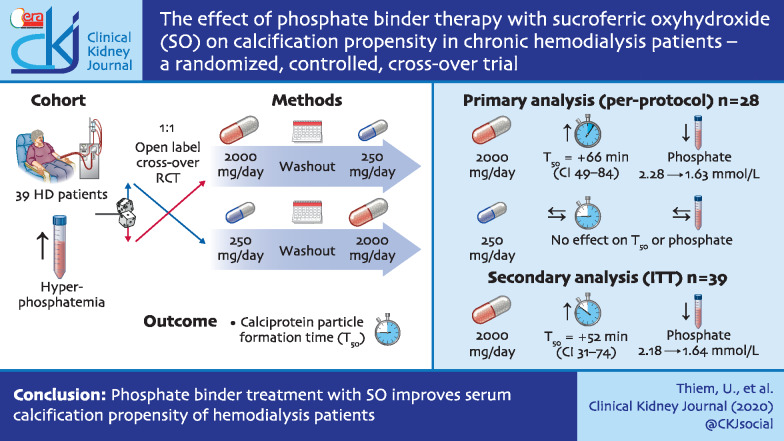
Graphical Abstract
Keywords: calcification propensity, haemodialysis, phosphate binder, sucroferric oxyhydroxide
Abstract
Background
Calcification propensity is associated with the risk for cardiovascular events and death in end-stage renal disease patients. Here we investigated the effect of lowering serum phosphate with oral phosphate binder therapy on calcification propensity.
Methods
We performed an open-label, randomized, controlled, crossover study in chronic haemodialysis patients with hyperphosphataemia. Patients (n = 39) were randomized in a 1:1 ratio to either low-dose (250 mg/day) sucroferric oxyhydroxide (SO) followed by high-dose (2000 mg/day) SO or vice versa, with washout phases before and after SO treatment. The primary endpoint was changed in calcification propensity as measured by calciprotein particle formation time (T50 test) between washout and high-dose SO treatment in patients with ≥85% adherence to the prescribed SO dose (per-protocol analysis).
Results
In the primary per-protocol analysis (n = 28), 2000 mg/day SO treatment resulted in a mean increase in T50 of 66 min (95% CI 49–84 min, P < 0.0001), from 243 ± 63 to 309 ± 74 min compared with phosphate binder washout. Serum phosphate decreased from 2.28 ± 0.5 to 1.63 ± 0.43 mmol/L (P < 0.0001). SO at 250 mg/day did not influence T50 (P = 0.4) or serum phosphate concentrations (P = 0.9) compared with phosphate binder washout. The secondary intention-to-treat analysis (n = 39) showed similar results: an increase in T50 of 52 min (95% CI 31–74 min, P < 0.0001) and a decrease in serum phosphate from 2.18 ± 0.5 to 1.64 ± 0.46 mmol/L. No major adverse cardiovascular event, case of calciphylaxis or death occurred during the study.
Conclusion
Phosphate binder treatment with SO improves serum calcification propensity of haemodialysis patients and might lead to improved outcomes.
INTRODUCTION
Disturbances in mineral and bone metabolism, which are summarized as chronic kidney disease–mineral and bone disorder (CKD-MBD) syndrome [1], are a major contributor of cardiovascular morbidity and mortality in CKD and especially dialysis patients [2]. In CKD-MBD, an imbalance of promoters and inhibitors of calciprotein crystal formation is thought to cause vascular damage, ultimately leading to vascular calcification [3]. Numerous promoters (e.g. phosphate and calcium [4–8]) and inhibitors (e.g. magnesium [9, 10], bicarbonate [11] and fetuin-A [12]) of calcification have been associated with mortality in dialysis patients. Despite widespread clinical use, there is still a paucity of data demonstrating the benefits of phosphate binder therapy on lowering cardiovascular risk.
The so-called T50-test integrates the complex biological interplay of promoters and inhibitors of calciprotein particle (CPP) formation in blood into a single readout. In brief, patient serum is supersaturated with calcium and phosphate, which triggers the transition from primary to secondary CPPs within hours under appropriate conditions in vitro [13]. The T50-test determines the time needed for half-maximum transition from primary to secondary CPPs, lending this assay its popular name. Short T50 times, indicating a low resistance of blood to the formation of crystalline secondary CPPs, have been repeatedly associated with an increased risk for cardiovascular events and mortality in large cohort studies in patients with CKD [14, 15], kidney transplant recipients [16–18] and dialysis patients [19]. Serum phosphate has been consistently shown to independently and inversely correlate with T50 in multivariable analyses in vitro [13] and in different patient cohorts [14, 17, 19–21]. The aim of this study was to investigate the effect of phosphate binder therapy using sucroferric oxyhydroxide (SO) on the propensity of serum for calcification as measured by the T50-test. We hypothesized that phosphate binder treatment with SO would increase T50.
