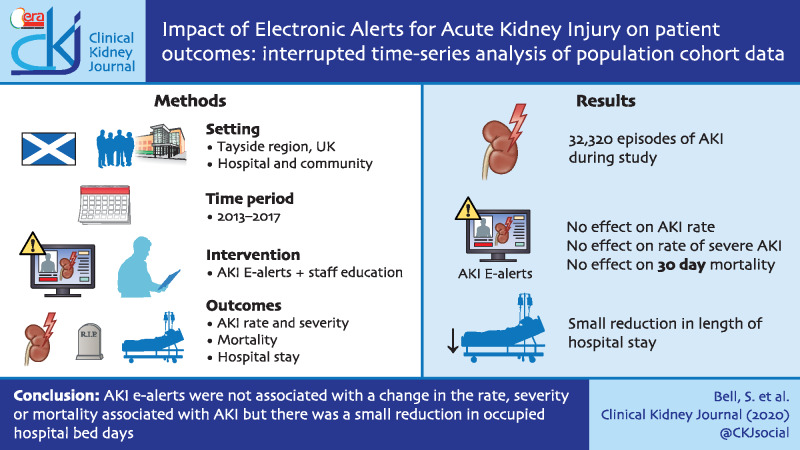
Graphical Abstract
Keywords: AKI, electronic alert, epidemiology, mortality, patient outcomes
Abstract
Background
Automated acute kidney injury (AKI) electronic alerts (e-alerts) are rule-based warnings triggered by changes in creatinine and are intended to facilitate earlier detection in AKI. We assessed the impact of the introduction in the Tayside region of UK in April 2015 of automated AKI e-alerts with an accompanying education programme.
Methods
Interrupted time-series analysis using segmented regression was performed involving all adults with AKI aged ≥18 years who had a serum creatinine measured between 1 April 2013 and 31 March 2017. Analysis evaluated associations of AKI e-alert introduction on rate and severity (Stages 2–3) of AKI as well as mortality and occupied hospital bed days per patient per month in the population with AKI.
Results
There were 32 320 episodes of AKI during the observation period. Implementation of e-alerts had no effect on the rate of any AKI [incidence rate ratio (IRR) 0.996, 95% confidence interval (CI) 0.991 to 1.001, P = 0.086] or on the rate of severe AKI (IRR 0.995, 95% CI 0.990 to 1.000, P = 0.061). Subgroup analysis found no impact on the rate or severity of AKI in hospital or in the community. Thirty-day mortality following AKI did not improve (IRR 0.998, 95% CI 0.987 to 1.009, P = 0.688). There was a slight reduction in occupied bed days (β-coefficient −0.059, 95% CI −0.094 to −0.025, P = 0.002).
Conclusions
Introduction of automated AKI e-alerts was not associated with a change in the rate, severity or mortality associated with AKI, but there was a small reduction in occupied hospital bed days.
INTRODUCTION
Acute kidney injury (AKI) affects 13–18% of all patients admitted to hospital [1]. There is increasing evidence showing that even mild, transient episodes of AKI are associated with adverse outcomes such as increased risk of death [2–4] and development of chronic kidney disease [5]. Once established, there are no proven effective treatments for AKI, although delays in diagnosis are associated with poorer outcomes [6]. The emphasis in policy is therefore prevention and earlier recognition. Electronic alerts (e-alerts) are automated alerts triggered by changes in serum creatinine that are intended to enable earlier detection of AKI and therefore expedite management. The effect of e-alerts on AKI for hospitalized patients remains unclear, with conflicting results from several studies [7, 8]. In the UK, there has been a national initiative aimed at improving the care of patients at risk for or with AKI [1, 9]. As part of this initiative, the National Health Service (NHS) in England mandated that all hospitals implement an e-alert for AKI based on the Kidney Disease: Improving Global Outcomes (KDIGO) definition for AKI [10].
