Abstract
Acute kidney injury (AKI) is the clinical term used for decline or loss of renal function. It is associated with chronic kidney disease (CKD) and high morbidity and mortality. However, not all causes of AKI lead to severe consequences and some are reversible. The underlying pathology can be a guide for treatment and assessment of prognosis. The Kidney Disease: Improving Global Outcomes guidelines recommend that the cause of AKI should be identified if possible. Renal biopsy can distinguish specific AKI entities and assist in patient management. This review aims to show the pathology of AKI, including glomerular and tubular diseases.
Keywords: acute tubular necrosis, AKI, hemoglobinuria, multiple myeloma, pathology, pathophysiology, review, rhabdomyolysis
AKI PERSPECTIVE
Acute kidney injury (AKI), previously called acute renal failure (ARF), is a condition of sudden kidney failure in patients with or without preexisting chronic kidney disease (CKD); severe kidney dysfunction within a few hours or days results in a significant decrease (oliguria) or complete elimination of urine (anuria), with electrolyte imbalance, often requiring hemodialysis.
While it is unclear when AKI was first recognized, incidences are scattered in the medical literature over the centuries (http://www.renalmed.co.uk). Most experts agree that the pathology was first described during World War II when four cases of crush injury characterized by diffuse acute tubular damage with pigmented casts followed by impaired renal function were reported [1]. Homer W. Smith introduced the term ‘ARF’ in 1951 [2]. In 2004, ARF was replaced by AKI [3, 4]. Before 2004 there were at least 35 ARF definitions. This situation of having various definitions has given rise to a wide range of incidence estimates for AKI from 1 to 25% of intensive care unit (ICU) patients and has led to mortality rate estimates from 15 to 60% [5, 6].
AKI is now defined by the RIFLE criteria (risk, injury, failure, loss, end-stage kidney disease) and is not just ARF. It incorporates the entire spectrum of the syndrome, from minor changes in renal function to the requirement for renal replacement therapy [7]. In practice, most nephrologists follow the Kidney Disease: Improving Global Outcomes (KDIGO) criteria, which recommend determining the cause of AKI whenever possible [5, 6]. The incidence of AKI on renal biopsy is not entirely known, but is common either as an isolated finding or concurrent with other diseases. This review is an account of the spectrum of entities identified on renal biopsy from patients presenting with AKI.
AKI clinical and pathologic classifications
It should be remembered that AKI is a clinical term. Pathologists use descriptive pathologic findings that cumulate to the term ‘acute tubular injury’ (ATI). Prerenal, intrarenal, postrenal and even unilateral insults can cause ATI. A dissociation between structural and functional changes was first recognized at autopsy of World War II soldiers with acute kidney failure and death who were found to have mild kidney findings (so-called shock kidneys) [1]. Examples of dissociation between clinical symptoms and histopathological findings include prerenal AKI caused by volume depletion as in cardiogenic, allergic or hemorrhagic shock. In such cases, ATI may be mild and/or even absent. Postrenal AKI is caused by urinary flow obstruction and can be unilateral or bilateral, e.g. unilateral hydronephrosis, lithiasis and/or pyelonephritis. The recent AKI classification that includes categories designated as declining renal function (glomerular filtration rate) instead of renal failure are in range and extent the histopathological ATI spectrum [6]. In practice, a semiquantitative histopathological scoring of ATI as mild, moderate or severe (or focal versus diffuse) is preferable instead of the term acute tubular necrosis (ATN), which was previously used despite the absence of necrosis in many cases.
Histopathological definitions of AKI
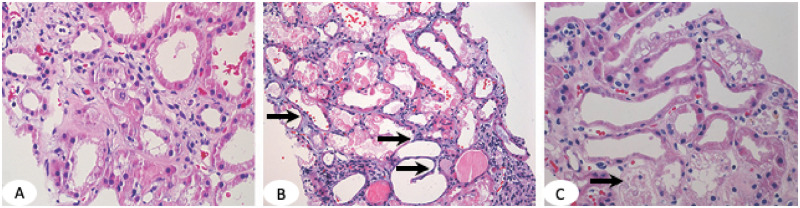
ATI is characterized by focal or diffuse tubular luminal dilatation, simplification of the lining epithelium, loss of the brush border in proximal tubules, loss of nuclei and/or the presence of nucleoli (Figure 1A). Epithelial cell mitoses and cytoplasmic basophilia can also be seen and are thought to represent epithelial cell regeneration. Both proximal and distal tubules can be affected by ATI. ATN is characterized by focal or diffuse tubular epithelial cell coagulative-type necrosis and detachment from the basement membrane (Figure 1B and C). Epithelial cell necrosis consists of cytoplasmic swelling (oncosis), degeneration of cytoplasmic organelles and a ghost-like tubular appearance staining dark pink on hematoxylin and eosin (H&E) stain. ATN is much less common compared with ATI and requires prolonged and sustained tubular injury that is usually absent in acute AKI. The exception is cortical necrosis caused by an acute ischemic process, leading to degeneration of large number of tubules (coagulation necrosis). ATI and ATN may coexist (Figure 1C).
FIGURE 1.
(A) ATI in proximal tubules shows luminal dilatation, simplification of the lining epithelium and loss of epithelial cell nuclei in some cells and loss of the brush border. (B) ATN is defined by tubular epithelial cell necrosis (dark pink fragmented cytoplasm with no nuclei) and denudation of the basement membrane (arrows). (C) ATI and ATN in the same renal biopsy. Arrow points to necrotic tubules. Dilated tubules are lined by a thin epithelial layer with no brush border. H&E,×100.
Intrarenal AKI is associated with numerous diseases, including glomerular, tubulointerstitial and vascular. Intrinsic toxic insults to tubular epithelial cells include heavy proteinuria, hematuria, interstitial nephritis and ischemia secondary to microvascular (endothelial) injury, e.g. renal vasculitis and thrombotic microangiopathies (TMAs). Glomerular diseases, acute or chronic, can be complicated by ATI. Examples include diabetic nephropathy, immunoglobulin A (IgA) nephropathy, hypertensive kidney disease, myeloma cast nephropathy, transplant rejection and TMAs.
A list of specific entities leading to intrarenal ATI is shown in Table 1. The pathology of the most common entities is described below.
Table 1.
