Abstract
Background
Tubulointerstitial fibrosis is a major pathological feature in chronic kidney disease (CKD) and collagen type III (COL3) is a major component of the renal fibrotic scar. We hypothesized that a dysregulated turnover of COL3 is an important determinant of CKD progression. We assessed the relationship between fragments reflecting active formation (PRO-C3) and degradation (C3M) of COL3 and CKD disease progression and mortality in a prospective cohort of CKD patients.
Methods
We measured PRO-C3 and C3M in urine (uPRO-C3 and uC3M) and serum (sPRO-C3 and sC3M) of 500 patients from the Renal Impairment in Secondary Care study. Disease progression was defined as a decline in estimated glomerular filtration rate >30% or the start of renal replacement therapy within 12 and 30 months.
Results
Levels of uC3M/creatinine decreased, whereas levels of uPRO-C3/creatinine and sPRO-C3 increased with increasing CKD stage. uC3M/creatinine was inversely and independently associated with disease progression by 12 months {odds ratio [OR] 0.39 [95% confidence interval (CI) 0.18–0.83]; P = 0.01 per doubling of uC3M/creatinine} with development of end-stage renal disease [hazard ratio (HR) 0.70 (95% CI 0.50–0.97); P = 0.03 per doubling of uC3M/creatinine]. sPRO-C3 at baseline was independently associated with increased mortality [HR 1.93 (95% CI 1.21–3.1); P = 0.006 per doubling of sPRO-C3] and disease progression by 30 months [OR 2.16 (95% CI 1.21–3.84); P = 0.009 per doubling of sPRO-C3].
Conclusions
Dynamic products of COL3 formation and degradation were independently associated with CKD progression and mortality and may represent an opportunity to link pathological processes with targeted treatments against fibrosis.
Keywords: biomarkers, CKD, ESRD, interstitial fibrosis, prognosis
INTRODUCTION
Renal fibrosis is the common histological manifestation of most forms of chronic kidney disease (CKD), irrespective of the initial cause of the disease [1]. During the progression of CKD, tubular atrophy, microvascular rarefaction and tissue hypoxia promote fibrosis, which can ultimately lead to end-stage renal disease (ESRD) [2]. Tubulointerstitial fibrosis, tubular atrophy and glomerular sclerosis are associated with worse kidney outcomes [3]. Despite the recent development of novel methods for the non-invasive monitoring of renal fibrosis [4, 5], their specificity is uncertain [6], and histological evaluation of renal biopsies is still the gold standard to assess the fibrotic burden in the kidneys. However, a kidney biopsy is a costly procedure, which puts patients at risk of bleeding and is not routinely applied to all CKD patients, especially patients with diabetic kidney disease and severe hypertension. Moreover, the sampled area may not be representative of the whole organ, and histology can only give a snapshot of the extent of fibrosis at the moment of sampling and therefore cannot be used to assess the highly dynamic process of tissue turnover. Non-invasive measures of renal stromal turnover may improve the clinical management of patients with CKD through improved risk stratification and may have a role as markers of response to interventions that target renal fibrosis [1, 7].
Renal fibrosis is a dynamic process that involves several extracellular matrix (ECM) components [8]. Collagen type III (COL3) is one of the most abundant ECM components in the human body [9]. Several fragments of COL3 were present at altered levels in the urine peptidome of patients with CKD compared with healthy individuals. COL3 was the second most abundant collagen identified in the urine of CKD patients after COL1 [10]. The amino-terminal pro-peptide of type III procollagen (PIIINP) was proposed as a potential urinary biomarker for CKD [11], and decreased urinary levels of a degradation fragment of COL3 (C3M) generated by matrix metallopeptidase 9 (MMP-9) were associated with the severity of CKD in patients with immunoglobulin A nephropathy [12] and in patients with kidney allograft failure [13].
In this study, we focused our attention on COL3, using biomarkers measuring COL3 formation and degradation (PRO-C3 and C3M, respectively) in urine and serum of patients with CKD and explored the relationship between altered turnover of COL3 and progression of CKD and mortality. PRO-C3 differs from PIIINP in measuring the neo-epitope of the released-pro-peptide, thus ensuring that the assay only detects fragments that are released as part of COL3 formation and deposition in the ECM [14].
MATERIALS AND METHODS
Study subjects
We included 500 participants of the Renal Impairment in Secondary Care (RIISC) Study (NCT01722383), a prospective observational cohort study designed to identify determinants of adverse outcomes in CKD. Detailed methodology of the RIISC study has previously been described [15]. The study subjects were recruited from Queen Elizabeth Hospital, Birmingham, UK. Patients were recruited if they had (i) an estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2, (ii) an eGFR 30–59 mL/min/1.73 m2 with a decline of ≥5 mL/min/1.73 m2/year or ≥10 mL/min/1.73 m2/5 years or (iii) a urinary albumin:creatinine ratio (ACR) ≥70 mg/mmol on three occasions. Exclusion criteria included the use of immunosuppression for immune-mediated kidney disease or renal replacement therapy (RRT). Patients consented to follow-up for 10 years or until the start of RRT or death. Ethical approval was granted by the South Birmingham Local Research Ethics Committee (reference 10/H1207/6). All patients provided written informed consent and the study was conducted in accordance with the Declaration of Helsinki. All patients received standard CKD management during the study. Data and samples were obtained from 6-month study visits, which occurred between April 2011 and September 2014, and time-to-event data were calculated from the 6-month study visit. Outcomes were captured up to 31 December 2017 and patients who had not reached a study endpoint were censored on this date. All participants had demographic, clinical and laboratory data collected at recruitment and during follow-up.
Laboratory analyses
Serum and urine were processed immediately after collection according to pre-defined standard operating procedures and stored at −80°C until analysis. Urine was frozen shortly after collection and was not centrifuged prior to storage. Biochemistry results from the local clinical laboratory were obtained from tests performed in accordance with the current standard of care.
