Abstract
Background
Similarities in clinicopathological presentations in immunoglobulin A (IgA) nephropathy and IgA vasculitis with nephritis (IgAVN) raise the question of the utility of the Oxford classification in the latter. The aim of this study was to evaluate the Oxford classification in IgAVN.
Methods
We conducted a retrospective cohort study and meta-analysis following systematic searching of the MEDLINE and Excerpta Medica Database (EMBASE) databases between January 2009 and September 2019. We modeled the association of 30 and 50% decline in estimated glomerular filtration rate or end-stage renal disease with pathologic lesions of the Oxford classification including mesangial hypercellularity (M), endocapillary hypercellularity (E), segmental glomerulosclerosis (S), interstitial fibrosis/tubular atrophy (T) and crescents (C). Results were pooled using random-effects meta-analysis.
Results
The cohort study included 132 patients, and only T lesion was an independently risk factor in IgAVN. The meta-analysis yielded six retrospective studies with 721 patients and 139 endpoints. In multivariate model, T lesion was significantly associated with renal outcome (hazard ratio = 2.45, P = 0.007). M and C lesions could not predict renal outcome without evidence of heterogeneity. E and S lesions could not predict renal outcome with evidence of heterogeneity (I2 = 66.6%; P = 0.01, and I2 = 65.8%; P = 0.03, respectively). Subgroup analysis showed that the possible reasons to the heterogeneity were from usage of immunosuppressant, sample size and follow-up time.
Conclusions
The study suggests that the Oxford classification could not be fully validated in IgAVN. Higher portion of immunosuppressant especially before renal biopsy might be the main confounder for the predictive value of Oxford classification in IgAVN.
Keywords: IgA vasculitis with nephritis, IgA nephropathy, meta-analysis, Oxford classification, systematic review
INTRODUCTION
Various studies have confirmed that renal involvement is the main prognostic factor in immunoglobulin A (IgA) vasculitis (IgAV; formerly known as Henoch–Schönlein purpura) [1–6]. About 20–25% of IgAV with nephritis (IgAVN) patients reached end-stage renal disease (ESRD) within 10 years after diagnosis [7, 8]. The diagnosis of IgAVN requires a renal biopsy, and histological features have an important role in determining prognosis and therapy selection. There are some histological classification systems used in IgAVN, including the International Study of Kidney Disease in Children schema [6] and other grouped and semi-quantitative systems [9–11]; however, there is presently no consensus on the implementation of a pathological classification for determining prognosis and guiding treatment in IgAVN.
IgAVN shares many common features with primary IgA nephropathy (IgAN) [7, 12, 13]. The Oxford classification was a global standardized pathological classification in IgAN [14, 15] and was recently updated [16]. The Oxford MEST-C score, defined by mesangial hypercellularity (M), endocapillary hypercellularity (E), segmental glomerulosclerosis (S), interstitial fibrosis/tubular atrophy (T) and crescentic lesions (C) scores in kidney biopsies, has been increasingly used to predict renal outcomes in IgAN individuals [17–19]. The working group does not recommend the use of MEST-C scores in IgAVN since cases of patients with this condition were not included in the validation cohort. In recent years, some studies have validated the Oxford classification in IgAVN; however, the Oxford classification has not been fully validated as a predictor of renal outcomes in IgAVN [20–22]. There are some possible reasons. First of all, most of the validation studies in IgAVN are single center with sample size <100, and the number of endpoints is also low. On the other hand, there are differences in cliniopathological features and treatment between IgAVN and IgAN. IgAVN patients tend to have more proteinuria at the onset of disease and histologically more active lesions, including E and C lesions , than IgAN patients, which result in earlier and more frequent administration of immunosuppression to IgAVN patients [7, 8, 23]. The latter may impact the prognostic value of the Oxford classification in these patients, as in IgAN patients, treatment with immunosuppression post-biopsy reduces or eliminates the association of these scores, other than T-score, with clinical outcomes [18, 19].
In order to investigate the usage of the Oxford classification in IgAVN, we first compare the predictive values of Oxford classification on kidney outcome in an IgAVN cohort and clinical and a pathological parameters-matched IgAN group in a single center. We then combined the IgAVN cohort with a meta-analysis of the relevant literature.
MATERIALS AND METHODS
Cohort study
There were 429 adult patients diagnosed with IgAVN from January 1997 through June 2016 in the Peking University First Hospital. The inclusion criteria were a clinical diagnosis of IgAV, following the 2010 European League Against Rheumatism (EULAR)/Paediatric Rheumatology International Trials Organisation (PRINTO)/Paediatric Rheumatology European Society (PRES) criteria [1]. The 2010 EULAR/PRINTO/PRES criteria are presented in Supplementary data, Appendix S1. IgAVN was diagnosed when hematuria, proteinuria and/or renal failure are associated with IgAV and predominant mesangial IgA immune deposits [23]. The exclusion criteria included systemic lupus erythematosus, anti-neutrophil cytoplasmic antibodies (ANCA)-associated vasculitis (AAV), cryoglobulinemia and thrombocytopenia. A total of 297 patients with follow-up <1 year or with an inadequate number of glomeruli (fewer than eight) were excluded. Ultimately, 132 IgAVN patients were included in the study.
