Abstract
Background
Serum anti-Müllerian hormone (AMH) is a biomarker of ovarian reserve. There are limited data to guide the clinical interpretation of AMH in women with chronic kidney disease (CKD). The purpose of this study was to examine AMH concentrations in women with CKD compared with women without CKD.
Methods
We conducted a prospective cohort study of serum AMH concentrations in 163 non-pregnant women with CKD. Serum AMH concentrations were compared with age-specific AMH centiles from 887 healthy female controls.
Results
Participants included 30 women with Stage 1 CKD, 37 women with Stage 2 CKD, 26 women with Stage 3a CKD, 31 women with Stage 3b CKD and 39 women with Stages 4 and 5 CKD. The median estimated glomerular filtration rate (eGFR) was 51 (interquartile range 31–80) mL/min/1.73 m2. Serum AMH concentrations were lower in all CKD stages compared with women without CKD. Women ages 20–24 years with CKD had comparable serum AMH concentrations (median 1.959 ng/mL) to women ages 35–39 years without CKD (median 1.995 ng/mL). There was no evidence that eGFR was an independent modifier of serum AMH concentrations. More than half of women with CKD (58%) were predicted to have a low response to gonadotrophin stimulation.
Conclusions
Women with CKD have a lower ovarian reserve and are predicted to have a lower ovarian response to gonadotrophin stimulation compared with women without CKD of a similar age. Women with CKD who fail to conceive within 6 months of regular unprotected intercourse should be considered for fertility assessment and intervention.
Keywords: anti-Müllerian hormone, fertility, infertility, renal insufficiency
INTRODUCTION
Anti-Müllerian hormone (AMH) is expressed in the granulosa cells of developing follicles. Serum concentrations of AMH thus reflect the number of small antral follicles and are considered to be the best currently available biomarker of ovarian reserve [1]. AMH is proposed to be clinically superior to other biomarkers because serum concentrations are unaffected by the growth of a dominant follicle in the latter half of the menstrual cycle, meaning that there is lower intra- and intercycle variability compared with other markers [2]. AMH concentrations can be used to predict response to fertility treatment and individualize dosing for ovarian stimulation [3–5] and there is evolving evidence demonstrating its use in the assessment of iatrogenic gonadotoxicity and prediction of the female reproductive lifespan [1, 6].
Chronic kidney disease (CKD) is estimated to affect 3% of women of reproductive age [7]. The mechanistic effects of CKD upon fertility include inhibition of the hypothalamic–pituitary axis due to low oestrogen levels, loss of the normal cyclical variation in luteinizing hormone concentration and hyperprolactinaemia [8]. Sexual dysfunction, voluntary childlessness and the use of cyclophosphamide, which is known to be gonadotoxic [9, 10], contribute to the complex clinical scenario in which women with CKD present for fertility advice and investigation. Yet the interpretation of circulating AMH concentration in women with CKD remains poorly understood. Published data regarding the assessment of ovarian reserve in women with CKD are limited to three small cohort studies [11, 12], which report higher AMH concentrations in haemodialysis patients compared with other stages of CKD. These studies are limited by manual methods of AMH quantification and the absence of assay-specific normal ranges [11, 13]. To date, no analysis of AMH according to the stage of CKD has been published. Serum AMH concentrations in women with early-stage CKD [estimated glomerular filtration rate (eGFR) >60 mL/min/1.73 m2] have never been described, yet these women represent the majority of women with CKD presenting for pregnancy advice in the UK [14].
The aim of this study was to investigate serum AMH concentration concentrations in women across the spectrum of CKD severity, including women on dialysis and with renal transplants, using an automated assay and age-specific normal ranges, to facilitate the interpretation of serum AMH concentrations in women with CKD.
MATERIALS AND METHODS
Non-pregnant women were recruited from specialist pre-pregnancy and general nephrology clinics at two UK centres (Guy’s and St Thomas’ NHS Foundation Trust and King’s College Hospital NHS Trust) between 2015 and 2017. Inclusion criteria were reproductive age with a known diagnosis of CKD based on Kidney Disease: Improving Global Outcomes criteria [15].
Participants were enrolled prospectively. Collected demographic data included age, ethnicity, renal disease aetiology, menstrual cycle and pregnancy history, mode of dialysis and medication including contraceptive use. eGFR was calculated from serum creatinine concentrations using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula, with classification according to CKD Stages: 1 (eGFR > 90 mL/min/1.73 m2), 2 (eGFR 60–89 mL/min/1.73 m2), 3a (eGFR 45–59 mL/min/1.73 m2), 3b (eGFR 30–44 mL/min/1.73 m2) and 4 and 5 (eGFR < 30 mL/min/1.73 m2).