Urine and serum concentrations of PRO-C3 and C3M (uPRO-C3 and uC3M when measured in urine, and sPRO-C3 and sC3M when measured in serum) were measured using competitive enzyme-linked immunosorbent assays (ELISAs) developed and produced at Nordic Bioscience (Herlev, Denmark). uPRO-C3 was measured in all patients, uC3M was measured in 499 patients and sPRO-C3 and sC3M were measured in 497 patients, due to different sample availability. The assay procedure was described previously [14, 16, 17]. The PRO-C3 assay used a monoclonal antibody (mAb) detecting the sequence CPTGPQNYSP (Nordic Bioscience, Herlev, Denmark), corresponding to the cleavage site of the pro-peptide from the mature collagen in position 153 of the COL3 α1 chain. The C3M assay used a mAb detecting the sequence KNGETGPQGP (Nordic Bioscience, Herlev, Denmark), corresponding to the cleavage site of MMP-9 in position 610 of COL3. Urine PRO-C3 and C3M concentrations were divided by urinary creatinine to adjust for urine concentration. Urinary creatinine was measured by the QuantiChrom Creatinine Assay Kit (BioAssay Systems, Hayward, CA, USA).
Serum creatinine measurements were performed on a Roche Modular Analyser using a blank rated and compensated Jaffe reaction, and eGFR was estimated using the creatinine-based Chronic Kidney Disease Epidemiology Collaboration equation [18]. Urinary ACR was measured using a Roche Hitachi 702 analyser. C-reactive protein (CRP) was measured using the Full Range C-Reactive Protein Kit on a SPA automated PLUS turbidimeter (Binding Site Group, Birmingham, UK). The normal range for CRP is between 0.1 and 9 mg/L, with 90% <3 mg/L [19]. Serum kappa (κ) and lambda (λ) free light chain (sFLC) concentrations were measured by nephelometry on a Dade-Behring BNTMII Analyser (Siemens AG, Erlangen, Germany) using particle enhanced high-specificity homogeneous immunoassays (Freelite; Binding Site Group). The normal reference ranges for sFLC concentrations have been previously described as κ 3.3 ± 19.4 mg/L and λ 5.7 ± 26.3 mg/L, with the assay sensitivity being demonstrated as <1 mg/L. Pulse wave velocity was measured using the Vicorder device [15].
Statistical analyses
The primary outcomes comprised: (i) CKD progression (defined as a > 30% decline in eGFR or start of RRT) at 12 and 30 months; (ii) time to ESRD (defined as the start of RRT) and (iii) time to death.
Differences between baseline characteristics in patients below and above the biomarker median were assessed using the chi-squared test for categorical variables and the Mann–Whitney test for continuous variables.
Urine and serum PRO-C3 and C3M data transformed in logarithm with base 2 had a distribution closer to normal than the untransformed biomarker (Supplementary data, Figure S1); therefore, all analyses were performed per doubling (log2). Z-scores of the markers were used to have them on the same scale.
The Mann–Whitney test was used to compare the values of the markers between two groups and the Kruskal–Wallis test was used to compare the values of the markers between three or more groups.
To evaluate the correlation between the markers and continuous clinical or biochemical parameters, non-parametric Spearman’s rank correlation or linear regression analysis on log2 transformed data were used.
Logistic regression models were used to analyse the association of the markers and score with CKD progression at 12 and 30 months.
Kaplan–Meier survival analysis and Cox proportional hazards regression analysis were used to analyse the association of the markers and score with death and progression to ESRD.
Multivariable regression models included possible confounding factors. The confounding factors included demographics, renal-associated variables and comorbidities and the variables that were associated in univariable analysis with the outcome of interest were included in the multivariable analysis. For CKD progression and development of ESRD, these were age, gender, eGFR, ACR, renal diagnosis (except when evaluating the outcome only in the diabetic group), presence of cancer and the use of gliptin, α-blockers and vasodilators. For 12-month CKD progression, they were age, gender, eGFR and ACR, renal diagnosis (except when evaluating the outcome only in the diabetic group) and the use of α-blockers. For 30-month CKD progression, they were age, gender, eGFR and ACR, renal diagnosis (except when evaluating the outcome only in the diabetic group), presence of cancer and the use of insulin. For death, they were age, gender, eGFR, ACR, diabetes (except when evaluating the outcome only in the diabetic group), cerebrovascular disease, ischaemic heart disease, peripheral artery disease and renal diagnosis (except when evaluating the outcome only in the diabetic or glomerulonephritis group).
The prognostic analyses were performed both on the whole cohort and on the subgroup of patients with diabetes mellitus.
All two-tailed P-values <0.05 were considered significant. Statistical analyses were performed using MedCalc (Ostend, Belgium), R (version 3.2.4; R Foundation for Statistical Computing, Vienna, Austria), GraphPad Prism (version 7.00; GraphPad Softaware, La Jolla, CA, USA) and Stata 15 (StataCorp, College Station, TX, USA).
RESULTS
Demographic and clinical parameters
The cohort demographic and clinical parameters are presented in Table 1 for patients stratified below or above the median value of the marker.
Table 1.
