Abstract
Background
The harm caused by the long interdialytic interval in three-times-per-week haemodialysis regimens (3×WHD) may relate to fluid accumulation and associated high ultrafiltration rate (UFR). Four-times-per-week haemodialysis (4×WHD) may offer a solution, but its impact on mortality, hospitalization and vascular access complications is unknown.
Methods
From the AROii cohort of incident in-centre haemodialysis patients, 3×WHD patients with a UFR >10 mL/kg/h were identified. The hazard for the outcomes of mortality, hospitalization and vascular access complications in those who switched to 4×WHD compared with staying on 3×WHD was estimated using a marginal structural Cox proportional hazards model. Adjustment included baseline patient and treatment characteristics with inverse probability weighting used to adjust for time-varying UFR and cardiovascular comorbidities.
Results
From 10 637 European 3×WHD patients, 3842 (36%) exceeded a UFR >10 mL/kg/h. Of these, 288 (7.5%) started 4×WHD and at baseline were more comorbid. Event rates while receiving 4×WHD compared with 3×WHD were 12.6 compared with 10.8 per 100 patient years for mortality, 0.96 compared with 0.65 per year for hospitalization and 14.7 compared with 8.0 per 100 patient years for vascular access complications. Compared with 3×WHD, the unadjusted hazard ratio (HR) for mortality on 4×WHD was 1.05 [95% confidence interval (CI) 0.78–1.42]. Following adjustment for baseline demographics, time-varying treatment probability and censoring risks, this HR was 0.73 (95% CI 0.50–1.05; P = 0.095). Despite these adjustments on 4×WHD, the HR for hospitalization remained elevated and vascular access complications were similar to 3×WHD.
Conclusions
This observational study was not able to demonstrate a mortality benefit in patients switched to 4×WHD. To demonstrate the true benefits of 4×WHD requires a large, well-designed clinical trial. Our data may help in the design of such a study.
Keywords: four-times-per-week haemodialysis, hospitalization, survival analysis, ultrafiltration, vascular access
INTRODUCTION
For the majority of in-centre haemodialysis (HD) patients, a three-times-per-week HD (3×WHD) schedule is unphysiological with interdialytic intervals of between 48 and 72 h. Harm associated with the accumulation of uraemic toxics, potassium and fluid may manifest in increases in arrhythmias [1], cardiac failure [2], hospitalization, mortality and symptom burden [3–5]. More intensive HD theoretically mitigates some of these harms. Observational data suggest that 3×WHD with a longer session length is associated with improved overall survival, but increases in mortality after the long interdialytic interval persist [6]. Obtaining outcome data on benefits of more frequent dialysis through clinical trials has proven challenging [7]. Six-times-per-week HD may be clinically beneficial and cost effective but is only practical in a subgroup of patients [8–11].
Recent analyses suggest that compared with the short interdialytic interval, the longer 2-day interdialytic interval is associated with increases in hospitalization and mortality of 80–100% [12]. Specifically targeting this period with an additional dialysis session may be attractive both to providers and to patients: one-fifth of patients who report being bothered by their fluid restriction state that they would accept an additional HD session if offered it [13]. Four-times-per-week HD (4×WHD) is the third most commonly prescribed HD frequency after three- and two-times-per-week schedules; however, previous evaluations have combined 4×WHD with other augmented regimes such as extended-hours dialysis, making the impact of the discrete removal of the long interdialytic interval challenging [14].
We present a target trial observational data analysis where a large dataset was used to emulate the desired clinical trial with inclusion and exclusion criteria, follow-up time, adherence and endpoints reflecting benefits and harms [15, 16]. Identifying high-risk individuals using the ultrafiltration rate (UFR) representing the clinical driver to initiate 4×WHD [17], it employs marginal structural modelling [18] to address the relationship between UF and the risk of initiating of 4×WHD and mortality, which may lead to biased estimates of the benefits of treatment. This study design recognizes the clinical indications to offer a 3×WHD patient a regular additional session, appropriately factoring in the variables that are associated with the time-varying risk of events the clinician and patient are trying to avoid.
MATERIALS AND METHODS
Cohort and data
The Analyzing Data, Recognizing Excellence and Optimizing Outcomes (ARO) cohort was a prospective observational cohort study of electronic medical records capturing anonymized longitudinal individual-level data for incident HD patients enrolled at 1 of the 312 Fresenius Medical Care (FMC) facilities across 15 European countries between 2007 and 2009 and followed up until the end of 2014. All local ethical and regulatory obligations concerning patient data for each of the 15 participating countries were met. These approvals encompass subsequent analyses including those described here. Informed consent was obtained from all patients by FMC (Europe).