Selected causes of AKI with distinct pathologic findings on renal biopsy
| Pigment-induced AKI |
| Rhabdomyolysis |
| Hemoglobin cast nephropathy |
| RBC casts: anticoagulation (warfarin) nephropathy, hematuric syndromes, vasculitis |
| Hemosiderosis: hemochromatosis, sickle cell disease, blood transfusions, sepsis |
| Bile nephropathy (cholemic nephrosis): hepatic disorders and hepatotoxic drugs |
| Malignancy-induced AKI |
| Myeloma cast nephropathy |
| Proximal tubulopathy |
| Lysozyme nephropathy |
| Crystal-induced AKI |
| Calcium oxalate nephropathy: hereditary, dietary, ethylene glycol, various medicinal drugs, malabsorption, bowel obstruction or small intestine/gastric bypass |
| Phosphate nephropathy |
| Cystinosis |
| 2,8-dihidroxiadeninuria |
| Cholesterol crystals |
| Crixivan/indinavir crystals |
| Acute urate nephropathy |
| Drug-induced AKI |
| Isometric vacuolization/osmotic nephrosis, contrast nephropathy |
| Antibiotics: e.g. aminoglycosides, vancomycin |
| Immunotherapy-based agents |
| Illicit drugs: cocaine |
| Over-the-counter supplements |
| Chemotherapy drugs |
| Infection-induced AKI |
| Urinary tract obstruction |
| Sepsis |
| Pyelonephritis |
| Interstitial nephritis |
| Influenza types A and B (most common) |
| COVID-19 |
| Parainfluenza virus |
| HIV |
| Coxsackievirus |
| Epstein–Barr virus |
| Echovirus |
| Cytomegalovirus |
| Adenovirus |
| Herpes simplex virus |
| Varicella-zoster virus |
| West Nile virus |
| Legionella |
| Generic ATN casts |
| TMA |
| Any glomerulonephritis |
ATI with distinct pathology
Rhabdomyolysis
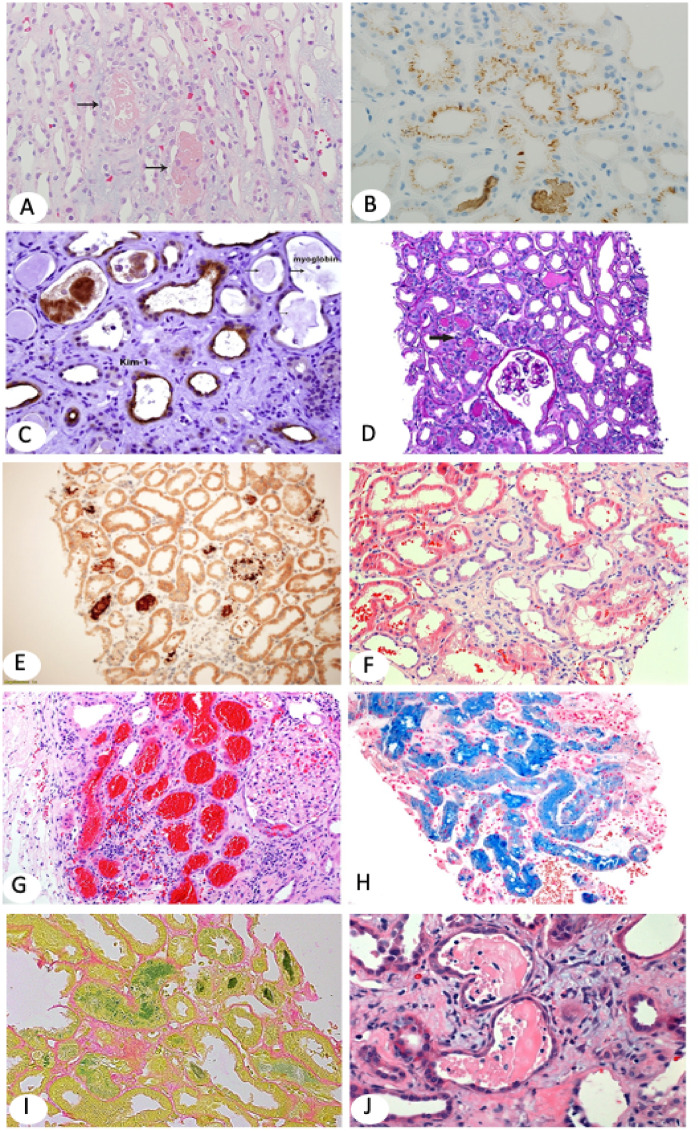
Rhabdomyolysis causes ARF in 7–15% of all AKI cases in the USA and affects 13–50% of hospitalized patients, with worse prognosis and greater mortality in critically ill patients [8]. In our recent study of renal biopsies accrued from 2011 through June 2014 among 27 850 renal biopsies in our search, 249 biopsies (∼1%) were positive for myoglobin casts [9]. On H&E stain, myoglobin casts are focal, light pink, almost translucent, but may vary from pink to dark red, granular or chain-like clumps (Figure 2A). Myoglobin casts are difficult to diagnose accurately because they have overlapping morphology with hemoglobin casts, myeloma casts and Tamm–Horsfall protein casts. Myoglobin immunohistochemistry is very helpful in arriving at a definitive diagnosis, highlighting greater numbers of injured tubules (not obvious on H&E) by staining luminal deposits (casts) and/or proximal and occasionally collecting duct epithelium (Figure 2B). Notably, ATI marked by the kidney injury molecule-1 (KIM-1) antibody is more widespread, highlighting the majority of tubules, compared with focal myoglobin staining (Figure 2C). KIM-1 is not currently routinely used to assess ATI in renal biopsies even though it is US Food and Drug Administration approved as a biomarker believed to participate in the process of both AKI and healing [10].
The pathogenesis of rhabdomyolysis is attributed to the release of myoglobin into the circulation, subsequently filtered by the glomeruli and cleared in the tubules where it accumulates either as tubular myoglobin casts or intraepithelial deposits with either a ropey or finely granular appearance [9]. Diagnosing rhabdomyolysis clinically is complicated by frequently absent classic clinical symptoms (triad of muscle pain, weakness and dark urine) and/or nondiagnostic values of laboratory tests such as creatine phosphokinase (CPK). CPK increases within 12 h of the onset of muscle injury, has a serum half-life of ∼36 h and declines 3–5 days after cessation of muscle injury [11]. At the time of biopsy, CPK may already have dissipated. The exact mechanism of ATI due to myoglobin pigment deposits is still debated but it is thought that myoglobin itself rarely leads to kidney injury in the absence of other risk factors such as ischemia, volume depletion and hypotension. Acid urine enhances the renal toxicity of myoglobin by converting heme in myoglobin to ferrihemate (hematin), shown to produce free hydroxy radicals that are directly toxic to renal tubular epithelial cells or via renal vasoconstriction due to inhibition of nitric oxide synthesis. In addition, the heme fraction of myoglobin induces the release of free radicals, further contributing to ischemic tubular damage [9].
Underlying etiologies of myoglobin casts include drugs of abuse (heroin, cocaine, opioids), infections [including human immunodeficiency virus (HIV)], bacterial sepsis, chemotherapy and immunosuppression (transplantation medicines, e.g. rapamycin), dehydration (intense exercise), malignant hypertension, trauma (surgery, traffic accidents) and myopathies [12] . The importance of making the correct diagnosis of rhabdomyolysis has prognostic implications. Full renal function recovery occurs in about half of the patients; the rest remain dialysis dependent or progress to CKD [9].
Hemoglobinuria and red blood cell casts, including Coumadin nephropathy and hemosiderosis
Heme proteins can cause AKI via at least three mechanisms: direct cytotoxicity of released hemoglobin products, decreased renal perfusion and interaction of the intratubular hemoglobin with Tamm–Horsfall protein (hemoglobin casts). Free hemoglobin is bound to serum haptoglobin; when haptoglobin is saturated, free plasma hemoglobin dissociates to dimeric molecules that filter more easily through the glomeruli. Hemoglobin is taken up by the megalin–cubilin receptors on the apical surface of tubular epithelium and deposits into proximal tubules [9]. Intracellular hemoglobin dissociates into heme and globin and heme is degraded by heme oxygenase (HO). The inducible HO-1 isoform increases rapidly, accompanied by increased intracellular ferritin. These intracellular reactions lead to binding of iron to ferritin. Even though the response is aimed to diminish damage to cytoplasmic organelles, mitochondrial injury occurs by impairment of mitochondrial oxygenation. Tubular epithelial cell apoptosis, oxidative stress and release of pro-inflammatory cytokines follow. Other organs, such as the liver and lungs, are more likely to be affected because the hemoglobin–haptoglobin complex is too large to be filtered by the glomerulus. Therefore hemoglobin deposits rarely cause AKI.
