Abstract
Primary or idiopathic focal segmental glomerulosclerosis (FSGS) is a kidney entity that involves the podocytes, leading to heavy proteinuria and in many cases progresses to end-stage renal disease. Idiopathic FSGS has a bad prognosis, as it involves young individuals who, in a considerably high proportion (∼15%), are resistant to corticosteroids and other immunosuppressive treatments as well. Moreover, the disease recurs in 30–50% of patients after kidney transplantation, leading to graft function impairment. It is suspected that this relapsing disease is caused by a circulating factor(s) that would permeabilize the glomerular filtration barrier. However, the exact pathologic mechanism is an unsettled issue. Besides its poor outcome, a major concern of primary FSGS is the complexity to confirm the diagnosis, as it can be confused with other variants or secondary forms of FSGS and also with other glomerular diseases, such as minimal change disease. New efforts to optimize the diagnostic approach are arising to improve knowledge in well-defined primary FSGS cohorts of patients. Follow-up of properly classified primary FSGS patients will allow risk stratification for predicting the response to different treatments. In this review we will focus on the diagnostic algorithm used in idiopathic FSGS both in native kidneys and in disease recurrence after kidney transplantation. We will emphasize those potential confusing factors as well as their detection and prevention. In addition, we will also provide an overview of ongoing studies that recruit large cohorts of glomerulopathy patients (Nephrotic Syndrome Study Network and Cure Glomerulonephropathy, among others) and the experimental studies performed to find novel reliable biomarkers to detect primary FSGS.
Keywords: biomarkers, diagnosis algorithm, focal segmental glomerulosclerosis, idiopathic nephrotic syndrome, primary FSGS
INTRODUCTION
Focal and segmental glomerulosclerosis (FSGS) is a histological pattern used in clinical practice to define a podocytopathy that develops with nephrotic-range proteinuria and segmental obliteration or collapse of glomerular capillary loops with increased extracellular matrix in some glomeruli. FSGS is the leading glomerulopathy responsible for end-stage renal disease (ESRD) in the USA [1]. It is the cause of ∼40% of the nephrotic syndrome in adults and 20% in children [2]. As in other kidney diseases, the incidence of FSGS is generally 1.2- to 1.5-fold higher in men than in women [3]. FSGS can be classified into two main forms: secondary, which includes genetic, adaptive, infection/inflammation and medication-associated FSGS, and primary or idiopathic FSGS [2, 4]. Primary FSGS is thought to be caused by an unknown circulating factor(s) that damages the podocytes and consequently permeabilizes the glomerular filtration barrier [2]. The major concerns of idiopathic FSGS are the poor renal prognosis with an absence of response to immunosuppressive therapies or relapses and its recurrence after kidney transplantation in ~30–50% of patients, which often leads to renal graft failure [5].
Primary FSGS should be suspected in patients with abrupt nephrotic proteinuria, severe hypoalbuminaemia, oedema and uniform podocyte foot process effacement (FPE) by electron microscopy (EM) of a renal biopsy [6]. A careful and detailed medical history and clinical examination are needed, as often it is not easy to distinguish between primary and secondary FSGS. From a practical point of view, it is worth mentioning that primary FSGS is often confused with other glomerulopathies such as minimal change disease (MCD). In fact, many authors believe that MCD and primary FSGS are the same disease, the second being a more advanced stage of the first, where glomerular lesions can be seen by light microscopy (LM) [7]. Diagnosis of primary FSGS is not a straightforward process and therefore several studies have focused on finding factors and markers that may be helpful for diagnosis of the disease and the detection of FSGS recurrence after kidney transplantation [8–10].
In this review we discuss the diagnosis algorithm of FSGS in the native kidney and its relapse after kidney transplantation. In addition, we will review studies focused on new biomarkers for early diagnosis and/or detection of FSGS relapse after kidney transplant. We will also cover new strategies aimed at identifying accurate biomarkers of disease activity and progression.
The primary FSGS diagnosis in the native kidney
FSGS is a histological term rather than a specific clinical disease. FSGS is a lesion resulting from a podocyte injury characterized by segmental (in parts) and focal (of some) sclerosis of the glomeruli. Thus the histological finding lends its name to the associated clinical disease. It can be classified into secondary FSGS or primary (idiopathic) FSGS. Secondary FSGS lesions are observed as an adaptive response to a reduction in nephron mass, genetic mutations, drug consumption or viral infections, among others (Table 1) [4]. In secondary FSGS cases (except for the genetic forms), treatment of the underlying cause of the disease can reverse or slow down the progression of the disease, depending on the extent of established renal damage (i.e. angiotensin-converting enzyme inhibitor or angiotensin receptor blocker treatment for hypertension-caused FSGS or antiviral treatment for virus-induced FSGS). In contrast, primary FSGS is thought to be caused by undefined circulating factors that cause abnormal glomerular permeability and is diagnosed after exclusion of any other identifiable cause of secondary FSGS [4]. This subtype of FSGS is associated with a poor renal prognosis when compared with secondary forms [12] and another primary glomerulonephritis [13] and requires immunosuppressive treatment. Therefore the distinction between primary and secondary forms of FSGS has therapeutic and prognostic implications. As the presence of an FSGS lesion itself in a kidney biopsy does not offer a precise diagnosis, it remains a clinical diagnosis. Additionally, other diseases may present similar clinical and histological findings, such as MCD or focal and global glomerulosclerosis (FGGS). Identifying each form properly is crucial to avoid unnecessary immunosuppressive-based therapeutic approaches and to establish the appropriate treatment for the FSGS patient.
Table 1.
