Abstract
Background
The relationships of sodium intake to kidney function within the population have been poorly investigated and are the objective of the study.
Methods
This observational, population-based, cross-sectional and longitudinal study targeted 4595 adult participants of the Gubbio study with complete data at baseline exam. Of these participants, 3016 participated in the 15-year follow-up (mortality-corrected response rate 78.4%). Baseline measures included sodium:creatinine ratio in timed overnight urine collection, used as an index of sodium intake, together with serum creatinine, sex, age and other variables. Follow-up measures included serum creatinine and other variables. Estimated glomerular filtration rate (eGFR, mL/min/1.73 m2) was calculated using serum creatinine, sex and age and was taken as an index of kidney function.
Results
The study cohort was stratified in sex- and age-controlled quintiles of baseline urine sodium:creatinine ratio. A higher quintile associated with higher baseline eGFR (P < 0.001). In multivariable analysis, the odds ratio (OR) of Stage1 kidney function (eGFR ≥90 mL/min/1.73 m2) was 1.98 times higher in Quintile 5 compared with Quintile 1 [95% confidence interval (CI) 1.50–2.59, P < 0.001]. The time from baseline to follow-up was 14.1 ± 2.5 years. Baseline to follow-up, the eGFR change was more negative along quintiles (P < 0.001). In multivariable analysis, the OR in Quintile 5 compared with Quintile 1 was 2.21 for eGFR decline ≥30% (1.18–4.13, P = 0.001) and 1.38 for worsened stage of kidney function (1.05–1.82, P = 0.006). Findings were consistent within subgroups.
Conclusions
Within the general population, an index of higher sodium intake associated cross-sectionally with higher kidney function but longitudinally with greater kidney function decline.
Keywords: diet, epidemiology, kidney function, salt, sodium
INTRODUCTION
Current recommendations suggest that adults’ intake of sodium should be <1–2 g/day for the prevention of chronic disease and cardiovascular risk [1, 2]. The intake of sodium is determined by various sources, including the intake of processed foods prepackaged with the addition of sodium, sodium added during cooking or at the table and the natural sodium content of foods [3]. Urine sodium is the most reliable index of sodium intake because the kidneys adapt urine sodium excretion to sodium intake [4]. Data about urine sodium in different countries indicate that sodium intake is usually well above the required intake [5, 6]. Several observations indicate that high sodium intake associates with hypertension, whereas sodium restriction favours hypertension control [7]. With regard to kidney disease, the available data indicate that the restriction of sodium intake reduces blood pressure and proteinuria [7, 8]. In 2017, the meta-analysis of Nomura et al. [9] concluded that ‘There is no robust evidence suggesting that long-term reduction of salt intake would prevent chronic kidney disease or delay its progression’. The meta-analysis did not include any population-based longitudinal study. The first population-based, longitudinal data set was reported in 2018 by Sogiura et al. [10], who analysed the sodium:creatinine ratio in spot urine of 12 126 subjects ≥20 years of age undergoing an annual check-up at the Nagoya Hospital (Japan). The results indicated that a higher urine sodium:creatinine ratio (uNa:Cr) associated with a higher incidence of impaired kidney function over time. The study did not investigate other dietary markers but demonstrated that the association was independent of sex, age, body mass index (BMI), blood pressure, serum lipids, diabetes, proteinuria and reported information on alcohol intake and smoking habit.
The Gubbio study is an observational, longitudinal, population-based study that measures urinary sodium at different exams for investigation of the relationships of sodium intake with cardiovascular diseases or kidney diseases [5, 11–16]. The present analysis was designed to investigate cross-sectional and longitudinal relationships of urine sodium with kidney function in adult examinees of the Gubbio study.
MATERIALS AND METHODS
The Gubbio study is a population-based project ongoing in Gubbio, Italy [11, 12]. The study adheres to the Declaration of Helsinki and includes an informed consent and approval by the institutional committee (CEAS-Umbria #2850/16). The study design, involvement of participants, response rates and characteristics of the Gubbio cohort were previously reported [11, 12]. The main exams of the study were performed in 1983–85, 1988–92 and 2001–07. Death certificates and information about initiation of chronic dialysis treatment were collected from local sections of national registries. The main analyses of the present article deal with data from the 1988–92 exam (hereafter baseline), data of the 2001–07 exam (hereafter follow-up) and registry data about mortality and initiation of chronic dialysis treatment. M.C. and M.L. analysed the data. The baseline exam comprised a timed urine collection under fed conditions from the first void after the evening meal to the first void at the morning wake-up included [17]; an early morning blood sample collected under fasting conditions after the completion of the overnight urine collection [12]; a morning timed urine collection under fasting conditions after blood sampling for assessment of kidney tubule functions [18] and a brief medical visit for measurements of anthropometry and blood pressure and for the administration of questionnaires on socio-economic status, medical history and lifestyles [12]. The follow-up exam comprised the collection of an early morning blood sample under fasting conditions and a brief medical visit for measurements of anthropometry and blood pressure and for the administration of questionnaires on medical history and lifestyles [12]. Blood pressure was measured at each visit by trained personnel following the specific World Health Organization Cardiovascular Survey Methods Manual [11, 12]. In subgroups of baseline examinees, additional urinary lab tests were performed 3–6 years before baseline [5, 11–16] and were used for ancillary analyses reported in the Supplementary Material.
The list of baseline data for the analysis included the following: date of exam and demographic data; BMI (weightkg/) and blood pressure; overnight urine creatinine excretion and estimated 24-h urine creatinine excretion [19]; overnight urine flow and urine concentration of sodium, urea nitrogen and potassium; haematocrit and serum concentration of creatinine, sodium and glucose; reported information on years of education, history of cardiovascular disease (myocardial infarction, stroke or heart failure), treatment with diuretics, treatment with renin–angiotensin system (RAS) inhibitor, smoking habit, habitual physical activity and habitual intake of alcohol, of milk or yogurt, of caffeine-containing beverages and of total fluids. Only for examinees with 45–64 years of age, baseline data also included overnight urine albumin as albumin:creatinine ratio [17]. Follow-up data included in the analysis were the date of exam, serum creatinine and glucose, BMI and blood pressure, history of cardiovascular disease (myocardial infarction, stroke or heart failure), treatment with diuretics, treatment with RAS inhibitor/blocker, smoking habit and habitual intake of alcohol.