On light microscopy, hemoglobin casts appear pale or granular and closely resemble myoglobin casts. Occasionally hemoglobin appears light brown. Immunohistochemistry with antibodies to hemoglobin is the only way to reliably distinguish from myoglobin casts (Figure 2D and E). Of note, renal biopsies with myoglobin-positive casts may also have evidence of hemolysis in the background. Intact red blood cells (RBCs) also stain with hemoglobin stain (internal control). Strenuous exercise, hemolysis secondary to infection (case shown in Figure 2D and E), incompatible blood transfusion and hematologic disorders are common causes of hemoglobinuria [13, 14]. Another reported cause of hemoglobinuria is transurethral prostate resection when distilled water is used as an irrigant [15].
FIGURE 2.
(A) Myoglobin casts involve focal tubules and appear light pink on H&E (×100). Arrows point to myoglobin casts. (B) Myoglobin stains tubular casts brown and may also stain tubular epithelial brush border and/or cytoplasm in a punctuate pattern. Immunohistochemistry (IHC) ×100. (C) KIM-1, a marker for AKI, is overexpressed in injured and simplified (thin) tubular epithelium [same biopsy as in (B)]. KIM-1 IHC ×200. (D, E) The biopsy shows ATI with focal translucent tubular casts (arrow in D). Hemoglobin IHC highlights the tubular casts (E). Myoglobin stain was negative. The patient in (D–E), a 72-year-old Caucasian man with severe coronary artery disease, hypertension (HTN) and type 2 diabetes developed recurrent infection on his right foot, treated with intravenous piperacillin/tazobactam and developed chills and shortness of breath. He also had hematuria and severe peripheral hemolysis. CPK was normal; creatinine increased to 7 mg/dL with low C3 and C4. Clinical diagnoses included all comorbidities, but hemoglobin nephropathy was least expected. Hemoglobin IHC ×100. (F) Patient with IgA nephropathy who presented with hematuria and AKI. Renal biopsy shows tubular dilatation, simplification of the epithelium and multifocal luminal RBCs (H&E ×100). (G) Large patch of subcapsular proximal tubules packed with RBCs. Renal biopsy is from a 79-year-old white woman who presented with AKI on CKD. She has a histroy of atrial fibrillation on Coumadin. (I) Faucet stain marking bilirubin casts (×100). The patient was a 50-year-old Caucasian man with kidney transplant and AKI. Serum creatinine was 3.9 mg/dL and bilirubin and liver function tests were increased. (H) Marked tubular iron deposits with Prussian blue stain. The patient is a 60-year-old African American man who presented with AKI, macroscopic hematuria, hemolysis 1+ and increased reticulocytes. He had a history of mitral valve replacement, congestive heart failure and anemia. The differential diagnosis included cardiac valve defect, sickle cell disease and/or supratherapeutic international normalized ratio (H&E ×100). (J–L) Diffuse ATI and typical multiple myeloma casts that appear as partially crumbled luminal protein deposits admixed with inflammatory cells.
Gross or microscopic hematuria manifested by large amounts of RBCs in the urine may cause ATI by tubular obstruction. Hematuric syndromes, e.g. IgA nephropathy (Figure 2F), or minimal change disease presenting with hematuria, vasculitis and anticoagulation are the most frequent causes of obstructive ATI caused by RBC casts.
Anticoagulation nephropathy has potentially fatal consequences, particularly in patients with CKD. Clinical presentation with AKI is sometimes without overt creatinine changes, thus so-called warfarin nephropathy can be clinically overlooked. The incidence and severity were only recently recognized [16, 17]. Renal biopsy typically shows large numbers of intratubular RBC casts associated with tubular epithelial thinning, luminal dilation and loss of brush border (Figure 2G).
Hemosiderosis is a known complication of chronic hemolytic anemias, including paroxysmal nocturnal hemoglobinuria, and mechanical cardiac valves with residual valvular regurgitation or perivalvular leak. ATI is due to hemosiderin, an iron storage complex. The breakdown of heme gives rise to biliverdin and iron. Released iron is trapped and stored as hemosiderin in tissues. Hemosiderin is also generated from the abnormal metabolic pathway of ferritin. With H&E, hemosiderin stains as brown and granular deposits within tubular epithelial cells. Prussian blue iron specifically stains hemosiderin deposits (Figure 2H). Additional causes of hemosiderosis include sepsis, iron overload as in hereditary hemochromatosis and multiple transfusions for sickle cell disease. Some cases of infectious hemosiderosis may be reversible. For example, while Clostridium difficile–induced hemolysis may be complicated by hemoglobinuria-induced ATI, rarely is hemosiderosis reported; these deposits may resolve with resolution of the infection [18]. Supratherapeutic doses of Coumadin and other blood thinners (e.g., dabigatran) should also be excluded in patients with artificial valves or heart disease since anticoagulation is routinely prescribed.
BILE CAST NEPHROPATHY (CHOLEMIC NEPHROSIS)
Bile cast nephropathy is an infrequent cause of ATI, typically observed in patients with liver disease and jaundice. There is a spectrum of histopathological findings in renal biopsies ranging from mild ATI to epithelial cell swelling and bile cast formation [19, 20]. The casts may vary in color from yellow to brown to green and stain dark green with Hall stain (Figure 2I). At autopsy of severely jaundiced patients, kidneys have a green discoloration. This is due to conversion of bilirubin to biliverdin after formalin fixation. Green streaks of bile casts may be seen grossly.
Numerous hepatic disorders in children and adults including biliary cirrhosis (alcoholic cirrhosis in particular), bile duct atresia, nonalcoholic hepatitis, sclerosing cholangitis, shock liver, hepatotoxic drugs (including anabolic steroids), fulminant autoimmune hepatitis and intrahepatic malignancy can lead to bile cast nephropathy. Hepatic disease may cause prerenal, intrarenal and rarely postrenal ATI. The umbrella term ‘cholemic nephrosis’ is used to cover the spectrum of etiologies. Prerenal AKI is due to nonvolume responsive hepatorenal syndrome causing rapid renal failure in patients with acute or chronic renal failure. Most authors agree that bile casts require sustained liver disease and high levels of serum bilirubin. The term bile cast nephropathy is used when bile or bilirubin casts obstruct the nephrons, usually the distal tubules. Whether bilirubin itself causes direct injury to tubular epithelia or additional factors (vasoconstriction and volume depletion) contribute to precipitation of bile in the tubules is debated [21].