In April 2015, the NHS Tayside region of Scotland introduced AKI e-alerts simultaneously for all patients in primary and secondary care. The e-alerts are generated by the laboratory reporting system using the same rules as the NHS England e-alerts. The AKI stage appears with creatinine results alongside a link that was added to signpost clinicians to local AKI guidelines. New guidelines tailored for primary care were created to supplement the AKI in hospital guidance that was already in place. These guidelines cover the assessment, investigations and management of patients with AKI including indications for referral to the renal team, and are available as supplementary figures (Supplementary data, Figures S1 and S2). The banner with the patients identifying information changes colour when an e-alert has been triggered, further highlighting to the clinician reviewing the results that an AKI has been detected. An automated email is also sent to clinicians for all AKI Stages 2 and 3. This was accompanied by both a community- and hospital-wide education programme aimed at raising awareness of AKI. An AKI awareness week was organized for the week preceding the introduction of the AKI e-alerts, with teaching carried out for junior doctors and nursing staff on every ward as well as through departmental meetings, hospital grand rounds and a stand at the main hospital entrance. An educational video [11] and lanyard cards [12] were also developed.
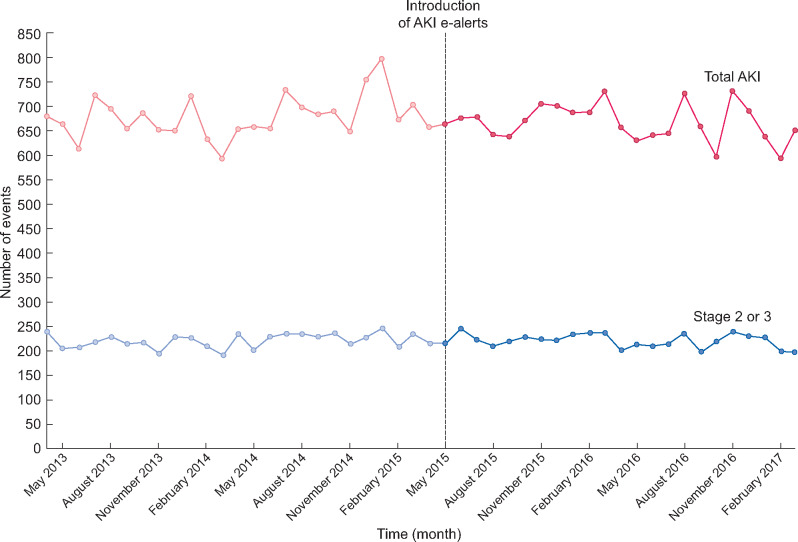
FIGURE 1:
Monthly count of all AKI and severe AKI (Stages 2–3) between 1 April 2013 and 31 March 2017 in NHS Tayside.
The aim of this study was to assess the impact of AKI e-alerts on patient outcomes by examining the rate and severity of AKI, mortality and occupied hospital bed days for patients with AKI.
MATERIALS AND METHODS
Study population
The study population comprised of all adults aged ≥18 years who were not receiving renal replacement therapy, and who had a creatinine measurement between 1 April 2013 and 31 March 2017 who resided in the NHS Tayside region of Scotland, UK.
Data sources
Data were provided by the Health Informatics Centre (HIC) at the University of Dundee [13]. HIC links individual patient data by means of the unique identifier used across NHS Scotland [the Community Health Index (CHI) number]. Data from the following datasets were linked: CHI register for patient demography; Scottish Morbidity Record of hospital admissions (SMR01) for length of inpatient stay; Scottish Renal Registry (SRR) to exclude people on renal replacement therapy [14]; the NHS laboratory providing all biochemistry measurement in Tayside for creatinine; and the General Registry Office (GRO) national death registration system for mortality. Community-acquired AKI was defined as occurring in the community or on the first day of hospital admission. Hospital-acquired AKI included all episodes of AKI that occurred during a hospital admission, excluding those that were first detected prior to admission or during the first 24 h of admission.
