Objectives:
To evaluate the effect of high-flow oxygen implementation on the respiratory rate as a first-line ventilation support in chronic obstructive pulmonary disease patients with acute hypercapnic respiratory failure.
Design:
Multicenter, prospective, analytic observational case series study.
Setting:
Five ICUs in Argentina, between August 2018 and September 2019.
Patients:
Patients greater than or equal to 18 years old with moderate to very severe chronic obstructive pulmonary disease, who had been admitted to the ICU with a diagnosis of hypercapnic acute respiratory failure, were entered in the study.
Interventions:
High-flow oxygen therapy through nasal cannula delivered using high-velocity nasal insufflation.
Measurements and Main Results:
Forty patients were studied, 62.5% severe chronic obstructive pulmonary disease. After the first hour of high-flow nasal cannula implementation, there was a significant decrease of respiratory rate compared with baseline values, with a 27% decline (29 vs 21 breaths/min; p < 0.001). Furthermore, a significant reduction of Paco2 (57 vs 52 mm Hg [7.6 vs 6.9 kPa]; p < 0.001) was observed. The high-flow nasal cannula application failed in 18% patients. In this group, the respiratory rate, pH, and Paco2 showed no significant change during the first hour in these patients.
Conclusions:
High-flow oxygen therapy through nasal cannula delivered using high-velocity nasal insufflation was an effective tool for reducing respiratory rate in these chronic obstructive pulmonary disease patients with acute hypercapnic respiratory failure. Early determination and subsequent monitoring of clinical and blood gas parameters may help predict the outcome.
Keywords: acute respiratory failure, chronic obstructive pulmonary disease, chronic obstructive pulmonary disease exacerbation, high-flow oxygen therapy
High-flow nasal cannula (HFNC) essentially consists of the delivery of a humidified and heated Fio2 at supraphysiologic flow rates (1). Its clinical efficacy in hypoxemic patients with acute respiratory failure (ARF) is based on the multiple physiologic effects of its application, from decreased inspiratory effort to an improved blood gas exchange (2). Its benefits were shown in various clinical studies, from which the use of HFNC has been extended to ICUs (3, 4). There is evidence that high-velocity nasal insufflation (HVNI) therapy, an advanced form of HFNC, delivering high flow at high velocity provides a more efficient flush mechanism for upper airway deadspace. This use of high velocity has been shown to be noninferior to noninvasive mechanical ventilation (NIMV) as a support strategy in ARF from various causes (5). HFNC has been suggested in successful management of patients with chronic obstructive pulmonary disease (COPD) (6). The results in a series of case reports on the use of HFNC in patients with COPD exacerbation were also encouraging (7–9). A subgroup analysis of Hypercapnic and COPD patients was performed from a larger study of HVNI in the management of undifferentiated respiratory failure, suggesting the ability to provide adequate ventilatory support by avoiding intubation (10). The efficacy and safety of the use of HVNI as a first-line support treatment strategy in COPD patients with hypercapnic respiratory failure are, however, still unknown.
The main objective of this pilot study was to evaluate the effect of HVNI on the respiratory rate (RR) when used as a first-line ventilation support in COPD patients with acute hypercapnic respiratory failure. The secondary objectives were to determine possible changes in the clinical signs of respiratory failure and in blood gas exchange and the presence of predictors for success or failure of treatment.
MATERIALS AND METHODS
This multicenter, prospective, analytic observational case series study was conducted in five ICUs in Argentina, between August 1, 2018, and September 30, 2019. Patients 18 years old or more with moderate to very severe COPD (in the primary physician’s judgment), who had been admitted to the ICU with a diagnosis of hypercapnic ARF (Paco2 > 45 mm Hg [6.0 kPa] and pH < 7.35), were included in the study. The patients’ diagnosis was determined at admission by arterial blood gas (ABG) analysis, taken while at rest receiving supplemental oxygen titrated to maintain an arterial blood oxygen concentration (Sao2) between 88% and 92%, and at least one of the following: RR greater than or equal to 25 breaths per minute, intercostal and/or supraclavicular inspiratory retraction, or thoracoabdominal asynchrony.