Baseline and follow-up plasma creatinine were measured in frozen samples by automated biochemistry (Express Plus, Bayer Diagnostics, Tarrytown, NY, USA) using a kinetic alkaline picrate assay with isotope-dilution mass spectrometry–traceable standardization [20]. Other lab variables were measured in fresh samples using automated biochemistry and quality controls [12].
Measurements
The sodium:creatinine ratio in baseline overnight urine collection (uNa:Cr) was used as the main independent variable and as an index of baseline dietary sodium [4, 10, 21]. uNa:Cr was preferred to sodium excretion to avoid the inaccuracy of timing and completeness of urine collection. The overnight urine sodium excretion rate (in mmol/h) and overnight urine flow were included as additional data. Measured overnight urine creatinine excretion and estimated 24-h urine creatinine excretion were analysed for evaluation of the influence of urine creatinine on uNa:Cr. The product of estimated 24-h urine creatinine excretion times the overnight uNa:Cr was used to extrapolate estimated 24-h urine sodium excretion. Additional subgroup data were used for investigation of the consistency of uNa:Cr over time and in different types of urine collection (Supplementary Material). Serum sodium and haematocrit were analysed as disequilibrium indices in sodium [22] and/or plasma volume [23].
Kidney function was assessed using the estimated glomerular filtration rate (eGFR) calculated by the Chronic Kidney Disease Epidemiology Collaboration equation [24, 25]. Baseline examinees who did not participate in the follow-up exam but initiated chronic dialysis treatment before completion of the follow-up exam were given the assigned value of 10 mL/min/1.73 m2 for follow-up eGFR. As recommended by the guidelines [26], eGFR was used for definition of five kidney function stages (Stages 1–5: ≥90, 89–60, 59–30, 29–15 and <15 mL/min/1.73 m2, respectively). Follow-up duration was calculated as the date of the follow-up exam minus the date of the baseline exam for examinees with a follow-up exam and as the date of dialysis initiation minus the date of the baseline exam for examinees without a follow-up exam and with the assigned eGFR.
The target cohort comprised 4679 examinees with adult age at the baseline exam. Of the baseline examinees, 84 were excluded from the analysis because of missing data for baseline variables. Of the baseline examinees with complete data, 747 died before follow-up completion and 3016 participated in the follow-up exam. The follow-up examinees had complete data for all variables in the analysis. Forty-one baseline examinees without a follow-up exam initiated chronic dialysis before follow-up completion and were included in the analyses with assigned values for follow-up eGFR and follow-up date of exam. Thus the cohort for baseline cross-sectional analyses consisted of 4595 persons with complete baseline data, whereas the cohort for longitudinal analyses consisted of 3057 individuals with complete data for baseline and follow-up variables.
Statistical analyses
Statistical analyses were based on quintiles of baseline uNa:Cr that were defined separately in the two sexes and, for each sex, in seven separate strata of ages (18–24, 25–34, 35–44, 45–54, 55–64, 65–74, ≥75 years). The sex- and age-stratified procedure was designed to obtain quintiles with progressively higher uNa:Cr but with similar sex and age.
First, cross-sectional analyses investigated with the use of analysis of variance (ANOVA), chi-squared analysis or logistic regression and the associations at baseline of the uNa:Cr quintile with eGFR and, for clinical implications, with kidney function stage. Cross-sectional multivariable models included as covariates sex and the following baseline variables: age, education, physical activity, obesity (BMI ≥30 kg/m2) or BMI, smoking habit, blood pressure or hypertension (systolic pressure ≥140 mm Hg or diastolic pressure ≥90 mm Hg or regular antihypertensive drug treatment), cardiovascular disease, diuretic treatment, treatment with angiotensin-converting enzyme (ACE) inhibitor, diabetes (serum glucose ≥7.0 mmol/L or regular antidiabetic treatment), protein intake (g/day) estimated using overnight urine excretion of urea nitrogen [27, 28], overnight urine excretion of potassium used as an index of dietary potassium [29], intake of alcohol, intake of milk or yogurt, intake of caffeine-containing beverages and intake of total fluids. In the subgroup with baseline ages 45–64 years only, analyses were also controlled for baseline overnight urine albumin:creatinine ratio that was log-transformed due to high skewness [17]. Interactions of the uNa:Cr quintile with given covariates were analysed, including in the multivariable logistic regression and the product between baseline uNa:Cr and the covariate. Multivariable models were analysed in selected subgroups for investigations on consistency of results.
Next, longitudinal analyses investigated with the use of ANOVA, chi-squared analysis or logistic regression and the associations at baseline of uNa:Cr quintile with eGFR change (continuous variable = follow-up eGFR − baseline eGFR), with eGFR change per year of follow-up (continuous variable = eGFR change/follow-up duration) and, for clinical implications, with eGFR change −30% or less of baseline eGFR [30] and with worsened kidney function stage (follow-up stage > baseline stage) [26]. As covariates, longitudinal multivariable models included sex, follow-up duration and baseline data of age, education, physical activity, obesity or BMI, smoking habit, blood pressure or hypertension, cardiovascular disease, diuretic treatment, treatment with ACE inhibitor, diabetes, protein intake, overnight urine excretion of potassium, intake of alcohol, intake of milk or yogurt, intake of caffeine-containing beverages and intake of total fluids. In the subgroup with baseline ages 45–64 years only, analyses were also controlled for log-transformed baseline overnight urine albumin:creatinine ratio. Interactions of baseline uNa:Cr quintile with given baseline covariates were analysed, including in the multivariable regression and the product between baseline uNa:Cr quintile and the baseline covariate. Additional multivariable models in Supplementary Material also included as covariates follow-up data for BMI or obesity, smoking habit, blood pressure or hypertension, cardiovascular disease, diuretic treatment, treatment with ACE inhibitor/blocker, diabetes and alcohol intake.
For further analyses about the role of baseline eGFR and about regression towards the mean, non-adjusted longitudinal analyses on eGFR change were rerun separately for examinees with baseline eGFR ≥90 mL/min/1.73 m2 and with baseline eGFR <90 mL/min/1.73 m2.
Statistical procedures were performed using SPSS Statistics 19 (IBM, Armonk, NY, USA). Data presentation included mean, standard deviation (SD), median, interquartile range (IQR), prevalence, incidence, odds ratio (OR) and 95% confidence interval (CI).