Main causes of secondary FSGS (adapted from KDIGO guidelines 2012 for glomerulonephritis [4])
| Causes of secondary FSGS | |
|---|---|
| Genetic | Mutations in the genes coding for crucial podocyte proteins: |
| Mutations in ACTN4 (α-actinin 4), mutations in NPHS1 (nephrin), mutations in NPHS2 (podocin), mutations in WT-1 (Wilms tumor 1), mutations in TRPC6 (transient receptor potential cation channel subfamily C member 6), mutations in SCARB2 (lysosomal integral membrane protein 2), mutations in INF2 (formin), mutations in CD2-associated protein, others: PAX-2 (paired box protein Pax-2), MYO1E (unconventional myosin-Ie), among others [11] | |
| Mitochondrial cytopathies | |
| Apolipoprotein L1 risk variants: associated to APOL1(apolipoprotein L1) polymorphisms | |
| Associated with infectious diseases | Secondary to viral infections: human immunodeficiency virus, parvovirus B19, hepatitis virus B and C, cytomegalovirus, Epstein–Barr virus, varicella zoster |
| Secondary to parasite or bacterial infections: malaria, syphilis, toxoplasmosis | |
| Medication | Medication or drug consumption induced: heroin, interferon-α, lithium, pamidronate/alendronate, anabolic steroids |
| Adaptive structural–functional responses likely due to hypertrophy or hyperfiltration | Reduced kidney mass: oligomeganephronia, unilateral kidney agenesis, kidney dysplasia,cortical necrosis, reflux nephropathy, surgical kidney ablation, chronic allograft nephropathy, any advanced kidney disease with reduction in functioning nephrons |
| Initially normal kidney mass: diabetes mellitus, hypertension, obesity, cyanotic congenital heart disease, sickle cell anaemia | |
| Malignancy | Associated mainly with lymphoma |
| Non-specific pattern of FSGS | Produced secondary to the scarring due to the presence of other glomerulopathies: focal proliferative glomerulonephritis (immunoglobulin A nephropathy, lupus nephritis, pauci-immune focal necrotizing and crescentic glomerulonephritis), hereditary nephritis (Alport syndrome), membranous glomerulopathy, thrombotic microangiopathy, associated with apolipoprotein L1 (ApoL1) polymorphisms |
FSGS is clinically noticed when patients present with heavy proteinuria. Depending on the degree of proteinuria, primary or secondary FSGS can be suspected. Patients with primary FSGS usually present with nephrotic-range proteinuria (>3.5 g/day) with complete nephrotic syndrome (severe hypoalbuminaemia), often associated with renal insufficiency, hypertension and microhaematuria [14]. In contrast, secondary FSGS patients usually present a broad range of proteinuria (including subnephrotic and nephrotic range) and in general do not develop complete nephrotic syndrome despite the presence of nephrotic-range proteinuria. Renal insufficiency is less common in secondary FSGS and is usually associated with a slow increase of proteinuria over time [15]. However, this is not applicable for genetic FSGS, which may be very aggressive, with early-age onset, even in utero, associated or not with extrarenal characteristic clinical features, with overall progression to ESRD [16].
Together with clinical and laboratory findings, kidney biopsy is key for disease identification. However, histological injury is difficult to evaluate in single kidney sections since the focal sclerotic lesions, which are present in many glomeruli, affect only 12.5% of the total glomerular volume [17]. Therefore it is essential to obtain representative biopsy specimens that include at least 10 glomeruli, both cortical and juxtamedullary, as sclerotic changes may occur earlier in the latter. Juxtamedullary glomeruli can sometimes be missed on the kidney biopsy. Furthermore, these samples should be processed in successive sections that allow observation of the whole glomerular tuft and evaluation in LM. The histology of FSGS is defined by a segmental increase of the mesangial matrix with obliteration of the capillaries, sclerosis, hyalinosis, segmental scarring and finally Bowman’s capsule adhesion to the glomerular tuft. Under LM, FSGS histological lesions can be classified into five subtypes according to the Columbia classification [18]: perihilar, tip, collapsing, cellular and not otherwise specified (NOS). NOS is the most common form in diverse series [19]. Prognosis may also be associated with the histological subtype. The tip variant has shown the highest rates of remission, while the collapsing variant is related to poor prognosis [20]. Of note, initial stages are only detectable by electronic microscopy where a degree of diffuse podocyte FPE should be observed. Generalized diffuse FPE (>80% of the analysed podocitary surface) can be associated with primary FSGS or MCD, while segmental and <80% FPE is usually related to secondary FSGS [21, 22]. FPE assessment using transmission electronic microscopy is to date the only established way to analyse the podocyte morphology, but it is far from being a standard method, as it is technically complex, requires ability and time and, moreover, deals with a geometric bias derived from physical sectioning [23]. Therefore, novel high-resolution microscopy techniques are in development to improve visualization of the glomerular filtration barrier compounds [23, 24].
When the onset of proteinuria with nephrotic syndrome is noted in paediatric patients, the diagnostic approach is very different. A kidney biopsy is discouraged as a first-line diagnostic procedure. When a child presents with proteinuria associated with nephrotic syndrome, treatment with steroids is started. The majority of patients are steroid-sensitive (∼85%) [25, 26] and a kidney biopsy is normally not performed unless the patient develops steroid resistance. In case of steroid resistance, a kidney biopsy and genetic testing are indicated. About 15% of the paediatric patients with nephrotic syndrome are corticosteroid resistant and, of these, ∼60% do not respond to any other therapeutic option. FSGS is detected in the kidney biopsy in >50% of steroid-resistant paediatric patients [26]. Moreover, most of the genetic forms of FSGS appear during childhood and are mainly associated with corticosteroid resistance. Mutations in the nephrin (NPHS-1), Wilms tumour 1 (WT-1) and podocin (NPHS-2) genes can explain ∼70% of the studied cases of corticosteroid-resistant nephrotic syndrome [26]. Furthermore, routine whole-exome sequencing has allowed not only the description of >30 genes related to steroid resistance in nephrotic syndrome [11, 27, 28], but also discarding of other kidney diseases with proteinuria and FSGS, but from a totally different aetiology (i.e. Alport syndrome [29, 30], kidney dysplasia [31] or congenital abnormalities of the kidney and the urinary tract [32, 33]). A positive genetic test will focus the therapy on an antiproteinuric and symptomatic treatment, avoiding exposure to immunosuppression, but a negative result does not fully exclude mutations not previously reported.
Relapse of FSGS after kidney transplantation
One of the major concerns of primary FSGS is that the disease can recur after kidney transplantation and it seems to be related to the permeabilization activity of the FSGS circulating factor(s). Post-transplant FSGS recurrence happens in ∼30–50% of patients and it can occur immediately or months to years after transplantation [5, 34]. The known risk factors for FSGS recurrence include younger patients, those who progress to ESRD within 3 years of diagnosis, a history of recurrence in a prior allograft and patients with higher proteinuria levels pre-transplantation [35]. Recurrent primary FSGS presents with nephrotic-range proteinuria and frequently has a rapid onset in the early post-transplant period. Patients usually have symptoms and signs of nephrotic syndrome, such as oedema, hypoalbuminaemia and hyperlipidaemia, together with some degree of graft dysfunction. Nevertheless, the presence of proteinuria is enough to raise the suspicion of FSGS recurrence in patients that do not develop the full clinical picture. The definitive diagnosis is performed by allograft biopsy, where the characteristic features of FSGS are identical to FSGS in the native kidney, although in the initial phases the histological findings are characterized by a normal kidney under the optical microscope and podocyte FPE using EM [36]. The treatment for FSGS recurrence, as in native kidney idiopathic FSGS patients, is not standardized and the results are variable [37]. Kidney transplanted patients are usually already under a tacrolimus-based immunosuppressive treatment, therefore, to manage the FSGS recurrence, a change to cyclosporine can be performed as a strategy to decrease proteinuria [38]. Currently, early plasmapheresis treatment combined with intensified immunosuppression is the most common treatment choice for primary FSGS recurrence [39]. However, the results obtained with this treatment approach are variable and, in addition, these treatments are not exempt from toxicity [34, 40, 41].
De novo FSGS may also occur in the transplanted kidney among patients who did not have FSGS as a cause of ESRD in the native kidney. This is often detected >12 months after transplantation and is associated with variable amounts of proteinuria (including the nephrotic range), hypertension and progressive deterioration of renal allograft function. Compensatory glomerular hyperfiltration in residual nephrons caused by nephron loss or low nephron number in the transplanted kidney (size discrepancies between the graft and the recipient) has been implicated in the pathogenesis of de novo FSGS. Therefore hypertension, diabetes, allograft rejection, immunosuppressive-induced allograft damage, BK polyomavirus or parvovirus B19 infection and any other condition leading to a loss of renal mass can be involved in the pathogenesis of de novo FSGS [42]. De novo FSGS should be closely monitored in patients with unknown aetiology of ESRD in the native kidney, as primary FSGS recurrence cannot be totally excluded.