All renal biopsies were processed for light microscopy (LM), immunofluorescence microscopy (IF) and electronic microscopy (EM). LM sections were stained with hematoxylin and eosin (H&E), periodic acid–Schiff (PAS), PAS with silver methenamine and Masson’s trichrome. The renal biopsies were independently reviewed by two pathologists who were blinded to the clinical data, using the updated Oxford classification criteria [16]. Necrosis was defined as the presence of glomerular fibrin with associated glomerular basement membrane disruption and/or mesangiolysis. ESRD was defined as estimated glomerular filtration rate (eGFR) <15 mL/min/1.73 m2 using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [24], or renal replacement therapy. Microscopic hematuria was examined at high power under a bright-field microscope and quantified as red blood cells/high-power field. Mean arterial pressure (MAP) was calculated as diastolic blood pressure (BP) + 1/3 (systolic BP – diastolic BP). Immunosuppressant before renal biopsy was defined as any immunosuppressive agent within 1 year of renal biopsy, regardless of duration or dose. Immunosuppressant after renal biopsy was defined as any immunosuppressive agent, regardless of duration or dose. Renin–angiotensin system blockade (RASB) was defined as angiotensin-converting enzyme inhibitor and/or angiotensin receptor blocker after biopsy. The renal outcomes were survival from a combined event, which were defined as either ≥50% eGFR decline or ESRD.
Continuous variables were presented as the mean ± standard deviation (SD) for normally distributed data and were compared using Student’s t-test. Nonparametric continuous variables were presented as the median (interquartile range) and were compared using the Mann–Whitney test. For categorical variables, the results were expressed as percentages and were analyzed by the Chi-squared test or Fisher’s test. Survival from the combined event was estimated with the Kaplan–Meier method and compared with the log-rank test. The relationship between parameters and survival from the combined event was assessed using Cox regression analysis. The results were presented as hazard ratio (HR) with 95% confidence intervals (CIs). Statistical analysis was performed using SPSS (version 23.0.0.0, IBM Corporation).
Meta-analysis
This meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) [25], and the checklist for PRISMA is presented in Supplementary data, Appendix S2. The protocol for this review was registered with the prospective register of systematic reviews (PROSPERO) (CRD 42020144739). We identified relevant studies by searching the following databases using Ovid: MEDLINE and Excerpta Medica Database (EMBASE) (from January 2009 to September 2019), using relevant keywords and medical subject headings (Supplementary data, Appendix S3). The search was limited to studies validating the Oxford classification in IgAVN, without language restrictions. Study endpoints had to include ESRD, ≥30% or ≥50% decrease in eGFR. Risks of bias in each study were assessed according to the previously published methods [26]. The literature search, data extraction and quality assessment were done independently by two authors (B.Y. and S.S.) using a standardized approach. Any disagreements in abstracted data were adjudicated by a third reviewer (W.H.).
Individual study HRs and 95% CIs were extracted from each study before data pooling. Summary estimates of HRs were obtained from a random-effects model. We estimated the percentage of variability across studies attributable to heterogeneity beyond chance using the I2 statistic. Subgroup analysis was performed according to ethnicity, the number of patients, the number of endpoints, children or adults, length of follow-up and immunosuppressant. Funnel plots were applied to evaluate publication bias. Stata version 12.0 was used for all statistical analysis. Two-tailed P < 0.05 values were considered statistically significant.
RESULTS
Validation of the Oxford classification in cohort study
Baseline characteristics in IgAVN cohort
The IgAVN cohort included 132 patients, and the median age was 32 ± 13 years, with 54% female. The MAP was 93 (83–97) mmHg, eGFR was 104 ± 33 mL/min/1.73 m2 and proteinuria was 1.57 (0.78–2.80) g/day. For pathology findings, 33% of patients showed M1, 57% showed E1, 64% showed S1, 20% showed T1/2, 46% showed C1 and 17% showed C2; the mean percentage of crescents was 13%; and eight (6%) patients showed one or more glomeruli with necrosis. Overall, 94% of patients received RASB, 54% of patients received immunosuppression before renal biopsy and 62% of patients received immunosuppression after renal biopsy. The mean eGFR slope was –2.84 ± 2.40 mL/min/1.73 m2/year during a follow-up of 64 ± 45 months, 63 (48%) patients progressed to 30% eGFR decline or ESRD and 19 (14%) patients progressed to 50% eGFR decline or ESRD (Table 1).
Table 1.