Outcomes
For both NHS e-alerts and study outcomes, the diagnosis and stage of AKI were defined using the NHS England e-alert algorithm , which is based on the KDIGO creatinine-based criteria [10, 15]. Baseline creatinine was defined as either the median of all creatinine measurements taken between 8 and 365 days prior to the index creatinine measurement or the minimum creatinine taken in the preceding 7 days before the index creatinine measurement. AKI Stage 1 was defined as an increase in serum creatinine between 150% and <200% of baseline or a rise in serum creatinine of ≥26.5 μmol/L in 2 days; AKI Stage 2 was defined as an increase in serum creatinine between 200% and <300% of the baseline value; and AKI Stage 3 was defined as an increase in serum creatinine to ≥300% of baseline value or a serum creatinine of ≥354 µmol/L. AKI episodes were counted as unique events if there were >7 days between e-alerts being triggered. AKI stage was defined by the highest stage experienced during each AKI episode. We examined the impact of the introduction of AKI e-alerts on the total rate of AKI, and on the rate of severe AKI (Stages 2–3). Subgroup analysis was performed to establish whether e-alerts differentially affected rates of community-acquired AKI or hospital-acquired AKI.
Mortality within 30 days and 90 days of the occurrence of the highest stage of AKI episode was examined using death registration data from the General Register of Deaths. In those admissions where there was an AKI detected, length of stay was calculated using number of occupied bed days per patient. Occupied bed days for people admitted with AKI were summed for each month, and divided by the number of individuals with AKI who contributed to these stays to obtain the bed days per patient per month.
Statistical methods
The study design was an observational cohort study with segmented regression analysis of interrupted time-series (ITS) data [16]. We used 25 monthly time points before and 23 time points after the introduction of AKI e-alerts in April 2015 to assess the impact of the intervention in terms of both immediate impact (change in level of the outcome immediately after the intervention) and gradual impact (change in trend after the intervention). For all analyses, the presence of autocorrelation was evaluated using the Durbin–Watson statistic, and accounted for if necessary, by fitting a lagged variable.
The impact of the intervention on the number of unique AKI events per month was analysed using a Poisson segmented regression model. The impact of the intervention on death within 30 days and within 90 days was also analysed using a Poisson model where the total unique AKI episodes per month were used as an offset, converting the model outcome into a rate. The impact of the intervention on occupied bed days per month per patient with AKI was examined using a linear segmented regression model. All analyses were carried in IBM SPSS (v22), R (v3.2.5) and STATA MP (v14).
Ethical approval
Anonymized record linkage was conducted according to HIC Standard Operating Procedures (SOPs). The Tayside Research Ethics Committee does not require submission of individual studies that follow HIC SOPs, which are Caldicott Guardian approved. All data used in the project were secured on a Scottish Government accredited and ISO27001 certified safe haven.
RESULTS
Between 1 April 2013 and 31 March 2017, there were 32 320 separate episodes of AKI in 19 924 eligible individuals aged ≥18 years and not on renal replacement therapy (RRT). Of these, 21 712 (67.2%) were AKI Stage 1, 4880 (15.1%) were AKI Stage 2 and 5728 (17.7%) were AKI Stage 3 (Table 1). The mean number of AKI episodes per month was 673 (SD 41). The mean age of patients at each AKI episode was 71.0 (SD 16.9) and 52% were female. Totally, 18 227 AKI episodes occurred in the community and 14 093 were acquired in hospital. The overall mortality within 30 days and 90 days of an episode of AKI was 15.9% and 22.8%, respectively.
Table 1.