MYELOMA CAST NEPHROPATHY AND RELATED DISORDERS
About 50% of patients with multiple myeloma develop renal disease. AKI is increasingly recognized as the first presentation of multiple myeloma [22, 23]. The most common pathologic findings on renal biopsy are myeloma cast nephropathy, light chain proximal tubulopathy and light chain deposition disease (LCDD). Light microscopy can be unimpressive, but immunofluorescence is usually diagnostic. AKI complicating multiple myeloma is associated with worse 1-year survival and reduces the therapeutic options available to patients [22].
Myeloma casts are typically periodic acid–Schiff (PAS) negative and appear as fractured or crackled paper-like proteinaceous deposits. Tubular casts are engulfed by giant cells or are admixed with inflammatory cells, sometimes mimicking acute pyelonephritis or interstitial nephritis (Figure 2J). Other times, paraprotein casts are devoid of an inflammatory component, are pale and translucent, mimicking rhabdomyolysis casts. Monoclonality is determined by immunofluorescence staining for kappa and lambda light chains. Tubular epithelial injury presents as epithelial simplification, epithelial cell necrosis or giant cell formation. Less frequently, paraproteins take the form of crystal deposits within tubular epithelium or in the lumen (with or without Fanconi syndrome) [24]. Light chain proximal tubulopathy (Figure 2K and L) is characterized by tubular epithelial cytoplasmic droplets staining with monoclonal light chains, either kappa or lambda [25, 26]. Light chain proximal tubulopathy may appear as generic ATI on light microscopy and, unless carefully examined and interpreted by experienced renal pathologists, can be easily overlooked. In the absence of ATI, monoclonal light chains within the tubular epithelium may alternatively represent physiologic proteinuria due to overproduction of a monoclonal light chain. A third pattern of myeloma injury is the so-called monoclonal light chain deposition disease, characterized by linear staining of the glomerular basement membranes, tubular basement membranes or both, with either kappa or lambda restriction by immunofluorescence. In rare cases, multiple myeloma pathologies involving the kidney (e.g. cast nephropathy and LCDD) are concurrently present (case shown in Figure 2 J–L) [27]. Additional pathologies such as plasma cell infiltrates and amyloidosis concurrent with cast nephropathy or other combinations are also possible. AKI is invariably in the background.
HEMATOLOGIC MALIGNANCIES AND TUMOR LYSIS- AND LYSOZYME-INDUCED ATI
About two-thirds of critically ill patients with hematological malignancies develop AKI at some point during the course of their disease or following treatment. AKI secondary to malignancy may manifest alongside malignant infiltrates involving the kidney parenchyma (malignant plasma cells, leukemia/lymphoma infiltrates) or be precipitated by tumor cell lysis. Hemodynamic compromise (ischemic ATI), chemotherapy-induced (toxic ATI) and tumor lysis syndrome are part of the spectrum of oncologic AKI [28].
An exceptional type of ATI associated with malignancy is lysozyme nephropathy due to release of lysozyme from malignant cells [29, 30]. Lysozyme is produced in low levels by granulocytes, monocytes and histiocytes. In the kidney, it is stored in proximal tubules within lysosomes. The enzyme is excessively produced in pathologic conditions such as the myelomonocytic cells of chronic myelogenous leukemia (CML).
It is also associated with high macrophage turnover and secretion of lysozyme in the serum (such as in patients with sarcoidosis). Lysozyme filters through the glomeruli and is absorbed by tubular epithelial cells, which hold high affinity for lysozyme. Plasma levels decrease after treatment of CML and perhaps other conditions so that lysozyme-induced AKI may not be clinically apparent or with blood tests. A renal biopsy may then be performed. The unique constellation of histopathological findings includes intensely eosinophilic and silver-negative protein droplets in proximal tubules. On electron microscopy, membrane-bound lysosomal inclusions are identified. Staining with lysozyme confirms the diagnosis (Figure 2M and N). Nonspecific staining for Congo red may be seen.
ISOMETRIC VACUOLIZATION, OSMOTIC NEPHROSIS, CONTRAST MEDIA AND MITOCHONDRIAL INJURY-INDUCED ATI
Isometric tubular vacuolization is a distinct form of ATI characterized by focal or diffuse bubbly appearing tubules (Figure 2O). The isometric-appearing vacuoles in most cases are due to swollen lysosomes (seen by electron microscopy) or swollen mitochondria (see below) [31]. This is typically an acute toxicity of calcineurin inhibitors (CNIs), particularly in renal allografts [32]. Cyclosporine, tacrolimus, intravenous IgG, dextran and osmotically active substances can cause similar pathology. Low-osmolar and iso-osmolar radiographic (contrast) media such as iotrolan and iodixanol (but also high-osmolality agents) cause intracellular vacuolization in tubular epithelial cells. It is hypothesized that these agents may interfere with physiologic protein reabsorption and are facilitated by hypoxia (patients with diabetes, atherosclerosis, CKD) [33]. The finding of isometric tubular vacuolization is nonspecific, but important to recognize, in order to prompt identification of a triggering agent and drug discontinuation, possibly reversing ATI. Recovery from the tubular injury will wean the patient off dialysis in many cases. The vacuoles may fade away or persist due to poorly understood mechanisms. Background disease such as diabetes and kidney ischemia may contribute to persistent vacuolization. Cyclosporine toxicity causes mitochondrial swelling (megamitochondria). Mitochondrial enlargement is responsible for the vacuolated cytoplasmic appearance as evidenced by electron microscopy. It was more common in the early era of cyclosporine therapy, but since regular drug monitoring was established, acute cyclosporine toxicity has become rare. The most common mitochondrial toxicity currently seen is with antiretroviral medications (tenofovir and related drugs) [34]. On renal biopsy, mitochondrial toxicity manifests with either isometric vacuolization or more rarely with giant mitochondria with abnormal cristae (dysmorphic), appearing as eosinophilic cytoplasmic inclusions in tubular epithelial cells on H&E (Figure 2P).
ATI ASSOCIATED WITH CRYSTALLOPATHIES
Calcium oxalate is the most common type of crystal nephropathy on renal biopsy (Helen Liapis,unpublished results). The extent of oxalate crystals varies from a few foci to massive amounts. Acute presentation shows colorless crystals in tubules and/or the interstitium associated with varying degrees of tubular injury, usually ATI without necrosis. Oxalate crystals, colorless on H&E, polarize under dark-field microscopy (Figure 2Q and R). Under normal conditions, calcium and oxalate form a complex in the colon and are excreted in the feces. In the absence of or with reduced luminal calcium, free oxalate increases, leading to enhanced absorption by the colonic epithelium and ultimately calcium oxalate crystals deposit in the kidney. Fat and/or bile acid malabsorption also facilitate oxalate uptake by colonic epithelial cells.
Entities leading to renal oxalosis include enteric hyperoxaluria (e.g. Crohn’s disease, celiac sprue, pancreatic insufficiency, gastric/small intestine bypass or resection, chronic pancreatitis or malabsorption syndromes), vitamin B6 deficiency, ethylene glycol toxicity, excess ingestion of vitamin C, a plethora of dietary products rich in oxalic acid (e.g. dark leafy vegetables, rhubarb, star fruit, tea, spinach, sesame seeds, almonds, beets, buckwheat flour, chocolate soy milk; www.OHF.org/docs/Oxalate2008.pdf), hereditary hyperoxalurias and ATI itself (Table 1). Other risk factors include the absence of enteric oxalate-degrading bacteria (i.e. Oxalobacter formigenes), aspergillosis and drugs (Orlistat, Praxilene). The insults can be irreversible and may be fatal in a fraction of patients [35, 36].