Data on demographics, comorbidities, laboratory results, hospitalizations, mortality and individual HD sessions were captured [19]. The presence of 11 comorbid conditions was identified using International Classification of Diseases, Tenth revision (ICD-10) codes from administrative data using existing schema (ischaemic heart disease, congestive heart failure, cerebrovascular accident, peripheral vascular disease, other cardiac disease, chronic obstructive pulmonary disease, gastrointestinal bleeding, liver disease, dysrhythmia, cancer and diabetes [20]). Hospitalization was defined as an admission to hospital lasting at least 1 day. Within these hospitalizations, ICD-10 codes for vascular access complications were identified (see Supplementary data, Table S1, for associated ICD-10 codes).
Eligibility, exposure, adherence and follow-up
The inclusion criteria of an UFR >10 mL/kg/h was based on the progressive mortality increase associated with this range of UF [17]. Patients were classed as eligible for 4×WHD and included in the analysis from the point they exceeded this UFR across three HD sessions while prescribed 3×WHD. The UFR was calculated for each session from the recorded difference in pre- and post-dialysis weights divided by treatment time in minutes. There were no clinical exclusion criteria. Exposure to 4×WHD was classed as receiving four HD sessions in week 1, four sessions per week 4 weeks later and a further four sessions per week 2 weeks after that. This was in order to address any incorrect identification of 4×WHD associated with rescheduling HD for elective admissions or ad hoc HD to address specific issues. Adherence was assessed while receiving 3×WHD and 4×WHD using the mean number of delivered sessions per week. Attendance for HD was defined as the presence of a recording for blood pressure, pre-dialysis weight and HD treatment time. Follow-up was up to 3 years from the date of first eligibility or until censoring for transplantation, moving to a non-FMC facility, changing dialysis modality, withdrawal of consent or death.
Statistical methods
During the course of the analysis, an individual’s dialysis frequency (moving from 3×WHD to 4×WHD) and the clinical parameters that are associated with this change in frequency and also the outcomes (e.g. increasing UFR) vary. Marginal structural Cox proportional hazards models were used to estimate the time-varying association between dialysis frequency (4×WHD versus 3×WHD) and the endpoints of mortality, hospitalization and hospitalization for vascular access complications. Marginal structural models are designed to account for confounding introduced by time-varying clinical parameters and their response to treatment over time by weighting an individual patient’s observations to create a pseudo-population where time-varying covariates are more evenly distributed between treatment arms. They still assume there are no unmeasured confounders. First, logistic regression was used to obtain probabilities of treatment (switching from 3×WHD to 4×WHD) and censoring (transplantation or being lost to follow-up) for each month from inclusion in the study by meeting the eligibility until the end of follow-up [18, 21]. Baseline covariates (comorbidities at eligibility, achieved dialysis session duration, dialysis catheter use, equilibrated Kt/V, time on dialysis, age, serum phosphate level and post-dialysis weight) and time-varying covariates (UFR, systolic blood pressure and the comorbidities of congestive heart failure and ischaemic heart disease, which varied within individuals during their follow-up) were included. Continuous variables were split by quantiles into five equal groups. Probabilities from these logistic regression models were converted into weights by dividing the probabilities estimated from the baseline covariates (numerator) by the probabilities estimated using the baseline and time-varying covariates (denominator). Treatment and censoring weights were calculated separately and multiplied together, resulting in stabilized weights with a mean of 1.006 [standard deviation (SD) 0.174]. The final marginal structural models were adjusted for baseline covariates because they appeared in both the numerator and the denominator of the stabilized weights [18, 21]. The time-varying data include a variable reflecting if the patient is receiving 3×WHD or 4×WHD and weights were set to 1 following the initiation of 4×WHD [18]. The hazard for the endpoints associated with the time-varying exposure to 4×WHD is reported following sequential adjustment: (1) adjusted for baseline covariates, which leaves residual confounding, because clinical parameters and their response to treatment vary over time; (2) adjusted for time-varying covariates using inverse probability weighting for treatment; and (3) employing weighting to address the time-varying risk of being censored by transplantation or lost to follow-up, which may be associated with the outcomes of interest. Proportional hazards assumptions were assessed graphically using Schoenfeld residuals. When time-varying laboratory and HD data were missing we used the last-observation-carried forward approach (2.97% and 1.42% of patient follow-up time beyond 35 and 3 days, respectively); as in clinical practice, decisions to initiate 4×WHD may be made from historical observations. Patients with missing data at the time of death were excluded entirely, which only affected 14 patients who never received 4×WHD.