RESULTS
Descriptive statistics
Baseline descriptive statistics are reported in Table 1 for the whole cohort and in Table 2 for the uNa:Cr quintiles. Women had lower eGFR, lower prevalence of Stage 1 kidney function and higher uNa:Cr due to lower urinary creatinine (Table 1). As per the definition, uNa:Cr quintiles differed for uNa:Cr but were similar for sex and age (Table 2). The uNa:Cr trend along quintiles was consistent in morning urine, 24-h urine and untimed spot samples additionally collected in some subgroups at baseline or years before baseline (Supplementary Material, Table S1). Urine sodium excretion rate paralleled uNa:Cr. Compared with Quintile 1, Quintile 5 had 3.7 times higher sodium excretion in the presence of minor differences in urine creatinine, either as measured overnight excretion or estimated 24-h excretion. The uNa:Cr quintile did not associate with serum sodium but associated inversely with haematocrit. A higher uNa:Cr quintile associated significantly with lower education, higher BMI, higher obesity prevalence, lower smoking prevalence, higher diabetes prevalence, higher protein intake, higher potassium intake, lower intake of milk or yogurt, lower intake of caffeine-containing beverages and higher fluids intake. In examinees with baseline ages 45–64 years, higher uNa:Cr associated weakly with higher urine albumin:creatinine ratio and significantly with more prevalent urine albumin:creatinine ratio ≥30 mg/g (Supplementary data, Table S2). The baseline uNa:Cr quintile associated with follow-up data for BMI, obesity, smoking habit and alcohol intake and similarly to baseline (Supplementary data, Table S3).
Table 1.
Baseline descriptive statistics of the study cohort: prevalence, mean±SD or median (IQR)a
| Variable | Men | Women |
|---|---|---|
| Baseline examinees, n | 2082 | 2513 |
| Age (years), mean ± SD | 48.6 ± 17.6 | 50.9 ± 17.8 |
| BMI (kg/m2), mean ± SD | 26.9 ± 3.7 | 26.5 ± 4.8 |
| Obese, % | 18.9 | 21.6 |
| Education (years of study), mean ± SD | 8.41 ± 4.26 | 7.37 ± 4.42 |
| Physical activity (h/day) | 0.14 (0.54) | 0.10 (0.25) |
| Smoker, % | 35.8 | 24.2 |
| Systolic pressure (mmHg), mean ± SD | 127.3 ± 17.7 | 127.6 ± 21.6 |
| Diastolic pressure (mmHg), mean ± SD | 75.0 ± 10.6 | 74.3 ± 11.0 |
| Hypertensive, % | 32.1 | 36.3 |
| Cardiovascular disease, % | 5.7 | 4.7 |
| Diuretic, % | 8.7 | 16.0 |
| Renin–angiotensin system inhibitor, % | 4.3 | 4.8 |
| Diabetic, % | 5.7 | 5.1 |
| Serum creatinine (mg/dL), median (IQR)a | 0.97 (0.14) | 0.83 (0.13) |
| eGFR (mL/min/1.73 m2), mean ± SD | 92 ± 16 | 83 ± 17 |
| Stage 1 kidney function, % | 57.3 | 34.5 |
| Stage 2 kidney function, % | 39.9 | 57.7 |
| Stage ≥3 kidney function, % | 2.8 | 7.8 |
| uNa:Cr (mmol/g), median (IQR)a | 94.7 (73) | 116 (88) |
| Overnight urine sodium excretion (mmol/h), median (IQR)a | 6.61 (5.37) | 5.46 (4.43) |
| Overnight urine flow (mL/h), mean ± SD | 55.8 ± 27.1 | 53.2 ± 28.1 |
| Overnight urine creatinine excretion (mg/h), mean ± SD | 73.7 ± 26.3 | 48.8 ± 17.2 |
| Estimated urine creatinine excretion (g/24 h), mean ± SD | 1.54 ± 0.17 | 1.00 ± 0.17 |
| Estimated urine sodium excretion (mmol/24 h), median (IQR)a,b | 144 (107) | 115 (89) |
| Protein intake (g/day), median (IQR)a,c | 72.4 (30.4) | 60.5 (29.0) |
| Overnight urine potassium excretion (mmol/h), median (IQR)a | 1.59 (0.92) | 1.34 (0.78) |
| Alcohol intake (g/day), median (IQR)a | 25.0 (37.5) | 12.5 (12.5) |
| Milk or yogurt intake (mL/day), median (IQR)a | 20 (125) | 63 (125) |
| Intake of caffeine-containing beverages (n/day), median (IQR)a | 1.4 (1.4) | 1.4 (1.3) |
| Intake of total fluids (mL/day), median (IQR)a | 1195 (646) | 845 (508) |
| Serum sodium (mmol/L), mean ± SD | 141.8 ± 2.6 | 141.3 ± 2.7 |
| Haematocrit (%), mean ± SD | 45.3 ± 3.0 | 40.5 ± 3.1 |
Table 2.