Demographic and anthropometric data, comorbidities, hospitalization duration, and clinical parameters were recorded. Patient comfort during HVNI therapy was recorded using a Visual Analog Scale ranked from one to five, one being “very comfortable” and five “very uncomfortable.”
Exclusion criteria include patients prescribed NIMV (before admission or at the time of evaluation), those requiring intubation and invasive mechanical ventilation (iMV) and those with pH less than 7.20, Paco2 greater than 80 mm Hg (10.7 kPa), degraded level of consciousness (Kelly-Matthay score [KMS] > 3) (11), unstable hemodynamics (systolic blood pressure < 90 mm Hg [12 kPa] or mean blood pressure < 65 mm Hg [8.7 kPa] with fluid intake), and/or contraindications to the use of HVNI (cannula placement impossible, profuse bleeding of nasal cavities, or ARF due to neuromuscular disease).
High-Flow Oxygen Therapy Delivery and Devices
The patient was placed half-seated, tilted at 45°. A Precision Flow Plus (Vapotherm, Exeter, NH) HVNI technology was used. Therapy was started at a flow rate of 40 L/min at a temperature of 43°C and Fio2 of 1.0, which was titrated targeting an Sao2 between 88% and 92%. Flow rate and temperature were adjusted to the individual patient’s work of breathing, comfort, and tolerance.
After the first hour of HVNI, ABG analysis was performed. At that time, the criteria for treatment interruption were tachypnea (RR > 35 breaths per minute), persistent intercostal and/or supraclavicular retraction, persistent thoracoabdominal asynchrony, worsening gas exchange (pH < 7.30 and/or 20% increase in Paco2, and/or Pao2 lower than 60 mm Hg [8 kPa] at an Fio2 of 1.0), or KMS less than 3. A need escalate treatment to NIMV or iMV was considered a “treatment failure.”
Absent failure, HVNI was given without interruption for 24 hours. Subsequent treatment suspension was authorized only in the presence of the following criteria for 2 consecutive hours: Fio2 less than 30% with Sao2 greater than or equal to 92% and RR less than 25 breaths per minute. During treatment suspensions, patients received low-flow oxygen therapy (mask or nasal cannula) to keep Sao2 88–92% and restarting HVNI was reevaluated every 30 minutes. HVNI was restarted if RR greater than 25 breaths per minute, intercostal and/or supraclavicular respiratory retraction, thoracoabdominal asynchrony, and increased oxygen support (increased ≥ 20% for longer than 5 min). In those patients in whom it had to be restarted, HVNI continued for a period of at least 12 hours until a subsequent evaluation.
HVNI suspension for a period of 24 consecutive hours or longer was considered a treatment success.
Other standard care used in management of these patients included: hydrocortisone (300 mg/d, 100 mg/8 hr), ampicillin/sulbactam (1.5 g every 6 hr), clarithromycin (500 mg every 12 hr), oseltamivir (75 mg every 12 hr), and aerosolized salbutamol and ipratropium bromide (every 6 hr or more frequently if necessary).
Statistical Analysis
Continuous data were expressed as mean and sd or as median and interquartile range (25–75). Normality was assessed by visual inspection and Shapiro-Wilk test. Categorical data were expressed as absolute values and/or percentages. A sample size of 20 patients was calculated for detecting a 15% difference in RR with 80% power and α value of 0.05, from previous studies (3). Nonparametric variables were compared using Friedman, McNemar, and Mann-Whitney U tests. A p value of less than 0.05 was considered significant. SPSS software Version 25.0, IBM Corp., Armonk, NY, was used to perform the statistical analysis.
Ethical Considerations
The study protocol was approved by centers’ Institutional Review Board (IRB) (Sanatorio Anchorena Recoleta IRB F004-02-A[04]2017) and an informed consent form was recorded. The study was registered with ClinicalTrials.gov (NCT04109560). This study did not receive any financial support. The HVNI equipment was provided by JAEJ S.A. (Buenos Aires, Argentina).
