinical outcomes.[4]
Although, the European Society of Cardiology and the European Association for Cardio-Thoracic Surgery (ESC/EACTS) in 2018 stated that routine PPI comedication with dual antiplatelet therapy (DAPT) is not recommended,[5] routine PPIs use seem popular reported in some studies. Furthermore, CYP2C19 allele which encode the key enzyme in the metabolism of clopidogrel differs among ethnics[6,7] which may exert a different effect on clinical outcomes among different ethnics. Therefore, we tried to make this meta-analysis to explore these problems.
2. Methods
This meta-analysis was designed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. (Supplement PRISMA checklist, http://links.lww.com/MD/F670).
2.1. Data sources and search strategy
Two reviewers (W-CS, LY) carefully searched EMBASE, PubMed/Medline databases, and the Cochrane library for Randomized Controlled Trials (RCTs) and observational studies. We used “proton pump inhibitor,” “clopidogrel,” “percutaneous coronary intervention,” “stent implantation” as key words to search in databases. Additionally, their abbreviation such as PPI, PCI, and DAPT were also used. In order to search more studies, we widen the key words and “omeprazole,” “pantoprazole,” “lansoprazole,” “esomeprazole,” “rabeprazole,” and “thienopyridine” were included in our search strategies.
2.2. Criteria for inclusion/exclusion
Original, research studies published or presented to April 2019 in English were eligible for inclusion. Studies comparing PPIs comedication with clopidogrel alone in patients after stent implantation were included. Population without definite stent implantation and clopidogrel medication were excluded. Follow-up <12 months and adverse cardiovascular outcomes (especially major adverse cardiovascular disease) were not reported as their clinical endpoints were also ineligible. Conference abstracts and studies where full articles could not be retrieved were not initially excluded from the search strategy but later excluded from the meta-analysis
2.3. Study selection
We imported identified studies into NoteExpress and duplicates were deleted. Two investigators (SWC and YL) independently screened all titles and abstracts returned by the search strategy. Then 2 investigators (SWC and YL) viewed all full text copies of potential relatively studies. A third investigator (YMY) resolved any discordance in assessments.
2.4. Study endpoints
The primary clinical endpoints chosen for this analysis was MACCE and individual endpoints reported such as all-cause death (ACD), cardiac death (CD), myocardial infarction (MI), stroke, stent thrombosis (ST), target vessel revascularization (TVR), and target lesion revascularization (TLR). We also reported safety endpoints including bleeding events and net adverse clinical events (NACE).
2.5. Data extraction
Two investigators (SWC and YL) independently used a standardized data form (supported by Microsoft Excel) to extracted study characteristics (author, study design, country), total number of patients, type of PPI, type of clinical endpoints, coronary risk factors, and follow-up. We extract data with propensity score matching (PSM) in priority if available.[8,9] Disagreements regarding the appropriateness of studies included for analysis were resolved by discussion and consultation with a third investigators of our group (YMY).
2.6. Risk of bias assessment
Two investigators (SWC and YL) assessed risk of bias, and a third investigator (YMY) resolved discrepancies by consensus. The New-castle-Ottawa Scale was used to assess the methodological quality of observational studies in terms of validating participant selection, population comparability, and outcome/exposure assessment. (Supplement 1, http://links.lww.com/MD/F606 and Supplement 2, http://links.lww.com/MD/F607). Only one post hoc analysis of randomized trial[10] was included and we used recommendations from the Cochrane Collaboration.
2.7. Data synthesis and analysis
Measures of association, including odds ratios (ORs), hazard ratios (HRs), and relative risks (RRs), with 95% confidence intervals (CIs), were extracted from included studies. We pooled adjusted ORs, HRs, and RRs because adverse cardiovascular events were rare. Review Manager version 5.3 for Windows was utilized to analyze the weighted mean, variance of the overall effect, 95% CI and P-value, and generate forest plots for exposure-outcome comparisons in each dataset. Separate analyses of primary endpoint and individual endpoints were prespecified. Considering the inherent differences between these study designs, a fixed effects model (I2 < 50%) or a random effects model (I2 > 50%) was used based on the value of I2 obtained and also a sensitivity analysis was conducted by individual exclusion of each study for each outcome to assess their effects on the pooled outcome. OR with 95% CIs were calculated and we measured significance using a P value of <.05. Publication bias was assessed by observing funnel plots.
2.8. Ethical review
Our manuscript is a pooled analysis of former published articles and no ethical approval is applicable.
3. Results
3.1. Study selection
A total of 523 articles were download after researching the databases by keywords mentioned above and there are 239 articles remained after removing duplicates. One hundred eighty five articles were excluded for reasons such as function and gene experiment, mechanism studies, case report, or review. Another 36 articles did not meet the including criteria we set for this study. Therefore we pooled 17 cohort studies[8,9,11–25] and one post-hoc analysis of RCT[10] together in the following meta-analysis finally (Fig. 1).