MATERIALS AND METHODS
Participants
The main criteria for study inclusion were age ≥18 years, chronic (≥3 months) treatment with haemodialysis or haemodiafiltration thrice weekly and hyperphosphataemia with or without current phosphate binder use. The main exclusion criteria were a history of calciphylaxis (calcific uraemic arteriolopathy), intact parathyroid hormone (iPTH) >800 pg/mL, parathyroidectomy planned or expected, significant gastrointestinal or hepatic disorders and pregnancy.
This study was approved by the Ethics Committee of Upper Austria (Study ID: A-VIII-16) and conducted according to regulations of the International Conference on Harmonization on Good Clinical Practice and the Declaration of Helsinki on Ethical Principles for Medical Research Involving Human Subjects. Patients were enrolled in this study after providing oral and written informed consent. This study was registered before initiation at the European Union clinical trials register (EudraCT 2016-004789-24) and ClinicalTrials.gov (NCT03010072).
Study design and intervention
This randomized, single-centre, open-label, controlled, crossover, proof-of-principle study was conducted at the dialysis facility of the Ordensklinikum Linz Elisabethinen Hospital from 2017 to 2018. An independent statistician generated a randomization list using permuted block randomization in SAS (SAS Institute, Cary, NC, USA) and prepared opaque, sealed and consecutively numbered envelopes containing the respective allocation. Block sizes of four and six in alternating sequence were used, starting with a block of four patients. Patients were randomized in a 1:1 ratio to either receive low-dose (250 mg/day as one 250 mg tablet) SO followed by high-dose (2000 mg/day as four 500 mg tablets) SO (sequence A–B) or to receive high-dose SO followed by low-dose SO (sequence B–A) with washout phases (no phosphate binder therapy at all) before and after every SO phase. A schematic overview of the study sequences is shown in Supplementary data, Figure S1. Each study phase lasted 2 weeks and two consecutive study visits were performed in the second week of each study phase during the second and third dialysis sessions of the week. An open-label design with a subtherapeutic dose of SO as a control treatment was chosen because creating a convincing placebo for SO is not feasible due to black discolouration of faeces during treatment as a consequence of the iron content of SO. The dose of 250 mg/day of SO was chosen as a control because it had no significant effect on serum phosphate levels in adult dialysis patients in previous studies [22, 23], allowing for comparison of a highly effective phosphate binder therapy (SO 2000 mg/day) and ineffective phosphate binder dosing (SO 250 mg/day) on T50. The dose of 2000 mg/day of SO was chosen because the mean dose necessary to achieve adequate serum phosphate control in a previous Phase III study in dialysis patients was 3.6 tablets/day [each tablet containing 500 mg of SO, which is the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) approved dose per tablet], equaling 1800 mg/day [23]. Concomitant medications, especially vitamin D and calcimimetics, as well as the composition of the dialysis bath, were held constant throughout the study as far as clinically justifiable as determined by the attending physicians.
Participant safety and rescue treatment
For adverse events (AEs) that were probably linked to SO treatment, a dose reduction algorithm was pre-specified in the study protocol. In case of patient-reported nausea, vomiting or diarrhoea, which appeared to be intolerable as judged by the patient, the SO dose was reduced from 250 mg/day to nil in the low-dose phase and from 2000 to 1500 mg/day in the high-dose phase and patients were allowed to continue study participation and were also included in the per-protocol population for statistical analysis. Patients who experienced further gastrointestinal AEs on 1500 mg of SO per day were excluded from further participation in the study and from the per-protocol analysis but were included in the intention-to-treat analysis (ITT). Adherence to study medication was defined as intake of ≥85% of prescribed pills in the SO high-dose phase as determined by pill counting. In case serum phosphate was >2.6 mmol/L in two consecutive measurements, rescue treatment consisting of sevelamer carbonate 1600 mg/day was initiated and titrated up to 3200 mg/day if necessary to achieve serum phosphate levels <2.6 mmol/L. Sevelamer rescue was then discontinued at the beginning of each subsequent study phase. Patients were excluded from further participation in the study if a serum phosphate level <2.6 mmol/L could not be achieved despite sevelamer rescue of 3200 mg/day due to suspicion of poor compliance.
Laboratory measurements
Blood was collected in separating gel tubes (BD Vacutainer, Becton Dickinson, Plymouth, UK) for serum and K3EDTA tubes (Vacuette, Greiner BioOne, Kremsmünster, Austria) for plasma. Samples were centrifuged at 1280 rcf for 15 min at room temperature and aliquots were transferred directly to −80°C for storage. T50 was measured in serum as described previously [19] at Calciscon, Nidau, Switzerland, without knowledge of randomization group or study phase. Routine blood tests were performed in the central laboratory facility of the Ordensklinikum Linz Elisabethinen Hospital using Cobas analyzer systems (Roche Diagnostics, Rotkreuz, Switzerland). Intact PTH was measured using the Elecsys PTH(1–84) assay on a Cobas system (Roche). Intact fibroblast growth factor 23 (iFGF23) was measured in ethylenediaminetetraacetic acid plasma samples using the Diasorin iFGF23 chemiluminescent immunoassay on a fully automated Liasion XL platform (DiaSorin, Saluggia, Italy).
