Abstract
Lung cancer is the most common type of cancer worldwide with a high mortality rate. The specific tyrosine kinase inhibitors of epidermal growth factor receptor (EGFR) have made enormous strides in non-small-cell lung cancer (NSCLC) treatment. The novel systemic immune-inflammation index (SII), a parameter that integrates lymphocytes, neutrophils, and platelets, has been found to play the vital role of a marker for predicting survival and recrudescence in various tumors.
We retrospectively examined 102 patients with different EGFR-mutant lung adenocarcinomas. Survival analysis was performed using the Kaplan-Meier method with the log-rank test. Cut-off points were identified using the receiver operating characteristic curves with the maximum log-rank values. The Cox proportional hazards regression, expressed as p value, hazards regression, and 95% confidence interval, was conducted to assess the prognostic values of variables in overall survival (OS)/ progression-free survival (PFS).
Lower SII was associated with prolonged survival in patients with different EGFR mutant lung adenocarcinomas in both variable and multivariable analyses.
SII before treatment was a powerful indicator for the PFS and OS of patients who received the first-generation EGFR-TKI.
Keywords: adenocarcinoma, epidermal growth factor receptor, prognosis, systemic immune-inflammation index
1. Introduction
Lung cancer is the most common type of cancer worldwide with a high mortality rate. The majority of patients are diagnosed at an advanced stage because the tumor is typically asymptomatic for a long time.[1,2] Primary lung cancer includes the following 2 pathological types: non-small-cell lung cancer (NSCLC) and small-cell lung cancer (SCLC). NSCLC accounts for the majority of all newly diagnosed lung cancers, which include many pathological subtypes, such as adenocarcinoma, squamous carcinoma, and large-cell carcinoma.[3,4] Chemotherapy and radiotherapy were mainly used to treat advanced metastatic lung cancer, before the advent of targeted therapy. After treatment, the mortality rate of patients with lung cancer has not changed to a great extent.[2] Epidermal growth factor receptor (EGFR)/ERBB1/HER1, which is a member of the EGFR family, is a transmembrane protein with cytoplasmic kinase activity.
The specific tyrosine kinase inhibitors (TKIs) of EGFR have made enormous strides in NSCLC treatment.[5] During the last decade, EGFR-TKI has been identified as a rising star for accurate lung cancer treatment. Lung adenocarcinoma, which is the most common form of lung cancer, has a higher EGFR mutation rate (about 30%–40%) among Asian patients.[6] Many clinical trials have suggested that lung adenocarcinomas harboring the EGFR mutation treated with EGFR-TKI (erlotinib, gefitinib, or afatinib) are associated with significant improvement in progression-free survival (PFS) and objective response rate (ORR) compared with tumors treated with standard first-line chemotherapy.[7–9]
For a long time, increasing research has corroborated the role of inflammatory factors that participate in tumor shaping and metastasis.[9] Several immune-inflammation-based indexes, such as the monocyte/lymphocyte ratio (MLR), the platelet/lymphocyte ratio (PLR), and the neutrophil/lymphocyte ratio (NLR), have been proven to predict cancer recurrence and prognosis in patients with various malignant solid tumors.[10–12] The novel systemic immune-inflammation index (SII), a parameter that integrates lymphocytes, neutrophils, and platelets, has been found to be more promising than PLR, MLR, or NLR.[13–15] Previous studies have demonstrated that the SII plays the vital role of a marker for predicting survival and recrudescence in various tumors, such as esophageal cancer,[16] hepatocellular carcinoma,[15] pancreatic cancer,[17] and prostate cancer.[18]
However, there is no predictive biomarker that can be easily detected and can accurately predict the prognosis of patients with NSCLC and EGFR mutation. Therefore, our research aimed to investigate the role of the SII in predicting the prognosis of advanced lung adenocarcinoma with different EGFR mutations.
2. Methods
2.1. Ethical statement
This research was approved by the Ethics Committee of Xijing Hospital, the First Hospital affiliated to the Fourth Military Medical University.