Clinicopathological features of IgAVN cohort
| Variable | IgAVN cohort (n = 132) |
|---|---|
| Clinical information | |
| Age, years | 32 ± 13 |
| Female, n (%) | 71 (54) |
| Macroscopic hematuria, n (%) | 28 (21) |
| eGFR at renal biopsy, mL/min/1.73 m2 | 104 ± 33 |
| Proteinuria at renal biopsy, g/day | 1.57 (0.78-2.80) |
| Microscopic hematuria at renal biopsy, red blood cells/high-power field | 20 (7–65) |
| MAP at renal biopsy, mmHg | 93 (83–97) |
| Prior immunosuppression, n (%) | 71 (54) |
| Pathological features, n (%) | |
| M1 | 43 (33) |
| E1 | 75 (57) |
| S1 | 85 (64) |
| T1/2 | 27 (20) |
| C0 | 49 (37) |
| C1 | 61 (46) |
| C2 | 22 (17) |
| Percentage of crescents, % | 13 |
| Necrosis, n (%) | 8 (6) |
| Follow-up | |
| Length of follow-up, months | 64 ± 45 |
| Treated with RASB, n (%) | 110 (94) |
| Treated with any immunosuppression, n (%) | 82 (62) |
| Rate of renal function decline, mL/min/1.73 m2 | –2.84 ± 2.40 |
| 30% eGFR decline or ESRD, n (%) | 63 (48) |
| 50% eGFR decline or ESRD, n (%) | 19 (14) |
| ESRD, n (%) | 8 (6) |
Data are n (%) or mean ± SD or median (interquartile range).
In the IgAVN cohort, E1 was strongly associated with higher proteinuria (r = 0.20, P = 0.02) and microscopic hematuria at renal biopsy (r = 0.25, P = 0.001). C1/2 was associated with more microscopic hematuria at renal biopsy (r = 0.16, P = 0.02). T1/2 was associated with a reduced eGFR at renal biopsy (r = –0.39, P < 0.001). Patients who received immunosuppression had more E lesions (68% versus 38%, P = 0.001), fewer S lesions (57% versus 76%, P = 0.03) and similar C1/2 lesions (66% versus 58%, P = 0.37) compared with untreated individuals.
Predictive value of MEST-C score to renal outcome in IgAVN cohort
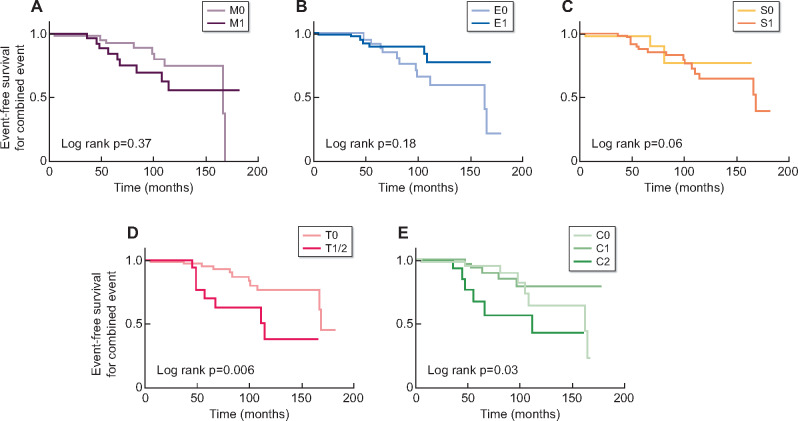
A Kaplan–Meier plot showed that T1/T2 (log-rank P = 0.006) were significantly associated with decreased renal survival. The kidney survival in C1 group was better than C0 and C2 groups (P = 0.03), and the kidney survival in E1 group was better than E0, but without statistical significance (P = 0.18) (Figure 1). By multivariate Cox regression analysis, only T lesion was significantly predictive of survival from the combined event (Table 2).
FIGURE 1.
Kaplan–Meier plots for renal event-free survival according to the Oxford classification in the IgAVN cohort.
Table 2.
Univariate and multivariate Cox regression models of factors associated with development of the combined event in the IgAVN cohort
| Variable | IgAVN cohort |
|
|---|---|---|
| Univariate HR (95% CI); P-value | Multivariate HR (95% CI); P-value | |
| Age | 1.03 (0.99–1.06); 0.21 | 1.02 (0.97–1.07); 0.48 |
| Gender (female vs male) | 0.54 (0.22–1.36); 0.19 | 0.69 (0.26–1.85); 0.46 |
| eGFR at renal biopsy | 0.99 (0.98–1.01); 0.32 | 1.001(0.98–1.02); 0.90 |
| Proteinuria at renal biopsy | 1.05 (0.91–1.21); 0.54 | 1.00 (0.85–1.18); 0.99 |
| MAP at renal biopsy | 1.03 (0.99–1.07); 0.20 | 1.02 (0.97–1.07); 0.45 |
| M0 | Reference | |
| M1 | 1.51 (0.6–3.79); 0.38 | |
| E0 | Reference | |
| E1 | 0.53 (0.21–1.36); 0.19 | |
| S0 | Reference | |
| S1 | 1.32 (0.38–4.64); 0.66 | |
| T0 | Reference | Reference |
| T1/2 | 3.53 (1.36–9.17); 0.01 | 2.86 (0.99–8.28); 0.05 |
| C0 | Reference | |
| C1/2 | 1.00 (0.40–2.50); 0.99 | |
Combined event, survival from a 50% eGFR decline or ESRD.