Figure 1.
Flow diagram for the study selection.
3.2. Population and baseline characteristics
A total of 79,670 patients (31,732 patients treated with clopidogrel plus PPIs and 47,938 patients treated with clopidogrel alone) aged 60 years or older were available for analyses. All of the patients take aspirin (75–100 mg) and clopidogrel as DAPT routinely recommended in guidelines. Six studies were conducted in Asian country (China[8,10] and Japan[9,17,18,25]), others were conducted in Europe and America such as US,[11,12,20,22,23] Italy,[11,12,20,22,23] Austria,[19] Greece,[24] and 3 multi-country studies.[13,15,16] The PPIs used in these studies included esomeprazole, omeprazole, pantoprazole rabeprazole, and lansoprazole (Table 1).
Table 1.
Studies included in present article.
| Study/Year | Location | Study design | Patients | No. of subjects PPI(+): PPI(−) | Age (years) PPI(+): PPI(−) | DM (%) PPI(+): PPI(−) | HTN (%) PPI(+): PPI(−) | Hyperlipidemia (%) PPI(+): PPI(−) | Smoking (%) PPI(+): PPI(−) | Aspirin dose, mg/d | PPI studied | Follow-up | MACE (composite outcome definition) | Individual outcomes reported |
| Pei Zhu/2017 | China | Cohort | PCI | 1966:1966 | 60.2 ± 10.6:57.7 ± 10.3 | 29.0: 30.3 | 64.0: 65.4 | 66.9: 71.7 | NM | 100 | NM | 2-year | ACD, MI, TVR, ST, stroke | ACD,MI, TVR, ST, stroke, bleeding, BARC 3 or 5, GI bleeding |
| Jaya/2017 | US/EU | Cohort | PCI with stent | 1062:3573 | 64.1 ± 11.4: 65.4 ± 11.1 | 33.0: 32.8 | 79.8: 80.4 | 77.5: 75.3 | 16.9: 20.2 | NM | NM | 2-year | CD, ST, MI, TLR | CD, ST, MI, TLR, BARC 3 or 5, NACE |
| Jackson/2016 | US | Cohort | AMI with PCI | 1636:7196 | 63 (55–70):59 (51–67) | 32.4: 25.2 | 76.1: 64.8 | 73.1: 63.9 | NM | NM | NM | 1-year | Death, MI, TVR, stroke | GUSTO moderate/severe bleeding |
| Yan Y/2016 | Multi | Cohort | ACS with PCI | 4814:4126 | 66.22 (56.39–73.80) 61.25 (52.00–71.00) | 26.1: 22.4 | 59.5: 49.6 | 48.4: 42.0 | NM | NM | NM | 1-year | ACD, re-infarction | ACD, re-infarction, bleeding (GUSTO moderate/severe bleeding) |
| Gargiulo G/2016 | Italia | Cohort | PCI | 375:612 | 71.8 (63.8–77.7) 67.9 (58.9–74.5) | 23.3: 24.8 | 72.5: 71.3 | 53.8: 55.3 | NM | 75-100 | NM | 2-year | ACD, MI, CVA | ACD, CD, MI, ST, BARC 3 or 5, GUSTO moderate/severe bleeding, NACE |
| Weisz /2015 | Multi | Cohort | PCI with DES | 2162:6419 | 64.4 ± 10.5 63.2 ± 11.0 | 34.8: 31.4 | 83.7: 77.8 | 76.9: 73.2 | 10.4: 6.5 | NM | NM | 2-year | CD, MI, TLR | CD, MI, TVR, ST, bleeding |
| Zou J/2014 | China | Post hoc analysis | PCI with DES | 6188:1456 | 66.2 ± 10.2 65.7 ± 10.6 | 25.8: 23.6 | 71.3: 70.4 | 60.2: 62.3 | 32.2: 31.0 | 100 | NM | 1-year | Death, MI, TVR, TLR, CABG, ST | ST, MI, death, TVR, TLR, CABG |
| Burkard K/2012 | NM | Cohort | PCI with stent | 109:692 | 66.5 ± 10.563.3 ± 11.3 | 29.6: 17.2 | 72.5: 65.0 | 73.4: 75.9 | 24.8: 29.8 | 100 | O, E, P, L | 3-year | CD, MI, TVR | CD, MI, TVR, ST |
| Chitose /2012 | Japan | Cohort | PCI | 187:443 | 69.7 ± 11.4: 69.6 ± 10.6 | 35.3: 33.7 | 77.9:79.0 | 61.9: 61.7 | 23.9: 26.2 | 100 | NM | 18-month | CD, MI, stroke | CD, MI, stroke, GI event |
| Aihara H/2012 | Japan | Cohort | PCI | 500:500 | 69 ± 11:68 ± 10 | 40.8: 39.4 | 71.2: 69.0 | 83.0: 83.8 | 44.6: 43.2 | 100 | NM | 3-year | ACD, MI | ACD, MI, stroke, ST, GI bleeding |