In the main analysis, patients who were exposed to 4×WHD were treated as receiving this until the end of follow-up, as adverse consequences of 4×WHD (which may manifest once a patient returns to 3×WHD) would then be captured. A sensitivity analysis was performed to explore any modification of the effect of 4×WHD accounting for patients who receive the treatment but return to 3×WHD, whereby patients who moved from 4×WHD back to 3×WHD were treated as on 3×WHD from 3 months after the observed treatment switch. Reverting to 3×WHD was defined as 3 consecutive non-hospitalized weeks receiving ≤3 sessions per week. This lagged per-protocol analysis assigned any events occurring within the 3 months of switching from 4×WD back to 3×WHD to the 4×WHD treatment. Statistical analyses were conducted in R version 3.4.4 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Patient characteristics
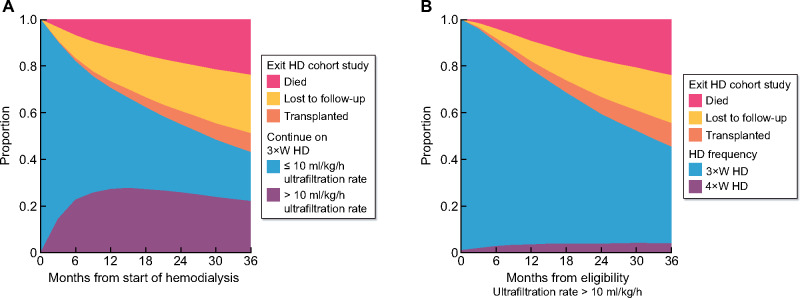
A total of 10 637 incident HD patients were screened for inclusion in the study, with 4009 (37.7%) patients meeting the inclusion criteria of an average UFR across three HD sessions >10 mL/kg/h following a median of 6.1 months of HD. Figure 1A shows the proportion of patients on 3×WHD with a UFR <10 mL/kg/h, 3×WHD with a UFR >10 mL/kg/h (eligible for 4×WHD), transplanted, lost to follow-up or died. The prevalence of patients eligible for 4×WHD stabilizes at 20–25% following 12 months of HD.
FIGURE 1:
(A) The prevalence of patients meeting the inclusion criteria (10 mL/kg/h UFR) for the four-times-per-week target trial. (B) The proportion of patients meeting the inclusion criteria who go on to receive 4×WHD, and other competing events.
Having exceeded a UFR of 10 mL/kg/h and classed as eligible for 4×WHD, 7.5% of patients subsequently went on to receive 4×WHD and were suitable for analysis. The prevalence of patients receiving 4×WHD stabilized at 4% after ~12 months, as new patients commenced 4×WHD and patients on 4×WHD left the study (Figure 1B). Patient flow through the screening process, inclusion criteria, subsequent treatment and inclusion in the analysis are shown in Figure 2. The demographics of patients who remained on 3×WHD or subsequently went onto 4×WHD having met the inclusion criteria are illustrated in Table 1, showing baseline differences in age (62.5 versus 60.8 years), diabetes (34.7% versus 48.3%), weight (64.5 and 68.2 kg) and heart failure (16.4% versus 21.5%). The numbers of patients from each country are available in the Supplementary Materials.
FIGURE 2:
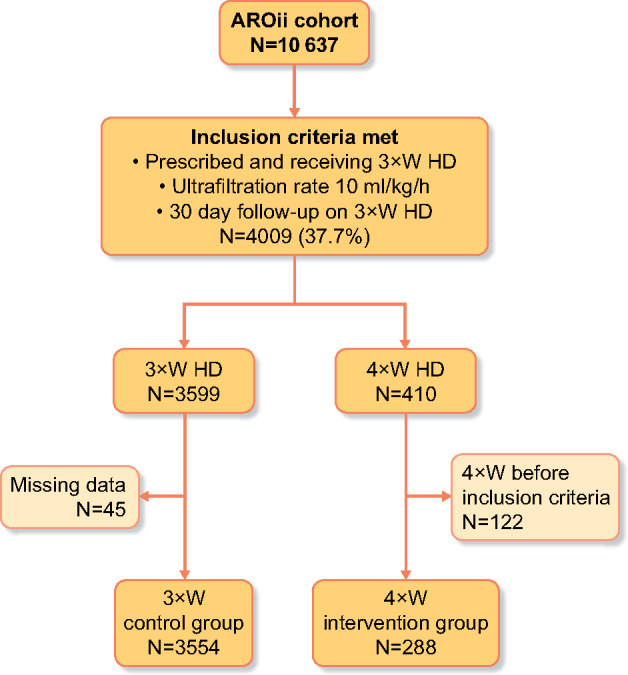
Incident patients screened throughout their follow-up and their flow through the analysis.
Table 1.