Definition of study endpoints
The primary study endpoint was changed in T50 between washout and high-dose (2000 mg/day) SO treatment. The means of all laboratory parameters including T50 were calculated from both study visits during a given study phase to decrease intra-individual variation, which may result from day-to-day changes in diet in this outpatient setting. The secondary study endpoint was changed in serum phosphate between washout and high-dose (2000 mg/day) SO treatment.
Sample size calculation
As changes in T50 in response to phosphate binder treatment in dialysis patients were unknown until this study, sample size calculation was based on estimations from previous studies [13, 14, 19–21] and the following assumptions: T50 in the washout phase was expected to be 220 min, T50 in the SO treatment phase was estimated to be 270 min, the standard deviation (SD) of differences in T50 between the washout and treatment phases was estimated to be 80 min and the dropout rate was expected to be 15%. Thus a target population of 34 patients (29 patients completing the study per-protocol) will have a 90% power to detect a difference in means of T50 minutes in the primary endpoint in a per-protocol analysis. The sample size calculation is based on a paired t-test with a 0.05 two-sided significance level and was performed using nQuery Advisor software (Statistical Solutions, Cork, Ireland).
Statistical analyses
The characteristics of patients at study baseline were described by mean and SD, median and interquartile range (IQR) or frequency and percentage for normally distributed variables, non-normally distributed variables and categorical variables, respectively.
The primary endpoint, change in T50 between washout and SO high-dose, was investigated per-protocol (pre-specified primary analysis, including only patients adhering to study medication) and ITT (pre-specified secondary analysis) applying the most conservative assumption of no treatment effect by inserting T50 values during washout (pretreatment phase value carried forward) in case of missing values. P-values <0.05 were considered statistically significant. The per-protocol approach for the primary analysis was chosen because this is a proof-of-principle study investigating the pharmacodynamic effects of SO on T50. The ITT approach was chosen only as a secondary analysis since it is based on the initial treatment assignment and not on the treatment actually received. ITT analysis ignores non-compliance, protocol deviations, withdrawal and anything that happens after randomization [24] and therefore is not the first choice for the analysis of proof-of-principle studies.
A linear mixed model was used to investigate the change in mean T50 between SO low-dose and SO high-dose corrected for the preceding washout phase as a dependent variable, treatment group and treatment period as fixed effects and the patient as a random effect in the model. A carry-over effect was assessed by testing the interaction of period and treatment group for statistical significance. In this study, no carry-over effect was detected (P = 0.3). Thus the interaction term for the carry-over effect was not kept in the model. Changes in T50 are reported as means and 95% confidence intervals (CIs). Data for serum phosphate (pre-specified secondary outcome parameter) were analysed the same way as T50. Results for other parameters are shown in a descriptive manner to avoid type I errors due to multiple testing.
Figures were prepared using GraphPad Prism version 8.3 (GraphPad Software, San Diego, CA, USA). Because no carry-over effect was detected, data from both study sequences were merged for statistical analysis and to allow for graphical illustration of study results in one figure per parameter (Supplementary data, Figure S1). Data sharing as defined by the Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals, updated in 2019, of the International Committee of Medical Journal Editors is not intended. M.C.H. was the leading statistician for this study and confirms that the analyses are reliable and vouches for the reliability of the findings.
RESULTS
Patients and intervention
Of 260 screened patients, 39 were randomized (ITT population) and 28 completed the study as defined by the study protocol (per-protocol population). A patient flow chart is given in Figure 1. The numbers of patients not fulfilling the inclusion/exclusion criteria, who were deemed to be unfit to participate in the study (e.g. mental disease, language barriers and low life expectancy), who declined to participate and who dropped out or did not adhere to the study protocol were all higher than anticipated. Therefore the study was closed due to feasibility reasons after 39 randomized patients, resulting in a per-protocol population of 28 instead of the intended 29 individuals. Patient baseline characteristics are presented in Table 1. Of the 28 patients representing the per-protocol population, 5 patients required dose reductions of SO in the high-dose phase from 2000 to 1500 mg/day, resulting in a mean prescribed SO dose of 1911 mg/day during the high-dose phase for the per-protocol cohort. Sevelamer rescue due to serum phosphate levels repeatedly measuring >2.6 mmol/L was taken by six patients at some point in time during the study in the per-protocol population.