Serum samples were collected at routine outpatient attendance or prior to dialysis in women receiving regular dialysis treatment. Samples were stored on ice before being centrifuged at 1500g for 10 min at 4°C. The separated supernatant was aliquoted and stored at −80°C. Serum AMH was quantified using a fully automated AMH electrochemiluminescence assay (Elecsys AMH assay, Roche Diagnostics, Rotkreuz, Switzerland) [16]. The interassay and intra-assay coefficients of variation were 1.4 and 2.4%, respectively.
Differences across CKD stages were assessed using a chi-square test for categorical variables and a Kruskal–Wallis test for continuous variables. Correlation of AMH concentrations with eGFR was examined using non-parametric Spearman correlation. In order to remove age as a confounding factor, AMH concentrations were converted from picomoles per litre to nanograms per millilitre using a multiplication factor of 0.14. Age-specific AMH centiles were then generated from data derived from 887 healthy women ages 20–50 years with regular menstrual cycles [16] using polynomial interpolation from percentile point estimates at 1–5% intervals provided by Roche Diagnostics. Mann–Whitney tests were used to examine for differences in age-corrected AMH centile between women with and without CKD. As study participants were recruited from tertiary centres that may not provide routine renal care, a history of cyclophosphamide use was not always available. To exclude cyclophosphamide use as a confounder of serum AMH concentration [17, 18], analyses were repeated with the exclusion of women with a diagnosis of lupus, non-lupus vasculitis and transplantation where the aetiology of renal failure was unknown. An assay-specific cut-off of ≤5.4 pmol/L was used to predict a low response to gonadotropins in accordance with National Institute of Health and Care Excellence recommendations [5]. Single linear regression was used to assess the effect on AMH centile attributable to CKD compared with healthy controls and to examine the effect of age, serum creatinine, eGFR, ethnicity, chronic hypertension, renal disease aetiology, transplantation, dialysis, regular menstruation (in the absence of oestrogen and progesterone use) and the use of oestrogen or progesterone-containing medication within 3 months of sample collection in women with CKD. Statistical analysis was performed using GraphPad Prism 7 (GraphPad Software, San Diego, CA, USA) and Stata version 15.1 (StataCorp, College Station, TX, USA).
Approval was provided by the UK Research Ethics Service and the Health Research Authority (15/WA/0009). The study was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants.
RESULTS
Serum AMH concentrations were measured in 163 women with CKD, including 30 women with Stage 1 CKD, 37 women with Stage 2 CKD, 26 women with Stage 3a CKD, 31 women with Stage 3b CKD and 39 women with Stages 4 and 5 CKD. The median eGFR was 51 mL/min/1.73 m2 [interquartile range (IQR) 31–80]. The most common cause of renal disease was non-lupus glomerulonephritis (26%), with reflux nephropathy more prevalent among women with higher stages of CKD. There were 37 (23%) women with a functioning renal transplant and 10 (6%) receiving dialysis therapy. Cohort demographics according to CKD stage are shown in Table 1. Women with more advanced CKD were older but there were no other measurable demographic differences between CKD stages.
Table 1.