Descriptive statistics: baseline overnight uNa:Cr and other baseline data in the whole study cohort and in sex- and age-matched control quintiles (mean or prevalence)
| Variable | Quintile of baseline uNa:Cr ratio |
P for trenda | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Baseline examinees, n | 913 | 919 | 924 | 922 | 917 | |
| Women, % | 54.9 | 54.5 | 54.5 | 54.7 | 54.9 | 0.984 |
| Age (years), mean | 49.7 | 49.6 | 50.2 | 49.8 | 50.1 | 0.561 |
| BMI (kg/m2), mean | 26.4 | 26.5 | 26.7 | 26.8 | 27.1 | <0.001 |
| Obese % | 18.4 | 19.4 | 19.9 | 20.7 | 23.6 | 0.006 |
| Education (years of study), mean | 8.54 | 8.11 | 7.69 | 7.57 | 7.30 | <0.001 |
| Physical activity (h/day), mean | 0.292 | 0.266 | 0.268 | 0.295 | 0.291 | 0.622 |
| Smoker, % | 35.7 | 31.1 | 32.1 | 24.9 | 23.3 | <0.001 |
| Systolic pressure (mmHg), mean | 128 | 126 | 127 | 127 | 129 | 0.104 |
| Diastolic pressure (mmHg), mean | 75 | 75 | 75 | 74 | 75 | 0.214 |
| Hypertensive, % | 35.0 | 32.3 | 33.1 | 33.6 | 37.9 | 0.152 |
| Cardiovascular disease, % | 4.8 | 4.5 | 5.4 | 4.9 | 6.2 | 0.172 |
| Diuretic, % | 11.5 | 10.4 | 12.8 | 14.3 | 14.5 | 0.004 |
| RAS inhibitor, % | 4.7 | 5.7 | 4.9 | 4.1 | 3.6 | 0.088 |
| Diabetic, % | 4.3 | 4.5 | 5.0 | 5.4 | 7.5 | 0.001 |
| uNa:Cr (mmol/g), mean | 50 | 82 | 109 | 141 | 221 | <0.001 |
| Overnight urine sodium excretion (mmol/h), mean | 3.07 | 4.81 | 6.35 | 8.14 | 11.28 | <0.001 |
| Overnight urine flow (mL/h), mean | 43 | 46 | 53 | 59 | 70 | <0.001 |
| Overnight urine creatinine excretion (mg/h), mean | 62.4 | 61.1 | 61.1 | 60.1 | 54.9 | <0.001 |
| Estimated urine creatinine excretion (g/24 h), mean | 1.23 | 1.25 | 1.24 | 1.25 | 1.25 | 0.216 |
| Estimated urine sodium excretion (mmol/24 h)b, mean | 60 | 98 | 130 | 169 | 262 | <0.001 |
| Protein intake (g/day)c, mean | 58.5 | 63.8 | 68.9 | 73.8 | 78.4 | <0.001 |
| Overnight urine potassium excretion (mmol/h), mean | 1.38 | 1.43 | 1.56 | 1.72 | 1.86 | <0.001 |
| Alcohol intake (g/day), mean | 21.7 | 23.7 | 21.6 | 21.4 | 20.8 | 0.164 |
| Milk or yogurt intake (mL/day), mean | 86.7 | 77.5 | 79.3 | 77.2 | 74.2 | 0.008 |
| Intake of caffeine-containing beverages (n/day), mean | 1.80 | 1.77 | 1.68 | 1.79 | 1.58 | 0.003 |
| Intake of total fluids (mL/day), mean | 1061 | 1083 | 1093 | 1092 | 1131 | 0.007 |
| Serum sodium (mmol/L), mean | 141.4 | 141.5 | 141.7 | 141.5 | 141.6 | 0.149 |
| Haematocrit (%), mean | 42.9 | 42.8 | 42.8 | 42.5 | 42.5 | 0.009 |
Cross-sectional analyses: uNa:Cr and kidney function at baseline
A higher uNa:Cr quintile associated with lower serum creatinine, higher eGFR and more prevalent Stage 1 kidney function in non-adjusted analyses and in multivariable analyses (Table 3). Complete results of multivariable logistic regression for Stage 1 kidney function were reported in Supplementary data, Table S4. Supplementary data, Figure S1, shows the relationship at baseline between estimated 24-h urine excretion and the OR of Stage 1 kidney function. The interaction test was borderline significant for diabetes (P = 0.052) but not significant for sex, age, obesity, hypertension and protein intake (P > 0.29). In multivariable models, the OR of Stage 1 kidney function in Quintile 5 compared with Quintile 1 was >1 in the whole cohort and all subgroups with the exception of diabetics (Figure 1). In the age 45–64 years subgroup, findings were also similar with the controls for log-transformed baseline urinary albumin:creatinine ratio [Quintile 5 to 1 OR 1.91 (95% CI 1.28–2.83), P = 0.001].
Table 3.
Cross-sectional analyses: baseline data of eGFR and prevalence of stage 1 kidney function (eGFR ≥ 90 mL/min × 1.73 m2) by sex- and age- controlled quintiles of baseline overnight uNa/Cr ratio (mean or prevalence)
| Variable | Quintile of baseline uNa/Cr ratio |
P for trenda | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Baseline examinees, n | 913 | 919 | 924 | 922 | 917 | |
| Serum creatinine (µmol/L), mean | ||||||
| Non-adjusted | 81.7 | 80.9 | 79.9 | 79.1 | 77.7 | <0.001 |
| Adjustedb | 81.2 | 80.9 | 79.9 | 79.3 | 78.0 | <0.001 |
| eGFR (mL/min/1.73 m2), mean | ||||||
| Non-adjusted | 84.8 | 85.9 | 86.9 | 87.9 | 89.3 | <0.001 |
| Adjustedb | 85.4 | 85.8 | 87.0 | 87.6 | 88.9 | <0.001 |
|
39.1% (357) | 42.7% (392) | 45.2% (418) | 46.6% (430) | 50.6% (464) | <0.001 |
| 1 (reference) | 1.16 (0.96/1.40) | 1.29 (1.07/1.55) | 1.34 (1.11/1.61) | 1.63 (1.35/1.96) | <0.001 | |
| Stage 1 kidney function, adjusted %b | 40.2 | 42.4 | 45.7 | 46.1 | 49.9 | <0.001 |
| Adjusted ORb | 1 (reference) | 1.15 (0.89/1.48) | 1.47 (1.13/1.90) | 1.48 (1.13/1.92) | 1.98 (1.50/2.59) | <0.001 |
By linear or logistic regression analysis.
Covariates in the multivariable model: sex and baseline data of age, education, physical activity, obesity, smoking, hypertension, cardiovascular disease, diuretic treatment, treatment with ACE inhibitor, diabetes, protein intake, overnight urine potassium, alcohol intake, milk or yogurt intake, intake of caffeine-containing beverages and intake of total fluids.
FIGURE 1:

Graphical and tabular presentation of adjusted OR with 95% CI of prevalence of Stage 1 kidney function (eGFR ≥90 mL/min/1.73 m2) at baseline in uNa:Cr Quintile 5 compared with Quintile 1 in the whole cohort and selected subgroups. The dotted line indicates an OR of 1. Covariates used for OR adjustment are listed in the footnote of Table 3. N Stage 1, n examinees with Stage 1 kidney function; total N, n examinees in the analysis.