Despite features that allow us to discern primary FSGS and its relapses from other variants of FSGS and other renal diseases (Table 2), none of these findings is pathognomonic and identifying primary FSGS continues to be challenging. It is important to highlight again that FSGS is a histological pattern rather than a specific disease and that not all causes leading to podocyte damage have been elucidated. It is unknown whether primary FSGS is the consequence of one circulating factor or the conjunction of different factors that damage the glomerular filtration barrier. Furthermore, it is not clear what determines the response to immunosuppressive treatment and if there are different variants of primary FSGS. Finally, although genomic analysis has allowed the description of many genetic mutations related to FSGS, it is possible that still unidentified genetic alterations are responsible for adult FSGS onset, a fact that would lead to a mistaken diagnosis. For all these reasons, during the last decades, different groups have tried to find different biomarkers to help us recognize and understand the pathways involved in primary FSGS.
Table 2.
Characteristics of various forms and diseases included in the differential diagnosis of FSGS
| Characteristics | Primary FSGS | Secondary FSGS | Genetic FSGS | MCD |
|---|---|---|---|---|
| Clinical history | Acute onset of nephrotic syndrome without risk factors or previous renal disease history | Risk factors are present, such as obesity, drug consumption, vesicoureteral reflux, renal agenesis or reduced nephron mass or viral infection | Family history of FSGS disease (although frequently there are not familiar records); proteinuria or nephrotic syndrome with onset in early childhood or adolescence | Acute onset of nephrotic syndrome without risk factors or previous renal disease history |
| Laboratory findings | Nephrotic syndrome: peripheral oedema, hypoalbuminaemia and >3.5 g of proteinuria in 24-h urine; haematuria is common | Non-nephrotic or nephrotic-range proteinuria, without nephrotic syndrome; normal serum albumin levels | Childhood-onset genetic FSGS: usually nephrotic syndrome is present; adolescence or adult-onset genetic FSGS: proteinuria without nephrotic syndrome | More rapid onset of nephrotic syndrome; peripherial oedema, hypoalbuminaemia and >3.5 g of proteinuria in 24-h urine |
| Pathological findings | LM: segmental areas of sclerosis, partial capillary collapse and hyaline depositsa | LM: normal glomeruli | ||
| IF: none or few immune deposits in sclerotic lesions positive to IgM and occasionally to C3 | IF: negative | |||
| EM: usually diffuse (>80%) podocyte FPE | EM: usually segmental (<80%) podocyte FPE | EM: either diffuse or segmental podocyte FPE | EM: diffuse (>80%) podocyte FPE | |
aDepending on the location of the lesions, tip and perihiliar variants are distinguished. Cellular and collapsing variant show their own characteristics. If not a quality biopsy, glomeruli may seem normal.
IF, immunofluorescence.
Towards a better understanding of FSGS
FSGS is a complex pathology not only because of the difficulties in diagnosis mentioned earlier, but also due to its low prevalence and the lack of clinical tools for its risk stratification, prediction of remission, treatment selection and monitoring of drug response. In addition, FSGS may show a slow progression, thus requiring years of follow-up to prove the effectiveness of an intervention. Therefore it has been necessary to join efforts and collect data from patients from different hospitals to obtain large cohorts to study the pathologic mechanism (among others) of FSGS and the response to different treatment approaches. Several registries have been created to this end, such as the Nephrotic Syndrome Study Network (NEPTUNE) and Cure Glomerulonephropathy (CureGN).
The NEPTUNE is a prospective observational study that began in 2010 and will recruit 450 paediatric and adult patients not only with FSGS, but also with MCD and membranous glomerulonephritis (MGN) at the time of the first renal biopsy clinically indicated for proteinuria. The enrolment includes 18 clinical centres in the USA and Canada and aims to find changes in urinary protein excretion and renal function and to assess the outcomes in life quality, development of new-onset diabetes, malignancies, infections, thromboembolic events, hospitalization, acute kidney injury and death [43]. From the NEPTUNE study cohort, a scoring strategy for renal biopsies has been designed to standardize morphological analysis. This scoring method provides a multilevel annotation of the glomeruli of a whole-slide image for multidimensional reconstruction. This method allows simultaneous review and scoring of the same glomeruli on each digital slide [44, 45]. The purpose of the authors is to redefine histopathological classification and link these novel criteria to clinical and molecular phenotypes of glomerular diseases [46–48]. Renal tissue and compartment-specific genetic profiles obtained by genome-wide meesenger RNA (mRNA) expression data [49–51] will be performed using the samples of the NEPTUNE cohort with the aim to obtain molecular scoring data that will facilitate multidisciplinary and transdisciplinary research exploration along with the genotype–phenotype continuum. Other relevant data that have also been obtained thanks to study of the NEPTUNE cohort are the poor correlation that exists between the spot urine urinary protein:creatinine ratio (which is usually used to estimate 24-h proteinuria) with the real 24-h urine protein excretion in glomerular diseases [52, 53], new statistical models to improve longitudinal CKD outcome biomarkers analysis [54] and the relevant factors that could predict nephrotic syndrome remission [55].
Another of the biggest studies ongoing is the CureGN, that began recruitment in 2014 and plans to enrol 2400 children and adults with MCD, FSGS, MGN or immunoglobulin A nephropathy (IgAN) (including IgA vasculitis). Patients are included if they have a first kidney biopsy–proven diagnosis within the last 5 years and, once enrolled, a prospective follow-up is performed under a common protocol. Patients with ESRD and those with secondary causes of glomerular disease are excluded. At least 30% of the cohort is expected to be paediatric. This large cohort will target a racially, ethnically and geographically diverse population with glomerular disease, which contrasts with other studies that included more homogeneous patients [56]. The hypothesis of the authors of the CureGN is that different disease mechanisms can result in similar pathologic and clinical phenotypes but show very different disease development and that the histopathology per se does not adequately define disease course and response to therapy for all individuals within a given diagnosis. The identification and characterization of the underlying mechanisms will have a broad influence on diagnostic classification, accurate prognostication, definition of patient cohorts for clinical trials and the indication of individualized therapies. Awaiting results in terms of diagnosis improvement and disease management, data from this cohort regarding health-related quality of life [57] and cardiovascular disease risk in paediatric patients [58] are available. Most of the patients in the NEPTUNE study meet the inclusion criteria of the CureGN study; this will allow us follow patients for years and to obtain long-term longitudinal information, which is very important in low-prevalence pathologies. Both NEPTUNE and CureGN data intend to complement relevant work from previous large registry studies that have provided important data on risk factors for disease progression [25, 59–67]. It is worthy of mention that the PodoNet, a paediatric cohort study that recruited steroid-resistant nephrotic syndrome patients, has provided valuable information regarding kidney histology, genetic defects, treatment response and outcomes of this complex clinical entity [25].