RESULTS
A total of 138 patients were admitted, of whom 40 were included in the study. Their mean age was 68 years (10 yr) and their median Simplified Acute Physiology Score II score 28 (21–36). The majority of patients had severe COPD (25%; n = 62.5) with exacerbation of nonspecific origin (n = 22, 55%) (Table 1).
TABLE 1.
Baseline Characteristics of the Enrolled Patients
| Variable | Value (n = 40) |
| Age (yr)a | 68 (± 10) |
| Gender (female), n (%) | 32 (80) |
| Height (cm)a | 158 ± 26 |
| Real weight (kg)a | 72 ± 24 |
| Charlson scoreb | 2 (1–3) |
| Simplified Acute Physiology Score IIb | 28 (21–36) |
| Type of admission | |
| Medical | 37 (92.5) |
| Surgical | 3 (7.5) |
| Chronic obstructive pulmonary disease classification, n (%) | |
| Moderate | 9 (22.5) |
| Severe | 25 (62.5) |
| Very severe | 6 (15) |
| Cause of exacerbation, n (%) | |
| Bacterial | 10 (25) |
| Viral | 8 (20) |
| Nonspecific | 22 (55) |
| Infiltrates on x-rayb | 1 (0–2) |
| LOS ICUb | 7 (4–10) |
| LOS hospitalb | 12 (9–15) |
LOS = length of stay.
aMean (± sd).
bMedian (interquartile range).
Clinical and Blood Gas Data
At admission, patients had a RR of 29 breaths per minute (27–31 breaths per minute), most of them using the accessory respiratory muscles (n = 36; 90%), many with thoracoabdominal asynchrony (n = 14; 35%). ABG showed a pH of 7.32 (7.29–7.34), a Pao2 of 67.5 mm Hg (59.9–76.5 mm Hg) (9.0 kPa [8–10.2 kPa]), a Paco2 of 57 (51–69) (7.5 kPa [6.8–9.2 kPa]), and Sao2 of 92% (89–94%).
RR was significantly reduced on HVNI, by 28% (29 vs 21 breaths/min; p < 0.001), after 1-hour of HVNI. A significant reduction of Paco2 (57 vs 52 mm Hg [7.6 vs 6.9 kPa]; p < 0.001) and pH increase (7.32 vs 7.36; p < 0.001) were observed (Fig. 1). These changes were maintained during the first 24 hours and until the discontinuation of HVNI (Fig. 2). There was also a statistically significant reduction in the percentage of patients who used their accessory muscles (n = 36 [9%] vs n = 15 [3%]; p < 0.001) and in those with thoracoabdominal asynchrony (n = 14 [35%] vs n = 3 [7%]; p < 0.001) with HVNI administration (Fig. 3). No differences were found in relation to Pao2 or Sao2. HVNI was delivered with a mean (sd) Fio2 in the first hour of therapy was 0.32 (0.08) and 0.29 (0.06) for the first 24 hours of therapy. The average HVNI flow in the first 24 hours was 32.3 L/min (6.7 L/min), delivered at a temperature of 36.9°C (1.5°C) and an Fio2 of 30% (6%).
Figure 1.
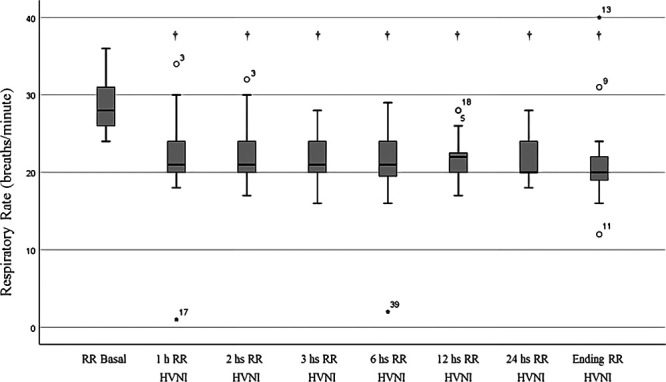
Respiratory rate (RR) over time (†p < 0.001 for all measurements compared with baseline). HVNI = high-velocity nasal insufflation.