In transplant renal biopsies, secondary causes of renal oxalosis include prolonged tubular injury, chronic pancreatic allograft rejection in kidney–pancreas recipients, hypocitraturia secondary to CNIs and mycophenolate mofetil (MMF)-induced malabsorption syndrome secondary to prolonged diarrhea. The anesthetic methoxyflurane is also reported to cause AKI secondary to oxalate nephropathy.
Other drugs can cause ATI with unique crystalline deposits beyond oxalate; for example, indinavir (not shown here).
Calcium phosphate is the second most common crystallopathy seen on renal biopsy. The deposits are usually focal and stain blue on H&E (Figure 2S) and black with von Kossa stain. Heavy deposits (nephrocalcinosis) are seen with primary or secondary hypercalcemia, including sarcoidosis, vitamin D intoxication, milk-alkali syndrome, ingestion of phosphate-containing medications [antacids, soft drinks, bowel preparations (e.g. oral sodium phosphate)—also called phosphate nephropathy] and stone disease [37]. Once again, drugs may be the culprit causing phosphate crystal deposits (bisphosphonates, ganciclovir and others).
Some unique causes of crystal deposition associated with AKI will be briefly mentioned here. These include cholesterol embolism presenting with AKI and cystinosis, a defective transport of cystine across lysosomal membranes resulting in systemic accumulation of cystine crystals, including in the kidney (glomeruli, tubules and interstitium). Cysteine crystals are difficult to identify in tissue because they dissolve during formalin processing.
Cholesterol crystals appear as empty spindle-shaped spaces (clefts) within vascular lumens surrounded by inflammatory cells. AKI and diffuse ATI are invariably present.
The mechanisms of crystallopathy-associated AKI remains an enigma [38, 39]. The fate of crystal deposition may be dependent on recruitment of phagocytes enabling crystal clearance from the interstitium, while intratubular deposits may dissolve or clear with urinary flow. Studies show that renal crystal deposits may be a transient phenomenon and disappear at a later time. For example, in rat and human kidneys, calcium oxalate and calcium phosphate crystals translocate into the interstitial space where infiltrating mononuclear cells contribute to crystal disintegration and clearance [36–38]. Recently the NLRP3 inflammasome was shown to trigger inflammation and AKI in oxalate nephropathy, raising the hypothesis of innate immunity possibly involved in this and other crystallopathies [38]. Resolution of inflammation and crystal removal may halt the deleterious chronic effects of crystal deposition within the kidney. There is clinical evidence from AKI recovery in humans that repair of injury is possible via a macrophage phenotype switch toward anti-inflammatory M2 macrophages [39].
AKI due to adenine phosphoribosyltransferase (APRT) deficiency is characterized by excessive production of 2,8-dihydroxyadenine (DHA). This is an autosomal recessive disorder due to complete loss of APRT. It manifests with AKI episodes, progressive CKD and nephrolithiasis. Renal biopsy reveals round, brown DHA crystals that polarize, mimicking oxalate. The diagnosis is confirmed by the absence of APRT enzyme activity in red cell lysates or identification of biallelic pathogenic variants. A low-purine diet, ample fluid intake and allopurinol therapy improve outcomes [40, 41].
Acute uric acid nephropathy typically presents with oliguric or anuric AKI and is most frequently associated with massive tumor lysis [42]. The chronic effects of uric acid nephropathy are known for granuloma formation (gouty nephropathy) and interstitial fibrosis.
INFECTION–RELATED AKI
Infections can cause obstructive AKI and ATI/ATN through white cell tubular cast formation or direct invasion of the microorganisms into the tubular epithelia. An associated interstitial nephritis is invariably present [35]. Examples include ATI in the setting of polyomavirus, cytomegalovirus, coronaviruses (including influenza and coronavirus disease 2019 (COVID-19) or adenovirus nephropathy in transplant or immunocompromised patients (Table 1) [43].
TMA ASSOCIATED WITH ATYPICAL HEMOLYTIC SYNDROME SYNDROMES, ANTIPHOSPHOLIPID SYNDROME, PREECLAMPSIA, DRUG TOXICITY
TMAs are life-threatening entities and have characteristic pathology of thrombi involving glomerular capillaries and/or arterioles (Figure 2T). Clinically, severe AKI is a frequent presenting symptom, while thrombocytopenia, peripheral schistocytes, elevated lactate dehydrogenase and decreased haptoglobin may be nondiagnostic. causing atypical hemolytic syndrome (aHUS). Antiphospholipid syndrome falls in the category of aHUS and on renal biopsy the findings range from subcortical necrosis to focal TMA. Renal biopsy pathology explains the acute presentation, demonstrating hemorrhagic ATN in severe cases or diffuse ATI adjacent to ‘focal’ thrombotic lesions. The emphasis here is on focal TMA manifesting either as single glomerular capillary thrombosis or endothelial swelling and narrowing of the arterioles, sometimes lacking bona fide thrombi. The main injury in TMA is endothelial and ATI is secondary to ischemia and RBC lysis. Mural fragmented RBCs in small arterioles may be present, but these are sufficient for histopathologic diagnosis of TMA. Preeclampsia, postpartum TMA and other causes of aHUS during or after pregnancy have emerged as significant AKI causes, frequently and definitively diagnosed best with renal biopsy. The nephrologists’ reaction to the renal biopsy findings in these cases, typically young women, may be surprise, followed by ambiguity regarding appropriate and immediate potentially lifesaving patient management [44]. This complex clinical setting requires both hematology and nephrology consultation.
The current COVID-19 pandemic brought to light the deleterious effects of viruses to endothelia, manifesting in the kidney as TMA, but also systemically (e.g. strokes) [45].
Last but not least, chemotherapy agents and monoclonal antibodies, e.g. immune checkpoint inhibitors, that target inhibitory receptors expressed on T cells and currently used for solid tumors or hematologic malignances are increasingly reported as causes of TMA-induced AKI. Other side effects to explain AKI in such patients include interstitial nephritis and generic ATI [46].
AKI pathophysiology
An increased understanding of the pathophysiology underlying AKI was revealed in the last few decades through molecular and animal studies that show oxidative stress [47], endothelial injury [48], mitochondrial injury (best described in the HIV) population treated with antiretroviral medications] [49] and innate immunity as central mechanisms [50], discussed briefly below.
AKI, previously thought to be a relatively benign process without significant long-term sequelae, is now considered a long-term risk factor for CKD, particularly in older patients with coexisting comorbidities, particularly sepsis, affecting 40–70% of patients in the ICU [51, 52].
Therapeutic or illicit drugs and toxins represent external insults. Numerous drugs can cause ATI/ATN. The most common are antibiotics (e.g. vancomycin), chemotherapeutics, angiotensin-converting enzyme inhibitors, lithium and over-the-counter supplements. Similar patterns of tubular injury have been reported in association with illicit drugs such as opioids and synthetic cannabinoids (Spice, K2, etc.) [49, 53–55]. Drugs are such a common cause of ATI/ATN that, above and beyond any other causes, drug exposure should first and foremost be clinically excluded.