| Kimura T/2011 | Japan | Cohort | PCI with stent | 3223:9223 | 69.0 ± 11.267.7 ± 10.9 | 39: 37 | 83: 82 | NM | 31: 32 | ≥81 | O, R, L | 3-year | CD. MI, stroke | CD, MI, stroke, ST, GUSTO moderate/severe bleeding, GI bleeding |
| Rossini R/2011 | Italy | Cohort | PCI with DES | 1158:170 | 64 ± 11 63 ± 11 | 27.1: 28.0 | 63.6: 65.2 | 65.8: 72.5 | 49.3: 49.7 | 100 | L, O, P | 1-year | Death, MI, destabilizing symptoms leading to hospitalization, and nonfatal stroke | Death, ST |
| Tentzeris/2010 | Austria | Cohort | PCI with stent | 691:591 | 64.11 ± 12.42:64.44 ± 11.87 | 18.7: 26.0 | 73.7: 78.2 | 76.4: 77.1 | 27.9: 23.1 | 100 | NM | 1-year | ACD, ACS, ST | ACD, CD, ACS, ST, |
| Kreutz/2010 | US | Cohort | PCI with stent | 6828:9862 | 67.5 ± 10.4:65.2 ± 10.6 | 25.9: 22.7 | 50.6: 46.5 | 67.8: 63.4 | NM | NM | O, E, P, L, R | 1-year | Stroke, TIA, ACS, CD, CABG, PCI | Stroke or TIA, MI or UA, CABG, PCI, CD, MI, UA, PCI, CABG |
| Gaglia/2010 | US | Cohort | PCI with DES | 318:502 | 63.8 ± 11.663.7 ± 11.6 | 36.3: 33.1 | 78.5: 74.8 | 87.7: 82.4 | 13.8: 18.9 | 325 | E, L, O, P, R | 1-year | ACD, MI, TVR, ST | ACD, MI, TVR, ST |
| Gupta/2010 | US | Cohort | PCI | 72:243 | 61.7 ± 1.262 ± 0.7 | 36: 30 | 76: 68 | 67: 60 | 25: 33 | NM | NM | 4-year | Death, MI, target vessel failure | Death, TLR, target vessel failure |
| Zairis M/2010 | Greece | Cohort | PCI with stent | 340:248 | 62.1 ± 10.5 61.7 ± 10.8 | 30.0: 26.2 | 50.9: 46.4 | 66.5: 65.3 | 49.7: 50.8 | 100-325 | O | 1-year | CD, MI | CD, MI, ST, |
| Yasu/2010 | Japan | Cohort | PCI with DES | 188:103 | 69.0 ± 9.667.4 ± 10.1 | 35.0: 39.7 | 64.1: 64.8 | 68.0: 57.8 | 24.3: 27.1 | NM | R | 395-day | CD, ACS, ST, TLR | CD, ACS, ST, TLR |
ACD = any-cause death, ACS = acute coronary syndrome, BARC 3 or 5 = bleeding academic research consortium 3 or 5, DES = drug eluting stents, DM = diabetes mellitus, E = esomeprazole, GI bleeding = gastrointestinal bleeding, HTN = hypertension, L = lansoprazole, MI = myocardial infarction, NM = not mentioned, O = omeprazole, P = pantoprazole, PCI = percutaneous coronary intervention, R = rebeprazole, ST = stent thrombosis, TLR = target lesion revascularization, TVR = target vessel revascularization.
3.3. Risk of bias assessment
All 18 studies included for meta-analysis showed good over-all methodological quality. The descriptions of population selection, exposure, and outcome ascertainment are clear mentioned. Most studies had a high competence of follow-up with outcome data. However, few studies used prescription and pharmacy dispensing record databases to ascertain exposure and current procedural terminology fourth edition (CPT-4) codes.[20] In addition, choice of antiplatelet therapy and PPIs use were left to the discretion of the individual treating physicians in accordance with practice guideline recommendations and local standards of care in all studies.
3.4. Heterogeneity assessment
We found no heterogeneity among MACCE (X2 = 29.10, P = .03; I2 = 42%), ACD (X2 = 11.52, P = .12; I2 = 39%), MI (X2 = 18.79, P = .07; I2 = 41%), ST (X2 = 4.71, P = .97; I2 = 0%), TVR (X2 = 7.95, P = .16; I2 = 37%), TLR (X2 = 6.16, P = .10; I2 = 51%), and bleeding events except gastrointestinal bleeding (X2 = 11.25, P = .01; I2 = 73%). However statistically significant heterogeneity were observed in CD (X2 = 28.29, P = .005; I2 = 58%), and stroke (X2 = 16.50, P = .002; I2 = 76%).