Many authors agree that recent advances have led to the discovery of biomarkers of glomerular disease, including discovery of the M-type phospholipase A2 receptor [68] and thrombospondin type-1 domain-containing 7A [69] as the target antigens in most patients with primary MGN, studies of galactose-deficient IgA1 and antiglycan response in IgAN [70] and associating mutations in apolipoprotein L1 to the development of kidney disease in patients of African ancestry [71, 72]. But although the number of genetic mutations identified in patients with FSGS has also expanded in the last decade [73], the causal mechanism of the primary FSGS form is still unknown. The registry data such as that obtained with the NEPTUNE or the CureGN cohorts will most probably provide a platform for genetic testing and the identification and validation of new biomarkers. These large studies may be helpful to better understand and manage primary FSGS as well as other glomerulonephritis.
Novel biomarkers to diagnose primary FSGS
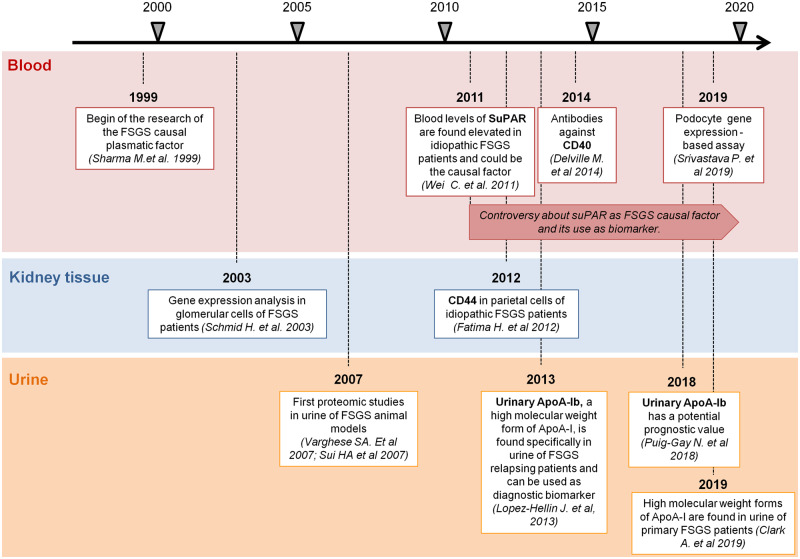
As mentioned before, sometimes it is difficult to distinguish between different forms of FSGS and even to differentiate it from other glomerular diseases. The major challenge in FSGS diagnosis is early detection of the primary or idiopathic forms in order to decide the best therapeutic option. Several approaches have been used in order to improve our understanding of the disease and help in diagnosis and management (Figure 1).
FIGURE 1.
Milestones for primary FSGS biomarker identification. Timeline of research studies focused on finding putative biomarkers to detect primary FSGS in blood, kidney tissue and urine.
As the disease is mainly a glomerular dysfunction, efforts have been made to establish which proteins are differentially expressed in glomerular cells of primary FSGS patients that could potentially be used as markers. In 2003, gene expression analysis of common podocyte proteins (ACTN4, GLEPP-1, WT-1, synaptopodin, dystroglycan, nephrin, podoplanin and podocin) of microdissected glomeruli suggested that the podocin:synaptopodin expression ratio may be useful to distinguish FSGS from MCD and nephrotic syndrome, although not from MGN [74]. CD44+ staining in the glomerular parietal cells has also been related to FSGS. Although initially it seemed that it was specifically associated to primary FSGS forms [75, 76], it has also been detected in secondary forms [77], suggesting that CD44 expression in the parietal cells is related to a common FSGS development mechanism. Furthermore, kidney tissue microRNA (miRNA) expression has also been analysed in FSGS patients. Although several miRNAs are upregulated in glomeruli of primary FSGS patients [78], it seems that miRNA-193a is relevant for development of the disease, as it decreases the expression of WT-1, which compromises the podocyte function [79]. miRNA-193a is also increased in urinary exosomes of FSGS patients when compared with MCD patients [80], thus this miRNA could be suitable to detect primary FSGS, although this needs further validation.
Although study of the proteins specifically expressed in FSGS in the kidney tissue has helped in understanding the disease, to date the clue to better diagnose idiopathic FSGS has not been found. Therefore a specific non-invasive biomarker would be very useful to discriminate different forms of FSGS. Biomarkers have been searched both in blood and in urine samples. The search for plasma biomarkers has been basically focused on the permeabilizing factor. Research on the putative plasma factor began about two decades ago [81, 82], and although some candidates have been proposed [83, 84], it has not been firmly demonstrated that any of them causes idiopathic FSGS. Certainly, one of the most promising proposed circulating factors was soluble urokinase receptor (suPAR), which was found to be increased in the serum of FSGS patients [8]. In a previous work, Wei et al. [85] showed that the urokinase receptor could be involved in the FPE of podocytes via activation of the αβ3-integrin pathway in a urokinase plasminogen activator receptor (uPAR) knockout mouse model [85]. Therefore they hypothesized that maybe the soluble uPAR (suPAR) could be the FSGS causal circulating factor. suPAR levels were measured in serum samples of 78 subjects with FSGS, 25 with MCD, 16 with MN, 7 with pre-eclampsia and in 22 healthy individuals. Blood suPAR levels were higher in the FSGS patient group than in the rest of the studied groups and, moreover, suPAR levels were also higher in the FSGS patients that relapsed after kidney transplantation. In addition, the authors demonstrated that the uPAR recombinant form injected into a knockout mouse model induced proteinuria, reinforcing the idea of suPAR is the plasma circulating factor related to FSGS [8]. Unfortunately, later studies were unable to demonstrate the same results in independent cohorts [86–88], nor could it be firmly demonstrated that it was the cause of the disease [89, 90]. Moreover, plasma suPAR levels have also been found to be elevated in several extrarenal pathologies that can potentially be concomitant to FSGS, such as cancer or inflammatory disorders, among others [91–95]. Recent studies suggest that both urinary levels of suPAR [96, 97] and uPAR detection in kidney biopsies [98] may be useful in the diagnosis of primary FSGS, but further investigation is needed. Anti-CD40 blood levels have also been associated with FSGS, with 78% accuracy to predict post-transplant FSGS recurrence [99]; however, further studies are required to confirm these results. Recently it has been shown that plasma of relapsing FSGS patients induces the expression of several specific genes upon cultured podocytes and the authors suggest that this could be used to distinguish FSGS recurrence from other types of renal disease. As an example, analysis of interleukin-1β gene expression induced by serum of recurrent FSGS patients shows >80% sensitivity and specificity to discriminate relapsing FSGS patients from other nephropathies [100]. Although the study was elegantly designed, it represents an indirect method on cultured podocytes, cells that need several days to differentiate (from 7 to 14 days) [101], hence it would not allow a fast diagnosis of FSGS relapse, making it difficult to use in current clinical practice. Finally, plasma miRNAs have also been explored as potential biomarkers to detect primary FSGS and several of these have been associated to the disease [102–104].