Cohort demographics, serum AMH concentrations and age-corrected AMH centiles according to CKD stage
| Demographics and AMH results | All CKD | CKD 1 | CKD 2 | CKD 3a | CKD 3b | CKD 4–5 |
|---|---|---|---|---|---|---|
| n | 163 | 30 | 37 | 26 | 31 | 39 |
| Age (years) | 36.5 | 33.2 | 38.3 | 38.0 | 34.8 | 41.7 |
| (29.9–42.9) | (25.9–36.8) | (30.0–44.8) | (30.6–42.3) | (30.7–44.1) | (34.5–44.9) | |
| Ethnicity, n (%) | ||||||
| White European | 84 (62) | 16 (64) | 17 (63) | 16 (67) | 17 (63) | 18 (55) |
| Black | 30 (22) | 5 (20) | 5 (19) | 6 (25) | 5 (19) | 9 (27) |
| Southeast Asian | 22 (16) | 4 (16) | 5 (19) | 2 (8) | 5 (19) | 6 (18) |
| eGFR (mL/min/1.73 m2) | 51 (31–80) | 116 (109–140) | 72 (65–80) | 52 (48–56) | 38 (34–41) | 15 (9–23) |
| Renal disease aetiology, n (%) | ||||||
| Non-lupus glomerular disease | 42 (26) | 11 (37) | 9 (24) | 6 (23) | 9 (29) | 7 (18) |
| Lupus vasculitis | 28 (17) | 10 (33) | 3 (8) | 4 (15) | 3 (10) | 8 (21) |
| Hereditary/congenital | 21 (13) | 2 (7) | 11 (30) | 1 (4) | 2 (6) | 5 (13) |
| Reflux | 14 (9) | 0 (0) | 3 (8) | 2 (8) | 4 (13) | 5 (13) |
| Diabetic nephropathy | 13 (8) | 2 (7) | 1 (3) | 2 (8) | 4 (13) | 4 (10) |
| Hypertensive/renovascular | 8 (5) | 0 (0) | 2 (5) | 3 (12) | 2 (6) | 1 (3) |
| Other | 15 (9) | 0 (0) | 4 (11) | 3 (12) | 2 (6) | 6 (15) |
| Unknown | 22 (13) | 5 (17) | 4 (11) | 5 (19) | 5 (16) | 3 (8) |
| Renal transplant, n (%) | ||||||
| Functioning | 37 (23) | 1 (3) | 3 (8) | 10 (38) | 14 (45) | 9 (23) |
| Non-functioning | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (3) |
| Current dialysis, n (%) | 10 (6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 10 (56) |
| Haemodialysis | 7 (4) | 7 (39) | ||||
| Peritoneal dialysis | 3 (2) | 3 (17) | ||||
| Regular menstruation, n/N (%) | 35/51 (69) | 8/11 (73) | 5/8 (64) | 5/8 (64) | 8/12 (67) | 9/12 (75) |
| Oestrogen-containing drug use, n/N (%) | 9/133 (7) | 2/24 (8) | 5/30 (17) | 0/20 (0) | 0/25 (0) | 2/34 (6) |
| Progesterone-containing contraceptive use, n/N (%) | 25/131 (19) | 4/24 (17) | 5/29 (17) | 3/20 (15) | 7/25 (28) | 6/33 (18) |
| Serum AMH (pmol/L) | 6.33 (1.90–15.65) | 10.06 (4.62–20.73) | 6.02 (1.79–12.85) | 4.27 (1.44–8.79) | 8.89 (4.99–19.36) | 5.29 (1.27–14.77) |
| Serum AMH (ng/mL) | 0.88 (0.27–2.19) | 1.41 (0.65–2.90) | 0.84 (0.25–1.80) | 0.60 (0.20–1.23) | 1.25 (0.70–2.71) | 0.74 (0.18–2.07) |
| AMH centile | 19 (8–53) | 30 (4–55) | 28 (10–53) | 11 (4–31) | 24 (14–59) | 15 (7–60) |
| AMH predictive of low response to gonadotrophin stimulation (AMH ≤5.4 pmol/L), n (%)a | 95 (58) | 9 (30) | 18 (47) | 17 (65) | 8 (26) | 20 (51) |
Values are median (IQR) unless stated.
National Institute for Health and Care Excellence advise that AMH ≤5.4 pmol/L (Beckman-Coulter assay) is used to predict a low ovarian response to gonadotrophin stimulation [5].
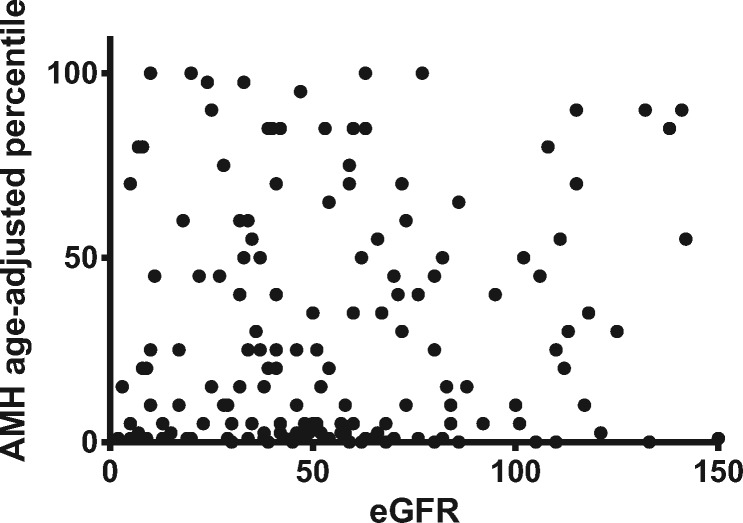
AMH concentrations were lower and maternal age was higher with increasing CKD stage (Table 1). However, there was no measurable difference in age-corrected AMH centiles across CKD stages or correlation between eGFR and AMH centile (Figure 1).