3.5. MACCE and individual outcomes
Concomitant therapy showed a statistically significant increase in composite MACCE compared with clopidogrel monotherapy (OR = 1.38; 95% CI = 1.28–1.49) (Fig. 2). Although the definition of MACCE were divergent in different studies, as for individual components of MACCE, concomitant therapy were associated with increased risk for ACD (OR = 1.54; 95% CI = 1.31–1.80), CD (OR = 1.35; 95% CI = 1.19–1.53), MI (OR = 1.30; 95% CI = 1.19–1.41), ST (OR = 1.53; 95% CI = 1.27–1.83), TVR (OR = 1.27; 95% CI = 1.18–1.35), TLR (OR = 1.14; 95% CI = 1.04–1.25), and stroke (OR = 1.26; 95% CI = 1.08–1.46) (Fig. 3).
Figure 2.
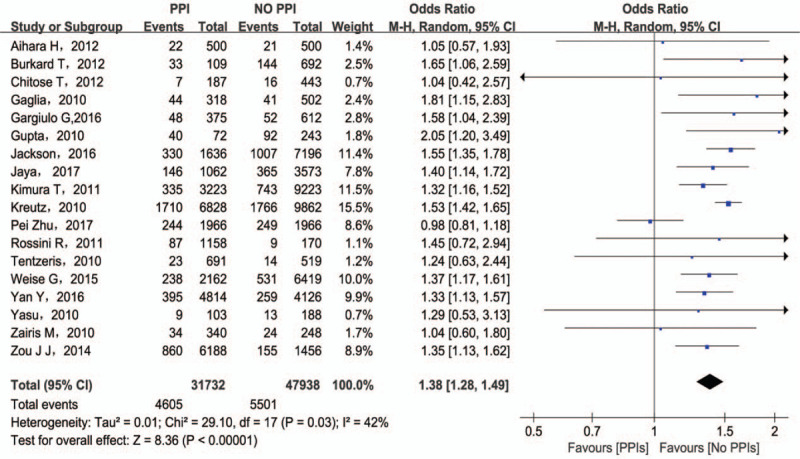
MACCE associated with the concomitant use of clopidogrel and PPIs. MACCE = major adverse cardiovascular and cerebrovascular events; PPI = proton pump inhibitors.
Figure 3.

Results for individual endpoints of MACCE. MACCE = major adverse cardiovascular and cerebrovascular events.
3.6. Bleeding outcomes
The main purpose of combined treatment is to prevent GI bleeding. However, the use of PPIs did not decrease the risk for GIB (OR = 1.50; 95% CI = 1.21–1.87) (Fig. 4). Although a significant heterogeneity was found among these 4 studies reporting GIB, only exclusion of Kimura (2011)[11] changed the results indicating that PPIs medication had no effect on GIB (OR = 1.00; 95% CI = 0.65–1.55). The differences in baseline clinical characteristics among these studies are responsible for the results. As for BARC 3/5 bleeding and GUSTO moderate/severe bleeding events, PPIs are related to bleeding events with OR of 2.80 (95% CI = 1.98–3.96) and OR of 1.66 (95% CI = 1.44–1.91) respectively (Fig. 4).
Figure 4.

Results for bleeding events and NACE. NACE = net adverse clinical events.
3.7. NACE
Net adverse clinical event (NACE) could evaluate comprehensive effect of concomitant therapy which is defined as a composite endpoint including bleeding events and MACCE. Only 2 studies report NACE and the pooled analysis is in favor of clopidogrel monotherapy (OR = 1.35; 95% CI = 1.13–1.60). (Fig. 4).
3.8. Subgroup analysis
We made a subgroup analysis considering the discrepancy in ethnics in order to illuminate the potential difference between Caucasians and Asian population. We found Asian patients treated with PPIs concurrently showed no significant difference in MACE (OR = 1.20; 95% CI = 0.99–1.39), ACD (OR = 1.24; 95% CI = 0.74–2.06), CD (OR = 1.29; 95% CI = 0.64–2.57), stroke (OR = 1.00; 95% CI = 0.82–1.21), TVR (OR = 1.10; 95% CI = 0.93–1.29), and TLR (OR = 1.09; 95% CI = 0.98–1.20). (Fig. 5A) However, the results in Caucasians population support the monotherapy of PPI for all endpoints (Fig. 5B).
Figure 5.
