Abstract
Background
Disordered bone and mineral metabolism are a common complication of chronic kidney disease (CKD). Phosphate binders are often prescribed in advanced CKD, when hyperphosphataemia develops. Little is known about the role of these drugs in earlier stages, when serum phosphorus levels are kept in the normal range by increased urinary excretion.
Methods
A retrospective, controlled observational study was conducted on a cohort of 78 pre-dialysis patients. Subjects had CKD Stage 3 or 4, normal serum phosphorus levels and increased urinary fractional excretion of phosphate. Thirty-eight patients receiving calcium carbonate for 24 months were compared with 40 patients under no phosphate binders, regarding mineral metabolism parameters and vascular calcification scores.
Results
Calcium carbonate decreased mean urinary fractional excretion of phosphate and median 24-h urine phosphorus, whereas no significant change was seen in the control group. Mean serum phosphorus and median serum intact parathyroid hormone (iPTH) remained stable in treated patients but increased in the control group. Vascular calcification, assessed by Kauppila and Adragão scores, worsened under calcium carbonate with no significant change in the control group.
Conclusions
Calcium carbonate reduced urinary phosphate excretion and prevented the rise in phosphorus and iPTH serum levels in a cohort of normophosphataemic pre-dialysis patients. However, treatment was associated with increased vascular calcification, suggesting that calcium-based phosphate binders are not a safe option for CKD patients.
Keywords: calcium carbonate, phosphate binders, pre-dialysis patients, urinary fractional excretion of phosphate, vascular calcification
INTRODUCTION
Chronic kidney disease (CKD) is frequently associated with disordered mineral and bone metabolism (CKD-mineral and bone disorder, CKD-MBD). This complication is linked to increased morbidity and mortality risks in dialysis patients, but there is little data regarding the effects of CKD-MBD in patients with CKD Stages 3 and 4 [1, 2].
Hyperphosphataemia is a hallmark of CKD-MBD, usually occurring in the advanced stages of the disease. In fact, urinary fractional phosphate excretion (FEPi) rises gradually in the course of CKD, led by the action of regulators such as parathyroid hormone (PTH) and fibroblast growth factor 23 (FGF23), to avoid phosphorus retention. However, this adaptive process ultimately fails, leading to overt hyperphosphataemia [2, 3]. Phosphate binders are often prescribed at this stage, usually in the form of calcium-based salts, which are widely available and inexpensive.
There is evidence to suggest that pre-dialysis patients have an increased risk of CKD progression, vascular calcification and death with serum phosphate levels still in the upper limits of the normal range [4–6]. This points to the potential benefits associated with the early treatment of CKD-MBD but, unfortunately, few studies have addressed the issue in normophosphataemic pre-dialysis patients [7, 8].
The purpose of this retrospective, controlled observational study is to report the efficacy and safety of calcium carbonate to prevent phosphate retention in a cohort of normophosphataemic patients with CKD Stages 3 and 4.
MATERIALS AND METHODS
Patients and study design
A cohort of patients from our pre-dialysis clinic was retrospectively analysed. Subjects attending the clinic between January 2015 and July 2016 were consecutively recruited. Inclusion criteria were ≥18 years of age, glomerular filtration rate (GFR) between 15 and 60 mL/min/1.73 m2, serum phosphorus levels within the normal range (2.7–4.5 mg/dL) and increased FEPi (>20%). We excluded patients previously exposed to drugs interfering with bone metabolism, namely calcium or aluminium salts, native or analogue vitamin D agents, and steroids or bisphosphonates. The treatment group consisted of patients starting calcium carbonate at the time of enrolment according to the decision of the attending physician. The control group was composed of subjects on no phosphate binder throughout the study period. Patients were followed-up for 2 years. The study protocol was approved by the Ethics Committee of Centro Hospitalar Universitário São João.