FIGURE 1.
Scatter plot of age-corrected AMH centiles against eGFR in women with CKD.
For women <35 years of age, serum AMH concentrations were lower in women with CKD compared with those without CKD (Figure 2). Women 20–24 years of age with CKD had comparable AMH concentrations [median 1.959 ng/mL (IQR 1.126–2.717)] to women 35–39 years of age without CKD [median 1.995 ng/mL (IQR 0.889–4.185), P = 0.741]. For women ≥35 years of age, age was a more important determinant of AMH concentrations than CKD stage.
FIGURE 2.
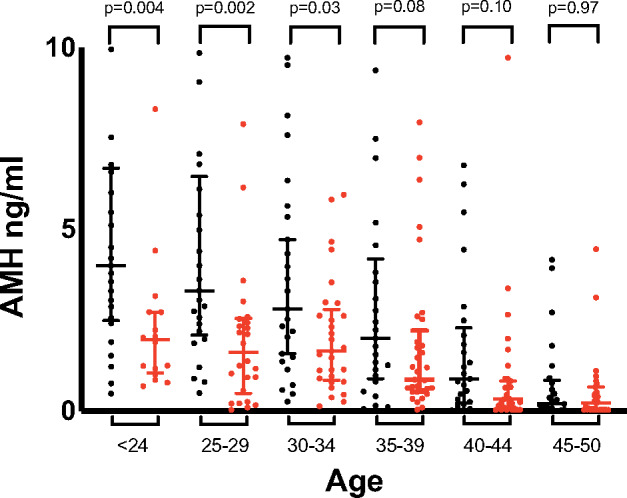
Serum AMH concentrations in women with CKD (red) compared with age-matched women without CKD (black). Bars show median (IQR) values. Serum AMH concentrations for women without CKD were obtained from Anckaert et al. [16].
AMH centiles were lower in both early and late stages of CKD compared to women without CKD (Figure 3). This difference was apparent even with the exclusion of women with a confirmed or possible history of previous cyclophosphamide exposure, including women with lupus, non-lupus vasculitis and transplantation where the aetiology of renal failure was unknown (P = 0.004).
FIGURE 3.
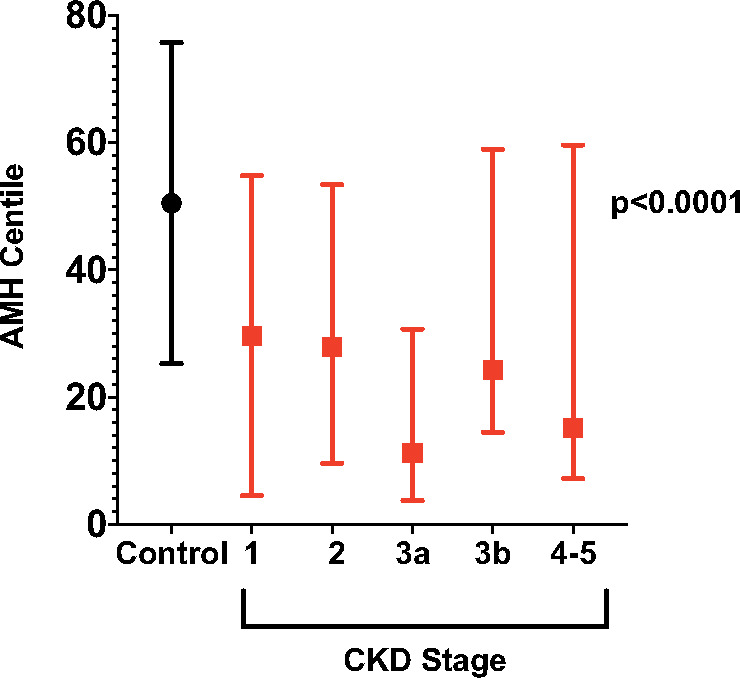
AMH centiles in women without CKD (control) and in women with CKD Stages 1–5. Bars show the median (IQR) centile value. P < 0.0001 is a measure of the difference in the distribution of AMH centile across all groups shown.
Low ovarian response to gonadotrophin stimulation was predicted in 95 (58%) women with CKD, with no significant difference detectable between CKD stages (Table 1).