Clinical characteristics of the study population at baseline categorized according marker levels below or above the median
| Characteristics | uPRO-C3/creatinine (ng/µg) |
P-value | uC3M/creatinine (ng/µg) |
P-value | PRO-C3 (ng/mL) |
P-value | C3M (ng/mL) |
P-value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤0.47 (n = 253) | >0.47 (n = 247) | ≤2.54 (n = 257) | >2.54 (n = 242) | ≤12.3 (n = 251) | >12.3 (n = 246) | ≤11.8 (n = 252) | >11.8 (n = 245) | |||||
| Age (years) | 61 (16) | 64 (16) | 0.052 | 63 (16) | 62 (17) | 0.92 | 62 (16) | 63 (17) | 0.16 | 63 (16) | 63 (16) | 0.89 |
| Gender (male) | 184 (72.7) | 124 (50.2) | 0.0006 | 184 (71.6) | 124 (51.2) | 0.0006 | 161 (64.1) | 145 (58.9) | 0.36 | 164 (65.1) | 142 (58.0) | 0.21 |
| Ethnicity: White | 176 (69.6) | 185 (74.9) | 0.63 | 179 (69.6) | 181 (74.8) | 0.9 | 182 (72.5) | 178 (72.3) | 0.8 | 193 (76.6) | 167 (68.2) | 0.2 |
| Ethnicity: Black | 30 (11.8) | 14 (5.6) | 0.02 | 27 (10.5) | 17 (7.0) | 0.1 | 19 (7.6) | 24 (9.7) | 0.4 | 13 (5.2) | 30 (12.2) | 0.01 |
| Ethnicity: South Asian | 45 (17.8) | 45 (18.2) | 1.0 | 50 (19.4) | 40 (16.5) | 0.3 | 46 (18.3) | 43 (17.5) | 0.7 | 44 (17.5) | 45 (18.4) | 0.9 |
| Ethnicity: other | 2 (0.8) | 3 (1.2) | 0.6 | 1 (0.4) | 4 (1.6) | 0.2 | 4 (1.6) | 1 (0.4) | 0.2 | 2 (0.8) | 3 (1.2) | 0.6 |
| Body mass index (kg/m)2 | 29.8 (6.7) | 29.3 (8.0) | 0.89 | 29.6 (6.9) | 29.5 (7.7) | 0.45 | 28.2 (6.4) | 30.9 (8.0) | <0.0001 | 28.5 (6.4) | 30.5 (8.0) | 0.005 |
| eGFR (mL/min/1.73 m2) | 32.0 (18.0) | 26.9 (13.9) | 0.0008 | 24.2 (9.8) | 35.1 (19.7) | <0.0001 | 32.3 (18.2) | 26.6 (13.6) | <0.0001 | 32.0 (18.0) | 26.9 (13.9) | 0.14 |
| Urinary ACR (mg/mmol) | 91.5 (129.6) | 91.9 (137.8) | 0.69 | 101.2 (138.7) | 81.2 (127.5) | 0.02 | 85.0 (129.3) | 96.9 (136.9) | 0.28 | 89.5 (134.0) | 92.3 (132.5) | 0.82 |
| CRP (mg/L) | 7.5 (16.6) | 7.4 (12.9) | 0.10 | 7.1 (14.7) | 7.9 (15.1) | 0.58 | 6.3 (13.0) | 8.7 (16.6) | 0.08 | 3.3 (3.3) | 11.7 (20.0) | <0.0001 |
| Serum κ FLC (mg/L) | 45.5 (31.4) | 73.3 (299.1) | 0.08 | 57.7 (69.4) | 60.9 (296.0) | <0.0001 | 47.0 (66.4) | 72.0 (293.9) | 0.01 | 45.8 (69.2) | 73.3 (293.7) | <0.0001 |
| Serum λ FLC (mg/L) | 74.8 (667.0) | 36.2 (26.2) | 0.10 | 80.0 (661.6) | 30.0 (21.2) | <0.0001 | 32.3 (21.5) | 79.8 (676.3) | 0.03 | 73.0 (668.5) | 38.2 (24.2) | <0.0001 |
| Systolic blood pressure (mmHg) | 124 (19) | 130 (22) | 0.0006 | 128 (21) | 126 (21) | 0.30 | 124.7 (19.7) | 129.8 (5.1) | 0.006 | 127 (21) | 127 (21) | 0.50 |
| Diastolic blood pressure (mmHg) | 75 (11) | 75 (12) | 0.61 | 75 (12) | 75 (11) | 1.00 | 75.0 (11.3) | 75.1 (11.6) | 0.68 | 75 (12) | 75 (11) | 0.91 |
| Pulse wave velocity (m/s) | 9.9 (4.0) | 9.3 (3.3) | 0.82 | 9.4 (3.4) | 9.8 (3.9) | 0.10 | 9.4 (3.3) | 9.7 (4.0) | 0.12 | 9.3 (3.4) | 9.9 (4.0) | 0.11 |
| Pulse pressure (mmHg) | 48.8 (17.5) | 55.5 (20.7) | 0.0002 | 52.5 (19.2) | 51.5 (19.4) | 0.53 | 49.6 (18.7) | 54.7 (20.0) | 0.003 | 51.7 (19.4) | 52.6 (19.5) | 0.40 |
| Diagnosis: ischaemia/hypertension | 59 (23.3) | 71 (28.7) | 0.3 | 64 (24.9) | 66 (27.3) | 0.9 | 61 (24.3) | 67 (27.2) | 0.6 | 60 (23.8) | 68 (27.7) | 0.5 |
| Diagnosis: diabetes | 21 (8.3) | 27 (10.9) | 0.4 | 29 (11.3) | 18 (7.4) | 0.1 | 14 (5.6) | 34 (13.8) | 0.004 | 19 (7.5) | 29 (11.8) | 0.1 |
| Diagnosis: glomerulonephritis | 59 (23.3) | 25 (10.1) | 0.0002 | 47 (18.3) | 37 (15.3) | 0.3 | 54 (21.5) | 30 (12.2) | 0.009 | 44 (17.5) | 40 (16.3) | 0.7 |
| Diagnosis: polycystic kidney disease | 11 (4.3) | 18 (7.3) | 0.2 | 17 (6.6) | 12 (5.0) | 0.3 | 17 (6.8) | 12 (4.9) | 0.3 | 18 (7.1) | 11 (44.9) | 0.2 |
| Diagnosis: other/uncertain | 78 (30.8) | 88 (35.6) | 0.4 | 75 (29.2) | 91 (37.6) | 0.2 | 87 (34.7) | 78 (31.7) | 0.5 | 83 (32.9) | 82 (33.5) | 0.9 |
| Comorbidities: diabetes mellitus | 83 (32.8) | 100 (40.5) | 0.21 | 81 (31.5) | 101 (41.7) | 0.14 | 77 (30.7) | 106 (43.1) | 0.03 | 80 (31.7) | 103 (42.0) | 0.09 |
| Comorbidities: cerebrovascular disease | 24 (9.5) | 30 (12.1) | 0.41 | 19 (7.4) | 35 (14.5) | 0.03 | 25 (10.0) | 28 (11.4) | 0.68 | 27 (10.7) | 26 (10.6) | 0.89 |
| Comorbidities: ischaemic heart disease | 52 (20.5) | 60 (24.3) | 0.45 | 48 (18.7) | 64 (26.4) | 0.13 | 51 (20.7) | 61 (12.6) | 0.34 | 51 (20.2) | 61 (24.9) | 0.34 |
| Comorbidities: peripheral vascular disease | 25 (9.9) | 26 (10.5) | 0.89 | 23 (8.9) | 28 (11.6) | 0.48 | 21 (8.3) | 29 (11.8) | 0.26 | 19 (7.5) | 31 (12.6) | 0.09 |
| Comorbidities: cancer | 39 (15.4) | 33 (13.4) | 0.48 | 26 (10.1) | 46 (19.0) | 0.02 | 34 (13.5) | 37 (15.0) | 0.72 | 34 (13.5) | 37 (15.1) | 0.72 |
| Comorbidities: chronic obstructive pulmonary fibrosis | 23 (9.0) | 37 (15.0) | 0.07 | 23 (8.9) | 37 (15.3) | 0.07 | 31 (12.3) | 29 (11.8) | 0.80 | 27 (10.7) | 33 (13.5) | 0.44 |
Values are presented as n (%).