Demographics of patients at inclusion (10 mL/kg/hr UFR) and at exposure to 4×WHD
| Characteristics | 3×WHD at eligibility | 4×WHD at eligibility | 4×WHD at initiation |
|---|---|---|---|
| Patients, n | 3554 | 288 | 288 |
| Age (years), mean (SD) | 62.5 (14.9) | 60.8 (15.3) | 62.40 (15.37) |
| Male, n (%) | 2148 (60.4) | 159 (55.2) | 159 (55.2) |
| Days on dialysis, mean (SD) | 355.2 (417.0) | 221.7 (221.6) | 803.9 (596.6) |
| Ischaemic heart disease, n (%) | 563 (15.8) | 40 (13.9) | 52 (18.1) |
| Cancer, n (%) | 260 (7.3) | 22 (7.6) | 26 (9.0) |
| Heart failure, n (%) | 582 (16.4) | 62 (21.5) | 71 (24.7) |
| Chronic obstructive pulmonary disease, n (%) | 234 (6.6) | 20 (6.9) | 23 (8.0) |
| Cerebrovascular disease, n(%) | 350 (9.8) | 34 (11.8) | 40 (13.9) |
| Depression, n (%) | 69 (1.9) | 8 (2.8) | 8 (2.8) |
| Diabetes, n (%) | 1232 (34.7) | 139 (48.3) | 142 (49.3) |
| Arrhythmia, n (%) | 335 (9.4) | 37 (12.8) | 50 (17.4) |
| Gastrointestinal disease, n (%) | 76 (2.1) | 5 (1.7) | 6 (2.1) |
| Liver disease, n (%) | 132 (3.7) | 13 (4.5) | 15 (5.2) |
| Other cardiac disease, n (%) | 39 (1.1) | 5 (1.7) | 7 (2.4) |
| Peripheral vascular disease, n (%) | 542 (15.3) | 43 (14.9) | 68 (23.6) |
| Ultrafiltration volume (L), mean (SD) | 2.72 (1.00) | 2.92 (0.80) | 2.48 (1.01) |
| UFR (mL/kg/h), mean (SD) | 11.00 (3.59) | 11.37 (2.83) | 9.61 (3.72) |
| Equilibrated Kt/V, mean (SD) | 1.42 (0.30) | 1.36 (0.30) | 1.44 (0.29) |
| Phosphate, mean (SD) | 1.55 (0.47) | 1.61 (0.48) | 1.54 (0.52) |
| HD session duration (min), mean (SD) | 231.91 (16.98) | 228.95 (17.46) | 230.59 (20.50) |
| Loop diuretic use, n(%) | 329 (9.3) | 34 (11.8) | 37 (12.8) |
| Weight (kg), mean (SD) | 64.48 (12.83) | 68.22 (14.13) | 68.09 (14.24) |
| Systolic blood pressure (mmHg), mean (SD) | 137.16 (22.90) | 141.10 (23.96) | 138.81 (25.58) |
| Diastolic blood pressure (mmHg), mean (SD) | 71.17 (13.72) | 71.64 (13.96) | 69.42 (14.71) |
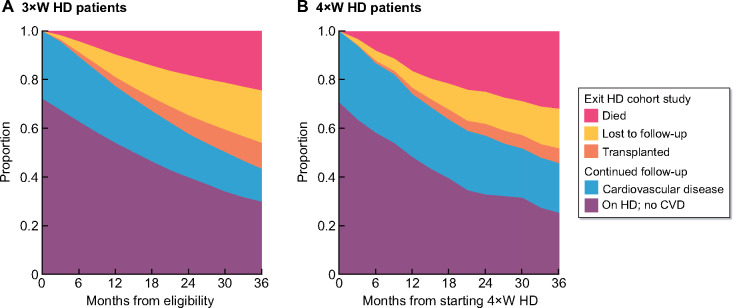
Both at baseline and during follow-up, there were differences in the prevalence of cardiovascular comorbidity and the proportion of patients who were transplanted or lost to follow-up between the time patients received 3×WHD (Figure 3A) and 4×WHD (Figure 3B). These time-varying associations support the use of inverse probability weighting methods for treatment and censoring. The demographics of patients according to their reasons for exiting the study are listed in Supplementary data, Table S2.
FIGURE 3:
The prevalence of comorbid conditions and censoring events according to dialysis frequency: (A) 3×WHD and (B) 4×WHD.
Treatment initiation and adherence
In those who received it, 4×WHD was initiated a median of 12.5 months from the time patients first became eligible. From 6 weeks following the initiation of 4×WHD treatment, the median time from the initiation of 4×WHD to the end of follow-up or 3 consecutive weeks receiving less than four sessions per week was 6.1 months. For the duration of follow-up, the average number of sessions delivered per week, excluding hospitalized time, was 3.46 sessions per week in the 4×WHD arm and 2.96 sessions per week in the 3×WHD arm. The mean session duration was 229 min while receiving 4×WHD and 235 min while receiving 3×WHD.