Among women with CKD, there was no measurable relationship between AMH centile and serum creatinine concentration, eGFR, CKD stage, ethnicity, chronic hypertension, glomerular disease, transplantation, regular menstruation or oestrogen and progesterone-containing drug use (Table 2).
Table 2.
Linear regression analysis of age-corrected AMH centiles in women with CKD
| Variable | Simple linear regression coefficient (95% confidence interval) | P-value |
|---|---|---|
| CKDa | −17.82 (−25.42 to −10.21) | <0.001 |
| Serum creatinine | −0.01 (−0.03–0.01) | 0.424 |
| eGFR | 0.016 (−0.112–0.145) | 0.803 |
| CKD stage | −1.16 (−5.18–2.87) | 0.570 |
| Ethnicityb | 2.08 (−2.21–6.38) | 0.339 |
| Black ethnicityc | −7.82 (−20.23–4.58) | 0.215 |
| Chronic hypertension | 3.27 (−6.53 to 13.06) | 0.511 |
| Renal transplantation | 1.08 (−10.40–12.56) | 0.853 |
| Glomerular disease | −2.53 (−13.04–7.98) | 0.635 |
| Regular menstruationd | 2.94 (−36.04–41.91) | 0.872 |
| Oestrogen-containing drug use | −4.070 (−25.752–17.611) | 0.711 |
| Progesterone-containing drug use | −9.560 (−23.401–4.281) | 0.174 |
The coefficient is a measure of the difference in AMH centile that can be attributed to that variable.
Compared with women without CKD.
White European/Black/Southeast Asian.
Compared to non-black ethnicity.
In the absence of oestrogen and progesterone use.
DISCUSSION
Serum AMH concentrations in women <35 years aged with CKD are substantially lower than in women without CKD. For example, women 20–24 years of age with CKD have comparable serum AMH concentrations to women ≥35 years of age without CKD. Lower serum AMH concentrations are evident across all CKD stages, even with the exclusion of women exposed to cyclophosphamide therapy. There is no evidence that eGFR is an independent modifier of serum AMH concentrations or that serum AMH concentrations are higher in women on dialysis. More than half of women with CKD would be anticipated to have a low ovarian response to gonadotrophin stimulation.
To our knowledge, following a literature search, this is the largest study to date examining serum AMH concentrations in women with CKD and the first to assess serum AMH levels across the spectrum of CKD severity including both mild and severe disease. The study uses a fully automated, precise, sensitive AMH assay [16], which avoids the variability and poor reproducibility encountered in historic studies [19], is unaffected by complement activity [16] and provides an accurate and validated quantification of samples previously stored at −80°C [20]. The strength of this study is the use of assay-specific, age-specific centiles, as age is a known confounder of both low serum AMH concentrations [21] and impaired renal function [22].
This study shows a reduction in age-corrected AMH centiles in women with CKD, including those on dialysis. This is consistent with the molecular size of AMH, which at 140 kDa is too large to be substantially influenced by glomerular filtration or dialytic clearance. Despite this, previously published cohorts reported higher serum AMH concentrations in 26 women receiving dialysis compared with women with earlier-stage CKD [12] and a reduction in serum AMH concentrations after the successful renal transplantation of 10 women previously on dialysis [11]. These findings were based on limited control data [11] and a small number of age-corrected values [12]. Whether there are modality-specific effects in haemo dialysis and peritoneal dialysis remain unknown and a larger study is needed.
Reliable menstrual history was only available for 31% (51/163) of women with CKD in this study. However, this is unlikely to have impacted the study findings given that menstrual irregularity is thought to lead to an increase in AMH via impaired folliculogenesis and polycystic ovarian syndrome [16] and therefore cannot explain the lower AMH concentrations in women with CKD compared with women without CKD. Hormonal contraceptives were used by 24% (32/131) of women in this cohort. The impact of hormonal contraceptive use on AMH concentrations remains unclear [23], with studies both suggesting [24, 25] and refuting [26, 27] an association with AMH concentrations. Our study provides no evidence that oestrogen-containing or progesterone-only contraceptive use in the 3 months prior to the study sample were significant modifiers of the age-specific AMH centile in women with CKD.
The finding of a reduction in serum AMH concentrations in women with CKD is similar to the AMH profile described in other chronic diseases, including Crohn’s disease [28], coeliac disease [29], chronic viral hepatitis [30], psoriasis [31], multiple sclerosis [32] and neuromyelitis [33]. Lower AMH concentrations in women with chronic inflammatory disease can be hypothesized to be an appropriate physiological response, reducing fertility where pregnancy may be detrimental to maternal health and/or the survival of the offspring, and this may be relevant to women with CKD.