Meta-analysis
Eligible studies and study characteristics
The literature search yielded 910 articles. Five studies [20–22, 27, 28] including 589 patients with 76 endpoints were eligible for inclusion. Reasons for exclusion are listed in Figure 2. Data from the current cohort were included in the meta-analysis. Thus, six studies including 721 patients with 139 endpoints were included in the meta-analysis (Figure 2). All studies were retrospective, with a mean follow-up of 40 ± 82 months. In three studies [21, 22, 27], more than two independent pathologists were blinded to the clinical outcomes according to the Oxford classification. Characteristics of the included studies are summarized in Table 3. Five studies (646 patients) were of Asian populations, including Chinese, Japanese and Korean; only one study was from Turkey (75 patients). Two studies with 179 participants included children and four studies with 542 participants included adults only. Percentage of patients receiving immunosuppression after renal biopsy ranged from 42.6% to 100%. All studies used multivariate regression models for statistical analysis.
FIGURE 2.
Flowchart for study selection.
Table 3.
Characteristics of the studies included in the systematic review and meta-analyses
| Variable | Yu | Kim | Xu | Cakici | Inagaki | Huang |
|---|---|---|---|---|---|---|
| Center | Single center, China | Single center, Korea | Single center, China | Single center, Turkey | Single center, Japan | Single center, China |
| Ethnicity | 100% Asian | 100% Asian | 100% Asian | European | 100% Asian | 100% Asian |
| Number of patients | 132 | 61 | 104 | 75 | 74 | 275 |
| F/U, months | 64 ± 45 | 49 (25–102) | 40 (12–145) | 82 (12–194) | 68.0 ± 33.0 | 56 (30–86) |
| Age, years | 32 ± 13 | 34.1 ± 16.4 | 10 (4–17) | 10.1 ± 2.54 | 47.8 ± 17.4 | 33 ± 17 |
| M:F | 0.85:1 | 1.2:1 | 1.26:1 | 1.6:1 | 0.9:1 | 1.08:1 |
| Proteinuria, g/day | 1.6 (0.8–2.8) | 1.69 ± 2.27 | 1.7 (0.1–10.8) | NA | 1.40 (0.7–2.4) | NA |
| eGFR, mL/min/1.73 m2 | 104 ± 33 | 92.6 ± 22.4 | 161 ± 48 | NA | 76.4 ± 25.8 | NA |
| MAP, mmHg | 93 (83–97) | 91.5 ± 9.5 | 82 ± 11 | NA | NA | NA |
| HT, % | 31 | 30 | NA | NA | 35.1 | NA |
| RASB, % | 94 | 53 | NA | NA | 75.7 | 35 |
| Immunosuppression after renal biopsy, % | 62 | 50 | 100 | 42.60 | 82.4 | 72.70 |
| Immunosuppression before renal biopsy | Yes | NA | NA | No | NA | NA |
| Endpoint definition | ≥50% eGFR decline or ESRD | eGFR <60, ≥30% eGFR decline or ESRD | ≥50% eGFR decline or eGFR <90 | eGFR <90 or ≥50% eGFR decline | ≥30% eGFR decline or ESRD | ≥30% eGFR decline, doubling of Scr or ESRD |
| Number of endpoints | 63 | 13 | 8 | 11 | 14 | 30 |
| Number of ESRD events | 8 | 3 | NA | 5 | 1 | 12 |
| Number of pathologists | 2, blinded | 1, blinded | 2, blinded | 2, blinded | 2 | 1, blinded |
| M0/M1 | 67/33 | 85/15 | 49/51 | 38.7/61.3 | 93.2/6.8 | 85.1/14.9 |
| E0/E1 | 43/57 | 85/15 | 74/26 | 56/44 | 48.6/51.4 | 70.2/29.8 |
| S0/S1 | 36/64 | 66/34 | 52/48 | 77.4/22.6 | 48.6/51.4 | 45.8/54.2 |
| T0/T1/T2 | 80/13/7 | 87/13 (T1/2) | 82/18 (T1/2) | 86.7/9.3/4 | 75.7/18.9/5.4 | 97.1/2/0.9 |
| C0/C1/C2 | 37/46/17 | NA | 37/63 (C1/2) | 48/52 (C1/2) | 29.7/47.3/23 | 36/51.3/12.7 |
| Statistical methods | Cox regression | Cox regression | Cox regression | Cox regression | Cox regression | Cox regression |
| Multivariate analysis | MEST-C | ETC | MEST-C | EST | ETC | ESTC |
| Adjusted factors | Age, gender, MAP, proteinuria, eGFR | Proteinuria, hypertension, eGFR | Proteinuria, MAP, eGFR | Proteinuria, eGFR | Age, proteinuria | Age, proteinuria, eGFR |
F/U, follow-up; NA, not available; HT, hypertension; Scr, serum creatinine.
By current standards [26], we found risks of bias existed in the included studies (Supplementary data, Appendix S4). Prognostic factor measurement was incomplete or unclear in three studies, and only three studies adequately reported the association of MEST-C lesions with kidney survival. Outcome measurement across the included studies was not consistent: three studies used 50% eGFR decline or ESRD as renal outcome and three studies reported 30% eGFR decline or ESRD. Data reporting immunosuppressant before renal biopsy (confounding measurement) were incomplete or unclear in four studies. Inclusion of variables (analysis) was incomplete in three studies. Age was adjusted for in three studies, baseline eGFR in five studies and MAP in two studies.