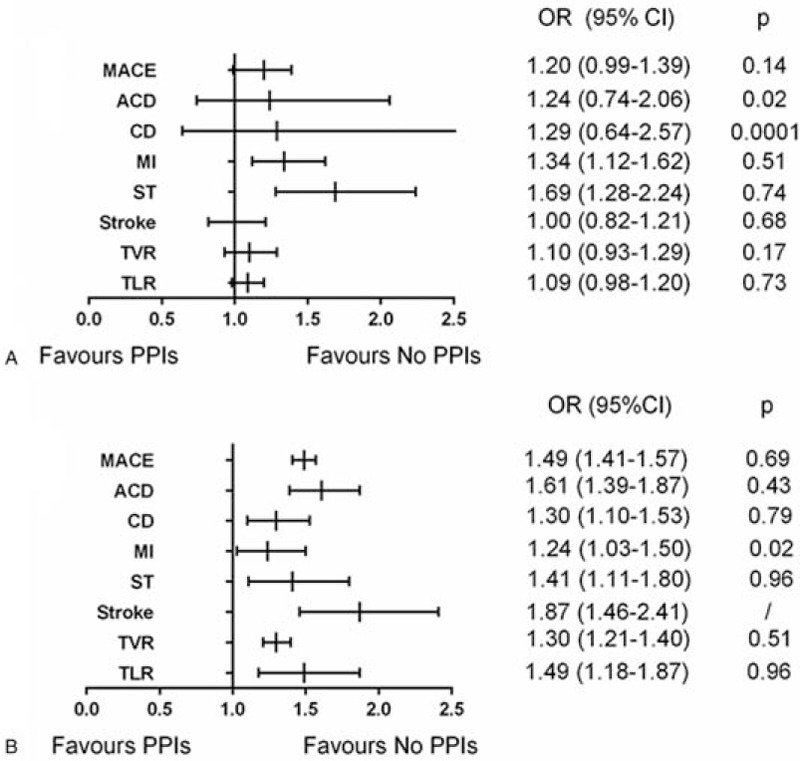
Pooled results for Asian population (A) and Caucasian population (B).
3.9. Sensitivity analysis
In a sensitivity analysis by single study exclusion for each outcome, Kimura (2011) cause the heterogeneity among CD, GI, and stroke. However, the results did not change while the exclusion of Kimura (2011). In Caucasians population during the subgroup analysis, Kreutz (2010)[20] resulted the heterogeneity in MI and changed the outcome opposite to the concomitant use (OR = 1.11; 95% CI = 0.96–1.27). For other outcomes, the exclusion of any study did not significantly alter the results or the heterogeneity.
4. Discussion
In this meta-analysis pooling 18 studies, we found no benefits in all clinical endpoints for patients with PPIs comedication. But a divergent result after subgroup analysis according to ethnics was identified.
Clopidogrel is a prodrug that depends on cytochrome P450 (CYP) with isoenzyme CYP2C19 playing a major role to generate an active metabolite,[26] PPIs also interact with the CYP, which may inhibit the conversion of clopidogrel to its active metabolite and potentially alter its antiplatelet properties.[4] Some pharmacokinetic experiments show an interaction between PPIs and clopidogrel which would attenuate its antiplatelet effect.[27–30] In addition, recent study has demonstrated that patients with reduced-function CYP2C19 allele lead to reduced levels of active clopidogrel metabolites, which are associated with worse cardiovascular outcomes, including stent thrombosis.[30] In our study, the results seemed support the theory.
Moreover, several mechanisms impairing endothelial function have been reported to account for the complications of PPI use.[31] Dimethylarginine dimethylamino-hydrolase (DDAH) is present in all cells, degrading asymmetric dimethylarginine (ADMA), which inhibits the endothelial enzyme nitric oxide synthase (eNOS). While PPI use would inhibit DDAH and increase ADMA, this would reduce levels of vasodilator nitric oxide (NO). Vascular NO inhibits thrombosis and vascular inflammation.[32] Therefore, PPI use was associated with a broad impairment in endothelial function which would be expected to increase major adverse cardiovascular events.
A series studies demonstrate a hypofunctional CYP2C19 metabolic phenotypes variance from 13% to 23% in healthy East Asian populations to only 2% to 5% in Caucasians.[7] Therefore we made a subgroup analysis to demonstrate the ethnic variance on the effect of concomitant use. The results showed the concomitant use did not bring statistically adverse effect among Asian population on MACE, ACD, CD, stroke, TVR, and TLR, but the effect on MI, ST still remained. It seemed that patients with hypofunctional CYP2C19 metabolic phenotypes might benefit more from DAPT-PPIs combination therapy. In China, physicians prescribed DAPT (especially clopidogrel as first-line medicine) to patients after stent implantation, and study reported that GIB incidence is higher in Chinese AMI population.[33,34] Therefore the PPIs usage is prevalent in China. But we found no study focused on this topic and Chinese physicians tend to prescribe PPIs on recommendation from guideline based on studies from Caucasians population. Our study indicated that the adverse effect of concomitant use seemed less in Asian population. In addition, a small recent RCT show that prescription of PPI was associated with higher compliance with DAPT and decreases the risk of recurrent cardiovascular events.[35] Therefore Asian population might benefit from concomitant use to some degree. However, the definite mechanism and effect of ethnic variation on this topic still unknown, further pharmacokinetic and pharmacodynamic studies, and largescale clinical trials especially RCTs were warranted.