Data collection
Biochemical parameters measured in fasted condition were recorded at the time of enrolment, after 12 months and at the end of study. Serum calcium, phosphorus, albumin, creatinine and alkaline phosphatase (ALP) were measured using an Olympus AU5400 analyzer (Olympus America, Center Valley, PA, USA). Serum intact PTH (iPTH) and 25-hydroxyvitamin D [25(OH)D] levels were determined through an electrochemiluminescence immunoassay with a Cobas E411 analyzer (Roche Diagnostics, Mannheim, Germany). Serum bicarbonate was measured with a Siemens RapidLab 1265 gas analyzer (Siemens Healthcare Diagnostics, Tarrytown, NY, USA). Calcium was corrected for albumin using the formula: corrected calcium = measured calcium + [0.8 × (4 − serum albumin)]. A creatinine assay traceable to isotope dilution mass spectrometry allowed the estimation of GFR through Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [9]. FEPi was calculated using the formula: (urinary phosphate × serum creatinine)/(serum phosphate × urine creatinine) × 100.
Plain radiographs of the pelvis, hands and lateral lumbar spine were taken at start and end of study. They allowed the calculation of semi-quantitative vascular calcification scores as proposed by Adragão et al. and Kauppila et al [10, 11]. Adragão score (AS) estimates iliac, femoral, radial and digital arteries calcification, ranging from 0 to 8. Kauppila score (KS) specifies lumbar aorta calcification and ranges from 0 to 24. Vascular calcification is considered prominent if AS is > 3 or KS is ≥ 7. These cut-off values are associated with higher mortality and higher tomographic coronary calcification, respectively, in dialysis patients [12, 13]. Radiographs were read by two independent observers, blinded to group allocation and baseline score.
Statistical analysis
Continuous data were expressed as mean and standard deviation (SD) (for normally distributed variables) or median and interquartile range (IQR) (for non-normally distributed variables). Categorical variables were expressed as frequencies and percentages. Mann–Whitney test and Wilcoxon rank-sum test were used to compare the differences between and within groups, respectively. The association between continuous variables was assessed by Spearman's rank correlation testing because of data skewness. Data analysis was carried out with SPSS version 25 (IBM SPSS Statistics, Chicago, IL, USA) and Graphpad Prism 8.0 (GraphPad Software, San Diego, CA, USA). P < 0.05 were considered statistically significant.
RESULTS
Baseline characteristics
The study included 78 patients, divided into the treatment (n = 38) and control (n = 40) groups. Clinical and biochemical characteristics at baseline are presented in Table 1. Most subjects were male (n = 51, 65.4%) with mean age of 71.8 ± 10.9 years old. Twenty-nine patients (37.2%) were diabetic. Most cases of CKD were due to hypertension (n = 28, 35.9%), followed by diabetes (n = 10, 12.8%), chronic pyelonephritis (n = 8, 10.3%) and chronic glomerulonephritis (n = 7, 8.9%). Aetiology was unknown in 13 cases (16.7%). Eleven patients (14.1%) were on warfarin, three in the treatment group and eight in the control group. The difference between groups was not statistically significant (P = 0.194). Mean serum creatinine was 2.22 ± 0.45 mg/dL with mean estimated GFR of 27.6 ± 7.7 mL/min/1.73 m2. There were 49 patients (62.8%) with Stage G4 CKD (24 in the treatment group and 25 in the control group) and 29 (37.2%) with Stage G3 CKD (14 in the treatment group and 15 the in control group), according to KDIGO classification [14]. Mean serum calcium and phosphorus were 9.2 ± 0.4 and 3.3 ± 0.5 mg/dL, respectively. Median (IQR) serum iPTH and 25(OH)D were 121 pg/mL (69–165 pg/mL) and 19 ng/mL (11–27 ng/mL), respectively. Median (IQR) urinary phosphate was 549 mg/day (474–751 mg/day) and mean FEPi 33.9 ± 9.3%. Median (IQR) Kauppila and Adragão calcification scores were 5 (2–9) and 1 (0–3), respectively. There were no statistically significant differences between the two groups at baseline, except for higher serum 25(OH)D levels in the treatment group. The mean prescribed dose of calcium carbonate in the treatment group was 2947 ± 226 mg (1178 ± 90 mg of elemental calcium) per day.