Applicability of the Oxford classification pathologic lesions
Mesangial hypercellularity
Five studies with 647 adult patients and 125 endpoints reported the association of M lesions with kidney survival. M1 was not statistically a risk factor for renal outcome (HR = 1.22, 95% CI 0.9–1.66; P = 0.2), with no evidence of heterogeneity (I2 = 0%; P = 0.43; Figure 3). Exclusion of the present cohort (accounting for 70.76% of the weight) did not affect association of M lesion with kidney survival (HR = 1.34, 95% CI 0.71–2.54; P = 0.37). Funnel plots showed no statistical evidence of publication bias by Egger’s test (P = 0.38).
FIGURE 3.
HRs of kidney failure for patients with versus without M, E, S, T and C. Weights are from random-effects analysis. Yu 2019 represents the present study.
Endocapillary hypercelluarity
Six studies with 721 adult patients and 139 endpoints reported the association of E lesions with kidney survival. E1 was not statistically a risk factor for renal outcome (HR = 1.54, 95% CI 0.63–3.77; P = 0.34) with moderate heterogeneity (I2 = 66.6%; P = 0.01; Figure 3), which is mostly attributable to the result of the Xu et al.’s [21] study. Exclusion of the Xu et al.’s [21] study resulted in a risk increase (HR = 1.94, 95% CI 0.82–4.58; P = 0.13) with moderate evidence of heterogeneity (I2 = 64.2%; P = 0.03). Exclusion of the present cohort (accounting for 25.38% of the weight) resulted in a risk increase (HR = 1.95, 95% CI 0.64–5.99; P = 0.24) with moderate evidence of heterogeneity (I2 = 61.2%; P = 0.04). As shown in Figure 4, subgroup analysis revealed that E lesions were associated with worse renal outcome only in studies with a small sample size (HR = 3.85, 95% CI 1.24–11.93; P = 0.006). No interaction between immunosuppression and E lesions could be found in subgroup analysis (P = 0.41). Funnel plots showed no statistical evidence of publication bias by Egger’s test (P = 0.39).
FIGURE 4.
Subgroup analysis of HRs of kidney failure for patients with E and S. Weights are from random-effects analysis.
Segmental glomerulosclerosis
Data about the effects of S1 lesion on kidney survival were available from four studies including 586 participants and 112 endpoints. S1 was not statistically a risk factor for renal outcome (HR = 1.48, 95% CI 0.44–4.97; P = 0.52), with moderate heterogeneity (I2 = 65.8%; P = 0.03; Figure 3). Exclusion of the present cohort (accounting for 37.38% of the weight) resulted in a risk increase (HR = 2.61, 95% CI 0.89–7.65; P = 0.08) with a much reduced I2 value of 11.7%. Subgroup analysis suggested the presence of heterogeneity in the effect of immunosuppression, patient number and follow-up time. S lesions were associated with worse renal outcome only in studies with more immunosuppression, large sample size and shorter follow-up time (HR = 4.41, 95% CI 1.34–14.5; P = 0.004) (Figure 4). Funnel plots showed no statistical evidence of publication bias by Egger’s test (P = 0.25).
Tubular atrophy and interstitial fibrosis
Six studies with 721 adult patients and 139 endpoints reported the association of T lesions with kidney survival. T1/2 lesion was associated strongly with progression to kidney failure (HR = 2.45, 95% CI 1.28–4.69; P = 0.007), without evidence of major heterogeneity (I2 = 26.3%; P = 0.24; Figure 3). Exclusion of the present cohort (accounting for 39.15% of the weight) resulted in a risk increase (HR = 3.65, 95% CI 1.74–7.63; P = 0.001) with a much reduced I2 value of 0%. Funnel plots showed no statistical evidence of publication bias by Egger’s test (P = 0.19).
Cellular/fibrocellular crescents
One study [22] reported the association of a score of C1 and C2 with kidney survival, respectively. One study [20] described the association of crescents ≥50% (crescents <50% as reference) with kidney survival. Data about the effects of C1/2 on kidney survival were available from three studies including 511 participants and 101 endpoints. These studies showed that C1/2 was not statistically a risk factor for renal outcome (HR = 1.23, 95% CI 0.8–1.88; P = 0.35), with no evidence of heterogeneity (I2 = 0%; P = 0.45; Figure 3). Funnel plots showed no statistical evidence of publication bias by Egger’s test (P = 0.77).
DISCUSSION
The applicability of the Oxford classification in IgAVN patients is controversial. Although many studies have tried to evaluate the use of the Oxford classification, validation is difficult. This is due partly to the small sample size in most studies. Recently, no systematic review examining the Oxford classification in IgAVN has been published.