As presented in Table 2, all markers were significantly associated with baseline eGFR; this relationship remained significant after adjustment for age, gender and ACR. However, the direction of the association for uPRO-C3/creatinine, sPRO-C3 and sC3M changed, while uC3M/creatinine maintained a strong positive association with baseline eGFR. No markers correlated with baseline ACR. uPRO-C3/creatinine, uC3M/creatinine and sPRO-C3 were also independently associated with 12- and 30-month eGFR after adjustment for age, gender and ACR.
Table 2.
Linear regression analysis for baseline and 12- and 30-month eGFR and baseline ACR and the markers
| eGFR |
ACR |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Biomarker | Model | Baseline | P-value | 12-month | P-value | 30-month | P-value | Baseline | P-value |
| Log2_uPRO-C3/creatinine | Unadjusted | 27.18±1.02 | 0.001 | 27.48±1.30 | 0.002 | 26.17±1.43 | 0.07 | 86.14±8.48 | 0.35 |
| Adjusted | −2.20±0.75 | 0.003 | −3.22±0.93 | 0.0006 | −2.30±1.07 | 0.03 | −2.83±6.43 | 0.65 | |
| Log2_uC3M/creatinine | Unadjusted | 13.09±1.56 | <0.0001 | 10.89±1.96 | <0.0001 | 9.88±2.35 | <0.0001 | 106.53±14.15 | 0.24 |
| Adjusted | 12.54±1.00 | <0.0001 | 14.28±1.29 | <0.0001 | 13.18±1.55 | <0.0001 | −12.42±11.37 | 0.27 | |
| Log2_sPRO-C3 | Unadjusted | 50.80±4.74 | <0.0001 | 55–33±5.73 | <0.0001 | 46.37±6.88 | <0.0001 | 72.29±39.31 | 0.63 |
| Adjusted | −4.79±1.21 | 0.0001 | −5.88±1.49 | 0.0001 | −15.47±6.21 | 0.01 | 8.83±10.56 | 0.40 | |
| Log2_sC3M | Unadjusted | 43.55±5.19 | 0.006 | 43.02±6.46 | 0.05 | 38.01±7.66 | 0.18 | 70.30±42.49 | 0.62 |
| Adjusted | −4.12±1.35 | 0.002 | −3.30±1.71 | 0.05 | −8.78±7.00 | 0.21 | 7.00±11.67 | 0.55 | |
The β estimates represent a doubling of the markers. Adjustment included age, gender, eGFR (only for association with ACR) and ACR (only for association with eGFR).
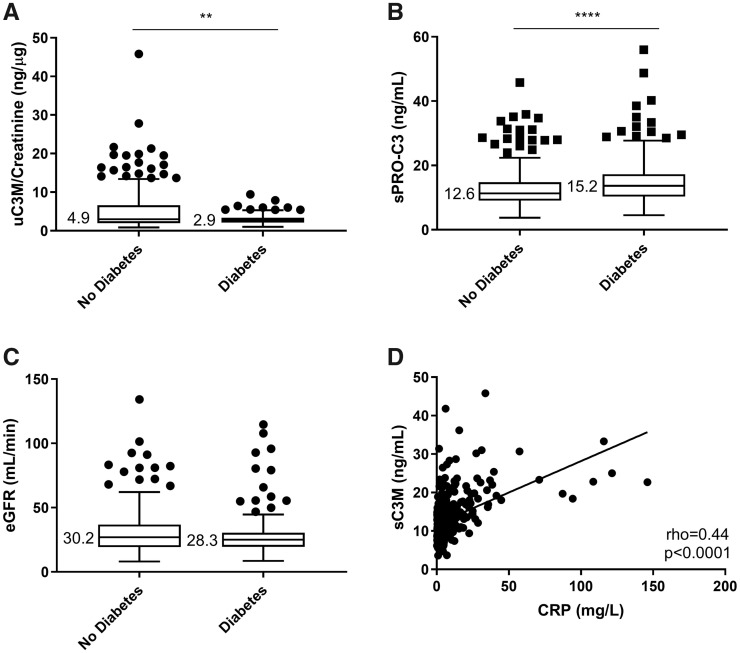
uC3M/creatinine was lower and sPRO-C3 was higher in patients with diabetes (Figure 1A and B). However, eGFR did not differ significantly between patients with diabetes and patients without diabetes (Figure 1C). sPRO-C3 correlated with levels of haemoglobin A1c (ρ = 0.16, P = 0.0006). sC3M correlated with the inflammatory markers CRP (ρ = 0.44, P < 0.0001; Figure 1D) and serum κ and λ FLCs (ρ = 0.24, P < 0.0001 and ρ = 0.23, P < 0.0001, respectively).
FIGURE 1.
(A) Levels of uC3M/creatinine in diabetic and non-diabetic CKD patients. (B) Levels of sPRO-C3 in diabetic and non-diabetic CKD patients. (C) eGFR in diabetic and non-diabetic CKD patients. (D) Correlation of sC3M with CRP. Statistical significance: **P < 0.01; ****P < 0.0001. (A–C) are presented as Tukey box plots.