Table 1.
Baseline characteristics of the study population
| All | Treatment group | Control group | P-valuea | |
|---|---|---|---|---|
| n | 78 | 38 | 40 | |
| Age, years | 71.8 ± 10.9 | 72.3 ± 9.9 | 71.1 ± 11.9 | 0.768 |
| Male, % | 65.4 | 65.8 | 65.0 | 0.999 |
| Diabetes, % | 37.2 | 26.3 | 47.5 | 0.064 |
| ACCI | 4 (3–5) | 4 (3–5) | 5 (3–5) | 0.084 |
| Body mass index, kg/m2 | 28.6 ± 4.6 | 28.6 ± 5.2 | 28.6 ± 4.1 | 0.780 |
| SBP, mmHg | 134 ± 18 | 130 ± 22 | 138 ± 12 | 0.054 |
| DBP, mmHg | 78 ± 12 | 78 ± 10 | 77 ± 14 | 0.722 |
| Serum creatinine, mg/dL | 2.22 ± 0.45 | 2.30 ± 0.45 | 2.15 ± 0.44 | 0.177 |
| GFR, mL/min/1.73 m2 | 27.6 ± 7.7 | 26.4 ± 7.0 | 28.8 ± 8.3 | 0.220 |
| Serum calcium, mg/dL | 9.2 ± 0.4 | 9.3 ± 0.5 | 9.2 ± 0.4 | 0.526 |
| Serum phosphorus, mg/dL | 3.3 ± 0.5 | 3.3 ± 0.5 | 3.3 ± 0.6 | 0.722 |
| Serum magnesium, mEq/L | 1.7 ± 0.3 | 1.7 ± 0.3 | 1.7 ± 0.2 | 0.968 |
| Serum iPTH, pg/mL | 121 (69–165) | 121 (88–145) | 106 (61–183) | 0.881 |
| Serum ALP, U/L | 91 (66–114) | 91 (64–130) | 82 (68–108) | 0.396 |
| Serum 25(OH)D, ng/mL | 19 (11–27) | 22 (14–31) | 16 (7–22) | 0.008 |
| Serum bicarbonate, mmol/L | 26.0 ± 3.0 | 25.8 ± 2.9 | 26.3 ± 3.0 | 0.541 |
| C-reactive protein, mg/L | 4 (2–9) | 3 (2–7) | 5 (2–9) | 0.214 |
| Serum LDL cholesterol, mg/dL | 98 (78–118) | 91 (74–112) | 106 (86–126) | 0.061 |
| Serum haemoglobin, g/dL | 12.9 ± 1.8 | 12.9 ± 1.7 | 13.0 ± 1.9 | 0.924 |
| Serum ferritin, ng/mL | 155 (106–288) | 163 (109–288) | 147 (104–297) | 0.764 |
| Serum albumin, g/dL | 4.2 ± 0.3 | 4.2 ± 0.3 | 4.2 ± 0.3 | 0.332 |
| Serum BNP, pg/mL | 86 (41–181) | 76 (44–181) | 106 (34–180) | 0.851 |
| Urinary calcium, mg/dayb | 68 (44–112) | 72 (51–109) | 64 (44–112) | 0.444 |
| Urinary phosphate, mg/dayb | 549 (474–751) | 547 (443–751) | 575 (476–762) | 0.376 |
| FEPi, % | 33.9 ± 9.3 | 33.3 ± 8.1 | 34.5 ± 10.3 | 0.814 |
| Urinary protein, mg/dayb | 270 (128–1155) | 320 (108–1175) | 230 (140–1188) | 0.799 |
| Kauppila calcification score | 5 (2–9) | 5 (2–9) | 5 (2–9) | 0.956 |
| Adragão calcification score | 1 (0–3) | 1 (0–3) | 1 (0–3) | 0.844 |
ACCI, age-adjusted Charlson comorbidity index; SBP, systolic blood pressure; DBP, diastolic blood pressure; BNP, brain natriuretic peptide; LDL, low-density lipoprotein. GFR was estimated by CKD-EPI equation. Data are reported as mean ± SD for normally distributed variables, median (IQR) for non-normally distributed variables or percentage for categorical variables.