2.2. Patients and follow-up
We consecutively collected the data on patients with NSCLC who harbored EGFR mutations and received EGFR-TKI treatment from January 2014 to December 2016 at Xijing Hospital. The inclusion criteria were as follows:
-
1.
exon 19 deletion and Leu858Arg point mutation in exon 21 (L858R);
-
2.
age range, 30 to 80 years;
-
3.
adenocarcinoma, adenocarcinoma with squamous differentiation, or adenosquamous carcinoma;
-
4.
EGFR-TKIs as the first-line treatment and patients who did not receive another treatment before targeted therapy;
-
5.
Eastern Cooperative Oncology Group Performance Status (ECOG PS) from 0 to 2;
-
6.
stages IIIb and IV.
The clinical stage evaluation at least included computed tomography scans of the chest and upper abdomen, bone, and brain. All of the patients were pathologically diagnosed according to the World Health Organization pathology classification.[19] All of the patients were routinely administered the first-generation EGFR-TKI, including gefitinib, erlotinib, or icotinib, until disease progression or intolerance to adverse effects. Ultimately, 102 patients with advanced stage adenocarcinoma (entirely or partially) were included in our study. All of the patients were followed up through their medical records at the hospital or by telephone until March 2019. PFS referred to survival from the initiation of EGFR-TKI treatment to disease progression, which was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) (Version 1.1), or death from other causes.[20] A total of 89 subjects showed disease progression during the follow-up, and PFS varied from 1 month to 48 months. Overall survival (OS) referred to survival from the initiation of EGFR-TKI treatment to death from any cause.
2.3. Statistical analyses
Survival analysis was performed using the Kaplan–Meier method with the log-rank test. Cutoff points were identified using the receiver operating characteristic curves with the maximum log-rank values. Systematic immune indexes included NLR (NLR = neutrophil count/lymphocyte count), PLR (PLR = platelet count/lymphocyte count), MLR (MLR = monocyte count/lymphocyte count), and SII (SII = platelet count × neutrophil count/lymphocyte count). The Cox proportional hazards regression, expressed as p value, hazards regression, and 95% confidence interval, was conducted to assess the prognostic values of variables in OS/PFS. The variables that were statistically significant in univariate Cox analysis were selected for the multivariate Cox analysis. All reported P values were two-sided. A P < .05 was considered statistically significant. All statistical analyses were performed using SPSS 22.0 for Windows (SPSS Inc, Chicago, IL).
3. Results
3.1. The SII as a prognostic factor
Ultimately, 102 patients were included in our analysis (Table 1). Among these patients, 88 subjects had pure adenocarcinoma, whereas 14 subjects had mixed carcinoma (mainly adenosquamous carcinoma). The median age was 59.50 years, ranging from 30 years to 80 years. First-generation EGFR-TKI in our analysis included gefitinib (66 patients), erlotinib (15 patients), and icotinib (21 patients). Among these patients, females and never-smokers constituted the majority.
Table 1.
Clinical characteristics of all 102 lung cancer patients.
| Characteristic | Data |
| No. of patients | 102 |
| Age (years) Mean ± SD (range; median) | 58.37 ± 10.51 (30–80; 59.50) |
| Gender | |
| Male | 41 (40.2%) |
| Female | 61 (59.8%) |
| Smoke status | |
| Never smoker | 76 (74.5%) |
| Smoker | 26 (25.5%) |
| Histology | |
| entire ADC | 88 (86.3%) |
| partial ADC | 14 (13.7%) |
| TNM stage | |
| III | 23 (22.5%) |
| IV | 79 (77.5%) |
| Drug | |
| Icotinib | 21 (20.6%) |
| Erlotinib | 15 (14.7%) |
| Gefitinib | 66 (64.7%) |
| PLR Mean ± SD (range; median) | 175.31 ± 90.05 (60.85–748.57; 156.11) |
| MLR Mean ± SD (range; median) | 0.33 ± 0.23 (0.11–1.75; 0.26) |
| NLR Mean ± SD (range; median) | 3.41 ± 1.67 (0.98–8.33; 2.95) |
| SII Mean ± SD (range; median) | 855.31 ± 560.89 (130.21–3480.86; 712.76) |
| PFS (months) Median/Mean ± SD | 11.00/12.79 ± 10.74 |
| OS (months) Median/Mean ± SD | 20.05/22.63 ± 13.03 |
Using Kaplan–Meier curves, high SII values indicated a poor PFS (P = .028) (Fig. 1) and OS (P = .014) (Fig. 2). Otherwise, patients >57 years old had a longer PFS (P = .004) (Fig. 1). Patients with a more advanced stage of cancer had a shorter OS (P = .015) (Fig. 2).