In addition, PPIs were analysis in our study as a class. Several studies indicated that PPIs are metabolized by CYP2C19, but to a varying degree.[36] Clopidogrel response were measured by VASP in Thomas study[37] showed that patients receiving pantoprazole had a significantly better platelet response to clopidogrel compared with omeprazole. Another study focused on esomeprazole and rabeprazole demonstrated no association with impaired response to clopidogrel by testing VASP.[38] However, the most suitable PPI for Asians is still unclear and more prospective studies are warranted.
Overall, ethnic variation on concomitant therapy indicated that a lower threshold for PPIs prescription might be suitable in Asian population. What's more, our research provides a perspective for future research that ethnic differences are potential factors that may affect drug metabolism and clinical outcomes, and we should increase our concern on it.
4.1. Limitations
Several limitations in this meta-analysis affect the conclusions. No randomized trials included may affected the results and because of this reason, the bias risk of the studies was not assessed using recommendations from the Cochrane Collaboration. The definition and standard for diagnose for MACCE, ST, and GIB are different among included studies which may potentially affect the conclusion. Few studies focus on the bleeding endpoints such as GI bleeding and NACE, and subgroup analysis were not made in these endpoints, therefore the ethnic variance on bleeding events and NACE were short in this study. PPI used in different studies were analyzed as a class in our meta-analysis, and PPIs with varied inhibition of CYP2C19 might exert different clinical effect. Because the choice of PPI use was left to the discretion of the individual physicians in involved studies, it's hard to make separate analysis showing differential response with ethnic variance.
5. Conclusions
The present systematic review and meta-analysis found consistent evidence of an association between concomitant drug-use and adverse clinical outcomes, we also identified an ethnic variance on clinical outcomes. The results suggested that prescription for PPIs among Asian patients may suitable. New evidence focus on ethnic variance are warranted.
Acknowledgments
The authors thank all co-authors, especially YJG and YMY who have supported this manuscript.
Author contributions
Conceptualization: Mengyue Yu.
Data curation: Wence Shi, Lu Yan.
Formal analysis: Wence Shi.
Funding acquisition: Mengyue Yu.
Investigation: Lu Yan.
Methodology: Lu Yan, Jingang Yang.
Project administration: Jingang Yang, Mengyue Yu.
Software: Wence Shi, Lu Yan.
Supervision: Lu Yan, Mengyue Yu.
Validation: Mengyue Yu.
Writing – original draft: Wence Shi.
Writing – review & editing: Jingang Yang, Mengyue Yu.
Glossary
Abbreviations: ACD = all-cause death, ACS = acute coronary syndrome, BARC 3 or 5 = bleeding academic research consortium 3 or 5, DES = drug eluting stents, E = esomeprazole, GI bleeding = gastrointestinal bleeding, L = lansoprazole, MI = myocardial infarction, NM = not mentioned, O = omeprazole, P = pantoprazole, PCI = percutaneous coronary intervention, R = rebeprazole, ST = stent thrombosis, TLR = target lesion revascularization, TVR = target vessel revascularization.