Figure 2.
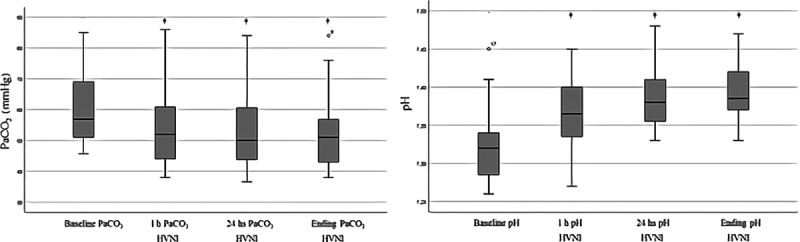
Paco2 and pH over time (†p < 0.001 compared with baseline). HVNI = high-velocity nasal insufflation.
Figure 3.
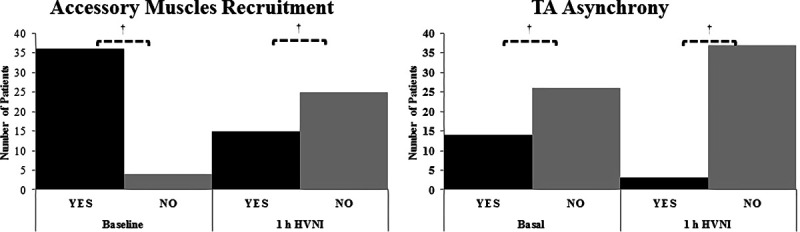
Evolution of clinical patterns. A, Total number of patients exhibiting use of accessory muscles recruitment to breathe, comparing baseline to the first hour from start of high-flow oxygen therapy (†p < 0.001 compared with baseline). B, Total number of patients with thoracoabdominal (TA) asynchrony, comparing baseline to the first hour from start of high-flow oxygen therapy (†p < 0.001 compared with baseline). HVNI = high-velocity nasal insufflation.
Tolerance to High-Velocity Nasal Insufflation Therapy, Its Duration, and Scheduling
HVNI treatment was comfortable for the patients, with the values being 1.5 (1–2) at the start of treatment and 1 (1–2) at the completion (p = not significant). Intolerance was not recorded as the cause of failure in any patient and no unexpected adverse events of any kind were observed. The duration of HVNI was 48 hours (34.5–96.2), the longest recorded time being 194 hours (8 d).
Patient Results and Stratified Analysis of Success/Failure
HVNI application failed in seven patients (17%), requiring NIMV within a median of 12 hours (1–36 hr); one progressing to iMV. Stratified analysis of the group of patients with a HVNI failure showed no significant improvement from baseline in the first hour of treatment of RR, Paco2, or pH, while such changes were seen in successful HVNI patients (Table 2). HVNI failure was associated with persistent respiratory acidosis within the hour after the start of HVNI (pH 7.37 vs 7.31, respectively; p = 0.022) (Table 2).
TABLE 2.
Changes in Respiratory Rate, Paco2, and pH After 1 Hour of High-Velocity Nasal Insufflation According to the Result of the Therapy
| Variable | Success | Failure | ||||
|---|---|---|---|---|---|---|
| Baseline | 1 hr HVNI | p | Baseline | 1 hr HVNI | p | |
| Respiratory rate (breaths/min)a | 29 (28–31) | 22 (20–24) | < 0.001 | 30 (25–31) | 23 (22–29) | 0.379 |
| Paco2 (mm Hg)a | 56 (51–67) | 51 (44–59) | < 0.001 | 66 (56–77) | 72 (68–78) | 0.392 |
| pHa,b | 7.32 (7.28–7.34) | 7.37 (7.34–7.40)b | 0.006 | 7.31 (7.29–7.32) | 7.31 (7.28–7.35)b | 0.56 |
HVNI = high-velocity nasal insufflation.
aValues expressed as median (interquartile range).
bp = 0.022 for pH at the time of HVNI therapy (Mann-Whitney U test).