Longitudinal analyses: baseline uNa:Cr and kidney function changes
The mortality-corrected response rate at the follow-up exam was 78.4% and did not differ along uNa:Cr quintiles (Supplementary data, Table S5). Compared with other examinees, the subgroup of 41 baseline examinees with the initiation of chronic dialysis without a follow-up exam differed for sex distribution and several baseline variables (Supplementary data, Table S6). The eGFR change was a decrease for 83.4% of the cohort. The eGFR decrease was much greater in subgroups with an eGFR decline ≥30% or with a worsened stage of kidney function (Supplementary data, Tables S7–S8). Sex distribution and baseline age of examinees in longitudinal analyses did not differ along uNa:Cr quintiles (Supplementary data, Table S9).
In non-adjusted analyses and in multivariable adjusted analyses, higher baseline uNa:Cr quintile associated with a more negative eGFR change and with a higher incidence of eGFR decline ≥30% and of a worsened stage of kidney function (Table 4). The complete results of the multivariable logistic regression for eGFR decline ≥30% are reported in Supplementary data, Table S10. The interaction test was not significant for sex and baseline values of age, obesity, eGFR, hypertension, diabetes and protein intake (P > 0.11). In multivariable models, the OR of eGFR decline ≥30% in Quintile 5 compared with Quintile 1 was >1 in the whole cohort and all subgroups, including men and women, youngsters and elderly, individuals with reduced kidney function, non-hypertensives, obese and non-obese individuals, non-diabetics and individuals with high protein intake and without high protein intake (Figure 2). Findings in the subgroup with ages 45–64 years were similar with controls for baseline log-transformed urine albumin:creatinine ratio [OR 2.26 (95% CI 0.96–5.30)]. Findings were consistent in non-adjusted analyses excluding the 41 baseline examinees with assigned eGFR (Supplementary data, Table S11) and in multivariable analyses with controls for follow-up data (Supplementary data, Table S12). Supplementary data, Figure S2 shows the relationship between baseline estimated 24-h urine excretion and the OR of eGFR decline ≥30% or of worsened stage of kidney function.
Table 4.
Longitudinal analyses: follow-up duration, follow-up eGFR and indices of kidney function changes from baseline to follow-up by sex- and age-matched quintiles of baseline overnight uNa:Cr ratio
| Variable | Quintile of baseline uNa:Cr ratio |
P for trenda | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Examinees, n | 591 | 603 | 629 | 643 | 591 | |
| Follow-up duration (years), mean | 14.1 | 14.2 | 14.0 | 14.0 | 14.1 | 0.439 |
| Follow-up eGFR (mL/min/1.73 m2), mean | 79.9 | 79.6 | 78.0 | 78.8 | 79.6 | 0.537 |
| eGFR change (mL/min/1.73 m2), non-adjusted, mean | −8.2 | −9.5 | −11.4 | −11.7 | −13.2 | <0.001 |
| eGFR change (mL/min/1.73 m2), adjustedb, mean | −9.5 | −10.6 | −11.4 | −11.3 | −11.4 | 0.001 |
| eGFR change per year (mL/min/1.73 m2), non-adjusted, mean | −0.63 | −0.72 | −1.09 | −1.63 | −1.30 | <0.001 |
| eGFR change per year (mL/min/1.73 m2), adjusted, mean | −0.71 | −0.80 | −1.06 | 1.57 | −1.23 | 0.037 |
|
2.7% | 2.5% | 6.4% | 7.9% | 8.1% | <0.001 |
| 1 (reference) | 0.92 (0.45/1.87) | 2.44 (1.35/4.41) | 3.10 (1.75/5.49) | 3.18 (1.78/5.66) | ||
|
3.7% | 3.4% | 6.2% | 7.5% | 6.8% | 0.001 |
| 1 (reference) | 0.96 (0.46/2.01) | 1.96 (1.05/3.66) | 2.40 (1.30/4.44) | 2.21 (1.18/4.13) | ||
|
27.1% | 30.7% | 36.9% | 36.1% | 38.9% | <0.001 |
| 1 (reference) | 1.19 (0.93/1.53) | 1.57 (1.23/2.01) | 1.52 (1.19/1.94) | 1.72 (1.34/2.19) | ||
|
29.4% | 32.5% | 36.6% | 35.4% | 35.8% | 0.013 |
| 1 (reference) | 1.19 (0.91/1.55) | 1.45 (1.12/1.88) | 1.37 (1.05/1.78) | 1.38 (1.05/1.82) | ||
By linear or logistic regression analysis.
Covariates in the multivariable model: sex, follow-up duration and baseline values of age, education, physical activity, obesity, smoking, hypertension, cardiovascular disease, diuretic treatment, treatment with ACE inhibitor, diabetes, eGFR, protein intake, overnight urine potassium, alcohol intake, milk or yogurt intake, intake of caffeine-containing beverages and intake of total fluids.
FIGURE 2:

Graphical and tabular presentation of adjusted OR with 95% CI of incidence of eGFR ≥30% of baseline eGFR in uNa:Cr Quintile 5 compared with Quintile 1 in the whole cohort and selected subgroups. The dotted line indicates an OR of 1. Covariates used for OR adjustment are listed in the footnote of Table 4. N 30% decline, n examinees with incidence of eGFR ≥30% of baseline eGFR; total N, n examinees in the analysis. OR and 95% CI are incalculable in the diabetic subgroup due to the low number.
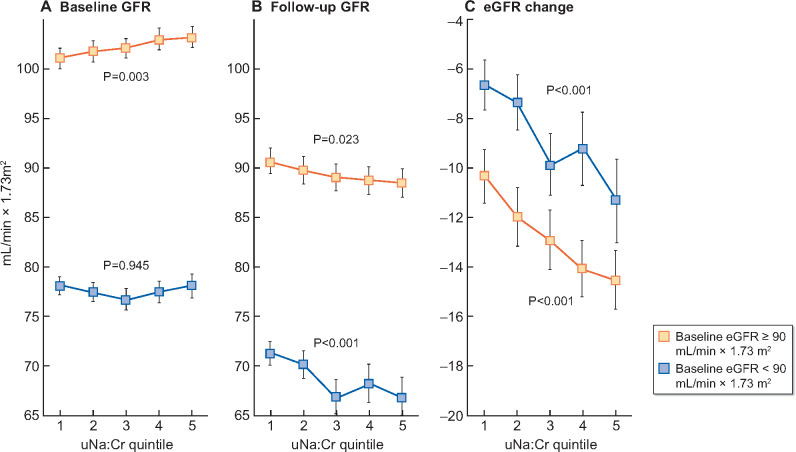
Non-adjusted analyses in eGFR subgroups showed that higher uNa/Cr quintile associated with lower follow-up eGFR and more negative eGFR change both in the subgroup with baseline eGFR ≥90 mL/min × 1.73 m2 and in the subgroup with baseline eGFR < 90 mL/min × 1.73 m2 (Figure 3).