Figure 1.
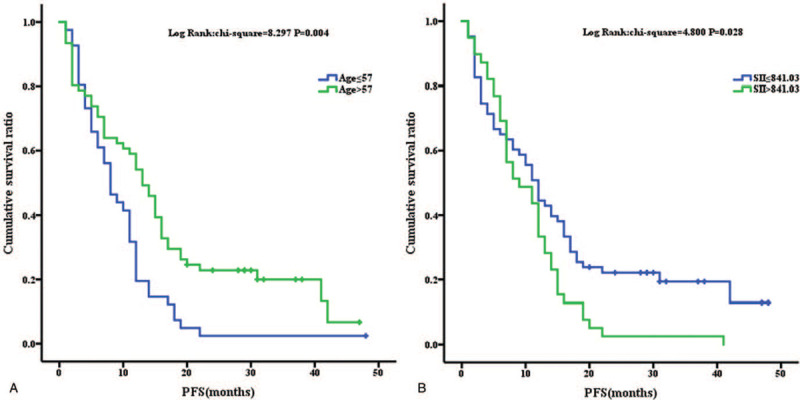
Kaplan–Meier analysis for PFS analysis for age (1A) and SII (1B).
Figure 2.
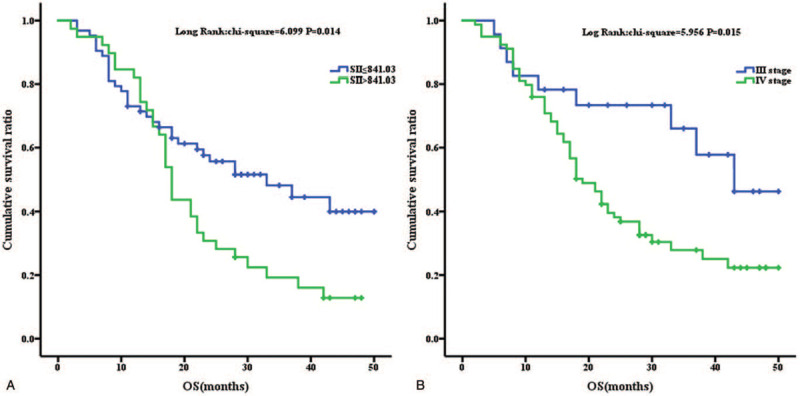
Kaplan–Meier analysis for OS analysis for SII (2A) and Stage (2B).
3.2. Univariate and multivariate analyses of clinical characteristics related to long-term outcome of EGFR-TKI treatment
The cut-off values in our analyses were 57 (for age), 172.88 (for PLR), 0.23 (for MLR), 2.72 (for NLR), and 841.03 (for the SII), respectively, in this study. The Cox analysis results showed that among the inflammatory indexes, the SII was significantly associated with PFS (Univariate: P = .036, hazards ratio [HR] (confidence interval) [CI], 1.577 (1.030–2.415); Multivariate: P = .043, HR (CI), 1.555 (1.014–2.385)), whereas NLR, PLR, and MLR were not associated with PFS. Interestingly, drug selection (gefitinib, erlotinib, or icotinib) did not affect the PFS. There was no significant difference in the progression time between stages III and IV. However, elderly patients had a lower risk of early progression (Univariate: P = .006, HR (CI), 0.550 (0.358–0.843); Multivariate: P = .007, HR (CI), 0.555 (0.361–0.853)).
For the OS analysis, stage and SII were independent prognostic factors for patients who received EGFR-TKI treatment (P = .043 and .049, respectively) (Table 3). Neither smoking status nor gender had a significant influence on the OS. Similarly, any kind of first-generation EGFR-TKI was available (P = .322). Once the EGFR mutant status was suitable, pure adenocarcinoma and partial adenocarcinoma did not exhibit a significant difference in the long-term prognosis (Tables 2 and 3).
Table 3.