Of the 40 patients entered in the study, three died (7%); two of them subsequently died after successful HVNI treatment, while the third patient, with very severe COPD, died during iMV. The median durations of ICU stay and hospital stay were 7 and 12 days, respectively.
DISCUSSION
This study evaluated the effects of HVNI administration in patients with COPD and acute hypercapnic respiratory failure. The principal results were as follows: 1) HVNI causes early and sustained changes in the clinical and blood gas parameters; 2) RR, Paco2, and pH appear to be early prognostic factors of treatment success, with acidosis at 1 hour of onset of HVNI associated with treatment failure; 3) HVNI was successful as supportive treatment in 83% of cases; and 4) HVNI was well tolerated.
Currently, COPD patients with ARF who have a pH of 7.20–7.35 (absent metabolic etiology) are considered good candidates for the application of NIMV, leaving iMV as a second-line treatment option in the case of failure (12, 13). Early improvement of pH and/or RR is a good predictor of favorable NIMV outcome, with a response observed almost universally within the first 2 hours of initiation (14). During the administration of HVNI, we found a 27% decrease of RR during the first hour of treatment, similar to that reported for NIMV (15). The decrease in RR was accompanied by a fall of Paco2, suggesting a reduction of Paco2 possibly linked to either an increase in tidal volume or a decrease of functional dead space. This behavior of both RR and Paco2 could be useful as an indicator of favorable outcome. In this context, persistent acidosis at the start of HVNI administration was a prognostic factor for treatment failure.
“Accessory muscle” recruitment due to structural and functional alterations are typical of COPD patients, particularly in ARF (16, 17). In these patients, the increase in respiratory effort is due to air trapping produced by flow obstruction, placing a mechanical overburden on the respiratory musculature (18). RR decrease with HFNC has been suggested to improve pulmonary emptying through an increase in expiratory time (19), allowing improved diaphragm function by optimizing contraction length (20, 21). The positive end-expiratory pressure effect of HVNI could also have played a small role in this direction by counterbalancing the load resulting from air trapping (22, 23).
HFNC treatment of COPD patients has been described in several reports of stable or NIMV-intolerant patients (6–9, 24). For this study, it was decided to use HVNI as first-line supportive treatment, based on the available physiologic (14, 16) and clinical data (5–10, 24) as reported by various studies (15, 25–28). Failure for NIMV in COPD patients with hypercapnic ARF and pH less than 7.35 is approximately 15%, and 25% for patients with a pH less than 7.30 (15, 29). In this study, HVNI failed as a supportive therapy 17% for hypercapnic COPD patients, similar to that reported for NIMV used for this patient population (15, 29). The average mortality reported for COPD patients with hypercapnic ARF treated using NIMV is approximately 6% (15, 27–30), compared mortality among patients in this study was 7%. The length of hospital stay was 12 days for this HVNI patient group, which compares favorably to the length of stay reported for a similar population treated using NIMV (15).
Patient comfort during NIMV is one of the known factors to consider for successful therapy (31). Comfort is substantially better with the use of high-flow cannulas as compared with NIMV masks (16, 32, 33). HVNI application was well tolerated in our study; the technique was described as being “very comfortable” or “comfortable” in all cases. There was no interruption of HVNI due to patient discomfort. Cannula-based high-flow therapy, compared with NIMV, also removes any asynchrony (34, 35), reduces the caregiver interventions, and lowers risk of pressure injury due to therapy interface (36).
The average flow with HVNI in our study, as well as that of Doshi et al (5), was lower (32.5 and 30 L/min, respectively), compatible with the recently published data describing 30 L/min as the optimal flow rate to reduce work of breathing in COPD patients, comparable to NIMV at an inspiratory pressure of 11 cm H2O (11–13 cm H2O) and an expiratory pressure of 5 cm H2O (5 cm H2O) (22). Cannula type may play a mechanistic role in the effect of therapy. HVNI administration employs a small-bore nasal cannula prong, imparting greater velocity to the gas flow. This has been demonstrated to provide a mechanistic advantage to flush the accessible deadspace in the upper airway, likely through creation of increased turbulent kinetic energy (37–39).