CKD stages
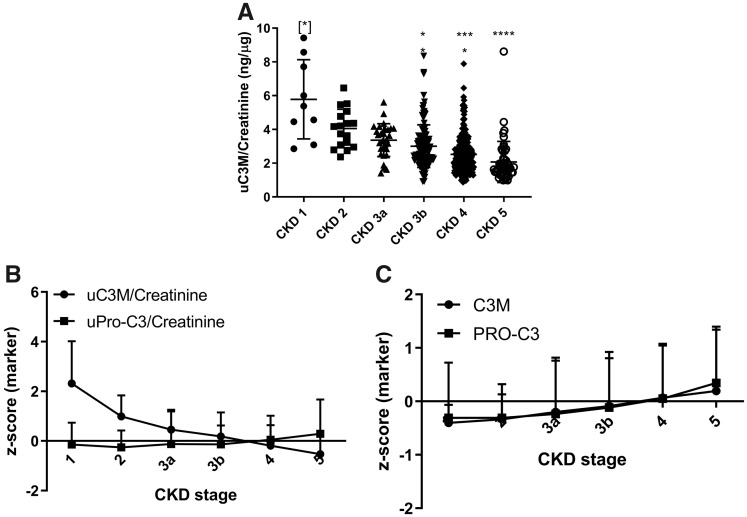
uC3M/creatinine decreased with increasing CKD stages (Figure 2A,B). uPRO-C3/creatinine and sPRO-C3 increased only in Stages 4 and 5 CKD (Figure 2B and C), while sC3M slightly increased in late CKD stages (Figure 2C).
FIGURE 2.
Levels of the markers at different CKD stages. (A) uC3M/creatinine at different CKD stages. (B) Z-score of the urinary markers at different CKD stages. (C) Z-score of the serum markers at different CKD stages. Statistical significance: *P < 0.05; ***P < 0.001; ****P < 0.0001. (A) Data are presented as a scatter plot, (B) and (C) data are reported as mean and SD.
CKD progression at 12 and 30 months
Data on progression at 12 months were available from 417 study participants due to some patients dying before the 12-month follow-up or not attending the follow-up visit. A total of 46 (11%) participants had CKD progression at 12 months. Data on progression at 30 months were available from 324 study participants. A total of 140 (43%) had CKD progression at 30 months. When comparing patients that did not have follow-up data with patients with available follow-up data, patients without follow-up were older and therefore at higher risk of mortality. A higher uC3M/creatinine was associated with a lower risk of CKD progression at 12 months after adjustment for age, gender, eGFR, ACR, renal diagnosis and the use of α-blockers (Table 3). High sPRO-C3 levels were independently associated with the risk of CKD progression at 30 months (Table 3). None of the other markers had independent associations with CKD progression (Table 3).
Table 3.
Risk of CKD progression at 12 and 30 months in relation to the markers in the RIISC cohort
| Biomarker | CKD progression at 12 months |
CKD progression at 30 months |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Univariate |
Multivariate |
Univariate |
Multivariate |
|||||||
| Events, n (%) | OR (95% CI) | P-value | OR (95% CI) | P-value | Events, n (%) | OR (95% CI) | P-value | OR (95% CI) | P-value | |
| uPRO-C3/ creatinine | 46 (11.0) | 1.22 (0.30–1.65) | 0.19 | 1.22 (0.83–1.79) | 0.3 | 140 (43.2) | 0.98 (0.78–1.23) | 0.86 | 0.91 (0.66–1.25) | 0.56 |
| uC3M/ creatinine | 46 (11.1) | 0.29 (0.17–0.51) | <0.0001 | 0.39 (0.18–0.83) | 0.01 | 140 (43.2) | 0.29 (0.19–0.45) | <0.0001 | 0.63 (0.34–1.17) | 0.15 |
| sPRO-C3 | 46 (11.1) | 1.90 (1.15–3.17) | 0.01 | 1.45 (0.74–2.85) | 0.28 | 138 (43.0) | 1.99 (1.33–2.98) | 0.0008 | 2.16 (1.21–3.84) | 0.009 |
| sC3M | 46 (11.1) | 1.5 (0.87–2.69) | 0.14 | 1.09 (0.52–2.27) | 0.83 | 138 (43.0) | 1.64 (1.05–2.56) | 0.03 | 1.56 (0.83–2.91) | 0.16 |
Values are OR (95% CI) per doubling of the marker (log2). Adjustment for 12-month CKD progression in the multivariate model includes age, gender, eGFR, ACR, renal diagnosis and use of α-blockers. Adjustment for 30-month CKD progression in the multivariate model includes age, gender, eGFR, ACR, renal diagnosis, presence of cancer and use of insulin.
Development of ESRD
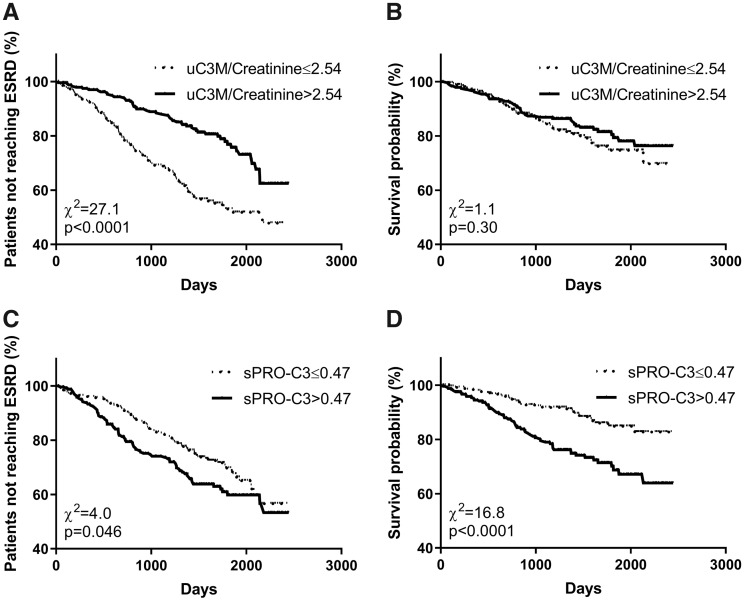
In the study, 158 (31.6%) participants progressed to ESRD. Kaplan–Meier curves are shown in Figure 3A and C. Patients with uC3M/creatinine levels below the median had an increased risk of ESRD {Figure 3A; logrank test P < 0.0001, unadjusted hazard ratio [HR] 2.8 [95% confidence interval (CI) 1.94–3.96]}, and patients with sPRO-C3 levels above the median had an increased risk of ESRD [Figure 3C; logrank test P = 0.046, unadjusted HR 1.58 (95% CI 1.10–2.26)].