References
- [1].Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines With Non-ST-Elevation Acute Coronary Syndromes. Circulation 2016;134:e123–55. [DOI] [PubMed] [Google Scholar]
- [2].Hermiller JB, Krucoff MW, Kereiakes DJ, et al. Benefits and Risks of Extended Dual Antiplatelet Therapy After Everolimus-Eluting Stents. JACC Cardiovasc Interv 2016;9:138–47. [DOI] [PubMed] [Google Scholar]
- [3].Shivaraju A, Patel V, Fonarow GC, et al. Temporal trends in gastrointestinal bleeding associated with percutaneous coronary intervention: analysis of the 1998-2006 Nationwide Inpatient Sample (NIS) database. Am Heart J 2011;162:1062–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Parri MS, Gianetti J, Dushpanova A, et al. Pantoprazole significantly interferes with antiplatelet effect of clopidogrel: results of a pilot randomized trial. Int J Cardiol 2013;167:2177–81. [DOI] [PubMed] [Google Scholar]
- [5].Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018;39:213–60. [DOI] [PubMed] [Google Scholar]
- [6].Zou D, Goh KL. East Asian perspective on the interaction between proton pump inhibitors and clopidogrel. J Gastroenterol Hepatol 2017;32:1152–9. [DOI] [PubMed] [Google Scholar]
- [7].Goldstein JA, Ishizaki T, Chiba K, et al. Frequencies of the defective CYP2C19 alleles responsible for the mephenytoin poor metabolizer phenotype in various Oriental, Caucasian, Saudi Arabian and American black populations. Pharmacogenetics 1997;7:59–64. [DOI] [PubMed] [Google Scholar]
- [8].Zhu P, Gao Z, Tang XF, et al. Impact of Proton-pump Inhibitors on the Pharmacodynamic Effect and Clinical Outcomes in Patients Receiving Dual Antiplatelet Therapy after Percutaneous Coronary Intervention: A Propensity Score Analysis. Chin Med J (Engl) 2017;130:2899–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Aihara H, Sato A, Takeyasu N, et al. Effect of individual proton pump inhibitors on cardiovascular events in patients treated with clopidogrel following coronary stenting: results from the Ibaraki Cardiac Assessment Study Registry. Catheter Cardiovasc Interv 2012;80:556–63. [DOI] [PubMed] [Google Scholar]
- [10].Zou JJ, Chen SL, Tan J, et al. Increased risk for developing major adverse cardiovascular events in stented Chinese patients treated with dual antiplatelet therapy after concomitant use of the proton pump inhibitor. PLoS One 2014;9:e84985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jackson LR, Peterson ED, McCoy LA, et al. Impact of Proton Pump Inhibitor Use on the Comparative Effectiveness and Safety of Prasugrel Versus Clopidogrel: Insights From the Treatment With Adenosine Diphosphate Receptor Inhibitors: Longitudinal Assessment of Treatment Patterns and Events After Acute Coronary Syndrome (TRANSLATE-ACS) Study. J Am Heart Assoc 2016;5:e003824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chandrasekhar J, Bansilal S, Baber U, et al. Impact of proton pump inhibitors and dual antiplatelet therapy cessation on outcomes following percutaneous coronary intervention: Results From the PARIS Registry. Catheter Cardiovasc Interv 2017;89:E217–25. [DOI] [PubMed] [Google Scholar]
- [13].Yan Y, Wang X, Fan JY, et al. Impact of concomitant use of proton pump inhibitors and clopidogrel or ticagrelor on clinical outcomes in patients with acute coronary syndrome. J Geriatr Cardiol 2016;13:209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gargiulo G, Costa F, Ariotti S, et al. Impact of proton pump inhibitors on clinical outcomes in patients treated with a 6- or 24-month dual-antiplatelet therapy duration: Insights from the PROlonging Dual-antiplatelet treatment after Grading stent-induced Intimal hyperplasia studY trial. Am Heart J 2016;174:95–102. [DOI] [PubMed] [Google Scholar]
- [15].Weisz G, Smilowitz NR, Kirtane AJ, et al. Proton Pump Inhibitors, Platelet Reactivity, and Cardiovascular Outcomes After Drug-Eluting Stents in Clopidogrel-Treated Patients: The ADAPT-DES Study. Circ Cardiovasc Interv 2015;8:e001952. [DOI] [PubMed] [Google Scholar]
- [16].Burkard T, Kaiser CA, Brunner-La Rocca H, et al. Combined clopidogrel and proton pump inhibitor therapy is associated with higher cardiovascular event rates after percutaneous coronary intervention: a report from the BASKET trial. J Intern Med 2012;271:257–63. [DOI] [PubMed] [Google Scholar]
- [17].Kimura T, Morimoto T, Furukawa Y, et al. Association of the use of proton pump inhibitors with adverse cardiovascular and bleeding outcomes after percutaneous coronary intervention in the Japanese real world clinical practice. Cardiovasc Interv Ther 2011;26:222–33. [DOI] [PubMed] [Google Scholar]
- [18].Chitose T, Hokimoto S, Oshima S, et al. Clinical outcomes following coronary stenting in Japanese patients treated with and without proton pump inhibitor. Circ J 2012;76:71–8. [DOI] [PubMed] [Google Scholar]