Characteristics of AKI
| Community | Hospital | Total | |
|---|---|---|---|
| n = 18 227 | n = 14 093 | n = 32 320 | |
| AKI Stage 1 | 10908 | 10804 | 21712 |
| AKI Stage 2 | 2813 | 2067 | 4880 |
| AKI Stage 3 | 4506 | 1222 | 5728 |
| Mean (SD) age, years | |||
| AKI Stage 1 | 67.23 (19.1) | 74.91 (14.7) | 71.05 (17.4) |
| AKI Stage 2 | 70.44 (17.0) | 73.39 (14.6) | 71.69 (16.1) |
| AKI Stage 3 | 69.54 (15.8) | 71.57 (14.6) | 69.97 (15.6) |
| All AKI | 68.30 (18.0) | 74.39 (14.7) | 70.96 (16.9) |
| Sex, M (%)/F (%) | |||
| AKI Stage 1 | 4605 (42.2)/6303 (57.8) | 5277 (48.8)/5527 (51.2) | 9882 (45.5)/11830 (54.5) |
| AKI Stage 2 | 1289 (45.8)/1524 (54.2) | 953 (46.1)/1114 (53.9) | 2242 (45.9)/2638 (54.1) |
| AKI Stage 3 | 2842 (63.1)/1664 (36.9) | 652 (53.4)/570 (46.6) | 3494 (61)/2234 (39) |
| All AKI | 8736 (47.9)/9491 (52.1) | 6882 (48.8)/7211 (51.2) | 15618 (48.3)/16702 (51.7) |
| 30-day mortality post AKI (%) | 2201 (12.1) | 2926 (20.8) | 5127 (15.9) |
| 90-day mortality post AKI (%) | 3318 (18.2) | 4048 (28.7) | 7366 (22.8) |
AKI
Results from the segmented regression analysis showed there were no prior trends up or down in any of the outcomes. Following the intervention, there was no evidence of an immediate impact, known as the level change, on the rate of AKI intervention [incidence rate ratio (IRR) 0.972, 95% confidence interval (CI) 0.908–1.041]. There was also no change in the trend of AKI rates (IRR 0.996 per month, 95% CI 0.991–1.001). Similarly, there was no change in the level (IRR 1.005, 95% CI 0.934–1.081) or trend (IRR 0.995 per month, 95% CI 0.990–1.000) of Stages 2–3 AKI (Table 2, Figure 1). Subgroup analysis found no change in the rate of total AKI in the community (level change IRR 1.022, 95% CI 0.931–1.122; trend change IRR 0.998 per month, 95% CI 0.991–1.005) or in the rate of severe AKI in the community (Stages 2–3 AKI IRR level change 1.037, 95% CI 0.945–1.138; trend change IRR 0.996 per month, 95% CI 0.990–1.003). Similarly, for hospital-acquired AKI, there was no change in the rate of total AKI (level change IRR 0.929, 95% CI 0.841–1.027; trend change IRR 0.994 per month, 95% CI 0.986–0.002) or in the rate of severe AKI (Stage 2–3 IRR level change 0.939, 95% CI 0.815–1.082; trend change 0.991 per month, 95% CI 0.981–1.001).
Table 2.
Association of AKI e-alerts implementation with changes in AKI, mortality associated with AKI and occupied hospital days per patient
| Variable | Baseline trend | Level change | Trend change | |
|---|---|---|---|---|
| Results using the Poisson model with number of unique AKI events as outcome | ||||