FIGURE 1:
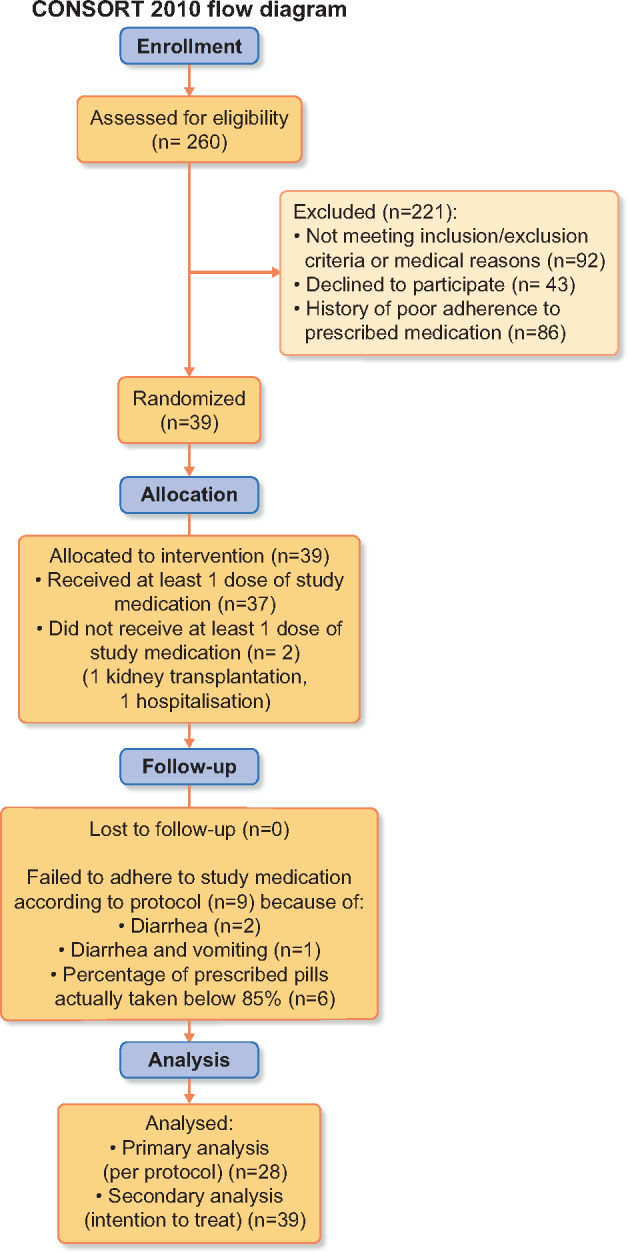
Participant flow diagram according to Consolidated Standards of Reporting Trials recommendations.
Table 1.
Baseline demographic data of patients (n = 39)
| Age (years), mean ± SD | 63 ± 27 |
| Sex (male), n (%) | 29 (74.3) |
| Primary renal disease, n (%) | |
| Diabetes | 6 (15.4) |
| Hypertensive/vascular | 13 (33.3) |
| ADPKD | 8 (20.5) |
| Glomerulonephritis | 9 (23.1) |
| Other | 3 (7.7) |
| Dialysis vintage (months), median (IQR) | 24 (16–36) |
| Previous kidney transplants, n (%) | |
| None | 35 (89.7) |
| 1 | 2 (5.1) |
| 2 | 1 (2.6) |
| 3 | 1 (2.6) |
| Vascular access, n (%) | |
| AV fistula | 34 (87.2) |
| Catheter | 5 (12.8) |
| Baseline laboratory parameters | |
| Calcium ionized (mmo/L) | 1.09 ± 0.06 |
| Calcium, albumin corrected (mmol/L) | 2.22 ± 0.15 |
| Phosphate (mmol/L) | 1.77 ± 0.53 |
| Bicarbonate (mmol/L) | 22.9 ± 2.3 |
| Magnesium (mmol/L) | 1.03 ± 0.14 |
| iPTH (pg/dL), median (IQR) | 309 (167–425) |
| iFGF23 (pg/mL), median (IQR) | 4035 (1523–9273) |
| Hypertension, n (%) | 29 (74.4) |
| Diabetes mellitus, n (%) | 12 (30.8) |
| Coronary artery disease, n (%) | 18 (46.2) |
| History of myocardial infarction, n (%) | 4 (10.3) |
| History of congestive heart failure, n (%) | 19 (48.7) |
| Atrial fibrillation, n (%) | 12 (30.8) |
| Peripheral occlusive vascular disease or amputation, n (%) | 16 (41.0) |
| Cerebrovascular disease, TIA or stroke, n (%) | 15 (38.5) |
| Cigarette smoking, n (%) | 9 (23.1) |
| Dialysate composition, n (%) | |
| Calcium (mmol/L) | |
| 1.25 | 37 (94.9) |
| 1.5 | 0 |
| 1.75 | 2 (5.1) |
| Bicarbonate (mmol/L) | |
| 32 | 36 (92.3) |
| 35 | 3 (7.7) |
| Magnesium (mmol/L) | |
| 0.5 | 39 (100) |
| Dialysis modality, n (%) | |
| HD | 12 (30.8) |
| HDF | 27 (69.2) |
| Medication, n (%) | |
| Phosphate binders | |
| Sevelamer carbonate | 29 (74.3) |
| Calcium-containing | 0 |
| Other | 8 (20.5) |
| Vitamin K antagonists | 7 (17.9) |
| Active vitamin D | 25 (64.1) |
| Cinacalcet | 14 (35.9) |
| Etelcalcetide | 2 (5.1) |
| Statin | 4 (10.3) |
| Antihypertensives | 37 (94.8) |
| Erythropoiesis-stimulating agent | 23 (58.9) |
ADPKD: autosomal dominant polycystic kidney disease; AV: arteriovenous; TIA: transitory ischaemic attack; HD: haemodialysis; HDF: haemodiafiltration.