Interesting mechanisms of infection-induced ischemic AKI continue to be found. For example, neutrophil extracellular traps damage the kidney through neutrophil arginine deiminase 4 [56, 57].
Animal models of AKI
A significant amount of research has been directed at investigating AKI pathophysiology and developing AKI therapeutics in animal models [58, 59]. However, none of these therapies have translated into clinical care to date. One of the most widely used animal models of AKI is the ischemia–reperfusion model. A warm ischemia–reperfusion study is typically performed by unilateral or bilateral clamping of the renal vasculature for 30–45 min followed by reperfusion for 1–2 days [59, 60]. This model was extensively studied in pigs, dogs, rabbits, rats and mice. Toxin exposure is a known cause of AKI and has been used to study AKI pathophysiology in vivo. Cisplatin, folic acid, aristolochic acid and warfarin are common nephrotoxins utilized to induce AKI in animal models [51–65]. Rhabdomyolysis is a specific clinical condition that may be reproduced in animals using a glycerol model of AKI. Glycerol injected into the hind leg muscles of rats produces rapid AKI and rhabdomyolysis [66, 67]. The unilateral ureteral obstruction model is a reproducible animal model whereby a single ureter is ligated, resulting in mechanical stress and inflammation in one kidney. This model is used to study the AKI to CKD transition. Sepsis is another well-documented cause of AKI [51, 68]. Studying this process in animals may be performed by lipopolysaccharide injection or by using the more clinically relevant cecal ligation and puncture (CLP) model [69, 70]. Although the CLP model is more typical of the human condition, it is less reproducible and more technically challenging. Animal models are a useful tool to investigate the pathophysiology of AKI. However, the dearth of new clinically useful therapeutics developed using these animal models highlights the disconnect between human clinical AKI and preclinical studies. This underscores the point that clinical AKI in humans is a diverse process with multiple etiologies and varying pathophysiology such that single treatment options are unlikely to prove effective.
AKI biomarkers
Current clinical practice utilizes serum creatinine and urine output to identify patients with AKI, regardless of the underlying etiology. A significant achievement has been standardizing AKI diagnostic criteria by the KDIGO [5, 71, 72]. Serum creatinine may not increase until days following injury, may change in cases without structural kidney damage and may not change despite injury in patients with significant renal reserve [73–75]. Due to these known imperfections, a troponin-like biomarker for AKI is desired. The hope is to facilitate early diagnosis in order to implement current management strategies aimed at preventing further injury. Earlier diagnosis may facilitate reexamination of therapeutics that previously failed clinical trials, possibly due to delayed treatment using creatinine for therapeutic initiation.
The last decade has seen a significant effort to identify sensitive and specific urine and plasma AKI biomarkers. AKI biomarkers may be functional (cystatin C), related to damage (myo-inositol oxygenase, N-acetyl-β-glucosaminidase, glutathione S-transferase, alkaline phosphatase), inflammatory (interleukins-18, -6, -10 and -5), upregulated in the proximal tubule following injury (KIM-1), upregulated in the distal tubule following injury (neutrophil gelatinase–associated lipocalin) or cell cycle arrest indicators (tissue inhibitor metalloproteinase-2 and insulin-like growth factor binding protein-7) [76, 77]. Despite extensive research and development of standardized assays for some biomarkers, AKI biomarkers have predominantly been restricted to research use and have not yet permeated clinical practice. One reason for this discrepancy is the use of creatinine as a flawed gold standard for biomarker qualification [76]. Another drawback is their lack of specificity for renal disease [7]. One biomarker, myo-inositol oxygenase, is reportedly restricted to renal tissue and shows promise as a renal-specific proximal tubular damage indicator but has yet to undergo significant investigation [76]. Utilizing other criteria such as need for dialysis and mortality has helped to identify biomarkers that complement clinical assessment [78–80]. Despite these shortcomings, recent studies indicate a possible role for biomarkers in discriminating true AKI from prerenal azotemia, hepatorenal syndrome and cardiorenal syndrome [78]. Future studies will need to assess the ability of AKI biomarkers to improve patient outcomes in order to be widely adopted in clinical practice [77].
CONCLUSIONS
The pathology of AKI is as diverse as the entities causing it. Renal biopsy illuminates this diversity and provides specific diagnoses using available immunohistochemical or histochemical stains to complement routine pathologic evaluation. Interpretation and effective consultation require highly skilled and sophisticated renal pathologists and clear communication with the treating nephrologists. Renal biopsy pathology is frequently the fastest and most accurate procedure in determining the specific cause of AKI, as shown below. Furthermore, in spite of the existing clinical AKI criteria and worldwide validation, there is still inconsistency in the application of criteria confounded by the limitations of serum creatinine and urine output as AKI biomarkers.
CONFLICT OF INTEREST STATEMENT
None declared. The results presented in this article have not been published previously in whole or part, except in abstract format.
REFERENCES
- 1. Brun CC, Munck O. Lesions of the kidney in acute renal failure following shock. Lancet 1957; 269: 603–607 [DOI] [PubMed] [Google Scholar]
- 2. Smith HM. The Kidney: Structure and Function in Health and Disease. 1st edn. Oxford: Oxford University Press, 1951 [Google Scholar]
- 3. Bellomo R, Kellum JA, Ronco C. Defining acute renal failure: physiological principles. Intensive Care Med 2004; 30: 33–37 [DOI] [PubMed] [Google Scholar]
- 4. Ronco C, Bellomo R, Kellum JA. Acute kidney injury. Lancet 2019; 394: 1949–1964 [DOI] [PubMed] [Google Scholar]
- 5. Kidney Disease: Improving Global Outcomes Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl 2012; 2: 1–138 [Google Scholar]
- 6. Okusa MD, Davenport A. Reading between the (guide)lines—the KDIGO practice guidelines on acute kidney injury in the individual patient. Kidney Int 2014; 85: 39–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thomas ME, Blaine C, Dawnay A et al. The definition of acute kidney injury and its use in practice. Kidney Int 2015; 87: 62–73 [DOI] [PubMed] [Google Scholar]
- 8. Heard H, Barker J. Recognizing, diagnosing, and treating rhabdomyolysis. J Am Acad Physician Assist 2016; 29: 29–32 [DOI] [PubMed] [Google Scholar]
- 9. Liapis H, Boils C, Hennigar R et al. Myoglobin casts in renal biopsies: immunohistochemistry and morphologic spectrum. Hum Pathol 2016; 54: 25–30 [DOI] [PubMed] [Google Scholar]