| Total AKI episodes | IRR (95% CI) | 1.003 (0.999 to 1.006) | 0.972 (0.908 to 1.041) | 0.996 (0.991 to 1.001) |
| P-value | 0.118 | 0.425 | 0.086 | |
| Stages 2–3 AKI episodes | IRR (95% CI) | 1.002 (0.999 to 1.006) | 1.005 (0.934 to 1.081) | 0.995 (0.990 to 1.000) |
| P-value | 0.188 | 0.898 | 0.061 | |
| Community-acquired total AKI episodes | IRR (95% CI) | 1.001 (0.996 to 1.005) | 1.022 (0.931 to 1.122) | 0.998 (0.991 to 1.005) |
| P-value | 0.793 | 0.645 | 0.616 | |
| Community-acquired Stages 2–3 AKI episodes | IRR (95% CI) | 1.002 (0.997 to 1.006) | 1.037 (0.945 to 1.138) | 0.996 (0.990 to 1.003) |
| P-value | 0.477 | 0.450 | 0.311 | |
| Hospital-acquired total AKI episodes | IRR (95% CI) | 1.004 (0.999 to 1.009) | 0.929 (0.841 to 1.027) | 0.994 (0.986 to 1.002) |
| P-value | 0.113 | 0.158 | 0.130 | |
| Hospital-acquired Stages 2–3 AKI episodes | IRR (95% CI) | 1.004 (0.997 to 1.011) | 0.939 (0.815 to 1.082) | 0.991 (0.981 to 1.001) |
| P-value | 0.250 | 0.389 | 0.097 | |
| Results using Poisson model with number of deaths within 30 and 90 days from AKI detection as outcome | ||||
| 30-day mortality | IRR (95% CI) | 1.008 (0.999 to 1.016) | 0.878 (0.757 to 1.017) | 0.998 (0.987 to 1.009) |
| P-value | 0.084 | 0.091 | 0.688 | |
| 90-day mortality | IRR (95% CI) | 1.006 (1.000 to 1.012) | 0.918 (0.828 to 1.018) | 0.999 (0.991 to 1.006) |
| P-value | 0.075 | 0.111 | 0.745 | |
| Results using the linear model with occupied hospital bed days per month per patient with AKI as outcome | ||||
| Occupied hospital bed days | Beta (95% CI) | −0.015 (−0.038 to 0.008) | 0.774 (0.293 to 1.255) | −0.059 (−0.094 to −0.025) |
| P-value | 0.200 | 0.003 | 0.002 | |
Mortality
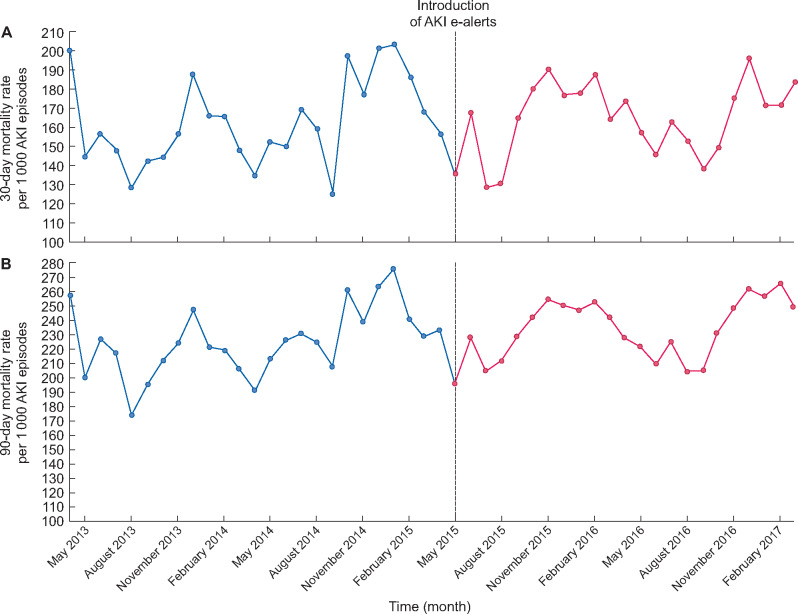
There was no change in the 30-day mortality associated with an AKI episode following the intervention (level change IRR 0.878, 95% CI 0.757–0.017, P = 0.091; trend change IRR 0.998, 95% CI 0.987–1.009, P = 0.688) (Figure 2A). Similarly, there was no change in 90-day mortality (level change IRR 0.918, 95% CI 0.828–1.018, P = 0.111; trend change IRR 0.999, 95% CI 0.991–1.006, P = 0.745) (Figure 2B).
FIGURE 2:
Rate of (A) 30-day mortality and (B) 90-day mortality associated with AKI between 1 April 2013 and 31 March 2017 in NHS Tayside.
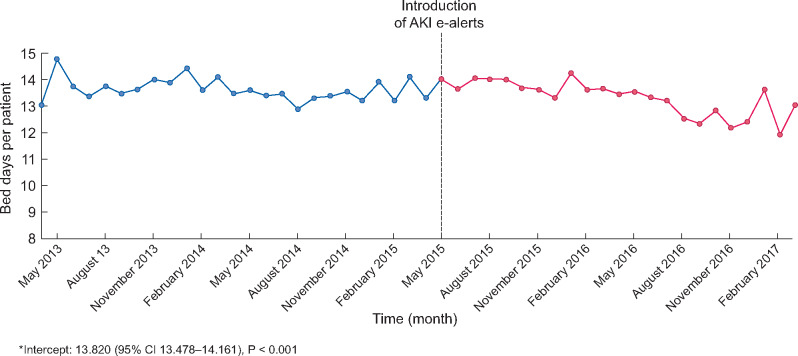
Occupied hospital bed days
There was a small reduction in occupied bed days per patient per month for patients with AKI following the implementation of e-alerts (β-coefficient −0.059, 95% CI −0.094 to −0.025, P = 0.002) (Figure 3).