Univariate and multivariate proportional hazards (Cox) regression analyses according to OS.
| Univariate Cox analysis | Multivariate Cox analysis | |||
| Variables in the equation | P | Hazard ratio (95% CI) | P | Hazard ratio (95.0% CI) |
| Age | ||||
| <57 | – | – | ||
| >57 | .360 | 0.793 (0.483–1.303) | ||
| Gender | ||||
| Male | – | – | ||
| Female | .572 | 0.865 (0.523–1.430) | ||
| Smoke status | ||||
| Never | – | – | ||
| Smoker | .946 | 1.020 (0.572–1.818) | ||
| Histology | ||||
| ADC | – | – | ||
| Others | .462 | 0.745 (0.340–1.633) | ||
| TNM stage | ||||
| III | – | – | – | – |
| IV | .020 | 2.326 (1.145–4.724) | 0.043 | 2.096 (1.022–4.299) |
| PLR | ||||
| <172.88 | – | – | ||
| >172.88 | .053 | 1.621 (0.993–2.646) | ||
| MLR | ||||
| <0.23 | – | – | ||
| >0.23 | .055 | 1.690 (0.989–2.887) | ||
| NLR | ||||
| <2.72 | – | – | ||
| >2.72 | .140 | 1.469 (0.881–2.448) | ||
| SII | ||||
| <841.03 | – | – | – | – |
| >841.03 | .017 | 1.817 (1.114–2.961) | 0.049 | 1.644 (1.002–2.696) |
| Drug | ||||
| Icotinib | .322 | – | ||
| Erlotinib | .141 | 0.509 (0.207–1.251) | ||
| Gefitinib | .324 | 0.742 (0.411–1.341) | ||
Table 2.
Univariate and multivariate proportional hazards (Cox) regression analyses according to PFS.
| Univariate Cox analysis | Multivariate Cox analysis | |||
| Variables in the equation | P | Hazard ratio (95%CI) | P | Hazard ratio (95.0% CI) |
| Age | ||||
| <57 | – | – | – | – |
| >57 | .006 | 0.550 (0.358–0.843) | .007 | 0.555 (0.361–0.853) |
| Gender | ||||
| Male | – | – | ||
| Female | .410 | 0.837 (0.549–1.277) | ||
| Smoke status | ||||
| Never | – | – | ||
| Smoker | .313 | 1.273 (0.796–2.034) | ||
| Histology | ||||
| ADC | – | – | ||
| Others | .391 | 0.766 (0.416–1.409) | ||
| TNM stage | ||||
| III | – | – | ||
| IV | .500 | 1.191 (0.717–1.978) | ||
| PLR | ||||
| <172.88 | – | – | ||
| >172.88 | .137 | 1.385 (0.902–2.127) | ||
| MLR | ||||
| <0.23 | – | – | ||
| >0.23 | .055 | 1.557 (0.990–2.450) | ||
| NLR | ||||
| <2.72 | – | – | ||
| >2.72 | .092 | 1.453 (0.940–2.246) | ||
| SII | ||||
| <841.03 | – | – | – | – |
| >841.03 | .036 | 1.577 (1.030–2.415) | 0.043 | 1.555 (1.014–2.385) |
| Drug | ||||
| Icotinib | .792 | – | ||
| Erlotinib | .736 | 0.884 (0.431–1.812) | ||
| Gefitinib | .464 | 0.832 (0.490–1.413) | ||
3.3. Subgroup analyses
For PFS, the SII was significant in never-smokers (P = .031) and had edge significance in the female subgroup (P = .050), whereas SII demonstrated no statistical significance in the other subgroups with the limitation of sample size (Table 4). This meant that the SII might be more significant in females and never-smokers who received EGFR-TKIs for predicting disease progression. However, for OS, the SII was a significant predictive factor in male and stage IV patients. Interestingly, the SII was more effective in stage IV patients, which indicated that the inflammatory status was more essential for the OS of patients with more advanced stages of cancer.