P-values were calculated using Mann–Whitney test for continuous variables and Chi-squared test for categorical variables.
Twenty-four-hour urine collections were adjusted for adequacy using urinary creatinine excretion.
Laboratory outcomes
Biochemical results obtained at baseline, and 12 and 24 months are presented in Table 2. At the end of study, calcium carbonate had decreased mean FEPi by 20.4% (P = 0.001) and median 24-h urine phosphorus by 23.0% (P < 0.001), with no significant change seen in the control group. On the other hand, mean serum phosphorus and median serum iPTH increased by 9.1% and 18.9%, respectively, in the control group (P < 0.05 for both) but remained stable in the treatment group (Figure 1). At the end of study, no significant differences between groups were seen in serum calcium, serum ALP, 25(OH)D levels, serum creatinine or urinary calcium excretion.
Table 2.
Biochemical measurements at baseline, and 12 and 24 months in the two study groups
| Treatment group |
Control group |
|||||
|---|---|---|---|---|---|---|
| Baseline | Month 12 | Month 24 | Baseline | Month 12 | Month 24 | |
| Serum calcium, mg/dL | 9.3 ± 0.5 | 9.2 ± 0.5 | 9.5 ± 0.5 | 9.2 ± 0.4 | 9.0 ± 0.5 | 9.2 ± 0.6 |
| Serum phosphorus, mg/dL | 3.3 ± 0.5 | 3.3 ± 0.5 | 3.5 ± 0.7 | 3.3 ± 0.6 | 3.4 ± 0.8 | 3.6 ± 1.2a |
| Serum iPTH, pg/mL | 121 (88–145) | 104 (79–148) | 127 (88–163) | 106 (61–183) | 117 (68–203)a | 126 (80–214)a |
| Serum ALP, U/L | 91 (64–130) | 94 (71–112) | 90 (75–106) | 82 (68–108) | 95 (71–114) | 90 (70–108) |
| Serum 25(OH)D, ng/mL | 22 (14–31)b | 23 (15–29) | 19 (14–26)a | 16 (7–22)b | 16 (13–27)a | 16 (13–22) |
| Serum creatinine, mg/dL | 2.30 ± 0.45 | 2.35 ± 0.64 | 2.53 ± 0.79 | 2.15 ± 0.44 | 2.30 ± 0.76 | 2.45 ± 1.18 |
| GFR, mL/min/1.73 m2 | 26.4 ± 7.0 | 26.4 ± 8.7 | 24.6 ± 9.1 | 28.8 ± 8.3 | 27.7 ± 9.1 | 27.4 ± 8.8 |
| Urinary calcium, mg/day | 72 (51–109) | 84 (42–128) | 74 (47–113) | 64 (44–112) | 76 (34–115) | 72 (44–120) |
| Urinary phosphate, mg/day | 547 (443–751) | 446 (310–539)a,b | 421 (342–500)a,b | 575 (476–762) | 563 (441–698)a,b | 638 (526–735)b |
| FEPi, % | 33.3 ± 8.1 | 27.2 ± 9.5a | 26.5 ± 9.0a, b | 34.5 ± 10.3 | 31.8 ± 8.9a | 34.7 ± 11.0b |
Data are reported as mean ± SD or median (IQR). GFR estimated by CKD-EPI equation.
Within-group change from baseline (P < 0.05).
Between-group change (P < 0.05).
FIGURE 1.
Changes in biochemical parameters by group over the study period. Change in median (IQR) of (A) urinary FEPi, (B) 24-h urine phosphorus, (C) serum phosphorus and (D) serum iPTH. *Within-group change from baseline (P < 0.05). **Between-group change (P < 0.05).