FIGURE 3:
Non-adjusted mean with 95% CI of baseline eGFR (left panel), follow-up eGFR (centre panel) and eGFR change (right panel) by quintile of baseline overnight uNa:Cr in the subgroup with baseline eGFR ≥90 mL/min/1.73 m2 (open symbols) and in the subgroup with baseline eGFR <90 mL/min/1.73 m2 (closed symbols). Number of examinees from Quintile 1 to Quintile 5: subgroup with baseline eGFR ≥90 mL/min/1.73 m2 = 261, 290, 316, 330 and 347 and subgroup with baseline eGFR <90 mL/min/1.73 m2 = 330, 313, 313, 313 and 244. P-values for trend are from non-adjusted ANOVA.
DISCUSSION
The study results indicate that, in a general population sample, higher overnight uNa:Cr associated cross-sectionally with higher eGFR and longitudinally with greater long-term decline in eGFR. These associations were independent of sex, age and other variables, including hypertension, other dietary markers, lifestyles, obesity and diabetes.
The main study limitation was the use of a single overnight urine collection. This implied two possible causes of misclassification: misclassification of habitual sodium intake due to high day-to-day intra-individual variability in sodium intake [4, 31, 32] and misclassification due to circadian rhythms in urinary sodium [33]. Data in the Supplementary Material indicate that the misclassification was minor at the level of quintiles because linear trends in uNa:Cr were consistent in morning urine, 24-h urine and untimed spot urine collected at baseline or years before the baseline exam [5, 13, 14]. The independence of results of blood pressure status and their significance in non-hypertensives indicated that the hypertensives’ greater night-time sodium excretion played a minor bias [34]. The lack of ambulatory blood pressure measurements precluded investigation about the possible role of dipping/non-dipping hypertension. The lack of information on all nutrients limited the analyses on dietary factors. Nonetheless, the availability of urea data gave information about protein intake, that is, the key dietary modulator of kidney function [35]. The lack of albuminuria assessment for all age strata limited to individuals with ages 45–64 years and the evidence that the cross-sectional and longitudinal associations of urinary sodium with eGFR were independent of albuminuria. Ethnic-dependent differences could not be investigated. Study strengths were the high participation rate of the Gubbio people and the use of outcomes with validated prognostic power for kidney failure, cardiovascular and all-cause mortality [26, 30].
Urine sodium is the sole objective and measurable index of sodium intake in individuals on unrestricted diet [4, 29]. Baseline data for serum sodium and haematocrit supported the interpretation that higher overnight uNa:Cr reflected higher dietary sodium. If higher overnight uNa:Cr was not supported by higher sodium intake, morning lab tests after the overnight collection should have disclosed a trend along uNa:Cr quintiles towards relative hyponatraemia and/or isotonic hypovolaemia [22]. Altogether, the lack of association with serum sodium, the inverse association with haematocrit and the positive association with urine flow and fluids intake supported the view that high uNa:Cr associated with a slight relative isotonic hypervolaemia [22]. The interpretation that higher overnight uNa:Cr reflected higher sodium intake was supported also by the positive cross-sectional associations at baseline of uNa:Cr with other diet-related indices such as urine urea nitrogen (protein intake) [27, 28], urine potassium (potassium intake) [29], BMI and obesity.
Observational studies can hardly investigate mechanisms and are inevitably prone to confounders. For longitudinal results, a mere effect of regression towards the mean could be excluded because the inverse association of uNa:Cr with eGFR changes was also strong in the subgroup with baseline eGFR <90 mL/min/1.73 m2, that is, in the subgroup where a regression towards the mean should have driven towards an increase or towards a smaller decline in eGFR. The possibility that the association of uNa:Cr with kidney function could be mediated by sodium intake effects on blood pressure was not supported by the independence of results of hypertension at baseline and follow-up. The statistical insignificance of the 24% higher incidence of greater eGFR decline in individuals with baseline hypertension could reflect the high rate of hypertension control in the Gubbio cohort [36] and the inclusion in the multivariable model of hypertension determinants (i.e. obesity, physical activity, alcohol intake and an index of sodium intake). Theoretically the baseline cross-sectional positive association of overnight uNa:Cr with eGFR could reflect an upregulation of kidney function secondary to relative hypervolaemia and/or related adaptations. Thus sodium intake could play a contributory role in the post-meal increase in glomerular filtration defined as hyperfiltration [35, 37]. In this view, the contrast between the cross-sectional positive association and the longitudinal inverse association could be combined in a hypothetic model where sodium intake effects on kidney function vary over time, that is, a short-term hyperfiltration in the hours subsequent to high salt intake and a long-term detrimental acceleration of the kidney function decline, perhaps due to the chronic stimulation of hyperfiltration. Given that sodium intake accounts for a large percentage of total urine osmolality, another, not alternative, possibility could be high sodium intake contributes to kidney function decline through an osmotic overload [38]. Last, the cross-sectional association of urinary sodium with urine albumin could point to a mechanism mediated by high albuminuria [39]. It is impossible to exclude other mechanisms as, for instance, direct toxicity of sodium on glomerular, tubulointerstitial or vascular compartments of the kidney.
Study results could have at least two practical implications. Cross-sectional results prove that sodium intake in the hours prior to eGFR assessment may play a confounding role favouring higher eGFR in the case of high intake and, vice versa, lower eGFR in the case of low intake. Given that kidney function stage and eGFR decline ≥30% are powerful predictors of prognosis for kidney function [26, 30], longitudinal results indicate that medical practice and guidelines should consider high sodium intake as an independent determinant of accelerated kidney function decline in individuals with or at risk of kidney dysfunction. Thus the study supported the worldwide advice to reduce sodium intake to <100 mmol/day. Besides its key role in prevention and control of cardiovascular disease [1, 2, 7], low sodium intake could be useful also for risk reduction of acute renal hyperfiltration and of long-term decline of kidney function.