There were limitations of this study. It was not a randomized controlled trial (RCT). This study was designed as a pilot study for a subsequent RCT. Second, this study was not blinded to the investigators or to the subjects, which could add bias; however, due to the study design, it was impossible.
CONCLUSIONS
High-flow oxygen therapy using HVNI through a nasal cannula was an effective tool for reducing RR and providing oxygenation support of these COPD patients with acute hypercapnic respiratory failure. HVNI therapy in this study has a 17% failure rate, which may be comparable to NIMV. Clinical behavior and blood gas parameters may help predict the outcome of HVNI management for such patients.
Our study suggests that the use of HVNI as supportive treatment in COPD patients with acute hypercapnic respiratory failure warrants further randomized study comparing it to NIMV.
ACKNOWLEDGMENTS
We thank Juan Ignacio Mithieux and George C. Dungan for all your selfless support during the study.
Footnotes
Mr. Plotnikow conceived and designed the study, collected, interpreted, and analyzed data, searched literature, and wrote the article. Mr. Accoce, Mr. Fredes, Mr. Tiribelli, Mr. Setten, and Dr. Rodriguez designed the study, collected data, and critically revised the article content. Mr. Dorado, Dr. Guaymas, Dr. Ilutovich, Dr. Cesio, Dr. Scapellato, and Dr. Vasquez designed the study, collected data, searched literature, and critically revised the article. Dr. Scapellato designed the study, analyzed data, and critically revised the article.
Mr. Plotnikow reports receiving payment for consulting Vapotherm, Exeter, NH, not directly pertaining to the work presented herein. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Ethics approval: Yes (Institutional Review Board F004-02-A[04]2017).
REFERENCES
- 1.Roca O, Hernández G, Díaz-Lobato S, et al. ; Spanish Multidisciplinary Group of High Flow Supportive Therapy in Adults (HiSpaFlow). Current evidence for the effectiveness of heated and humidified high flow nasal cannula supportive therapy in adult patients with respiratory failure. Crit Care. 2016; 20:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mauri T, Turrini C, Eronia N, et al. Physiologic effects of high-flow nasal cannula in acute hypoxemic respiratory failure. Am J Respir Crit Care Med. 2017; 195:1207–1215 [DOI] [PubMed] [Google Scholar]
- 3.Sztrymf B, Messika J, Bertrand F, et al. Beneficial effects of humidified high flow nasal oxygen in critical care patients: A prospective pilot study. Intensive Care Med. 2011; 37:1780–1786 [DOI] [PubMed] [Google Scholar]
- 4.Frat JP, Thille AW, Mercat A, et al. ; FLORALI Study Group; REVA Network. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015; 372:2185–2196 [DOI] [PubMed] [Google Scholar]
- 5.Doshi P, Whittle JS, Bublewicz M, et al. High-velocity nasal insufflation in the treatment of respiratory failure: A randomized clinical trial. Ann Emerg Med. 2018; 72:73–83.e5 [DOI] [PubMed] [Google Scholar]
- 6.Lee MK, Choi J, Park B, et al. High flow nasal cannulae oxygen therapy in acute-moderate hypercapnic respiratory failure. Clin Respir J. 2018; 12:2046–2056 [DOI] [PubMed] [Google Scholar]
- 7.Lepere V, Messika J, La Combe B, et al. High-flow nasal cannula oxygen supply as treatment in hypercapnic respiratory failure. Am J Emerg Med. 2016; 34:1914.e1–e2 [DOI] [PubMed] [Google Scholar]
- 8.Plotnikow G, Thille AW, Vasquez D, et al. High-flow nasal cannula oxygen for reverting severe acute exacerbation of chronic obstructive pulmonary disease: A case report. Med Intensiva. 2017; 41:571–572 [DOI] [PubMed] [Google Scholar]