The clinical implications of lower AMH concentrations in women with CKD are uncertain. Although serum AMH is utilized as a quantitative marker of ovarian reserve, it does not measure the quality of ovarian follicles and cannot be used in isolation to determine likely reproductive success. Women with low circulating concentrations of AMH can and do conceive [34]. AMH concentration is a variable predictor of both time to conception [35–37] and time to menopause [38–40]. Age, rather than biomarker quantification, is advocated as the initial predictor of the likelihood of reproductive success [5]. The clinical significance and predictive value of a lower measured AMH in young women with CKD remain unclear.
AMH concentration is used to predict the response to ovarian stimulation in women undergoing assisted reproduction [5]. More than half of women with CKD in this cohort (58%) would be predicted to have a low response. Further work is needed to determine how these data should be used to inform gonadotropin dosing in women with CKD given the clinical implications of intravascular fluid depletion and superimposed acute kidney injury that may result from hyperstimulation.
CKD impacts on mechanistic, functional and psychological components of fertility and is associated with lower serum AMH concentrations compared with age-matched women without CKD. Although the trajectory of AMH decline across the reproductive lifespan shows marked interindividual variation [38], these data suggest that women with CKD who fail to conceive within 6 months of regular unprotected intercourse should be considered for fertility assessment and treatment. This mirrors guidelines for women >36 years of age without CKD [5] in whom serum AMH concentrations are comparable, although consideration of the risks of ovarian stimulation in women with CKD is warranted. These findings have potential implications for planning the timing of pregnancy for women with CKD, although prospective studies on natural reproductive success are needed.
ACKNOWLEDGEMENTS
The authors acknowledge the National Institute for Health Research (NIHR) Rare Diseases Translational Research Collaboration as well as the Biomedical Research Centre at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London for funding K.W. under the terms of a doctoral research fellowship. The views expressed are those of the authors and not necessarily those of the National Health Service (NHS), the NIHR or the UK Department of Health. The authors also acknowledge Roche Diagnostics for the provision of control data as well as the contribution of Jennifer Barsana, Aasmita Gautam., Julia Kopeika and Emma Wayman
FUNDING
K.W. is funded by the National Institute for Health Research Rare Diseases Translational Research Collaboration and the Biomedical Research Centre at Guy’s & St Thomas & King’s College London. L.C.C. is supported by a Research Professorship from the National Institute for Health Research (RP-2014-05-019).
AUTHORS’ CONTRIBUTIONS
K.W., E.A., J.G., L.C. and K.B. were involved in the concept and design. K.W., F.H., C.N.P., L.L., L.C. and K.B. were involved in the acquisition of data. K.W., E.A., J.G., L.C. and K.B. were involved in the analysis and interpretation of data. K.W., E.A., F.H., C.N.P., L.L., J.G., L.C. and K.B. contributed to manuscript drafting and critical revision. Final approval of the version to be published was provided by K.W., E.A., F.H., C.N.P., L.L., J.G., L.C. and K.B.
CONFLICT OF INTEREST STATEMENT
The results presented in this article have not been published previously in whole or part, except in abstract format. Control data were provided by E.A. and Roche Diagnostics. K.B. has received consultancy fees from Alexion and is a member of the speaker’s bureau for Alexion and Otsuka. C.N.P. has received consultancy fees from Alliance Pharma, Alexion and UCB Pharma and is a member of the speaker’s bureau for UCB Pharma and Sanofi.