T50 and serum phosphate
In the primary analysis (per-protocol population, n = 28), high-dose SO treatment (2000 mg/day, consisting of four 500 mg tablets) resulted in a mean increase in T50 (primary endpoint) of 66 min (95% CI 49–84 min, P < 0.0001), from 243 ± 63 to 309 ± 74 min compared with phosphate binder washout (Figure 2A). Simultaneously, serum phosphate decreased from 2.28 ± 0.5 to 1.63 ± 0.43 mmol/L (P < 0.0001; Figure 2B). Interestingly, we observed considerable interindividual variability with regard to changes in T50 between patients with comparable magnitudes of phosphate lowering (Supplementary data, Figure S2).
FIGURE 2:
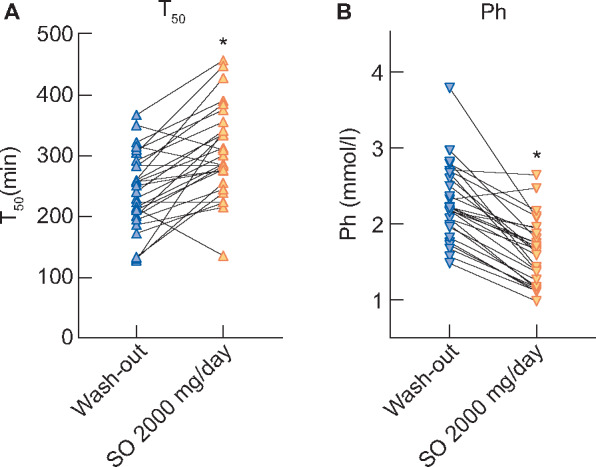
Effects of SO therapy on (A) T50and (B) serum phosphateon an individual patient level between washout and SO 2000 mg/day. Asterisks indicate statistically significant differences (all P < 0.0001) between SO 2000 mg/day and the preceding washout phase.
Δ: mean change between washout and SO 2000 mg/day.
SO at 250 mg/day did not influence T50 times (P = 0.4) or serum phosphate values (P = 0.9) compared with phosphate binder washout. In the linear mixed model analysis investigating the change in mean T50 between SO low-dose (control) and SO high-dose corrected for the preceding washout phase, an increase of 76 min (95% CI 49–103) for SO high-dose compared with SO low-dose was found.
Six patients had missing values (in two of them T50 values in both study phases were missing and in four only the last study phase was missing), which were replaced by inserting T50 values of the preceding washout phase for ITT analysis to avoid a risk of bias from overestimation of treatment effects. In the ITT analysis (all randomized patients, n = 39), treatment with high-dose (2000 mg/day) SO resulted in a mean increase in T50 times of 52 min (95% CI 31–74, P < 0.0001), while serum phosphate decreased from 2.18 ± 0.5 to 1.64 ± 0.46 mmol/L compared with washout. Again, no significant differences between washout and SO low-dose were found for changes in T50 (P = 0.4).
Other laboratory parameters
Results of other laboratory parameters of the per-protocol-population (n = 28) are presented in Table 2. When comparing washout to SO 2000 mg/day, ionized calcium (1.07 ± 0.08 to 1.11 ± 0.067 mmol/L), albumin (3.9 ± 0.27 to 4.0 ± 0.28 g/dL), magnesium (1.01 ± 0.12 to 1.05 ± 0.02 mmol/L) and bicarbonate (22.6 ± 2.28 to 23.4 ± 2.24 mmol/L) increased, while iPTH [median 319 (IQR 194–419) pg/mL] to 244 [IQR 141–348]} and iFGF-23 [median 4217 (IQR 2149–11 989) pg/mL to 2462 (1082–4612)] decreased. Differences in these parameters between washout and SO 250 mg/day tended to be in the same direction, but to a lesser extent (data not shown).
Table 2.