- 10. Zuk A, Bonventre JV. Acute kidney injury. Annu Rev Med 2016; 67: 293–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Simpson JP, Taylor A, Sudhan N et al. Rhabdomyolysis and acute kidney injury: creatine kinase as a prognostic marker and validation of the McMahon score in a 10-year cohort: a retrospective observational evaluation. Eur J Anaesthesiol 2016; 33: 906–912 [DOI] [PubMed] [Google Scholar]
- 12. Huerta-Alardín AL, Varon J, Marik PE. Bench-to-bedside review: rhabdomyolysis—an overview for clinicians. Crit Care 2005; 9: 158–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dvanajscak Z, Walker PD, Cossey LN et al. Hemolysis-associated hemoglobin cast nephropathy results from a range of clinicopathologic disorders. Kidney Int 2019; 96: 1400–1407 [DOI] [PubMed] [Google Scholar]
- 14. González I, Rais R, Gaut JP et al. Evans syndrome complicated by intratubular hemoglobin cast nephropathy. Case Rep Pediatr 2017; 2017: 1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen SS, Lin AT, Chen KK et al. TURP and intravascular hemolysis is another cause of hemoglobinuria hemolysis in transurethral resection of the prostate using distilled water as the irrigant. J Chin Med Assoc 2006; 69: 270–275 [DOI] [PubMed] [Google Scholar]
- 16. Wheeler DS, Giugliano RP, Rangaswami J. Anticoagulation-related nephropathy. J Thromb Haemost 2016; 14: 461–467 [DOI] [PubMed] [Google Scholar]
- 17. Brodsky SV, Mhaskar NS, Thiruveedi S et al. Acute kidney injury aggravated by treatment initiation with apixaban: another twist of anticoagulant-related nephropathy. Kidney Res Clin Pract 2017; 36: 387–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abou Arkoub R, Wang D, Zimmerman D. A rare cause of reversible renal hemosiderosis. Case Rep Nephrol 2015; 2015: 1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van Slambrouck CM, Salem F, Meehan SM et al. Bile cast nephropathy is a common pathologic finding for kidney injury associated with severe liver dysfunction. Kidney Int 2013; 84: 192–197 [DOI] [PubMed] [Google Scholar]
- 20. Betjes MG, Bajema I. The pathology of jaundice-related renal insufficiency: cholemic nephrosis revisited. J Nephrol 2006; 19: 229–233 [PubMed] [Google Scholar]
- 21. Romano TG, Vieira Junior JM. Do biliary salts have role on acute kidney injury development? J Clin Med Res 2015; 7: 667–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Leung N, Nasr SH. A patient with abnormal kidney function and a monoclonal light chain in the urine. Clin J Am Soc Nephrol 2016; 11: 1073–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Darmon M, Vincent F, Canet E et al. Acute kidney injury in critically ill patients with haematological malignancies: results of a multicentre cohort study from the Groupe de Recherche en Réanimation Respiratoire en Onco-Hématologie. Nephrol Dial Transplant 2015; 30: 2006–2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Herlitz LC, D'Agati VD, Markowitz GS. Crystalline nephropathies. Arch Pathol Lab Med 2012; 136:713–720 [DOI] [PubMed] [Google Scholar]
- 25. Larsen CP, Bell JM, Harris AA et al. The morphologic spectrum and clinical significance of light chain proximal tubulopathy with and without crystal formation. Mod Pathol 2011; 24: 1462–1469 [DOI] [PubMed] [Google Scholar]
- 26. Stokes MB, Valeri AM, Herlitz L et al. Light chain proximal tubulopathy: clinical and pathologic characteristics in the modern treatment era. J Am Soc Nephrol 2016; 27: 1555–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gokden N, Cetin N, Colakoglu N et al. Morphologic manifestations of combined light-chain deposition disease and light-chain cast nephropathy. Ultrastruct Pathol 200; 31: 141–149 [DOI] [PubMed] [Google Scholar]
- 28. Lam AQ, Humphreys BD. Onco-nephrology: AKI in the cancer patient. Clin J Am Soc Nephrol 2012; 7: 1692–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cossey LN, Larsen CP. Lysozyme-induced acute tubular injury: an under-recognized cause of acute renal failure. J Am Soc Nephrol 2013; 24(Suppl): SA-PO025 [Google Scholar]
- 30. Patel TV, Rennke HG, Sloan JM et al. A forgotten cause of kidney injury in chronic myelomonocytic leukemia. Am J Kidney Dis 2009; 54: 159–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dickenmann M, Oettl T, Mihatsch MJ. Osmotic nephrosis: acute kidney injury with accumulation of proximal tubular lysosomes due to administration of exogenous solutes. Am J Kidney Dis 2008; 51: 491–503 [DOI] [PubMed] [Google Scholar]
- 32. Laftavi MR, Weber-Shrikant E, Kohli R et al. Sirolimus-induced isometric tubular vacuolization: a new sirolimus histopathologic manifestation. Transplant Proc 2010; 42: 2547–2550 [DOI] [PubMed] [Google Scholar]
- 33. Kiss N, Hamar P. Histopathological evaluation of contrast-induced acute kidney injury rodent models. Biomed Res Int 2016; 2016: 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rodriguez-Nóvoa S, Alvarez E, Labarga P et al. Renal toxicity associated with tenofovir use. Expert Opin Drug Saf 2010; 9: 545–559 [DOI] [PubMed] [Google Scholar]
- 35. Colvin RB, Chang A, Farris B et al. Diagnostic Pathology: Kidney diseases, 2nd edn. Salt Lake City, UT: AMIRSYS, 2015
- 36. Vervaet BA, Verhulst A, Dauwe SE et al. An active renal crystal clearance mechanism in rat and man. Kidney Int 2009; 75: 41–51 [DOI] [PubMed] [Google Scholar]
- 37. Markowitz GS, Nasr SH, Klein P et al. Renal failure due to acute nephrocalcinosis following oral sodium phosphate bowel cleansing. Hum Pathol 2004; 35: 675–684 [DOI] [PubMed] [Google Scholar]
- 38. Mulay SR, Kulkarni OP, Rupanagudi KV et al. Calcium oxalate crystals induce renal inflammation by NLRP3-mediated IL-1β secretion. J Clin Invest 2013; 123: 236–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Anders HJ, Suarez-Alvarez B, Grigorescu M et al. The macrophage phenotype and inflammasome component NLRP3 contributes to nephrocalcinosis-related chronic kidney disease independent from IL-1-mediated tissue injury. Kidney Int 2018; 93: 656–669 [DOI] [PubMed] [Google Scholar]
- 40. Runolfsdottir HL, Palsson R, Agustsdottir IM et al. Kidney disease in adenine phosphoribosyltransferase deficiency. Am J Kidney Dis 2016; 67: 431–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nasr SH, Sethi S, Cornell LD et al. Crystalline nephropathy due to 2,8-dihydroxyadeninuria: an under-recognized cause of irreversible renal failure. Nephrol Dial Transplant 2010; 25: 1909–1915 [DOI] [PubMed] [Google Scholar]
- 42. Kjellstrand CM, Cambell DC II, von Hartitzsch B et al. Hyperuricemic acute renal failure. Arch Intern Med 1974; 133: 349–359 [PubMed] [Google Scholar]
- 43. Pei G, Zhang Z, Peng J et al. Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol 2020; doi: 10.1681/ASN.2020030276 [DOI] [PMC free article] [PubMed]
- 44. Fakhouri F. Pregnancy-related thrombotic microangiopathies: clues from complement biology. Transfus Apher Sci 2016; 54: 199–202 [DOI] [PubMed] [Google Scholar]