Table 4.
subgroup analyses according to PFS and OS.
| PFS | OS | |||
| Variables in the equation | P | Hazard ratio (95% CI) | P | Hazard ratio (95.0% CI) |
| Age | ||||
| <57 | .300 | 1.401 (0.740–2.653) | .054 | 2.153 (0.988–4.691) |
| >57 | .071 | 1.697 (0.956–3.013) | .191 | 1.534 (0.808–2.912) |
| Gender | ||||
| Male | .451 | 1.298 (0.659–2.557) | .015 | 2.709 (1.212–6.052) |
| Female | .050 | 1.754 (1.000–3.077) | .271 | 1.419 (0.761–2.647) |
| Smoke status | ||||
| Never | .031 | 1.730 (1.051–2.850) | .054 | 1.726 (0.990–3.012) |
| Smoker | .891 | 1.063 (0.444–2.548) | .014 | 2.185 (0.774–6.165) |
| Histology | ||||
| ADC | .130 | 1.423 (0.901–2.248) | .068 | 1.627 (0.965–2.743) |
| Others | .072 | 3.922 (0.885–17.376) | .134 | 3.570 (0.675–18.876) |
| TNM stage | ||||
| III | .177 | 2.268 (0.691–7.441) | .931 | 1.072 (0.221–5.199) |
| IV | .127 | 1.443 (0.901–2.311) | .041 | 1.740 (1.024–2.959) |
4. Discussion
The SII, a novel biomarker, has been shown to be correlated with poor prognosis in patients with lung cancer. As already known, the SII is calculated on the basis of the peripheral lymphocyte, neutrophil, and platelet blood counts; these 3 types of cells are involved in the inflammatory response of the human body. To date, many studies have confirmed that the inflammatory reaction in the human body is closely related to the occurrence and development of tumors.[21,22]
Therefore, the SII is regarded as a valuable and trustworthy noninvasive indicator for evaluating the survival and prognosis in patients with tumors.[13,16–18] In recent years, an increasing number of clinical studies have confirmed that the SII can predict the prognosis and the recurrence of lung cancer in patients. Li et al suggested that the decrease in SII values was associated with longer survival time in patients with brain metastasis from lung cancer, irrespective of whether there was an EGFR mutation.[23] Besides, Hong et al found that the patients with small-cell lung cancer who have SII >1600 × 109/L may have worse prognosis than patients with low SII values.[24] Consequently, scholars began to pay increased attention to the clinical significance of the SII in guiding the prognosis of patients with lung cancer.
Although EGFR-targeted therapy has produced prominent effects in alleviating tumor progression in patients with advanced NSCLC harboring the indicated driven-mutations,[25,26] several variations of molecular phenotypes may lead to tumor recurrence and poor prognosis in an acquired-drug resistance manner. The occurrence of a secondary mutation, especially the T790 M substitution in exon 20 of the EGFR kinase domain, acts as one of the most common mechanisms in mediating acquired resistance to gefitinib, the first-generation EGFR-TKI.[27] This abnormal gene expression weakens the combination between targeted drugs and the ATP-binding sites in EGFR, leading to continuous activation of EGFR-induced downstream signaling, and in turn, gives rise to resistance features.[28] The activation of an alternative pathway reveals another resistance mechanism, which activates the cytokine receptor on the membrane in an EGFR-independent manner and initiates the same signaling pathway with EGFR. The amplification of MET, a transmembrane tyrosine kinase receptor, has been viewed as a typical mutant oncogene in bypass activation.[29] Additionally, there is phenotype transformation from NSCLC to SCLC.[30]
In vitro experiments and clinical and epidemiologic studies, increasing evidence suggests that inflammatory response, immune microenvironment, and tumorigenesis are closely related to one another.[31,32] These theories might explain the mechanism of the high SII value associated with poor survival in patients with lung cancer. As early as the 19th century, Rudolf Virchow observed the presence of leukocytes within tumors. Subsequent studies found that inflammatory responses play a decisive role in nearly all stages of tumor development.[22,33–35] Current research reveals that hematopoiesis defects and immunosuppression may play an important role in tumor progression. In a stable state, the development of blood cell lines is strictly controlled by endogenous signals, which promote continuous differentiation of hematopoietic stem and progenitor cells (HSPCs).[36] Several studies found that HSPCs express receptors for various cytokines and microbial products. They inferred that hematopoiesis functions play a critical role in the primary immune response to tumors.[37–39] Therefore, it will lead to destruction of hematopoietic homeostasis in the process of tumor development, including myelopoiesis and leukocytosis. The present study showed that peripheral blood hematopoietic precursors could selectively lose lymphoid potential and skew towards granulocyte differentiation in patients with tumors.[40] Accordingly, increased number of peripheral neutrophils and NLR could serve as indicators for poor prognosis in patients with different types of tumors.