Changes in analyte concentrations compared with baseline
| Parameter | Baseline | SO 250 mg/day | SO 2000 mg/day |
|---|---|---|---|
| Mean ± SD | Change in mean (95% CI) | Change in mean (95% CI) | |
| Calcium ionized (mmol/L) | 1.09 ± 0.06 | −0.07 (−0.16–0.02) | 0.01 (−0.005–0.025) |
| Albumin (g/dL) | 3.9 ± 0.3 | 0.1 (0.0–0.1) | −0.1 (−0.4–0.2) |
| Bicarbonate (mmol/L) | 22.9 ± 2.3 | −1.2 (−3.1–0.7) | 0.7 (0.1–1.3) |
| Magnesium (mmol/L) | 1.03 ± 0.14 | −0.06 (−0.13–0.02) | −0.01 (−0.04–0.02) |
| iPTH (pg/dL) | 309 (167–425)a | 23 (−38–58)b | −24 (−36 to −9)b |
| iFGF23 (pg/mL) | 4035 (1523–9273)a | 652 (−22–2023)b | −72 (−387–782)b |
Median (IQR).
Change in median (95% CI).
Adverse events
At least one AE was reported by 30 of 39 patients. All patients reported discolouration of faeces during the SO study phases, which is suggestive of actual SO intake. The most common AE probably linked to SO therapy were gastrointestinal side effects such as loose stools/diarrhoea (n = 16), which either resolved spontaneously over a few days or responded to a dose reduction of SO. Three patients discontinued SO treatment in the 2000 mg/day phase because of diarrhoea (of which one patient reported diarrhoea and vomiting) after a median of 2 days on treatment. Three patients reported nausea and one patient reported vomiting during the 2000 mg/day SO phase. Seven patients reported nausea and four patients reported vomiting during the washout phases. No allergic reactions to SO or cases of acute liver injury were observed. No major adverse cardiovascular events, cases of calciphylaxis or death occurred during the study.
DISCUSSION
This study reports the effects of phosphate binder therapy on calcification propensity (T50) in chronic haemodialysis patients. The main finding is that phosphate binder therapy using 2000 mg/day SO significantly increased (i.e. improved) T50. Furthermore, control treatment with SO at 250 mg/day does not influence serum phosphate levels and T50. These results indicate that lowering serum phosphate is the major contributor to changes in T50 elicited by SO therapy. Based on the findings presented here, lowering of serum phosphate by 0.1 mmol/L leads to increases in T50 of ∼10 min. Of note, other parameters known to associate with T50in vitro or in patients in vivo, such as ionized calcium, albumin, magnesium and bicarbonate, slightly increased with SO 2000 mg/day treatment. For instance, serum magnesium was slightly higher (∼0.04 mmol/L) during SO 2000 mg/day treatment compared with the washout, which may be explained by reduced absorption of dietary magnesium due to complexation with dietary phosphate in the absence of phosphate binder treatment. As magnesium has been shown to increase T50in vitro [13] and in clinical trials [25, 26], this subtle increase may have contributed to the increase in T50 found with SO 2000 mg/day. It is thus possible that increases in T50 found with SO 2000 mg/day treatment might be partly attributable to changes in other laboratory parameters besides serum phosphate, but the magnitude of this contribution is probably small and difficult to quantify.
We found that T50 responded swiftly to SO treatment and phosphate binder washout, demonstrating that T50 values can be modulated in short periods of time and that changes in T50 in response to changes in serum phosphate are reversible.
So far, the largest body of evidence reporting on associations between T50 and morbidity and mortality of haemodialysis patients comes from a post hoc analysis of the Evaluation of Cinacalcet Hydrochloride Therapy to Lower Cardiovascular Events (EVOLVE) trial, where a statistically significant increase in the hazard ratio for the primary composite outcome (all-cause mortality, myocardial infarction, hospitalization for unstable angina, heart failure or peripheral vascular event) of 1.15 per 84 min lower T50 after multivariable adjustment was found [19]. Therefore increases in T50 of 66 min with SO 2000 mg/day monotherapy reported here might approach a clinically relevant range. Furthermore, as higher T50 has been associated with better patient outcomes [19], one might speculate that phosphate binder therapy in combination with other interventions aimed at further increasing T50, such as adding magnesium [26] or citrate [27] to the dialysis bath, might lead to relevant improvements in patient outcomes, especially in patients with low T50 at baseline. However, whether a combination of therapies aimed at increasing T50 values leads to improved patient outcomes remains to be studied.
AEs of SO treatment were within the expected spectrum. There seems to be some kind of adaptation of the intestinal tract to the laxative effect of SO, because the gastrointestinal side effects tended to resolve spontaneously within days in patients who continued the full SO dose, which has also been described before [23]. It might be appropriate to implement a dose-escalation strategy instead of starting with the full target dose in future studies with SO, to minimize gastrointestinal side effects and reduce dropout numbers.