In the present cohort study and meta-analysis, we have consistently confirmed that in patients with IgAVN, the presence of T lesion strongly predicts kidney disease outcomes independently of all clinical and laboratory parameters. M and C lesions were not an independently predictive factor to renal outcome in meta-analysis without heterogeneity. E and S lesions were not associated with renal outcome with significant heterogeneity, and further subgroup analysis showed that possible reasons to heterogeneity were from usage of immunosuppressant, sample size and follow-up time.
Acute inflammatory lesions including E and C lesions were more common in IgAVN than in IgAN patients [7, 23, 29, 30]. However, the predictive value of E and C lesions is controversial, and both of these lesions showed interaction with immunosuppressant. IgAVN patients, with more severe extrarenal manifestations, tended to have earlier administration of immunosuppression. In the present cohort study, 54% IgAVN patients received immunosuppression before renal biopsy, similar to previous studies [31, 32], and about half of IgAVN patients received immunosuppressant after renal biopsy, a much higher proportion than IgAN patients [29]. It also has been suggested that crescentic glomeruli can rapidly become sclerotic in untreated patients [6]. In addition, there is possibility of a different therapeutic response between IgAVN and IgAN patients, and remission in IgAVN can be achieved when adequately treated at an early stage [33–35]. Therefore, we speculate that the early and widespread use of immunosuppressant might have an important influence on the prognostic value of Oxford classification in IgAVN patients. This study may suggest the advantage of an early administration of immunosuppressant in IgAVN patients but needs to be confirmed in larger and randomized studies. Studies have shown that immunosuppression could decrease crescents and mesangial hypercellularity in repeated renal biopsy in IgAN. Whether the C and E lesions could be treated as a pathology marker to receive immunosuppressant but not to predict renal outcome needs further investigation.
Subgroup analysis of S lesion indicated that the predictive value of S lesion was correlated with proportion of immunosuppressant, which was not found in IgAN patients [36]. The different pathogenesis should be considered. Even though these two diseases showed similar presentation and underline the importance of the Gd-IgA1 molecule [13, 37]: IgAVN is vasculitis, which belongs to the autoimmune diseases; however, IgAN has been proven not to be an autoimmune disease, but an abnormal autoimmunity regulation disease [38]. In IgAN patients, S lesion has been considered to represent chronic and irreversible lesions and originate from glomerular inflammation, necrosis or as a consequence of hyperfiltration due to adaptive hemodynamic changes [18]; all these mechanism supported that S lesion had predictive value to renal outcome. S lesion was also refined to include the presence of podocytopathic features that were associated with greater initial proteinuria and worse renal survival compared with patients without these features, and immunosuppressant was associated with a better renal survival [39, 40]. However, the pathogenesis of S lesion in IgAVN is not clear, whether S lesion is the result of active inflammation and necrosis lesions or the podocytopathy needs further investigation. Different origins of S lesion in IgAVN might indicate different renal outcomes.
Furthermore, in this study, compared with patients in the IgAN cohort, patients in the IgAVN cohort were younger, and had higher baseline eGFR, lower MAP, more E lesion and fewer T1/2 lesions than IgAN patients. In a subgroup of the European Validation Study of the Oxford Classification of IgA nephropathy (VALIGA) cohort, there were 219 patients with IgAN and initial proteinuria <0.5 g/day, which is a subgroup not included in the original Oxford study. All MEST lesions were significantly less frequent in this subgroup compared with the remaining cohort, and no MEST score predicted renal outcome in multivariate analysis, even when age was included in the multivariate model [18]. So for IgAVN patients and the subgroup of IgAN patients at early phase and with mild clinicopathology presentations, new pathology markers need to be investigated to indicate renal outcome.
This study had several limitations. First, all the included studies were retrospective, thus we could not fully evaluate the interaction between pathological lesions and immunosuppressant, and immunosuppressant before renal biopsy was incomplete or unclear in four studies. Second, renal outcome across the included studies was not consistent, three studies used 50% eGFR decline or ESRD as renal outcome and three studies used 30% eGFR decline or ESRD as renal outcome. Third, clinical and pathological factors included in the multivariate regression model were not consistent, which can influence the effect sizes of Oxford classification. Fourthly, there were limited data on race and age, only one study was from a non-Asian population, and only two studies were about children; thus we could not evaluate the use of the Oxford classification according to race and age. Finally, in order to rule out the occurrence of publication bias because of the small number of studies included, we used the funnel plot to test the publication bias and found no evidence of publication bias in the present meta-analysis. There is also possibility of publication bias because some studies with negative results may not be published or are reported only at meetings. For this reason, we searched conference proceedings and consulted experts to try to identify unpublished studies. As immunosuppressive therapy was the biggest confounder of these results, we also want to analysis patients with and without immunosuppressant in a meta-analysis; however, we have been unable to obtain such data as yet.
In conclusion, this study confirms that T lesion is associated strongly with renal progression. The predictive value of MESC lesions might be influenced due to immunosuppressant. A larger multicenter study including adults and children across different races with a longer follow-up is needed to further examine the predictive value of the Oxford classification in IgAVN patients. New pathology lesions should also be investigated to predict renal outcome in IgAVN in the future.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to all the clinicians for their help in this study.