Radiological outcomes
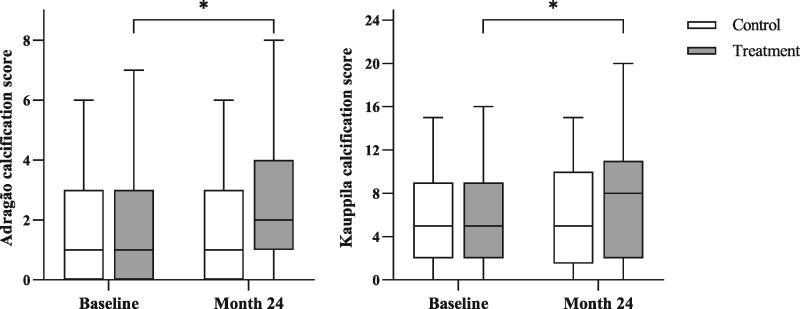
Vascular calcification was present in most patients at baseline (n = 68, 85.3%), and it was prominent (AS >3 or KS ≥7) in almost half (n = 28, 41.2%). Radiological data to assess changes in vascular calcification were available in 58 patients (33 in the treatment group and 25 in the control group). The scores were significantly correlated with each other both at the beginning (r = 0.564, P < 0.001) and at the end of study (r = 0.589, P < 0.001). Vascular calcification worsened significantly under calcium carbonate (Figure 2). Median (IQR) KS increased from 5 (2–9) to 8 (2–11) (P < 0.001) and median (IQR) AS from 1 (0–3) to 2 (1–4) (P < 0.001). Calcification scores did not change significantly in the control group.
FIGURE 2.
Vascular calcification scores by study group at baseline and after 24 months. Results are expressed as median (IQR). *Within-group change from baseline (P < 0.001).
DISCUSSION
Hyperphosphataemia is associated with cardiovascular disease and mortality risk in CKD patients, and there is evidence to suggest that this risk is already increased with phosphate levels within the upper normal range [15–17]. Vascular calcification, including coronary artery calcification, is highly prevalent in CKD patients and its progression, as measured by quantitative and semi-quantitative scores, has been shown to be predictive of cardiovascular events and death [12, 13].
Current guidelines consider that normophosphataemia may not be an indication to start phosphate-lowering treatments and suggest treating progressively or persistently elevated serum phosphate, restricting the dose of calcium-based binders [18]. Few studies have addressed the effects of phosphate binders on biochemical parameters and vascular calcification among pre-dialysis patients with normal or near-normal serum phosphorus. In a trial conducted by Oliveira et al. [7], 40 patients with CKD Stages 3 and 4 were randomized to either calcium acetate or sevelamer hydrochloride. After a 6-week treatment period, both binders reduced urinary fractional excretion of phosphate and serum iPTH. Sevelamer-treated patients also had a reduction in serum FGF23, with no difference observed in the calcium arm. No change on calcium or phosphorus serum levels was seen in either group. However, this study was limited by a small sample size, short duration and lack of control group. In a subsequent trial, Block et al. [8] randomized 148 patients with CKD Stages 3 b–4 to treatment with calcium acetate, lanthanum carbonate, sevelamer carbonate or placebo. After a period of 9 months, active therapy (all binders combined) reduced urinary FEPi and, albeit moderately, serum phosphorus levels. Serum iPTH remained stable, having increased in the placebo group. Curiously, no change was seen in serum FGF23 levels. Notably, active therapy increased calcification of the coronary arteries and abdominal aorta. Interpretation of these results is difficult, since the three binders, which are known to have different effects on mineral, bone and vascular metabolism, were analysed together due to small subgroup size [3]. However, the increase in calcification was mostly induced by the group treated with calcium acetate. Regarding vascular calcification, a previous study performed by Russo et al. [19] came to different conclusions. The authors randomized 90 pre-dialysis patients to one of the three treatment groups: low-phosphate diet alone, diet plus calcium carbonate or diet plus sevelamer. After a follow-up of 2 years, coronary artery calcification had increased with diet alone and, to a smaller extent, in calcium-treated patients. On the other hand, calcification did not progress in patients treated with sevelamer. Overall, evidence points to a benefit in treating CKD patients prematurely, before serum phosphorus levels rise above the normal range. Therefore, increased FEPi in the presence of normophosphataemia might be a target when treating CKD-MBD.