The major strength of this study is the controlled crossover design with intermittent washout phases, allowing for investigation of the effects of potent phosphate binder therapy compared with no phosphate binder therapy (washout versus SO 2000 mg/day), effective versus non-effective (SO 2000 versus SO 250 mg/day) phosphate lowering and also the reversibility of effects of phosphate binder therapy (SO 2000 mg/day versus washout) on T50. A specific feature of the study is the vigorous selection of patients adherent to oral study medication based on their medical history of adherence, supervision of actual SO intake (pill counting and surveying for black stools as signs of SO intake throughout the entire study) and the per-protocol (including only patients adherent to SO therapy) analysis of data. This approach ensures a reasonable level of confidence that the study drug was actually taken in the outpatient setting and data can be interpreted appropriately in this proof-of-principle study. However, this meticulous patient selection might also be viewed as a weakness of this study: adherence of dialysis patients to oral medication, and especially to phosphate binder therapy, is generally low [28, 29]. Therefore the data presented here are probably not generalizable to the entire dialysis population but may be applicable mainly to patients with good adherence as well as tolerance to SO. Furthermore, counting pills, which are returned by the patient at the end of the study phases in this outpatient setting, does not guarantee that the patient actually took the medication, and self-reported discolouration of stools does not necessarily mean the patient took every dose of SO as prescribed. Thus a certain degree of uncertainty regarding adherence to SO remains. Further limitations of this study are the open-label design, which may have influenced the dietary habits of patients and thus influenced serum phosphate levels in addition to SO treatment. Finally, effects of changes in calcification propensity induced by SO on clinical cardiovascular disease could not be assessed due to the short duration of the study.
In conclusion, we report that lowering serum phosphate with SO 2000 mg/day significantly increases T50 in prevalent haemodialysis patients.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
Supplementary Material
ACKNOWLEDGEMENTS
We wish to thank all dialysis patients who participated in the study and the dialysis staff of the Ordensklinikum Linz Elisabethinen Hospital for their support. We thank Dr Angelika Geroldinger for providing patient randomization. We thank Angela Kapsammer and Ondina Cejka for administrative and technical assistance.
FUNDING
This is an investigator-initiated study that was financially supported by Vifor Fresenius Medical Care Renal Pharma, St. Gallen, Switzerland (Vifor Pharma). Vifor Pharma had no role in the study design, data collection, data interpretation or preparation of the manuscript.
AUTHORS’ CONTRIBUTIONS
U.T., M.C.H., A.P. and D.C. contributed to the conception and design of the study. U.T., I.T., B.R., E.W., S.B., A.D., R.M., A.P., M.C.H. and D.C. contributed to data acquisition. U.T., M.C.H., A.P. and D.C. were responsible for data analysis and interpretation. U.T. and D.C. drafted the manuscript. U.T., I.T., B.R., E.W., S.B., A.D., R.M., A.P., M.C.H. and D.C. were responsible for manuscript revision. All authors approved the final version of the submitted manuscript and all authors accept accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
CONFLICT OF INTEREST STATEMENT
D.C. received speaker’s honoraria and travel grants from Vifor Pharma. A.P. is an employee of Calciscon, Nidau, Switzerland and holds stock in this company. All other authors declare no conflicts of interest. Results presented in this article have not been published previously in whole or part, except in abstract format.
REFERENCES
- 1.Kidney Disease: Improving Global Outcomes CKD-MBD Update Working Group. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 2017; 7: 1–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Block GA, Kilpatrick RD, Lowe KA. et al. CKD–mineral and bone disorder and risk of death and cardiovascular hospitalization in patients on hemodialysis. Clin J Am Soc Nephrol 2013; 8: 2132–2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pasch A, Jahnen-Dechent W, Smith ER.. Phosphate, calcification in blood, and mineral stress: the physiologic blood mineral buffering system and its association with cardiovascular risk. Int J Nephrol 2018; 2018:9182078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Block GA, Hulbert-Shearon TE, Levin NW. et al. Association of serum phosphorus and calcium × phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis 1998; 31: 607–617 [DOI] [PubMed] [Google Scholar]
- 5. Block GA, Klassen PS, Lazarus JM. et al. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 2004; 15: 2208–2218 [DOI] [PubMed] [Google Scholar]
- 6. Tentori F, Blayney MJ, Albert JM. et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 2008; 52: 519–530 [DOI] [PubMed] [Google Scholar]