Urine has also been exploited as a biomarker source. In 2007, Varghese et al. [105] performed two-dimensional electrophoresis (2DE) of urine samples from several groups of patients (FSGS, IgAN, MGN and diabetic nephropathy) with the aim of finding biomarkers to distinguish glomerulopathies. They quantified the relative abundance of each spot (that represents a protein) and, based on these data, they designed a prediction algorithm. Although this study was not focused on FSGS, it revealed that variations in the urinary proteome could be useful to discriminate kidney diseases with proteinuria. Similarly, in a rat model of induced FSGS, a serial analysis of urine samples using 2DE revealed that the proteomic profile changed along the course of the disease and that some proteins appeared before the sclerotic lesions, suggesting that they could be useful as early biomarkers [106]. To our knowledge, the first and only urinary biomarker that has been specifically associated with post-transplantation recurrent FSGS is apolipoprotein A-Ib (apoA-Ib), a modified form of apoA-I, described by Lopez-Hellín et al. in 2013 [107]. Results from two cohorts [10, 107] have shown that the presence of this form in urine allows the detection of recurrent FSGS patients with high specificity and sensitivity (>87% and >90%, respectively). ApoA-Ib also has a potential prognostic value, as it can be found in urine before the FSGS recurrence episodes [10]. Although the exact role of apoA-Ib in FSGS is unknown, other authors have also found increased levels of apoA-I [108] or the presence of high molecular weight forms of apoA-I [109] in urine of FSGS patients, reinforcing the idea that either apoA-I or the mechanisms that modify this lipoprotein are involved in the pathogenic mechanism of this disease [110]. The potential role of this biomarker to discriminate primary FSGS in native kidney is currently being pursued, but preliminary results obtained with idiopathic FSGS patients before kidney transplantation pointed out that the detection of apoA-Ib in native kidney patients is associated with a worse prognosis [10]. Regarding urinary mRNAs, several of them can be found differentially in urine of primary FSGS patients [111, 112], but their potential as biomarkers remains to be studied.
CONCLUSIONS
As reviewed in this work, the diagnosis and management of primary FSGS is not a trivial issue, as knowledge of this pathology holds ‘several types of truths’ [113], as every matter of study. Management of this rare disease with an unknown aetiology is based on empirical knowledge related to the response of primary FSGS to different treatments, which is per se extremely difficult. Experimental studies have provided fundamental data to better understand the disease and it is expected that the new biomarkers will allow an improved diagnostic algorithm for primary FSGS.
ACKNOWLEDGEMENTS
The authors want to thank the national federation Asociación para la Lucha Contra las Enfermedades del Riñón (ALCER) for their support. Vall d’Hebrón Hospital is a national reference centre for complex glomerular diseases of adults and children [Centros, Servicios y Unidades de Referencia (CSUR) de enfermedades glomerulares complejas de adultos y niños. Ministerio de Sanidad] and part of the autonomic network of clinical expertise in minoritary diseases [Xarxes d’unitats d’expertesa clínica (XUEC) en malalties minoritàries (MM), Servei Català de la Salut].
FUNDING
The authors are current recipients of research grants from the Fondo de Investigación Sanitaria-Feder, Instituto de Salud Carlos III (PI17/00257, PI18/01704 and PI18/01832) and REDinREN (RD16/0009/0030).
CONFLICT OF INTEREST STATEMENT
C.J.C. reports travel support from Retrophin. C.G.C. reports honoraria for conferences, advisory boards and travel support for professional meetings from AstraZeneca, Mundipharma, Boehringer Ingelheim, Astellas, Menarini, Novartis, Esteve, Sanofi, Chiesi and Novo Nordisk. G.A. has participated in advisory boards and educational activities for Alexion Pharmaceuticals, Chiesi, Advicenne, Kyowa-Kirim and Recordati Rare Diseases. She has received honoraria for research activities from those companies and from Oxthera and travel support for professional meetings from Alexion Pharmaceuticals, Advicenne, Kyowa-Kirim and Recordati Rare Diseases. M.J.S. has received speaker fees or travel support from Otsuka, Menarini, AstraZeneca, Boehringer Ingelheim, Janssen, Mundipharma, Novartis, Eli Lilly, Esteve and Novo Nordisk.
REFERENCES
- 1. D'Agati VD, Kaskel FJ, Falk RJ.. Focal segmental glomerulosclerosis. N Engl J Med 2011; 365: 2398–2411 [DOI] [PubMed] [Google Scholar]
- 2. Rosenberg AZ, Kopp JB.. Focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 2017; 12: 502–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. O’Shaughnessy MM, Hogan SL, Thompson BD. et al. Glomerular disease frequencies by race, sex and region: results from the International Kidney Biopsy Survey. Nephrol Dial Transplant 2018; 33: 661–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kidney Disease: Improving Global Outcomes Glomerulonephritis Work Group. KDIGO clinical practice guideline for glomerulonephritis. Kidnet Int Suppl 2012; 2: 1–274 [Google Scholar]
- 5. Cosio FG, Cattran DC.. Recent advances in our understanding of recurrent primary glomerulonephritis after kidney transplantation. Kidney Int 2017; 91: 304–314 [DOI] [PubMed] [Google Scholar]
- 6. Sprangers B, Meijers B, Appel G.. FSGS: diagnosis and diagnostic work-up. Biomed Res Int 2016; 2016:4632768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maas RJ, Deegens JK, Smeets B. et al. Minimal change disease and idiopathic FSGS: manifestations of the same disease. Nat Rev Nephrol 2016; 12: 768–776 [DOI] [PubMed] [Google Scholar]
- 8. Wei C, El Hindi S, Li J. et al. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med 2011; 17: 952–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maas RJ, Deegens JK, Wetzels JF.. Permeability factors in idiopathic nephrotic syndrome: historical perspectives and lessons for the future. Nephrol Dial Transplant 2014; 29: 2207–2216 [DOI] [PubMed] [Google Scholar]
- 10. Puig-Gay N, Jacobs-Cacha C, Sellarès J. et al. Apolipoprotein A-Ib as a biomarker of focal segmental glomerulosclerosis recurrence after kidney transplantation: diagnostic performance and assessment of its prognostic value – a multi-centre cohort study. Transpl Int 2019; 32: 313–322 [DOI] [PubMed] [Google Scholar]
- 11. Noone DG, Iijima K, Parekh R.. Idiopathic nephrotic syndrome in children. Lancet 2018; 392: 61–74 [DOI] [PubMed] [Google Scholar]
- 12. Kambham N, Markowitz GS, Valeri AM. et al. Obesity-related glomerulopathy: an emerging epidemic. Kidney Int 2001; 59: 1498–1509 [DOI] [PubMed] [Google Scholar]
- 13. Chou YH, Lien YC, Hu FC. et al. Clinical outcomes and predictors for ESRD and mortality in primary GN. Clin J Am Soc Nephrol 2012; 7: 1401–1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chun MJ, Korbet SM, Schwartz MM. et al. Focal segmental glomerulosclerosis in nephrotic adults: presentation, prognosis, and response to therapy of the histologic variants. J Am Soc Nephrol 2004; 15: 2169–2177 [DOI] [PubMed] [Google Scholar]