REFERENCES
- 1. Broer SL, Broekmans FJ, Laven JS et al. Anti-Müllerian hormone: ovarian reserve testing and its potential clinical implications. Hum Reprod Update 2014; 20: 688–701 [DOI] [PubMed] [Google Scholar]
- 2. van Disseldorp J, Lambalk CB, Kwee J et al. Comparison of inter- and intra-cycle variability of anti-Mullerian hormone and antral follicle counts. Hum Reprod 2010; 25: 221–227 [DOI] [PubMed] [Google Scholar]
- 3. Dewailly D, Andersen CY, Balen A et al. The physiology and clinical utility of anti-Mullerian hormone in women. Hum Reprod Update 2014; 20: 370–385 [DOI] [PubMed] [Google Scholar]
- 4. Iliodromiti S, Anderson RA, Nelson SM. Technical and performance characteristics of anti-Müllerian hormone and antral follicle count as biomarkers of ovarian response. Hum Reprod Update 2015; 21: 698–710 [DOI] [PubMed] [Google Scholar]
- 5.National Institute for Health and Care Excellence. Fertility Problems: Assessment and Treatment. Clinical Guideline [CG156]. 2013; updated 2017. https://www.nice.org.uk/guidance/cg156 (1 July 2019, date last accessed) [Google Scholar]
- 6. Iwase A, Osuka S, Goto M et al. Clinical application of serum anti-Müllerian hormone as an ovarian reserve marker: a review of recent studies. J Obstet Gynaecol Res 2018; 44: 998–1006 [DOI] [PubMed] [Google Scholar]
- 7. Piccoli GB, Attini R, Vasario E et al. Pregnancy and chronic kidney disease: a challenge in all CKD stages. Clin J Am Soc Nephrol 2010; 5: 844–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wiles KS, Nelson-Piercy C, Bramham K. Reproductive health and pregnancy in women with chronic kidney disease. Nat Rev Nephrol 2018; 14: 165–184 [DOI] [PubMed] [Google Scholar]
- 9. Ioannidis JPA, Katsifis GE, Tzioufas AG et al. Predictors of sustained amenorrhea from pulsed intravenous cyclophosphamide in premenopausal women with systemic lupus erythematosus. J Rheumatol 2002; 29: 2129–2135 [PubMed] [Google Scholar]
- 10. Boumpas DT, Austin HA, Vaughan EM et al. Risk for sustained amenorrhea in patients with systemic lupus erythematosus receiving intermittent pulse cyclophosphamide therapy. Ann Intern Med 1993; 119: 366–369 [DOI] [PubMed] [Google Scholar]
- 11. Sikora-Grabka E, Adamczak M, Kuczera P et al. Serum anti-Müllerian hormone concentration in young women with chronic kidney disease on hemodialysis, and after successful kidney transplantation. Kidney Blood Press Res 2016; 41: 552–560 [DOI] [PubMed] [Google Scholar]
- 12. Stoumpos S, Lees J, Welsh P et al. The utility of anti-Müllerian hormone in women with chronic kidney disease, on haemodialysis and after kidney transplantation. Reprod Biomed Online 2018; 36: 219–226 [DOI] [PubMed] [Google Scholar]
- 13. Szydłowska I, Marciniak A, Brodowska A et al. Assessment of ovarian reserve as an indicator of fertility and health consequences in patients with chronic kidney disease stages 3–4. Gynecol Endocrinol 2018; 34: 944–948 [DOI] [PubMed] [Google Scholar]
- 14. Wiles KS, Bramham K, Vais A et al. Pre-pregnancy counselling in chronic kidney disease: a retrospective analysis of nine years’ experience. BMC Nephrol 2015; 16: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Levin A, Stevens PE. Summary of KDIGO 2012 CKD guideline: behind the scenes, need for guidance, and a framework for moving forward. Kidney Int 2014; 85: 49–61 [DOI] [PubMed] [Google Scholar]
- 16. Anckaert E, Öktem M, Thies A et al. Multicenter analytical performance evaluation of a fully automated anti-Müllerian hormone assay and reference interval determination. Clin Biochem 2016; 49: 260–267 [DOI] [PubMed] [Google Scholar]
- 17. Mok CC, Chan PT, To CH. Anti-müllerian hormone and ovarian reserve in systemic lupus erythematosus. Arthritis Rheum 2013; 65: 206–210 [DOI] [PubMed] [Google Scholar]
- 18. Morel N, Bachelot A, Chakhtoura Z et al. Study of anti-Müllerian hormone and its relation to the subsequent probability of pregnancy in 112 patients with systemic lupus erythematosus, exposed or not to cyclophosphamide. J Clin Endocrinol Metab 2013; 98: 3785–3792 [DOI] [PubMed] [Google Scholar]
- 19. Rustamov O, Smith A, Roberts SA et al. Anti-Mullerian hormone: poor assay reproducibility in a large cohort of subjects suggests sample instability. Hum Reprod 2012; 27: 3085–3091 [DOI] [PubMed] [Google Scholar]
- 20. Gassner D, Jung R. First fully automated immunoassay for anti-Müllerian hormone. Clin Chem Lab Med 2014; 52: 1143–1152 [DOI] [PubMed] [Google Scholar]