Associations with mortality, hospitalization and vascular access complications
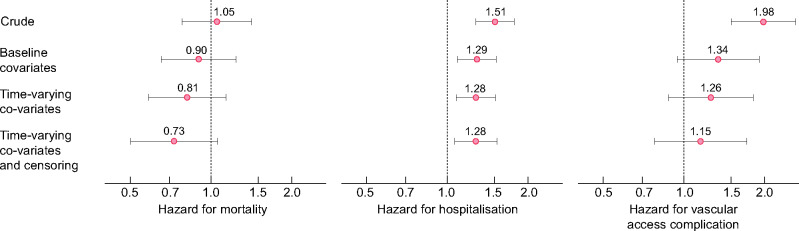
The crude mortality rate during follow-up was 10.8 per 100 patient years [95% confidence interval (CI) 10.1–11.6] while receiving 3×WHD and 12.6 per 100 patient years (95% CI 9.3–16.7) following the start of 4×WHD. The univariate hazard for survival while receiving 4×WHD compared with 3×WHD was 1.05 (95% CI 0.78–1.42; P = 0.735). The hazard ratio (HR) was 0.90 (95% CI 0.65–1.24; P = 0.518) after adjustment for baseline covariates and the final multivariable adjusted HR was 0.81 (95% CI 0.58–1.14; P = 0.229) after adjusting for time-varying covariates using inverse probability weighting for treatment. After weighting for censoring risk, this multivariable HR was 0.73 (95% CI 0.50–1.06; P = 0.096). These sequential adjustments are shown in Figure 4A.
FIGURE 4:
HR of 4×WHD compared with 3×WHD for the endpoints of mortality, hospitalization and vascular access complication. Adjustment for baseline covariates (comorbidities at eligibility, dialysis session duration, dialysis access type, equilibrated Kt/V, time on dialysis, age, serum phosphate level and post-dialysis weight) and time-varying covariates (UFR, systolic blood pressure and the comorbidities of congestive heart failure and ischaemic heart disease) are sequentially reported.
The crude hospitalization rate during follow-up was 0.65 (95% CI 0.64–0.67) per patient year while receiving 3×WHD and was 0.96 (95% CI 0.86–1.06) per patient year while receiving 4×WHD [univariate HR 1.51 (95% CI 1.28–1.77), P = 0.008]. Following adjustment for baseline and time-varying factors influencing treatment and censoring, 4×WHD had a multivariable HR of 1.28 (95% CI 1.06–1.53; P = 0.008).
The crude vascular access complication rate was 8.0 (95% CI 7.3–8.7) per 100 patient years while receiving 3×WHD and was 14.7 (95% CI 11.1–19.1) per 100 patient years while receiving 4×WHD [univariate HR 1.91 (95% CI 1.50–2.61), P < 0.001]. Sequential adjustments resulted in a final multivariable HR associated with 4×WHD of 1.15 (95% CI 0.78–1.72; P = 0.478) and are shown in Figure 4B and C.
The sensitivity analysis exploring the medium-term impact of switching from 4×WHD back to 3×WHD did not significantly alter the effect sizes for the HR associated with the 4×WHD treatment strategy (Figure 5).
FIGURE 5:
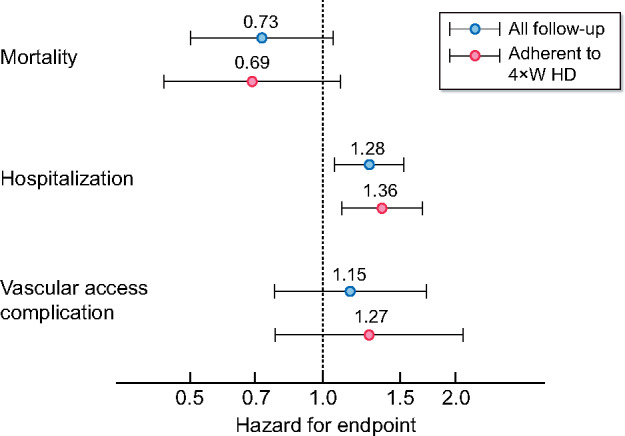
Sensitivity analysis comparing the HR for 4×WHD compared with thr3×WHD when patients who return to 3×WHD are treated as receiving this schedule from 3 months after the switch.
DISCUSSION
This study is the first to explore the association between 4×WHD and endpoints important to clinicians and patients [15, 18, 22]. Thirty-six percent of 3×WHD patients experienced the high UFRs associated with myocardial stunning and increased mortality [17, 23] and are the subjects of performance indicators for dialysis clinics [24]. Despite this, only 7.5% went on to receive 4×WHD for at least 6 weeks. Patient characteristics predicting mortality and transplantation were less favourable in those who received 4×WHD, and accounting for this, 4×WHD was not associated with an improvement in survival. Vascular access complications were comparable following adjustment and hospitalization remained elevated when compared with patients with high UF on 3×WHD.