- [19].Tentzeris I, Jarai R, Farhan S, et al. Impact of concomitant treatment with proton pump inhibitors and clopidogrel on clinical outcome in patients after coronary stent implantation. Thromb Haemost 2010;104:1211–8. [DOI] [PubMed] [Google Scholar]
- [20].Kreutz RP, Stanek EJ, Aubert R, et al. Impact of proton pump inhibitors on the effectiveness of clopidogrel after coronary stent placement: the clopidogrel Medco outcomes study. Pharmacotherapy 2010;30:787–96. [DOI] [PubMed] [Google Scholar]
- [21].Rossini R, Capodanno D, Musumeci G, et al. Safety of clopidogrel and proton pump inhibitors in patients undergoing drug-eluting stent implantation. Coron Artery Dis 2011;22:199–205. [DOI] [PubMed] [Google Scholar]
- [22].Gaglia MA, Torguson R, Hanna N, et al. Relation of proton pump inhibitor use after percutaneous coronary intervention with drug-eluting stents to outcomes. Am J Cardiol 2010;105:833–8. [DOI] [PubMed] [Google Scholar]
- [23].Gupta E, Bansal D, Sotos J, et al. Risk of adverse clinical outcomes with concomitant use of clopidogrel and proton pump inhibitors following percutaneous coronary intervention. Dig Dis Sci 2010;55:1964–8. [DOI] [PubMed] [Google Scholar]
- [24].Zairis MN, Tsiaousis GZ, Patsourakos NG, et al. The impact of treatment with omeprazole on the effectiveness of clopidogrel drug therapy during the first year after successful coronary stenting. Can J Cardiol 2010;26:e54–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yasu T, Ikee R, Miyasaka Y, et al. Efficacy and safety of concomitant use of rabeprazole during dual-antiplatelet therapy with clopidogrel and aspirin after drug-eluting stent implantation: a retrospective cohort study. Yakugaku Zasshi 2010;130:1743–50. [DOI] [PubMed] [Google Scholar]
- [26].Kim KA, Park PW, Hong SJ, et al. The effect of CYP2C19 polymorphism on the pharmacokinetics and pharmacodynamics of clopidogrel: a possible mechanism for clopidogrel resistance. Clin Pharmacol Ther 2008;84:236–42. [DOI] [PubMed] [Google Scholar]
- [27].Yun KH, Rhee SJ, Park HY, et al. Effects of omeprazole on the antiplatelet activity of clopidogrel. Int Heart J 2010;51:13–6. [DOI] [PubMed] [Google Scholar]
- [28].Sibbing D, Morath T, Stegherr J, et al. Impact of proton pump inhibitors on the antiplatelet effects of clopidogrel. Thromb Haemost 2009;101:714–9. [PubMed] [Google Scholar]
- [29].Gilard M, Arnaud B, Cornily JC, et al. Influence of omeprazole on the antiplatelet action of clopidogrel associated with aspirin: the randomized, double-blind OCLA (Omeprazole CLopidogrel Aspirin) study. J Am Coll Cardiol 2008;51:256–60. [DOI] [PubMed] [Google Scholar]
- [30].Mega JL, Close SL, Wiviott SD, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med 2009;360:354–62. [DOI] [PubMed] [Google Scholar]
- [31].Manolis AA, Manolis TA, Melita H, et al. Proton pump inhibitors and cardiovascular adverse effects: Real or surreal worries? Eur J Intern Med 2020;72:15–26. [DOI] [PubMed] [Google Scholar]
- [32].Yepuri G, Sukhovershin R, Nazari-Shafti TZ, et al. Proton Pump Inhibitors Accelerate Endothelial Senescence. Circ Res 2016;118:e36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Nikolsky E, Stone GW, Kirtane AJ, et al. Gastrointestinal bleeding in patients with acute coronary syndromes: incidence, predictors, and clinical implications: analysis from the ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) trial. J Am Coll Cardiol 2009;54:1293–302. [DOI] [PubMed] [Google Scholar]
- [34].Chen YL, Chang CL, Chen HC, et al. Major adverse upper gastrointestinal events in patients with ST-segment elevation myocardial infarction undergoing primary coronary intervention and dual antiplatelet therapy. Am J Cardiol 2011;108:1704–9. [DOI] [PubMed] [Google Scholar]
- [35].Jensen BES, Hansen JM, Larsen KS, et al. Randomized clinical trial: the impact of gastrointestinal risk factor screening and prophylactic proton pump inhibitor therapy in patients receiving dual antiplatelet therapy. Eur J Gastroenterol Hepatol 2017;29:1118–25. [DOI] [PubMed] [Google Scholar]
- [36].Hagymási K, Müllner K, Herszényi L, et al. Update on the pharmacogenomics of proton pump inhibitors. Pharmacogenomics 2011;12:873–88. [DOI] [PubMed] [Google Scholar]
- [37].Bhatt DL, Cryer BL, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. New England Journal of Medicine 2010;363:1909–17. [DOI] [PubMed] [Google Scholar]
- [38].Liu LP, Wang Y, Si R, et al. Esomeprazole and rabeprazole did not reduce antiplatelet effects of aspirin/clopidogrel dual therapy in patients undergoing percutaneous coronary intervention: a prospective, randomized, case-control study. Expert Opin Pharmacother 2016;17:7–16. [DOI] [PubMed] [Google Scholar]