FIGURE 3.
Kaplan–Meier survival curves for development of ESRD and mortality. (A) Development of ESRD for patients having uC3M/creatinine below and above the median. (B) Survival of patients having uC3M/creatinine below and above the median. (C) Development of ESRD for patients having sPRO-C3 below and above the median. (D) Survival of patients having sPRO-C3 below and above the median.
In the multivariable analyses, a higher uC3M/creatinine was associated with a lower risk of ESRD after adjustment for age, gender, eGFR, ACR, renal diagnosis, malignancy and the use of vasodilator, gliptin and α-blockers (Table 4). None of the other markers had independent associations with progression to ESRD (Table 4).
Table 4.
Risk of death and development of ESRD in relation to the markers in the RIISC cohort
| Biomarker | Mortality |
Development of ESRD |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate |
Multivariate |
Univariate |
Multivariate |
|||||||||
| Events, n (%) | HR (95% CI) | P-value | HR (95% CI) | P-value | Events, n (%) | HR (95% CI) | P-value | HR (95% CI) | P-value | |||
| uPRO-C3/ creatinine | 89 (17.8) | 1.21 (0.99–1.47) | 0.06 | 1.32 (1.01–1.73) | 0.04 | 158 (31.6) | 1.05 (0.89–1.23) | 0.58 | 0.91 (0.75–1.10) | 0.91 | ||
| uC3M/ creatinine | 89 (17.8) | 1.01 (0.72–1.42) | 0.94 | 1.87 (1.19–2.93) | 0.007 | 158 (31.7) | 0.37 (0.29–0.49) | <0.0001 | 0.70 (0.50–0.97) | 0.03 | ||
| sPRO-C3 | 89 (17.9) | 2.06 (1.45–2.93) | <0.0001 | 1.93 (1.21–3.1) | 0.006 | 156 (31.4) | 1.36 (1.04–1.78) | 0.03 | 1.15 (0.85–1.55) | 0.37 | ||
| sC3M | 89 (17.9) | 1.34 (0.91–2.00) | 0.14 | 1.23 (0.76–1.98) | 0.39 | 156 (31.4) | 1.23 (0.91–1.66) | 0.19 | 1.35 (0.96–1.91) | 0.08 | ||
Values are HR (95% CI) for mortality and development of ESRD per doubling of the marker (log2). Adjustment for mortality in the multivariate model includes age, gender, eGFR, ACR, presence of diabetes, presence of cerebrovascular disease, presence of ischaemic heart disease, presence of peripheral artery disease and renal diagnosis. Adjustment for ESRD in the multivariate model includes age, gender, eGFR, ACR, renal diagnosis, malignancy and use of vasodilator, gliptin and α-blockers.
Death
In the study, 89 (17.8%) participants died. There was no differences in risk of death in patients stratified by the median of uC3M/creatinine levels (Figure 3B). Patients with sPRO-C3 levels above the median had an increased risk of death [Figure 3D; logrank test P < 0.0001, unadjusted HR 2.43 (95% CI 1.60–3.69)]. In the multivariable analyses, a higher sPRO-C3 was associated with a higher risk of death after adjustment for age, gender, eGFR, ACR, presence of diabetes, presence of cerebrovascular disease, presence of ischaemic heart disease, presence of peripheral artery disease, renal diagnosis, comorbidities (Table 3).
Despite not showing an association for death in the univariate analysis, both uC3M/creatinine and uPRO-C3/creatinine were associated with death in the multivariable analysis (Table 3). We analysed the contribution of the various confounders to this association and we observed that only the addition of eGFR to the multivariate analysis led to an association of uC3M/creatinine with mortality and only the addition of ischaemic heart disease led to an association of uPRO-C3/creatinine with mortality.
Diabetic subgroup
We investigated the prognostic potential of the markers in the subgroup of patients with diabetes mellitus (n = 183). The clinical and demographic characteristics of the diabetic population compared with the rest of the RIISC cohort are reported in Supplementary data, Table S1. Patients with diabetes were older, with a higher body mass index (BMI), with higher levels of the inflammatory markers CRP and serum λ FLC, lower diastolic blood pressure and higher pulse pressure and pulse wave velocity. The incidence of comorbidities was also different in patients with and without diabetes (Supplementary data, Table S1). In patients with diabetes, uC3M/creatinine was associated with 30-month CKD progression [odds ratio (OR) 0.32 (95% CI 0.11–0.99); P = 0.02 per doubling of uC3M/creatinine] (Supplementary data, Table S2). sPRO-C3 was significantly associated with 30-month CKD progression [OR 2.57 (95% CI 1.09–6.06); P = 0.03 per doubling of sPRO-C3] (Supplementary data, Table S2) and ESRD [HR 1.64 (95% CI 1.01–2.66); P = 0.04 per doubling of sPRO-C3] after adjustment for confounding factors (Supplementary data, Table S3).
DISCUSSION
We have examined the turnover of COL3 in patients with CKD at high risk of disease progression by using non-invasive biomarkers of COL3 formation and degradation. We measured PRO-C3, a marker of COL3 formation, and C3M, a marker of COL3 degradation in both urine and serum.
Levels of PRO-C3 were similar across different CKD stages and slightly increased in both urine and serum at late stages. Urinary levels of C3M decreased with increasing CKD stages and slightly increased in serum at the latest stages. Levels of urinary C3M were inversely and independently associated with CKD progression by 12 and 30 months and with development of ESRD.