The favourable survival HR of 0.73 (95% CI 0.50–1.05; P = 0.095) observed with 4×WHD should be cautiously interpreted alongside HRs of 0.54, 3.88 and 0.91 for the 12-month interventions in the Frequent Hemodialysis Network (FHN) Frequent, Nocturnal and ACTIVE (A Clinical Trial of IntensiVE Dialysis) studies, respectively [8, 25, 26]. Statistical bodies and prominent journals recommend that estimates of effects and their margins of error should be interpreted together to inform clinicians and regulatory agencies regarding an intervention, with less reliance on the absolute P-value [27–29]. One of the primary reasons to initiate 4×WHD is to modify the increase in mortality after the 2-day break in 3×WHD [3, 4]. If this short-term increase is reduced to that of the rest of the week compared with a dialysis week with this short-term increase present, the HR is only 0.88 (Appendix 1 of Supplementary Materials).
The potential mechanisms through which the 4×WHD schedule improves outcomes could be through reductions in the UFR and hyperkalaemia, lower time-averaged volume overload and myocardial stunning and reduced arrhythmias in the build up to and during the first HD session of the dialysis week [1, 17, 23, 30, 31]. A previous 12-month randomized trial of alternate-day dialysis with no long interdialytic interval showed improvements in left ventricular mass and systolic blood pressure compared with 3×WHD [32]. The extended-hours intervention (predominantly extended sessions 3×WHD) in the ACTIVE trial showed reductions in left ventricular mass in those individuals who had a reduction in UFR, although UFR and patient survival were not improved in those randomized to extended hours [26, 33]. However, augmented HD may be associated with potential harms: the FHN short daily trial was associated with improved survival in contrast to the nocturnal study’s inferior survival [8, 25], and both tended towards a higher incidence of vascular access complications in the intervention arms [9, 34]. In our analysis, the increase in vascular access events in patients receiving 4×WHD was largely mitigated following adjustment, suggesting the patient characteristics that predict the vascular access complications and the clinical need for 4×WHD are similar.
The strengths of this analysis include the incident nature of the cohort and the use of highly granular data to define inclusion criteria, exposure to the intervention, adherence and outcomes, further capitalized on by the target trial methodology and marginal structural modelling approach. Although less prone to bias, the limitation of these methods is that they deal with observed confounders and residual unobserved differences between patients, which could introduce bias. Other weaknesses include the absence of information on residual kidney function and quality of life. Our definition of 4×WHD means that those who did not adhere within the first 6 weeks were not included. The median duration of 4×WHD was 6 months before the end of follow-up and we are unable to say with confidence what outcomes might be associated with longer treatment or sustained adherence. Our per-protocol analysis excluding follow-up time after 4×WHD patients returned to 3×WHD yielded similar effect sizes and statistical significance as the main analysis and suggests a legacy effect that was observed in the FHN daily trial but not the ACTIVE trial [8, 26].
Building on the existing observational data on UFR, this study could be used to advise patients on potential interventions once a UF threshold of 10 mL/kg/h is reached. However, in our study it took up to a year from patients meeting this threshold to the initiation of 4×WHD, suggesting other factors may inform the decision-making process, such as struggling with fluid restriction and subsequent hospitalization for fluid overload [6]. The mean duration of 6 months for 4×WHD suggests that some clinicians are using this treatment in response to subacute issues that then resolve. The more widespread sustained adoption of 4×WHD would have staffing and capacity implications, which may be offset by the increasing adoption of incremental HD start, with schedules of less than three sessions per week [35]. Other capacity-generating initiatives such as shared- and self-care HD programmes and the more widespread use of home HD may offer solutions [36]. Taken together with the finding of other augmented HD clinical trials [7, 10, 37], our results could help in the design of a prospective trial evaluation of 4×WHD: to demonstrate this HR with an 80% power, α = 0.05 and a 10% transplantation rate at 3 years would require 833 patients per arm, improving to 479 per arm by relaxing α (0.1) and significance to one-sided [38]. Sample sizes could be further reduced by adjustment for baseline variables such as cardiac failure [39], and stratification by suitability for transplantation should be considered.
With the mounting evidence of the range of harms associated with a long interdialytic interval, this study contributes to the supporting evidence for potential solutions; however, appropriately designed studies are required to ensure they are both clinically and cost effective, sustainable and acceptable to the patient.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared upon reasonable request to the corresponding author and approval of the ARO steering group.