- 9.Millar J, Lutton S, O’Connor P. The use of high-flow nasal oxygen therapy in the management of hypercarbic respiratory failure. Ther Adv Respir Dis. 2014; 8:63–64 [DOI] [PubMed] [Google Scholar]
- 10.Doshi PB, Whittle JS, Dungan G, 2nd, et al. The ventilatory effect of high velocity nasal insufflation compared to non-invasive positive-pressure ventilation in the treatment of hypercapneic respiratory failure: A subgroup analysis. Heart Lung. 2020; 49:610–615 [DOI] [PubMed] [Google Scholar]
- 11.Kelly BJ, Matthay MA. Prevalence and severity of neurologic dysfunction in critically ill patients. Influence on need for continued mechanical ventilation. Chest. 1993; 104:1818–1824 [DOI] [PubMed] [Google Scholar]
- 12.Keenan SP, Powers CE, McCormack DG. Noninvasive positive-pressure ventilation in patients with milder chronic obstructive pulmonary disease exacerbations: A randomized controlled trial. Respir Care. 2005; 50:610–616 [PubMed] [Google Scholar]
- 13.Thys F, Roeseler J, Reynaert M, et al. Noninvasive ventilation for acute respiratory failure: A prospective randomised placebo-controlled trial. Eur Respir J. 2002; 20:545–555 [DOI] [PubMed] [Google Scholar]
- 14.Plant PK, Owen JL, Elliott MW. Non-invasive ventilation in acute exacerbations of chronic obstructive pulmonary disease: Long term survival and predictors of in-hospital outcome. Thorax. 2001; 56:708–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brochard L, Mancebo J, Wysocki M, et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 1995; 333:817–822 [DOI] [PubMed] [Google Scholar]
- 16.Di Mussi R, Spadaro S, Stripoli T, et al. High-flow nasal cannula oxygen therapy decreases postextubation neuroventilatory drive and work of breathing in patients with chronic obstructive pulmonary disease. Crit Care. 2018; 22:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Supinski G, DiMarco A, Ketai L, et al. Reversibility of diaphragm fatigue by mechanical hyperperfusion. Am Rev Respir Dis. 1988; 138:604–609 [DOI] [PubMed] [Google Scholar]
- 18.Bellemare F, Grassino A. Force reserve of the diaphragm in patients with chronic obstructive pulmonary disease. J Appl Physiol Respir Environ Exerc Physiol. 1983; 55:8–15 [DOI] [PubMed] [Google Scholar]
- 19.Pisani L, Fasano L, Corcione N, et al. Change in pulmonary mechanics and the effect on breathing pattern of high flow oxygen therapy in stable hypercapnic COPD. Thorax. 2017; 72:373–375 [DOI] [PubMed] [Google Scholar]
- 20.Longhini F, Pisani L, Lungu R, et al. High-flow oxygen therapy after noninvasive ventilation interruption in patients recovering from hypercapnic acute respiratory failure: A physiological crossover trial. Crit Care Med. 2019; 47:e506–e511 [DOI] [PubMed] [Google Scholar]
- 21.Farkas GA, Roussos C:. Adaptability of the hámster diaphragm to exercise and/or emphysema. J Appl Physiol. 1982; 53:1263–1272 [DOI] [PubMed] [Google Scholar]
- 22.Rittayamai N, Phuangchoei P, Tscheikuna J, et al. Effects of high-flow nasal cannula and non-invasive ventilation on inspiratory effort in hypercapnic patients with chronic obstructive pulmonary disease: A preliminary study. Ann Intensive Care. 2019; 9:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nielsen KR, Ellington LE, Gray AJ, et al. Effect of high-flow nasal cannula on expiratory pressure and ventilation in infant, pediatric, and adult models. Respir Care. 2018; 63:147–157 [DOI] [PubMed] [Google Scholar]