Macrophages, lymphocytes, neutrophils, and dendritic cells belong to the leukocyte population. They take part in the invasion, metastasis, and angiogenesis of lung cancer cells by releasing a variety of cytokines, cytotoxic mediators, and chemokines, such as reactive oxygen species, metalloproteinases (MMPs), and membrane perforator agents, and soluble mediators of cell death, such as tumor necrosis factor (TNF)-α, interleukins (ILs), and interferons.[41] Karin et al. suggested that NF-κB and proinflammatory cytokines, such as IL-1β and TNF-α, are involved in promoting cancer cell proliferation by interacting with each other.[42] MMPs are a family of proteolytic enzymes that include MMP-1, -3, -7, and -9. Liu et al. reported that polymorphisms in the promoter regions of MMP-1, -3, -7, and -9 might be associated with metastasis in many cancers, such as lung cancer, breast cancer, and head/neck cancer.[43,44] Additional studies suggested that they not only participate in lung cancer metastasis but also in nearly all stages of cancer progression.[45]
However, this study has several limitations. This is a single-center, retrospective study. The number of samples included in the study is limited. The results of our study should be interpreted with caution. A multicenter, prospective study with a large population size is needed to confirm our results. Besides, tumor-associated macrophages (TAMs), which originate from circulatory monocytes, play an important part in the tumor microenvironment. Numerous data have revealed that TAMs are closely correlated with cancer progression.[46,47] Moreover, many previous studies have shown that the peripheral monocyte count is a useful prognostic marker.[48–50] However, monocytes are not included in the index. A more accurate index, which can combine them together, is needed to better predict the prognosis of EGFR-mutant lung adenocarcinoma. Nonetheless, to the best of our knowledge, this is the first study to evaluate the impact of relatively comprehensive inflammation-based scores (SII) on the prognosis of NSCLC with EGFR mutations in patients who received the first-generation EGFR-TKI.
5. Conclusion
Systemic immune and inflammation status was closely related to disease progression in patients with advanced lung adenocarcinoma. The SII before treatment was a powerful indicator for the PFS and OS in patients who received the first-generation EGFR-TKI.
Author contributions
Conceptualization: Qing Ju, Tingping Huang.
Data curation: Qing Ju, Tao Yan.
Formal analysis: Tingping Huang, Yong Zhang.
Methodology: Lei Wu, Jing Geng, Xiaoyan Mu, Jian Zhang.
Project administration: Qing Ju, Tingping Huang, Jian Zhang.
Writing – original draft: Qing Ju, Tingping Huang, Yong Zhang.
Writing – review & editing: Lei Wu, Jing Geng, Xiaoyan Mu, Tao Yan, Jian Zhang.
Glossary
Abbreviations: ECOG PS = Eastern Cooperative Oncology Group Performance Status, EGFR = epidermal growth factor receptor, HSPCs = hematopoietic stem and progenitor cells, ILs = interleukins, MLR = monocyte/lymphocyte ratio, MMPs = metalloproteinases, NLR = neutrophil/lymphocyte ratio, NSCLC = non-small-cell lung cancer, ORR = objective response rate, OS = overall survival, PFS = progression-free survival, PLR = platelet/lymphocyte ratio, RECIST = Response Evaluation Criteria in Solid Tumors, SCLC = small-cell lung cancer, SII = systemic immune-inflammation index, TKIs = tyrosine kinase inhibitors, TNF = tumor necrosis factor.