- 15. Praga M, Morales E, Herrero JC. et al. Absence of hypoalbuminemia despite massive proteinuria in focal segmental glomerulosclerosis secondary to hyperfiltration. Am J Kidney Dis 1999; 33: 52–58 [DOI] [PubMed] [Google Scholar]
- 16. Trautmann A, Schnaidt S, Lipska ZB. et al. Long-term outcome of steroid-resistant nephrotic syndrome in children. J Am Soc Nephrol 2017; 28: 3055–3065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fuiano G, Comi N, Magri P. et al. Serial morphometric analysis of sclerotic lesions in primary “focal” segmental glomerulosclerosis. J Am Soc Nephrol 1996; 7: 49–55 [DOI] [PubMed] [Google Scholar]
- 18. D’Agati VD, Fogo AB, Bruijn JA. et al. Pathologic classification of focal segmental glomerulosclerosis: a working proposal. Am J Kidney Dis 2004; 43: 368–382 [DOI] [PubMed] [Google Scholar]
- 19. Gipson DS, Trachtman H, Kaskel FJ. et al. Clinical trial of focal segmental glomerulosclerosis in children and young adults. Kidney Int 2011; 80: 868–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thomas DB, Franceschini N, Hogan SL. et al. Clinical and pathologic characteristics of focal segmental glomerulosclerosis pathologic variants. Kidney Int 2006; 69: 920–926 [DOI] [PubMed] [Google Scholar]
- 21. Sethi S, Zand L, Nasr SH. et al. Focal and segmental glomerulosclerosis: clinical and kidney biopsy correlations. Clin Kidney J 2014; 7: 531–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hommos MS, De Vriese AS, Alexander MP. et al. The incidence of primary vs secondary focal segmental glomerulosclerosis: a clinicopathologic study. Mayo Clin Proc 2017; 92: 1772–1781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Siegerist F, Endlich K, Endlich N.. Novel microscopic techniques for podocyte research. Front Endocrinol 2018; 9: 379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pullman JM. New views of the glomerulus: advanced microscopy for advanced diagnosis. Front Med 2019; 6: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Trautmann A, Bodria M, Ozaltin F. et al. Spectrum of steroid-resistant and congenital nephrotic syndrome in children: the PodoNet registry cohort. Clin J Am Soc Nephrol 2015; 10: 592–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Trautmann A, Lipska-Ziętkiewicz BS, Schaefer F.. Exploring the clinical and genetic spectrum of steroid resistant nephrotic syndrome: the PodoNet registry. Front Pediatr 2018; 6: 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sadowski CE, Lovric S, Ashraf S. et al. A single-gene cause in 29.5% of cases of steroid-resistant nephrotic syndrome. J Am Soc Nephrol 2015; 26: 1279–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lovric S, Ashraf S, Tan W, Hildebrandt F.. Genetic testing in steroid-resistant nephrotic syndrome: when and how? Nephrol Dial Transplant 2016; 31: 1802–1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wuttke M, Seidl M, Malinoc A. et al. A COL4A5 mutation with glomerular disease and signs of chronic thrombotic microangiopathy. Clin Kidney J 2015; 8: 690–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang P, Zhuo L, Zou Y. et al. COL4A5 mutation causes Alport syndrome with focal segmental glomerulosclerosis lesion: case report and literature review. Clin Nephrol 2019; 92: 98–102 [DOI] [PubMed] [Google Scholar]
- 31. Chen RY, Chang H.. Renal dysplasia. Arch Pathol Lab Med 2015; 139: 547–551 [DOI] [PubMed] [Google Scholar]
- 32. Hwang DY, Dworschak GC, Kohl S. et al. Mutations in 12 known dominant disease-causing genes clarify many congenital anomalies of the kidney and urinary tract. Kidney Int 2014; 85: 1429–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van der Ven AT, Connaughton DM, Ityel H. et al. Whole-exome sequencing identifies causative mutations in families with congenital anomalies of the kidney and urinary tract. J Am Soc Nephrol 2018; 29: 2348–2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ponticelli C. Recurrence of focal segmental glomerular sclerosis (FSGS) after renal transplantation. Nephrol Dial Transplant 2010; 25: 25–31 [DOI] [PubMed] [Google Scholar]
- 35. Shimizu A, Higo S, Fujita E. et al. Focal segmental glomerulosclerosis after renal transplantation. Clin Transplant 2011; 25: 6–14 [DOI] [PubMed] [Google Scholar]
- 36. Chang J-W, Pardo V, Sageshima J. et al. Podocyte foot process effacement in postreperfusion allograft biopsies correlates with early recurrence of proteinuria in focal segmental glomerulosclerosis. Transplant J 2012; 93: 1238–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cravedi P, Kopp JB, Remuzzi G.. Recent progress in the pathophysiology and treatment of FSGS recurrence. Am J Transplant 2013; 13: 266–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shishido S, Satou H, Muramatsu M. et al. Combination of pulse methylprednisolone infusions with cyclosporine-based immunosuppression is safe and effective to treat recurrent focal segmental glomerulosclerosis after pediatric kidney transplantation. Clin Transplant 2013; 27: E143–E150 [DOI] [PubMed] [Google Scholar]
- 39. Canaud G, Zuber J, Sberro R. et al. Intensive and prolonged treatment of focal and segmental glomerulosclerosis recurrence in adult kidney transplant recipients: a pilot study. Am J Transplant 2009; 9: 1081–1086 [DOI] [PubMed] [Google Scholar]
- 40. Mansur JB, Sandes‐Freitas TV, Kirsztajn GM. et al. Clinical features and outcomes of kidney transplant recipients with focal segmental glomerulosclerosis recurrence. Nephrology 2019; 24: 1179–1188 [DOI] [PubMed] [Google Scholar]
- 41. Rudnicki M. FSGS recurrence in adults after renal transplantation. BioMed Res Int 2016; 2016: 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ponticelli C, Moroni G, Glassock RJ.. De novo glomerular diseases after renal transplantation. Clin J Am Soc Nephrol 2014; 9: 1479–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gadegbeku CA, Gipson DS, Holzman LB. et al. Design of the Nephrotic Syndrome Study Network (NEPTUNE) to evaluate primary glomerular nephropathy by a multidisciplinary approach. Kidney Int 2013; 83: 749–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Barisoni L, Nast CC, Jennette JC. et al. Digital pathology evaluation in the multicenter Nephrotic Syndrome Study Network (NEPTUNE). Clin J Am Soc Nephrol 2013; 8: 1449–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Barisoni L, Troost JP, Nast C. et al. Reproducibility of the NEPTUNE descriptor-based scoring system on whole-slide images and histologic and ultrastructural digital images. Mod Pathol 2016; 29: 671–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nast CC, Lemley KV, Hodgin JB. et al. Morphology in the digital age: integrating high-resolution description of structural alterations with phenotypes and genotypes. Semin Nephrol 2015; 35: 266–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rosenberg AZ, Palmer M, Merlino L. et al. The application of digital pathology to improve accuracy in glomerular