FIGURE 3:
Bed days per patient per month for patients with AKI between 1 April 2013 and 31 March 2017 in NHS Tayside.
DISCUSSION
In this large observational study of all AKI episodes in adults in a population of >400 000 people over a 4-year period, we found that the introduction of AKI e-alerts for patients in hospital and in the community had no significant effect on the rate and severity of AKI, or on the mortality associated with AKI. There was a small reduction in occupied hospital bed days by people with AKI, which may not be clinically significant.
Previous studies have suggested a benefit from using AKI e-alerts on care processes including a reduction in time taken to modify or discontinue medications [17], and an increase in fluid assessments and prescribing in hospital [18, 19]. We have shown in a previous study that the introduction of AKI e-alerts in the community led not only to an increase in creatinine monitoring [20], but also to higher rates of hospitalization. Several studies have reported reductions in mortality associated with AKI following the introduction of e-alerts [21, 22] but have evaluated impact using an uncontrolled before or after comparison, which is at high risk of bias because it does not account for any underlying trend in the data (unlike the ITS analysis used in this study). In a single-centre randomized trial, evaluating the impact of AKI e-alerts, Wilson et al. found no reduction in mortality or in the need for renal replacement therapy [23]. Selby et al. [24] conducted a pragmatic stepped wedge cluster randomized trial in which e-alerts were introduced alongside an AKI bundle and education programme across five UK hospitals over sequential 3-month time periods. They found no improvement in 30-day mortality associated with AKI after the intervention. Furthermore, they demonstrated a reduced length of stay following the intervention, particularly in those with longer admissions.
While early recognition of AKI is desirable, it remains unclear whether AKI e-alerts improve patient outcomes. One reason could be that e-alerts based solely on creatinine rise (like the NHS England algorithm we used) are a late marker of disease since they may not rise until 48–72 h after kidney injury [25]. Reduction in urine output precedes creatinine rise and is part of the KDIGO diagnostic criteria for AKI, but is not recorded in the community and is often poorly recorded in hospital. Utilizing biomarkers that rise earlier in AKI may facilitate more timely intervention [26] but are not yet widely available in clinical practice. One study, however, examining the impact of AKI e-alerts in patients who were also reviewed by critical care (but based on physiological deterioration, not AKI) found that patients reviewed on the day the e-alert was triggered had a significantly improved mortality and reduction in need for RRT compared with patients reviewed by critical care on any subsequent day [27]. This suggests that changes in serum creatinine do provide a window of opportunity for intervention.
A second reason why e-alerts may not be effective is that they do not actually change clinical practice. In the NHS Tayside AKI e-alert, clinicians are encouraged to record their response, but it is not mandatory. We tested the use of an AKI care bundle in our acute medical admissions unit that used a sticker added to the notes of patients with AKI to demonstrate their care was in keeping with the guidance, but due to poor compliance, this was not adopted in other areas [12]. Notably, the results system delivering the AKI e-alert could continue to be used as normal whether the AKI care bundle was completed or not. In a meta-regression analysis, Roshanov et al. [28] found that requiring clinicians to provide a reason for overriding advice significantly improved the effectiveness of computerized clinical decision support systems in changing processes of care. Kolhe et al. [29] described an interruptive AKI e-alert that required clinicians to complete an AKI care bundle before they could request further blood tests or order medications unless a reason was given to override the alert. They found that this e-alert improved compliance with the AKI care bundle and that patients with the care bundle complete had a lower mortality and reduced progression of AKI to higher stages compared with a propensity score-matched group of patients with AKI where the care bundle was not complete [30]. McCoy et al. [31] examined the role of AKI e-alerts on medications management and found that while passive alerts had no significant impact, interruptive alerts led to medications being altered or discontinued more quickly. Passive AKI e-alerts, including the one used in this study, may be more likely to lead to alert fatigue, were clinicians exposed to a large number of alerts become desensitized to them. Notably, while no results reached statistical significance, the IRR was <1 for change in trend of AKI rates for all analyses (total AKI rate and severe AKI rate in hospital and in the community). The change in trend for severe AKI almost reached statistical significance (IRR 0.995 per month, P = 0.061), driven particularly by the impact on severe AKI in hospital (IRR 0.991 per month, P = 0.097). Clinicians in the community do not routinely review blood results on the day they are sent, so are less well placed to respond an AKI e-alert in a timely manner. A more assertive, interruptive e-alert may be more effective, particularly in AKI Stage 1, where early intervention could prevent progression to more severe AKI.