The present study showed that calcium carbonate effectively reduced urinary phosphorus excretion (by limiting intestinal phosphate absorption), delayed the onset of hyperphosphataemia and abated the progression to secondary hyperparathyroidism (SHPT). We speculate that intervention with calcium-free phosphate binders may also prove to decrease the progression to SHPT without the negative effect of increasing VC progression. It is important to note that no significant change in serum phosphorus was seen despite the impact of calcium carbonate on urinary phosphate excretion, but similar findings have been reported. In fact, circadian phosphate fluctuations are well recognized, which means that a single assessment may not adequately reveal time-averaged values [20, 21]. Our results thus suggest that serum phosphorus levels are not a reliable marker of dietary intake or binder efficacy in pre-dialysis patients. Disturbingly, treatment with about 1200 mg/day of elemental calcium (1000 mg of calcium carbonate three times daily) magnified vascular calcification, as assessed by simple scores based on plain radiographic films. Moreover, urinary calcium excretion did not increase in the treatment group, contrary to what one might have expected. Taken together, these findings hint that therapy leads to positive calcium balance and related soft tissue deposition, as supported by the metabolic studies published by Spiegel and Brady [22] and Hill et al. [23]. Notwithstanding the small sample size and limited duration, both studies showed that even modest calcium exposure (1000 and 800 mg/day, respectively) can lead to positive calcium balance in normophosphataemic pre-dialysis patients.
Despite its retrospective design, this study has several strengths, such as the inclusion of a control group and a relatively long follow-up period. All patients were naïve to drugs known to interfere with bone metabolism, thus eliminating an important confounding factor. Additionally, clinical and laboratory characteristics were well balanced between groups. It is worth mentioning that calcium-treated patients had higher serum 25(OH)D levels than controls at baseline, but this is unlikely to have affected study results. In fact, there is an association between low vitamin D levels and vascular calcification in pre-dialysis patients [24]. One would expect higher 25(OH)D levels to protect calcium-treated patients from exacerbation of vascular calcification, but this was not the case. Anyway, despite no supplementation, serum 25(OH)D levels did not differ between groups at the end of study. It is also meaningful that the potentially detrimental effect of calcium carbonate was observed despite a clear trend in the control group towards higher percentage of diabetics, age-adjusted Charlson comorbidity index score, systolic blood pressure and serum low-density lipoprotein cholesterol.
In conclusion, this study showed that prescription of calcium carbonate in normophosphataemic pre-dialysis patients reduced urinary phosphorus excretion, delayed the development of hyperphosphataemia and hampered the progression to SHPT. Nonetheless, despite its efficacy, calcium treatment worsened vascular calcification. Large, long-term prospective studies are needed to explore both the benefits and the safety of the early prescription of phosphate binders in CKD patients.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Levin A, Bakris GL, Molitch M et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int 2007; 71: 31–38 [DOI] [PubMed] [Google Scholar]
- 2. Vervloet MG, van Ballegooijen AJ. Prevention and treatment of hyperphosphatemia in chronic kidney disease. Kidney Int 2018; 93: 1060–1072 [DOI] [PubMed] [Google Scholar]
- 3. Drüeke TB, Massy ZA. Phosphate binders in CKD: bad news or good news? J Am Soc Nephrol 2012; 23: 1277–1280 [DOI] [PubMed] [Google Scholar]