- 21. Lanham M, Harlow S, Karvonen-Gutierrez C et al. The effects of age, lifestyle, and environment on longitüdinal anti-mullerian hormone levels in a population-based cohort of reproductive-aged women. Fertil Steril 2017; 108: e14 [Google Scholar]
- 22. Hill NR, Fatoba ST, Oke JL et al. Global prevalence of chronic kidney disease – a systematic review and meta-analysis. PLoS One 2016; 11: e0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. D’Arpe S, Di Feliciantonio M, Candelieri M et al. Ovarian function during hormonal contraception assessed by endocrine and sonographic markers: a systematic review. Reprod Biomed Online 2016; 33: 436–448 [DOI] [PubMed] [Google Scholar]
- 24. Kristensen SL, Ramlau-Hansen CH, Andersen CY et al. The association between circulating levels of antimüllerian hormone and follicle number, androgens, and menstrual cycle characteristics in young women. Fertil Steril 2012; 97: 779–785 [DOI] [PubMed] [Google Scholar]
- 25. van den Berg MH, van Dulmen-den Broeder E, Overbeek A et al. Comparison of ovarian function markers in users of hormonal contraceptives during the hormone-free interval and subsequent natural early follicular phases. Hum Reprod 2010; 25: 1520–1527 [DOI] [PubMed] [Google Scholar]
- 26. Streuli I, Fraisse T, Pillet C et al. Serum antimüllerian hormone levels remain stable throughout the menstrual cycle and after oral or vaginal administration of synthetic sex steroids. Fertil Steril 2008; 90: 395–400 [DOI] [PubMed] [Google Scholar]
- 27. Li HWR, Wong CYG, Yeung WSB et al. Serum anti-müllerian hormone level is not altered in women using hormonal contraceptives. Contraception 2011; 83: 582–585 [DOI] [PubMed] [Google Scholar]
- 28. Şenateş E, Çolak Y, Erdem ED et al. Serum anti-Müllerian hormone levels are lower in reproductive-age women with Crohn’s disease compared to healthy control women. J Crohns Colitis 2013; 7: e29–34 [DOI] [PubMed] [Google Scholar]
- 29. Cakmak E, Karakus S, Demirpence O et al. Ovarian reserve assessment in celiac patients of reproductive age. Med Sci Monit 2018; 24: 1152–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Karampatou A, Han X, Kondili LA et al. Premature ovarian senescence and a high miscarriage rate impair fertility in women with HCV. J Hepatol 2018; 68: 33–41 [DOI] [PubMed] [Google Scholar]
- 31. Aydogan Mathyk B,, Aslan Cetin B,, Bilici S et al. Evaluation of ovarian reserve in women with psoriasis. Gynecol Endocrinol 2019; 35: 608–611. [DOI] [PubMed] [Google Scholar]
- 32. Graves JS, Henry RG, Cree BAC et al. Ovarian aging is associated with gray matter volume and disability in women with MS. Neurology 2018; 90: e254–e260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thöne J, Lichtenberg S, Stahl A et al. Ovarian reserve in women with neuromyelitis optica spectrum disorder. Front Neurol 2018; 9: 446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pacheco A, Cruz M, García Velasco JA. Impact of very low anti-Müllerian hormone on pregnancy success. Curr Opin Obstet Gynecol 2017; 29: 131–135 [DOI] [PubMed] [Google Scholar]
- 35. Depmann M, Broer SL, Eijkemans MJC et al. Anti-Müllerian hormone does not predict time to pregnancy: results of a prospective cohort study. Gynecol Endocrinol 2017; 33: 644–648 [DOI] [PubMed] [Google Scholar]
- 36. Hagen CP, Vestergaard S, Juul A et al. Low concentration of circulating antimüllerian hormone is not predictive of reduced fecundability in young healthy women: a prospective cohort study. Fertil Steril 2012; 98: 1602–1608.e2. [DOI] [PubMed] [Google Scholar]
- 37. Steiner AZ, Herring AH, Kesner JS et al. Antimüllerian hormone as a predictor of natural fecundability in women aged 30-42 years. Obstet Gynecol 2011; 117: 798–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. de Kat AC, van der Schouw YT, Eijkemans MJ et al. Back to the basics of ovarian aging: a population-based study on longitudinal anti-Müllerian hormone decline. BMC Med 2016; 14: 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tehrani FR, Solaymani-Dodaran M, Azizi F. A single test of antimüllerian hormone in late reproductive-aged women is a good predictor of menopause. Menopause 2009; 16: 797–802 [DOI] [PubMed] [Google Scholar]
- 40. Broer SL, Eijkemans MJ, Scheffer GJ et al. Anti-Müllerian hormone predicts menopause: a long-term follow-up study in normoovulatory women. J Clin Endocrinol Metab 2011; 96: 2532–2539 [DOI] [PubMed] [Google Scholar]