A strength of our study was that we used a robust quasi-experimental design with a prolonged study period and clear intervention time. This allowed us to control for trends before the intervention and adjust for common biases such as seasonality and autocorrelation. AKI was defined using the KDIGO definition and the rate and stage of AKI were calculated using all available creatinine results from the study period, rather than by simply counting e-alerts before and after the intervention. We included all patients in hospital and in the community. Weaknesses include that we were unable to examine whether AKI e-alerts led to changed management of people with AKI. In addition, the population of the Tayside region of Scotland is predominantly of European ancestry with relatively low levels of ethnic diversity, which may limit the generalizability of our findings.
CONCLUSION
We have demonstrated that the introduction of a whole-system AKI e-alert and educational programme did not have a significant impact on the rate, severity or mortality associated with AKI. There was a small reduction in bed days per patient per month for patients with AKI associated with e-alert introduction. Our findings are consistent with other evidence from a single trial and other more robust observational studies, and add to the literature by examining impact of AKI e-alerts in the community as well as in hospital in a defined geographical population. Further research focusing on how AKI e-alerts alter clinicians’ behaviour and an evaluation of the impact this has on patient care is required to fully understand their effect on outcomes. In addition, work on how the delivery of the e-alert can be optimized is needed prior to further implementation. Evaluation of more active alerts in Stage 1 AKI would be of particular value.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
FUNDING
This work was funded by NHS Tayside Renal Research Endowment Fund.
AUTHORS’ CONTRIBUTIONS
S.B. conceived the idea. D.B., N.D.S., B.G. and S.B. designed the study. D.B., N.D.S., R.L. and H.W. acquired and analysed the data. D.B. and N.D.S. drafted the article. S.B. is guarantor for the study. All authors revised the article critically for important intellectual content and approved the final version of the manuscript. All authors had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis. S.B. attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
CONFLICT OF INTEREST STATEMENT
None declared.
Supplementary Material
REFERENCES
- 1.National Institute for Health and Care Excellence. Acute Kidney Injury: Prevention, Detection and Management Clinical guideline [CG169]. https://www.nice.org.uk/guidance/cg169 (10 July 2019, date last accessed) [PubMed]
- 2. Bucaloiu ID, Kirchner HL, Norfolk ER. et al. Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int 2012; 81: 477–485 [DOI] [PubMed] [Google Scholar]
- 3. Linder A, Fjell C, Levin A. et al. Small acute increases in serum creatinine are associated with decreased long-term survival in the critically ill. Am J Respir Crit Care Med 2014; 189: 1075–1081 [DOI] [PubMed] [Google Scholar]
- 4. Bell S, Dekker FW, Vadiveloo T. et al. Risk of postoperative acute kidney injury in patients undergoing orthopaedic surgery–development and validation of a risk score and effect of acute kidney injury on survival: observational cohort study. BMJ 2015; 351: h5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coca SG, Singanamala S, Parikh CR.. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int 2012; 81: 442–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yang L, Xing G, Wang L. et al. Acute kidney injury in China: a cross-sectional survey. Lancet 2015; 386: 1465–1471 [DOI] [PubMed] [Google Scholar]
- 7. Lachance P, Villeneuve PM, Rewa OG. et al. Association between e-alert implementation for detection of acute kidney injury and outcomes: a systematic review. Nephrol Dial Transplant 2017; 32: 265–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haase M, Kribben A, Zidek W. et al. Electronic alerts for acute kidney injury. Dtsch Arztebl Int 2017; 114: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.NHS England. Acute Kidney Injury (AKI) Programme, 2014. https://www.thinkkidneys.nhs.uk/ (10 July 2019, date last accessed)