FUNDING
This study was supported by the Natural Science Foundation of Beijing Municipality (Grant No. 7192209).
AUTHORS’ CONTRIBUTIONS
All authors have read and approved the manuscript as submitted, are qualified for authorship and take full responsibility for its content.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Ozen S, Pistorio A, Iusan SM. et al. ; for the Paediatric Rheumatology International Trials Organisation (PRINTO). EULAR/PRINTO/PRES criteria for Henoch-Schönlein purpura, childhood polyarteritis nodosa, childhood Wegener granulomatosis and childhood Takayasu arteritis: Ankara 2008. Part II: Final classification criteria. Ann Rheum Dis 2010; 69: 798–806 [DOI] [PubMed] [Google Scholar]
- 2. Jennette JC, Falk RJ, Bacon PA. et al. 2012 revised International Chapel Hill consensus conference nomenclature of vasculitides. Arthritis Rheum 2013; 65: 1–11 [DOI] [PubMed] [Google Scholar]
- 3. Hočevar A, Rotar Z, Jurčić V. et al. IgA vasculitis in adults: the performance of the EULAR/PRINTO/PRES classification criteria in adults. Arthritis Res Ther 2016; 18: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Saulsbury FT. Clinical update: Henoch-Schönlein purpura. Lancet 2007; 369: 976–978 [DOI] [PubMed] [Google Scholar]
- 5. Ronkainen J, Nuutinen M, Koskimies O.. The adult kidney 24 years after childhood Henoch-Schönlein purpura: a retrospective cohort study. Lancet 2002; 360: 666–670 [DOI] [PubMed] [Google Scholar]
- 6. Jelusic M, Sestan M, Cimaz R, Ozen S.. Different histological classifications for Henoch-Schönlein purpura nephritis: which one should be used? Pediatr Rheumatol 2019; 17: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Davin JC, Ten Berge IJ, Weening JJ.. What is the difference between IgA nephropathy and Henoch-Schönlein purpura nephritis? Kidney Int 2001; 59: 823–834 [DOI] [PubMed] [Google Scholar]
- 8. Coppo R, Mazzucco G, Cagnoli L. et al. Long-term prognosis of Henoch–Schönlein nephritis in adults and children. Nephrol Dial Transplant 1997; 12: 2277–2283 [DOI] [PubMed] [Google Scholar]
- 9. Koskela M, Ylinen E, Ukonmaanaho E-M. et al. The ISKDC classification and a new semiquantitative classification for predicting outcomes of Henoch-Schönlein purpura nephritis. Pediatr Nephrol 2017; 32: 1201–1209 [DOI] [PubMed] [Google Scholar]
- 10. Meadow SR, Glasgow EF, White RH. et al. Schonlein-Henoch nephritis. Q J Med 1972; 41: 241–258 [PubMed] [Google Scholar]
- 11. Coppo R, Andrulli S, Amore A. et al. Predictors of outcome in Henoch-Schönlein nephritis in children and adults. Am J Kidney Dis 2006; 47: 993–1003 [DOI] [PubMed] [Google Scholar]
- 12. Allen A, Willis F, Beattie T, Feehally J.. Abnormal IgA glycosylation in Henoch-Schonlein purpura restricted to patients with clinical nephritis. Nephrol Dial Transplant 1998; 13: 930–934 [DOI] [PubMed] [Google Scholar]
- 13. Kiryluk K, Moldoveanu Z, Sanders JT. et al. Aberrant glycosylation of IgA1 is inherited in pediatric IgA nephropathy and Henoch-Schönlein purpura nephritis. Kidney Int 2011; 80: 79–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cattran DC, Coppo R, Cook HT. et al. ; A Working Group of the International IgA Nephropathy Network and the Renal Pathology Society. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int 2009; 76: 534–545 [DOI] [PubMed] [Google Scholar]
- 15. Roberts IS, Cook HT, Troyanov S. et al. ; Working Group of the International IgA Nephropathy Network and the Renal Pathology Society. The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int 2009; 76: 546–556 [DOI] [PubMed] [Google Scholar]
- 16. Trimarchi H, Barratt J, Cattran DC. et al. Oxford classification of IgA nephropathy 2016: an update from the IgA nephropathy classification working group. Kidney Int 2017; 91: 1014–1021 [DOI] [PubMed] [Google Scholar]
- 17. Herzenberg AM, Fogo AB, Reich HN. et al. Validation of the Oxford classification of IgA nephropathy. Kidney Int 2011; 80: 310–317 [DOI] [PubMed] [Google Scholar]
- 18. Coppo R, Troyanov S, Bellur S. et al. ; on behalf of the VALIGA study of the ERA-EDTA Immunonephrology Working Group. VALIGA study of the ERA-EDTA Immunonephrology Working Group: validation of the Oxford classification of IgA nephropathy in cohorts with different presentations and treatments. Kidney Int 2014; 86: 828–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Haas M, Verhave JC, Liu ZH. et al. A multicenter study of the predictive value of crescents in IgA nephropathy. J Am Soc Nephrol 2017; 28: 691–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim CH, Lim BJ, Bae YS.. Using the Oxford classification of IgA nephropathy to predict long-term outcomes of Henoch–Schönlein purpura nephritis in adults. Modern Pathol 2014; 27: 972–982 [DOI] [PubMed] [Google Scholar]