enumeration in renal biopsies. PLoS One 2016; 11: e0156441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ju W, Nair V, Smith S. et al. Tissue transcriptome-driven identification of epidermal growth factor as a chronic kidney disease biomarker. Sci Transl Med 2015; 7: 316ra193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Harder JL, Menon R, Otto EA. et al. Organoid single cell profiling identifies a transcriptional signature of glomerular disease. JCI Insight 2019; 4: e122697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sampson MG, Robertson CC, Martini S. et al. Integrative genomics identifies novel associations with APOL1 risk genotypes in black NEPTUNE subjects. J Am Soc Nephrol 2016; 27: 814–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sampson MG, Hodgin JB, Kretzler M.. Defining nephrotic syndrome from an integrative genomics perspective. Pediatr Nephrol 2015; 30: 51–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hogan MC, Reich HN, Nelson PJ. et al. The relatively poor correlation between random and 24-hour urine protein excretion in patients with biopsy-proven glomerular diseases. Kidney Int 2016; 90: 1080–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Montero N, Soler MJ, Pascual MJ. et al. Correlation between the protein/creatinine ratio in spot urine and 24-hour urine protein. Nefrologia 2012; 32: 494–501 [DOI] [PubMed] [Google Scholar]
- 54. Liu Q, Smith AR, Mariani LH. et al. Methods for assessing longitudinal biomarkers of time-to-event outcomes in CKD. Clin J Am Soc Nephrol 2019; 14: 1315–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gipson DS, Troost JP, Lafayette RA. et al. Complete remission in the nephrotic syndrome study network. Clin J Am Soc Nephrol 2016; 11: 81–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mariani LH, Bomback AS, Canetta PA. et al. CureGN study rationale, design, and methods: establishing a large prospective observational study of glomerular disease. Am J Kidney Dis 2019; 73: 218–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Canetta PA, Troost JP, Mahoney S. et al. Health-related quality of life in glomerular disease. Kidney Int 2019; 95: 1209–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ashoor IF, Mansfield SA, O’Shaughnessy MM. et al. Prevalence of cardiovascular disease risk factors in childhood glomerular diseases. J Am Heart Assoc 2019; 8: e012143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Central Committee of the Toronto Glomerulonephritis Registry. Regional program for the study of glomerulonephritis. Can Med Assoc J 1981; 124: 158–161 [PMC free article] [PubMed] [Google Scholar]
- 60. Pei Y, Cattran D, Delmore T. et al. Evidence suggesting under-treatment in adults with idiopathic focal segmental glomerulosclerosis. Am J Med 1987; 82: 938–944 [DOI] [PubMed] [Google Scholar]
- 61. Falk RJ, Hogan S, Carey TS. et al. Clinical course of anti-neutrophil cytoplasmic autoantibody-associated glomerulonephritis and systemic vasculitis. Ann Intern Med 1990; 113: 656. [DOI] [PubMed] [Google Scholar]
- 62. Pelletier JH, Kumar KR, Engen R. et al. Correction to: recurrence of nephrotic syndrome following kidney transplantation is associated with initial native kidney biopsy findings. Pediatr Nephrol 2019; 34: 539–539 [DOI] [PubMed] [Google Scholar]
- 63. Barbour S, Beaulieu M, Gill J. et al. An overview of the British Columbia Glomerulonephritis Network and Registry: integrating knowledge generation and translation within a single framework. BMC Nephrol 2013; 14: 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Samuel S, Scott S, Morgan C. et al. The Canadian Childhood Nephrotic Syndrome (CHILDNEPH) Project: overview of design and methods. Can J Kidney Health Dis 2014; 1: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Smith JM, Stablein DM, Munoz R. et al. Contributions of the Transplant Registry: the 2006 Annual Report of the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS). Pediatr Transplant 2007; 11: 366–373 [DOI] [PubMed] [Google Scholar]
- 66. Denker M, Boyle S, Anderson AH. et al. Chronic Renal Insufficiency Cohort Study (CRIC): overview and summary of selected findings. Clin J Am Soc Nephrol 2015; 10: 2073–2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Osafo C, Raji YR, Burke D. et al. Human heredity and health (H3) in Africa kidney disease research network: a focus on methods in sub-Saharan Africa. Clin J Am Soc Nephrol 2015; 10: 2279–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Beck LH, Bonegio RGB, Lambeau G. et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 2009; 361: 11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tomas NM, Beck LH, Meyer-Schwesinger C. et al. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med 2014; 371: 2277–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tomana M, Novak J, Julian BA. et al. Circulating immune complexes in IgA nephropathy consist of IgA1 with galactose-deficient hinge region and antiglycan antibodies. J Clin Invest 1999; 104: 73–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Freedman BI, Limou S, Ma L. et al. APOL1-associated nephropathy: a key contributor to racial disparities in CKD. Am J Kidney Dis 2018; 72(5 Suppl 1): S8–S16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Friedman DJ, Kozlitina J, Genovese G. et al. Population-based risk assessment of APOL1 on renal disease. J Am Soc Nephrol 2011; 22: 2098–2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lepori N, Zand L, Sethi S. et al. Clinical and pathological phenotype of genetic causes of focal segmental glomerulosclerosis in adults. Clin Kidney J 2018; 11: 179–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Schmid H, Henger A, Cohen CD. et al. Gene expression profiles of podocyte-associated molecules as diagnostic markers in acquired proteinuric diseases. J Am Soc Nephrol 2003; 14: 2958–2966 [DOI] [PubMed] [Google Scholar]
- 75. Froes BP, de Almeida Araújo S, Bambirra EA. et al. Is CD44 in glomerular parietal epithelial cells a pathological marker of renal function deterioration in primary focal segmental glomerulosclerosis? Pediatr Nephrol 2017; 32: 2165–2169 [DOI] [PubMed] [Google Scholar]
- 76. Fatima H, Moeller MJ, Smeets B. et al. Parietal epithelial cell activation marker in early recurrence of FSGS in the transplant. Clin J Am Soc Nephrol 2012; 7: 1852–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kuppe C, Gröne HJ, Ostendorf T. et al. Common histological patterns in glomerular epithelial cells in secondary focal segmental glomerulosclerosis. Kidney Int 2015; 88: 990–998 [DOI] [PubMed] [Google Scholar]
- 78. Baker MA, Davis SJ, Liu P. et al. Tissue-specific microRNA expression patterns in four types of kidney disease. J Am Soc Nephrol 2017; 28: 2985–2992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Gebeshuber CA, Kornauth C, Dong L. et al. Focal segmental glomerulosclerosis is induced by microRNA-193a and its downregulation of WT1. Nat Med 2013; 19: 481–487 [DOI] [PubMed] [Google Scholar]