- 24.Bräunlich J, Wirtz H. Nasal high-flow in acute hypercapnic exacerbation of COPD. Int J Chron Obstruct Pulmon Dis. 2018; 13:3895–3897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kramer N, Meyer TJ, Meharg J, et al. Randomized, prospective trial of noninvasive positive pressure ventilation in acute respiratory failure. Am J Respir Crit Care Med. 1995; 151:1799–1806 [DOI] [PubMed] [Google Scholar]
- 26.Contou D, Fragnoli C, Córdoba-Izquierdo A, et al. Noninvasive ventilation for acute hypercapnic respiratory failure: Intubation rate in an experienced unit. Respir Care. 2013; 58:2045–2052 [DOI] [PubMed] [Google Scholar]
- 27.Avdeev SN, Tretiakov AV, Grigoriants RA, et al. Study of the use of noninvasive ventilation of the lungs in acute respiratory insufficiency due exacerbation of chronic obstructive pulmonary disease. Anesteziol Reanimatol. 1998; 3:45–51 [PubMed] [Google Scholar]
- 28.Celikel T, Sungur M, Ceyhan B, et al. Comparison of noninvasive positive pressure ventilation with standard medical therapy in hypercapnic acute respiratory failure. Chest. 1998; 114:1636–1642 [DOI] [PubMed] [Google Scholar]
- 29.Plant PK, Owen JL, Elliott MW. Early use of non-invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease on general respiratory wards: A multicentre randomised controlled trial. Lancet. 2000; 355:1931–1935 [DOI] [PubMed] [Google Scholar]
- 30.Bott J, Carroll MP, Conway JH, et al. Randomised controlled trial of nasal ventilation in acute ventilatory failure due to chronic obstructive airways disease. Lancet. 1993; 341:1555–1557 [DOI] [PubMed] [Google Scholar]
- 31.Hill SN. Where should noninvasive ventilation be delivered? Respir Care. 2009; 54:62–69 [PubMed] [Google Scholar]
- 32.Roca O, Riera J, Torres F, et al. High-flow oxygen therapy in acute respiratory failure. Respir Care. 2010; 55:408–413 [PubMed] [Google Scholar]
- 33.Schwabbauer N, Berg B, Blumenstock G, et al. Nasal high-flow oxygen therapy in patients with hypoxic respiratory failure: Effect on functional and subjective respiratory parameters compared to conventional oxygen therapy and non-invasive ventilation (NIV). BMC Anesthesiol. 2014; 14:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carteaux G, Lyazidi A, Cordoba-Izquierdo A, et al. Patient-ventilator asynchrony during noninvasive ventilation: A bench and clinical study. Chest. 2012; 142:367–376 [DOI] [PubMed] [Google Scholar]
- 35.Raux M, Ray P, Prella M, et al. Cerebral cortex activation during experimentally induced ventilator fighting in normal humans receiving noninvasive mechanical ventilation. Anesthesiology. 2007; 107:746–755 [DOI] [PubMed] [Google Scholar]
- 36.Sun J, Li Y, Ling B, et al. High flow nasal cannula oxygen therapy versus non-invasive ventilation for chronic obstructive pulmonary disease with acute-moderate hypercapnic respiratory failure: An observational cohort study. Int J Chron Obstruct Pulmon Dis. 2019; 14:1229–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller TL, Saberi B, Saberi S:. Computational fluid dynamics modeling of extrathoracic airway flush: Evaluation of high flow nasal cannula design elements. J Pulmon Respir Med. 2016; 6:376 [Google Scholar]
- 38.Dysart K, Miller TL, Wolfson MR, et al. Research in high flow therapy: Mechanisms of action. Respir Med. 2009; 103:1400–1405 [DOI] [PubMed] [Google Scholar]
- 39.Frizzola M, Miller TL, Rodriguez ME, et al. High-flow nasal cannula: Impact on oxygenation and ventilation in an acute lung injury model. Pediatr Pulmonol. 2011; 46:67–74 [DOI] [PMC free article] [PubMed] [Google Scholar]