References
- [1].Herbst RS, Heymach JV, Lippman SM. Lung cancer. The New England journal of medicine 2008;359:1367–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Saintigny P, Burger JA. Recent advances in non-small cell lung cancer biology and clinical management. Discovery medicine 2012;13:287–97. [PubMed] [Google Scholar]
- [3].Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA: a cancer journal for clinicians 2016;66:115–32. [DOI] [PubMed] [Google Scholar]
- [4].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA: a cancer journal for clinicians 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- [5].da Cunha Santos G, Shepherd FA, Tsao MS. EGFR mutations and lung cancer. Annual review of pathology 2011;6:49–69. [DOI] [PubMed] [Google Scholar]
- [6].Li C, Fang R, Sun Y, et al. Spectrum of oncogenic driver mutations in lung adenocarcinomas from East Asian never smokers. PloS one 2011;6:e28204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. The New England journal of medicine 2009;361:947–57. [DOI] [PubMed] [Google Scholar]
- [8].Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. The Lancet Oncology 2012;13:239–46. [DOI] [PubMed] [Google Scholar]
- [9].Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. The Lancet Oncology 2014;15:213–22. [DOI] [PubMed] [Google Scholar]
- [10].Takeuchi H, Kawanaka H, Fukuyama S. Comparison of the prognostic values of preoperative inflammation-based parameters in patients with breast cancer. PloS one 2017;12:e0177137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wu G, Yao Y, Bai C, et al. Combination of platelet to lymphocyte ratio and neutrophil to lymphocyte ratio is a useful prognostic factor in advanced non-small cell lung cancer patients. Thoracic cancer 2015;6:275–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Templeton AJ, Ace O, McNamara MG, et al. Prognostic role of platelet to lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2014;23:1204–12. [DOI] [PubMed] [Google Scholar]
- [13].Chen JH, Zhai ET, Yuan YJ, et al. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World journal of gastroenterology 2017;23:6261–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wang L, Wang C, Wang J, et al. A novel systemic immune-inflammation index predicts survival and quality of life of patients after curative resection for esophageal squamous cell carcinoma. Journal of cancer research and clinical oncology 2017;143:2077–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hu B, Yang XR, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clinical cancer research: an official journal of the American Association for Cancer Research 2014;20:6212–22. [DOI] [PubMed] [Google Scholar]
- [16].Geng Y, Shao Y, Zhu D, et al. Systemic Immune-Inflammation Index Predicts Prognosis of Patients with Esophageal Squamous Cell Carcinoma: A Propensity Score-matched Analysis. Scientific reports 2016;6:39482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Aziz MH, Sideras K, Aziz NA, et al. The Systemic-immune-inflammation Index Independently Predicts Survival and Recurrence in Resectable Pancreatic Cancer and its Prognostic Value Depends on Bilirubin Levels: A Retrospective Multicenter Cohort Study. Annals of surgery 2019;270:139–46. [DOI] [PubMed] [Google Scholar]
- [18].Lolli C, Caffo O, Scarpi E, et al. Systemic Immune-Inflammation Index Predicts the Clinical Outcome in Patients with mCRPC Treated with Abiraterone. Frontiers in pharmacology 2016;7:376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2015;10:1243–60. [DOI] [PubMed] [Google Scholar]
- [20].Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). European journal of cancer (Oxford, England : 1990) 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- [21].Gomes M, Teixeira AL, Coelho A, et al. The role of inflammation in lung cancer. Advances in experimental medicine and biology 2014;816:1–23. [DOI] [PubMed] [Google Scholar]
- [22].Hamilton G, Rath B. Smoking, inflammation and small cell lung cancer: recent developments. Wiener medizinische Wochenschrift (1946) 2015;165:379–86. [DOI] [PubMed] [Google Scholar]
- [23].Li H, Wang G, Zhang H, et al. Prognostic role of the systemic immune-inflammation index in brain metastases from lung adenocarcinoma with different EGFR mutations. Genes and immunity 2019;20:455–61. [DOI] [PubMed] [Google Scholar]
- [24].Hong X, Cui B, Wang M, et al. Systemic Immune-inflammation Index, Based on Platelet Counts and Neutrophil-Lymphocyte Ratio, Is Useful for Predicting Prognosis in Small Cell Lung Cancer. The Tohoku journal of experimental medicine 2015;236:297–304. [DOI] [PubMed] [Google Scholar]
- [25].Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. The New England journal of medicine 2010;362:2380–8. [DOI] [PubMed] [Google Scholar]