- 4. Toussaint ND, Pedagogos E, Tan SJ et al. Phosphate in early chronic kidney disease: associations with clinical outcomes and a target to reduce cardiovascular risk. Nephrology (Carlton) 2012; 17: 433–444 [DOI] [PubMed] [Google Scholar]
- 5. Scialla JJ, Wolf M. Roles of phosphate and fibroblast growth factor 23 in cardiovascular disease. Nat Rev Nephrol 2014; 10: 268–278 [DOI] [PubMed] [Google Scholar]
- 6. Vervloet MG, Sezer S, Massy ZA et al. The role of phosphate in kidney disease. Nat Rev Nephrol 2017; 13: 27–38 [DOI] [PubMed] [Google Scholar]
- 7. Oliveira RB, Cancela AL, Graciolli FG et al. Early control of PTH and FGF23 in normophosphatemic CKD patients: a new target in CKD-MBD therapy? Clin J Am Soc Nephrol 2010; 5: 286–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Block GA, Wheeler DC, Persky MS et al. Effects of phosphate binders in moderate CKD. J Am Soc Nephrol 2012; 23: 1407–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Levey AS, Stevens LA, Schmid CH et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Adragão T, Pires A, Lucas C et al. A simple vascular calcification score predicts cardiovascular risk in haemodialysis patients. Nephrol Dial Transplant 2004; 19: 1480–1488 [DOI] [PubMed] [Google Scholar]
- 11. Kauppila LI, Polak JF, Cupples LA et al. New indices to classify location, severity and progression of calcific lesions in the abdominal aorta: a 25-year follow-up study. Atherosclerosis 1997; 132: 245–250 [DOI] [PubMed] [Google Scholar]
- 12. Adragão T, Pires A, Birne R et al. A plain X-ray vascular calcification score is associated with arterial stiffness and mortality in dialysis patients. Nephrol Dial Transplant 2009; 24: 997–1002 [DOI] [PubMed] [Google Scholar]
- 13. Bellasi A, Ferramosca E, Muntner P et al. Correlation of simple imaging tests and coronary artery calcium measured by computed tomography in hemodialysis patients. Kidney Int 2006; 70: 1623–1628 [DOI] [PubMed] [Google Scholar]
- 14.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl 2013; 3: 1–150 [Google Scholar]
- 15. Dhingra R, Sullivan LM, Fox CS et al. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med 2007; 167: 879–885 [DOI] [PubMed] [Google Scholar]
- 16. Kestenbaum B, Sampson JN, Rudser KD et al. Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol 2005; 16: 520–528 [DOI] [PubMed] [Google Scholar]
- 17. Tonelli M, Sacks F, Pfeffer M et al. Cholesterol And Recurrent Events Trial Investigators. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation 2005; 112: 2627–2633 [DOI] [PubMed] [Google Scholar]
- 18.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 2017; 7: 1–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Russo D, Miranda I, Ruocco C et al. The progression of coronary artery calcification in predialysis patients on calcium carbonate or sevelamer. Kidney Int 2007; 72: 1255–1261 [DOI] [PubMed] [Google Scholar]
- 20. Isakova T, Ix JH, Sprague SM et al. Rationale and approaches to phosphate and fibroblast growth factor 23 reduction in CKD. J Am Soc Nephrol 2015; 26: 2328–2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Becker GJ, Walker RG, Hewitson TD et al. Phosphate levels–time for a rethink? Nephrol Dial Transplant 2009; 24: 2321–2324 [DOI] [PubMed] [Google Scholar]
- 22. Spiegel DM, Brady K. Calcium balance in normal individuals and in patients with chronic kidney disease on low- and high-calcium diets. Kidney Int 2012; 81: 1116–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hill KM, Martin BR, Wastney ME et al. Oral calcium carbonate affects calcium but not phosphorus balance in stage 3-4 chronic kidney disease. Kidney Int 2013; 83: 959–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. García-Canton C, Bosch E, Ramírez A et al. Vascular calcification and 25-hydroxyvitamin D levels in non-dialysis patients with chronic kidney disease stages 4 and 5. Nephrol Dial Transplant 2011; 26: 2250–2256 [DOI] [PubMed] [Google Scholar]