Study results agreed with two observational clinical studies in nephropathic patients [21, 40] and with the population-based study of Sogiura et al. [10]. In relation to that study, the major strengths were the inclusion in the analysis of several dietary indices besides urine sodium for a well-characterized population sample [12]. The average values of 121 mmol/g uNa:Cr and of a 6.72 mmol/h urine sodium excretion rate pointed to a daily urine sodium excretion of 150–160 mmol/24 h, in agreement with previous data in the Gubbio cohort and in other Italian cohorts [5, 13]. On the other hand, study results disagreed with the results of interventional clinical trials in patients at high cardiovascular risk [41] and could seem in contrast with the results of a Dutch observational population-based study [42]. The disagreement with clinical trials reasonably reflected substantial differences not only in the study cohorts (i.e. high cardiovascular risk patients and general population), but also in drug treatment and follow-up duration. The prevalence of treatment with RAS inhibitors or blockers was indeed >90% in the trials, whereas in the Gubbio cohort it was <5% at baseline and <25% at follow-up. Moreover, follow-up duration averaged <5 years in the trials and almost 15 years in the Gubbio cohort. The seeming contrast in the conclusions between the Dutch study and the present study reasonably reflected methodological differences in the selection of incident outcomes: chronic kidney disease defined as eGFR <60 mL/min/1.73 m2 in that study and the extent of eGFR decline in the present study. With the use of an outcome defined as incident eGFR <60 mL/min/1.73 m2, the events could be unrelated to the extent of eGFR decline in many cases. For example, a 3-mL eGFR decline close to the threshold of 60 mL/min/1.73 m2, i.e. from 62 to 59 mL/min/1.73 m2, would be an incident event with that definition, although it was a minor eGFR change. In contrast, a 30-mL eGFR decline above or below the threshold of 60 mL/min/1.73 m2, e.g. from 92 to 62 mL/min/1.73 m2 or from 59 to 29 mL/min/1.73 m2, would not be an incident event for that definition, although it was a substantial eGFR decline.
Briefly, this observational cohort study reported that in the general population, a urine marker of high sodium intake associated with higher kidney function at baseline but predicted a greater long-term decline of kidney function independent of sex, age, baseline kidney function, hypertension and other covariates. Thus the study results are in accordance with the idea that high salt intake could be an independent determinant of kidney function decline over time and support the need for interventional studies to investigate the renoprotective effects of dietary salt restriction.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
Supplementary Material
ACKNOWLEDGEMENTS
The Gubbio Study was made possible thanks to the enthusiasm of the people of the town of Gubbio and to the support of its municipal and health authorities and community leaders.
FUNDING
In the past, economic support to the Gubbio study was provided by Merck, Sharp and Dohme, Italy; the US National Heart, Lung and Blood Institute (grant R01HL-40397-02) and the Ministero Italiano di Università e Ricerca (grant 068034, PRIN 2004). None of the sponsors or funders had any role in the study design; in the collection, analysis and interpretation of data; in the writing of the report or in the decision to submit the article for publication.
AUTHORS’ CONTRIBUTIONS
The Gubbio study was conducted by the Centro Studi Epidemiologici di Gubbio in collaboration with Gubbio Hospital, Federico II University of Naples, University of Milan, Northwestern University of Chicago and Istituto Superiore di Sanità. The present analysis was planned by M.C. in collaboration with M.L. The first draft of the manuscript was prepared by M.C. M.C. and M.L. take responsibility for the overall content of the work and/or the conduct of the study, had access to the data and controlled the decision to publish. All authors provided substantial contributions to the following: acquisition, analysis or interpretation of data; drafting the manuscript or revising it critically for important intellectual content; final approval of the version to be published and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflicts of interest.
REFERENCES
- 1.Joint World Health Organization/Food and Agriculture Organization of the United Nations Expert Consultation. Diet, Nutrition and the Prevention of Chronic Disease Geneva: World Health Organization, 2003
- 2.World Health Organization. Prevention of Cardiovascular Disease: Guidelines for Assessment and Management of Cardiovascular Risk. Geneva: World Health Organization, 2007
- 3. James WP, Ralph A, Sanchez-Castillo CP.. The dominance of salt in manufactured food in the sodium intake of affluent societies. Lancet 1987; 1: 426–429 [DOI] [PubMed] [Google Scholar]
- 4. Cogswell ME, Maalouf J, Elliott P. et al. Use of urine biomarkers to assess sodium intake: challenges and opportunities. Annu Rev Nutr 2015; 35: 349–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stamler J, Rose G, Elliott P. et al. Findings of the international cooperative INTERSALT study. Hypertension 1991; 17(1 Suppl): I19–I15 [DOI] [PubMed] [Google Scholar]
- 6. Powles J, Fahimi S, Micha R. et al. Global, regional and national sodium intakes in 1990 and 2010: a systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide. BMJ Open 2013; 3: e003733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aburto NJ, Ziolkovska A, Hooper L. et al. Effect of lower sodium intake on health: systematic review and meta-analyses. BMJ 2013; 346: f1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McMahon EJ, Campbell KL, Bauer JD. et al. Altered dietary salt intake for people with chronic kidney disease. Cochrane Database Syst Rev 2015; 2: CD010070. [DOI] [PubMed] [Google Scholar]
- 9. Nomura K, Asayama K, Jacobs L. et al. Renal function in relation to sodium intake: a quantitative review of the literature. Kidney Int 2017; 92: 67–78 [DOI] [PubMed] [Google Scholar]
- 10. Sugiura T, Takase H, Ohte N. et al. Dietary salt intake is a significant determinant of impaired kidney function in the general population. Kidney Blood Press Res 2018; 43: 1245–1254 [DOI] [PubMed] [Google Scholar]