These results suggest that an impaired degradation of COL3 is associated with a higher risk of disease progression, as a consequence of a shift in the balance between formation and degradation of COL3 towards accumulation. These results are in accordance with findings reported from proteomic studies, in which many fragments from COL3 (including the fragment recognized by C3M) were present in lower concentrations in urine of CKD patients compared with controls and of patients with a progressive phenotype [20]. This is consistent with the paradigm of fibrosis progression, in which collagens are accumulated and cross-linked and become resistant to proteolytic degradation [21]. In the patients analysed in this cohort, the shift can be identified after CKD Stage 3 b, as depicted in Figure 2B. At this turning point, the increasing formation of COL3 by myofibroblasts is not contrasted by increased activity of the proteases degrading the scar tissue, as described by a decreased release of the degradation fragment C3M in the urine. In fact, a high level of collagen breakdown is associated with a lower risk of progression. We have previously observed a tight relationship between urinary C3M and kidney function, with low levels of uC3M/creatinine associated with worse kidney function and higher risk of progression [12, 13]; here we confirm these findings in a larger cohort of patients with a range of causes of CKD. The fact that only uC3M/creatinine maintained a strong positive association with eGFR after adjustment for confounding factors (while the others changed the direction of association after adjustment, probably due to colinearity between the confounding factors, the markers and eGFR) points in the direction that this is the marker that is mostly related to the status of the kidney. The strong association between uC3M/creatinine with baseline eGFR may explain the attenuated, although still significant, association of uC3M/creatinine in the multivariate analyses (that included eGFR as confounding factor) compared with the univariate analyses for CKD progression and development of ESRD. We also found that levels of uC3M/creatinine were significantly lower in patients with diabetes compared with patients without diabetes; however, a separate analysis in the diabetic subgroup revealed that the prognostic value of the marker is more powerful in the general CKD population. We observed the same in a separate analysis in the group of patients with glomerulonephritis (data not shown). Other clinical measurements that were different in patients with marker levels below or above the median were a higher BMI in patients with higher levels of the serum markers of COL3 remodelling, which may be related to a metabolic phenotype presenting a high pro-fibrotic and pro-inflammatory profile, and a higher systolic and pulse pressure in patients with higher levels of PRO-C3 in both serum and urine, which may link this marker with a hypertensive phenotype.
As urine is in close contact with the kidneys, urinary C3M is more likely to originate from the kidneys and may reflect fibrosis-associated remodelling in the renal stroma. In contrast, serum levels of C3M may reflect a systemic process, and this would explain why levels are unaltered with increasing CKD stages. Although sC3M was not associated with a clinical endpoint in this study, we observed a correlation with CRP and sFLC, consistent with previous observations that serum C3M may be increased by systemic inflammation [12]. Both CRP and the serum λ FLCs were more elevated in patients with diabetes, as previously observed [22], indicating that the inflammatory burden is higher in patients with diabetes and kidney disease. The significantly different levels of sFLC in patients with uC3M/creatinine and PRO-C3 below and above the median suggests a connection between these two markers and increasing inflammation, which would not be surprising considering how inflammation plays a major role in fibrosis onset and progression.
Urinary PRO-C3 was not associated with an increased risk of progression in CKD or mortality, but it increased in CKD Stage 5, consistent with previously reported findings [12]. Serum PRO-C3 was independently associated with mortality and is likely to reflect a contribution from different organs, which is supported by the finding that patients with higher BMI and diabetes had higher levels of sPRO-C3. For example, sPRO-C3 levels may partly reflect the diffuse myocardial fibrosis that has been shown to develop early in CKD [23]. sPRO-C3 has also been extensively studied as a marker for liver fibrosis [24, 25] and has been identified as an independent predictor of transplant-free survival in primary sclerosing cholangitis [26]. As patients with diabetes are at increased risk of developing liver-related disease [27], the increased sPRO-C3 in these patients may at least in part be a result of an increased COL3 formation in the liver.
This study illustrates that different fragments coming from the same protein and measured in different biological fluids can provide different information on the same disease. The findings also highlight the need to further dissect the processes of collagen formation and degradation to understand the complex and dynamic process of fibrogenesis and study its relation to adverse outcome. Due to the lack of data on other biomarkers measured in this cohort, it was not possible to benchmark the prognostic ability of the COL3 biomarkers with established biomarkers of kidney injury and disease progression. Future perspectives include assessment of these biomarkers in large established cohorts of CKD patients where other biomarkers have been measured, to compare our data with those of biomarkers of kidney injury and damage. Moreover, the prognostic value of the markers discovered in this derivation cohort will need to be evaluated in an independent validation cohort.
Antifibrotic treatments for halting disease progression in CKD have not yet been successfully developed, and the search for novel antifibrotic drugs is not one of the main focuses of the pharmaceutical industry working in nephrology. Our study points towards fibrosis as an important factor in disease progression and that treating patients before the shift towards irreversible fibrosis happens is crucial to succeed in halting the disease. The biomarkers described in this work can aid antifibrotic drug development, guiding informed decisions about patient selection for clinical trials and efficacy of intervention. It is believed that patients with active and rapidly progressing disease are more likely to benefit from the treatment in the limited time of a clinical trial, and biomarkers directly describing the fibrotic process can be important tools to detect target engagement and efficacy.
In conclusion, we have identified two processing products of COL3 that were associated with adverse outcome in CKD. Urinary C3M was independently associated with CKD progression and development of ESRD, which may reflect an imbalance in COL3 turnover in the kidney with less collagen degradation, faster progression of fibrosis and accelerated CKD progression. Serum PRO-C3 was independently associated with the risk of death, possibly reflecting an association with systemic pro-fibrotic processes.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Sophie Birot for her statistical consultancy.
FUNDING
We thank the Danish Research Foundation for supporting this work.
CONFLICT OF INTEREST STATEMENT
F.G., D.G.K.R. and M.A.K. are full-time employees at Nordic Bioscience. Nordic Bioscience is a privately owned, small to medium-size enterprise partly focused on the development of biomarkers. None of the authors received fees, bonuses or other benefits for the work described in this article. F.G. and M.A.K. hold stock in Nordic Bioscience. The patents for the ELISAs used in this work are owned by Nordic Bioscience. The funder provided support in the form of salaries for authors F.G., D.G.K.R. and M.A.K., but did not have any additional role in the study design, data collection and analysis, decision to publish or preparation of the manuscript. All other authors report no competing financial interests relevant to this article.