- 21. Xu K, Zhang L, Ding J. et al. Value of the Oxford classification of IgA nephropathy in children with Henoch–Schönlein purpura nephritis. J Nephrol 2018; 31: 279–286 [DOI] [PubMed] [Google Scholar]
- 22. Inagaki K, Kaihan AB, Hachiya A. et al. Clinical impact of endocapillary proliferation according to the Oxford classification among adults with Henoch Schönlein purpura nephritis: a multicenter retrospective cohort study. BMC Nephrol 2018; 19: 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pillebout E, Thervet E, Hill G. et al. Henoch-Schonlein purpura in adults: outcome and prognostic factors. J Am Soc Nephrol 2002; 13: 1271–1278 [DOI] [PubMed] [Google Scholar]
- 24. Ma YC, Zuo L, Chen JH. et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol 2006; 17: 2937–2944 [DOI] [PubMed] [Google Scholar]
- 25. Liberati A, Altman DG, Tetzlaff J. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009; 151: W–94 [DOI] [PubMed] [Google Scholar]
- 26. Hayden JA, Côté P, Bombardier C.. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med 2006; 144: 427–437 [DOI] [PubMed] [Google Scholar]
- 27. Çakıcı EK, Gür G, Yazılıtaş F. et al. A retrospective analysis of children with Henoch-Schonlein purpura and re-evaluation of renal pathologies using Oxford classification. Clin Exp Nephrol 2019; 23: 939–947 [DOI] [PubMed] [Google Scholar]
- 28. Huang X, Ma L, Ren P. et al. Updated Oxford classification and the international study of kidney disease in children classification: application in predicting outcome of Henoch-Schönlein purpura nephritis. Diagn Pathol 2019; 14: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shi SF, Wang SX, Jiang L. et al. Pathologic predictors of renal outcome and therapeutic efficacy in IgA nephropathy: validation of the Oxford Classification. Clin J Am Soc Nephrol 2011; 6: 2175–2184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shrestha S, Sumingan N, Tan J. et al. Henoch Schonlein purpura with nephritis in adults: adverse prognostic indicators in a UK population. Q J Med 2006; 99: 253–265 [DOI] [PubMed] [Google Scholar]
- 31. Peruzzi L, Tarizzo F, Gianoglio B. et al. Better outcome of Henoch-Schonlein purpura nephritis in children: analysis from the European HSPN registry. Pediatr Nephrol 2017; 32: 1643–183428840254 [Google Scholar]
- 32. Peruzzi L, Gianoglio B, Pecoraro C. et al. A European registry of Henoch-schonlein purpura nephritis in children to detect risk factors for progression. Nephrol Dial Transplant 2016; 31 (Suppl 1): i79–i81 [Google Scholar]
- 33. Chakera A, MacEwen C, Bellur SS. et al. Prognostic value of endocapillary hypercellularity in IgA nephropathy patients with no immunosuppression. J Nephrol 2016; 29: 367–375 [DOI] [PubMed] [Google Scholar]
- 34. Davin JC, Coppo R.. Pitfalls in recommending evidence-based guidelines for a protean disease like Henoch–Schönlein purpura nephritis. Pediatr Nephrol 2013; 28: 1897–1903 [DOI] [PubMed] [Google Scholar]
- 35. Audemard-Verger A, Pillebout E, Guillevin L. et al. IgA vasculitis (Henoch-Shönlein purpura) in adults: diagnostic and therapeutic aspects. Autoimmun Rev 2015; 14: 579–585 [DOI] [PubMed] [Google Scholar]
- 36. Lv J, Shi S, Xu D. et al. Evaluation of the Oxford Classification of IgA nephropathy: a systematic review and meta-analysis. Am J Kidney Dis 2013; 62: 891–899 [DOI] [PubMed] [Google Scholar]
- 37. Suzuki H, Yasutake J, Makita Y. et al. IgA nephropathy and IgA vasculitis with nephritis have a shared feature involving galactose-deficient IgA1-oriented pathogenesis. Kidney Int 2018; 93: 700–705 [DOI] [PubMed] [Google Scholar]
- 38. Suzuki H, Kiryluk K, Novak J. et al. The pathophysiology of IgA nephropathy. J Am Soc Nephrol 2011; 22: 1795–1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Trimarchi H, Coppo R.. Podocytopathy in the mesangial proliferative immunoglobulin A nephropathy: new insights into the mechanisms of damage and progression. Nephrol Dial Transplant 2019; 34: 1280–1285 [DOI] [PubMed] [Google Scholar]
- 40. Bellur SS, Lepeytre F, Vorobyeva O. et al. International IgA Nephropathy Working Group. Evidence from the Oxford Classification cohort supports the clinical value of subclassification of focal segmental glomerulosclerosis in IgA nephropathy. Kidney Int 2017; 91: 235–243 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.