- 11. Laurenzi M, Cirillo M, Angeletti M. et al. Gubbio population study: baseline findings. Nutr Metab Cardiovasc Dis 1991; 1: S1–S18 [Google Scholar]
- 12. Cirillo M, Terradura-Vagnarelli O, Mancini M. et al. Cohort profile: the Gubbio population study. Intern J Epidemiol 2014; 43: 713–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Farinaro E, Trevisan M, Jossa F. et al. INTERSALT in Italy: findings and community health implications. J Hum Hypertens 1991; 5: 15–19 [PubMed] [Google Scholar]
- 14. Cirillo M, Laurenzi M, Panarelli W. et al. Urinary sodium to potassium ratio and urinary stone disease. The Gubbio Population Study Research Group. Kidney Int 1994; 46: 1133–1139 [DOI] [PubMed] [Google Scholar]
- 15. Cirillo M, Ciacci C, Laurenzi M. et al. Salt intake, urinary sodium, and hypercalciuria. Miner Electrolyte Metab 1997; 23: 265–268 [PubMed] [Google Scholar]
- 16. Brown IJ, Dyer AR, Chan Q. et al. Estimating 24-hour urinary sodium excretion from casual urinary sodium concentrations in western populations – the INTERSALT study. Am J Epidemiol 2013; 177: 1180–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cirillo M, Lanti MP, Menotti A. et al. Definition of kidney dysfunction as a cardiovascular risk factor: use of urinary albumin excretion and estimated glomerular filtration rate. Arch Intern Med 2008; 168: 617–624 [DOI] [PubMed] [Google Scholar]
- 18. Cirillo M, Ciacci C, De Santo NG.. Age, renal tubular phosphate reabsorption, and serum phosphate levels in adults. N Engl J Med 2008; 359: 864–866 [DOI] [PubMed] [Google Scholar]
- 19. Ix JH, Wassel CL, Stevens LA. et al. Equations to estimate creatinine excretion rate: the CKD Epidemiology Collaboration. Clin J Am Soc Nephrol 2011; 6: 184–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Myers GL, Miller WG, Coresh J. et al. Recommendations for improving serum creatinine measurement: a report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem 2006; 52: 5–18 [DOI] [PubMed] [Google Scholar]
- 21. Lambers Heerspink HJ, Holtkamp FA, Parving HH. et al. Moderation of dietary sodium potentiates the renal and cardiovascular protective effects of angiotensin receptor blockers. Kidney Int 2012; 82: 330–337 [DOI] [PubMed] [Google Scholar]
- 22. Skorecki K, Ausiello D.. Disorders of sodium and water homeostasis In: Goldman L, Schafer AI (eds). Goldman’s Cecil Medicine. 24th edn, vol. 1. Philadelphia, PA: Elsevier Saunders, 2011: 720–734 [Google Scholar]
- 23. Johansen LB, Videbæk R, Hammerum M. et al. Underestimation of plasma volume changes in humans by hematocrit/hemoglobin method. Am J Physiol 1998; 274: R126–R130 [DOI] [PubMed] [Google Scholar]
- 24. Levey AS, Stevens LA, Schmid CH. et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cirillo M, Lombardi C, Luciano MG. et al. Estimation of GFR: a comparison of new and established equations. Am J Kidney Dis 2010; 56: 802–804 [DOI] [PubMed] [Google Scholar]
- 26. Kidney Disease: Improving Global Outcomes CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3: 1–150 [DOI] [PubMed] [Google Scholar]
- 27. Bingham SA. Urine nitrogen as a biomarker for the validation of dietary protein intake. J Nutr 2003; 133: 921S–924S [DOI] [PubMed] [Google Scholar]
- 28. Cirillo M, Lombardi C, Chiricone D. et al. Protein intake and kidney function in the middle-age population: contrast between cross-sectional and longitudinal data. Nephrol Dial Transplant 2014; 29: 1733–1740 [DOI] [PubMed] [Google Scholar]
- 29. Caggiula AW, Wing RR, Nowalk MP. et al. The measurement of sodium and potassium intake. Am J Clin Nutr 1985; 42: 391–398 [DOI] [PubMed] [Google Scholar]
- 30. Coresh J, Turin TC, Matsushita K. et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA 2014; 311: 2518–2531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Elliott P, Stamler J, Nichols R. et al. INTERSALT revisited: further analyses of 24 hour sodium excretion and blood pressure within and across populations. BMJ 1996; 312: 1249–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sakaki M, Tsuchihashi T, Arakawa K. et al. Long-term variability of urinary salt excretion and blood pressure in hypertensive patients. Hypertens Res 2014; 37: 939–943 [DOI] [PubMed] [Google Scholar]
- 33. Firsov D, Bonny O.. Circadian regulation of renal function. Kidney Int 2010; 78: 640–645 [DOI] [PubMed] [Google Scholar]
- 34. Dyer AR, Stamler R, Grimm R. et al. Do hypertensive patients have a different diurnal pattern of electrolyte excretion? Hypertension 1987; 10: 417–424 [DOI] [PubMed] [Google Scholar]
- 35. King AJ, Levey AS.. Dietary protein and renal function. J Am Soc Nephrol 1993; 3: 1723–1737 [DOI] [PubMed] [Google Scholar]
- 36. Menotti A, Lanti M, Puddu PE. et al. A twenty-year cardiovascular and all-cause mortality trends and changes in cardiovascular risk factors in Gubbio, Italy: the role of blood pressure changes. J Hypertens 2009; 27: 266–274 [DOI] [PubMed] [Google Scholar]
- 37. Santo D, Anastasio P, Cirillo M. et al. Sequential analysis of variation in glomerular filtration rate to calculate the haemodynamic response to a meat meal. Nephrol Dial Transplant 1995; 10: 1629–1636 [PubMed] [Google Scholar]
- 38. Bankir L, Bouby N, Ritz E.. Vasopressin: a novel target for the prevention and retardation of kidney disease? Nat Rev Nephrol 2013; 9: 223–239 [DOI] [PubMed] [Google Scholar]
- 39. De Jong PE, Brenner BM.. From secondary to primary prevention of progressive renal disease: the case for screening for albuminuria. Kidney Int 2004; 66: 2109–2118 [DOI] [PubMed] [Google Scholar]
- 40. He J, Mills KT, Appel LJ. et al. Urinary sodium and potassium excretion and CKD progression. J Am Soc Nephrol 2016; 27: 1202–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Smyth A, Dunkler D, Gao P. et al. The relationship between estimated sodium and potassium excretion and subsequent renal outcomes. Kidney Int 2014; 86: 1205–1212 [DOI] [PubMed] [Google Scholar]
- 42. Kieneker LM, Bakker SJL, de Boer RA. et al. Low potassium excretion but not high sodium excretion is associated with increased risk of developing chronic kidney disease. Kidney Int 2016; 90: 888–896 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.