- 80. Huang Z, Zhang Y, Zhou J. et al. Urinary exosomal MIR-193a can be a potential biomarker for the diagnosis of primary focal segmental glomerulosclerosis in children. Biomed Res Int 2017; 2017: 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sharma M, Sharma R, McCarthy ET. et al. “The FSGS factor”: enrichment and in vivo effect of activity from focal segmental glomerulosclerosis plasma. J Am Soc Nephrol 1999; 10: 552–561 [DOI] [PubMed] [Google Scholar]
- 82. Sharma M, Sharma R, McCarthy ET. et al. The focal segmental glomerulosclerosis permeability factor: biochemical characteristics and biological effects.Exp Biol Med (Maywood) 2004; 229: 85–98 [DOI] [PubMed] [Google Scholar]
- 83. Reiser J, Nast CC, Alachkar N.. Permeability factors in focal and segmental glomerulosclerosis. Adv Chronic Kidney Dis 2014; 21: 417–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wada T, Nangaku M.. A circulating permeability factor in focal segmental glomerulosclerosis: the hunt continues. Clin Kidney J 2015; 8: 708–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wei C, Möller CC, Altintas MM. et al. Modification of kidney barrier function by the urokinase receptor. Nat Med 2008; 14: 55–63 [DOI] [PubMed] [Google Scholar]
- 86. Spinale JM, Mariani LH, Kapoor S. et al. A reassessment of soluble urokinase-type plasminogen activator receptor in glomerular disease. Kidney Int 2015; 87: 564–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wada T, Nangaku M, Maruyama S. et al. A multicenter cross-sectional study of circulating soluble urokinase receptor in Japanese patients with glomerular disease. Kidney Int 2014; 85: 641–648 [DOI] [PubMed] [Google Scholar]
- 88. Maas RJH, Wetzels JFM, Deegens J.. Serum-soluble urokinase receptor concentration in primary FSGS. Kidney Int 2012; 81: 1043–1044 [DOI] [PubMed] [Google Scholar]
- 89. Cathelin D, Placier S, Ploug M. et al. Administration of recombinant soluble urokinase receptor per se is not sufficient to induce podocyte alterations and proteinuria in mice. J Am Soc Nephrol 2014; 25: 1662–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Harel E, Shoji J, Abraham V. et al. Further evidence that the soluble urokinase plasminogen activator receptor does not directly injure mice or human podocytes. Transplantation 2019;104:54–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Rotbain Curovic V, Theilade S, Winther SA. et al. Soluble urokinase plasminogen activator receptor predicts cardiovascular events, kidney function decline, and mortality in patients with type 1 diabetes. Diabetes Care 2019; 42: 1112–1119 [DOI] [PubMed] [Google Scholar]
- 92. Desmedt S, Desmedt V, Delanghe JR. et al. The intriguing role of soluble urokinase receptor in inflammatory diseases. Crit Rev Clin Lab Sci 2017; 54: 117–133 [DOI] [PubMed] [Google Scholar]
- 93. Thunø M, Macho B, Eugen-Olsen J.. suPAR: the molecular crystal ball. Dis Mark 2009; 27: 157–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Hodges GW, Bang CN, Wachtell K. et al. suPAR: a new biomarker for cardiovascular disease? Can J Cardiol 2015; 31: 1293–1302 [DOI] [PubMed] [Google Scholar]
- 95. Wrotek A, Jackowska T.. The role of the soluble urokinase plasminogen activator (suPAR) in children with pneumonia. Respir Physiol Neurobiol 2015; 209: 120–123 [DOI] [PubMed] [Google Scholar]
- 96. Franco Palacios CR, Lieske JC, Wadei HM. et al. Urine but not serum soluble urokinase receptor (suPAR) may identify cases of recurrent FSGS in kidney transplant candidates. Transplant J 2013; 96: 394–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Huang J, Liu G, Zhang Y. et al. Urinary soluble urokinase receptor levels are elevated and pathogenic in patients with primary focal segmental glomerulosclerosis. BMC Med 2014; 12: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Pereira LHd M, da Silva CA, Monteiro MLGDR et al. Podocin and uPAR are good biomarkers in cases of focal and segmental glomerulosclerosis in pediatric renal biopsies. PLoS One 2019; 14: e0217569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Delville M, Sigdel TK, Wei C. et al. A circulating antibody panel for pretransplant prediction of FSGS recurrence after kidney transplantation. Sci Trans Med 2014; 6: 256ra136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Srivastava P, Solanki AK, Arif E. et al. Development of a novel cell-based assay to diagnose recurrent focal segmental glomerulosclerosis patients. Kidney Int 2019; 95: 708–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Saleem MA, Ni L, Witherden I. et al. Co-localization of nephrin, podocin, and the actin cytoskeleton: evidence for a role in podocyte foot process formation. Am J Pathol 2002; 161: 1459–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Xiao B, Wang L-N, Li W. et al. Plasma microRNA panel is a novel biomarker for focal segmental glomerulosclerosis and associated with podocyte apoptosis. Cell Death Dis 2018; 9: 533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Zhang C, Zhang W, Chen HM. et al. Plasma microRNA-186 and proteinuria in focal segmental glomerulosclerosis. Am J Kidney Dis 2015; 65: 223–232 [DOI] [PubMed] [Google Scholar]
- 104. Cai X, Xia Z, Zhang C. et al. Serum microRNAs levels in primary focal segmental glomerulosclerosis. Pediatr Nephrol 2013; 28: 1797–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Varghese SA, Powell TB, Budisavljevic MN. et al. Urine biomarkers predict the cause of glomerular disease. J Am Soc Nephrol 2007; 18: 913–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Shui HA, Huang TH, Ka SM. et al. Urinary proteome and potential biomarkers associated with serial pathogenesis steps of focal segmental glomerulosclerosis. Nephrol Dial Transplant 2007; 23: 176–185 [DOI] [PubMed] [Google Scholar]
- 107. Lopez-Hellin J, Cantarell C, Jimeno L. et al. A form of apolipoprotein A-I is found specifically in relapses of focal segmental glomerulosclerosis following transplantation. Am J Transplant 2013; 13: 493–500 [DOI] [PubMed] [Google Scholar]
- 108. Nafar M, Kalantari S, Samavat S. et al. The novel diagnostic biomarkers for focal segmental glomerulosclerosis. Int J Nephrol 2014; 2014: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Clark AJ, Jabs K, Hunley TE. et al. Urinary apolipoprotein AI in children with kidney disease. Pediatr Nephrol 2019; 34: 2351–2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Jacobs-Cachá C, López-Hellín J.. Should high molecular weight forms of apolipoprotein A-I be analyzed in urine of relapsing FSGS patients? Pediatr Nephrol 2019; 34: 2423–2424 [DOI] [PubMed] [Google Scholar]
- 111. Zhang W, Zhang C, Chen H. et al. Evaluation of microRNAs miR-196a, miR-30a-5p, and miR-490 as biomarkers of disease activity among patients with FSGS. Clin J Am Soc Nephrol 2014; 9: 1545–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Ramezani A, Devaney JM, Cohen S. et al. Circulating and urinary microRNA profile in focal segmental glomerulosclerosis: a pilot study. Eur J Clin Invest 2015; 45: 394–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Baggini J. A Short History of Truth: Consolations for a Post-Truth World London: Quercus, 2017