The results presented in this article have not been published previously in whole or part, except in abstract format.
REFERENCES
- 1. Boor P, Ostendorf T, Floege J. Renal fibrosis: novel insights into mechanisms and therapeutic targets. Nat Rev Nephrol 2010; 6: 643–656 [DOI] [PubMed] [Google Scholar]
- 2. Liu Y. Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol 2011; 7: 684–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barbour SJ, Reich HN. Risk stratification of patients with IgA nephropathy. Am J Kidney Dis 2012; 59: 865–873 [DOI] [PubMed] [Google Scholar]
- 4. Takahashi T, Wang F, Quarles CC. Current MRI techniques for the assessment of renal disease. Curr Opin Nephrol Hypertens 2015; 24: 217–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhao J, Wang ZJ, Liu M et al. Assessment of renal fibrosis in chronic kidney disease using diffusion-weighted MRI. Clin Radiol 2014; 69: 1117–1122 [DOI] [PubMed] [Google Scholar]
- 6. Boor P, Perkuhn M, Weibrecht M et al. Diffusion-weighted MRI does not reflect kidney fibrosis in a rat model of fibrosis. J Magn Reson Imaging 2015; 42: 990–998 [DOI] [PubMed] [Google Scholar]
- 7. Miyata T, Ando T, Hiragi H et al. Drug discovery in renal disease—towards a more efficient framework. Nat Rev Nephrol 2014; 10: 290–296 [DOI] [PubMed] [Google Scholar]
- 8. Genovese F, Manresa AA, Leeming DJ et al. The extracellular matrix in the kidney: a source of novel non-invasive biomarkers of kidney fibrosis? Fibrogenesis Tissue Repair 2014; 7: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gelse K, Pöschl E, Aigner T. Collagens—structure, function, and biosynthesis. Adv Drug Deliv Rev 2003; 55: 1531–1546 [DOI] [PubMed] [Google Scholar]
- 10. Good DM, Zürbig P, Argilés A et al. Naturally occurring human urinary peptides for use in diagnosis of chronic kidney disease. Mol Cell Proteomics 2010; 9: 2424–2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Soylemezoglu O, Wild G, Dalley AJ et al. Urinary and serum type III collagen: markers of renal fibrosis. Nephrol Dial Transplant 1997; 12: 1883–1889 [DOI] [PubMed] [Google Scholar]
- 12. Genovese F, Boor P, Papasotiriou M et al. Turnover of type III collagen reflects disease severity and is associated with progression and microinflammation in patients with IgA nephropathy. Nephrol Dial Transplant 2016; 31: 472–479 [DOI] [PubMed] [Google Scholar]
- 13. Stribos EGD, Nielsen SH, Brix S et al. Non-invasive quantification of collagen turnover in renal transplant recipients. PLoS One 2017; 12: e0175898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nielsen MJ, Nedergaard AF, Sun S et al. The neo-epitope specific PRO-C3 ELISA measures true formation of type III collagen associated with liver and muscle parameters. Am J Transl Res 2013; 5: 303–315 [PMC free article] [PubMed] [Google Scholar]
- 15. Stringer S, Sharma P, Dutton M et al. The natural history of, and risk factors for, progressive chronic kidney disease (CKD): the Renal Impairment in Secondary Care (RIISC) study; rationale and protocol. BMC Nephrol 2013; 14: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Papasotiriou M, Genovese F, Klinkhammer BM et al. Serum and urine markers of collagen degradation reflect renal fibrosis in experimental kidney diseases. Nephrol Dial Transplant 2015; 30: 1112–1121 [DOI] [PubMed] [Google Scholar]
- 17. Barascuk N, Veidal SS, Larsen L et al. A novel assay for extracellular matrix remodeling associated with liver fibrosis: an enzyme-linked immunosorbent assay (ELISA) for a MMP-9 proteolytically revealed neo-epitope of type III collagen. Clin Biochem 2010; 43: 899–904 [DOI] [PubMed] [Google Scholar]
- 18. Levey AS, Stevens LA, Schmid CH et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sakkinen PA, Macy EM, Callas PW et al. Analytical and biologic variability in measures of hemostasis, fibrinolysis, and inflammation: assessment and implications for epidemiology. Am J Epidemiol 1999; 149: 261–267 [DOI] [PubMed] [Google Scholar]
- 20. Schanstra JP, Zurbig P, Alkhalaf A et al. Diagnosis and prediction of CKD progression by assessment of urinary peptides. J Am Soc Nephrol 2015; 26: 1999–2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int 2006; 69: 213–217 [DOI] [PubMed] [Google Scholar]
- 22. Hutchison CA, Cockwell P, Harding S et al. Quantitative assessment of serum and urinary polyclonal free light chains in patients with type II diabetes: an early marker of diabetic kidney disease? Expert Opin Ther Targets 2008; 12: 667–676 [DOI] [PubMed] [Google Scholar]
- 23. Edwards NC, Moody WE, Yuan M et al. Diffuse interstitial fibrosis and myocardial dysfunction in early chronic kidney disease. Am J Cardiol 2015; 115: 1311–1317 [DOI] [PubMed] [Google Scholar]
- 24. Nielsen MJ, Veidal SS, Karsdal MA et al. Plasma Pro-C3 (N-terminal type III collagen propeptide) predicts fibrosis progression in patients with chronic hepatitis C. Liver Int 2015; 35: 429–437 [DOI] [PubMed] [Google Scholar]
- 25. Harrison SA, Rinella ME, Abdelmalek MF et al. NGM282 for treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2018; 391: 1174–1185 [DOI] [PubMed] [Google Scholar]
- 26. Nielsen MJ, Thorburn D, Leeming DJ et al. Serological markers of extracellular matrix remodeling predict transplant-free survival in primary sclerosing cholangitis. Aliment Pharmacol Ther 2018; 48: 179–189 [DOI] [PubMed] [Google Scholar]
- 27. Marcellin P, Kutala BK. Liver diseases: a major, neglected global public health problem requiring urgent actions and large-scale screening. Liver Int 2018; 38: 2–6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.