- 10.NHS England. Acute Kidney Injury (AKI) Algorithm, 2014. https://www.england.nhs.uk/akiprogramme/aki-algorithm/ (10 July 2019, date last accessed)
- 11. Norris R, Sloan J, Bell S.. NHS Tayside: Acute Kidney Injury, 2017. https://www.youtube.com/watch? v=gW0pgXrIdgo&t=3s (10 October 2019, date last accessed)
- 12. Logan R, Davey P, Davie A. et al. Care bundles for acute kidney injury: a balanced accounting of the impact of implementation in an acute medical unit. BMJ Open Qual 2018; 7: e000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.University of Dundee. Health Informatics Centre. https://www.dundee.ac.uk/hic (10 July 2019, date last accessed)
- 14.Public Health Scotland. The Scottish Renal Registry. https://www.srr.scot.nhs.uk/ (10 July 2019, date last accessed)
- 15.KDIGO AKI Work Group. Section 2: AKI definition. Kidney Int Suppl 2012; 2: 19–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wagner AK, Soumerai SB, Zhang F. et al. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther 2002; 27: 299–309 [DOI] [PubMed] [Google Scholar]
- 17. Rind DM, Safran C, Phillips RS. et al. Effect of computer-based alerts on the treatment and outcomes of hospitalized patients. Arch Intern Med 1994; 154: 1511–1517 [PubMed] [Google Scholar]
- 18. Ebah L, Hanumapura P, Waring D. et al. A multifaceted quality improvement programme to improve acute kidney injury care and outcomes in a large teaching hospital. BMJ Quality 2017; 6: u219176.w7476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Colpaert K, Hoste EA, Steurbaut K. et al. Impact of real-time electronic alerting of acute kidney injury on therapeutic intervention and progression of RIFLE class. Crit Care Med 2012; 40: 1164–1170 [DOI] [PubMed] [Google Scholar]
- 20. Aiyegbusi O, Witham MD, Lim M. et al. Impact of introducing electronic acute kidney injury alerts in primary care. Clin Kidney J 2019; 12: 253–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chandrasekar T, Sharma A, Tennent L. et al. A whole system approach to improving mortality associated with acute kidney injury. QJM 2017; 110: 657–666 [DOI] [PubMed] [Google Scholar]
- 22. Al-Jaghbeer M, Dealmeida D, Bilderback A. et al. Clinical decision support for in-hospital AKI. J Am Soc Nephrol 2018; 29: 654–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wilson FP, Shashaty M, Testani J. et al. Automated, electronic alerts for acute kidney injury: a single-blind, parallel-group, randomised controlled trial. Lancet 2015; 385: 1966–197425726515 [Google Scholar]
- 24. Selby NM, Casula A, Lamming L. et al. An organizational-level program of intervention for AKI: a pragmatic stepped wedge cluster randomized trial. J Am Soc Nephrol 2019; 30: 505–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Portilla D, Dent C, Sugaya T. et al. Liver fatty acid-binding protein as a biomarker of acute kidney injury after cardiac surgery. Kidney Int 2008; 73: 465–472 [DOI] [PubMed] [Google Scholar]
- 26. Slocum JL, Heung M, Pennathur S.. Marking renal injury: can we move beyond serum creatinine? Transl Res 2012; 159: 277–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Prendecki M, Blacker E, Sadeghi-Alavijeh O. et al. Improving outcomes in patients with acute kidney injury: the impact of hospital based automated AKI alerts. Postgrad Med J 2016; 92: 9–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Roshanov PS, Fernandes N, Wilczynski JM. et al. Features of effective computerised clinical decision support systems: meta-regression of 162 randomised trials. BMJ 2013; 346: f657. [DOI] [PubMed] [Google Scholar]
- 29. Kolhe NV, Staples D, Reilly T. et al. Impact of compliance with a care bundle on acute kidney injury outcomes: a prospective observational study. PLoS One 2015; 10: e0132279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kolhe NV, Reilly T, Leung J. et al. A simple care bundle for use in acute kidney injury: a propensity score-matched cohort study. Nephrol Dial Transplant 2016; 31: 1846–1854 [DOI] [PubMed] [Google Scholar]
- 31. McCoy AB, Waitman LR, Gadd CS. et al. A computerized provider order entry intervention for medication safety during acute kidney injury: a quality improvement report. Am J Kidney Dis 2010; 56: 832–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.