SUPPLEMENTARY DATA
Supplementary data are available at ndt online.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Bruno Fouqueray and Karly Louie from Amgen for their assistance with this analysis.
FUNDING
The ARO CKD Research Initiative is a joint observational research commitment from Amgen and FMC (Europe), fully funded by Amgen (Europe), Rotkreuz, Switzerland. This article presents independent research funded by the National Institute for Health Research (NIHR). The views expressed are those of the authors and not necessarily those of the National Health Service, the NIHR or the Department of Health and Social Care. J.F. has received speaker honoraria from FMC and conducts research funded by Vifor Pharma and Novartis. This specific research is funded by a National Institute for Health Research Clinician Scientist Fellowship awarded to J.F. (CS-2015-15-008). J.F. has received consultancy honoraria from Amgen, Fresenius and Vifor. D.C.W. has received consultancy fees from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Janssen, GlaxoSmithKline, Mundipharma, Mitsubishi, Napp and Vifor Fresenius. Results presented in this article have not been published previously in whole or part, except in abstract format.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Roy-Chaudhury P, Tumlin JA, Koplan BA et al. Primary outcomes of the monitoring in dialysis study indicate that clinically significant arrhythmias are common in hemodialysis patients and related to dialytic cycle. Kidney Int 2018; 93: 941–951 [DOI] [PubMed] [Google Scholar]
- 2. Obokata M, Negishi K, Marwick TH et al. Comparison of different interdialytic intervals among hemodialysis patients on their echocardiogram-based cardiovascular parameters. Am Heart J 2015; 169: 523–530.e2 [DOI] [PubMed] [Google Scholar]
- 3. Foley RN, Gilbertson DT, Murray T et al. Long interdialytic interval and mortality among patients receiving hemodialysis. N Engl J Med 2011; 365: 1099–1107 [DOI] [PubMed] [Google Scholar]
- 4. Fotheringham J, Fogarty DG, El Nahas M et al. The mortality and hospitalization rates associated with the long interdialytic gap in thrice-weekly hemodialysis patients. Kidney Int 2015; 88: 569–575 [DOI] [PubMed] [Google Scholar]
- 5. Ju A, Unruh M, Davison S et al. Establishing a core outcome measure for fatigue in patients on hemodialysis: a Standardized Outcomes in Nephrology–Hemodialysis (SONG-HD) consensus workshop report. Am J Kidney Dis 2018; 72: 104–112 [DOI] [PubMed] [Google Scholar]
- 6. Fotheringham J, Sajjad A, Stel VS et al. The association between longer haemodialysis treatment times and hospitalization and mortality after the two-day break in individuals receiving three times a week haemodialysis. Nephrol Dial Transplant 2019; 34: 1577–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dember LM, Lacson E, Brunelli SM et al. The TiME trial: a fully embedded, cluster-randomized, pragmatic trial of hemodialysis session duration. J Am Soc Nephrol 2019; 30: 890–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chertow GM, Levin NW, Beck GJ et al. Long-term effects of frequent in-center hemodialysis. J Am Soc Nephrol 2016; 27: 1830–1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chertow GM, Levin NW, Beck GJ et al. In-center hemodialysis six times per week versus three times per week. N Engl J Med 2010; 363: 2287–2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sergeyeva O, Gorodetskaya I, Ramos R et al. Challenges to enrollment and randomization of the Frequent Hemodialysis Network (FHN) Daily Trial. J Nephrol 2012; 25: 302–309 [DOI] [PubMed] [Google Scholar]
- 11. Liu FX, Treharne C, Arici M et al. High-dose hemodialysis versus conventional in-center hemodialysis: a cost-utility analysis from a UK payer perspective. Value Health 2015; 18: 17–24 [DOI] [PubMed] [Google Scholar]
- 12. Fotheringham J, Smith MT, Froissart M et al. Hospitalization and mortality following non-attendance for hemodialysis according to dialysis day of the week: a European cohort study. BMC Nephrol 2020; 21: 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Flythe JE, Mangione TW, Brunelli SM et al. Patient-stated preferences regarding volume-related risk mitigation strategies for hemodialysis. Clin J Am Soc Nephrol 2014; 9: 1418–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marshall MR, Polkinghorne KR, Kerr PG et al. Intensive hemodialysis and mortality risk in Australian and New Zealand populations. Am J Kidney Dis 2016; 67: 617–628 [DOI] [PubMed] [Google Scholar]
- 15. Hernan MA, Robins JM. Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol 2016; 183: 758–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hernán MA, Robins JM. Per-protocol analyses of pragmatic trials. N Engl J Med 2017; 377: 1391–1398 [DOI] [PubMed] [Google Scholar]