- 45. Gavriilaki E, Brodsky RA. Severe COVID-19 infection and thrombotic microangiopathy: success doesn't come easily. Br J Haematol 2020; 189; e227–e230 [DOI] [PubMed] [Google Scholar]
- 46. Cortazar FB, Marrone KA, Troxell ML et al. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int 2016; 90: 638–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Heyman SN, Rosen S, Rosenberger C. A role for oxidative stress. Contrib Nephrol 2011; 174: 138–148 [DOI] [PubMed] [Google Scholar]
- 48. Patschan D, Kribben A, Müller GA. Postischemic microvasculopathy and endothelial progenitor cell-based therapy in ischemic AKI: update and perspectives. Am J Physiol Renal Physiol 2016; 311: F382–F394 [DOI] [PubMed] [Google Scholar]
- 49. Tsuji N, Tsuji T, Ohashi N et al. Role of mitochondrial DNA in septic AKI via Toll-like receptor 9. J Am Soc Nephrol 2016; 27: 2009–2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mulay SR, Holderied A, Kumar SV et al. Targeting inflammation in so-called acute kidney injury. Semin Nephrol 2016; 36: 17–30 [DOI] [PubMed] [Google Scholar]
- 51. Takasu O, Gaut JP, Watanabe E et al. Mechanisms of cardiac and renal dysfunction in patients dying of sepsis. Am J Respir Crit Care Med 2013; 187: 509–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Perazella MA, Luciano RL. Review of select causes of drug-induced AKI. Expert Rev Clin Pharmacol 2015; 8: 367–371 [DOI] [PubMed] [Google Scholar]
- 53. Pendergraft WF 3rd, Herlitz LC, Thornley-Brown D et al. Nephrotoxic effects of common and emerging drugs of abuse. Clin J Am Soc Nephrol 2014; 9: 1996–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Belen C, Budhiraja P, Bracamonte E et al. Biopsy-proven acute tubular necrosis associated with vancomycin in an adult patient. Ren Fail 2012; 34: 502–505 [DOI] [PubMed] [Google Scholar]
- 55. Bhanushali GK, Jain G, Fatima H et al. AKI associated with synthetic cannabinoids: a case series. Clin J Am Soc Nephrol 2013; 8: 523–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Raup-Konsavage WM, Wang Y, Wang WW et al. Neutrophil peptidyl arginine deiminase-4 has a pivotal role in ischemia/reperfusion-induced acute kidney injury. Kidney Int 2018; 93: 365–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nakazawa D, Kumar SV, Marschner J et al. Histones and neutrophil extracellular traps enhance tubular necrosis and remote organ injury in ischemic AKI. J Am Soc Nephrol 2017; 28: 1753–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ortiz A, Sanchez-Niño MD, Izquierdo MC et al. Translational value of animal models of kidney failure. Eur J Pharmacol 2015; 759: 205–220 [DOI] [PubMed] [Google Scholar]
- 59. Skrypnyk NI, Siskind LJ, Faubel S et al. Bridging translation for acute kidney injury with better preclinical modeling of human disease. Am J Physiol Renal Physiol 2016; 310: F972–F984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lieberthal W, Nigam SK. Acute renal failure. II. Experimental models of acute renal failure: imperfect but indispensable. Am J Physiol Renal Physiol 2000; 278: F1–F12 [DOI] [PubMed] [Google Scholar]
- 61. Szczypka MS, Westover AJ, Clouthier SG et al. Rare incorporation of bone marrow-derived cells into kidney after folic acid-induced injury. Stem Cells 2005; 23: 44–54 [DOI] [PubMed] [Google Scholar]
- 62. Heyman SN, Lieberthal W, Rogiers P et al. Animal models of acute tubular necrosis. Curr Opin Crit Care 2002; 8: 526–534 [DOI] [PubMed] [Google Scholar]
- 63. Molitoris BA, Dagher PC, Sandoval RM et al. siRNA targeted to p53 attenuates ischemic and cisplatin-induced acute kidney injury. J Am Soc Neprol 2009; 20: 1754–1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Matsui K, Kamijo-Ikemori A, Hara M et al. Clinical significance of tubular and podocyte biomarkers in acute kidney injury. Clin Exp Nephrol 2011; 15: 220–225 [DOI] [PubMed] [Google Scholar]
- 65. Ware LB, Herridge M. Acute lung injury. Semin Respir Crit Care Med 2013; 34: 439–440 [DOI] [PubMed] [Google Scholar]
- 66. Thiel G, Wilson DR, Arce ML et al. Glycerol induced hemoglobinuric acute renal failure in the rat. II. The experimental model, predisposing factors, and pathophysiologic features. Nephron 1967; 4: 276–297 [DOI] [PubMed] [Google Scholar]
- 67. Curry SC, Chang D, Connor D. Drug- and toxin-induced rhabdomyolysis. Ann Emerg Med 1989; 18: 1068–1084 [DOI] [PubMed] [Google Scholar]
- 68. Schrier RW, Wang W. Acute renal failure and sepsis. N Engl J Med 2004; 351: 159–169 [DOI] [PubMed] [Google Scholar]
- 69. Remick DG, Bolgos GR, Siddiqui J et al. Six at six: interleukin-6 measured 6 h after the initiation of sepsis predicts mortality over 3 days. Shock 2002; 17: 463–467 [DOI] [PubMed] [Google Scholar]
- 70. Gaut JP, Yeh GC, Tran HD et al. Neutrophils employ the myeloperoxidase system to generate antimicrobial brominating and chlorinating oxidants during sepsis. Proc Natl Acad Sci USA 2001; 98: 11961–11966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Fliser D, Laville M, Covic A et al. A European Renal Best Practice (ERBP) position statement on the Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelines on acute kidney injury: part 1: definitions, conservative management and contrast-induced nephropathy. Nephrol Dial Transplant 2012; 27: 4263–4272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mehta RL, Kellum JA, Shah SV et al. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007; 11: R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Waikar SS, Betensky RA, Emerson SC et al. Imperfect gold standards for kidney injury biomarker evaluation. J Am Soc Nephrol 2012; 23: 13–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Blantz RC. Pathophysiology of pre-renal azotemia. Kidney Int 1998; 53: 512–523 [DOI] [PubMed] [Google Scholar]
- 75. Bosch JP, Saccaggi A, Lauer A et al. Renal functional reserve in humans. Effect of protein intake on glomerular filtration rate. Am J Med 1983; 75: 943–950 [DOI] [PubMed] [Google Scholar]
- 76. Gaut JP, Crimmins DL, Ohlendorf MF et al. Development of an immunoassay for the kidney-specific protein myo-inositol oxygenase, a potential biomarker of acute kidney injury. Clin Chem 2014; 60: 747–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Vanmassenhove J, Kielstein J, Jörres A et al. Management of patients at risk of acute kidney injury. Lancet 2017; 389: 2139–2151 [DOI] [PubMed] [Google Scholar]
- 78. Parikh CR, Mansour SG. Perspective on clinical application of biomarkers in AKI. J Am Soc Nephrol 2017; 28: 1677–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Parikh CR, McSweeney P, Schrier RW. Acute renal failure independently predicts mortality after myeloablative allogeneic hematopoietic cell transplant. Kidney Int 2005; 67: 1999–2005 [DOI] [PubMed] [Google Scholar]
- 80. Liangos O, Perianayagam MC, Vaidya VS et al. Urinary N-acetyl-beta-(D)-glucosaminidase activity and kidney injury molecule-1 level are associated with adverse outcomes in acute renal failure. J Am Soc Nephrol 2007; 18: 904–912 [DOI] [PubMed] [Google Scholar]