- [26].Wu YL, Cheng Y, Zhou X, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. The Lancet Oncology 2017;18:1454–66. [DOI] [PubMed] [Google Scholar]
- [27].Sos ML, Rode HB, Heynck S, et al. Chemogenomic profiling provides insights into the limited activity of irreversible EGFR Inhibitors in tumor cells expressing the T790M EGFR resistance mutation. Cancer research 2010;70:868–74. [DOI] [PubMed] [Google Scholar]
- [28].Yun CH, Mengwasser KE, Toms AV, et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proceedings of the National Academy of Sciences of the United States of America 2008;105:2070–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Onitsuka T, Uramoto H, Nose N, et al. Acquired resistance to gefitinib: the contribution of mechanisms other than the T790M, MET, and HGF status. Lung cancer (Amsterdam, Netherlands) 2010;68:198–203. [DOI] [PubMed] [Google Scholar]
- [30].Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clinical cancer research : an official journal of the American Association for Cancer Research 2013;19:2240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ribeiro R, Araújo A, Lopes C, et al. Immunoinflammatory mechanisms in lung cancer development: is leptin a mediator? Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2007;2:105–8. [DOI] [PubMed] [Google Scholar]
- [32].Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. The Journal of clinical investigation 2007;117:1175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:883–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature 2008;454:436–44. [DOI] [PubMed] [Google Scholar]
- [35].Karin M. Nuclear factor-kappaB in cancer development and progression. Nature 2006;441:431–6. [DOI] [PubMed] [Google Scholar]
- [36].Kondo M, Wagers AJ, Manz MG, et al. Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annual review of immunology 2003;21:759–806. [DOI] [PubMed] [Google Scholar]
- [37].Cortez-Retamozo V, Etzrodt M, Newton A, et al. Origins of tumor-associated macrophages and neutrophils. Proceedings of the National Academy of Sciences of the United States of America 2012;109:2491–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Essers MA, Offner S, Blanco-Bose WE, et al. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature 2009;458:904–8. [DOI] [PubMed] [Google Scholar]
- [39].Solito S, Falisi E, Diaz-Montero CM, et al. A human promyelocytic-like population is responsible for the immune suppression mediated by myeloid-derived suppressor cells. Blood 2011;118:2254–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Engblom C, Pfirschke C, Zilionis R, et al. Osteoblasts remotely supply lung tumors with cancer-promoting SiglecF(high) neutrophils. Science (New York, NY) 2017;358: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Karin M. The IkappaB kinase - a bridge between inflammation and cancer. Cell research 2008;18:334–42. [DOI] [PubMed] [Google Scholar]
- [43].Liu D, Guo H, Li Y, et al. Association between polymorphisms in the promoter regions of matrix metalloproteinases (MMPs) and risk of cancer metastasis: a meta-analysis. PloS one 2012;7:e31251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].González-Arriaga P, Pascual T, García-Alvarez A, et al. Genetic polymorphisms in MMP 2, 9 and 3 genes modify lung cancer risk and survival. BMC cancer 2012;12:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Gregory AD, Houghton AM. Tumor-associated neutrophils: new targets for cancer therapy. Cancer research 2011;71:2411–6. [DOI] [PubMed] [Google Scholar]
- [46].Sica A, Schioppa T, Mantovani A, et al. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. European journal of cancer (Oxford, England : 1990) 2006;42:717–27. [DOI] [PubMed] [Google Scholar]
- [47].Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nature reviews Cancer 2004;4:71–8. [DOI] [PubMed] [Google Scholar]
- [48].Shigeta K, Kosaka T, Kitano S, et al. High Absolute Monocyte Count Predicts Poor Clinical Outcome in Patients with Castration-Resistant Prostate Cancer Treated with Docetaxel Chemotherapy. Annals of surgical oncology 2016;23:4115–22. [DOI] [PubMed] [Google Scholar]
- [49].Takada K, Shimokawa M, Tanaka K, et al. Association between peripheral blood markers and immune-related factors on tumor cells in patients with resected primary lung adenocarcinoma. PloS one 2019;14:e0217991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Parikh K, Kumar A, Ahmed J, et al. Peripheral monocytes and neutrophils predict response to immune checkpoint inhibitors in patients with metastatic non-small cell lung cancer. Cancer immunology, immunotherapy : CII 2018;67:1365–70. [DOI] [PMC free article] [PubMed] [Google Scholar]