- 17. Flythe JE, Kimmel SE, Brunelli SM. Rapid fluid removal during dialysis is associated with cardiovascular morbidity and mortality. Kidney Int 2011; 79: 250–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fewell Z, Wolfe F, Choi H et al. Controlling for time-dependent confounding using marginal structural models. Stata J 2004; 4: 402–420 [Google Scholar]
- 19. Steil H, Amato C, Carioni C et al. EuCliD—a medical registry. Methods Inf Med 2004; 43: 83–88 [PubMed] [Google Scholar]
- 20. Liu J, Huang Z, Gilbertson DT et al. An improved comorbidity index for outcome analyses among dialysis patients. Kidney Int 2010; 77: 141–151 [DOI] [PubMed] [Google Scholar]
- 21. Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol 2008; 168: 656–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Evangelidis N, Tong A, Manns B et al. Developing a set of core outcomes for trials in hemodialysis: an international Delphi survey. Am J Kidney Dis 2017; 70: 464–475 [DOI] [PubMed] [Google Scholar]
- 23. Burton JO, Jefferies HJ, Selby NM et al. Hemodialysis-induced cardiac injury: determinants and associated outcomes. Clin J Am Soc Nephrol 2009; 4: 914–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kramer H, Yee J, Weiner DE et al. Ultrafiltration rate thresholds in maintenance hemodialysis: an NKF-KDOQI controversies report. Am J Kidney Dis 2016; 68: 522–532 [DOI] [PubMed] [Google Scholar]
- 25. Rocco MV, Daugirdas JT, Greene T et al. Long-term effects of frequent nocturnal hemodialysis on mortality: the Frequent Hemodialysis Network (FHN) Nocturnal Trial. Am J Kidney Dis 2015; 66: 459–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Smyth B, Zuo L, Gray NA et al. No evidence of a legacy effect on survival following randomization to extended hours dialysis in the ACTIVE Dialysis trial. Nephrology 2020; 25: 792–800. [DOI] [PubMed] [Google Scholar]
- 27. Wasserstein RL, Lazar NA. The ASA statement on p-values: context, process, and purpose. Am Stat 2016; 70: 129–133 [Google Scholar]
- 28. Wasserstein RL, Schirm AL, Lazar NA. Moving to a world beyond “p < 0.05”. Am Stat 2019; 73(Suppl 1): 1–19 [Google Scholar]
- 29. Harrington D, D’Agostino RB, Gatsonis C et al. New guidelines for statistical reporting in the Journal N Engl J Med 2019; 381: 285–286 [DOI] [PubMed] [Google Scholar]
- 30. Moissl U, Arias-Guillén M, Wabel P et al. Bioimpedance-guided fluid management in hemodialysis patients. Clin J Am Soc Nephrol 2013; 8: 1575–1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brunelli SM, Du Mond C, Oestreicher N et al. Serum potassium and short-term clinical outcomes among hemodialysis patients: impact of the long interdialytic interval. Am J Kidney Dis 2017; 70: 21–29 [DOI] [PubMed] [Google Scholar]
- 32. Katopodis KP, Dounousi E, Challa A et al. Switch from conventional to every other day hemodialysis: a comparison pilot study. ASAIO J 2009; 55: 41–46 [DOI] [PubMed] [Google Scholar]
- 33. Smyth B, Chan CT, Grieve SM et al. Predictors of change in left-ventricular structure and function in a trial of extended hours hemodialysis. J Card Fail 2020; 26: 482–491 [DOI] [PubMed] [Google Scholar]
- 34. Rocco MV, Lockridge RS Jr, Beck GJ et al. The effects of frequent nocturnal home hemodialysis: the Frequent Hemodialysis Network Nocturnal Trial. Kidney Int 2011; 80: 1080–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kalantar-Zadeh K, Unruh M, Zager PG et al. Twice-weekly and incremental hemodialysis treatment for initiation of kidney replacement therapy. Am J Kidney Dis 2014; 64: 181–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fotheringham J, Barnes T, Dunn L et al. Rationale and design for SHAREHD: a quality improvement collaborative to scale up Shared Haemodialysis Care for patients on centre based haemodialysis. BMC Nephrol 2017; 18: 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jardine MJ, Zuo L, Gray NA et al. A trial of extending hemodialysis hours and quality of life. J Am Soc Nephrol 2017; 28: 1898–1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Parmar MKB, Sydes MR, Morris TP. How do you design randomised trials for smaller populations? A framework. BMC Med 2016; 14: 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kahan BC, Jairath V, Doré CJ, Morris TP. The risks and rewards of covariate adjustment in randomized trials: an assessment of 12 outcomes from 8 studies. Trials 2014; 15: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared upon reasonable request to the corresponding author and approval of the ARO steering group.