- 7. Fernandez-Martin JL, Martinez-Camblor P, Dionisi MP. et al. Improvement of mineral and bone metabolism markers is associated with better survival in haemodialysis patients: the COSMOS study. Nephrol Dial Transplant 2015; 30: 1542–1551 [DOI] [PubMed] [Google Scholar]
- 8. Lamina C, Kronenberg F, Stenvinkel P. et al. Association of changes in bone mineral parameters with mortality in haemodialysis patients: insights from the ARO cohort. Nephrol Dial Transplant2020; 35: 478–487. [DOI] [PubMed] [Google Scholar]
- 9. Sakaguchi Y, Fujii N, Shoji T. et al. Hypomagnesemia is a significant predictor of cardiovascular and non-cardiovascular mortality in patients undergoing hemodialysis. Kidney Int 2014; 85: 174–181 [DOI] [PubMed] [Google Scholar]
- 10. Lacson E Jr, Wang W, Ma L. et al. Serum magnesium and mortality in hemodialysis patients in the United States: a cohort study. Am J Kidney Dis 2015; 66: 1056–1066 [DOI] [PubMed] [Google Scholar]
- 11. Bommer J, Locatelli F, Satayathum S. et al. Association of predialysis serum bicarbonate levels with risk of mortality and hospitalization in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 2004; 44: 661–671 [PubMed] [Google Scholar]
- 12. Ketteler M, Bongartz P, Westenfeld R. et al. Association of low fetuin-A (AHSG) concentrations in serum with cardiovascular mortality in patients on dialysis: a cross-sectional study. Lancet 2003; 361: 827–833 [DOI] [PubMed] [Google Scholar]
- 13. Pasch A, Farese S, Graber S. et al. Nanoparticle-based test measures overall propensity for calcification in serum. J Am Soc Nephrol 2012; 23: 1744–1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Smith ER, Ford ML, Tomlinson LA. et al. Serum calcification propensity predicts all-cause mortality in predialysis CKD. J Am Soc Nephrol 2014; 25: 339–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bundy JD, Cai X, Mehta RC. et al. Serum calcification propensity and clinical events in CKD. Clin J Am Soc Nephrol 2019; 14: 1562–1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Keyzer CA, de Borst MH, van den Berg E. et al. Calcification propensity and survival among renal transplant recipients. J Am Soc Nephrol 2016; 27: 239–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dahle DO, Asberg A, Hartmann A. et al. Serum calcification propensity is a strong and independent determinant of cardiac and all-cause mortality in kidney transplant recipients. Am J Transplant 2016; 16: 204–212 [DOI] [PubMed] [Google Scholar]
- 18. Bostom A, Pasch A, Madsen T. et al. Serum calcification propensity and fetuin-a: biomarkers of cardiovascular disease in kidney transplant recipients. Am J Nephrol 2018; 48: 21–31 [DOI] [PubMed] [Google Scholar]
- 19. Pasch A, Block GA, Bachtler M. et al. Blood calcification propensity, cardiovascular events, and survival in patients receiving hemodialysis in the EVOLVE trial. Clin J Am Soc Nephrol 2017; 12: 315–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bielesz B, Reiter T, Marculescu R. et al. Calcification propensity of serum is independent of excretory renal function. Sci Rep 2017; 7: 17941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aigner C, Cejka D, Sliber C. et al. Oral sodium bicarbonate supplementation does not affect serum calcification propensity in patients with chronic kidney disease and chronic metabolic acidosis. Kidney Blood Press Res 2019; 44: 188–112 [DOI] [PubMed] [Google Scholar]
- 22. Wüthrich RP, Chonchol M, Covic A. et al. Randomized clinical trial of the iron-based phosphate binder PA21 in hemodialysis patients. Clin J Am Soc Nephrol 2013; 8: 280–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Floege J, Covic AC, Ketteler M. et al. A phase III study of the efficacy and safety of a novel iron-based phosphate binder in dialysis patients. Kidney Int 2014; 86: 638–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gupta SK. Intention-to-treat concept: a review. Perspect Clin Res 2011; 2: 109–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bressendorff I, Hansen D, Schou M. et al. Oral magnesium supplementation in chronic kidney disease stages 3 and 4: efficacy, safety, and effect on serum calcification propensity—a prospective randomized double-blinded placebo-controlled clinical trial. Kidney Int Rep 2017; 2: 380–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bressendorff I, Hansen D, Schou M. et al. The effect of increasing dialysate magnesium on serum calcification propensity in subjects with end stage kidney disease: a randomized, controlled clinical trial. Clin J Am Soc Nephrol 2018; 13: 1373–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lorenz G, Mayer CC, Bachmann Q. et al. Acetate-free, citrate-acidified bicarbonate dialysis improves serum calcification propensity—a preliminary study. Nephrol Dial Transplant2018; 33: 2043–2051 [DOI] [PubMed] [Google Scholar]
- 28. Chiu Y-W, Teitelbaum I, Misra M. et al. Pill burden, adherence, hyperphosphatemia, and quality of life in maintenance dialysis patients. Clin J Am Soc Nephrol 2009; 4: 1089–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ghimire S, Castelino RL, Lioufas NM. et al. Nonadherence to medication therapy in haemodialysis patients: a systematic review. PLoS One 2015; 10: e0144119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.